Abstract
To investigate the impact of total flavonoids of Epimedium (TFE) on the development of follicles of laying hens, 3 types of follicles including primary, prehierarchical, and preovulatory follicles were selected to obtain the follicular granulosa cells cultured in vitro. First, extraction of TFE was conducted by alcohol-soluble and ultrasonic methods. The effects of TFE on activity and proliferation of follicular granulosa cells were detected by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and measuring the expression of proliferating cell nuclear antigen mRNA through real-time quantitative polymerase chain reaction, and the expression of the follicle-stimulating hormone receptor, luteinizing hormone receptor, steroidogenic acute regulatory protein, and cytochrome P450 family 11 subfamily A member 1 mRNA was detected to study the functions of TFE affecting the differentiation and hormone secretion by granulosa cells. The results showed that TFE significantly improved the proliferation of 3 types of granulosa cells and promoted the differentiation of granulosa cells and accelerated the conversion of primary follicles to prehierarchical follicles. Total flavonoids of Epimedium played an important role in promoting progesterone secretion by prehierarchical and preovulatory granulosa cells. The results indicated that TFE could promote proliferation and differentiation of follicular granulosa cells and improve hormone secretion and follicle development, which provided reference data for TFE used as a feed additive or safe Chinese veterinary medicine to promote the laying rate.
Key words: total flavonoids of Epimedium, laying hen, follicular granulosa cell, proliferation and differentiation
Introduction
The cyclic recruitment of a follicle in the laying hen's ovary represents such a process that a single follicle is selected to enter the rapid growth phase and undergo final maturation before ovulation (Johnson and Lee, 2016). Primordial follicles in chicken ovary are activated and then develop into growing follicles to form primary, prehierarchical, and preovulatory follicles (Lin et al., 2019). The prehierarchical follicles contain small white follicles (2–4 mm in diameter), large white follicles (4–6 mm in diameter), and small yellow follicles (6–8 mm in diameter). Small white follicles start to accumulate yellow yolk and are selected and enter the preovulatory phase when the follicles grow rapidly and reach an enormous size (Lin et al., 2019) to form preovulatory follicules. Preovulatory follicles are arranged and maintained in a size hierarchy. A follicle in chicken ovary contains germ cells (oocyte) in the center and 2 types of somatic cells (theca cells and granulosa cells) in the outer layers. The outer thecal layer is usually separated from the inner granulosa cells by a basement membrane (van Montfoort et al., 2014). Synthesis of steroid hormones mainly occurs in theca cells and granulosa cells (Huang et al., 2013). Interaction exists between the 2 types of somatic cells and between the somatic cells and the oocyte until ovulation.
Synthesis of sex steroids by embryonic gonads in avian species is regulated by luteinizing hormone (LH) and follicle-stimulating hormone (FSH) (Grzegorzewska et al., 2009). Follicle-stimulating hormone is the main hormone promoting follicular growth, development, and maturation. The follicle-stimulating hormone receptor (FSHR) plays a central role in promoting follicle maturation through the FSH-mediated cAMP pathway in animals (Xia et al., 2020). Studies have revealed that there are FSHR gene mRNAs in prehierarchical follicles. When the expression level of FSHR is increased, it is an important marker for particle cell differentiation, selection of dominant follicles, and establishment of hierarchy (Johnson 2015; Ghanem and Johnson, 2018). When high levels of luteinizing hormone receptor (LHR) are expressed in prehierarchical follicles, the prehierarchical follicles are about to become preovulatory follicles, which induce synthesis of progesterone. The granulosa cells stimulated initially by FSH and next by LH start to express a steroidogenic acute regulatory protein (StAR) and cytochrome P450 family 11 subfamily A member 1 (CYP11A1) (Johnson and Bridgham, 2001; Johnson and Woods, 2009; Sechman et al., 2014). During the synthesis of progesterone, free cholesterol is transported into the inner membrane from the outer membrane of mitochondria by StAR (Stocco, 2001). Then, under the action of CYP11A1, cholesterol is converted into pregnenolone (Guo et al., 2007; Fiedler et al., 2008), and finally, pregnenolone is converted into progesterone under the action of 3β-hydroxysteroid dehydrogenase (Kazeto et al., 2003). Both StAR and CYP11A1 are expressed in prehierarchical follicular granulosa cells in chicken ovary.
The egg production rate is directly related to the economic benefits of farmers, and the residues of hormone drugs in eggs are directly related to human health, the Chinese herbal medicines were selected to improving laying rate for their safety and minor side effects.
Epimedium is a genus of about 52 species of plants in the family Berberidaceae (Ma et al., 2011). Epimedium is one of the most famous herbal resources frequently used as an aphrodisiac, tonic, and antirheumatic in China for over 2000 yr (Zhang et al., 2008, 2013). This herbal medicine is used in China, Japan, and Korea (Ma et al., 2011) for curing reproductive diseases both in male and female animals (Zhang et al., 2005). The main components of Epimedium include herba icariin, herba icariin B, herba icariin C, and total flavonoids (Liang et al., 2012; Zhang et al., 2013). In the Chinese pharmacopoeia, content of total flavonoids is considered an index of quality of Epimedium (Zhang et al., 2013).
Many plants of the Epimedium species have been proven to possess therapeutic efficacy on sexual dysfunction and osteoporosis (Zhang et al., 2013; Li et al., 2018). More than 260 compounds have been isolated from Epimedium; among them, total flavonoids are the major constituents possessing wide pharmacological properties, especially in strengthening Kidney Yang (enhancing reproductive function) (Ma et al., 2011). It is widely used for the treatment of impotence (Huang et al., 2013), osteoporosis (Xu et al., 2016), immunosuppression (Fan et al., 2015), cardiovascular diseases (Inokuchi et al., 1984; Zhang et al., 2005; Johnson and Woods, 2009), and cancer (Zhang et al., 2005). The total flavonoids of Epimedium (TFE) play a role such as that of estrogen in improving the coefficient of uterine weight and making the endometrium thick in ovariectomized mice (Wang et al., 2012). Total flavonoids of Epimedium effectively reduce DNA oxidative damage in the testis of aging rats (Zhao et al., 2017). Total flavonoids of Epimedium exert beneficially protective effects on the damage of the reproductive system male mice and reduce apoptosis in spermatogenic cells (Yuan et al., 2014). The extraction methods of TFE include ultrasonic extraction, microwave extraction, alkali solution extraction, hot water extraction, organic solvent extraction, and supercritical fluid extraction (Li et al., 2014). Compared with the hot water extraction method, ultrasonic extraction reduced extraction time, extraction temperature, and solvent consumption (Zhang et al., 2009).
According to the existing data, the most rapid proliferation period of follicular granulosa cells is the primary follicular stage. After reaching the prehierarchical follicular stage, the proliferation of granulosa cells in most follicles slows down (Sharum et al., 2017). The proliferating cell nuclear antigen (PCNA) and apoptosis regulator Bax gene expression had significant effects on primary follicular granulosa cells (Gupta et al., 2015; Dai et al., 2016). The previous studies demonstrated that TFE could improve the reproductive performance and promote the secretion of endogenous estrogen in laying hens (Huo et al., 2018); however, the mechanism of TFE promoting follicular development is unknown. To reveal the impacts of TFE on follicles, 3 types of granulosa cells of the ovary were cultured, and FSHR, LHR, StAR, and CYP11A1 genes were used as differentiation markers of prehierarchical and preovulatory follicular granulosa cells. The PCNA gene was used as a proliferation marker for primary follicular granulosa cells to reveal the functions of TFE on the development of granulosa cells. The research will provide reference data for TFE used as a feed additive or safe Chinese veterinary medicine to improve the egg production rate.
Materials and methods
Extraction and Determination of TFE
First, Epimedium was ground into powder, and then, 65% ethanol aqueous solution was added in the ratio of 1:30, and the resultant was left for 60 min at room temperature. The active components of Epimedium were extracted by the ultrasonic method using an ultrasonic purifying device operated at 200 W power at 60 °C for 60 min. The solution was filtered using a filter paper and then concentrated to 1 mg/mL using a rotary evaporator at 80°C. The concentrate was extracted using petroleum ether, and the sediment was preserved at 4°C.
The quantitative analysis of TFE was conducted by colorimetry at 496 nm, using rutin as the reference and aluminum nitrate as the chromogenic agent (Guo and Zhang, 2019). Two milligrams of rutin standard products was added with 25 mL of 95% ethanol and then diluted to 0.04 mg/mL, 0.02 mg/mL, 0.01 mg/mL, and 0.005 mg/mL. Total flavonoid content was determined by the spectrophotometric method using rutin as the standard sample. One milliliter of 5% sodium nitrite solution was added to all samples, and the resultant was shaken and left for 6 min at room temperature; then, 1.0 mL of 10% aluminum nitrate solution was added, and the resultant was shaken and placed for 6 min; finally, 10.0 mL of 4% sodium hydroxide solution was added, and the resultant was shaken and placed for 12 min. The solution without samples was used as the blank reference. The absorbance was measured at 510 nm.
Culture and Grouping of Follicular Granulosa Cells
This research on live animals complies with guidelines approved by the institutional animal care and use committee. Healthy 200-day-old Hy-Line brown laying hens (provided by Dingnong Corporation of Hebei, Baoding, China) were killed by cardiopuncturing. The ovaries were taken out under aseptic conditions; then, primary follicles (0.8–2 mm in diameter), prehierarchical follicles (6–8 mm in diameter), and preovulatory follicles (10–40 mm in diameter) were collected from hens based on their different sizes (Johnson and Woods, 2007; Lin et al., 2019). Layers of the granulosa cells in 3 types of follicles were separated from follicular theca in cold phosphate-buffered saline (HyClone; Gibco BRL, Bethesda, MD) using sterile needles. The granulosa cells were suspended in 0.1% collagenase II at 37°C for 30 min by gentle agitation in a constant-temperature shaker; then, serum-containing culture fluid was added to terminate the digestion and filtered using a 200-mesh sieve. After centrifugation, the granulosa cells were washed twice with a serum-free medium and then suspended in Dulbecco's Modified Eagle Medium (Gibco BRL, Bethesda, MD) with 10% fetal bovine serum and subsequently placed in Petri dishes or 96-well plates at a density of 1 × 106 cells/mL. The viable cells were estimated using trypan blue. The cells were cultured at 39°C in a water-saturated atmosphere of 95% air and 5% CO2. The granulosa cells of 3 types of follicles were divided into the control group and experimental groups. After 24 h of incubation in the culture medium, the cells were washed twice with a serum-free medium and then treated with different doses of TFE (10−2, 10−2.5, 10−3.5, 10−4.5,10−5, and 10−6 mg/mL) and human chorionic gonadotropin (hCG) (1 IU/mL) for 24 h. At the end of incubation, the granulosa cells and supernatant fraction were collected from the plates and used for polymerase chain reaction (PCR) and determination of progesterone levels.
Growth and Viability of Granulosa Cells
Granulosa cells from the primary follicles (1 × 106 cells/mL) were placed in 96-well culture plates. After 24 h of incubation in Dulbecco's Modified Eagle Medium with 10% fetal bovine serum, the culture medium was changed with phenol red–free and serum-free medium; the control group was treated with phosphate-buffered saline, and the other groups were treated with different doses of TFE for 24 h. At the end of incubation, the morphology and growth condition of granulosa cells in different groups were observed under inverted light microcopy; then, the culture medium was filtered out, and the granulosa cells were treated with 5 mg/ml of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide at a density of 20 μl/well for 4 h; then, 180 μL of dimethyl sulphoxide was added, and the resultant was incubated at 37°C in a constant-temperature shaker for 30 min. The absorbance of the converted dye was measured at a wavelength of 570 nm, with a background subtraction at 650 nm (Wang et al., 2010).
Granulosa cells (1 × 106 cells/mL) from the primary, prehierarchical, and preovulatory follicles were treated with TFE. After 12 h of incubation with the culture medium, the cells were observed and photographed using an inverted microscope and then were detached from the Petri dish and counted using a blood count board.
Expression of PCNA, FSHR, LHR, StAR, and CYP11A1 mRNA in Granulosa Cells
Granulosa cells were scraped from culture dishes at the end of incubation. The total RNA was extracted using the Trizol reagent of a commercial RNA assay kit obtained from Invitrogen (Carlsbad, California), according to the manufacturer's instructions. One millileter of Trizol reagent and 200 μl of chloroform were added to granulosa cells with sufficient mixing; after standing on ice for 10 min, the mixture was centrifuged at 12,000 × g for 15 min at 4°C. An equal volume of isopropanol was added to the supernatant and centrifuged at 12,000 × g for 15 min at 4°C. The precipitate was washed using 1 mL of 75% ethanol and centrifuged at 10,000 × g for 5 min at 4°C. Twenty microliter of RNase-free water was added to dissolve the total RNA, and then, the total RNA concentration was measured using an ultraviolet spectrophotometer. Reverse transcription was performed using 25 μL of the reaction mixtures containing 10 μL of total RNA extraction solution, 2 μL of Olig (dT), 2 μL of RNase inhibitor, 5 μL of dNTPs, 5 μL of 5× M-MLV buffer, and 1 μL of M-MLV reverse transcriptase, and cDNA was synthesized at 42°C for 1 h.
Expression of FSHR and LHR was detected by reverse transcription PCR. The process was performed using 25 μL of the reaction mixtures containing 2 μL of cDNA, 0.5 μL of forward and reverse primer (Sangon Biotech [Shanghai] company, Shanghai, China, see Table 1), 10 × 2.5 μL of buffer, 2 μL of dNTPs, 0.5 μL of Taqase, and 17 μL of H2O. The PCR reaction process of this study was conducted under the following cycling conditions: predenaturation at 94°C for 2 min; amplification for 35 cycles with denaturation at 94°C for 1 min; annealing at the same temperature for 1 min; extension at 65°C for 1 min; followed by a final extension at 72°C for 5 min (Joensuu, 1990; Mowa and Iwanaga, 2000). Then, in accordance with the conventional method, the PCR products were electrophoresed and photographed using a gelatin imager, and the results were observed and calculated to determine the relative value of the target band to beta-actin using an AlphaImager 2200 device acquired from Alpha Innotech Corporation of San Leandro, California.
Table 1.
Primers used for detection of the follicle-stimulating hormone receptor (FSHR) and luteinizing hormone receptor (LHR) gene by reverse transcription polymerase chain reaction.
| Gene | Primer sequences | Amplicon size (bp) | Annealing temperature (°C) | Accession number |
|---|---|---|---|---|
| β-actin-F1 | ACGTCGCACTGGATTTCGAG | 282 | 58 | NM_205518.1 |
| β-actin-R2 | TGTCAGCAATGCCAGGGTAC | |||
| FSHR-F3 | AAGAGCGAGGTCTACATACA | 414 | 52 | XM_025148544.1 |
| FSHR-R4 | GTGGTGTTCCCAGTGATAG | |||
| LHR-F5 | GCTGCTCATTGCTTCGG | 713 | 54 | U92082.1 |
| LHR-R6 | GCTCTGCTCGGCTCTTAC |
Refers to the forward primer and reverse primer of beta-actin (β-actin, a housekeeping gene used as a control for normalization).
Indicates the forward primer and reverse primer of the FSHR.
Indicates the forward primer and reverse primer of the LHR.
Expression of PCNA, StAR, and CYP11A1 was detected by real-time quantitative PCR (q-PCR). The q-PCR was conducted using a fluorescence ration PCR instrument (CFX96, Bio-Rad, CA). The q-PCR reaction process was performed using 25 μL of the reaction mixtures containing 12.5 μL of 2× M5 Hiper SYBR Premix Es Taq (Mei5 Biotechnology, Beijing, China), 0.5 μL of each forward and reverse primer (Table 2), 2 μL of cDNA, and 9.5 μL of ddH2O, according to the following program: 95°C for 3 min; 95°C for 5 s, and 60°C for 30 s for 40 cycles. Melting curves were used to confirm the specificity of each product; the PCR efficiency was close to 100%, allowing the use of the 2−ΔΔCt method for the calculation of relative gene expression levels (Zhao et al., 2018). All samples were amplified in triplicate, and the data were normalized to glyceraldehyde phosphate dehydrogenase expression.
Table 2.
Primers used for detection of proliferating cell nuclear antigen (PCNA), steroidogenic acute regulatory protein (StAR), and cytochrome P450 family 11 subfamily A member 1 (CYP11A1) gene by real-time quantitative polymerase chain reaction.
| Gene | Primer sequences | Amplicon size (bp) | Annealing temperature (°C) | Accession number |
|---|---|---|---|---|
| GAPDH-F1 | ACGTCGCACTGGATTTCGAG | 82 | 60 | NM_204305 |
| GAPDH-R2 | TGTCAGCAATGCCAGGGTAC | |||
| PCNA-F3 | GCAGATGTTCCTCTCGTTGTGGAG | 95 | 60 | NM_204170.2 |
| PCNA-R4 | GAGCCTTCCTGCTGGTCTTCAATC | |||
| StAR-F5 | CGCTGCCATCTCCTACCAACAC | 197 | 60 | NM_204686.2 |
| StAR-R6 | AGGACATCTCCATCTCGCTGAAGG | |||
| CYP11A1-F7 | CCGCCACCTCAACACCAAGAC | 157 | 60 | NM_001001756.1 |
| CYP11A1-R8 | CACAAGGAGGCTGAAGAGGATGC |
Refers to the forward primer and reverse primer glyceraldehyde phosphate dehydrogenase (GAPDH; a housekeeping gene used as a control for normalization).
Indicates the forward primer and reverse primer of PCNA.
Indicates the forward primer and reverse primer of StAR.
Indicates the forward primer and reverse primer of CYP11A1.
Determination of Progesterone Secreted by Granulosa Cells
A volume of 0.1 mL of the culture medium from each group was taken from 60 × 60 cell culture dishes at different times (1, 4, 8, and 12 h) after 1% TFE was added and stored at −20°C for detection. Progesterone hormone was detected using the enzyme immunoassay kit of chicken progesterone (MLBIO, Shanghai, China) in the enzyme-linked immunosorbent assay instrument. All procedures were conducted according to the manufacturer's protocol.
Data Analysis
All the experiments were repeated at least 3 times, and the results were expressed as means ± SE. Statistical analyses were performed using the SPSS software package version 11.5 (SPSS Inc., Chicago, IL). All data were analyzed using one-way analysis of variance to determine the differences among the groups. In the study, the groups were considered significantly difference if P < 0.05.
Results
Standard Curve of Rutin and Concentration of Total Flavonoids
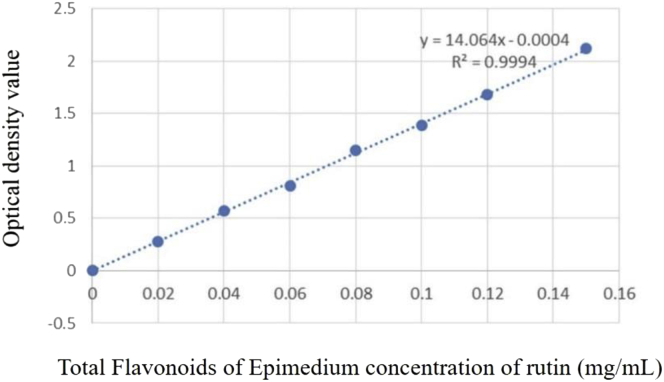
According to the relation curves of optical density and TFE concentration of rutin (Figure 1), the linear regression equation of absorbance y and concentration x is y = 14.064x + 0.0004, R2 = 0.9994. The average optical density value of Epimedium-extracted solution was brought into the linear regression equation to calculate the concentration. The result was 16.5 ± 0.32 mg/mL.
Figure 1.
The relation curves of optical density value and total flavonoids of Epimedium concentration of rutin. The drafting of total flavonoids of Epimedium standard curves was using rutin as the standard sample; the absorbance measurement was determined at 496 nm.
Effect of Different Concentration of TFE on the Viability of Primary Follicular Granulosa Cells
The effect of different doses of TFE on the viability of primary follicular granulosa cells was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. The viability of granulosa cells was improved with increase in the concentration of TFE. The most effective concentration of TFE acting on granulosa cell proliferation was 5 × 10−3 mg/mL (Table 3).
Table 3.
Effects of different concentrations of total flavonoids of Epimedium on the viability of primary follicular granulosa cells.
| Total flavonoids of Epimedium concentration (mg/mL) | Optical density value |
|---|---|
| Control (0) | 0.42 ± 0.024A,a |
| 10−6 | 0.40 ± 0.012A,a |
| 10−5 | 0.49 ± 0.014A,b |
| 10−4.5 | 0.58 ± 0.003B,c |
| 10−3.5 | 0.70 ± 0.014C |
| 10−2.5 | 1.17 ± 0.0152D |
| 10−2 | 1.08 ± 0.008E |
a,b,cIndicates significant differences (P <0.05) (n = 3).
A,B,C,D,EMeans extremely significant differences (P <0.01) (n = 3).
Effects of TFE on the Proliferation of Three Types of Granulosa Cells
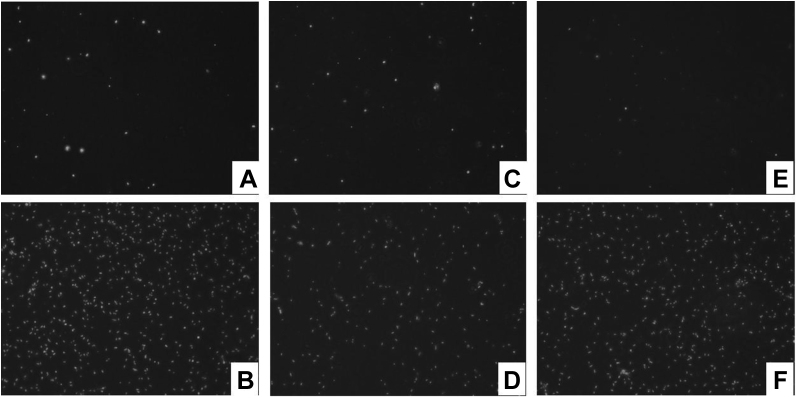
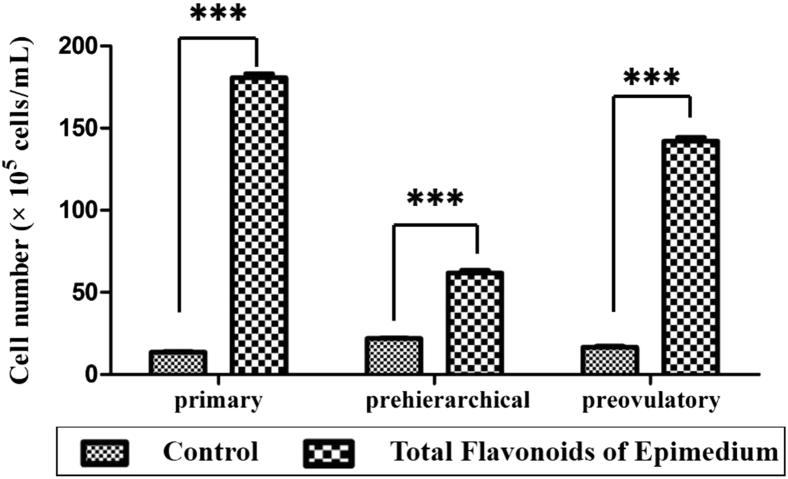
Granulosa cells from the primary, prehierarchical, and preovulatory follicles were treated with TFE (5 × 10−3 mg/mL) for 12 h. The proliferation of the 3 types of follicular granulosa cells was significantly enhanced compared with the control group (P < 0.001). The results proved the actions of TFE enhancing the proliferation of granulosa cells (Figures 2, 3).
Figure 2.
The effects of total flavonoids of Epimedium on the proliferation of granulosa cells from primary, prehierarchical and preovulatory follicles. (A, B) Control and experimental group of primary follicular granulosa cells; (C, D) control and experimental group of prehierarchical follicular granulosa cells; (E, F) control and experimental group of preovulatory follicular granulosa cells.
Figure 3.
The effects of total flavonoids of Epimedium on the proliferation of granulosa cells from primary, prehierarchical, and preovulatory follicles (n = 3). The proliferation of the 3 types of follicular granulosa cells extremely significant differences (∗∗∗P < 0.001). The control group was treated with phosphate-buffered saline. The total flavonoids of Epimedium group was treated with total flavonoids of Epimedium (5 × 10−3 mg/mL, diluted by phosphate-buffered saline).
Effects of TFE on the Expression of FSHR and LHR mRNA in Follicular Granulosa Cells From Prehierarchical and Preovulatory Follicles
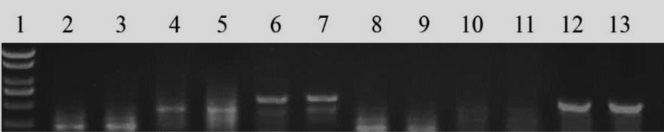
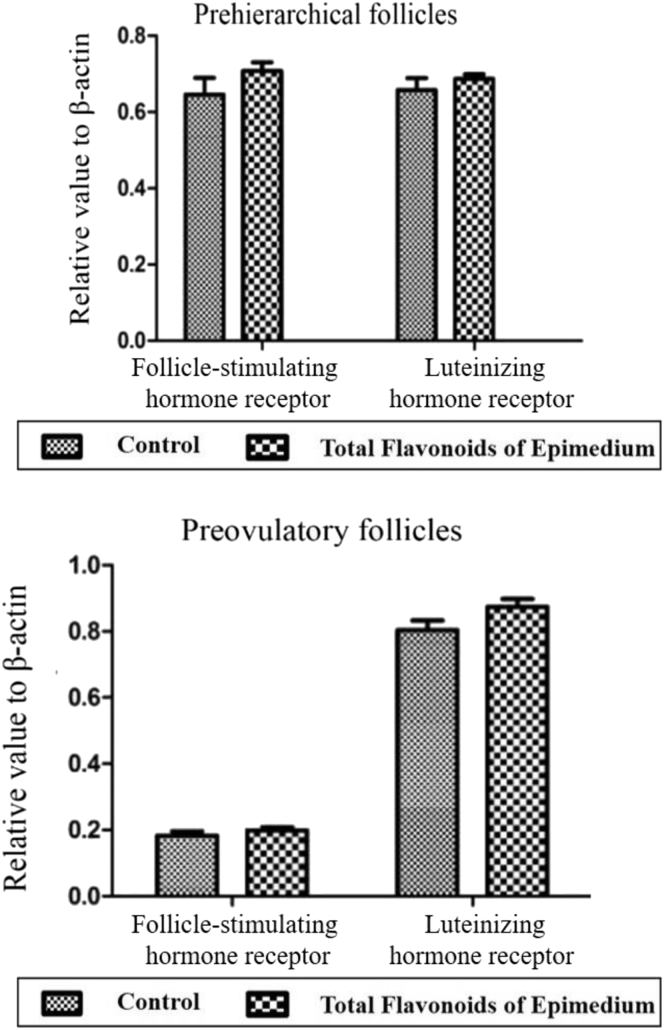
The results showed that TFE had no effects on the expression of FSHR and LHR mRNA of granulosa cells both in prehierarchical follicles and preovulatory follicles (Figures 4, 5), although the expression of FSHR mRNA reduced from prehierarchical follicles to preovulatory follicles. The results implied that effects on follicular granulosa cells were not dependent on the expression of FSHR and LHR.
Figure 4.
The expression of follicle-stimulating hormone receptor and luteinizing hormone receptor mRNA in prehierarchical and preovulatory follicular granulosa cells by reverse transcription polymerase chain reaction. 1DL2000 marker; 2, 3polymerase chain reaction products of beta-actin in prehierarchical follicles of the control and experimental group; 4, 5polymerase chain reaction products of the follicle-stimulating hormone receptor in prehierarchical follicles of the control and experimental group; 6, 7polymerase chain reaction products of the luteinizing hormone receptor in prehierarchical follicles of the control and experimental group; 8, 9polymerase chain reaction products of beta-actin in preovulatory follicles of the control and experimental group; 10, 11polymerase chain reaction products of the follicle-stimulating hormone receptor in preovulatory follicles of the control and experimental group; 12, 13polymerase chain reaction products of the luteinizing hormone receptor in preovulatory follicles of the control and experimental group.
Figure 5.
The expression of follicle-stimulating hormone receptor and luteinizing hormone receptor mRNA in prehierarchical and preovulatory follicular granulosa cells by reverse transcription polymerase chain reaction. No asterisk indicates no significant differences between the control and total flavonoids of Epimedium group (P > 0.05) (n = 3). Expression of follicle-stimulating hormone receptor and luteinizing hormone receptor mRNA of granulosa cells in prehierarchical follicles and preovulatory follicles.
Effects of TFE on the Expression of PCNA, StAR, and CYP11A1 mRNA in Granulosa Cells
The results showed that the relative value of PCNA mRNA to glyceraldehyde phosphate dehydrogenase of the TFE group was higher than that of the control group in granulosa cells of primary follicles (P < 0.01) (Table 4), the expression of StAR mRNA of the TFE group was significantly improved both in prehierarchical and preovulatory follicular granulosa cells, and the expression of CYP11A1 mRNA was also extremely improved in the TFE group (P < 0.01) (Table 5). The results indicated that TFE could improve the proliferation of primary follicular granulosa cells and improve the differentiation of granulosa cells in prehierarchical and preovulatory follicles.
Table 4.
Effects of total flavonoids of Epimedium on the expression of proliferating cell nuclear antigen (PCNA), steroidogenic acute regulatory protein (StAR), and cytochrome P450 family 11 subfamily A member 1 (CYP11A1) mRNA in primary follicular granulosa cells.
| Gene | Control | Total flavonoids of Epimedium |
|---|---|---|
| PCNA | 1.00 ± 0.10A | 2.17 ± 0.13B |
| StAR | 1.00 ± 0.09A | 2.71 ± 0.10B |
| CYP11A1 | 1.01 ± 0.14A | 2.80 ± 0.44B |
A,BMeans extremely significant differences (P < 0.01) (n = 3).
Table 5.
Effects of total flavonoids of Epimedium on the expression of steroidogenic acute regulatory protein (StAR) and cytochrome P450 family 11 subfamily A member 1 (CYP11A1) mRNA in prehierarchical follicular granulosa cells.
| Gene | Control | Total flavonoids of Epimedium |
|---|---|---|
| StAR | 1.00 ± 0.07a | 1.52 ± 0.11b |
| CYP11A1 | 1.04 ± 0.22A | 2.10 ± 0.23B |
a,bIndicates significant differences (P < 0.05), A, B means extremely significant differences (P < 0.01) (n = 3).
Effects of TFE on the Progesterone Secretion by Granulosa Cells
The results showed that TFE and hCG had no effects on the progesterone secretion by granulosa cells of primary follicles; there was no significant difference in the progesterone concentration in the control, TFE, and hCG groups after TFE was added at 1 h, 4 h, 8 h, and 12 h (P > 0.05) (Table 6). For prehierarchical follicles, the level of progesterone concentration in the TFE group was higher than that in the control group after TFE was added at 1 h (P < 0.05) and extremely improved after TFE was added at 8 h and 12 h (P < 0.01). The progesterone level in the hCG group was higher than that in the control group after TFE was added at 1 h (P < 0.05), but there were no significant differences when compared with the control group after hCG was added at 4 h, 8 h, and 12 h (P > 0.05) (Table 7). In the preovulatory follicles, the progesterone concentration in the TFE group was no different after TFE was added at 1 h, was higher at 4 h and 8 h (P < 0.05), and extremely improved after TFE was added at 12 h (P < 0.01). The level of progesterone in prehierarchical follicles from the hCG group showed significant difference when compared with the control group after 1 h (P < 0.05) and remarkable difference after 12 h (P < 0.01) (Table 8). These results implied that TFE could enhance the progesterone secretion by granulosa cells in prehierarchical and preovulatory follicles and could not affect the progesterone secretion by primary follicular granulosa cells.
Table 6.
Progesterone concentration of primary follicular granulosa cells.
| Groups | Concentration of progesterone (Pmol/L) |
|||
|---|---|---|---|---|
| 1 h | 4 h | 8 h | 12 h | |
| Control1 | 48.9 ± 1.15 | 61.1 ± 2.92 | 61.3 ± 6.06 | 57.6 ± 5.54 |
| Total flavonoids of Epimedium2 | 57.3 ± 5.86 | 58.4 ± 0.28 | 65.6 ± 2.90 | 65.2 ± 2.40 |
| Human chorionic gonadotropin3 | 47.9 ± 1.15 | 51.9 ± 4.04 | 62.3 ± 0.87 | 56.3 ± 3.71 |
No superscript letter indicates no significant differences in the control, total flavonoids of Epimedium, and human chorionic gonadotropin group (P > 0.05) (n = 3).
The control group was treated with phosphate-buffered saline.
The total flavonoids of Epimedium group was treated with total flavonoids of Epimedium at the dose of 5 × 10−3 mg/mL.
The human chorionic gonadotropin group was treated with human chorionic gonadotropin at the dose of 1 IU/mL.
Table 7.
Progesterone concentration of prehierarchical follicular granulosa cells.
| Groups | Concentration of progesterone (Pmol/L) |
|||
|---|---|---|---|---|
| 1 h | 4 h | 8 h | 12 h | |
| Control | 57.6 ± 5.27a | 71.3 ± 3.75a | 83.3 ± 3.75A | 94.6 ± 3.47A |
| Total flavonoids of Epimedium | 78.3 ± 0.87b | 106.3 ± 8.94b | 118.3 ± 3.17B | 127.6 ± 1.75B |
| Human chorionic gonadotropin | 71.6 ± 1.19b | 78.6 ± 8.08a | 93.6 ± 2.32A | 95.6 ± 3.47A |
a,b,cIndicates significant differences in the control, total flavonoids of Epimedium, and human chorionic gonadotropin group (P < 0.05).
A,B,CIndicates extremely significant differences in the control, total flavonoids of Epimedium, and human chorionic gonadotropin group (P < 0.01) (n = 3).
Table 8.
Progesterone concentration of preovulatory follicular granulosa cells.
| Groups | Concentration of progesterone (Pmol/L) |
|||
|---|---|---|---|---|
| 1 h | 4 h | 8 h | 12 h | |
| Control | 75.9 ± 1.15a | 79.3 ± 4.90a | 94.2 ± 8.99a | 95.6 ± 7.51A |
| Total flavonoids of Epimedium | 71.9 ± 4.04a | 94.3 ± 6.06b | 120.6 ± 6.08b | 147.9 ± 5.19B |
| Human chorionic gonadotropin | 84.9 ± 1.15b | 95.3 ± 8.25b | 101.6 ± 3.47c | 176.6 ± 5.20C |
a,b,cIndicates significant differences in the control, total flavonoids of Epimedium, and human chorionic gonadotropin group (P < 0.05).
A,B,CIndicates extremely significant differences in the control, total flavonoids of Epimedium, and human chorionic gonadotropin group (P < 0.01) (n = 3).
Discussion
In the present study, the improvement in impact of TFE on the proliferation and differentiation of follicular granulosa cells was verified in laying hens. The results indicate that the main cause of TFE increasing the egg laying rates is associated with improving the development of follicular granulosa cells.
In the study, TFE was obtained from Epimedium by soaking in ethanol, extracted by the ultrasonic method, filtered using petroleum ether, and concentrated by decompression (Guo and Zhang, 2019). The TFE extracted from Epimedium in this study was a dark yellow crystalline powder, which is difficult to dissolve in water and easy to dissolve in ethanol. Using the above method to extract TFE, the extraction rate is 4.15%.
The development of granulosa cells in follicles is very important for follicular growth and choosing of dominant follicles (Schmidt et al., 2004). The effects of different doses of TFE on the activity of primary follicular granulosa cells were detected, and the most effective concentration of TFE was found to be 5 × 10−3 mg/mL. Interestingly, using the concentration of TFE acting on the granulosa cells from primary, prehierarchical, and preovulatory follicles, we found that the proliferation of 3 types of granulosa cells was all improved significantly (P < 0.01). Proliferating cell nuclear antigen is an essential component of DNA replication, cell cycle regulation, and epigenetic inheritance (Dai et al., 2016). In the present study, TFE enhanced the expression of PCNA mRNA in primary follicular granulosa cells. The study indicates that estradiol enhances PCNA mRNA expression in human breast cancer MCF7 cells (Wang et al., 2008). The authors of the present study speculate that some components of TFE might have estradiol-like functions of promoting the proliferation of granulosa cells, which needs more investigations for verification.
The differentiation of granulosa cells and progesterone secretion is connected with the expression of LHR, FSHR, StAR, and CYP11A1 (Palermo, 2007). Granulosa cells from primary follicles without differentiation have no such function of secreting progesterone (Wang, 2017). The results of the present study demonstrated that both TFE and hCG did not affect progesterone secretion by primary follicular granulosa cells. To investigate the effects of TFE on the differentiation of granulosa cells, the expression of LHR, FSHR, StAR, and CYP11A1 was detected in prehierarchical and preovulatory follicular granulosa cells. In the gonadotrophin-dependent stage of follicular development, FSH and LH signaling pathways play an obligatory role in follicle differentiation, selection, and survival (Palermo, 2007). Follicle-stimulating hormone induces prehierarchical follicular selection of some follicles for the next development. The increase in plasma FSH levels during luteal–follicular transition is the basis for follicle selection (Palermo, 2007). Luteinizing hormone causes follicular ovulation and promotes the formation of the corpus luteum on the basis of FSH. In the present study, the novel thing was that TFE significantly enhanced progesterone secretion by prehierarchical and preovulatory follicular granulosa cells, but did not affect the expression of FSHR and LHR (P > 0.05). From the results, we postulated that TFE acted on the progesterone secretion by prehierarchical and preovulatory follicular granulosa cells, which was independent of FSHR and LHR mediation.
The granulosa cells stimulated initially by FSH and next by LH start to express StAR and P450scc/CYP11A1 (Johnson and Bridgham, 2001; Johnson and Woods, 2009; Sechman et al., 2014). Therefore, LH, StAR, and CYP11A1 combine to promote progesterone secretion. In the primary follicles, progesterone was basically absent, and TFE did not affect progesterone secretion, whereas TFE stimulated the proliferation of granulosa cells in this period. The authors found that both in the prehierarchical and preovulatory follicular granulosa cells, TFE extremely improved the expression of StAR and CYP11A1 mRNA (P < 0.01). In the prehierarchical follicles, the effects of TFE on progesterone secretion by granulosa cells were stronger than those of hCG. In the preovulatory follicles, TFE enhanced progesterone secretion by granulosa cells such as hCG. The results coincided with the speculation that TFE acted on the granulosa cells independent of FSHR and LHR mediation. The prehierarchical follicular granulosa cells are yet fully differentiated; the addition of TFE accelerates the differentiation, increases the expression of LHR, CYP11A1, and StAR, and promotes the secretion of progesterone. Preovulatory follicular granulosa cells are completely differentiated and can autonomously secrete progesterone, so the effect of promotion is not as obvious as that of prehierarchical follicular granulosa cells.
Total flavonoids of Epimedium can effectively promote the proliferation of granulosa cells in laying hen's follicles, and the optimal concentration is 5 × 10−3 mg/mL. Moreover, TFE can promote granulosa cell differentiation and progesterone secretion.
Acknowledgments
This project was financially supported by Fund for Scientific Research and Development of Agricultural University of Hebei, project no: JY2018005.
Conflict of Interest Statement: The authors declare there is no conflict of interest with regard to publication of this manuscript.
References
- Dai M., Jiang S., Yuan X., Yang W., Yang Z., Huang L. Effects of zearalenone-diet on expression of ghrelin and PCNA genes in ovaries of post-weaning piglets. Anim. Reprod. Sci. 2016;168:126–137. doi: 10.1016/j.anireprosci.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Fan Y., Ren M., Hou W., Guo C., Tong D., Ma L., Zhang W., He M., Song X. The activation of Epimedium polysaccharide-propolis flavone liposome on Kupffer cells. Carbohydr. Polym. 2015;133:613–623. doi: 10.1016/j.carbpol.2015.07.044. [DOI] [PubMed] [Google Scholar]
- Fiedler S.D., Carletti M.Z., Hong X., Christenson L.K. Hormonal regulation of MicroRNA expression in periovulatory mouse mural granulosa cells. Biol. Reprod. 2008;79:1030–1037. doi: 10.1095/biolreprod.108.069690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanem K., Johnson A.L. Follicle dynamics and granulosa cell differentiation in the Turkey hen ovary. Poult. Sci. 2018;97:3755–3761. doi: 10.3382/ps/pey224. [DOI] [PubMed] [Google Scholar]
- Grzegorzewska A.K., Sechman A., Paczoska-Eliasiewicz H.E., Rząsa J. The expression of pituitary FSHβ and LHβ mRNA and gonadal FSH and LH receptor mRNA in the chicken embryo. Biol. Reprod. 2009;9:253–269. doi: 10.1016/s1642-431x(12)60030-8. [DOI] [PubMed] [Google Scholar]
- Guo H., Zhang Y. Determination of total flavonoids content in wuling capsules by ultraviolet-visible spectrophotometry. J. Guangzhou Univ. Tradit Chin Med. 2019;36:1074–1079. (in Chinese) [Google Scholar]
- Guo I.C., Shih M.C., Lan H.C., Hsu N.C., Hu M.C., Chung B.C. Transcriptional regulation of human CYP11A1 in gonads and adrenals. J. Biomed. Sci. 2007;14:509–515. doi: 10.1007/s11373-007-9177-z. [DOI] [PubMed] [Google Scholar]
- Gupta M., Dangi S.S., Singh G., Sarkar M. Expression and localization of ghrelin and its receptor in ovarian follicles during different stages of development and the modulatory effect of ghrelin on granulosa cells function in buffalo. Gen. Comp. Endocrinol. 2015;210:87–95. doi: 10.1016/j.ygcen.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Huang D., Yang J., Lu X., Deng Y., Xiong Z., Li F. An integrated plasma and urinary metabonomic study using UHPLC–MS: Intervention effects of Epimedium koreanum on ‘Kidney-Yang Deficiency syndrome’ rats. J. Pharm. Biomed. Anal. 2013;76:200–206. doi: 10.1016/j.jpba.2012.12.022. [DOI] [PubMed] [Google Scholar]
- Huo S., Kang J., Li Y., Wu X. Effects of "YI MU SAN" on fallopian tube repair and egg laying performance of laying hens. China Poult. 2018;40:53–56. (in Chinese) [Google Scholar]
- Inokuchi J., Okabe H., Yamauchi T., Nagamatsu A. Inhibitors of Angiotensin converting enzyme in Crude drugs. Chem. Pharm. Bull. (Tokyo) 1984;32:3615–3619. doi: 10.1248/cpb.32.3615. [DOI] [PubMed] [Google Scholar]
- Joensuu T.K. Chick oviduct differentiation. The effect of estrogen and progesterone on the expression of progesterone receptor. Cell Differ Dev. 1990;30:207–218. doi: 10.1016/0922-3371(90)90140-r. [DOI] [PubMed] [Google Scholar]
- Johnson A.L. Ovarian follicle selection and granulosa cell differentiation. Poult. Sci. 2015;94:781–785. doi: 10.3382/ps/peu008. [DOI] [PubMed] [Google Scholar]
- Johnson A.L., Bridgham J.T. Regulation of steroidogenic acute regulatory protein and luteinizing hormone receptor messenger ribonucleic acid in hen granulosa cells. Endocrinology. 2001;142:3116–3124. doi: 10.1210/endo.142.7.8240. [DOI] [PubMed] [Google Scholar]
- Johnson A.L., Woods D.C. Reproductive Biology and Phylogeny of Aves. Science publishers, inc.; 2007. Chapter 6. Ovarian dynamics and follicle development; pp. 243–277. [Google Scholar]
- Johnson A.L., Woods D.C. Dynamics of avian ovarian follicle development: Cellular mechanisms of granulosa cell differentiation. Gen. Comp. Endocrinol. 2009;163:12–17. doi: 10.1016/j.ygcen.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Johnson A.L., Lee J. Granulosa cell Responsiveness to follicle stimulating hormone during early growth of hen ovarian follicles. Poult. Sci. 2016;95:108–114. doi: 10.3382/ps/pev318. [DOI] [PubMed] [Google Scholar]
- Kazeto Y., Ijiri S., Matsubara H., Adachi S., Yamauchi K. Molecular cloning and characterization of 3beta-hydroxysteroid dehydrogenase/Delta5-Delta4 isomerase cDNAs from Japanese eel ovary. J. Steroid Biochem. Mol. Biol. 2003;85:49–56. doi: 10.1016/s0960-0760(03)00138-9. [DOI] [PubMed] [Google Scholar]
- Li B., Zhang N., Wang D.X., Jiao L., Tan Y., Wang J., Li H., Wu W., Jiang D.C. Structural analysis and antioxidant activities of neutral polysaccharide isolated from Epimedium koreanum Nakai. Carbohydr. Polym. 2018;196:246–253. doi: 10.1016/j.carbpol.2018.05.037. [DOI] [PubMed] [Google Scholar]
- Li Q., Li X., Wei Y., Fan Y., Chen L., Zhang H., Zhao T. Extraction of total flavonoid from herba Epimedii Maxim using alkali solution. Nat. Prod. Res. Dev. 2014;26:1141–1144. (in Chinese) [Google Scholar]
- Liang Q., Wei G., Chen J., Wang Y., Huang H. Variation of medicinal components in a Unique Geographical Accession of Horny Goat weed epimedium sagittatum Maxim. (Berberidaceae) Molecules. 2012;17:13345–13356. doi: 10.3390/molecules171113345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Ma Y., Qian T., Yao J., Mi Y., Zhang C. Basic fibroblast growth factor promotes prehierarchical follicle growth and yolk deposition in the chicken. Theriogenology. 2019;139:90–97. doi: 10.1016/j.theriogenology.2019.07.025. [DOI] [PubMed] [Google Scholar]
- Ma H., He X., Yang Y., Li M., Hao D., Jia Z. The genus Epimedium: an ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2011;134:519–541. doi: 10.1016/j.jep.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Mowa C.N., Iwanaga T. Developmental changes of the oestrogen receptor-alpha and -beta mRNAs in the female reproductive organ of the rat--an analysis by in situ hybridization. J. Endocrinol. 2000;167:363–369. doi: 10.1677/joe.0.1670363. [DOI] [PubMed] [Google Scholar]
- Palermo R. Differential actions of FSH and LH during folliculogenesis. Reprod. Biomed. Online. 2007;15:326–337. doi: 10.1016/s1472-6483(10)60347-1. [DOI] [PubMed] [Google Scholar]
- Schmidt D., Ovitt C.E., Anlag K., Fehsenfeld S., Gredsted L., Treier A.C., Treier M. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development. 2004;131:933–942. doi: 10.1242/dev.00969. [DOI] [PubMed] [Google Scholar]
- Sechman A., Antos P., Katarzyńska D., Grzegorzewska A., Wojtysiak D., Hrabia A. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on secretion of steroids and STAR, HSD3B and CYP19A1 mRNA expression in chicken ovarian follicles. Toxicol. Lett. 2014;225:264–274. doi: 10.1016/j.toxlet.2013.12.021. [DOI] [PubMed] [Google Scholar]
- Sharum I.B., Granados-Aparici S., Warrander F.C., Tournant F.P., Fenwick M.A. Serine threonine kinase receptor associated protein regulates early follicle development in the mouse ovary. Reproduction. 2017;153:221–231. doi: 10.1530/REP-16-0612. [DOI] [PubMed] [Google Scholar]
- Stocco D.M. StAR protein and the regulation of steroid hormone Biosynthesis. Annu. Rev. Psychol. 2001;63:193–213. doi: 10.1146/annurev.physiol.63.1.193. [DOI] [PubMed] [Google Scholar]
- van Montfoort A.P., Plösch T., Hoek A., Tietge U.J. Impact of maternal cholesterol metabolism on ovarian follicle development and fertility. J. Reprod. Immunol. 2014;104-105:32–36. doi: 10.1016/j.jri.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Wang C., Yu J., Kallen C.B. Two estrogen response element sequences near the PCNA gene are not responsible for its estrogen-enhanced expression in MCF7 cells. PLoS One. 2008;3:e3523. doi: 10.1371/journal.pone.0003523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Chen D., Luo H., Liu J., Ji X., Fan J., Cui S. Low-dose ethanol suppresses 17beta-estradiol activity in GH4C1 pituitary tumor cells. Cell Biol Toxicol. 2010;26:265–277. doi: 10.1007/s10565-009-9129-7. [DOI] [PubMed] [Google Scholar]
- Wang J. Huazhong Agric Univ; Doctor: 2017. Study on the Molecular Mechanism of FOXL2 in Chicken Granulosa Cell Differentiation. (in Chinese) [Google Scholar]
- Wang j., Zhang G., Ren G. Effect of epimedin C and icariin on uterus of ovariectomized mice. China J. Clin Pharmacol. 2012;28 (in Chinese) [Google Scholar]
- Xia Y., Wang Q., He X.D., Chen Y., JiGe M.T., Zi X.D. Cloning and expression analysis of follicle-stimulating receptor (FSHR) gene in the reproductive axis of female yaks (Bos grunniens) Domest. Anim. Endocrinol. 2020;70:106383. doi: 10.1016/j.domaniend.2019.07.011. [DOI] [PubMed] [Google Scholar]
- Xu F., Ding Y., Guo Y., Liu B., Kou Z., Xiao W., Zhu J. Anti-osteoporosis effect of Epimedium via an estrogen-like mechanism based on a system-level approach. J. Ethnopharmacol. 2016;177:148–160. doi: 10.1016/j.jep.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Yuan D., Wang H., He H., Jia L., He Y., Wang T., Zeng X., Li Y., Li S., Zhang C. Protective effects of total flavonoids from Epimedium on the male mouse reproductive system against cyclophosphamide-induced oxidative injury by up-regulating the expressions of SOD3 and GPX1. Phytother Res. 2014;28:88–97. doi: 10.1002/ptr.4956. (in Chinese) [DOI] [PubMed] [Google Scholar]
- Zhang H.F., Yang T.S., Li Z.Z., Wang Y. Simultaneous extraction of epimedin A, B, C and icariin from Herba Epimedii by ultrasonic technique. Ultrason. Sonochem. 2008;15:376–385. doi: 10.1016/j.ultsonch.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Zhang H.F., Yang X.H., Zhao L.D., Wang Y. Ultrasonic-assisted extraction of epimedin C from fresh leaves of Epimedium and extraction mechanism. Innovative Food Sci. 2009;10:54–60. [Google Scholar]
- Zhang H.F., Zhang X., Yang X.H., Qiu N.X., Wang Y., Wang Z.Z. Microwave assisted extraction of flavonoids from cultivated Epimedium sagittatum: extraction yield and mechanism, antioxidant activity and chemical composition. Ind. Crops Prod. 2013;50:857–865. [Google Scholar]
- Zhang X., Li Y., Yang X., Wang K., Ni J., Qu X. Inhibitory effect of Epimedium extract on S-adenosyl-l-homocysteine hydrolase and biomethylation. Life Sci. 2005;78:180–186. doi: 10.1016/j.lfs.2005.04.057. [DOI] [PubMed] [Google Scholar]
- Zhao D., Leghari I.H., Li J., Mi Y., Zhang C. Isolation and culture of chicken growing follicles in 2- and 3-dimensional models. Theriogenology. 2018;111:43–51. doi: 10.1016/j.theriogenology.2018.01.012. [DOI] [PubMed] [Google Scholar]
- Zhao H., Song L., Huang W., Liu J., Yuan D., Wang Y., Zhang C. Total flavonoids of Epimedium reduce ageing-related oxidative DNA damage in testis of rats via p53-dependent pathway. Andrologia. 2017;49:e12756. doi: 10.1111/and.12756. [DOI] [PubMed] [Google Scholar]