Supplemental Digital Content is available in the text.
Keywords: atherothrombotic stroke, cardiac emboli, collateral circulation, endovascular treatment, ischemic stroke
Background and Purpose—
Due to chronic hypoperfusion, cervical atherosclerosis may promote cerebral collateral circulation. We hypothesized that patients with ischemic stroke due to cervical carotid atherosclerosis have a more extensive collateral circulation and better outcomes than patients with cardioembolism. We tested this hypothesis in a population of patients who underwent endovascular treatment for large vessel occlusion.
Methods—
From the MR-CLEAN Registry (Multicenter Randomized Controlled Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands), we selected consecutive adult endovascular treatment patients (March 2014 to June 2016) with acute ischemic stroke due to anterior circulation large vessel occlusion and compared patients with cervical carotid artery stenosis >50% to those with cardioembolic etiology. The primary outcome was collateral score, graded on a 4-point scale. Secondary outcomes included the modified Rankin Scale (mRS) score and mortality at 90 days. We performed multivariable regression analyses and adjusted for potential confounders.
Results—
Of 1627 patients in the Registry, 190 patients with cervical carotid atherosclerosis and 476 with cardioembolism were included. Patients with cervical carotid atherosclerosis were younger (median 69 versus 76 years, P<0.001), more often male (67% versus 47%, P<0.001), more often had an internal carotid artery terminus occlusion (33% versus 18%, P<0.001), and a lower prestroke mRS (mRS score, 0–2; 96% versus 85%, P<0.001), than patients with cardioembolism. Stroke due to cervical carotid atherosclerosis was associated with higher collateral score (adjusted common odds ratio, 1.67 [95% CI, 1.17–2.39]) and lower median mRS at 90 days (adjusted common odds ratio, 1.45 [95% CI, 1.03–2.05]) compared with cardioembolic stroke. There was no statistically significant difference in proportion of mRS 0–2 (aOR, 1.36 [95% CI, 0.90–2.07]) or mortality at 90 days (aOR, 0.80 [95% CI, 0.48–1.34]).
Conclusions—
Patients with stroke due to cervical carotid atherosclerosis had a more extensive cerebral collateral circulation and a slightly better median mRS at 90 days than patients with cardioembolic stroke.
Underlying etiology contributes to the outcome of patients after ischemic stroke. In general, patients with ischemic stroke of cardioembolic origin have worse functional outcomes,1,2 higher recurrence rates, and a higher risk of death than patients with ischemic stroke of other origin. However, little is known on the impact of stroke etiology on functional outcome of patients with stroke who underwent endovascular treatment (EVT).3
In patients with ischemic stroke caused by an occlusion of a proximal intracranial artery treated with EVT, higher collateral scores are associated with a greater chance of a better functional outcome,4–6 presumably because intracranial (leptomeningeal and pial) collateral arteries contribute to prolonged preservation of ischemic brain tissue at risk of infarction.7,8 Experimental studies in an animal model of bilateral common carotid artery occlusion have found that chronic cerebral hypoperfusion promotes formation of new and recruitment of existing intracranial collateral arteries.9 Cervical carotid atherosclerosis in humans develops over decades and is often accompanied by arterial stenosis. Theoretically, this might promote the cerebral collateral circulation. In contrast, since cardioembolic stroke is not accompanied by chronic cerebral hypoperfusion, collateral artery formation and recruitment are less likely in these patients.
We hypothesized that patients with ischemic stroke due to cervical stenotic carotid atherosclerosis have a more extensive collateral circulation than patients with stroke due to cardioembolism. We explored this hypothesis in a large sample of patients who underwent EVT for acute ischemic stroke with large vessel occlusion (LVO). We further assessed whether the presumed cause of stroke was associated with clinical, radiological, and procedural outcomes after EVT.
Methods
Data will not be made available to other researchers, as no patient approval was obtained for sharing coded data. However, syntax and output files of statistical analyses may be made available on request.
Patient Selection
We used data of the MR-CLEAN Registry (Multicenter Randomized Controlled Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands), a prospective, nationwide registry of consecutive stroke patients treated with EVT in the Netherlands. For the current study, data of patients who underwent EVT between March 16, 2014, and June 15, 2016, were used. We included adult patients with an LVO of the anterior circulation (internal carotid artery/internal carotid artery terminus [ICA/ICA-T], middle cerebral artery [M1/M2], anterior cerebral artery [A1/A2]), confirmed by computed tomography angiography (CTA), who were treated in a MR-CLEAN trial hospital, and had a cervical carotid stenosis greater than 50% due to atherosclerosis, or a cardiac source of stroke. The study protocol has been evaluated by the medical ethics committee of the Erasmus University Medical Center in Rotterdam, and permission to carry out the study as a registry was granted. All imaging was assessed by an imaging core laboratory, whose members were blinded to clinical findings, except for side of symptoms. Detailed methods of the MR-CLEAN Registry have been reported previously.10
Stroke Etiology Assessment
All patients underwent CTA of the cervical arteries and 12-lead electrocardiography. Additional etiologic work-up was performed according to local protocols. Stroke etiology was determined from information in discharge letters and from reports of the imaging core laboratory. We used a modification of the TOAST (Trial of ORG 10172 in Acute Stroke Treatment) criteria11 to categorize etiology into cervical large-artery atherosclerosis, cardioembolism, stroke of other determined cause, or undetermined cause (2 or more causes identified, negative, or incomplete evaluation). A patient was considered to have stroke due to cervical carotid atherosclerosis if there was >50% atherosclerotic stenosis or occlusion at the bifurcation of the carotid artery on the symptomatic side, as confirmed by core lab adjudication. Patients with high- or medium-risk cardioembolic stroke sources were classified as having cardioembolic stroke.11
Assessment of Collateral Circulation, Outcomes, and Thrombus Perviousness
Our primary outcome was collateral score, graded on baseline CTA by the imaging core laboratory on a 4-point scale, with 0 for absent collaterals (0% filling of the occluded vascular territory), 1 for poor (>0% and ≤50% filling), 2 for moderate (>50% and <100% filling), and 3 for good collaterals (100% filling), as used previously.5,6,12 We also dichotomized the collateral scores into poor (grade 0–1) versus good (grade 2–3).
Clinical outcomes were the difference between National Institutes of Health Stroke Scale (NIHSS) score at baseline and at 24 to 48 hours (ΔNIHSS); modified Rankin Scale (mRS) score at 90 days; functional independence at 90 days (defined as an mRS score of 0–2); mortality at 90 days; and symptomatic intracranial hemorrhage. Intracranial hemorrhage was considered symptomatic if patients died or deteriorated neurologically (a decline of at least 4 points on the NIHSS), and the hemorrhage was related to the clinical deterioration (according to the Heidelberg criteria13).
Radiological outcomes were the proportion of patients with an extended Thrombolysis in Cerebral Infarction (eTICI) score of ≥2B and ≥2C.14 Procedural outcomes were the number of passes with a stent retriever; first-pass effect,15 defined as single pass/use of the device as first line of EVT, resulting in complete reperfusion (eTICI 3) of the LVO and its downstream territory and no use of rescue therapy after use of the device; and EVT procedure duration from groin puncture to successful reperfusion (eTICI ≥2B) or last contrast bolus (when successful reperfusion was not achieved or no target occlusion was observed during the intervention).
To explore differences in thrombus imaging characteristics between cervical carotid atherosclerosis patients and patients with cardioembolism, we compared thrombus perviousness on baseline CTA. Thrombus perviousness is an imaging biomarker that estimates the extent to which a thrombus allows flow through the thrombus. This is measured as the thrombus attenuation increase (TAI or Δ) in Hounsfield units in the thrombus on CTA compared to noncontrast CT (Δ=ρthrombusCTA−ρthrombusNCCT).16
Statistical Analysis
For the main analysis, we compared patients with cervical carotid atherosclerosis to patients with cardioembolic stroke. In line with an analysis previously performed in the NASCET (North American Symptomatic Carotid Endarterectomy Trial) in a nonacute ischemic stroke population with carotid artery stenosis,17 in a sensitivity analysis we compared collateral status and clinical outcomes of patients with moderate (51%–70%) to those with severe (71%–99%) stenosis within the sample of patients with cervical carotid atherosclerosis. Last, we analyzed clinical outcome between patients with cervical carotid atherosclerosis and cardioembolic stroke patients, within the sample of patients with incomplete reperfusion (eTICI 0–2A), since these patients would theoretically be most reliant on their collateral flow for preserving penumbral tissue.
Baseline characteristics were described using standard statistics. The shift on the full mRS, measured with a common odds ratio (cOR), was estimated with ordinal logistic regression. We performed binary logistic regression for dichotomous outcome measures and linear regression for continuous outcome measures. Variables for adjustment were chosen based on theoretical identification using directed acyclic graphs.18 For associations with collateral status, we adjusted for age, history of stroke, and occlusion location. For clinical outcomes (ΔNIHSS, mRS, functional independence, and mortality), we adjusted for age, history of peripheral artery disease, history of myocardial infarction, prior use of anticoagulant medication (vitamin K antagonists or direct oral anticoagulants), occlusion location, onset-to-groin-puncture time and hyperdense artery sign. For symptomatic intracranial hemorrhage, we adjusted for history of myocardial infarction. For successful reperfusion and procedural outcomes, we adjusted for age and occlusion location.
Missing data were imputed using multiple imputation based on relevant covariates and outcome. Adjusted (a)ORs and betas (β) are reported with 95% CI, and all P values are 2-sided. Statistical analyses were performed using IBM SPSS Statistics for Windows, version 24.0.
Results
Of the 1627 patients in the MR-CLEAN Registry, 198 were excluded because of age under 18 years, posterior circulation occlusion, treatment in a non–MR-CLEAN trial hospital or because their discharge letter was not available to determine stroke etiology (Figure 1). Of the remaining 1429 patients, 190 (13%) had cervical carotid atherosclerosis, and 476 (33%) had cardioembolism. Among the patients with cardioembolism, 362 (76%) had atrial fibrillation (newly diagnosed in 111). Other causes of cardioembolic stroke are listed in Table I in the online-only Data Supplement. Stroke of other determined etiology occurred in 67 (5%) patients, of whom 44 had carotid artery dissection. In 696 (49%) patients, the cause was undetermined; 78 had more than one potential cause and in 618 the assessment was negative or incomplete.
Figure 1.
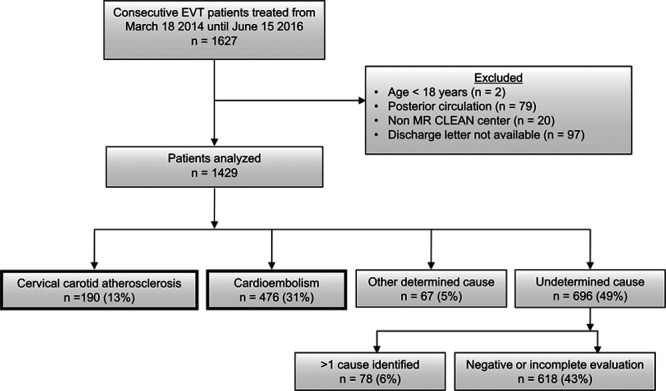
Flowchart of patient selection. Patients with cervical carotid atherosclerotic and cardioembolic stroke cause were included in the study. EVT indicates endovascular treatment; and MR CLEAN Registry, Multicenter Randomized Controlled Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands.
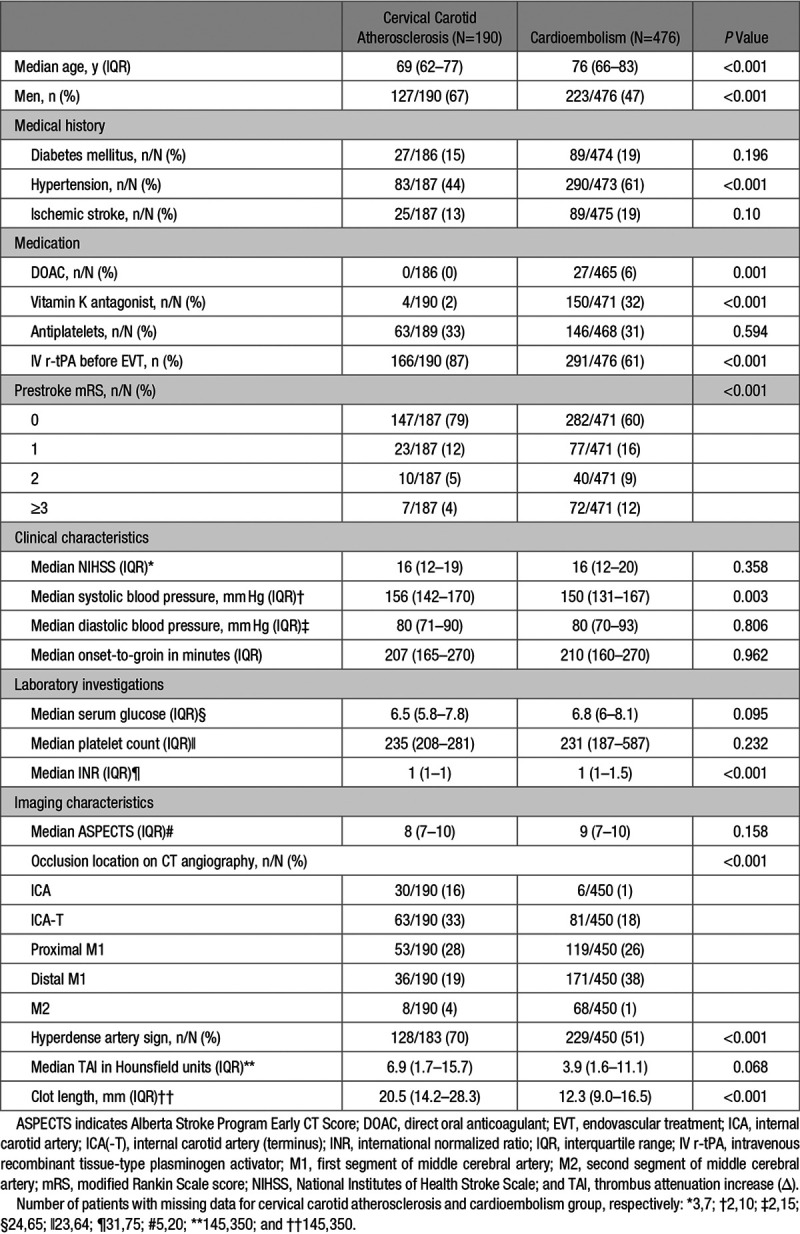
Patients with cervical carotid atherosclerosis were younger (median 69 versus 76 years, P<0.001) and more often male (127/190 [67%] versus 223/476 [47%], P<0.001); had lower prestroke mRS scores (mRS score of 0–2, 180/187 [96%] versus 399/471 [85%], P<0.001), and more often had an ICA/ICA-T occlusion (93/190 [49%] versus 87/450 [19%], P<0.001), than patients with cardioembolic stroke; Table 1.
Table 1.
Baseline Characteristics
We found a significant shift towards better collateral scores in favor of stroke due to cervical carotid atherosclerosis (adjusted common odds ratio, 1.67 [95% CI, 1.17–2.39]; Figure 2). Also when scores were dichotomized into good (grade 2–3) and poor (grade 0–1), patients with cervical carotid atherosclerosis had significantly more often good collateral scores than those with cardioembolic stroke (130/184 [71%] versus 266/441 [60%], aOR, 1.84 [95% CI, 1.15–2.94]).
Figure 2.
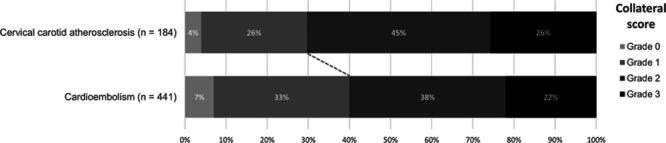
Collateral circulation for patients with stroke due to cervical carotid atherosclerosis vs stroke due to cardioembolism. Collateral score was graded by the imaging core laboratory on a 4-point scale, with 0 for absent (0% filling of the occluded vascular territory), 1 for poor (>0% and ≤50% filling), 2 for moderate (>50% and <100% filling), and 3 for good collaterals (100% filling).
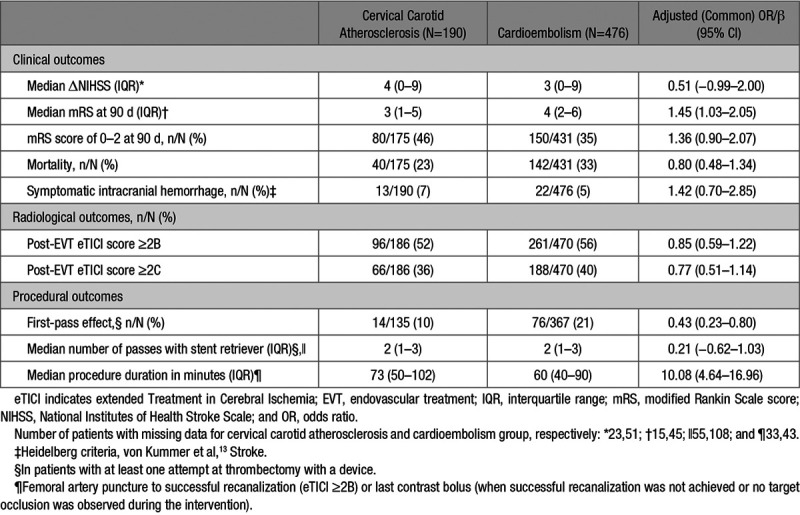
Patients with cervical carotid atherosclerotic stroke had a lower median mRS at 90 days than cardioembolic stroke patients (3 versus 4, adjusted common odds ratio, 1.45 [95% CI, 1.03–2.05]; Table 2). There were no statistically significant differences in the proportions of patients with mRS score of 0–2 (46% versus 35%, aOR, 1.36 [95% CI, 0.90–2.07]) or mortality (23% versus 33%, aOR, 0.80 [95% CI, 0.48–1.34]) at 90 days between cervical carotid atherosclerotic and cardioembolic stroke. In patients with cervical carotid atherosclerosis a first-pass effect was achieved less frequently (10% versus 21%, aOR, 0.43 [95% CI, 0.23–0.80]), and median procedure duration was longer (73 versus 60 minutes, adjusted β=10.08 [95% CI, 4.64–16.96]) compared to patients with stroke because of cardioembolism. There were no significant differences in any of the other clinical or radiological outcomes. Among the 82 patients with cervical carotid atherosclerosis who had a 51% to 99% stenosis, a slightly larger proportion of patients with a severe (71%–99%) stenosis had a good (grade 2–3) collateral status compared with those with a moderate (51%–70%) stenosis (75% versus 67%, P=0.423), but this difference disappeared after adjustment for confounders (aOR, 1.06 [95% CI, 0.39–2.90]). A larger proportion of patients with a severe stenosis had mRS score of 0–2 at 90 days, although this difference was not statistically significant (62% versus 41%, aOR, 1.66 [95% CI, 0.49–5.57]). Finally, in 299 patients with incomplete reperfusion, functional outcome at 90 days was better for patients with cervical carotid atherosclerosis than for cardioembolic stroke patients (median mRS score of 4 versus 5, adjusted common odds ratio, 2.12 [95% CI, 1.17–3.83]; Tables II through V and Figure I in the online-only Data Supplement).
Table 2.
Clinical, Radiological, and Procedural Outcomes
Discussion
In line with our hypothesis, we found that patients who underwent EVT for anterior circulation LVO caused by cervical large-artery atherosclerosis had a more extensive cerebral collateral circulation and a better functional outcome at 90 days than those with cardioembolic stroke. We found no statistically significant difference in functional independence (mRS score of 0–2) or mortality between the groups.
The association between cervical large-artery atherosclerosis and better collateral circulation compared with cardioembolic stroke has been suggested previously in 2 small cohort studies (N=15819 and 12220, respectively). However, both studies did not provide analyses adjusted for confounders for this association, which limits the interpretation of the results. In addition, one of these studies20 only examined patients with atrial fibrillation and did not include other cardioembolic sources of stroke. Furthermore, our study differs from these studies in terms of patient population (proportion of patients with LVO) and use of a different collateral grading scale.
In our study, patients with cervical carotid atherosclerotic stroke were younger and more often male than patients with cardioembolic stroke, which is consistent with previous studies.21 Prestroke mRS scores were lower in cervical carotid atherosclerosis patients, possibly, in part, due to younger age and less comorbidity. Patients with cervical carotid atherosclerotic stroke received IV r-tPA (intravenous recombinant tissue-type plasminogen activator) more frequently than patients with cardioembolic stroke, which is explained by oral anticoagulation use in the latter group. Notably, there was no difference in baseline NIHSS score between cervical carotid atherosclerotic and cardioembolic stroke patients. In studies using data of non-EVT populations, cardioembolic stroke is generally reported to present with more severe deficits than stroke of other origins.1,2 This is explained by the fact that cardioembolic stroke is usually associated with relatively large thrombi resulting more often in LVO compared with stroke of other etiology.22 As our study population consisted solely of patients with a LVO, this likely explains why we did not observe a difference in severity of deficits between cervical large-artery atherosclerosis and cardioembolic stroke. In fact, in our study, we found a higher occurrence of intracranial internal carotid artery and terminal internal carotid artery occlusions in patients with cervical large-artery atherosclerosis, similar to a distribution previously found in a study comparing these 2 groups who underwent EVT.20
The association between collateral status and 90-day mRS scores and mortality is well established in EVT patients.4,5 In line with these observations, we found a small statistically significant difference in median mRS in favor of patients with cervical carotid atherosclerosis. However, this result should be interpreted with caution because there was no statistically significant difference in functional independence nor in mortality and the difference in mRS only just reached statistical significance. Similarly, a MR-CLEAN subgroup analysis comparing EVT patients with and without atrial fibrillation found no significant differences in outcome.23 In further support of our hypothesis, when only selecting those patients with incomplete reperfusion (eTICI 0–2A), patients with carotid atherosclerosis did have a better functional outcome than patients with cardioembolism. This may suggest that in patients who are truly dependent on their collaterals, patients with cervical carotid atherosclerosis have a small benefit. However, despite adjusting for potential confounders, several baseline imbalances remained in this subgroup analysis (ie, eTICI 0–2A patients with cardioembolism more often had a worse prestroke mRS, a medical history of ischemic stroke and hypertension, and less often received IV r-tPA) and we, therefore, cannot rule out residual confounding. We must also emphasize the explorative nature of this analysis. Finally, our study and its subgroup analyses may be underpowered to detect a true difference.
However, collateral status may not be the main deciding factor when studying the association between stroke etiology and outcome. For one, the procedural outcomes in both groups, which were in favor of cardioembolic stroke patients, may in part explain the lack of significant differences in clinical and radiological outcomes. Patients with cervical carotid atherosclerotic stroke had longer procedure duration than patients with cardioembolic stroke, which could reflect difficulties in gaining intracranial access (eg, due to cervical stenosis) or performance of percutaneous transluminal angioplasty. Also, in patients with cervical carotid atherosclerosis, eTICI 3 on first pass was achieved less often. Perhaps this is due to differences in thrombus length. Patients with cervical carotid atherosclerosis more often had ICA/ICA-T occlusions, and longer/larger thrombi are more difficult to remove in one attempt.15 Thrombus composition may also be a factor in achieving first-pass effect.24,25 Although we do not have histological data on thrombus composition, in our study we found that patients with cervical carotid atherosclerosis more often had a hyperdense artery sign, but there was no statistically significant difference in thrombus perviousness between the 2 groups.
Our study has several limitations. First, a large group of patients had an undetermined stroke etiology (49% compared with ≈25% in most studies).26 The higher proportion of patients with stroke of undetermined etiology is partially explained by the absence of patients with small vessel disease in a cohort of patients treated with EVT. Undetermined cause (excluding those with more than one possible cause) can be the result of negative evaluation or of incomplete evaluation. The majority of the patients with cardioembolic stroke etiology in our study had atrial fibrillation. Atrial fibrillation generally only accounts for about half of all cardioembolic causes.27 Atrial fibrillation may be relatively more prevalent than other cardioembolic sources in patients with LVO. Alternatively, the work-up for other cardioembolic sources may have been incomplete,28 and a proportion of patients with undetermined etiology may have had a cardioembolic source.26 Unfortunately, detailed data on electrocardiography, rhythm monitoring, and echocardiography were unavailable for some patients, which is a result of a registry of daily clinical practice.
A second limitation is that all patients underwent single-phase CTA instead of multiphase CTA, which could have led to underestimation of collateral status in the case of delayed filling in combination with an early acquisition phase.29,30 This underestimation may disproportionally affect patients with occlusion due to cervical large-artery atherosclerosis, who more often had ICA-T occlusions than patients with cardioembolism, which may lead to slower or less contrast flow in anterior and middle cerebral artery territories. Still, if this were the case, the true difference in collateral status between patients with cervical large-artery atherosclerosis and cardioembolic stroke would be even more pronounced. Furthermore, current methods for collateral circulation assessment on CTA are rather coarse. Conventional digital subtraction angiography is generally considered the golden standard.8 More quantitative CTA scores have the potential to be more discriminative.31
Third, important considerations when studying stroke etiology, collateral circulation and outcomes, are thrombus size and thrombus composition.32 Smaller thrombi may allow for increased pial collateral flow, increasing collateral score.33 In patients with larger clots, this might have led to underestimation of collateral circulation. Although we did not analyze thrombus histopathology, we did have thrombus perviousness at our disposal. If cervical large-artery atherosclerotic thrombi are more pervious than cardioembolic thrombi, this would allow for better vessel opacification in stroke due to cervical large-artery atherosclerosis, leading to an overestimation of the difference in collateral score between the 2 groups. In our study, we did not find a statistical difference in TAI between cervical large-artery atherosclerotic and cardioembolic stroke.
Conclusions
In patients who underwent EVT because of LVO of the anterior circulation, stroke due to cervical carotid atherosclerosis was associated with better collateral status and a slightly better functional outcome at 90 days compared to cardioembolic stroke. However, there was no statistically significant difference in functional independence nor in mortality between patients with cervical carotid atherosclerotic stroke and those with cardioembolic stroke. This discrepancy may be partially explained by better procedural outcomes in cardioembolic stroke patients.
Sources of Funding
This study was funded and carried out by the Erasmus University Medical Centre, the Amsterdam UMC, location AMC, and the Maastricht University Medical Centre. The study was additionally funded by the Applied Scientific Institute for Neuromodulation (Toegepast Wetenschappelijk Instituut voor Neuromodulatie), which played no role in trial design and patient enrollment, nor in data collection, analysis, or writing of the article.
Disclosures
Dr Majoie reports grants from CVON/Dutch Heart Foundation, European Commission, TWIN Foundation and Stryker, outside the submitted work (paid to institution). In addition, Drs Majoie, Jansen, and Marquering are shareholders of Nico.lab, a company that focuses on the use of artificial intelligence for medical image analysis. Dr Roos reports stockholdings from Nico.lab outside the submitted work. Dr Dippel reports grants from the Dutch Heart Foundation, the Brain Foundation Netherlands, The Netherlands Organization for Health Research and Development, Health Holland Top Sector Life Sciences & Health, Stryker European Operations BV, Penumbra Inc, Medtronic, and Thrombolytic Science, LLC, outside the submitted work. Dr van der Worp reports speaker’s fees from Boehringer Ingelheim and Bayer, serving as a consultant to Boehringer Ingelheim and grants from the European Union outside the submitted work. Dr Coutinho reports grants from Medtronic outside the submitted work. Drs Majoie, Roos, Coutinho, Treurniet, and Dr LeCouffe are (co-)investigators of the MR-CLEAN-NO IV trial (ISRCTN80619088). The other authors report no conflicts.
Supplementary Material
Appendix
Rotterdam, July 19, 2018
MR CLEAN Registry Investigators—Group Authors
Executive Committee
Diederik W.J. Dippel, Department of Neurology, Erasmus MC University Medical Center; Aad van der Lugt, Department of Radiology, Erasmus MC University Medical Center; Charles B.L.M. Majoie, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Yvo B.W.E.M. Roos, Department of Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam; Robert J. van Oostenbrugge, Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); Wim H. van Zwam, Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); Jelis Boiten, Department of Neurology, Haaglanden MC, the Hague; Jan A. Vos, Department of Radiology, Sint Antonius Hospital, Nieuwegein.
Study Coordinators
Ivo G.H. Jansen, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Maxim J.H.L. Mulder, Department of Neurology and Department of Radiology, Erasmus MC University Medical Center; Robert-Jan B. Goldhoorn, Department of Neurology and Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); Kars C.J. Compagne, Department of Radiology, Erasmus MC University Medical Center; Manon Kappelhof, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam.
Local Principal Investigators
Wouter J. Schonewille, Department of Neurology, Sint Antonius Hospital, Nieuwegein; Jan A. Vos, Department of Radiology, Sint Antonius Hospital, Nieuwegein; Charles B.L.M. Majoie, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Jonathan M. Coutinho, Department of Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam; Marieke J.H. Wermer, Department of Neurology, Leiden University Medical Center; Marianne A.A. van Walderveen, Department of Radiology, Leiden University Medical Center; Julie Staals, Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); Wim H. van Zwam, Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); Jeannette Hofmeijer, Department of Neurology, Rijnstate Hospital, Arnhem; Jasper M. Martens, Department of Radiology, Rijnstate Hospital, Arnhem; Geert J. Lycklama à Nijeholt, Department of Radiology, Haaglanden MC, the Hague; Jelis Boiten, Department of Neurology, Haaglanden MC, the Hague; Bob Roozenbeek, Department of Neurology, Erasmus MC University Medical Center; Bart J. Emmer, Department of Radiology, Erasmus MC University Medical Center; Sebastiaan F. de Bruijn, Department of Neurology, HAGA Hospital, the Hague; Lukas C. van Dijk, Department of Radiology, HAGA Hospital, the Hague; H. Bart van der Worp, Department of Neurology, University Medical Center Utrecht; Rob H. Lo, Department of Radiology, University Medical Center Utrecht; Ewoud J. van Dijk, Department of Neurology, Radboud University Medical Center, Nijmegen; Hieronymus D. Boogaarts, Department of Neurosurgery, Radboud University Medical Center, Nijmegen; Paul L.M. de Kort, Department of Neurology, Sint Elisabeth Hospital, Tilburg; Jo P. Peluso, Department of Radiology, Sint Elisabeth Hospital, Tilburg; Jan S.P. van den Berg, Department of Neurology, Isala Klinieken, Zwolle; Boudewijn A.A.M. van Hasselt, Department of Radiology, Isala Klinieken, Zwolle; Leo A.M. Aerden, Department of Neurology, Reinier de Graaf Gasthuis, Delft; René J. Dallinga, Department of Radiology, Reinier de Graaf Gasthuis, Delft; Maarten Uyttenboogaart, Department of Neurology, University Medical Center Groningen; Omid Eshghi, Department of Radiology, University Medical Center Groningen; Tobien H.C.M.L. Schreuder, Department of Neurology, Atrium Medical Center, Heerlen; Roel J.J. Heijboer, Department of Radiology, Atrium Medical Center, Heerlen; Koos Keizer, Department of Neurology, Catharina Hospital, Eindhoven; Lonneke S.F. Yo, Department of Radiology, Catharina Hospital, Eindhoven; Heleen M. den Hertog, Department of Neurology, Isala Klinieken, Zwolle; Emiel J.C. Sturm, Department of Radiology, Medical Spectrum Twente, Enschede.
Imaging Assessment Committee
Charles B.L.M. Majoie (chair), Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Wim H. van Zwam, Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); Aad van der Lugt, Department of Radiology, Erasmus MC University Medical Center; Geert J. Lycklama à Nijeholt, Department of Radiology, Haaglanden MC, the Hague; Marianne A.A. van Walderveen, Department of Radiology, Leiden University Medical Center; Marieke E.S. Sprengers, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Sjoerd F.M. Jenniskens, Department of Radiology, Radboud University Medical Center, Nijmegen; René van den Berg, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Albert J. Yoo, Department of Radiology, Texas Stroke Institute, Texas; Ludo F.M. Beenen, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Alida A. Postma, Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); Stefan D. Roosendaal, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Bas F.W. van der Kallen, Department of Radiology, Haaglanden MC, the Hague; Ido R. van den Wijngaard, Department of Radiology, Haaglanden MC, the Hague; Adriaan C.G.M. van Es, Department of Radiology, Erasmus MC University Medical Center; Bart J. Emmer, Department of Radiology, Erasmus MC University Medical Center and Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Jasper M. Martens, Department of Radiology, Rijnstate Hospital, Arnhem; Lonneke S.F. Yo, Department of Radiology, Catharina Hospital, Eindhoven; Jan A. Vos, Department of Radiology, Sint Antonius Hospital, Nieuwegein; Joost Bot, Department of Radiology, Amsterdam UMC, Vrije Universiteit van Amsterdam, Amsterdam; Pieter-Jan van Doormaal, Department of Radiology, Erasmus MC University Medical Center.
Writing Committee
Diederik W.J. Dippel (chair), Department of Neurology, Erasmus MC University Medical Center; Aad van der Lugt, Department of Radiology, Erasmus MC University Medical Center; Charles B.L.M. Majoie, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Yvo B.W.E.M. Roos, Department of Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam; Robert J. van Oostenbrugge, Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); Wim H. van Zwam, Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); Geert J. Lycklama à Nijeholt, Department of Radiology, Haaglanden MC, the Hague; Jelis Boiten, Department of Neurology, Haaglanden MC, the Hague; Jan A. Vos, Department of Radiology, Sint Antonius Hospital, Nieuwegein; Wouter J. Schonewille, Department of Neurology, Sint Antonius Hospital, Nieuwegein; Jeannette Hofmeijer, Department of Neurology, Rijnstate Hospital, Arnhem; Jasper M. Martens, Department of Radiology, Rijnstate Hospital, Arnhem; H. Bart van der Worp, Department of Neurology, University Medical Center Utrecht; Rob H. Lo, Department of Radiology, University Medical Center Utrecht.
Adverse Event Committee
Robert J. van Oostenbrugge (chair), Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); Jeannette Hofmeijer, Department of Neurology, Rijnstate Hospital, Arnhem; H. Zwenneke Flach, Department of Radiology, Isala Klinieken, Zwolle.
Trial Methodologist
Hester F. Lingsma, Department of Public Health, Erasmus MC University Medical Center.
Research Nurses/Local Trial Coordinators
Naziha el Ghannouti, Department of Neurology, Erasmus MC University Medical Center; Martin Sterrenberg, Department of Neurology, Erasmus MC University Medical Center; Corina Puppels, Department of Neurology, Sint Antonius Hospital, Nieuwegein; Wilma Pellikaan, Department of Neurology, Sint Antonius Hospital, Nieuwegein; Rita Sprengers, Department of Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam; Marjan Elfrink, Department of Neurology, Rijnstate Hospital, Arnhem; Joke de Meris, Department of Neurology, Haaglanden MC, the Hague; Tamara Vermeulen, Department of Neurology, Haaglanden MC, the Hague; Annet Geerlings, Department of Neurology, Radboud University Medical Center, Nijmegen; Gina van Vemde, Department of Neurology, Isala Klinieken, Zwolle; Tiny Simons, Department of Neurology, Atrium Medical Center, Heerlen; Cathelijn van Rijswijk, Department of Neurology, Sint Elisabeth Hospital, Tilburg; Gert Messchendorp, Department of Neurology, University Medical Center Groningen; Hester Bongenaar, Department of Neurology, Catharina Hospital, Eindhoven; Karin Bodde, Department of Neurology, Reinier de Graaf Gasthuis, Delft; Sandra Kleijn, Department of Neurology, Medical Spectrum Twente, Enschede; Jasmijn Lodico, Department of Neurology, Medical Spectrum Twente, Enschede; Hanneke Droste, Department of Neurology, Medical Spectrum Twente, Enschede; M. Wollaert, Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); D. Jeurrissen, Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); Ernas Bos, Department of Neurology, Leiden University Medical Center; Yvonne Drabbe, Department of Neurology, HAGA Hospital, the Hague; Nicoline Aaldering, Department of Neurology, Rijnstate Hospital, Arnhem; Berber Zweedijk, Department of Neurology, University Medical Center Utrecht; Mostafa Khalilzada, Department of Neurology, HAGA Hospital, the Hague.
PhD/Medical Students
Esmee Venema, Department of Public Health, Erasmus MC University Medical Center; Vicky Chalos, Department of Neurology and Department of Public Health, Erasmus MC University Medical Center; Ralph R. Geuskens, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Tim van Straaten, Department of Neurology, Radboud University Medical Center, Nijmegen; Saliha Ergezen, Department of Neurology, Erasmus MC University Medical Center; Roger R.M. Harmsma, Department of Neurology, Erasmus MC University Medical Center; Daan Muijres, Department of Neurology, Erasmus MC University Medical Center; Anouk de Jong, Department of Neurology, Erasmus MC University Medical Center; Wouter Hinsenveld, Department of Neurology and Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); Olvert A. Berkhemer, Department of Neurology, Erasmus MC University Medical Center, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam, and Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM); Anna M.M. Boers, Department of Radiology and Nuclear Medicine and Department of Biomedical Engineering & Physics, Amsterdam UMC, University of Amsterdam, Amsterdam; J. Huguet, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; P.F.C. Groot, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Marieke A. Mens, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Katinka R. van Kranendonk, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Kilian M. Treurniet, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Manon L. Tolhuijsen, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam; Heitor Alves, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam.
Footnotes
Drs Guglielmi and LeCouffe are joint first authors.
A list of all MR-CLEAN Registry participants is given in the Appendix.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.119.026299.
Contributor Information
Aad van der Lugt, Department of Radiology, Erasmus MC University Medical Center.
Wim H. van Zwam, Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)
Jelis Boiten, Department of Neurology, Haaglanden MC, the Hague.
Jan A. Vos, Department of Radiology, Sint Antonius Hospital, Nieuwegein.
Maxim J.H.L. Mulder, Department of Neurology and Department of Radiology, Erasmus MC University Medical Center
Robert-Jan B. Goldhoorn, Department of Neurology and Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)
Manon Kappelhof, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam.
Wouter J. Schonewille, Department of Neurology, Sint Antonius Hospital, Nieuwegein
Jan A. Vos, Department of Radiology, Sint Antonius Hospital, Nieuwegein
Marieke J.H. Wermer, Department of Neurology, Leiden University Medical Center
Marianne A.A. van Walderveen, Department of Radiology, Leiden University Medical Center
Julie Staals, Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM).
Wim H. van Zwam, Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)
Jeannette Hofmeijer, Department of Neurology, Rijnstate Hospital, Arnhem.
Jasper M. Martens, Department of Radiology, Rijnstate Hospital, Arnhem
Geert J. Lycklama à Nijeholt, Department of Radiology, Haaglanden MC, the Hague
Jelis Boiten, Department of Neurology, Haaglanden MC, the Hague.
Bob Roozenbeek, Department of Neurology, Erasmus MC University Medical Center.
Sebastiaan F. de Bruijn, Department of Neurology, HAGA Hospital, the Hague
Lukas C. van Dijk, Department of Radiology, HAGA Hospital, the Hague
Rob H. Lo, Department of Radiology, University Medical Center Utrecht
Ewoud J. van Dijk, Department of Neurology, Radboud University Medical Center, Nijmegen
Hieronymus D. Boogaarts, Department of Neurosurgery, Radboud University Medical Center, Nijmegen
Paul L.M. de Kort, Department of Neurology, Sint Elisabeth Hospital, Tilburg
Jo P. Peluso, Department of Radiology, Sint Elisabeth Hospital, Tilburg
Jan S.P. van den Berg, Department of Neurology, Isala Klinieken, Zwolle
Boudewijn A.A.M. van Hasselt, Department of Radiology, Isala Klinieken, Zwolle
Leo A.M. Aerden, Department of Neurology, Reinier de Graaf Gasthuis, Delft
René J. Dallinga, Department of Radiology, Reinier de Graaf Gasthuis, Delft
Maarten Uyttenboogaart, Department of Neurology, University Medical Center Groningen.
Omid Eshghi, Department of Radiology, University Medical Center Groningen.
Tobien H.C.M.L. Schreuder, Department of Neurology, Atrium Medical Center, Heerlen
Roel J.J. Heijboer, Department of Radiology, Atrium Medical Center, Heerlen
Koos Keizer, Department of Neurology, Catharina Hospital, Eindhoven.
Lonneke S.F. Yo, Department of Radiology, Catharina Hospital, Eindhoven
Heleen M. den Hertog, Department of Neurology, Isala Klinieken, Zwolle
Emiel J.C. Sturm, Department of Radiology, Medical Spectrum Twente, Enschede
Wim H. van Zwam, Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)
Aad van der Lugt, Department of Radiology, Erasmus MC University Medical Center.
Geert J. Lycklama à Nijeholt, Department of Radiology, Haaglanden MC, the Hague
Marianne A.A. van Walderveen, Department of Radiology, Leiden University Medical Center
Marieke E.S. Sprengers, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam
Sjoerd F.M. Jenniskens, Department of Radiology, Radboud University Medical Center, Nijmegen
René van den Berg, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam.
Albert J. Yoo, Department of Radiology, Texas Stroke Institute, Texas
Ludo F.M. Beenen, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam
Alida A. Postma, Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)
Stefan D. Roosendaal, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam
Bas F.W. van der Kallen, Department of Radiology, Haaglanden MC, the Hague
Ido R. van den Wijngaard, Department of Radiology, Haaglanden MC, the Hague
Adriaan C.G.M. van Es, Department of Radiology, Erasmus MC University Medical Center
Jasper M. Martens, Department of Radiology, Rijnstate Hospital, Arnhem
Lonneke S.F. Yo, Department of Radiology, Catharina Hospital, Eindhoven
Jan A. Vos, Department of Radiology, Sint Antonius Hospital, Nieuwegein
Joost Bot, Department of Radiology, Amsterdam UMC, Vrije Universiteit van Amsterdam, Amsterdam.
Pieter-Jan van Doormaal, Department of Radiology, Erasmus MC University Medical Center.
Aad van der Lugt, Department of Radiology, Erasmus MC University Medical Center.
Wim H. van Zwam, Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)
Geert J. Lycklama à Nijeholt, Department of Radiology, Haaglanden MC, the Hague
Jelis Boiten, Department of Neurology, Haaglanden MC, the Hague.
Jan A. Vos, Department of Radiology, Sint Antonius Hospital, Nieuwegein
Wouter J. Schonewille, Department of Neurology, Sint Antonius Hospital, Nieuwegein
Jeannette Hofmeijer, Department of Neurology, Rijnstate Hospital, Arnhem.
Jasper M. Martens, Department of Radiology, Rijnstate Hospital, Arnhem
Rob H. Lo, Department of Radiology, University Medical Center Utrecht
Jeannette Hofmeijer, Department of Neurology, Rijnstate Hospital, Arnhem.
H. Zwenneke Flach, Department of Radiology, Isala Klinieken, Zwolle.
Hester F. Lingsma, Department of Public Health, Erasmus MC University Medical Center
Naziha el Ghannouti, Department of Neurology, Erasmus MC University Medical Center.
Martin Sterrenberg, Department of Neurology, Erasmus MC University Medical Center.
Corina Puppels, Department of Neurology, Sint Antonius Hospital, Nieuwegein.
Wilma Pellikaan, Department of Neurology, Sint Antonius Hospital, Nieuwegein.
Rita Sprengers, Department of Neurology, Amsterdam UMC, University of Amsterdam, Amsterdam.
Marjan Elfrink, Department of Neurology, Rijnstate Hospital, Arnhem.
Joke de Meris, Department of Neurology, Haaglanden MC, the Hague.
Tamara Vermeulen, Department of Neurology, Haaglanden MC, the Hague.
Annet Geerlings, Department of Neurology, Radboud University Medical Center, Nijmegen.
Gina van Vemde, Department of Neurology, Isala Klinieken, Zwolle.
Tiny Simons, Department of Neurology, Atrium Medical Center, Heerlen.
Cathelijn van Rijswijk, Department of Neurology, Sint Elisabeth Hospital, Tilburg.
Gert Messchendorp, Department of Neurology, University Medical Center Groningen.
Hester Bongenaar, Department of Neurology, Catharina Hospital, Eindhoven.
Karin Bodde, Department of Neurology, Reinier de Graaf Gasthuis, Delft.
Sandra Kleijn, Department of Neurology, Medical Spectrum Twente, Enschede.
Jasmijn Lodico, Department of Neurology, Medical Spectrum Twente, Enschede.
Hanneke Droste, Department of Neurology, Medical Spectrum Twente, Enschede.
M. Wollaert, Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)
D. Jeurrissen, Department of Neurology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)
Ernas Bos, Department of Neurology, Leiden University Medical Center.
Yvonne Drabbe, Department of Neurology, HAGA Hospital, the Hague.
Nicoline Aaldering, Department of Neurology, Rijnstate Hospital, Arnhem.
Berber Zweedijk, Department of Neurology, University Medical Center Utrecht.
Mostafa Khalilzada, Department of Neurology, HAGA Hospital, the Hague.
Esmee Venema, Department of Public Health, Erasmus MC University Medical Center.
Vicky Chalos, Department of Neurology and Department of Public Health, Erasmus MC University Medical Center.
Ralph R. Geuskens, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam
Tim van Straaten, Department of Neurology, Radboud University Medical Center, Nijmegen.
Saliha Ergezen, Department of Neurology, Erasmus MC University Medical Center.
Roger R.M. Harmsma, Department of Neurology, Erasmus MC University Medical Center
Daan Muijres, Department of Neurology, Erasmus MC University Medical Center.
Anouk de Jong, Department of Neurology, Erasmus MC University Medical Center.
Wouter Hinsenveld, Department of Neurology and Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM).
Olvert A. Berkhemer, Department of Neurology, Erasmus MC University Medical Center, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam, and Department of Radiology, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM)
Anna M.M. Boers, Department of Radiology and Nuclear Medicine and Department of Biomedical Engineering & Physics, Amsterdam UMC, University of Amsterdam, Amsterdam
J. Huguet, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam
P.F.C. Groot, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam
Marieke A. Mens, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam
Katinka R. van Kranendonk, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam
Manon L. Tolhuijsen, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam
Heitor Alves, Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam.
Collaborators: the MR-CLEAN Registry Investigators, Aad van der Lugt, Wim H. van Zwam, Jelis Boiten, Jan A. Vos, Maxim J.H.L. Mulder, Robert-Jan B. Goldhoorn, Manon Kappelhof, Wouter J. Schonewille, Jan A. Vos, Marieke J.H. Wermer, Marianne A.A. van Walderveen, Julie Staals, Wim H. van Zwam, Jeannette Hofmeijer, Jasper M. Martens, Geert J. Lycklama à Nijeholt, Jelis Boiten, Bob Roozenbeek, Sebastiaan F. de Bruijn, Lukas C. van Dijk, Rob H. Lo, Ewoud J. van Dijk, Hieronymus D. Boogaarts, Paul L.M. de Kort, Jo P. Peluso, Jan S.P. van den Berg, Boudewijn A.A.M. van Hasselt, Leo A.M. Aerden, René J. Dallinga, Maarten Uyttenboogaart, Omid Eshghi, Tobien H.C.M.L. Schreuder, Roel J.J. Heijboer, Koos Keizer, Lonneke S.F. Yo, Heleen M. den Hertog, Emiel J.C. Sturm, Wim H. van Zwam, Aad van der Lugt, Geert J. Lycklama à Nijeholt, Marianne A.A. van Walderveen, Marieke E.S. Sprengers, Sjoerd F.M. Jenniskens, René van den Berg, Albert J. Yoo, Ludo F.M. Beenen, Alida A. Postma, Stefan D. Roosendaal, Bas F.W. van der Kallen, Ido R. van den Wijngaard, Adriaan C.G.M. van Es, Jasper M. Martens, Lonneke S.F. Yo, Jan A. Vos, Joost Bot, Pieter-Jan van Doormaal, Aad van der Lugt, Wim H. van Zwam, Geert J. Lycklama à Nijeholt, Jelis Boiten, Jan A. Vos, Wouter J. Schonewille, Jeannette Hofmeijer, Jasper M. Martens, Rob H. Lo, Jeannette Hofmeijer, H. Zwenneke Flach, Hester F. Lingsma, Naziha el Ghannouti, Martin Sterrenberg, Corina Puppels, Wilma Pellikaan, Rita Sprengers, Marjan Elfrink, Joke de Meris, Tamara Vermeulen, Annet Geerlings, Gina van Vemde, Tiny Simons, Cathelijn van Rijswijk, Gert Messchendorp, Hester Bongenaar, Karin Bodde, Sandra Kleijn, Jasmijn Lodico, Hanneke Droste, M. Wollaert, D. Jeurrissen, Ernas Bos, Yvonne Drabbe, Nicoline Aaldering, Berber Zweedijk, Mostafa Khalilzada, Esmee Venema, Vicky Chalos, Ralph R. Geuskens, Tim van Straaten, Saliha Ergezen, Roger R.M. Harmsma, Daan Muijres, Anouk de Jong, Wouter Hinsenveld, Olvert A. Berkhemer, Anna M.M. Boers, J. Huguet, P.F.C. Groot, Marieke A. Mens, Katinka R. van Kranendonk, Manon L. Tolhuijsen, and Heitor Alves
References
- 1.Tu HT, Campbell BC, Christensen S, Desmond PM, De Silva DA, Parsons MW, et al. EPITHET-DEFUSE Investigators. Worse stroke outcome in atrial fibrillation is explained by more severe hypoperfusion, infarct growth, and hemorrhagic transformation. Int J Stroke. 2015;10:534–540. doi: 10.1111/ijs.12007. doi: 10.1111/ijs.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henninger N, Goddeau RP, Jr, Karmarkar A, Helenius J, McManus DD. Atrial fibrillation is associated with a worse 90-day outcome than other cardioembolic stroke subtypes. Stroke. 2016;47:1486–1492. doi: 10.1161/STROKEAHA.116.012865. doi: 10.1161/STROKEAHA.116.012865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giray S, Ozdemir O, Baş DF, İnanç Y, Arlier Z, Kocaturk O. Does stroke etiology play a role in predicting outcome of acute stroke patients who underwent endovascular treatment with stent retrievers? J Neurol Sci. 2017;372:104–109. doi: 10.1016/j.jns.2016.11.006. doi: 10.1016/j.jns.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Leng X, Fang H, Leung TW, Mao C, Miao Z, Liu L, et al. Impact of collaterals on the efficacy and safety of endovascular treatment in acute ischaemic stroke: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2016;87:537–544. doi: 10.1136/jnnp-2015-310965. doi: 10.1136/jnnp-2015-310965. [DOI] [PubMed] [Google Scholar]
- 5.Berkhemer OA, Jansen IG, Beumer D, Fransen PS, van den Berg LA, Yoo AJ, et al. MR CLEAN Investigators. Collateral status on baseline computed tomographic angiography and intra-arterial treatment effect in patients with proximal anterior circulation stroke. Stroke. 2016;47:768–776. doi: 10.1161/STROKEAHA.115.011788. doi: 10.1161/STROKEAHA.115.011788. [DOI] [PubMed] [Google Scholar]
- 6.Román LS, Menon BK, Blasco J, Hernández-Pérez M, Dávalos A, Majoie CBLM, et al. HERMES Collaborators. Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data. Lancet Neurol. 2018;17:895–904. doi: 10.1016/S1474-4422(18)30242-4. doi: 10.1016/S1474-4422(18)30242-4. [DOI] [PubMed] [Google Scholar]
- 7.Liebeskind DS. Collateral circulation. Stroke. 2003;34:2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- 8.Shuaib A, Butcher K, Mohammad AA, Saqqur M, Liebeskind DS. Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. Lancet Neurol. 2011;10:909–921. doi: 10.1016/S1474-4422(11)70195-8. doi: 10.1016/S1474-4422(11)70195-8. [DOI] [PubMed] [Google Scholar]
- 9.Jing Z, Shi C, Zhu L, Xiang Y, Chen P, Xiong Z, et al. Chronic cerebral hypoperfusion induces vascular plasticity and hemodynamics but also neuronal degeneration and cognitive impairment. J Cereb Blood Flow Metab. 2015;35:1249–1259. doi: 10.1038/jcbfm.2015.55. doi: 10.1038/jcbfm.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jansen IGH, Mulder MJHL, Goldhoorn RB MR CLEAN Registry Investigators. Endovascular treatment for acute ischaemic stroke in routine clinical practice: prospective, observational cohort study (MR CLEAN Registry). BMJ. 2018;360:k949. doi: 10.1136/bmj.k949. doi: 10.1136/bmj.k949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 12.Tan IY, Demchuk AM, Hopyan J, Zhang L, Gladstone D, Wong K, et al. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol. 2009;30:525–531. doi: 10.3174/ajnr.A1408. doi: 10.3174/ajnr.A1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Kummer R, Broderick JP, Campbell BC, Demchuk A, Goyal M, Hill MD, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46:2981–2986. doi: 10.1161/STROKEAHA.115.010049. doi: 10.1161/STROKEAHA.115.010049. [DOI] [PubMed] [Google Scholar]
- 14.Goyal M, Fargen KM, Turk AS, Mocco J, Liebeskind DS, Frei D, et al. 2C or not 2C: defining an improved revascularization grading scale and the need for standardization of angiography outcomes in stroke trials. J Neurointerv Surg. 2014;6:83–86. doi: 10.1136/neurintsurg-2013-010665. doi: 10.1136/neurintsurg-2013-010665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaidat OO, Castonguay AC, Linfante I, Gupta R, Martin CO, Holloway WE, et al. First pass effect: a new measure for stroke thrombectomy devices. Stroke. 2018;49:660–666. doi: 10.1161/STROKEAHA.117.020315. doi: 10.1161/STROKEAHA.117.020315. [DOI] [PubMed] [Google Scholar]
- 16.Santos EM, Marquering HA, den Blanken MD, Berkhemer OA, Boers AM, Yoo AJ, et al. MR CLEAN Investigators. Thrombus permeability is associated with improved functional outcome and recanalization in patients with ischemic stroke. Stroke. 2016;47:732–741. doi: 10.1161/STROKEAHA.115.011187. doi: 10.1161/STROKEAHA.115.011187. [DOI] [PubMed] [Google Scholar]
- 17.Henderson RD, Eliasziw M, Fox AJ, Rothwell PM, Barnett HJ. Angiographically defined collateral circulation and risk of stroke in patients with severe carotid artery stenosis. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. Stroke. 2000;31:128–132. doi: 10.1161/01.str.31.1.128. doi: 10.1161/01.str.31.1.128. [DOI] [PubMed] [Google Scholar]
- 18.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 19.Zhang X, Zhang M, Ding W, Yan S, Liebeskind DS, Lou M. Distinct predictive role of collateral status on clinical outcome in variant stroke subtypes of acute large arterial occlusion. Eur J Neurol. 2018;25:293–300. doi: 10.1111/ene.13493. doi: 10.1111/ene.13493. [DOI] [PubMed] [Google Scholar]
- 20.Rebello LC, Bouslama M, Haussen DC, Grossberg JA, Dehkharghani S, Anderson A, et al. Stroke etiology and collaterals: atheroembolic strokes have greater collateral recruitment than cardioembolic strokes. Eur J Neurol. 2017;24:762–767. doi: 10.1111/ene.13287. doi: 10.1111/ene.13287. [DOI] [PubMed] [Google Scholar]
- 21.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke. 2001;32:2735–2740. doi: 10.1161/hs1201.100209. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- 22.Arboix A, Alió J. Cardioembolic stroke: clinical features, specific cardiac disorders and prognosis. Curr Cardiol Rev. 2010;6:150–161. doi: 10.2174/157340310791658730. doi: 10.2174/157340310791658730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heshmatollah A, Fransen PSS, Berkhemer OA, Beumer D, van der Lugt A, Majoie C, et al. Endovascular thrombectomy in patients with acute ischaemic stroke and atrial fibrillation: A mr clean subgroup analysis. EuroIntervention. 2017;13:996–1002. doi: 10.4244/EIJ-D-16-00905. doi: 10.4244/EIJ-D-16-00905. [DOI] [PubMed] [Google Scholar]
- 24.De Meyer SF, Andersson T, Baxter B, Bendszus M, Brouwer P, Brinjikji W, et al. Clot Summit Group. Analyses of thrombi in acute ischemic stroke: A consensus statement on current knowledge and future directions. Int J Stroke. 2017;12:606–614. doi: 10.1177/1747493017709671. doi: 10.1177/1747493017709671. [DOI] [PubMed] [Google Scholar]
- 25.Brinjikji W, Duffy S, Burrows A, Hacke W, Liebeskind D, Majoie CBLM, et al. Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome: a systematic review. J Neurointerv Surg. 2017;9:529–534. doi: 10.1136/neurintsurg-2016-012391. doi: 10.1136/neurintsurg-2016-012391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O’Donnell MJ, et al. Cryptogenic Stroke/ESUS International Working Group. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429–438. doi: 10.1016/S1474-4422(13)70310-7. doi: 10.1016/S1474-4422(13)70310-7. [DOI] [PubMed] [Google Scholar]
- 27.Freeman WD, Aguilar MI. Stroke prevention in atrial fibrillation and other major cardiac sources of embolism. Neurol Clin. 2008;26:1129–60, x. doi: 10.1016/j.ncl.2008.07.001. doi: 10.1016/j.ncl.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Perera KS, Vanassche T, Bosch J, Giruparajah M, Swaminathan B, Mattina KR, et al. ESUS Global Registry Investigators. Embolic strokes of undetermined source: prevalence and patient features in the ESUS Global Registry. Int J Stroke. 2016;11:526–533. doi: 10.1177/1747493016641967. doi: 10.1177/1747493016641967. [DOI] [PubMed] [Google Scholar]
- 29.Menon BK, d’Esterre CD, Qazi EM, Almekhlafi M, Hahn L, Demchuk AM, et al. Multiphase CT angiography: a new tool for the imaging triage of patients with acute ischemic stroke. Radiology. 2015;275:510–520. doi: 10.1148/radiol.15142256. doi: 10.1148/radiol.15142256. [DOI] [PubMed] [Google Scholar]
- 30.Jansen IG, Mulder MJ, Goldhoorn RB, Boers AM, van Es AC, Yo LS, et al. MR CLEAN Registry Investigators. Impact of single phase CT angiography collateral status on functional outcome over time: results from the MR CLEAN Registry. J Neurointerv Surg. 2019;11:866–873. doi: 10.1136/neurintsurg-2018-014619. doi: 10.1136/neurintsurg-2018-014619. [DOI] [PubMed] [Google Scholar]
- 31.Boers AMM, Sales Barros R, Jansen IGH, Berkhemer OA, Beenen LFM, Menon BK, et al. MR CLEAN Investigators. Value of quantitative collateral scoring on CT angiography in patients with acute ischemic stroke. AJNR Am J Neuroradiol. 2018;39:1074–1082. doi: 10.3174/ajnr.A5623. doi: 10.3174/ajnr.A5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sporns PB, Hanning U, Schwindt W, Velasco A, Minnerup J, Zoubi T, et al. Ischemic stroke: what does the histological composition tell us about the origin of the thrombus? Stroke. 2017;48:2206–2210. doi: 10.1161/STROKEAHA.117.016590. doi: 10.1161/STROKEAHA.117.016590. [DOI] [PubMed] [Google Scholar]
- 33.Alves HC, Treurniet KM, Dutra BG, Jansen IGH, Boers AMM, Santos EMM, et al. MR CLEAN Trial Investigators. Associations between collateral status and thrombus characteristics and their impact in anterior circulation stroke. Stroke. 2018;49:391–396. doi: 10.1161/STROKEAHA.117.019509. doi: 10.1161/STROKEAHA.117.019509. [DOI] [PubMed] [Google Scholar]


