Supplemental Digital Content is available in the text.
Keywords: biomarker, collateral circulation, electrode, ganglia, hand strength, nasolacrimal duct, pinch strength
Background and Purpose—
Two large, randomized trials indicated that sphenopalatine ganglion (SPG) stimulation improves final disability outcome in acute anterior circulation patients with ischemic stroke with confirmed cortical involvement. This study evaluated 2 refinements in SPG stimulation treatment technique: (1) SPG electrode placement with real-time optical tracking guidance; and (2) stimulation intensity comfortable tolerance level selection using non-noxious facial physiological markers.
Methods—
This study was a single, active arm trial at 4 centers, enrolling patients with anterior circulation ischemic stroke, National Institutes of Health Stroke Scale 1 to 6 including arm weakness subitem score ≥1, not receiving recanalization therapies, and within 24 hours of onset. Stimulation level was set based on ipsilateral facial tingling sensation or lacrimation. SPG stimulation effects were assessed by measuring volumetric blood flow in the ipsilateral common carotid artery by ultrasound and grasp and pinch strength in the affected hand before and during stimulation, and by change in National Institutes of Health Stroke Scale from day 1 to 7.
Results—
Among 50 enrolled patients, age was median 66 years (interquartile range, 60–74), 44% were female, National Institutes of Health Stroke Scale median was 5 (interquartile range, 4–5), and median onset-to-screening time was 18 hours (interquartile range, 9–20). Median implantation skin-to-skin time was 4 minutes (interquartile range, 3–7), and all 50 implants were placed correctly. Comfortable tolerance level was found based on physiological biomarkers in 96% of patients, including 86% in the optimal, low-medium intensity range. SPG stimulation significantly increased common carotid artery peak systolic and end-diastolic blood flow (44%, P<0.0001; and 52%, P<0.0001) and improved pinch strength (42%, P<0.0001) and grasp strength (26%, P<0.0001). Degree of National Institutes of Health Stroke Scale recovery by day 7 was greater than in matched historic controls, median 75% versus 50%, P=0.0003.
Conclusions—
SPG stimulator placement with real-time optical tracking guidance was fast and accurate, and selection of stimulation intensity levels based on non-noxious facial tingling and lacrimation was feasible in nearly all patients. SPG stimulation led to cervico-cranial blood flow augmentation and improved hand motor function.
Clinical Trial Registration—
URL: https://www.clinicaltrials.gov. Unique identifier: NCT03551093.
Acute ischemic stroke is a leading cause of long-term disability and patient mortality.1 Timely reperfusion is associated with improved neurological outcomes, and recanalization therapies, including intravenous thrombolysis and endovascular thrombectomy, are recommended by current clinical guidelines.2,3 The majority of AIS patients, however, are not eligible for these therapies.4 Reperfusion may be achieved not only by recanalization of an occluded vessel but also by enhancement of blood flow through collateral vessels.5
The sphenopalatine ganglion (SPG) is the source of parasympathetic innervation to the anterior cerebral circulation.6 Preclinical studies demonstrated that activation of the SPG leads to profound increase in ipsilateral collateral blood flow, augmentation of tissue perfusion,7 and improved outcome in AIS models.7–10 Two large, randomized, sham-controlled clinical trials in AIS patients provided evidence that SPG stimulation improved final disability outcome in anterior circulation ischemic stroke patients with confirmed cortical involvement at entry—the ImpACT-24A (Implant for Augmentation of Cerebral Blood Flow Trial A) and ImpACT-24B.11,12 In pooled analysis of the 2 trials, SPG stimulation in patients with confirmed cortical involvement increased 3-month favorable disability outcomes from 38.3% to 49.7% (P=0.004). In addition, the benefit accrued in an inverted U-shaped dose-response pattern, with the greatest improvement in disability outcomes at low to medium stimulation intensities.12
The current ImpACT-24M (Implant for Augmentation of Cerebral Blood Flow Trial in Mild Strokes) was undertaken to further refine SPG stimulation for AIS, with 3 goals.
The first aim of the trial was to evaluate the speed and accuracy of electrode implant placement using an advanced software algorithm that automatically registered the patient’s preprocedure computed tomography (CT) scan with the navigation system’s real-time optical tracking information.
The second aim of the trial was to determine if initial SPG stimulation intensity dose-selection in patients can be achieved when guided by non-noxious physiological effects (facial tingling and lacrimation) rather than noxious physiological effects (mild facial discomfort). As SPG stimulation intensities are increased, physiological effects include not only ipsilateral vasodilatation but also tingling sensation over the cheek or nose bridge, unilateral lacrimation, and facial discomfort. As stimulation level increases, the tingling sensation is the first biomarker to appear, followed by lacrimation, and finally, discomfort and pain.13 In the ImpACT-24A and 24B trials, in every treatment session, SPG stimulation intensities were started at a low level and gradually increased until mild facial discomfort occurred. The intensities were then reduced until facial discomfort resolved and the resulting intensity level was deemed the patient’s comfortable tolerance level (CTL). That intensity level was then used for the duration of the treatment. In the 24A and 24B trials, facial discomfort had to be used as the indicator to determine the CTL, rather than facial tingling because to maintain patient blinding to treatment assignment, the transmitter applied to all study patients, both active stimulation and sham, was vibrated to mimic a tingling sensation. It is, therefore, desirable to perform a study in patients not receiving masking vibrations to determine if setting stimulation intensity at the level at which non-noxious facial tingling or lacrimation is encountered permits determination of an effective CTL. Such an approach would enhance patient comfort during initial dose-finding by avoiding any occurrence of facial discomfort and would potentially reduce selection of CTL intensities at high settings beyond the optimum midrange of the dose-response curve.
The third aim of the current trial was to determine if SPG stimulation would produce immediate improvements in neurological motor function. The prior trials had examined outcomes after stimulation, with emphasis upon long-term disability outcome 3 months after stroke. The immediate effect during the stimulation sessions upon neurological deficits had not been delineated.
Methods
Study Data Availability
The authors will deposit the data 1 year after publication in the Virtual International Stroke Trial Archive (http://www.virtualtrialsarchives.org/vista/), to be maintained by Virtual International Stroke Trial Archive as available for use by other researchers. The investigators will provide further details of stimulation parameters to interested academic investigators.
Study Design and Participants
ImpACT-24M was a prospective, multicenter, single-arm trial performed in 4 centers. The study included patients aged 18 to 80 years, with National Institutes of Health Stroke Scale (NIHSS) scores between 1 and 6 including arm weakness subitem score of at least 1 point, clinical evidence of stroke in the anterior circulation, and ability to start treatment up to 24 hours from stroke onset (time last known well). Patients were excluded if they had radiological evidence of intracranial hemorrhage, massive (>2/3 of middle cerebral artery territory), or posterior circulation acute infarcts, or were eligible for treatment with intravenous thrombolysis or endovascular thrombectomy per national and institutional standard of care.
The protocol was approved by institutional review boards in all participating centers, and written informed consent was obtained from all participants or their legally authorized representatives.
Procedures
The study design included an implantation phase and a treatment phase, which included identification of the CTL and 5 days of SPG stimulation. Patients received medical therapy according to the standard of care for AIS throughout their study participation.
Implantation was a bedside, minimally invasive procedure in which the neurostimulator electrode (23 mm long, 2 mm in diameter) was injected into the pterygopalatine canal near the SPG.11,12 The implant placement used an advanced software algorithm that automatically registered the patient’s preprocedure CT scan with the navigation system’s real-time optical tracking information (Figure I in the online-only Data Supplement). A CT-opaque marker and an optical reference marker were attached to the patient at a fixed relative displacement using a dental impression of the upper palate and teeth (Figure II in the online-only Data Supplement). The navigation software automatically and instantaneously detected the unique shape of the CT marker and mapped the optical navigation data to the CT coordinate space. The software also facilitated verification of accuracy, and if needed, refinement of the registration. Compared with the software guiding placements in the final segment of the prior ImpACT-24B trial, the automatic detection of registration errors and automatic refinement of the registration was new functionality.
After implantation, active stimulation was administered in daily 4-hour sessions, beginning immediately following the placement procedure and continuing for 5 consecutive days.
In the first treatment session, stimulation parameters started at a low level and were incrementally advanced until physiological evidence for SPG activation was observed: patient-reported tingling sensation over the nose bridge or ipsilateral cheek, ipsilateral lacrimation, or both (Figure 1). Stimulation was then delivered at this CTL in the remainder of the first and all subsequent treatment sessions.
Figure 1.
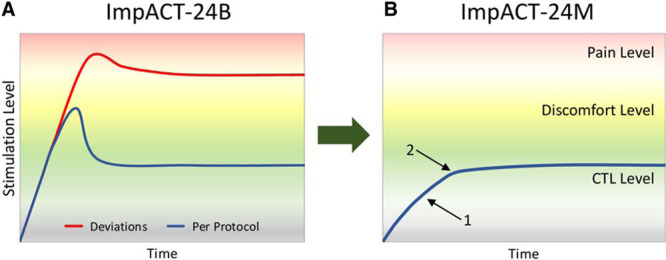
Schematic overview of protocols to determine stimulation intensity comfortable tolerance levels (CTL). A, Protocol used in prior ImpACT-24B trial (Implant for Augmentation of Cerebral Blood Flow Trial B) with escalation to mild facial pain and then de-escalation. Blueline: planned treatment protocol, redline: deviations from treatment protocol observed in 24.9% of patients, who received ongoing stimulation at levels that occasionally evoked facial discomfort and were associated with reduced final disability outcome efficacy. B, Protocol used in current ImpACT-24M (Implant for Augmentation of Cerebral Blood Flow Trial in Mild Strokes) trial with escalation to non-noxious physiological biomarkers of tingling sensation (1) or unilateral lacrimation (2).
At the second treatment day, the ipsilateral cervico-cranial blood flow effect of the stimulation at the CTL was measured by the quantitative common carotid artery (CCA) blood flow technique,14,15 measuring absolute blood flow through the ipsilateral CCA before and during stimulation. With B-mode ultrasound, the diameter of the CCA lumen was quantified 1 cm proximal to the carotid bifurcation. With Doppler ultrasound, the peak systolic and end-diastolic flow velocities were assessed at the same level, averaged over 3 cardiac cycles. From these measures, the absolute blood flow in cc per second through the CCA was calculated (Figure III in the online-only Data Supplement). The carotid studies were performed in each center by a single experienced ultrasonographer. As this was a single-arm trial, the ultrasonographer was not blinded to treatment assignment.
Additionally, at the same treatment session, hand and finger motor strength were quantified before and during stimulation (after 4 hours of stimulation) by measuring hand grasp and thumb pinch force using a hand dynamometer (Baseline Hydraulic Hand Dynamometers, Fabrication Enterprises Inc, White Plains, NY). For comparison, grasp and thumb pinch force were also measured in the nonaffected hand at the same time points.
Following the last treatment on day 5, CT imaging of the pterygoid fossa was performed to assess the attained implant position, rated by a central positioning evaluator blinded to all outcome data. After the day 5 imaging, the implant was removed with fine forceps. Neurological function after treatment was assessed using the NIHSS scale. Final patient follow-up was at 7 days.
Outcomes
The accuracy of electrode implantations using the revised implantation procedure was quantified as the proportion of patients with correct location of implants on anatomic imaging, defined as electrode center point within 5 mm of the pterygopalatine fossa (within which the SPG lies). Speed of electrode implantation was measured as the skin-to-skin time for the placement procedure.
To assess whether an effective SPG stimulation intensity dose-selection for patients can be achieved when guided by non-noxious physiological effects (facial tingling and lacrimation), the physiological efficacy end point assessed was increased ipsilateral cervico-cranial blood flow during stimulation on quantitative CCA assessment.
To assess whether SPG stimulation could produce immediate improvements in neurological motor function, the end points assessed were (1) hand grip strength before and after 2 and 4 hours of stimulation and (2) finger pincer strength before and after 2 and 4 hours of stimulation, evaluated in both the affected and unaffected hands.
To assess the attainability of non-noxious signals of substantial SPG stimulation, the percent of patients with unilateral lacrimation, nasal secretion, or facial redness on the stimulation side during stimulation intensity escalation was evaluated. To probe the intermediate-term clinical effect of SPG stimulation, the efficacy outcome assessed was normalized change in NIHSS between baseline and day 7, comparing enrolled patients with age- and deficit- matched historical controls from the NINDS rt-PA Study (National Institute of Neurological Disorders and Stroke Recombinant Tissue-Plasminogen Activator) control group.16 NIHSS scoring in ImpACT-24M patients was performed by the same certified evaluators at day 1 and day 7. Matching to NINDS-Study control patients was performed using 1:1 optimal inverse variance matching, an algorithmic approach highly proficient in balancing covariate values between groups. Calipers were set for age at ±5 years and for NIHSS at ±1. The NIHSS scores were matched for time from onset. ImpACT-24M patients 4 to 12 hours post-onset were matched to NINDS control patients based on NIHSS scores 2 hours after placebo drug (3.5–5 hours after onset); ImpACT-24M patients 12.1 to 24 hours post-onset were matched to NINDS control patients based on NIHSS scores 24 hours after onset.
For safety analysis, rates of the following outcomes by day 7 were compared with those in the active and sham stimulation groups of the ImpACT-24B trial: all serious adverse events; implantation-related adverse events; stimulation-related adverse events; mortality; neurological deterioration (defined as worsening of the NIHSS by ≥4 points); and symptomatic intracranial hemorrhage (defined as neurological worsening of any degree assessed by the local clinician-investigator as causally related to intracranial hemorrhage of any degree).
Statistical Analysis
A sample size of 50 patients was judged sufficient to characterize speed and accuracy of electrode placement. If a learning curve was noted with improving accuracy or accelerating speed during initial procedures, up to 50 additional patients would be recruited until stable performance on 50 consecutive patients was attained or a total of 100 patients recruited.
Patients enrolled with known atrial fibrillation did not undergo CCA measurements and were excluded from the blood flow analysis because of waveform variability making quantification less accurate; they did contribute to all other study outcomes. Patients who were not able to cooperate with the dynamometer motor strength testing were excluded from the motor function analysis. Changes in CCA flow and in fine motor function were assessed as continuous variables (using paired t test) and as dichotomized variables using a 20% change threshold for presence of moderate to substantial alteration (using χ2 analysis). The 20% threshold accords with that commonly used to guide induced hypertension in patients with vasospasm after subarachnoid hemorrhage, the most common cerebral blood flow augmentation clinical treatment setting.17
Results
Fifty patients were enrolled between May 2018 and September 2018.
Median age was 66 years (interquartile range [IQR], 60–74), 44% were female, baseline deficit severity on the NIHSS was median 5 (IQR, 4–5), and time from last known well to screening was median 18 hours (IQR, 9–20). Four patients (8%) had a medical history of atrial fibrillation.
Within the 7-day follow-up period, there were no cases of mortality, neurological deterioration, symptomatic intracranial hemorrhage, or stimulation-related adverse events (including pain). One serious adverse event of a new stroke was reported, unrelated to the implantation or treatment. Nine nonserious adverse events were reported, 1 related to the implantation (transient mild nausea).
All 50 implantations were completed successfully (100% on-target placement), with median skin-to-skin time of 4 minutes (IQR, 3–7), and all patients completed 5 treatment sessions.
Non-noxious facial physiological responses were evoked as stimulation intensity was increased in 96% (48/50) of the patients, including 64% (32/50) who had ipsilateral tingling sensation and lacrimation and 32% (16/50) who had tingling sensation only. In 4% (2/50), stimulation intensity escalation reached 100% of electrode output without evoking facial tingling, lacrimation, or discomfort.
The distribution of stimulation intensities at which non-noxious physiological responses identifying a CTL encountered is shown in Figure 2. The mean CTL was 35% (±SD 20%) of electrode maximum. Overall, the CTL in 86% (43/50) of the patients fell within the intensity range associated in the ImpACT-24B trial with a 1.5-fold or greater increase in the odds ratio of a favorable outcome associated with SPG stimulation.
Figure 2.
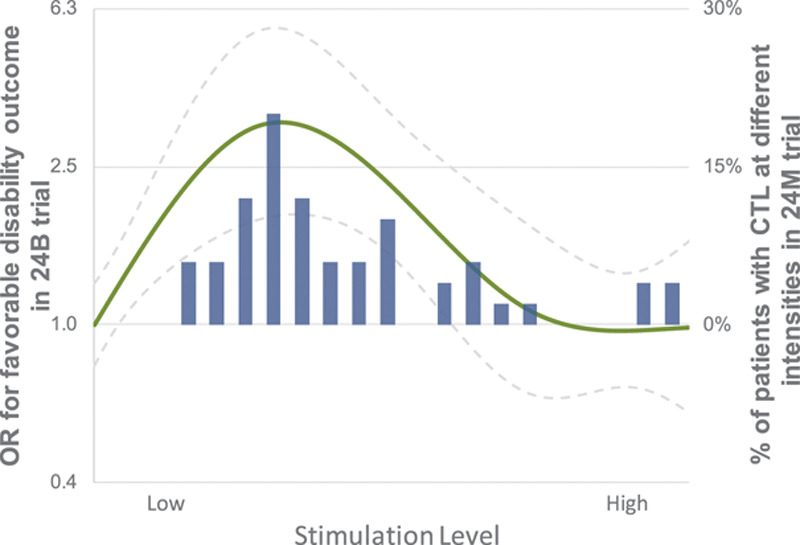
Distribution of attained comfortable tolerance level (CTL) stimulation intensities in ImpACT-24M (Implant for Augmentation of Cerebral Blood Flow Trial in Mild Strokes) and clinical efficacy dose-response curve (for favorable 3-month disability outcome) observed in ImpACT-24B trial (Implant for Augmentation of Cerebral Blood Flow Trial B). Blue bar histogram shows ImpACT-24M CTL stimulation intensities from 0-100% of electrode maximum in bin sizes of 5%. Solid green line shows odds ratios (OR) for favorable 3-month disability outcome at different stimulation intensities in the ImpACT-24B (and dotted lines show 95% CI).
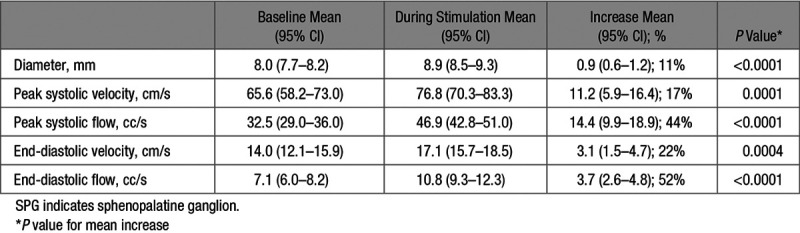
Among the 46 patients eligible for quantitative CCA blood flow measurements, 45/46 (98%) had valid measurements before and during treatment at their CTL. Stimulation was associated with increase in CCA vessel diameter and increase in flow velocity and flow volume in the CCA during both peak systole and end-diastole (Table 1).
Table 1.
Changes in Ipsilateral Common Carotid Artery Diameter, Flow Velocity, and Flow Volume With SPG Stimulation
Forty-seven patients (94%) underwent grasp and pinch motor evaluation before stimulation, after 2 hours of stimulation, and after 4 hours of stimulation (Figure 3). Mean pinch force in the affected hand increased by 1.3 (95% CI, 0.9–1.7) lbs (30%) after 2 hours of stimulation and by 1.8 (95% CI, 1.3–2.2) lbs (42%) after 4 hours of stimulation (both P<0.0001). Mean grasp force in the affected hand increased by 2.5 (95% CI, 1.4–3.7) lbs (15%) after 2 hours of stimulation and by 4.5 (95% CI, 3.2–5.8) lbs (26%) after 4 hours of stimulation (both P<0.0001). In contrast, in the unaffected hand, mean pinch force did not increase (changed by 0.2 and −0.1 lbs; P=0.77) and mean grasp force did not increase (changed by −0.2 and 1.1 lbs; P=0.10).
Figure 3.
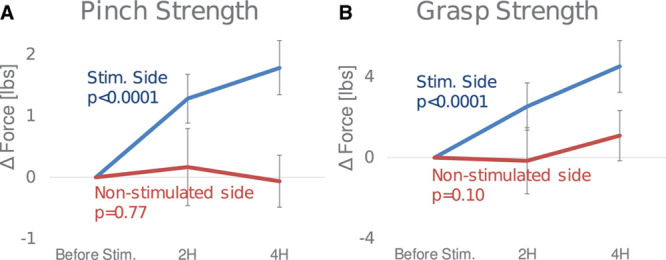
Effect of sphenopalatine ganglion stimulation on hand strength on affected and nonaffected sides. A, Pinch strength change from baseline to 2 h and change from baseline to 4 h, (B) grasp strength change from baseline to 2 h and change from baseline to 4 h. Error bars indicate the 95% CI of the difference compared with baseline measurement.
A significant relation was observed between the degree of improvement in blood flow augmentation and the degree of improvement in hand motor strength (Table 2; Figure 4).
Table 2.
Relation Between Substantial Improvement in Affected Hand Strength and Increases in Cranial Blood Flow During SPG Stimulation*
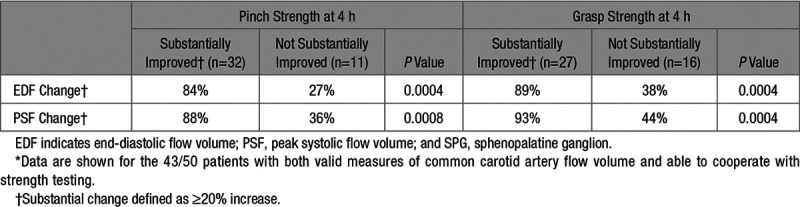
Figure 4.
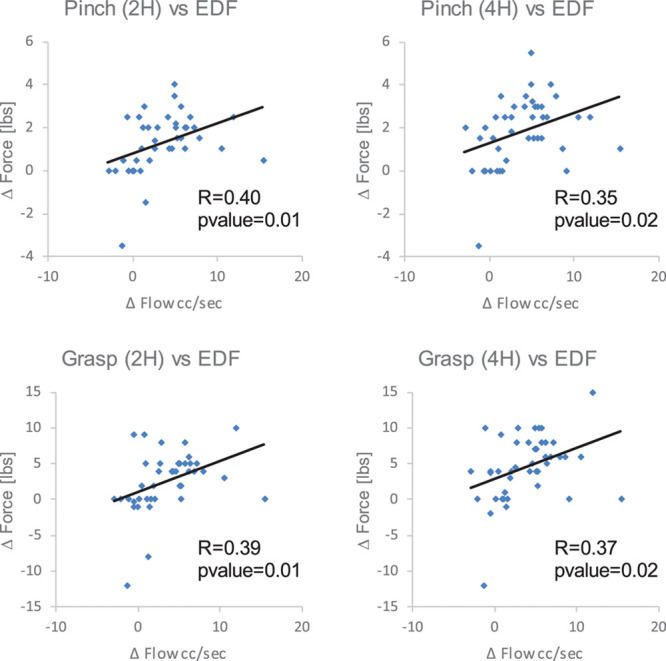
Scatterplots and linear trend lines showing relation between increase in common carotid artery diastolic blood flow volume and hand strength: (A): pinch strength, (B) grasp strength. EDF indicates end-diastolic flow volume.
In the NIHSS evolution analysis, matching yielded 98 patients, including 49 out of 50 patients treated with SPG stimulation in the current trial and 49 out of 312 treated with supportive care in the NINDS rt-PA Study. The SPG stimulation and control patients were well balanced in age (mean 66.9±8.4 versus 67.3±8.7) and in day 1 NIHSS [median 5 (IQR, 4–5) versus 5 (IQR, 4–6). Evolution of the NIHSS in the SPG stimulation patients was from median 5 (IQR, 4–5) on day 1 to median 1 (IQR, 1–2) on day 7; evolution of the NIHSS in the control patients was from median 5 (IQR, 4–6) on day 1 to median 2 (IQR, 2–4) on day 7. The normalized change in NIHSS from day 1 to day 7 was significantly more favorable in the SPG stimulation than control patients: median 75% (IQR, 60%–80%) versus 50% (IQR, 0%–67%), P=0.0003.
Discussion
In this study, sphenopalatine electrode placement using a refined automatic registration system was highly effective, with correct electrode position obtained in all patients in a rapidly performed procedure lasting less than 5 minutes on average. In addition, the trial found that non-noxious physiological end points of ipsilateral facial tingling or lacrimation could be used to determine a comfortable and effective stimulation intensity level for each patient, with no events of facial discomfort and a mean 44% increase in ipsilateral cervico-cranial blood flow at the selected intensities. Furthermore, the stimulation was associated with substantial improvement in grasp and pincer motor strength in the affected hand during the stimulation period.
The dose-escalation protocol to determine the CTL for intensity of SPG stimulation in the current trial has 2 important advantages over that used in the prior ImpACT-24A and 24B trials. First, it avoids the elicitation of even mild facial discomfort during the initial dose ramp-up, improving procedural tolerability for patients. Second, it lessens the chance that too high a final CTL level will be selected. In the ImpACT-24B trial, when high-stimulation intensities were used as the CTL, some patients developed facial discomfort during later treatment sessions, although the intensities were not associated with discomfort initially. Moreover, high-stimulation intensities were associated with less or null effect on improving final disability outcome, as they fell on the descending upper portion of the inverted U-shaped dose-response curve for clinical benefit. In the current study, with selection based on non-noxious facial physiological biomarkers, compared with the ImpACT-24B trial, CTL levels were more often in the intensity range associated with a ≥1.5-fold increase in the odds of improved final outcome, 86% versus 44%, P<0.0001.
The current study additionally provides insight into the biological mechanisms underlying the improved final disability outcomes associated with SPG stimulation in prior trials. SPG stimulation produces a robust increase in cervico-cranial blood flow, supporting that cerebral vasodilatation induced by neuronal stimulation leads to collateral enhancement and improved perfusion. The correlation of degree of improvement in hand motor strength during stimulation with degree of cervico-cranial blood flow increase during stimulation suggests that the improved collateral blood flow can restore neuronal function in ischemic fields. The study also affords new information related to the pragmatics of delivering SPG stimulation in acute stroke clinical settings. With the real-time optical tracking mapped to each individual’s pterygoid fossa anatomy visualized on CT, stimulator electrodes were placed by study physicians highly accurately and rapidly. In the first studies of SPG stimulation in patients with ischemic stroke, the electrode placement procedure took around 40 minutes. But this time has been substantially reduced, and placement accuracy enhanced, through several serial technological advancements. With the further refinement of real-time optical tracking used in this study, procedure time was shortened to just 5 minutes. Such efficient procedure conduct is desirable for integration of SPG stimulation into care workflow for acute cerebral ischemia patients. For example, the achieved rapidity of placement makes feasible a strategy of initiating SPG stimulation at a primary stroke center before patient transfer for endovascular intervention at a thrombectomy stroke center. A randomized trial is now underway, testing this bridging collateral enhancement treatment strategy.
This study has limitations. First, it was not a randomized, controlled trial. However, selecting a CTL of stimulation by use of evoked, non-noxious facial physiological effects can only be achieved in active SPG-stimulated patients. Second, consequently, the cervico-cranial blood flow and hand motor strength outcomes were assessed in a nonblinded manner. However, that the affected hand strength improvements reflect a genuine amelioration in motor function is supported by the absence of improvements in the contralateral hand and the correlation of the degree of strength improvement with degree of blood flow increase. Third, the cervico-cranial blood flow measurements were performed only ipsilaterally, and therefore, the results might be influenced by systemic hemodynamic effects, in addition to ipsilateral vasodilatory effects. However, a large prior trial found no significant change in heart rate or blood pressure during SPG stimulation.13 Fourth, quantitative CCA is known to tend to overestimate flow volume,14 but that would not affect the assessment of proportional change from baseline to stimulation. Fifth, differences in NIHSS outcome by day 7 between the SPG stimulation and historical control patients may, in part, reflect differences in concomitant supportive care that have developed over intervening years in addition to effects of stimulation.
In conclusion, this study found that SPG stimulator placement with real-time optical tracking guidance was speedy and accurate, and selection of stimulation intensity levels based on non-noxious facial tingling and lacrimation was feasible in nearly all patients. The great preponderance of selected stimulation intensities fell within the optimal stimulation range for improved final disability outcome observed in the ImpACT-24B pivotal trial. The current study further validated that SPG stimulation at CTL leads to a substantial increase in cervico-cranial blood flow and improvement in hand motor function. The results indicate that SPG stimulation can be deployed in a simple and practical manner in the clinical setting, facilitating potential widespread availability of this emerging therapy.
Acknowledgments
We thank the participating patients and families and the research staff at the participating trial sites.
Sources of Funding
The study was funded by BrainsGate Ltd, Caesarea, Israel.
Disclosures
Drs Saver and Bornstein received contracted hourly payments from BrainsGate for service on the ImpACT-24 (Implant for Augmentation of Cerebral Blood Flow Trial) steering committee. Dr Solberg is a paid employee of BrainsGate with ownership of stock options and patents assigned to BrainsGate; his institution, Brainsgate Ltd is the study sponsor. The institutions of Drs Kharaishvili, Janelidze, Beridze, and Zarqua received payments for study services on the basis of clinical trial contracts with BrainsGate.
Supplementary Material
Appendix
The ImpACT-24M Investigators include the following Site Principal Investigators and Co-Investigators: Rustavi Central Hospital, Rustavi, Georgia: Natia Khachidze, MD, Ivane Avazashvili, MD; Kutaisi Referral Hospital, Kutaisi, Georgia: Paata Meshviliani, MD, Ekaterine Mamardashvili, MD; First University Clinic, Tbilisi, Georgia: Nino Beridze, MD, Ivane Avazashvili, MD; Zugdidi Referral Hospital, Zugdidi, Georgia: Tamaz Gulua, MD, Giorgi Phiphia, MD.
Footnotes
A list of all IMPACT-24M Trial Investigators is given in the Appendix.
Guest Editor for this article was Sean I. Savitz, MD.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.119.027177.
Contributor Information
Natia Khachidze, Rustavi Central Hospital, Rustavi, Georgia.
Ivane Avazashvili, Rustavi Central Hospital, Rustavi, Georgia.
Paata Meshviliani, Kutaisi Referral Hospital, Kutaisi, Georgia.
Ekaterine Mamardashvili, Kutaisi Referral Hospital, Kutaisi, Georgia.
Nino Beridze, First University Clinic, Tbilisi, Georgia.
Ivane Avazashvili, First University Clinic, Tbilisi, Georgia.
Tamaz Gulua, Zugdidi Referral Hospital, Zugdidi, Georgia.
Giorgi Phiphia, Zugdidi Referral Hospital, Zugdidi, Georgia.
Collaborators: the IMPACT-24M Trial Investigators, Natia Khachidze, Ivane Avazashvili, Paata Meshviliani, Ekaterine Mamardashvili, Nino Beridze, Ivane Avazashvili, Tamaz Gulua, and Giorgi Phiphia
References
- 1.Krishnamurthi RV, Feigin VL, Forouzanfar MH, Menash GE, Connor M, Bennett DA, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the global burden of disease study 2010. Lancet Glob Health. 2013;1:e259–e281. doi: 10.1016/S2214-109X(13)70089-5. doi: 10.1016/S2214-109X(13)70089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, et al. European Stroke Organisation (ESO)- European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg. 2019;11:535–538. doi: 10.1136/neurintsurg-2018-014568. doi: 10.1136/neurintsurg-2018-014568. [DOI] [PubMed] [Google Scholar]
- 3.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. American Heart Association Stroke Council. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. doi: 10.1161/STR.0000000000000158. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 4.Rai AT, Seldon AE, Boo S, Link PS, Domico JR, Tarabishy AR, et al. A population-based incidence of acute large vessel occlusions and thrombectomy eligible patients indicates significant potential for growth of endovascular stroke therapy in the USA. J Neurointerv Surg. 2017;9:722–726. doi: 10.1136/neurintsurg-2016-012515. doi: 10.1136/neurintsurg-2016-012515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shuaib A, Butcher K, Mohammad AA, Saqqur M, Liebeskind DS. Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. Lancet Neurol. 2011;10:909–921. doi: 10.1016/S1474-4422(11)70195-8. doi: 10.1016/S1474-4422(11)70195-8. [DOI] [PubMed] [Google Scholar]
- 6.Seylaz J, Hara H, Pinard E, Mraovitch S, MacKenzie ET, Edvinsson L. Effect of stimulation of the sphenopalatine ganglion on cortical blood flow in the rat. J Cereb Blood Flow Metab. 1988;8:875–878. doi: 10.1038/jcbfm.1988.145. doi: 10.1038/jcbfm.1988.145. [DOI] [PubMed] [Google Scholar]
- 7.Levi H, Schoknecht K, Prager O, Chassidim Y, Weissberg I, Serlin Y, et al. Stimulation of the sphenopalatine ganglion induces reperfusion and blood-brain barrier protection in the photothrombotic stroke model. PLoS One. 2012;7:e39636. doi: 10.1371/journal.pone.0039636. doi: 10.1371/journal.pone.0039636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diansan S, Shifen Z, Zhen G, Heming W, Xiangrui W. Resection of the nerves bundle from the sphenopalatine ganglia tend to increase the infarction volume following middle cerebral artery occlusion. Neurol Sci. 2010;31:431–435. doi: 10.1007/s10072-010-0238-0. doi: 10.1007/s10072-010-0238-0. [DOI] [PubMed] [Google Scholar]
- 9.Henninger N, Fisher M. Stimulating circle of Willis nerve fibers preserves the diffusion-perfusion mismatch in experimental stroke. Stroke. 2007;38:2779–2786. doi: 10.1161/STROKEAHA.107.485581. doi: 10.1161/STROKEAHA.107.485581. [DOI] [PubMed] [Google Scholar]
- 10.Bar-Shir A, Shemesh N, Nossin-Manor R, Cohen Y. Late stimulation of the sphenopalatine-ganglion in ischemic rats: improvement in N-acetyl-aspartate levels and diffusion weighted imaging characteristics as seen by MR. J Magn Reson Imaging. 2010;31:1355–1363. doi: 10.1002/jmri.22110. doi: 10.1002/jmri.22110. [DOI] [PubMed] [Google Scholar]
- 11.Bornstein NM, Saver JL, Diener HC, Gorelick PB, Shuaib A, Solberg Y, et al. Sphenopalatine ganglion stimulation to augment cerebral blood flow: a randomized, sham-controlled trial. Stroke. 2019;50:2108–2117. doi: 10.1161/STROKEAHA.118.024582. [DOI] [PubMed] [Google Scholar]
- 12.Bornstein NM, Saver JL, Diener HC, Gorelick PB, Shuaib A, Solberg Y, et al. ImpACT-24B investigators. An injectable implant to stimulate the sphenopalatine ganglion for treatment of acute ischaemic stroke up to 24 h from onset (ImpACT-24B): an international, randomised, double-blind, sham-controlled, pivotal trial. Lancet. 2019;394:219–229. doi: 10.1016/S0140-6736(19)31192-4. doi: 10.1016/S0140-6736(19)31192-4. [DOI] [PubMed] [Google Scholar]
- 13.Robbines MS, Robertson CE, Kaplan E, Ailani J, Chrleston L, Kuruvilla D, et al. The sphenopalatine ganglion: anatomy, pathophysiology, and therapeutic targeting in headache. Headache. 2012;2:240–258. doi: 10.1111/head.12729. doi: 10.1111/head.12729. [DOI] [PubMed] [Google Scholar]
- 14.Schöning M, Walter J, Scheel P. Estimation of cerebral blood flow through color duplex sonography of the carotid and vertebral arteries in healthy adults. Stroke. 1994;25:17–22. doi: 10.1161/01.str.25.1.17. doi: 10.1161/01.str.25.1.17. [DOI] [PubMed] [Google Scholar]
- 15.Scheel P, Ruge C, Petruch UR, Schöning M. Color duplex measurement of cerebral blood flow volume in healthy adults. Stroke. 2000;31:147–150. doi: 10.1161/01.str.31.1.147. doi: 10.1161/01.str.31.1.147. [DOI] [PubMed] [Google Scholar]
- 16.Saver JL, Gornbein J, Starkman S. Graphic reanalysis of the two NINDS-tPA trials confirms substantial treatment benefit. Stroke. 2010;41:2381–2390. doi: 10.1161/STROKEAHA.110.583807. doi: 10.1161/STROKEAHA.110.583807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhar R, Scalfani MT, Zazulia AR, Videen TO, Derdeyn CP, Diringer MN. Comparison of induced hypertension, fluid bolus, and blood transfusion to augment cerebral oxygen delivery after subarachnoid hemorrhage. J Neurosurg. 2012;116:648–656. doi: 10.3171/2011.9.JNS11691. doi: 10.3171/2011.9.JNS11691. [DOI] [PMC free article] [PubMed] [Google Scholar]


