Abstract
The objective of this study was to investigate the effects of replacing antibiotics with a combination of plant essential oils on the growth performances and gastrointestinal health of broilers. A total of 720 1-day-old male AA broilers were randomly divided into 3 treatments: the control treatment (CON), the Aureomycin supplementation treatment (AGP), and the combined plant oils supplementation treatment (POC), with a 42-D period feeding procedure. Growth performances, carcass performances, intestinal sections, and cecal microbiota were investigated. Results indicated that POC supplementation decreased the feed conversion ratio compared with CON and AGP treatments, though not significantly. No significant differences were found for feed intake, BW gain, and culling rate among the 3 treatments (P > 0.05). In addition, no significant differences were seen on carcass performance. For the aspects of intestinal section, POC supplementation did not make significant effects on intestinal wall thickness, villus heights, crypt depths, and the ratio of villus heights/crypt depths compared with CON and AGP treatments. Cecal microbiota results demonstrated that bacterial diversity and some representative probiotic bacteria were significantly increased in numbers (P < 0.05) after POC supplementation. In conclusion, the combination of essential oils promoted intestinal health through improving gut bacterial diversity and probiotic bacteria, as well as improving feed conversion ratio of broilers. These results indicated that the combination of essential oils may benefit the gastrointestinal health and be applied as an antibiotic alternative.
Key words: broiler, antibiotic alternative, plant essential oil, gastrointestinal health
Introduction
Antibiotics have been ubiquitously included in broiler production over past decades and provided significant enhancement (Chapman and Johnson, 2002). However, continuous use of antibiotics disturbed gastrointestinal metabolism and caused serious antimicrobial resistance (Oliveira et al., 2010), which lead to a negative impact on animal health and further economic loss. Moreover, antibiotic residue in food animals might result in potential threat to human health. Thus, bans of antibiotics used as feed additives in husbandry production have been enacted (Wasch et al., 1998; Claudie et al., 2009). The investigation of suitable alternative supplements is of vital importance for broiler production.
Fortunately, antibiotic alternatives applied in broiler production have been investigated in the past few years, which included plant essential oils, probiotics, and antimicrobial peptides (Kähkönen et al., 1999; Shim et al., 2010; Wang et al., 2015). Plant essential oils drew general attention for the presence of bioactive ingredients that express antimicrobial, antioxidant, and anti-inflammatory activities such as oligosaccharides, polyphenols, and saponins (Fernandez et al., 2002; Brenes et al., 2008) and offered considerably protection and improvement on broiler production (Botsoglou et al., 2002; Amerah et al., 2012; Sivarajan et al., 2017). Eucalyptus oil (Nameghi et al., 2019) and carvacrol (Luna et al., 2010) are the representative plant oils used in the broiler productions and effectively prevent the occurrence of necrotic enteritis (Sivarajan et al., 2017). Cinnamyl aldehyde and capsaicin were also shown to present anti-inflammatory functions (Dong et al., 2019). The effects of combined essential oils on broiler production were seldom acquired, and whether combinations of essential oils performed a better than when not combined needs further examination. Therefore, a combination of eucalyptus oil, carvacrol, cinnamyl aldehyde, and capsaicin was applied in the present study to investigate their effects on growth performance and gastrointestinal health of broilers.
Gastrointestinal bacteria was well documented to play important roles in animal gastrointestinal health and animal growth (Pan and Yu, 2014). Antibiotic alternatives interacted with intestinal bacteria and affected gut morphology, nutrient absorption, and immune responses of the host (Apajalahti and Vienola, 2016). The microbial community in gastrointestinal tract may further interact with the intestinal epithelium and ultimately regulate the absorptivity of intestine and production performances of broilers (Cui et al., 2017). Therefore, the hypothesis was made that the combination of essential oils may regulate intestinal probiotic bacteria, improve the intestinal epithelium, and ultimately promote the production performance of broilers.
Materials and methods
Ethics Statement
This study was performed in accordance with local ethical guidelines and met the requirements of the institutional animal care and use committee.
Experimental Design and Birds
In the present study, 720 1-day-old male AA broilers with the similar birth weight (42 ± 1 g) were randomly divided into 3 treatments: the control treatment (CON), the Aureomycin supplementation treatment (AGP), and the supplementation of plant oils combination treatment (POC). Each treatment contained 8 replicates, and 30 broilers were included in each replicate. All chickens were housed in the battery pens (100 cm × 70 cm) with plastic wire floors; each replicate comprised 3 successive pens, and each pen was allocated with 10 broilers. All birds were provided with a 3-phase feeding program (day 0–21 as a starter phase, day 22–35 as a grower phase 2, and day 36–42 as a finisher phase), Aureomycin supplementation was stopped at the finisher phase. Feed and water were provided ad libitum throughout the experiment. The room temperature was maintained at 37°C for the first week and then reduced by 3°C each week until reaching 24°C. The lighting schedule was 23L:1D during the experiment period.
Plant oils supplements with the standard combination bioactive ingredients of eucalyptus oil (25%), carvacrol (35%), cinnamyl aldehyde (25%), capsaicin (10%), and some other prebiotics (5%) that were coated by long-chain fatty acids were acquired from EW Nutrition GmbH Ltd., Visbek, Germany. During the whole feeding period, supplements were added as the recommended dosage of 100 g/t in the diet of broilers. Aureomycin for feed additives (15% Aureomycin content) was acquired from Huameng Jinhe Industrial Co. Ltd, Inner Mongolia, China. https://www.etlong.com/nmghmj/. For the experimental diets in each phase, a master batch of the basal diet (negative control) will be prepared in mash, and antibiotics or the additives will be added afterward. The composition of the experimental diets and the nutrients are shown in Table 1.
Table 1.
Composition of the experimental diets for broilers.
| Ingredient | Starter phase | Grower phase | Finisher phase |
|---|---|---|---|
| Corn | 59.7 | 60.4 | 60.4 |
| Soy oil | 1.45 | 2.98 | 2.98 |
| SBM, CP 43% | 34.6 | 32.68 | 32.68 |
| L-Lys HCl, (98%) | 0.17 | 0.18 | 0.18 |
| DL-Met | 0.24 | 0.23 | 0.23 |
| CaCO3 | 1.2 | 1 | 1 |
| Calcium hydrophosphate (2 water) DCP | 1.86 | 1.8 | 1.8 |
| Salt | 0.4 | 0.4 | 0.4 |
| Choline HCl (50%) | 0.15 | 0.1 | 0.1 |
| Primix Vitamin1 | 0.03 | 0.03 | 0.03 |
| Primix mineral2 | 0.2 | 0.2 | 0.2 |
| Total | 100 | 100 | 100 |
| Level of nutrients (calculated) | |||
| ME (kcal/kg) | 2,950 | 3,020 | 3,020 |
| CP | 21 | 20 | 20 |
| Ca | 1.01 | 0.9 | 0.9 |
| P | 0.45 | 0.43 | 0.43 |
| DLys | 1.15 | 1.1 | 1.1 |
| DMet | 0.5 | 0.48 | 0.48 |
| DCys | 0.29 | 0.28 | 0.28 |
| dM + C | 0.86 | 0.82 | 0.82 |
Vitamin content: VA, 12,000IU/kg; VD, 33,000IU/kg; VE, 7.5IU/kg; VK, 31.50 mg/kg; VB1, 0.6 mg/kg; VB2, 4.8 mg/kg; VB6, 1.8 mg/kg; VB12, 10 mg/kg; Folic acid, 0.15 mg/kg; niacinamide, 30 mg/kg; pantothenic acid, 10.5 mg/kg.
Fe 80 mg, Cu 8 mg, Mn 80 mg, Zn 60 mg, Se 0.15 mg, I 0.35 mg.
Growth Performances
Broiler chicken weights and feed consumption were determined by pen on the hatching day, day 21, and day 42, to assess BW gain (BWG), feed intake (FI), and feed conversion ratio (FCR). Broilers were inspected thoroughly each d to record and remove any dead birds; mortality and culling rate was calculated based on dead and culling birds. The FI was adjusted for dead broiler chickens. European Index (EPI) was calculated for each treatment based on the following equation: EPI = (100-mortality%) × live weight (kg) × 100/age (d) × FCR (Maiorano et al., 2017), where age = 42 D, mortality, FCR was calculated based on the aforementioned result.
Carcass Performances and Immune Organs Index
On day 42, 3 birds per replication (24 per treatment) were randomly selected for measurement of carcass characteristics after 12-h fasting. Eviscerated yield was calculated as a percentage of BW. Breast muscle, thigh muscle, and abdominal fat were separated and weighed. Breast and thigh muscle yields were calculated as percentages of eviscerated weight. Abdominal fat percentage was calculated as abdominal fat weight/(abdominal fat weight + eviscerated weight). The immune organs spleen, thymus gland, and bursa of Fabricius were separated and weighted. The immune organs indexes were calculated as the percentage of immune organ weight to BW.
Morphologic Examination of Intestinal Wall
On day 42, a total of 72 birds (24 birds per treatment) were numbered serially 1–72 with no group information marked before slaughtering. The ileum and jejunum were collected for paraffin section. Ten villi of each intestinal segment were chosen in a random order to avoid bias for measuring the villus height and crypt depth. The ratio of villus height to crypt depth (V/C) was calculated.
Cecal Sampling and Microbiota Analysis
On day 42, cecal samples were collected from 1 bird per replication and dispensed into 3 nonenzymatically sterilized cryotubes, quickly frozen in liquid nitrogen, and then stored at −80°C for further cecal microbiota analysis. DNA from each sample was extracted using cetyltrimethyl ammonium bromide/sodium dodecyl sulfate (CTAB/SDS) method (Aristóteles et al., 2005). DNA concentration and purity were monitored on 1% agarose gels (Guo et al., 2018). The 16S rRNA gene V4 region was amplified using primer pairs F515 and R806, (F: GTGCCAGCMGCCGCGGTAA and R: GGACTACVSGGGTATCTAAT) (Gungor et al., 2016). All PCR reactions were carried out with Phusion High-Fidelity PCR Master Mix (New England Biolabs (Beijing) Biotech., Ltd., Beijing, China). Samples with bright main strip between 400–450 bp were chosen for further analysis. The mixture of PCR products was purified using the Qiagen Gel Extraction Kit (Qiagen, Hilden, Germany) and subsequently, sequencing libraries were generated using TruSeq DNA PCR-Free Sample Preparation Kit (Illumina Inc., San Diego). The library quality was assessed on the Qubit@ 2.0 Fluorometer (Thermo Scientific (China) Co. Ltd., Shanghai, China) and Agilent Bioanalyzer 2100 system (Agilent Technologies, Inc. Palo Alto, CA). At last, the library was sequenced on Illumina HiSeq 4000 platform (Illumina Inc.).
Quality filtering of raw tags were performed under specific filtering conditions to obtain high-quality clean tags in accordance with the QIIME (version 1.7.0; San Diego, CA; https://qiime.org/) quality control process. Sequences within similarity >97% were assigned into the same operational taxonomic unit (OTU). For each representative sequence, the Green Gene Database (http://greengenes.secondgenome.com.) was applied based on RDP classifier algorithm to annotate taxonomic information.
Based on the taxonomy results, sequences with >97% similarity were assigned to the same OTUs (Xue et al., 2019). Subsequent analysis of alpha diversity and beta diversity were all examined based on OUT results. Alpha diversity is applied in analyzing complexity of species diversity for a sample through Chao1, Shannon, Simpson, ACE indexes. All indices in our samples were calculated with QIIME (version 1.7.0) and displayed with R software (version 3.15.3; R Core Team, Vienna, Austria). Beta diversity was to evaluate differences of species complexity among different treatments, and calculated by QIIME software (version 1.7.0). the results were displayed with R software.
Statistical Analysis
For the differential analysis of growth performances, carcass performances and gastrointestinal morphology, normal distribution test was conducted using SAS (SAS Institute, Inc., Cary, NC). procedure “proc univariate data = test normal” and subsequently, a one-way ANOVA Student-Newman-Keuls test was applied to investigate the differences among the 3 treatments. Results were presented as mean ± SEM. OTU abundances of cecal bacteria were conducted with a transformation of normal distribution using log2, and then, a one-way ANOVA Student-Newman-Keuls test of SAS 9.2 was applied for the differential analysis. Alpha diversity and beta diversity in our samples were calculated with QIIME (version 1.7.0) and displayed with R software (version 3.15.3). Principle coordinate analysis was constructed using the WGCNA package, stat packages, and ggplot2 package in R software (version 3.15.3). P-value < 0.05 was considered to be significant, and 0.05 ≤ P < 0.10 was considered as a tendency. Spearman correlations between bacteria communities and fermentable and digestibility parameters were assessed using the PROC CORR procedure of SAS 9.2, and then, a correlation matrix was created and visualized in a heatmap format using R software (version 3.15.3).
Results
Animal Production Performances and Immune Organ Indexes
The differential analysis of FI, BWG, FCR, and culling rate are summarized in Table 2. Whether during the starter phase or grower phase or during the entire grow out phase (day 0–day 42), no significant differences were found for FI, BWG, FCR, and culling rate among the 3 treatments. However, POC supplementation decreased (P > 0.05) the FCR compared with CON and AGP treatments. In addition, the EPI which is applied to evaluate the broilers' production level indicated there was no significant differences among the 3 treatments; however, POC supplementation could increase the EPI (P > 0.05) to a certain degree.
Table 2.
Effects of combined plant essential oils on growth performances of AA broiler chickens.
| Growth phase | Items | CON | POC | AGP | SEM | P-value |
|---|---|---|---|---|---|---|
| Starter phase | FI, g/bird | 1,161 | 1,121 | 1,162 | 17.98 | 0.223 |
| BWG, g/bird | 904 | 890 | 920 | 22.13 | 0.081 | |
| FCR | 1.28 | 1.26 | 1.26 | 0.04 | 0.565 | |
| Mortality + culling rate% | 0.83 | 0.42 | 2.08 | 1.23 | 0.187 | |
| Grower and finisher phases | FI, g/bird | 2,963 | 2,646 | 3,004 | 171.21 | 0.154 |
| BWG, g/bird | 1,595 | 1,531 | 1,550 | 124.64 | 0.662 | |
| FCR | 1.65 | 1.60 | 1.76 | 0.11 | 0.434 | |
| Mortality + culling rate % | 7.54 | 5.89 | 5.09 | 3.12 | 0.575 | |
| Whole phase | FI, g/bird | 4,270 | 3,897 | 4,289 | 201.24 | 0.147 |
| BWG, g/bird | 2,486 | 2,388 | 2,465 | 114.27 | 0.389 | |
| FCR | 1.51 | 1.47 | 1.57 | 0.08 | 0.487 | |
| Mortality + culled rate % | 8.33 | 6.25 | 7.08 | 4.21 | 0.699 | |
| European index | 388.5 | 391.9 | 375.9 | 42.53 | 0.796 |
Abbreviations: AGP, the Aureomycin supplementation treatment; BWG, BW gain; CON, the control treatment; FCR, feed conversion ratio; FI, feed intake; POC, the combined plant essential oils treatment.
Carcass characteristics and immune organ indexes of the broilers are presented in Table 3. Based on the results, no significant differences for carcass performances were detected among CON, POC, and AGP treatments. Immune organs were sampled and weighed after slaughtered, and the results indicated that the broiler chickens from different treatments did not show significant differences in immune organ indexes.
Table 3.
Effects of combined plant essential oils on carcass characteristics and immune organs of AA broilers.
| Items (%) | CON | POC | AGP | SEM | P-value |
|---|---|---|---|---|---|
| Dressed yield | 92.47 | 92.44 | 92.14 | 1.172 | 0.349 |
| Eviscerated yield | 75.43 | 75.88 | 75.53 | 1.684 | 0.665 |
| Breast muscle | 32.29 | 32.37 | 32.34 | 2.342 | 0.996 |
| Thigh muscle | 25.38 | 22.99 | 23.72 | 4.243 | 0.310 |
| Abdominal fat | 1.21 | 1.17 | 1.08 | 0.361 | 0.534 |
| Spleen index | 0.11 | 0.11 | 0.11 | 0.020 | 0.534 |
| Thymus index | 0.20 | 0.19 | 0.18 | 0.070 | 0.534 |
| Bursa of Fabricius | 0.15 | 0.18 | 0.16 | 0.040 | 0.534 |
Abbreviations: AGP, the Aureomycin supplementation treatment; CON, the control treatment; POC, the combined plant essential oils treatment.
Morphologic Examination of the Gut Wall
The morphologic characteristics of the jejunum and ileum were examined to investigate the effects of POC on the gut development. Results were shown in Table 4. The POC supplementation did not have significant effects on intestinal wall thickness, villus heights, crypt depths, and the V/C compared with CON and AGP treatments. However, the V/C was highest (P > 0.05) in the POC supplementation treatment.
Table 4.
Effect of combined plant essential oils on morphologic development of the gut wall.
| Items | CON | POC | AGP | SEM | P-value | |
|---|---|---|---|---|---|---|
| Ileum | Villus height (μm) | 747.4 | 737.3 | 661.0 | 93.246 | 0.426 |
| Crypt depth (μm) | 92.84 | 87.81 | 88.98 | 21.314 | 0.355 | |
| Thickness (μm) | 237.3 | 245.1 | 226.9 | 67.675 | 0.534 | |
| Villus/Crypt | 8.47 | 9.08 | 7.65 | 2.142 | 0.827 | |
| Jejunum | Villus height (μm) | 904.4 | 938.5 | 837.2 | 145.341 | 0.117 |
| Crypt depth (μm) | 105.32 | 99.73 | 86.01 | 36.214 | 0.798 | |
| Thickness(μm) | 195.9 | 198.1 | 176.9 | 56.324 | 0.783 | |
| Villus/Crypt | 9.92 | 10.40 | 9.85 | 2.465 | 0.183 |
Abbreviations: AGP, the Aureomycin supplementation treatment; CON, the control treatment; POC, the combined plant essential oils treatment.
Cecal Microbiota
One sample from each repeat (8 samples in each treatment and total of 24 samples) was chosen for the 16S rRNA gene amplicon sequencing process to investigate the effects of POC and AGP supplementation treatments on gastrointestinal microbiota. Taxonomy results of all bacteria are shown in Supplementary File 1. To simply state, the effective tags of each sample ranged from 60,000 to 72,000 after quality control filtering, and the average length of a sequence read was about 410 bases. After taxonomy analysis, 10 phyla and more than 200 genera were identified in the present study and were subsequently used for further analysis.
Alpha Diversity
Alpha diversity was applied in analyzing the complexity of species diversity through Chao1, Shannon, Simpson, and ACE indexes, and all results are displayed in Table 5. The Shannon index for AGP treatment was significantly increased (P < 0.05), whereas the Simpson index was significantly decreased (P < 0.05) compared with CON treatment. No significant differences (P > 0.05) were detected between POC and AGP for the Shannon and Simpson indexes. The ACE index and Chao index of POC reached the highest among the 3 treatments, and the Chao index for POC exhibited a significant increase (P < 0.05) when compared with CON and AGP treatments.
Table 5.
Effects of combined plant essential oils on α-diversity of cecal contents bacterial communities.
| Items | CON | POC | AGP | SEM | P-value |
|---|---|---|---|---|---|
| Shannon | 3.87b | 4.06a,b | 4.23a | 0.057 | 0.031 |
| Simpson | 0.07a | 0.05a,b | 0.04b | 0.005 | 0.026 |
| Ace | 485.2 | 515.1 | 489.3 | 8.852 | 0.364 |
| Chao | 488.8b | 535.6a | 499.7b | 9.139 | 0.047 |
a,bMeans within a row with different letters differed significantly (P < 0.05).
Abbreviations: AGP, the Aureomycin supplementation treatment; CON, the control treatment; POC, the combined plant essential oils treatment.
Beta Diversity
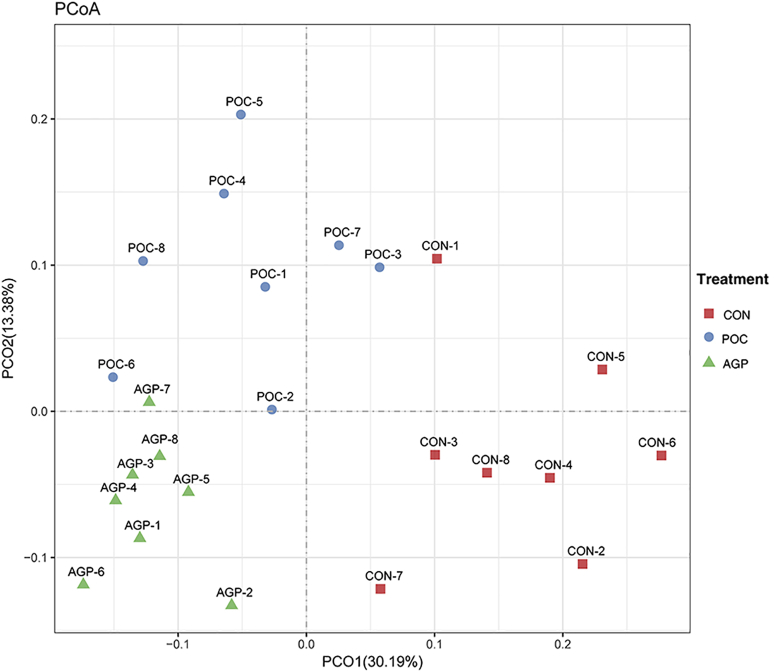
Principle coordinate analysis was conducted to compare bacterial profiles among the 3 treatments. As shown in Figure 1, principle coordinate analysis axes 1 and 2 accounted for 30.19 and 13.38% of the total variation, respectively. Based on the results, bacteria in POC and AGP treatments could be clearly separated from CON by PCo1, whereas bacteria in POC treatment could be separated from those in AGP treatment by PCo2.
Figure 1.
Principal coordinate analysis on community structures of the cecal microbiota in the different treatments. Abbreviations: AGP, the Aureomycin supplementation treatment; CON, the control treatment; POC, the combined plant essential oils treatment.
Subsequently, differential analysis on the abundances of different bacteria at the phyla and genera levels were chosen to investigate effects of POC supplementation on gut bacteria. Results are shown in Tables 6 and 7. At the level of phyla, Firmicutes was the dominant phylum in the cecal microbiota and accounted for 70–80% of the total microbiota in all 3 treatments. Bacteroidetes and Proteobacteria accounted for the second and third most abundant phylum of the total microbiota, respectively. The combined plant oils supplementation treatment and AGP significantly (P < 0.05) promoted the relative abundances of Bacteroidetes, whereas suppressed the abundances of Firmicutes. In addition, Tenericutes was found to significantly (P < 0.05) increase in AGP treatment compared with CON and POC.
Table 6.
Effects of combined plant essential oils on the relative abundances of cecal microbiota at the level of phyla.
| Phyla | CON | POC | AGP | SEM | P-value |
|---|---|---|---|---|---|
| Firmicutes | 15.64a | 15.38b | 15.43b | 0.047 | 0.046 |
| Bacteroidetes | 11.71b | 12.41a | 12.74a | 0.173 | 0.038 |
| Proteobacteria | 9.84 | 10.94 | 11.17 | 0.273 | 0.097 |
| Actinobacteria | 6.22 | 5.66 | 6.29 | 0.239 | 0.522 |
| Cyanobacteria | 6.04 | 5.70 | 5.45 | 0.469 | 0.894 |
| Tenericutes | 8.65b | 9.12b | 10.04a | 0.192 | 0.005 |
| Verrucomicrobia | 4.40 | 5.67 | 3.30 | 0.896 | 0.552 |
Sequences relative abundances were transformed using log2.
a,bMeans (n = 8) within rows and with different letters differed significantly (P < 0.05).
Abbreviations: AGP, the Aureomycin supplementation treatment; CON, the control treatment; POC, the combined plant essential oils treatment.
Table 7.
Effects of combined plant essential oils treatment on the relative abundances of cecal microbiota at the level of genera.
| Genera | CON | POC | AGP | SEM | P-value |
|---|---|---|---|---|---|
| Faecalibacterium | 13.85a | 12.59b | 12.17b | 0.185 | <0.001 |
| Ruminococcaceae | 11.86b | 12.45a | 12.83a | 0.117 | 0.001 |
| Alistipes | 12.00 | 12.05 | 12.51 | 0.164 | 0.395 |
| Ruminococcus | 12.08 | 12.09 | 12.52 | 0.116 | 0.216 |
| Lachnospiraceae | 11.76 | 11.98 | 11.93 | 0.123 | 0.771 |
| Subdoligranulum | 10.77 | 10.37 | 9.51 | 0.327 | 0.285 |
| Lactobacillus | 10.08 | 10.52 | 9.51 | 0.337 | 0.492 |
| Escherichia-Shigella | 11.39a | 7.35c | 10.07b | 0.463 | <0.001 |
| Blautia | 10.88a | 10.21a | 9.21b | 0.232 | 0.007 |
| Anaerotruncus | 10.31 | 10.09 | 10.17 | 0.124 | 0.787 |
| Lachnoclostridium | 9.75b | 9.90b | 10.58a | 0.117 | 0.004 |
| Butyricicoccus | 9.81 | 10.15 | 9.65 | 0.167 | 0.470 |
| Erysipelatoclostridium | 9.82a | 8.73b | 9.83a | 0.205 | 0.034 |
| Eisenbergiella | 8.93b | 9.95a | 9.66a | 0.157 | 0.017 |
| Clostridiales | 8.08c | 8.76b | 10.30a | 0.277 | 0.001 |
| Sellimonas | 9.93a | 9.14b | 9.09b | 0.152 | 0.031 |
| Shuttleworthia | 8.25b | 8.24b | 10.39a | 0.237 | <0.001 |
| Romboutsia | 8.82a | 7.13b | 5.35c | 0.481 | 0.007 |
| Parasutterella | 6.73b | 6.88b | 8.66a | 0.339 | 0.027 |
| Anaerostipes | 8.10 | 7.69 | 6.50 | 0.307 | 0.082 |
| Pseudomonas | 6.82b | 6.99b | 8.35a | 0.275 | 0.037 |
| Streptococcus | 6.19b | 7.69a | 4.23c | 0.574 | 0.040 |
| Enterococcus | 8.62a | 7.17b | 9.19a | 0.324 | 0.024 |
| Flavonifractor | 6.86 | 7.00 | 6.86 | 0.189 | 0.946 |
| Ruminiclostridium | 9.17 | 9.02 | 9.20 | 0.165 | 0.904 |
| Christensenellaceae | 8.32 | 8.78 | 8.57 | 0.227 | 0.730 |
| Others | 11.21 | 11.58 | 11.95 | 0.079 | <0.001 |
Sequences relative abundances were transformed using log2.
a,b,cMeans (n = 8) within rows and with different letters differed significantly (P < 0.05).
Abbreviations: AGP, the Aureomycin supplementation treatment; CON, the control treatment; POC, the combined plant essential oils treatment.
At the genera level, Faecalibacterium, Ruminococcaceae, Alistipes, Ruminococcus, and Lachnospiraceae accounted for the most abundant in all the treatments. Compared with CON, POC, and AGP supplementation significantly increased (P < 0.05) the abundances of Ruminococcaceae, Eisenbergiella, and Clostridiales, whereas these treatments significantly decreased (P < 0.05) Faecalibacterium, Escherichia-Shigella, and Sellimona. In particular, Blautia and Streptococcus relative numbers were found to be significantly increased (P < 0.05). Meanwhile, relative number for Escherichia-Shigella, Erysipelatoclostridium, and Enterococcus was significantly decreased (P < 0.05) after POC supplementation compared with the other 2 treatments.
No significant changes (P > 0.05) were seen among other genera for the 3 treatments.
The most abundant genera were selected for the correlation analysis between bacteria and production performance, carcass performance, and intestinal development parameters. Based on the results shown in Figure 2, the most abundant genus Faecalibacterium was positively correlated with culling rate, villus height, crypt depth, abdominal fat, and thymus index, whereas being negatively correlated with bursa of Fabricius. The second most abundant genus Ruminococcaceae showed an inverse correlation compared with Faecalibacterium. Clostridiales, Erysipelatoclostridium, and Lachnoclostridium were positively correlated with FCR, whereas negatively correlated with intestinal wall thickness. Two probiotics, Streptococcus and Bifidobacterium presented positive correlation with eviscerated yield and negative correlations with culling rate and crypt depth. No significant correlations were found for other parameters.
Figure 2.
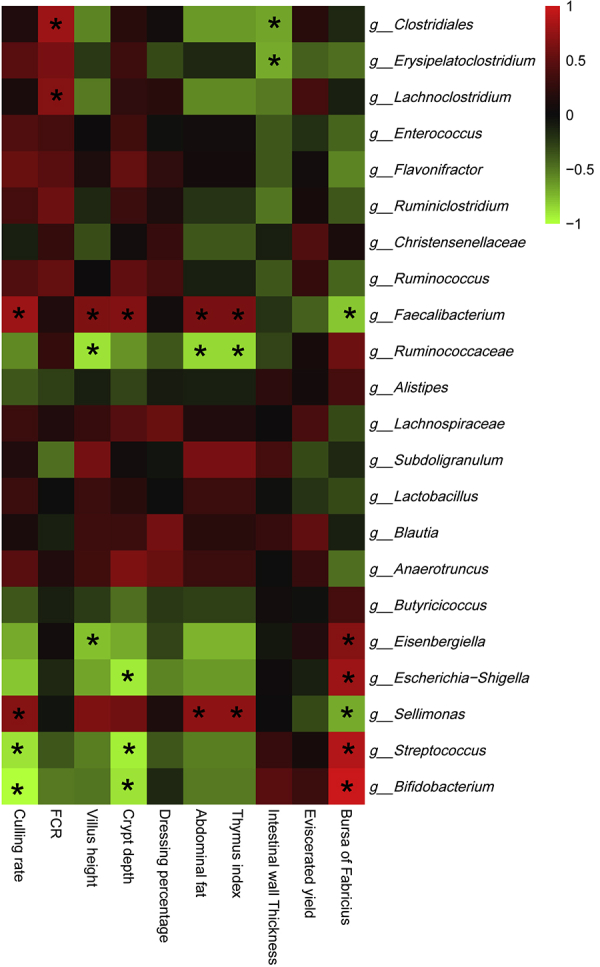
Correlation analyses between relative abundances of cecal bacteria and growth performances, carcass performances, and intestinal development parameters at the level of genera. The red color represents positive correlation, whereas the green color represents a negative correlation. ∗Significant correlation (|r| > 0.55, P < 0.05). Abbreviation: FCR, feed conversion ratio.
Effects of Plant Oils Supplementation on Gastrointestinal Probiotics
Probiotics such as Lactobacillus, Streptococcus, and Bifidobacterium (Guarner and Schaafsma, 1998) were then picked out to examine the effects of POC supplementation on gastrointestinal health. Results are shown in Table 8. All probiotics were seen to increase in relative numbers after POC supplementation, particularly, Streptococcus, and Bifidobacterium increased significantly (P < 0.05).
Table 8.
Effects of combined plant essential oils supplementation on the relative abundances of intestinal probiotics.
| Probiotics | CON | POC | AGP | SEM | P-value |
|---|---|---|---|---|---|
| Lactobacillus | 9.97 | 10.63 | 9.51 | 0.34 | 0.413 |
| Butyricicoccus | 9.63 | 10.33 | 9.64 | 0.17 | 0.148 |
| Streptococcus | 6.19b | 7.69a | 4.23c | 0.53 | 0.028 |
| Bifidobacterium | 0.13c | 1.50a | 0.63b | 0.11 | 0.047 |
Sequences relative abundances were transformed using log2.
a,b,cMeans (n = 8) within a row with different letters differed significantly (P < 0.05).
Abbreviations: AGP, the Aureomycin supplementation treatment; CON, the control treatment; POC, the combined plant essential oils treatment.
Discussion
Effects of Plant Oils Supplementation on Production Performances of Broilers
Antibiotic alternatives such as plant extract, probiotics, and antimicrobial peptides were well investigated over the past few years (Miles et al., 2006). Compared with other alternatives, plant essential oils were broadly applied because of their easy acquisition and broad-spectrum antimicrobial properties (Long et al., 2018). In the present study, the POC supplementation slightly promoted the FCR and reduced mortality and culling rates, these results were in line with those of the study by Amad et al. (2011). In practice, significant enhancement of BWG and FCR was always difficult to achieve through feed additives (Oviedo-Rondón et al., 2019). Reduction of mortality and culling rate because of the antioxidant and anti-inflammatory properties of the bioactive components (Engster et al., 2002) in POC supplementation treatment, and the increased EPI, may also indicate the enhancement of production performances.
Effects of Plant Oils Supplementation on Cecal Microbiology
In the present study, the α-diversity and β-diversity of cecal microbiota in the POC and AGP treatments showed a significant difference compared with those in the CON, which indicated that the POC and AGP supplementation modified the cecal microbiota. The microbiota in the cecum express high metabolic activity in the gastrointestinal tract of chickens (Xu et al., 2016), and the composition and diversity of cecal microbiota can be altered by diet composition and dietary manipulations such as the use of feed additives (Owens et al., 2008). Enrichment or inhibition of certain bacterial members depended on the antibiotic application–shifted gut microbiota community structure (Rad-Spice, 2015). Similarly, supplementation of plant oils inhibited the colonization of pathogens (Hovorkova et al., 2018), and prebiotics provided more substrates for gut microbiota (Ohimain and Ofongo, 2012), which led to a significant increase in total bacterial diversity (Johnson et al., 2015). Moreover, prebiotics are exclusively fermented by beneficial bacteria such as Lactobacillus, Bifidobacteria, and Bacteroides (Ohimain and Ofongo, 2012), which modified the microbial community structure in the gut, and partly demonstrated the increased relative numbers of these bacteria in the present study.
Besides, relative numbers of probiotics were significantly increased, whereas pathogens significantly decreased after POC supplemented. Plant essential oils can promote the proliferation of probiotics as reported by Çabuk et al. (2006) and as demonstrated 2 by probiotics Streptococcus and Bifidobacterium in the present study. Bactericidal properties of phenolic acids–abundant oils such as thymol and eugenol inhibited growth of pathogens Escherichia coli, Clostridium perfringens, and Salmonella Enteritidis (Thapa et al., 2012), which expressed a consistent results with the present study. The content of carvacrol in POC contains similar phenolic acids structure and might play similar antimicrobial property to the decreased abundances of Escherichia-Shigella and Erysipelatoclostridium. The inhibited pathogens in the intestinal environment contributed to the healthy growth of chicken.
Effects of POC Supplementation on Intestinal Morphology and Gastrointestinal Health
The development and structural integrity of intestinal mucosa reflected gastrointestinal health, which in the poultry industry is of great importance to achieve target growth rates and feed efficiency (Xu et al., 2003). A shorter villus and a deeper crypt always lead to the reduction of nutrient absorption and disease resistivity (Xu et al., 2003). In the present study, the V/C reached to the most in both ileum and jejunum in POC treatment, which might increase the nutrient absorption and disease resistivity and then account for the promoted FCR in POC treatment. This result was in line with that of the study by Liu et al. (2019) in which intestinal villus height after carvacrol essential oils supplementation increased significantly. The richness of polyphenols and essential oil content in the POC contributed to the development of gut epithelium. Polyphenols were reported to affect intestinal ecology by accumulation in the gut of undigested and unabsorbed compounds and phenol metabolites that affected the material transportation of intestinal wall, which may further influence the growth of intestine (Bravo, 2010). Besides, in broiler chickens, the addition of essential oils such as eucalyptus oil have been well proven to increase the transepithelial electrical resistance and stimulate the immune system response by enhancing the phagocytic activity of monocytes (Shiffman et al., 2017), which expressed splendid anti-inflammatory property of the intestine and prevented being attacked by pathogens. In addition, the active ingredient cineole in the eucalyptus controls the secretions of mucus in the epithelial layer of the respiratory system air passages (Juergens, 2014), which may also benefit the growth of intestine wall. Furthermore, carvacrol essential oils exerted positive effects on intestinal barriers function of broilers (Liu et al., 2018), and thus enhanced gastrointestinal development. These reasons contributed to the development of intestinal mucosa and enhancement of intestinal health.
In summary, although no enhancement of production performances was detected, supplementation of the combination of essential oils strengthened the intestinal wall and improved the intestinal health through improved relative abundances of gut microbiota diversity and probiotics. These results indicated the combination of essential oils could benefit the gastrointestinal health and work as antibiotic alternative.
Acknowledgments
Financial support of this study was provided by the Chinese Agricultural Research System (No. CARS-40), the National Key Research and Development Program of China (No. 2017YFD0502004 and 2016YFD0500502), and the Agricultural Science and Technology Innovation Program (No. ASTIPIAS04).
Conflict of Interest Statement: All authors declare that they do not have a conflict of interest.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.05.030.
Supplementary data
References
- Amad A.A., Manner K., Wendler K.R., Neumann K., Zentek K. Effects of a phytogenic feed additive on growth performance and ileal nutrient digestibility in broiler chickens. Poult. Sci. 2011;90:2811–2816. doi: 10.3382/ps.2011-01515. [DOI] [PubMed] [Google Scholar]
- Amerah A.M., Mathis G., Hofacre C.L. Effect of xylanase and a blend of essential oils on performance and Salmonella colonization of broiler chickens challenged with Salmonella Heidelberg. Poult. Sci. 2012;91:943–947. doi: 10.3382/ps.2011-01922. [DOI] [PubMed] [Google Scholar]
- Apajalahti J., Vienola K. Interaction between chicken intestinal microbiota and protein digestion. Anim. Feed Sci. Technol. 2016;221:323–330. [Google Scholar]
- Aristóteles G.N., Clarice L.L., Guerrero R.T. DNA extraction from frozen field-collected and dehydrated herbarium fungal basidiomata: performance of SDS and CTAB-based methods. Biotemas. 2005;18:18–32. [Google Scholar]
- Botsoglou N.A., Florou-Paneri P., Christaki E., Fletouris D.J., Spais A.B. Effect of dietary oregano essential oil on performance of chickens and on iron-induced lipid oxidation of breast, thigh and abdominal fat tissues. Br. Poult. Sci. 2002;43:223–230. doi: 10.1080/00071660120121436. [DOI] [PubMed] [Google Scholar]
- Bravo L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 2010;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Brenes A., Viveros A., Goni I., Centeno C., Sáyago-Ayerdy S.G., Arija I., Saura-Calixto F. Effect of grape pomace concentrate and vitamin E on digestibility of polyphenols and antioxidant activity in chickens. Poult. Sci. 2008;87:307. doi: 10.3382/ps.2007-00297. [DOI] [PubMed] [Google Scholar]
- Çabuk M., Bozkurt M., Alçiçek A., Akbaþ Y., Küçükyýlmaz K. Effect of a herbal essential oil mixture on growth and internal organ weight of broilers from young and old breeder flocks. South Afr. J. Anim. Sci. 2006;36:135–141. [Google Scholar]
- Chapman H.D., Johnson Z.B. Use of antibiotics and roxarsone in broiler chickens in the USA: analysis for the years 1995 to 2000. Poult. Sci. 2002;81:356–364. doi: 10.1093/ps/81.3.356. [DOI] [PubMed] [Google Scholar]
- Claudie B., Fatoumata D., Roland B., Luke M., Edward T., Diarra M.S. Pathotype and antibiotic resistance gene distributions of Escherichia coli isolates from broiler chickens raised on antimicrobial-supplemented diets. Appl. Environ. Microbiol. 2009;75:6955–6962. doi: 10.1128/AEM.00375-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Wang Q., Liu S., Sun R., Zhou Y., Li Y. Age-related variations in intestinal microflora of Free-Range and Caged Hens. Front Microbiol. 2017;8:1310. doi: 10.3389/fmicb.2017.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H., He J., Xiao K., Li C. Temperature: ensitive polyurethane (TSPU the experimental diets for broilers) film incorporated with carvacrol and cinnamyl aldehyde: antimicrobial activity, sustained release kinetics and potential use as food packaging for Cantonese-style moon cake. Int. J. Food Sci. Technol. 2019;55 [Google Scholar]
- Engster H.M., Marvil D., Stewart-Brown B. The effect of withdrawing growth promoting antibiotics from broiler chickens: a long-term commercial industry study. J. Appl. Poult. Res. 2002;11:431–436. [Google Scholar]
- Fernandez F., Hinton M., Gils B., Van Dietary mannan-oligosaccharides and their effect on chicken caecal microflora in relation to Salmonella Enteritidis colonization. Avian Pathol. 2002;31:49–58. doi: 10.1080/03079450120106000. [DOI] [PubMed] [Google Scholar]
- Guarner G.J., Schaafsma Probiotics. Int. J. Food Microbiol. 1998;39:237–245. doi: 10.1016/s0168-1605(97)00136-0. [DOI] [PubMed] [Google Scholar]
- Gungor B., Adiguzel E., Gursel I., Yilmaz B., Gursel M. Intestinal microbiota in patients with spinal cord injury. PLoS One. 2016;11:e0145878. doi: 10.1371/journal.pone.0145878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Xue S., Nasir M., Lv J., Gu J. Role of Bentonite on the mobility of antibiotic resistance genes, and microbial community in Oxytetracycline and Cadmium contaminated soil. Front. Microbiol. 2018;9:1–11. doi: 10.3389/fmicb.2018.02722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovorkova Petra, Lalouckova Klara, Skrivanova Eva. Determination of in vitro antibacterial activity of plant oils containing medium-chain fatty acids against gram-positive pathogenic and gut commensal bacteria. Czech J. Anim. Sci. 2018;63:119–125. [Google Scholar]
- Johnson L.P., Walton G.E., Psichas A., Frost G.S., Gibson G.S., Barraclough T.G. Prebiotics modulate the effects of antibiotics on gut microbial diversity and functioning in vitro. Nutrients. 2015;7:4480–4497. doi: 10.3390/nu7064480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juergens U.R. Anti-inflammatory properties of the monoterpene 1.8-cineole: current evidence for co-medication in inflammatory airway diseases. Drug Res. 2014;64:638–646. doi: 10.1055/s-0034-1372609. [DOI] [PubMed] [Google Scholar]
- Kähkönen M.P., Hopia A.I., Vuorela H.J., Rauha J.P., Pihlaja K., Kujala T.S., Heinonen M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999;47:3954–3962. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- Liu S., Cho J., Yun W., Lee J., Lee C., Kwak W., Oh H., Lee D. PSII-3 Effects of carvacrol essential oils on intestine barrier function in broilers. J. Anim. Sci. 2018;96:75–76. doi: 10.1111/jpn.12944. [DOI] [PubMed] [Google Scholar]
- Liu S.D., Song M.H., Yun W., Lee J.H., Lee C.H., Kwak W.G., Oh H.J., Kim H.B., Cho J.H. Effects of oral administration of various essential oils on blood metabolites, intestine development, microbial enumeration and meat quality in broilers. Indian J. Anim. Res. 2019;53:762–767. [Google Scholar]
- Long S., Xu Y., Wang C., Li C., Liu D., Piao X.S. Effects of dietary supplementation with a combination of plant oils on performance, meat quality and fatty acid deposition of broilers. Asian-Australas. J. Anim. Sci. 2018;31:11. doi: 10.5713/ajas.18.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna A., Labaque M.C., Zygadlo J.A., Marin R.H. Effects of thymol and carvacrol feed supplementation on lipid oxidation in broiler meat. Poult. Sci. 2010;89:366–370. doi: 10.3382/ps.2009-00130. [DOI] [PubMed] [Google Scholar]
- Maiorano G., Stadnicka K., Tavaniello S., Abiuso C., Bogucka J., Bednarczyk M. In ovo validation model to assess the efficacy of commercial prebiotics on broiler performance and oxidative stability of meat. Poult. Sci. 2017;96:511–518. doi: 10.3382/ps/pew311. [DOI] [PubMed] [Google Scholar]
- Miles R.D., Butcher G.D., Henry P.R., Littell R.C. Effect of antibiotic growth promoters on broiler performance, intestinal growth parameters, and quantitative morphology. Poult. Sci. 2006;85:476–485. doi: 10.1093/ps/85.3.476. [DOI] [PubMed] [Google Scholar]
- Nameghi A.H., Edalatian O., Bakhshalinejad R. Effects of a blend of thyme, peppermint and eucalyptus essential oils on growth performance, serum lipid and hepatic enzyme indices, immune response and ileal morphology and microflora in broilers. J. Anim. Physiol. Anim. Nutr. 2019;103 doi: 10.1111/jpn.13122. [DOI] [PubMed] [Google Scholar]
- Ohimain E.I., Ofongo R.T.S. The effect of probiotic and prebiotic feed supplementation on chicken health and gut microflora: a review. Int. J. Anim. Vet. Adv. 2012;4:135–143. [Google Scholar]
- Oliveira M., Santos V., Fernandes A., Bernardo F., Vilela C.L. Antimicrobial resistance and in vitro biofilm-forming ability of enterococci from intensive and extensive farming broilers. Poult. Sci. 2010;89:1065–1069. doi: 10.3382/ps.2008-00436. [DOI] [PubMed] [Google Scholar]
- Oviedo-Rondón E.O., Hume M.E., Barbosa N.A., Sakomura N.K., Weber G., Wilson J.W. Ileal and caecal microbial Populations in broilers Given specific essential oil Blends and probiotics in two Consecutive grow-Outs. Avian Biol. Res. 2019;3:157–169. [Google Scholar]
- Owens B., Tucker L., Collins M.A., Mccracken K.J. Effects of different feed additives alone or in combination on broiler performance, gut microflora and ileal histology. Br. Poult. Sci. 2008;49:202–212. doi: 10.1080/00071660802004890. [DOI] [PubMed] [Google Scholar]
- Pan D., Yu Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes. 2014;5:108–119. doi: 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rad-Spice M. PhD Diss; 2015. Antibiotics and Gut Microbiome. Accessed July 2020. https://www.researchgate.net/publication/279203636_Antibiotics_and_Gut_Microbiome. [Google Scholar]
- Shiffman M.E., Soo R.M., Dennis P.G., Morrison M., Tyson G.W., Hugenholtz P. Gene and genome-centric analyses of koala and wombat fecal microbiomes point to metabolic specialization forEucalyptusdigestion. Peerj. 2017;5:e4075. doi: 10.7717/peerj.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim Y., Shinde H., Choi P.L., KIM J.Y., Seo J.S., Kwon I.K. Evaluation of multi-microbial probiotics produced by submerged liquid and solid substrate fermentation methods in broilers. Asian Australas. J. Anim. Sci. 2010;23:521–529. [Google Scholar]
- Sivarajan M., Lalithapriya U., Mariajenita P., Vajiha B.A., Harini K., Madhushalini D., Sukumar M. Synergistic effect of spice extracts and modified atmospheric packaging towards non-thermal preservation of chicken meat under refrigerated storage. Poult. Sci. 2017;96:2839–2844. doi: 10.3382/ps/pex057. [DOI] [PubMed] [Google Scholar]
- Thapa D., Losa R., Zweifel B., Wallace R.J. Sensitivity of pathogenic and commensal bacteria from the human colon to essential oils. Microbiology. 2012;158:2870–2877. doi: 10.1099/mic.0.061127-0. [DOI] [PubMed] [Google Scholar]
- Wang S., Zeng X.F., Wang Q.W., Zhu J.L., Peng Q., Hou C.L., Thacker P., Qiao S.Y. The antimicrobial peptide sublancin ameliorates necrotic enteritis induced by in broilers. J. Anim. Sci. 2015;93:4750–4760. doi: 10.2527/jas.2015-9284. [DOI] [PubMed] [Google Scholar]
- Wasch K., De Okerman L., Croubels S., Brabander H., De Hoof Van J., Backer P. De. Detection of residues of tetracycline antibiotics in pork and chicken meat: correlation between results of screening and confirmatory tests. Analyst. 1998;123:2737–2741. doi: 10.1039/a804909b. [DOI] [PubMed] [Google Scholar]
- Xu Y., Yang H., Zhang L., Su Y., Shi D., Xiao H., Tian Y. High-throughput sequencing technology to reveal the composition and function of cecal microbiota in Dagu chicken. BMC Microbiol. 2016;16:259. doi: 10.1186/s12866-016-0877-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Hu C., Xia M., Zhan X.A., Wang M. Effects of dietary Fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of growing Pigs. Poult. Sci. 2003;82:1030–1036. doi: 10.1093/ps/82.6.1030. [DOI] [PubMed] [Google Scholar]
- Xue F., Sun F., Jiang L., Hua D., Wang Y., Nan X., Zhao Y., Xiong B. Effects of partial replacment of dietary forage using Kelp powder (Thallus Laminariae) on ruminal fermentation and lactation performances of dairy Cows. Animals (Basel) 2019;9:852. doi: 10.3390/ani9100852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.