Supplemental Digital Content is available in the text.
Keywords: accelerometry, physical activity, rehabilitation, secondary prevention, sedentary behavior, stroke
Background and Purpose—
Movement behaviors, that is, both physical activity and sedentary behavior, are independently associated with health risks. Although both behaviors have been investigated separately in people after stroke, little is known about the combined movement behavior patterns, differences in these patterns between individuals, or the factors associated with these patterns. Therefore, the objectives of this study are (1) to identify movement behavior patterns in people with first-ever stroke discharged to the home setting and (2) to explore factors associated with the identified patterns.
Methods—
Cross-sectional design using data from 190 people with first-ever stroke discharged to the home setting. Movement, behavior was measured over 2 weeks using an accelerometer. Ten movement behavior outcomes were calculated and compressed using principal component analysis. Movement behavior patterns were identified using a k-means clustering algorithm. Demographics, stroke, care, physical functioning, and psychological, cognitive and social factors were obtained. Differences between and factors associated with the patterns were investigated.
Results—
On average, the accelerometer was worn for 13.7 hours per day. The average movement behavior of the participants showed 9.3 sedentary hours, 3.8 hours of light physical activity, and 0.6 hours of moderate-vigorous physical activity. Three patterns and associated factors were identified: (1) sedentary exercisers (22.6%), with a relatively low age, few pack-years, light drinking, and high levels of physical functioning; (2) sedentary movers (45.8%), with less severe stroke symptoms, low physical functioning and high levels of self-efficacy; and (3) sedentary prolongers (31.6%), with more severe stroke symptoms, more pack-years, and low levels of self-efficacy.
Conclusions—
The majority of people with stroke are inactive and sedentary. Three different movement behavior patterns were identified: sedentary exercisers, sedentary movers, and sedentary prolongers. The identified movement behavior patterns confirm the hypothesis that an individually tailored approach might be warranted with movement behavior coaching by healthcare professionals.
Globally, stroke affects 16 million individuals every year. Patients who survive a stroke are at high risk for recurrent stroke and other cardiovascular events.1 In the next decades, the prevalence of stroke is expected to increase worldwide,2 highlighting the need for effective disease management and secondary prevention strategies. Sufficient amounts of physical activity (PA) can reduce the risk of first-ever stroke,3 risk of recurrent stroke, and other vascular events.4
International guidelines recommend at least 150 minutes per week of accumulated moderate-vigorous physical activity (MVPA).5 Only 17% of people with stroke meet these guidelines and spend only half of the recommended time being physically active compared with healthy persons.6,7 Therefore, stimulation of a physically active lifestyle forms a key element for secondary prevention. Furthermore, recent studies show that sedentary time in stroke survivors within the community setting ranges between 63% and 87% during waking hours. Additionally, it was found that these individuals are over 1 hour more sedentary than healthy persons.6,7 Research has also shown that even when older adults are sufficiently active, prolonged periods of sedentary behavior (SB) are independently associated with all-cause and cardiometabolic disease-related mortality.8 Therefore, SB can also be considered an important risk factor for stroke survivors.
Recently, international consensus was reached on a new term, movement behavior which includes SB and all levels of PA.9 This term includes the daily behavior pattern of a person about body postures, movements, and daily activities in the person’s own environment. PA can be classified based on metabolic equivalents (METs) at 3 intensity levels: light PA (LPA; >1.5–3.0 METs), moderate PA (3.0–6.0 METs), and vigorous PA (>6.0 METs). Persons are defined as physically inactive if they do not reach sufficient amounts of MVPA.5 Notably, inactivity is not the same as SB. SB is defined as any waking activity characterized by an energy expenditure of ≤1.5 METs and a sitting or reclining posture.10
A lack of MVPA and high amounts of SB are independent risk factors for all-cause mortality, cardiovascular diseases, and functional decline.3,4,8 Although the independent health risks of these single behaviors are highlighted in research, these behaviors are not self-contained but cluster in patterns (eg, high MVPA/high LPA/low SB or low MVPA/low LPA/high SB).11 It could be suggested that a movement behavior pattern with sufficient MVPA, high amounts of LPA, and low amounts of SB leads to optimal health.11 The distribution of single movement behaviors within the total pattern are important because the health benefits of 1 single behavior could be counteracted by the risks of another. For example, if someone engages in at least 150 minutes per week of moderate physical activity but is sedentary for the rest of the time, the health risks are still high.8 Additionally, the accumulation of SB is important since long prolonged sedentary bouts are damaging health and interrupting SB with LPA has shown cardiovascular health benefits.12
Currently, specific movement behavior patterns in people with stroke and the associated long-term health impact are unknown. Therefore, research on the identification of commonly distinct movement behavior patterns in people with stroke is needed. Insight into movement behavior patterns in people with stroke will ultimately enable more targeted interventions in people with unhealthy movement behavior patterns (eg, low MVPA, low LPA, and high amounts of SB). Additionally, insight into the characteristics of people with specific movement behavior patterns enables identification of the right persons for interventions after discharge from facility-based care. Therefore, the objectives of the present study were (1) to identify movement behavior patterns in people with first-ever stroke discharged from hospital or inpatient rehabilitation to the home setting and (2) to explore characteristics associated with the identified patterns.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Participants and Study Design
This cross-sectional study is part of the RISE-study. Participants were recruited from 4 participating stroke units in the Netherlands between February 2015 and April 2017 and were included when they had returned home. Patients were deemed eligible to participate when: presenting with a clinically confirmed first-ever stroke, expected to return home (with or without inpatient rehabilitation before returning home), activities of daily living independent before stroke (Barthel Index>18),13 >18 years old, able to maintain a conversation (score >4 on the Utrecht Communication Assessment14), and at least able to walk with supervision when they returned home (score ≥3 in the Functional Ambulation Categories15). Participants were excluded if their life expectancy was <2 years. All participants gave written informed consent. The study was approved by the Medical Ethics Research Committee of the University Medical Centre Utrecht (study number 14/76). Demographic, stroke, and care characteristics were obtained from medical health records. Within 3 weeks after discharge from inpatient care, participants were visited at home by trained researchers. Before the participant was visited at home, a postal questionnaire was sent to obtain psychological characteristics. Data on cognition, activities, and participation outcomes were obtained, and participants received an accelerometer during the visit to objectify movement behavior. The participants were given instructions to wear the accelerometer in the front pocket of their trousers on the unaffected leg throughout the whole day during waking time. Accelerometers were worn for 2 consecutive weeks, after which participants sent the devices back by mail.
Dependent Variables
Movement behavior was objectively measured with the Activ8, a 3-axial accelerometer (30 mm×32 mm×10 mm and 20 g). The Activ8 is worn on the thigh and can detect SB (lying and sitting), standing, walking, cycling, and running and yields MET values.16 The Activ8 has been validated to distinguish between different postures in community ambulatory people with stroke.17 Ten different movement behavior modes were calculated; mean time spent sedentary (h/d), LPA (h/d), and MVPA (h/d), mean time spent in sedentary bouts (uninterrupted periods of sitting and lying down) ≥5 minutes per day, ≥30 minutes per day, and ≥60 minutes per day, mean time MVPA in bouts ≥10 minutes, weighted median sedentary bout length, maximum sedentary bout length, and fragmentation index.18 Weighted median sedentary bout length is the length of the sedentary bout corresponding to 50% of the total sedentary time.18 Bouts are ordered from shortest to the longest. For example, if an individual has spent 8 hours being sedentary, the weighted median sedentary bout length represents the length of the bout that contains the 4 hours’ time point. A bout length of 20 minutes would indicate that individuals engage in SB for 50% of the time in bouts ≥20 minutes. The lower the weighted median sedentary bout is, the more interrupted the SB. The fragmentation index is the ratio of the number of sedentary bouts ≥5 minutes divided by total sedentary time.18 A higher fragmentation index indicates more interrupted SB. Participants filled out diaries with a start and stop time. Nonwear time was removed from the data files by comparing start and stop time from the diaries with the device’s internal clock. Valid data were considered to hold at least 7 days of at least 10 hours of movement behavior per day.19
Independent Variables
Demographic characteristics included age, sex, educational level, living situation, body mass index, smoking (pack-years), alcohol consumption (light [0–1 drink/day], moderate [1–2 drink/day], and heavy [>2 drinks/day] drinking20), PA before stroke, and comorbidities. Height and weight to calculate body mass index were objectively measured, and other measures were self-reported. Educational level was asked using the Dutch classification system and dichotomized into low (score 1–5, up to completed secondary education) and high (score 6–7, completed secondary professional education, university or higher).21 Prestroke physical activity was assessed with the Physical Activity Assessment scale (range, 0–8; <4 indicating insufficient amounts of MVPA). The Physical Activity Assessment scale contains 1 question regarding moderate PA and 1 question regarding the amount of vigorous PA during the week.22 Comorbidity was assessed by the Cumulative Illness Rating Scale (range, 0–52, a higher score indicates more comorbidities).23 Item 11 was not included because stroke is included in this item.
Stroke characteristics obtained from medical records included type, location, severity of stroke symptoms, and discharge destination. The severity of stroke symptoms was measured with the National Institutes of Health Stroke Scale (range, 0–42) and was divided into: (1) no stroke symptoms (0 points); (2) minor stroke symptoms (1–4 points); and (3) moderate to severe stroke symptoms (≥5 points).24
Balance was tested with the Berg Balance Scale (range, 0–56, higher scores indicate better functioning).25 Walking speed was measured with the 5-meter walking test, calculated in meter per second (<0.93 m/s indicating limited community walker).26 Activity limitations were assessed using the Late-Life Function and Disability Instrument Computerized Adaptive Test (scores range from 0 to 100, and higher scores indicate better functioning).27 The Late-Life Function and Disability Instrument Computerized Adaptive Test contains 137 questions, which are selected based on the answer to the preceding question. The stopping rule was set for 10 questions.
Cognitive functioning was assessed with the Montreal Cognitive Assessment (range, 0–30; <26 indicating impaired cognitive function).28 The Checklist for individual strength—fatigue assesses the amount of fatigue using 8 items. Each item is rated on a 7-point Likert-scale (range, 8–56, >40 represents severely fatigued).29 Anxiety and depression were assessed with the Hospital Anxiety and Depression Scale (range, 0–21, ≥8 presence of depression or anxiety symptoms).30 The Hospital Anxiety and Depression Scale consists of 14 items, 7 about anxiety and 7 about depression. Each question has a 4-point rating scale (0–3). Self-efficacy was evaluated with the Self-Efficacy for Symptom Management Scale which consists of 13 items (range, 13–130, <115 indicates low/moderate self-efficacy).31 Passive coping was assessed with the subscale of the Utrecht Coping List-Passive reaction pattern (range, 0–28, <16 indicates high passive coping),32 consisting of 7 questions with a 4-point Likert scale. All measurement tools used were valid and reliable.
Data Analysis
Data were analyzed with SPSS version 25.0. Principal component analysis (PCA) was used to compress the information on movement behavior variables to a lower subspace, resulting in components accounting for the desired variance in 60% of the data.33 Movement behavior variables were standardized using z-scores and contributed to one or more components. The compressed components were used to identify the patterns using the k-means clustering algorithm.33 K-means clustering defines that each individual can only be allocated into one pattern only by identifying cluster centers using repeated iteration. In this study, a maximum of ten iterations was used.33 The number of patterns was determined based on the interpretability of the patterns and a scree plot.33
Descriptive variables were presented. Differences between the patterns were evaluated using ANOVA, the Kruskal-Wallis test (nonnormally distributed variables), or the χ2 test (categorical and nominal data). Post hoc analyses were performed for multiple comparisons. Differences between 2 patterns were evaluated with the independent t test, a Mann-Whitney U test for non-normally distributed variables or a χ2 test in cases of categorical and nominal data. Statistical significance was set at P<0.05.
To determine factors associated with a single movement behavior pattern, logistic regression analyses were performed. Odds ratios were calculated to identify candidate factors using univariate analyses. The related variables were tested for multicollinearity (Pearson r<0.70) and effect modification (variance inflation factor >4).34 Significantly associated variables (P<0.1) were entered in multiple backward logistic regression analysis.
Results
In total, 200 participants were included (Figure). The movement behavior data of 10 participants were missing. Therefore, 190 participants were included in the analysis. The participants’ characteristics are presented in Table 1. The mean age at onset of stroke was 68.1 years, 64.7% were male, 91.5% had an infarction, 54.2% had minor stroke symptoms, and 73.7% of the participants were discharged directly to the home setting.
Table 1.
Participant Characteristics and Characteristics per Movement Behavior Pattern Expressed as Mean±SD, Median (IQR), or n (%)
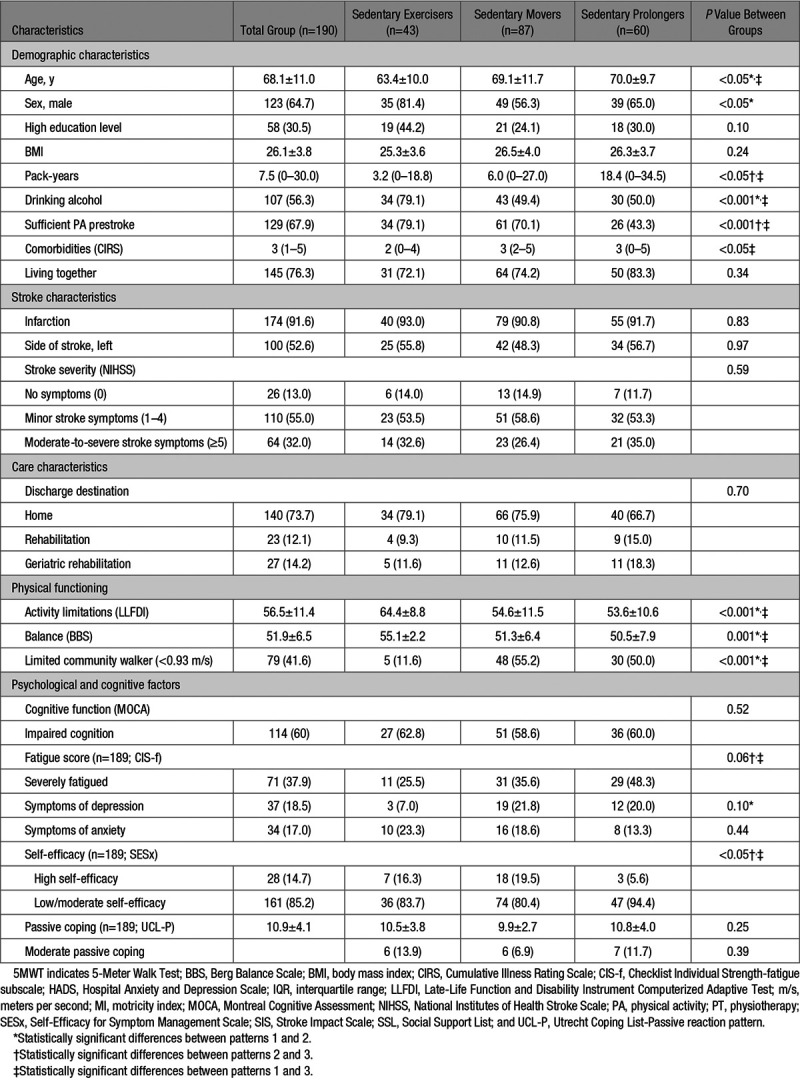
Figure.
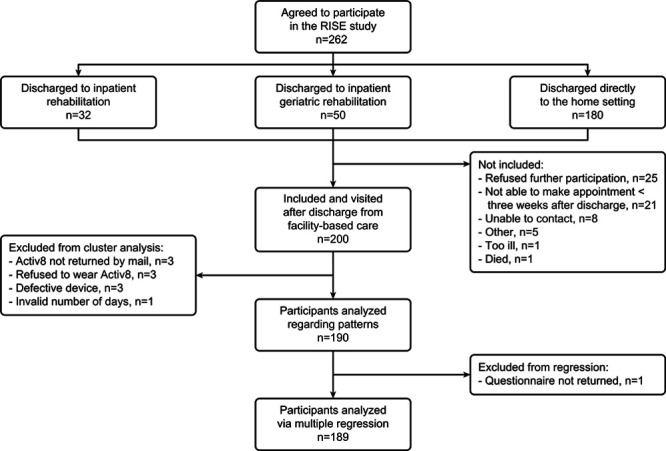
Flow diagram of participants.
The accelerometer was worn 90.4% of all days. The mean wear time was 13.7 hours per day. The mean sedentary time per day was 9.3 hours (67.8%), LPA, 3.8 hours (27.7%), and MVPA 0.6 hours (4.6%). The weighted median sedentary bout length was 22.1 minutes and MVPA accumulated in bouts >10 minutes was 13.8 minutes per day.
Through the use of using PCA, 3 components were identified accounting for 88% of the variance. The first component (58% of the variance) included mean sedentary time, mean sedentary time in bouts ≥5 minutes, mean time LPA, mean sedentary time in bouts ≥30 minutes, and mean sedentary time in bouts ≥60 minutes. The second component (18% of the variance) included mean time MVPA and mean time MVPA in bouts ≥10 minutes, and the third component (11% of the variance) included weighted median sedentary bout length, maximum sedentary bout, and fragmentation index. Scatterplots are presented in Figure IA through IC in the online-only Data Supplement.
Three movement behavior patterns were identified. The characteristics of these patterns are presented in Table 1, and movement behavior differences between individual patterns in Table 2. The results of the univariate analyses per pattern are presented in the online-only Data Supplement. The results of the multiple logistic regression analyses per pattern are shown in Table 3.
Table 2.
Participant Movement Behavior Outcomes and Movement Behavior Outcomes per Pattern
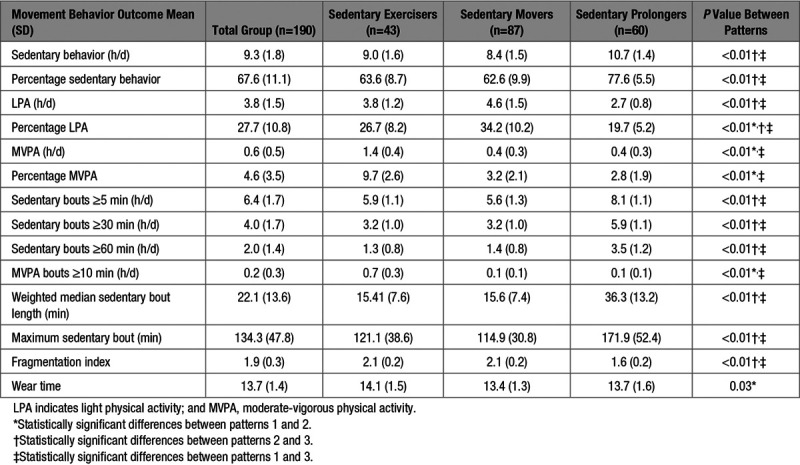
Table 3.
Associated Factors per Movement Behavior Pattern Using Multiple Logistic Regression
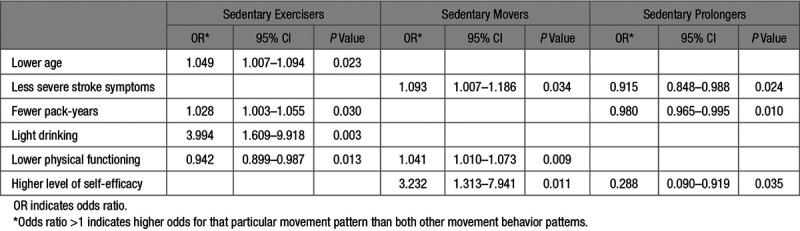
Pattern 1 (n=43; 22.6%), sedentary exercisers, was characterized by interrupted sedentary and active patterns. Participants assigned to pattern 1 were less sedentary (9.0 hours±1.6), had interrupted sedentary time, and reached sufficient amounts of MVPA (0.7 hours per day in bouts ≥10 minutes). Factors associated were younger age, fewer pack-years, light drinking, and fewer activity limitations.
Pattern 2 (n=87; 45.8%) sedentary movers were characterized by interrupted sedentary and inactive patterns. Participants assigned to pattern 2 showed similar results regarding total sedentary time and interrupted sedentary time but did not reach sufficient amounts of MVPA during the day (<0.5 hours per day in MVPA bouts ≥10 minutes). Factors associated were less severe symptoms of stroke, higher activity limitations, and higher levels of self-efficacy.
Pattern 3 (n=60; 31.6%), sedentary prolongers, was characterized by a prolonged and highly sedentary and inactive pattern. Participants assigned to pattern 3 were sedentary 10.7 hours±1.4 per day, had long prolonged sitting bouts and insufficient amounts of MVPA during the day. Factors associated with sedentary prolongers were more pack-years, lower levels of self-efficacy, and more severe stroke symptoms.
Discussion
This study is the first to investigate movement behavior patterns during waking hours, instead of single aspects of movement behavior. Our results indicated that the distribution of SB, as well as the accumulation of SB (interrupted or prolonged SB), LPA, and MVPA differed during waking hours within the sample, resulting in sedentary exercisers, sedentary movers, and sedentary prolongers. Although sedentary exercisers were physically active, they were still sedentary for almost 10 hours per day. This finding confirms the indication that MVPA and SB are 2 independent behaviors. Therefore, research should focus on movement behavior patterns instead of the separate aspects of movement behavior (eg, MVPA or SB only).
The comparison of SB between studies is difficult because in most studies, sleeping time was included in sedentary time.35 However, the recently introduced definition of SB excludes sleeping time.9 Only one study investigated SB excluding sleeping time in people with stroke36; this study found eight percent more SB during waking hours than our results. However, only participants who received inpatient rehabilitation were included. Those participants had more severe stroke symptoms and had comparable characteristics and movement behavior outcomes to the sedentary prolongers in our sample. When comparing our results to a general older population in the Netherlands, participants in all 3 movement behavior patterns in our study were more sedentary than age-matched peers, especially sedentary prolongers who showed far more sedentary time.37 Additionally, sedentary movers and sedentary prolongers demonstrated lower levels of MVPA. In line with other literature, people with stroke in the Netherlands seem to be more sedentary and, in general, more inactive than healthy peers.6,37
More research is needed regarding the accumulation of SB. Prolonged SB is an independent factor for increased health risks, but clear cut-off values are lacking.38 In general, it seems that the participants in this cohort, except for the sedentary prolongers, were interrupting their SB. As a result of the absence of MVPA, the high amount of SB and the accumulation of their SB, sedentary prolongers are at high risk for negative health consequences.
Important associating factors were found. The level of self-efficacy clearly discriminates between sedentary movers and sedentary prolongers. Therefore, lower self-efficacy might be an important target for future interventions to reduce prolonged SB. A lower age was associated with the sedentary exercisers. Older age has been associated with low MVPA levels in people with stroke.39 Earlier research in an elderly population showed that age was a predictor for low MVPA levels but not for the amount of LPA.40 Therefore, although sedentary prolongers are older, higher levels of LPA seem to be feasible. Additionally, sedentary prolongers had significantly more severe stroke symptoms. It seems evident that people with stroke who suffer from physical impairments have more difficulties in being physically active. However, more research is needed to explore the cause of a movement behavior pattern in people with stroke. Since the strongest associating factor with sedentary prolongers was low amounts of self-efficacy, further exploration of personal and psychological factors is needed.
To identify movement behavior patterns, 10 outcomes were used based on the recommendations of Byrom et al.18 Not all 10 outcomes seem to be relevant when monitoring in daily practice. SB, LPA, and MVPA should be measured to objectify the distribution during waking hours.9 Mean time MVPA in bouts ≥10 minutes should be included because people are classified as active when they spend 150 minutes per week in MVPA in bouts ≥10 minutes, according to the World Health Organization.5 To distinguish between prolonged and interrupted SB, the weighted median sedentary bout length seems to be the most meaningful outcome and is sensitive to change over time.35
Both the associated factors and movement behavior patterns give direction for future interventions and clinical practice. Identifying movement behavior patterns will make it possible to offer individuals physical activity options that are tailored to their needs and preferences to maximize health benefits for individuals. Healthcare professionals should focus on how to interrupt and decrease SB for sedentary exercisers and sedentary movers to reach an optimal level of movement behavior. In addition to reducing SB, the health benefits of MVPA should not be overlooked. Sedentary movers should be encouraged to reach sufficient amounts of MVPA, and sedentary exercisers should maintain their MVPA levels. For sedentary prolongers, a focus on interrupting and decreasing SB seems to be a more achievable goal. Changing sedentary daily routines with at least LPA, for example, walking in their own environment or making their own coffee, could lead to a reduction in SB. Personalized movement behavior profiling is essential to tailor future coaching interventions. Since behavioral change is needed, interventions should be theory driven and include at least important behavior change techniques such as self-monitoring of behavior, personalized feedback within the context of the individual, and action planning.41
A strength of our study was the use of a thigh worn accelerometer that allowed detailed analyses and identification of movement behavior patterns. Participants wore the device for 14 days. This method accurately reflected the habitual movement behavior of people with first-ever stroke. In general, our sample had slow to normal waking speeds. A previous study found that the Activ8 is a valid measurement tool for a free-living population comparable to our sample.17 Therefore, the results derived from the Activ8 are reliable and accurate. We investigated movement behavior as time spent sedentary, in LPA and in MVPA. These movement behavior outcomes are based on METs, and these measures were determined in healthy people. Therefore, it could be that LPA levels were overestimated and MVPA levels were underestimated.42 However, in one study, no significant differences in energy expenditures were found between people with stroke and healthy controls when using self-selected speeds.43 These findings indicate that classification during the day was probably correct as most people walk at a self-selected speed. Additionally, participants in our study mainly had mild stroke symptoms supporting the hypothesis that the estimated levels of PA are probably correct. Nevertheless, more research is needed regarding energy expenditure and the intensity of MVPA in people with stroke.42
Conclusions
The majority of people with stroke are inactive and sedentary. Three different movement behavior patterns in people with stroke were identified: sedentary exercisers, sedentary movers and sedentary prolongers. The identified movement behavior patterns confirm the hypothesis that an individually tailored approach might be warranted with movement behavior coaching by health care professionals, based on objectively monitoring the individuals’ movement patterns and associated factors.
Acknowledgments
We thank all participants and participating hospitals. We thank Thirsa Koebrugge and Joeri Polman, who helped with the data collection.
Sources of Funding
This article was funded by Dutch Organization for Scientific Research (NWO), Doctoral Grant for Teachers, 023.003.136.
Disclosures
None.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.119.027013.
References
- 1.Touzé E, Varenne O, Chatellier G, Peyrard S, Rothwell PM, Mas JL. Risk of myocardial infarction and vascular death after transient ischemic attack and ischemic stroke: a systematic review and meta-analysis. Stroke. 2005;36:2748–2755. doi: 10.1161/01.STR.0000190118.02275.33. doi: 10.1161/01.STR.0000190118.02275.33. [DOI] [PubMed] [Google Scholar]
- 2.Johnson CO, Nguyen M, Roth GA, Nichols E, Alam T, Abate D, et al. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. doi: 10.1016/S1474-4422(19)30034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee CD, Folsom AR, Blair SN. Physical activity and stroke risk: a meta-analysis. Stroke. 2003;34:2475–2481. doi: 10.1161/01.STR.0000091843.02517.9D. doi: 10.1161/01.STR.0000091843.02517.9D. [DOI] [PubMed] [Google Scholar]
- 4.Hackam DG, Spence JD. Combining multiple approaches for the secondary prevention of vascular events after stroke: a quantitative modeling study. Stroke. 2007;38:1881–1885. doi: 10.1161/STROKEAHA.106.475525. doi: 10.1161/STROKEAHA.106.475525. [DOI] [PubMed] [Google Scholar]
- 5.Organization WH. Global Recommendations on Physical Activity for Health. Geneva, Switzerland: 2010. [PubMed] [Google Scholar]
- 6.English C, Manns PJ, Tucak C, Bernhardt J. Physical activity and sedentary behaviors in people with stroke living in the community: a systematic review. Phys Ther. 2014;94:185–196. doi: 10.2522/ptj.20130175. doi: 10.2522/ptj.20130175. [DOI] [PubMed] [Google Scholar]
- 7.Butler EN, Evenson KR. Prevalence of physical activity and sedentary behavior among stroke survivors in the United States. Top Stroke Rehabil. 2014;21:246–255. doi: 10.1310/tsr2103-246. doi: 10.1310/tsr2103-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Ploeg HP, Chey T, Korda RJ, Banks E, Bauman A. Sitting time and all-cause mortality risk in 222 497 Australian adults. Arch Intern Med. 2012;172:494–500. doi: 10.1001/archinternmed.2011.2174. doi: 10.1001/archinternmed.2011.2174. [DOI] [PubMed] [Google Scholar]
- 9.Tremblay MS, Aubert S, Barnes JD, Saunders TJ, Carson V, Latimer-Cheung AE, et al. SBRN Terminology Consensus Project Participants. Sedentary Behavior Research Network (SBRN) - terminology consensus project process and outcome. Int J Behav Nutr Phys Act. 2017;14:75. doi: 10.1186/s12966-017-0525-8. doi: 10.1186/s12966-017-0525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sedentary Behaviour Research Network. Letter to the editor: standardized use of the terms “sedentary” and “sedentary behaviours.". Appl Physiol Nutr Metab. 2012;37:540–542. doi: 10.1139/h2012-024. doi: 10.1139/h2012-024. [DOI] [PubMed] [Google Scholar]
- 11.Chaput JP, Carson V, Gray CE, Tremblay MS. Importance of all movement behaviors in a 24 hour period for overall health. Int J Environ Res Public Health. 2014;11:12575–12581. doi: 10.3390/ijerph111212575. doi: 10.3390/ijerph111212575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.English C, Janssen H, Crowfoot G, Bourne J, Callister R, Dunn A, et al. Frequent, short bouts of light-intensity exercises while standing decreases systolic blood pressure: Breaking Up Sitting Time after Stroke (BUST-Stroke) trial. Int J Stroke. 2018;13:932–940. doi: 10.1177/1747493018798535. doi: 10.1177/1747493018798535. [DOI] [PubMed] [Google Scholar]
- 13.Collin C, Wade DT, Davies S, Horne V. The barthel ADL Index: a reliability study. Int Disabil Stud. 1988;10:61–63. doi: 10.3109/09638288809164103. [DOI] [PubMed] [Google Scholar]
- 14.Pijfers EM, Vries LAd, Messing-Petersen H. Het Utrechts Communicatie Onderzoek. Westervoort: 1985. [Google Scholar]
- 15.Holden MK, Gill KM, Magliozzi MR. Gait assessment for neurologically impaired patients. Standards for outcome assessment. Phys Ther. 1986;66:1530–1539. doi: 10.1093/ptj/66.10.1530. doi: 10.1093/ptj/66.10.1530. [DOI] [PubMed] [Google Scholar]
- 16.Activ8 accelerometer—Activ8all.com. Available at: http://www.activ8all.com/. Accessed 12 june 2019.
- 17.Fanchamps MHJ, Horemans HLD, Ribbers GM, Stam HJ, Bussmann JBJ. The accuracy of the detection of body postures and movements using a physical activity monitor in people after a stroke. Sensors (Switzerland) 2018;18:2167–2177. doi: 10.3390/s18072167. doi: 10.3390/s18072167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byrom B, Stratton G, Mc Carthy M, Muehlhausen W. Objective measurement of sedentary behaviour using accelerometers. Int J Obes (Lond) 2016;40:1809–1812. doi: 10.1038/ijo.2016.136. doi: 10.1038/ijo.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews CE, Hebert JR, Freedson PS, Stanek EJ, 3rd, Merriam PA, Ebbeling CB, et al. Sources of variance in daily physical activity levels in the seasonal variation of blood cholesterol study. Am J Epidemiol. 2001;153:987–995. doi: 10.1093/aje/153.10.987. doi: 10.1093/aje/153.10.987. [DOI] [PubMed] [Google Scholar]
- 20.Kadlecová P, Andel R, Mikulík R, Handing EP, Pedersen NL. Alcohol consumption at midlife and risk of stroke during 43 years of follow-up: cohort and twin analyses. Stroke. 2015;46:627–633. doi: 10.1161/STROKEAHA.114.006724. doi: 10.1161/STROKEAHA.114.006724. [DOI] [PubMed] [Google Scholar]
- 21.Verhage F. Intelligentie en leeftijd: onderzoek bij Nederlanders van twaalf tot zevenzeventig jaar [Intelligence and Age: study with Dutch people aged 12 to 77] Assen: Van Gorcum; 1964. [Google Scholar]
- 22.Marshall AL, Smith BJ, Bauman AE, Kaur S. Reliability and validity of a brief physical activity assessment for use by family doctors. Br J Sports Med. 2005;39:294–7. doi: 10.1136/bjsm.2004.013771. discussion 294. doi: 10.1136/bjsm.2004.013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity. a critical review of available methods. J Clin Epidemiol. 2003;56:221–229. doi: 10.1016/s0895-4356(02)00585-1. doi: 10.1016/s0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- 24.Meyer BC, Hemmen TM, Jackson CM, Lyden PD. Modified national institutes of health stroke scale for use in stroke clinical trials: prospective reliability and validity. Stroke. 2002;33:1261–1266. doi: 10.1161/01.str.0000015625.87603.a7. doi: 10.1161/01.str.0000015625.87603.a7. [DOI] [PubMed] [Google Scholar]
- 25.Blum L, Korner-Bitensky N. Usefulness of the berg balance scale in stroke rehabilitation: a systematic review. Phys Ther. 2008;88:559–566. doi: 10.2522/ptj.20070205. doi: 10.2522/ptj.20070205. [DOI] [PubMed] [Google Scholar]
- 26.Fulk GD, He Y, Boyne P, Dunning K. Predicting home and community walking activity poststroke. Stroke. 2017;48:406–411. doi: 10.1161/STROKEAHA.116.015309. doi: 10.1161/STROKEAHA.116.015309. [DOI] [PubMed] [Google Scholar]
- 27.Wondergem R, Pisters MF, Wouters EM, de Bie RA, Visser-Meily JM, Veenhof C. Validation and responsiveness of the late-life function and disability instrument computerized adaptive test in community-dwelling stroke survivors. Eur J Phys Rehabil Med. 2019;55:424–432. doi: 10.23736/S1973-9087.18.05359-5. doi: 10.23736/S1973-9087.18.05359-5. [DOI] [PubMed] [Google Scholar]
- 28.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 29.van der Werf SP, van den Broek HL, Anten HW, Bleijenberg G. Experience of severe fatigue long after stroke and its relation to depressive symptoms and disease characteristics. Eur Neurol. 2001;45:28–33. doi: 10.1159/000052085. doi: 10.1159/000052085. [DOI] [PubMed] [Google Scholar]
- 30.Spinhoven P, Ormel J, Sloekers PP, Kempen GI, Speckens AE, Van Hemert AM. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med. 1997;27:363–370. doi: 10.1017/s0033291796004382. doi: 10.1017/s0033291796004382. [DOI] [PubMed] [Google Scholar]
- 31.Cicerone KD, Azulay J. Perceived self-efficacy and life satisfaction after traumatic brain injury. J Head Trauma Rehabil. 2007;22:257–266. doi: 10.1097/01.HTR.0000290970.56130.81. doi: 10.1097/01.HTR.0000290970.56130.81. [DOI] [PubMed] [Google Scholar]
- 32.Stoilkova A, Janssen DJ, Franssen FM, Spruit MA, Wouters EF. Coping styles in patients with COPD before and after pulmonary rehabilitation. Respir Med. 2013;107:825–833. doi: 10.1016/j.rmed.2013.03.001. doi: 10.1016/j.rmed.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 33.von Luxburg U. Clustering stability: an overview. Found Trends Mach Learn. 2010;2:235–274. [Google Scholar]
- 34.Miles JNV, Shevlin ME. Applying Regression and Correlation: A Guide for Students and Researchers. Sage Publications; 2001. p. 253. [Google Scholar]
- 35.Tieges Z, Mead G, Allerhand M, Duncan F, van Wijck F, Fitzsimons C, et al. Sedentary behavior in the first year after stroke: a longitudinal cohort study with objective measures. Arch Phys Med Rehabil. 2015;96:15–23. doi: 10.1016/j.apmr.2014.08.015. doi: 10.1016/j.apmr.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 36.Ezeugwu VE, Manns PJ. Sleep duration, sedentary behavior, physical activity, and quality of life after inpatient stroke rehabilitation. J Stroke Cerebrovasc Dis. 2017;26:2004–2012. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.009. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 37.van Ballegooijen AJ, van der Ploeg HP, Visser M. Daily sedentary time and physical activity as assessed by accelerometry and their correlates in older adults. Eur Rev Aging Phys Act. 2019;16:3. doi: 10.1186/s11556-019-0210-9. doi: 10.1186/s11556-019-0210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis LK, Hunt T, Williams MT, English C, Olds TS. Sedentary behavior in people with and without a chronic health condition: how much, what and when? AIMS Public Health. 2016;3:503–519. doi: 10.3934/publichealth.2016.3.503. doi: 10.3934/publichealth.2016.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olsson OA, Persson HC, Alt Murphy M, Sunnerhagen KS. Early prediction of physical activity level 1 year after stroke: a longitudinal cohort study. BMJ Open. 2017;7:e016369. doi: 10.1136/bmjopen-2017-016369. doi: 10.1136/bmjopen-2017-016369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takagi D, Nishida Y, Fujita D. Age-associated changes in the level of physical activity in elderly adults. J Phys Ther Sci. 2015;27:3685–3687. doi: 10.1589/jpts.27.3685. doi: 10.1589/jpts.27.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maher JP, Conroy DE. A dual-process model of older adults’ sedentary behavior. Health Psychol. 2016;35:262–272. doi: 10.1037/hea0000300. doi: 10.1037/hea0000300. [DOI] [PubMed] [Google Scholar]
- 42.Compagnat M, Mandigout S, David R, Lacroix J, Daviet JC, Salle JY. Compendium of physical activities strongly underestimates the oxygen cost during activities of daily living in stroke patients. Am J Phys Med Rehabil. 2019;98:299–302. doi: 10.1097/PHM.0000000000001077. doi: 10.1097/PHM.0000000000001077. [DOI] [PubMed] [Google Scholar]
- 43.Kramer S, Johnson L, Bernhardt J, Cumming T. Energy expenditure and cost during walking after stroke: a systematic review. Arch Phys Med Rehabil. 2016;97:619–632.e1. doi: 10.1016/j.apmr.2015.11.007. doi: 10.1016/j.apmr.2015.11.007. [DOI] [PubMed] [Google Scholar]


