Abstract
This study examined the effects a synbiotic feed additive (PoultryStar meUS) on performance and intestinal health parameters in turkey poults administered a mixed Eimeria inoculation. The synbiotic feed additive consisted of Lactobacillus reuteri, Enterococcus faecium, Bifidobacterium animalis, Pediococcus acidilactici and a fructo-oligosaccharide prebiotic. Dietary treatments began on day of hatch, and poults were placed on a normal starter, starter containing Clinacox, or starter containing PoultryStar until the conclusion of the experiment on day 42. In addition, on day of hatch, all poults, with exception of the negative control, were orally inoculated with Salmonella enterica Enteritidis. On day 16, poults in inoculated treatment groups received an oral dose of Eimeria adenoides and Eimeria meleagrimitis oocysts resulting in a 2 × 3 factorial arrangement of treatments. BW were measured at weekly intervals after challenge, and fecal samples were collected from all pens during day 21 to day 33 to monitor fecal shedding and calculate oocyst per gram of feces. Five day after Eimeria inoculation, inoculated PoultryStar-fed (I-PS) and inoculated Clinacox-fed (I-CL) poults, on average, weighed and gained significantly more weight (P < 0.05) than inoculated controls (I-CON) and were similar to uninoculated treatments. Between day 21 and day 28, I-PS and I-CL poults showed a 23% improvement (P < 0.001) in percent change in BW gained relative to I-CON, and overall weight gain as a percentage was similar to the uninoculated control. Overall incidence of macroscopic intestinal lesions on day 21 and day 28 was low, but I-PS and I-CL poults were generally less positive than I-CON, and no oocysts were detected in the feces of any group except I-CON which cycled as expected. From this study, it can be concluded that incorporating PoultryStar into the diet of poults reared to 6 wk ameliorates and prevents aspects of performance loss and negative impacts on gut health seen with mixed Eimeria inoculation.
Key words: synbiotic, Eimeria adenoides, Eimeria meleagrimitis, turkey, probiotic
Introduction
Poultry currently represents one of the most consumed meats worldwide and demand is predicted to rise with time, making effective economic production practices essential (OECD and FAO, 2018). One of the largest threats to poultry production and profitability are direct and indirect costs of enteric diseases such as coccidiosis (Mot et al., 2014). Although coccidiosis is regarded as more problematic in chickens, it can also pose a challenge in young turkey poults though clinical signs are often more subtle. Despite the resistance against coccidial infection that turkeys develop as they age, especially in the presence of repeat exposure, poults can be particularly susceptible to the damaging and growth-inhibiting life cycle of this parasite (Chapman, 2008). Six Eimeria spp. associated with disease in turkeys include Eimeria innocua, Eimeria meleagridis, Eimeria dispersa, Eimeria gallopavonis, Eimeria meleagrimitis, and Eimeria adenoides with the latter 3 considered highly pathogenic (Chapman, 2008).
Methods of controlling coccidial infection include rotation programs between live oocyst vaccination and anticoccidial drugs or ionophores such as diclazuril (Clinacox), monensin, and salinomycin, though it should be noted salinomycin can be toxic to turkeys as they age (Potter et al., 1986). In addition, probiotics, prebiotics, and combination of the 2 (synbiotics) have become popular growth-promoting agents within livestock production and may be able to mitigate the impact of coccidiosis in the poultry industry. A probiotic can be defined as a feed additive containing live microbial organisms that benefit host health when administered at sufficient doses, whereas prebiotics are compounds that beneficially stimulate particular organisms in probiotics but are resistant to host digestion (Fuller, 1989; Gibson and Roberfroid, 1995). Synbiotics incorporate both prebiotic and probiotic components to optimize overall health and growth performance benefits to the host via microflora interactions. Probiotic mechanisms of action include a shift toward beneficial microflora, immune stimulation, competitive exclusion regarding free nutrients and adhesion sites used by pathogens, and direct antagonism of pathogens (Lloyd et al., 1977; Hume, 2011). Added benefits from a production standpoint are lack of withdrawal time, improvements to carcass quality, and a broader sense of consumer acceptance (Pelicano et al., 2003; Park et al., 2016).
A wide range of microbial species have been used as probiotics including Bacillus, Bifidobacterium, Enterococcus, Escherichia coli, Lactobacillus, Lactococcus, Streptococcus, yeasts, and mixed cultures (reviewed by Patterson and Burkholder, 2003). With regards to coccidial infection, probiotics have been shown to exert indirect inhibition of the Eimeria parasite via stimulation of the host immune response (Peek and Landman, 2011). This study compared Clinacox against a synbiotic product, PoultryStar, that contained the bacterial strains Lactobacillus reuteri, Enterococcus faecium, Bifidobacterium animalis, and Pediococcus acidilactici and a fructo-oligosaccharide prebiotic. The aims of this study were to examine the effects of this synbiotic on performance parameters and Eimeria oocyst fecal shedding when fed to turkey poults administered a mixed Eimeria inoculation.
Materials and methods
Animal Housing and Handling
Day-of-hatch (DoH) commercial male turkey poults (n = 1,080) were placed into floor pens with fresh pine shaving litter at The Ohio State University Ohio Agriculture Research and Development Center turkey facility. Poults were provided ad libitum access to feed and water (NRC, 1994), and ambient temperature was maintained within an age-appropriate range for the duration of the experiment (Aviagen Turkeys, 2015). Throughout the first wk, poults were given 24 h of light after which 1 h of darkness was added until a cycle of 20L:4D was reached. All experimental procedures were approved by the Institutional Animal Care and Use Committee of The Ohio State University.
Experimental Design
Diets included a nonmedicated basal turkey starter, starter containing synbiotic feed additive PoultryStar meUS (1.0 kg/metric ton), and starter containing Clinacox (1 ppm) all of which were administered for the duration of the experiment. Treatment groups consisted of an uninoculated untreated control (CON), uninoculated PoultryStar (PS), uninoculated Clinacox (CL), inoculated untreated (I-CON), inoculated PoultryStar (I-PS), and inoculated Clinacox (I-CL). Poults were initially placed into treatment respective floor pens (n = 90) with 2 replicates each from DoH through day 16. All poults in inoculated groups received an oral inoculation of Salmonella enterica serovar Enteritidis on DoH. On day 16, each poult in I-CON, I-PS, and I-CL received an inoculation of E. adenoides and E. meleagrimitis oocysts. All poults were then further divided by treatment into 6 replicate pens (n = 30) which created a 2 × 3 factorial arrangement. BW for all poults were collected on DoH, day 16, day 21, day 28, day 35, and day 42 to calculate BW gain (BWG), percent BWG (%BWG), and percent change in BWG (%ΔBWG) relative to respective controls (CON for uninoculated groups and I-CON for inoculated groups) as described by Wilson and coauthors (2018). Daily fecal samples were collected from each pen on day 21 to day 33 to monitor oocysts shedding that is represented as oocysts per gram of feces (OPG). Five poults per pen were weighed and euthanized via cervical dislocation on day 21 and day 28 to measure lesion scores in the duodenum, jejunum, ileum, and ceca with Eimeria infection using a 0 to 4 scale as described by El-Sherry et al. (2014). The lesion score evaluator was blinded to treatment.
Inoculation Organisms
Bacterial Culture Preparation
Salmonella Enteritidis was prepared as described by Shivaramaiah and co-authors (2011). The approximate concentration of Salmonella Enteritidis was quantified spectrophotometrically (Spectronic 200E, Thermo Scientific, Madison, WI), followed by serial dilutions in sterile saline to reach a concentration of approximately 104 CFU/poult. Exact concentration was measured retrospectively by serial dilution plating on tryptic soy agar and determined to be 6 × 103 CFU/poult.
Eimeria Preparation
Purified suspensions of E. adenoides and E. meleagrimitis were stored at 4°C until day of inoculation. For enumeration, oocysts were floated in a saturated salt solution, quantified in a McMaster chamber, and the number of sporulated oocysts were calculated as number of oocysts per mL solution. Oocysts were then resuspended to a dose of 6.25 × 103 sporulated oocysts per poult and 2.5 × 104 sporulated oocysts per poult of E. adenoides and E. meleagrimitis, respectively, in distilled water.
Evaluation of Fecal Shedding
Beginning 5 day after Eimeria inoculation, fecal samples were collected daily to approximate OPG. From each pen, 10 to 12 fresh, moist fecal droppings were collected and placed in sterile sample bags. A 3-fold w:v dilution with 2% potassium dichromate (Sigma-Aldrich, St. Louis, MO) was added to each bag, followed by storage at room temperature until oocysts were quantified in McMaster chambers. All samples that were negative for Eimeria oocysts by McMaster's method were evaluated by a direct fecal float in saturated salt solution to confirm absence of oocysts.
Statistical Analysis
All BW data were analyzed as a completely randomized factorial design with fixed effects of diet, inoculation challenge, and challenge by diet interactions. Treatment effects were assessed using an ANOVA in the JMP Pro 14 statistical software (SAS Institute Inc., Cary, NC, 2016), and statistical differences between means were determined using Tukey's honestly significant difference test with the exception of %ΔBWG that used the Student t test. The %BWG was calculated respective to period as (BWG/BWinitial), whereas %ΔBWG, relative to CON for PS and CL groups and relative to I-CON for I-PS and I-CL groups, was calculated as {(%BWG/Mean%BWG of (I-)CON)-1} × 100. Lesion scores were reported as the percentage of positive scores of total poults scored and statistical differences among the means were determined using chi-square analysis (χ2 > 3.841). All BW, BWG, and %BWG values reported are expressed as treatment mean ± SE. All %ΔBWG values are reported as mean ± SD.
Results
Oocyst Shedding
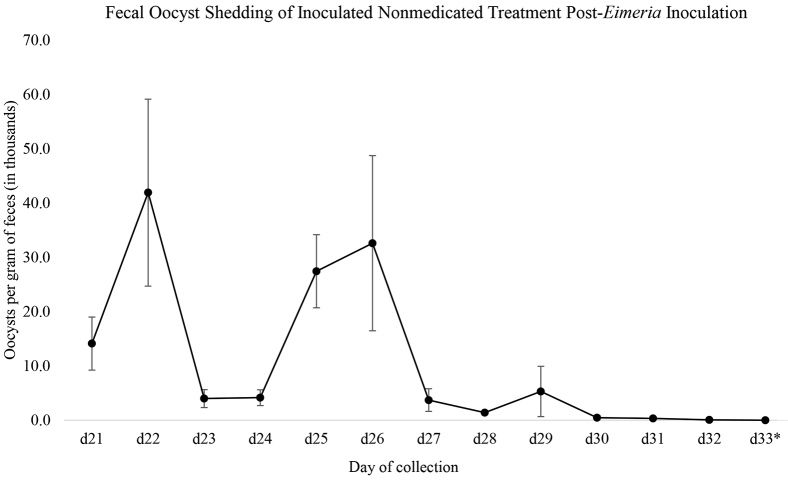
Fecal collections began 5 day after inoculation (day 21) and continued until 17 day after inoculation (day 33). Oocysts were detected only in I-CON and observed shedding levels cycled as expected in accordance with the Eimeria life cycle. Peak oocyst shedding was observed on day 22 with approximately 40,000 OPG and on day 26 with approximately 33,000 OPG. By day 33, oocysts were at undetectable levels (Figure 1). Effectiveness of Clinacox and PS at preventing detectable Eimeria infection is noted through the lack of oocyst shedding in these groups throughout the duration of the experiment. However, it should be noted that a lack of detectable levels of Eimeria does not signify a lack of infection as reported with lesion scores.
Figure 1.
Fecal shedding of Eimeria adenoides and Eimeria meleagrimitis oocysts during day 21 to day 33 in I-CON. On day 16, poults were inoculated with 6.25 × 103 E. adenoides and 2.50 × 104 E. meleagrimitis oocysts each, as indicated. Beginning on day 21, fresh feces were collected from each replicate pen once daily, and each sample was independently evaluated. No oocysts were detected in any groups except for I-CON. Values represent treatment-specific mean oocysts per gram of feces ± SE. ∗No oocysts detected. Abbreviation: I-CON, inoculated untreated.
Lesion Scores
On day 21 and day 28, 30 poults per treatment were lesion scored by a single evaluator blinded to treatment. Eimeria lesions were scored independently in the duodenum, jejunum, ileum and ceca, and poults receiving a score greater than zero were considered positive for coccidiosis. Few lesions were observed in the ileum on either day, with the exception of day 21 I-CON and I-PS poults in which 23.3% and 3.3% of poults were considered positive, respectively (Table 1). On day 21, 3.3% of I-PS poults were positive for lesions in the duodenum, which was significantly lower than 20% positive in I-CON. On the same day, incidence of lesions was significantly lower for both I-PS and I-CL in the jejunum and ileum than for I-CON, but all inoculated groups were similar and between 36.7 and 56.7% positive for lesions in the ceca (Table 1). No differences in presence of lesions were observed between I-PS and I-CON poults for any intestinal section on day 28, though I-CL had significantly lower incidence of lesions in the ceca. Interestingly, lesions were also observed in uninoculated groups on both day 21 and day 28, but incidence was lower than inoculated groups for all intestinal sections and time points with the exception of CL for day 21 duodenum. This suggests contamination within the rearing facility, but parasite levels were low enough that no oocysts were detected in the feces (Table 1 and Figure 1).
Table 1.
Percent of poults with Eimeria lesions after inoculation on day 16.
| Day 21 (%) |
Day 28 (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Duodenum | Jejunum | Ileum | Ceca | Duodenum | Jejunum | Ileum | Ceca | |
| CON | 10.0a,b | 3.3c | 0.0b | 16.7c,d | 10.0a,b | 3.3b | 0.0 | 10.0c |
| PS | 6.7a,b | 0.0c | 0.0b | 16.7c,d | 10.0a,b | 6.7b | 0.0 | 13.3b,c |
| CL | 3.3b | 6.7b,c | 0.0b | 10.0d | 3.3b | 3.3b | 0.0 | 13.3b,c |
| I-CON | 20.0a | 70.0a | 23.3a | 56.7a | 16.0a,b | 40.0a | 0.0 | 32.0a,b |
| I-PS | 3.3b | 20.0b | 3.3b | 36.7a,b | 23.3a | 16.7a,b | 0.0 | 40.0a |
| I-CL | 13.3a,b | 10.0b,c | 0.0b | 40.0a,b | 16.7a,b | 10.0b | 0.0 | 40.0a |
| SEM | 0.03 | 0.11 | 0.04 | 0.09 | 0.03 | 0.06 | – | 0.06 |
a-cPositive percentage values with different superscript letters within a column indicate a significant difference as determined by χ2 analysis (χ2 > 3.841).
Abbreviations: CL, uninoculated Clinacox; CON, uninoculated untreated; I-CL, inoculated Clinacox; I-CON, inoculated untreated; I-PS, inoculated PoultryStar; PS, uninoculated PoultryStar.
On day 21 (5 D after inoculation) and day 28 (12 D after inoculation), 5 poults from each replicate pen (for a total of 30 poults) were euthanized and evaluated for lesions consistent with Eimeria infection. Poults receiving a score greater than zero were considered positive for coccidiosis. Values are presented as percentage of poults positive for lesions for each section of the GIT evaluated.
Body Weight
All BW and BWG time points, after inoculation, showed significant (P < 0.05) diet and diet by challenge interactions (Table 2). On day 21, BW of I-PS (601.46 ± 5.34 g) and I-CL (595.73 ± 5.18 g) were similar to those of uninoculated groups and had significantly higher mean BW than I-CON, 571.34 ± 4.72 g. This trend continued throughout the experiment with I-CON poults having the lowest final mean BW of 2,415.88 ± 23.50 g on day 45, which was lower (P < 0.001) than all other groups and differed numerically by a minimum of 178 g. Final BW for PS and I-PS were slightly higher than CON, though not significant at 11.61 g and 10.12 g, respectively (Table 2). On day 21, 5 day after inoculation, CL had the lowest mean BW, 556.4 g, and the lowest BWG day16 to day 21, 190.9 g, which was comparable to I-CON (Table 2). BWG day 21to day 28 for I-PS, 406.57 ± 4.27 g, and I-CL, 408.14 ± 8.14 g, was significantly greater than PS, CL, and I-CON and exceeded I-CON by over 95 g. During this same week, I-CON had the lowest BWG, 311.3 ± 5.55 g, and from day 28 to day 35, I-PS and I-CL poults had significantly greater mean BWG than CON and I-CON (Table 2). In the final period of the experiment, day 35 to 45, mean BWG for I-CL was significantly greater than I-CON and I-PS and numerically exceeded all uninoculated groups (Table 2). All BW and BWG time points had significant diet and diet by challenge interactions.
Table 2.
BW and BW gain.
| BW (g) | Day of age |
|||||
|---|---|---|---|---|---|---|
| Day 0 | Day 16 | Day 21 | Day 28 | Day 35 | Day 45 | |
| CON | 54.26 ± 0.36a | 391.82 ± 3.70a,b | 592.75 ± 4.92a | 983.93 ± 8.86a,b | 1,531.10 ± 16.01b | 2,619.96 ± 27.00a |
| PS | 54.35 ± 0.33a | 386.38 ± 3.65a,b | 591.68 ± 5.08a | 967.92 ± 9.14b,c | 1,543.15 ± 16.23a,b | 2,631.57 ± 24.09a |
| CL | 54.02 ± 0.36a | 365.45 ± 3.08c | 556.37 ± 4.57b | 940.68 ± 8.32c | 1,513.00 ± 13.89b | 2,597.21 ± 22.04a |
| I-CON | 55.23 ± 0.35a | 381.88 ± 3.24b | 571.34 ± 4.72b | 885.67 ± 9.33d | 1,410.67 ± 13.93c | 2,415.88 ± 23.50b |
| I-PS | 54.87 ± 0.34a | 398.10 ± 3.48a | 601.46 ± 5.34a | 1,012.61 ± 8.81a | 1,592.06 ± 15.23a | 2,630.08 ± 24.51a |
| I-CL | 54.84 ± 0.32a | 395.15 ± 3.39a,b | 595.73 ± 5.18a | 996.13 ± 9.31a,b | 1,592.01 ± 15.19a | 2,683.88 ± 24.67a |
| SEM | 0.15 | 4.84 | 7.06 | 18.70 | 27.32 | 37.93 |
| P-value | 0.12 | 0.001 | 0.048 | 0.006 | 0.049 | <0.001 |
| Source of Variation |
P-value |
|||||
| Diet | – | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 |
| Challenge | – | <0.001 | 0.023 | 0.931 | 0.840 | 0.047 |
| Diet × Challenge | – | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| BWG (g) | Period |
||||
|---|---|---|---|---|---|
| Day 0–Day16 | Day 16–Day 21 | Day 21–Day 28 | Day 28–Day 35 | Day 35–Day 45 | |
| CON | 337.05 ± 3.59a,,b,c | 200.93 ± 2.52a,b,c | 395.88 ± 4.30a,b, | 549.99 ± 7.02c,d | 1,088.86 ± 12.40a,b |
| PS | 322.61 ± 5.73c,d | 204.28 ± 2.28a,b | 379.43 ± 5.34b | 577.33 ± 8.54a,b,c | 1,088.42 ± 10.48a,b |
| CL | 310.98 ± 3.06d | 190.92 ± 2.19c | 380.91 ± 4.06b | 566.42 ± 5.85b,c | 1,084.20 ± 10.03a,b |
| I-CON | 326.49 ± 3.24b,c | 191.65 ± 2.62b,c | 311.3 ± 5.55c | 538.12 ± 6.41d | 1,005.21 ± 11.69c |
| I-PS | 342.3 ± 3.56a | 209.48 ± 5.33a | 406.57 ± 4.27a | 587.10 ± 5.93a,b | 1,043.12 ± 13.37b,c |
| I-CL | 339.67 ± 3.44a,b | 198.30 ± 2.83a,b | 408.14 ± 8.14a | 596.07 ± 6.63a | 1,092.91 ± 11.36a |
| SEM | 4.91 | 2.95 | 14.69 | 9.04 | 14.48 |
| P-value | 0.050 | 0.038 | 0.009 | 0.044 | 0.033 |
| Source of Variation | P-value | ||||
| Diet | – | <0.001 | <0.001 | <0.001 | 0.002 |
| Challenge | – | 0.669 | 0.024 | 0.097 | <0.001 |
| Diet × Challenge | – | 0.015 | <0.001 | 0.009 | <0.001 |
a-cMean values with different superscript letters within a column indicate a significant difference (P ≤ 0.05).
Abbreviations: BWG, BW gain; CL, uninoculated Clinacox; CON, uninoculated untreated; I-CL, inoculated Clinacox; I-CON = inoculated untreated; I-PS, inoculated PoultryStar; PS, uninoculated PoultryStar.
Poults were weighed on day of hatch, day 16, day 21, day 28, day 35, and day 45, and weight gain was calculated between these intervals. Data are presented as mean ± SE.
During the inoculation period, day 16 to day 21, %BWG of all inoculated groups were similar to that of CON and had a significant challenge interaction (Table 3). During day 21 to day 28, %BWG of I-PS and I-CL (67.39 ± 0.68% and 67.03 ± 0.74%, respectively) were significantly greater than that of I-CON (54.80 ± 0.92%) and similar to that of CON (67.25 ± 0.64%). In addition, all interactions for %BWG during day 21to day 28 were significant (Table 3). No differences were observed for %ΔBWG during day 16 to day 21 indicating statistically similar growth rates relative to respective controls. Regarding %ΔBWG for day 21 to day 28, PS poults gained less (P = 0.004) than CON poults with −3.98 ± 13.67% relative change. However, %ΔBWG for I-PS and I-CL was significantly higher (P < 0.001) than I-CON with at least a 23% improvement in weight gain relative to I-CON during this period (Table 3).
Table 3.
Percent BW gain and percent change in BW gain.
| %BWG (%) | ||
|---|---|---|
| Period |
||
| Day 16–Day 21 | Day 21–Day 28 | |
| CON | 51.86 ± 0.64a,b | 67.25 ± 0.64a,b |
| PS | 52.80 ± 0.63a | 64.85 ± 0.86b |
| CL | 52.62 ± 0.57a | 67.93 ± 0.60a |
| I-CON | 50.11 ± 0.55b | 54.80 ± 0.92c |
| I-PS | 50.61 ± 0.59a,b | 67.39 ± 0.68a,b |
| I-CL | 51.03 ± 0.53a,b | 67.03 ± 0.74a,b |
| SEM | 0.45% | 2.06% |
| P-value | 0.028 | 0.039 |
| Source of Variation |
P-value |
|
| Diet | 0.134 | <0.001 |
| Challenge | <0.001 | <0.001 |
| Diet × Challenge | 0.912 | <0.001 |
| %ΔBWG (%) | ||
| Period |
||
| d16-d21 | d21-d28 | |
| CON | 0.00 ± 15.84a | 0.00 ± 10.88a |
| PS | 2.29 ± 13.79a | -3.98 ± 13.67b |
| CL | 1.31 ± 13.32a | 1.10 ± 9.56a |
| SEM | 0.66% | 1.54% |
| P-value | 0.319 | 0.004 |
| I-CON | 0.00 ± 12.97a | 0.00 ± 18.36b |
| I-PS | 1.85 ± 14.97a | 23.76 ± 13.82a |
| I-CL | 1.78 ± 12.66a | 23.13 ± 14.24a |
| SEM | 0.61% | 7.82% |
| P-value | 0.419 | <0.001 |
a-cMean values with different superscript letters within a column indicate a significant difference (P ≤ 0.05).
Abbreviations: CL, uninoculated Clinacox; CON, uninoculated untreated; I-CL, inoculated Clinacox; I-CON, inoculated untreated; I-PS, inoculated PoultryStar; N.S., not significant; PS, uninoculated PoultryStar.
The %BWG represents BW gain as a percentage of initial BW respective to time period, and value represent mean ± SE. The %ΔBWG represents change in %BWG relative to the control group and was therefore calculated separately for uninoculated groups and inoculated groups, and values represent mean ± SD. The %BWG and %ΔBWG were calculated only for the inoculation period and the week after inoculation to show effects of treatment on Eimeria challenge.
Discussion
A critical physiologic effect of coccidiosis is depressed weight gain, which results in large-scale economic losses for producers (Williams, 2005). Here, BWG was calculated for weekly intervals beginning 5 day after Eimeria inoculation when the greatest impact on BW was expected (Chapman, 2008). It was also calculated for the 5-day inoculation period, between administration of Eimeria and peak lesions (day 16–day 21), to measure the impact of inoculation on growth. Clarkson (1958, 1959) reported that inoculation with 2.5 × 104 oocysts of either E. adenoides or E. meleagrimitis resulted in depressed weight gain in poults approximately 3 wk of age. However, over the course of this experiment, BWG for I-PS and I-CL poults was similar to that of unchallenged groups, indicating that treatments were able to prevent aspects of growth depression associated with a direct Eimeria challenge. In particular, a 23.76% increase in %ΔBWG for I-PS compared with that for I-CON illustrated efficacy of PoultryStar at ameliorating performance losses observed over the course of coccidial infection. Despite beneficial influences on growth performance, no statistical differences were observed for feed conversion ratio at any point over the course of this study (data not shown).
Identification of gross macroscopic intestinal lesions represents another diagnostic method to assess coccidial infection within flocks, although this system is more thoroughly defined in chickens as opposed to turkeys (Johnson and Reid, 1970; Chapman et al., 2013). It is important to note, however, that while gross lesions are frequently visualized in Eimeria-infected broilers, this is not always the case with turkeys, which often show few observable lesions, even during severe infection (Madden and Ruff, 1979). In addition, the effectiveness of lesion scoring as a diagnostic tool is reliant on timing. In turkeys, the window of visibility for intestinal lesions is between 5 and 7 D after infection depending on the species (Vrba and Pakandl, 2014). The location of lesions along the gastrointestinal tract (GIT) can also vary by Eimeria species. With regards to E. meleagrimitis, lesions have been visualized between 5 and 6 D after infection primarily in the duodenum, jejunum, and upper ileum, whereas E. adenoides lesions are more prominently observed in the lower ileum and ceca, and both species are represented in the lesion-positive segments observed in this study (Hein, 1969; Chapman, 2008; El-Sherry et al., 2014; Vrba and Pakandl, 2014). Lesions were also noted in the Clinacox-treated groups, which suggested some parasite drug resistance, but overall, inoculated poults fed Clinacox or PoultryStar were less positive for lesions on day 21 and to a lesser extent, day 28 as compared with I-CON. Low levels of lesions present in uninoculated groups suggest some level of contamination within the rearing facility, though these levels were low enough that no oocysts were detected in feces. Notably, despite the presence of lesions, mortality was insignificant between treatments and less than 2% throughout the entire study (data not shown).
Quantification of fecal oocyst shedding is commonly used to assess severity of coccidial infection. Infective oocysts are intermittently shed in the feces and re-ingested by birds at higher numbers with each consecutive cycle (Chapman et al., 2013). Although there is a positive correlation between oocyst shedding and inoculation dose, it is also important to consider that oocysts recovered in the feces are proportional to the ability of the parasite to undergo replication in the GIT and not solely reliant on parasite load (Zhu et al., 2000). Owing to a complete lack of detection of Eimeria oocysts in the feces of the treated groups, it could be concluded that PoultryStar was able to inhibit some aspect of reproduction of Eimeria within the GIT. This could be a result of 1, or a combination of, aforementioned mechanisms by which synbiotics function in the intestinal environment. After ingestion, sporulated oocysts reach the small intestine where internal sporocysts release sporozoites. The motile sporozoites invade nearby enterocytes where they undergo asexual and eventual sexual reproduction resulting in mature gametes and, in turn, infective oocysts (Allen and Fetterer, 2002; Sharman et al., 2010). Given that incidence of Eimeria lesions were low and oocyst shedding was undetected within 5 D of infection, 1 hypothesis is that PoultryStar targeted the Eimeria life cycle between the sporozoite and schizogeny stages, where invasion of cells on the mucosal surface of the GIT occurs before the formation and shedding of infective oocysts, though it was not tested in this experiment. Inhibition of invasive and reproductive stages of Eimeria is critical as these periods are primarily responsible for observed enterocyte damage, macroscopic lesions, and performance losses (Allen and Fetterer, 2002; Chapman et al., 2013).
The results of performance parameters measured in this study indicate that the synbiotic product, PoultryStar, was able to prevent clinical signs associated with Eimeria challenge in poults and alleviate performance losses when included in the diet, especially regarding oocyst shedding. Notably, I-PS performed similar to CL. These findings likely result from the combined action of probiotics and prebiotics in PoultryStar that could include competitive exclusion for nutrients and attachment sites, beneficial modulation of the immune response, and potential modulation of microflora toward more growth-promoting or beneficial bacterial populations (Saarela et al., 2000; Koenen et al., 2004; Nava et al., 2005). Despite a lack of specificity surrounding the exact parasite–host physiologic mechanisms, it can be concluded that PoultryStar offers a promising alternative approach for control of coccidiosis in young turkey flocks.
Acknowledgemnets
The authors would like to thank BIOMIN for funding this research. Additional thanks are also extended to the OARDC Poultry Research Farm members including Keith Patterson, Jarrod Snell, Jack Sidle, and Jordan Welsh for contributing to animal husbandry throughout this study. Finally, the authors would like to acknowledge laboratory technician, Whitney Briggs, for helping to facilitate this project.
Conflict of Interest Statement: The authors declare no conflict of interest.
References
- Allen P.C., Fetterer R.H. Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry. Clin. Microbiol. Rev. 2002;15:58–65. doi: 10.1128/CMR.15.1.58-65.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviagen Turkeys . 2015. Management Guidelines Raising Commercial Turkeys. Aviagen Turkeys, East Lewisburg, WV. [Google Scholar]
- Chapman H.D. Coccidiosis in the Turkey. Avian Pathol. 2008;37:205–223. doi: 10.1080/03079450802050689. [DOI] [PubMed] [Google Scholar]
- Chapman H.D., Barta J.R., Blake D., Gruber A., Jenkins M., Smith N.C., Suo X., Tomley F.M. Advances in Parasitology. Academic Press; Cambridge, MA: 2013. A selective review of advances in coccidiosis research; pp. 93–171. [DOI] [PubMed] [Google Scholar]
- Clarkson M.J. Life history of and pathogenicity of Eimeria adenoeides Moore and Brown, 1951, in the Turkey poult. Parasitiology. 1958;48:70–88. doi: 10.1017/s0031182000021065. [DOI] [PubMed] [Google Scholar]
- Clarkson M.J. The life history and pathogenicity of Eimeria meleagrimitis Tyzzer 1929, in the Turkey poult. Parasitiology. 1959;49:70–82. doi: 10.1017/s0031182000026718. [DOI] [PubMed] [Google Scholar]
- El-Sherry S., Rathinam T., Hafeez M.A., Ogedengbe M.E., Chapman H.D., Barta J.R. Biological re-description of a genetically typed, single oocyst line of the Turkey coccidium, Eimeria meleagrimitis Tyzzer 1929. Parasitol. Res. 2014;113:1135–1146. doi: 10.1007/s00436-014-3751-x. [DOI] [PubMed] [Google Scholar]
- Fuller R. Probiotics in man and animals. J. Appl. Bacteriol. 1989;66:365–378. [PubMed] [Google Scholar]
- Gibson G.R., Roberfroid M.B. Dietary modulation of the human colonie microbiota: introducing the concept of prebiotics. J. Nutr. 1995;125:391–395. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- Hein H. Eimeria adenoeides and E. meleagrimitis: pathogenic effect in Turkey poults. Exp. Parasitol. 1969;24:163–170. doi: 10.1016/0014-4894(69)90153-2. [DOI] [PubMed] [Google Scholar]
- Hume M.E. Historic perspective: prebiotics, probiotics, and other alternatives to antibiotics. Poult. Sci. 2011;90:2663–2669. doi: 10.3382/ps.2010-01030. [DOI] [PubMed] [Google Scholar]
- Johnson J., Reid W.M. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970;28:30–36. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Koenen M.E., Kramer J., van der Hulst R., Heres L., Jeurissen S.H.M., Boersma W.J.A. Immunomodulation by probiotic lactobacilli in layer- and meat-type chickens. Br. Poult. Sci. 2004;45:355–366. doi: 10.1080/00071660410001730851. [DOI] [PubMed] [Google Scholar]
- Lloyd A.B., Cumming R.B., Kent R.D. Prevention of Salmonella Typhimurium infection in poultry by pretreatment of chickens and poults with intestinal extracts. Aust. Vet. J. 1977;53:82–87. doi: 10.1111/j.1751-0813.1977.tb14891.x. [DOI] [PubMed] [Google Scholar]
- Madden P.A., Ruff M.D. Eimeria dispersa, E. Adenoeides, and E. Meleagrimitis: intestinal mucosal Disruption in turkeys as seen with Scanning Electron microscopy. J. Parasitol. 1979;65:234. [PubMed] [Google Scholar]
- Mot D., Timbermont L., Haesebrouck F., Ducatelle R., Van Immerseel F. Progress and problems in vaccination against necrotic enteritis in broiler chickens. Avian Pathol. 2014;43:290–300. doi: 10.1080/03079457.2014.939942. [DOI] [PubMed] [Google Scholar]
- National Research Council . 9th rev. ed. Natl. Acad. Press; Washington, D.C: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Nava G.M., Bielke L.R., Callaway T.R., Castañeda M.P. Probiotic alternatives to reduce gastrointestinal infections: the poultry experience. Anim. Health Res. Rev. 2005;6:105–118. doi: 10.1079/ahr2005103. [DOI] [PubMed] [Google Scholar]
- OECD and FAO. OECD-FAO Agricultural Outlook 2018-2027. OECD Publishing, Paris/Food and Agriculture Organization of the United Nations; Rome, Italy: 2018. [Google Scholar]
- Park Y.H., Hamidon F., Rajangan C., Soh K.P., Gan C.Y., Lim T.S., Abdullah W.N.W., Liong M.T. Application of probiotics for the production of safe and high-quality poultry meat. Korean J. Food Sci. Anim. Resour. 2016;36:567–576. doi: 10.5851/kosfa.2016.36.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J., Burkholder K. Application of prebiotics and probiotics in poultry production. Poult. Sci. 2003;82:627–631. doi: 10.1093/ps/82.4.627. [DOI] [PubMed] [Google Scholar]
- Peek H.W., Landman W.J.M. Coccidiosis in poultry: anticoccidial products, vaccines and other prevention strategies. Vet. Q. 2011;31:143–161. doi: 10.1080/01652176.2011.605247. [DOI] [PubMed] [Google Scholar]
- Pelicano E., de Souza P., de Souza H., Oba A., Norkus E., Kodawara L., de Lima T. Effect of different probiotics on broiler carcass and meat quality. Revista Brasileira de Ciência Avícola. 2003;5:207–214. [Google Scholar]
- Potter L.M., Blake J.P., Blair M.E., Bliss B.A., Denbow D.M. Salinomycin toxicity in turkeys. Poult. Sci. 1986;65:1955–1959. doi: 10.3382/ps.0651955. [DOI] [PubMed] [Google Scholar]
- Saarela M., Mogensen G., Fondén R., Mättö J., Mattila-Sandholm T. Probiotic bacteria: safety, functional and technological properties. J. Biotechnol. 2000;84:197–215. doi: 10.1016/s0168-1656(00)00375-8. [DOI] [PubMed] [Google Scholar]
- Sharman P.A., Smith N.C., Wallach M.G., Katrib M. Chasing the golden egg: vaccination against poultry coccidiosis. Parasite Immunol. 2010;32:590–598. doi: 10.1111/j.1365-3024.2010.01209.x. [DOI] [PubMed] [Google Scholar]
- Shivaramaiah S., Wolfenden R.E., Barta J.R., Morgan M.J., Wolfenden A.D., Hargis B.M., Téllez G. The role of an early Salmonella Typhimurium infection as a predisposing factor for necrotic enteritis in a laboratory challenge model. Avian Dis. 2011;55:319–323. doi: 10.1637/9604-112910-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Vrba V., Pakandl M. Coccidia of Turkey: from isolation, characterisation and comparison to molecular phylogeny and molecular diagnostics. Int. J. Parasitol. 2014;44:985–1000. doi: 10.1016/j.ijpara.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Williams R.B. Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian Pathol. 2005;34:159–180. doi: 10.1080/03079450500112195. [DOI] [PubMed] [Google Scholar]
- Wilson K.M., Chasser K.M., Duff A.F., Briggs W.N., Latorre J.D., Barta J.R., Bielke L.R. Comparison of multiple methods for induction of necrotic enteritis in broilers. J. Appl. Poult. Res. 2018;27:577–589. doi: 10.3382/ps/pez405. [DOI] [PubMed] [Google Scholar]
- Zhu J.J., Lillehoj H.S., Allen P.C., Yun C.-H., Pollock D., Sadjadi M., Emara M.G. Analysis of disease resistance-associated parameters in broiler chickens challenged with Eimeria maxima. Poult. Sci. 2000;79:619–625. doi: 10.1093/ps/79.5.619. [DOI] [PubMed] [Google Scholar]