Abstract
It has been demonstrated that vitamin D (Vit D) included in diets offers a beneficial effect by improving innate immune responses in chickens. However, its mechanisms of action and the effect on immunosuppressive pathogens, such as infectious bursal disease virus, are not yet known. In the present study, we have studied the immunomodulatory effect of Vit D on the innate immune response in 3 cell lines: fibroblast cells (DF-1), macrophages (HD11), and B cells (DT-40) infected with IBDV (intermediate vaccine) at 2 multiplicity of infections (MOI) (1 and 0.1). Genes associated with innate immune responses (TLR-3, TLR-21, MDA-5, MyD88, TRIF, IRF-7, INF-α, INF-β, PKR, OAS, viperin, IL-1β, IL-6, and IL-12) were evaluated at different time points (3, 6, 12, 24, and 36 h after infection, h.p.i). Virus production reached a maximum at 24 h.p.i., which was significantly (P < 0.05) higher in DF-1 cells, followed by HD-11 and DT-40 cells. Mainly in HD-11 cells, there was a significant (P < 0.05) effect of Vit D supplementation on receptors TLR-3, TLR-21, and MDA-5 after 12 h.p.i, independent of MOI. DT-40 cells showed the highest antiviral activity, with a significant (P < 0.05) effect on IRF-7, IFN-β, OAS, and PKR gene expression, where expression of IRF-7 and IFN-β correlated positively with Vit D supplementation, while OAS and PKR were independent of Vit D. Proinflammatory cytokines were significantly (P < 0.05) upregulated and found to be Vit D and MOI dependent. In conclusion, this study demonstrated the capacity of IBDV to trigger a strong innate immune response in chicken cells and contributes to the understanding of the activation pathways of innate immunity induced by IBDV and further shows the benefitial effect of Vit D supplementation as an immunomodulator.
Key words: vitamin D, innate immunity, IBDV, macrophage, B cell
Introduction
Vitamin D (Vit D) can modulate immune responses by selective suppression of effector functions such as proinflammatory cytokine production and leukocyte infiltration into inflammatory sites (Helming et al., 2005; Bahar-Shany et al., 2010; Jadhav et al., 2018). On the contrary, its deficiency has been associated with an increase in CD4+/CD8+ cell ratio (Zofková and Kancheva, 1997), reducing the ability of the immune system to activate T lymphocytes such as CD8+ and therefore the cytotoxic function to eliminate cells infected with viruses (Pender, 2012). In general, the mechanisms of antiviral activity of Vit D have been divided into 5 parts: induction of antimicrobial peptides, immunoregulatory function, interaction with key cellular and viral factors, induction of autophagy and apoptosis, and epigenetics elements and genetic polymorphisms (Teymoori-Rad et al., 2019). Viral infections may also upregulate CYP27B1 gene, which encodes 25-hydroxyvitamin D-1α-hydroxylase, the enzyme responsible for the final and rate-limiting step in the synthesis of the active form of Vit D, and this synergy may be involved in the induction of antimicrobial peptides (Hansdottir et al., 2008).
In chickens, the immunomodulatory effect of Vit D has been studied from different approaches. We previously showed that in broiler chickens fed an optimal diet in calcium and deficient in phosphorus, supplementation of Vit D considerably augmented transcription of TLR2b, TLR4, CATH1, and CATHB1 with a predominantly Th2 cytokines profile observed in the spleen (Rodriguez-Lecompte et al., 2016). In chicken macrophages treated with 1,25 (OH) 2D3, increased ability of macrophages to respond to stimuli and production of nitric oxide were observed in the presence of TLR ligands (Shojadoost et al., 2015). Vit D alone did not activate macrophages and mainly resulted in the downregulation of CD86, MHC-II, CXCL8, and IL-1β (Shojadoost et al., 2015).
Infectious bursal disease is a highly contagious viral infection caused by infectious bursal disease virus (IBDV) and causes severe economic losses in the global poultry industry (Jackwood, 2017). Chickens are most susceptible to IBDV infection at 3 to 6 wk of age, where the virus carries out a lytic replicative cycle on B lymphocytes in the bursa of Fabricius (BF) and in other secondary lymphoid and nonlymphoid tissues (Abdul et al., 2013). The virus can also infect and replicate in macrophages in the BF (Khatri et al., 2005). This leads to death, disease, or immunosuppression leading to secondary infections with opportunistic pathogens and vaccination failures (Rautenschlein and Alkie, 2016). Viruses are generally detected through pathogen-associated molecular patterns of the innate immune system followed by the induction of antiviral responses. At the endosomal level, several TLRs recognize viral pathogen-associated molecular patterns, which includes TLR3 that detects double-stranded RNA (dsRNA). Activation of TLR-3 by IBDV results in the expression of antiviral interferon (INF)-β and the chemockine IL-8, and this is correlated well with the virulence of virus (He et al., 2017). At the cytoplasmic level, the recognition of IBDV RNA occurs mainly through the chicken melanoma differentiation-associated protein 5 (MDA-5), and its expression activates the INF-β and Mx promoters via IRF-7–dependent pathways resulting in inhibition of IBDV replication in cells (Ouyang et al., 2018). Few studies have addressed the effect of Vit D on IBDV infection, particularly determining the immunomodulatory capacity on the innate immune response at the cellular level; therefore, the aim of the present study was to evaluate innate immune response profile of cells susceptible to IBDV and potential immunomodulatory effect of Vit D supplementation after IBDV infection.
Materials and methods
Cells Cultures
For the development of the experiments, 3 cell lines, DF-1 (chicken fibroblast cell line), HD-11 (chicken macrophages cell line), and DT-40 (chicken lymphoid cell line), were used. All cell lines were cultured in Dulbecco's Modified Eagle's Medium (DMEM; ATCC 30-2002) supplemented with 1% penicillin/streptomycin and maintained at 37°C and 5% CO2. Particularly, the DT-40 cells were cultured on suspension and supplemented with 10% tryptophan phosphate broth, 10% bovine serum, 5% chicken serum, and 50 μM 2-mercaptoethanol. DF-1 cells were supplemented with 10% bovine serum, and HD-11 cells were supplemented with 8% bovine serum and 2% chicken serum.
Virus and Titration
A modified live vaccine UNIVAX-BD (mild strain [ST-12] of Bursal Disease Vaccine-Live virus) from Merck Animal Health was reconstituted in 10 mL of phosphate-buffered saline, and aliquots of 500 μL were made and stored at −70°C. To replicate the virus, an aliquot of the vaccine was inoculated onto DF-1 cells for 1 h in a T-25 cell culture flask at 37°C with 5% CO2, and then 5 mL of medium was added and the cells were incubated under the same conditions for 72 h. Subsequently, the supernatant was recovered and centrifuged at 1,000 × g for 10 min, and aliquots of 500 μL were made and stored at −70°C. The viral titration was performed using the median tissue culture infectious dose (TCID50) on DF-1 cells. For this, 2 × 104 cells per well were seeded in a 96-well plate (100 μL), and 10 viral logarithmic dilutions were made. This was incubated at 37°C with 5% CO2 for 72 h. The Sperber-Karber method was used to determine the virus titre. The titre obtained was of 2 × 108.25/mL.
Cloning and Amplification of Plasmids
To obtain the standard curves for real-time PCR, specific sequences of the genes to be evaluated were cloned into plasmids. Some of these (MDA-5, IL-1β, IRF-7, INF-α, PKR, OAS, and viperin) were kindly donated by Dr. Shayan Sharif from Ontario Veterinary College, University of Guelph, Ontario, Canada. The remaining (β-actin, VP2 IBDV, Vit D receptor [VDR], TLR-3, TLR-21, MyD88, TRIF, INF-β, IL-6, IL-12) were developed in our laboratory. For the latter, specific primers (Table 1) were used, and conventional PCR was performed to amplify the sequence of each gene. The concentrations used of each reagent for the PCRs were 1x Promega Master mix (12.5 μL), 1 μmol of each primer (1 μL), and 2 μL of DNA. The PCR conditions were 94°C for 2 min followed by 35 cycles at 94°C for 30 s, 55°C for 45 s, 72°C for 45 s, and a final extension at 72°C for 5 min. Positive controls were cells of each type infected with IBDV with a dose that corresponded to that used for each assay (MOI 0.1 and 1). The amplicons sizes were determined by electrophoresis on a 1% agarose gel. For cloning, the TOPO cloning TA Invitrogen kit (ThermoFisher Scientific, Burlington, ON, Canada) was used following the manufacturer's instructions.
Table 1.
Sequences of primers used for quantitative reverse transcription-polymerase chain reaction for detection of chicken and IBDV genes of interest.
| Gene name | Primer sequence | Annealing temperature (°C) | Amplicon size (bp) | Accesion number or reference |
|---|---|---|---|---|
| β-actin | F: 5′-CAACACAGTGCTGTCTGGTGGTA-3′ R: 5′-ATCGTACTCCTGCTTGCTGATCC-3′ |
61 | 205 | X00,182 |
| IBDV-VP2 | F: 5′-CTGACTACCGGCATCGACA-3′ R: 5′-CCACTTGCCGACCATGA-3′ |
60 | 149 | AF498631 |
| VDR | F: 5′-AGAAGCAAATTCAGCAGCAGGA-3′ R: 5′-AAGGCATCGGAGCCAAAGAC-3′ |
60 | 101 | NM205098.1 |
| TLR-3 | F: 5′-TGCATAAGAAGGAGCAGGAAG-3′ R: 5′-CTGGCCAGTTCAAGATGCAG-3′ |
60 | 263 | NM001011691 |
| TLR-21 | F: 5′-TCAGCTACACCAAAATGTTCAACC-3′ R: 5′-CGTGATTTTGCCTGTGAGC-3′ |
60 | 249 | NM204278 |
| MDA5 | F: 5′-GCAAAACCAGCACTGAATGGG-3′ R: 5′-CGTAAATGCTGTTCCACTAACGG-3′ |
59 | 178 | (Alkie et al., 2015) |
| TRIF | F: 5′-GCTGACCAAGAACTTCCTGTGC-3′ R: 5′-AGAGTTCTCATCCAAGGCCACC-3′ |
60 | 120 | NM001081506.1 |
| MyD88 | F: 5′-AGAAGGTGTCGGAGGATGGTG-3′ R: 5′-GGGCTCCAAATGCTGACTGC-3′ |
57 | 365 | NM001030962 |
| IRF7 | F: 5′-CTCCCCTCCTCCAAAAGCTG-3′ R: 5′-CTGGGAGCGAAGGAGGAATG-3′ |
60 | 127 | (Alkie et al., 2015) |
| INF-α | F: 5′-ATCCTGCTGCTCACGCTCCTTCT-3′ R: 5′-GGTGTTGCTGGTGTCCAGGATG-3′ |
60 | 197 | (Alkie et al., 2015) |
| INF-β | F: 5′-GCCTCCAGCTCCTTCAGAATACG-3′ R: 5′-CTGGATCTGGTTGAGGAGGCTGT-3′ |
60 | 223 | (Alkie et al., 2015) |
| OAS | F: 5′-AGAACTGCAGAAGAACTTTGTC-3′ R: 5′-GCTTCAACATCTCCTTGTACC-3′ |
58 | 91 | (Alkie et al., 2015) |
| PKR | F: 5′-GGAGGCGGGAATGGAGAAAA-3′ R: 5′-GAGCACATCCGCAGGTAGAG-3′ |
60 | 144 | (Alkie et al., 2015) |
| Viperin | F: 5′-GGAGGCGGGAATGGAGAAAA-3′ R: 5′-CAGCTGGCCTACAAATTCGC-3′ |
58 | 78 | (Alkie et al., 2015) |
| IL-1β | F: 5′-AACCCGACCAGGTCAACA-3′ R: 5′-CGGTACATACGAGATGGAAAC-3′ |
61 | 101 | AJ245728.1 |
| IL-12 | F: 5′-TCTGCTAAGACCCACGAGA-3′ R: 5′-TTGACCGTATCATTTGCCCAT-3′ |
60 | 82 | NM213571 |
| IL-6 | F: 5′-CAGGACGAGATGTGCAAGAA-3′ R: 5′-TAGCACAGAGACTCGACGTT-3′ |
59 | 232 | AJ309540 |
Abbreviations: IBDV, infectious bursal disease virus; IL, interleukin; INF, interferon; MDA-5, melanoma differentiation-associated protein 5.
Standarization of Curves for Real-time PCR
A standard curve was established for each of the 17 genes evaluated by making dilutions in log 10 base to each of the plasmids. For the real-time PCR, the SsoAdvanced Universal SYBR Green Supermix mix (BioRad, Mississauga, ON, Canada) was used and made in the CFX96 thermocycler. The standardization of the curves was carried out independently for each gene using 1x Universal SYBR Green Supermix (5 μL), 1 μmol of each primer (0.2 μL), and 1 μL of DNA in a total volume of 10 μL.
Cell Infection and Vit D Treatment
Two viral infection levels at a multiplicity of infection (MOI) of 1 and 0.1 and 2 Vit D (1,25(OH)2D3) levels (0 nmol and 100 nmol of Vit D) (Sigma-Aldrich, Oakville, ON, Canada) were used on each cell type. Initially, each of the cell lines (DF-1, HD-11, and DT-40) were seeded separately in 3 plates of 24 wells at a density of 5 × 105 cells/well and maintained in DMEM medium (ATCC 30-2002) supplemented with 10% bovine serum and 1% penicillin/streptomycin and incubated at 37°C at 5% CO2 for 16 h before infection. In the case of DT40 cells, the medium was additionally supplemented with mercaptoethanol and tryptophan phosphate broth. In addition, the cells treated with Vit D (1,25(OH)2D3) received this at a concentration of 100 nmol per well supplemented in the culture medium. After 16 h, the culture medium was removed, and 200 μl of inoculum containing MOI 1 or 0.1 was added per well leaving 1 h for adsorption. After this, the inoculum was removed and DMEM supplementation medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin or, in the case of cells treated with Vit D, the same supplementation with 100 nmol of Vit D was used. The cells were incubated at 37°C at 5% CO2.
From a 24-well plate, each well was recovered separately at the respective time (3, 6, 12, 24, and 36 h) after infection. Uninfected cells were maintained as negative control (cell control) and cells without Vit D (Vit D control). These controls recovered at 36 h along with the last infection time. At the end of the experiment, there were 24 samples per plate (12 treated with Vit D and 12 without Vit D) taken in 5 times (3, 6, 12, 24, and 36 h), and 3 plates were mounted for each cell line. From each 24-well plate, half of the samples were taken for processing, one representing each repetition, that is, 12 per plate. As 3 plates were used for each cell line, 36 samples were obtained from each cell type, and as 2 MOIs (0.1 and 1) were used, there were 72 samples for each cell line.
Subsequently, the cells and supernatant from each well were recovered in Eppendorf tubes and centrifuged at 10,000 × g/10 min. Finally, the cell pellet was recovered for RNA extraction.
Real-time PCR Evaluation
RNA extractions were performed using Trizol-LS (Invitrogen, Burlington, ON, Canada) following the manufacturer's recommendations. Once the RNA was recovered, it was quantified by using a NanoDrop Spectrophotometer (ThermoFisher Scientific, Burlington, ON, Canada) and were stored at −70°C for later use. Subsequently, cDNA synthesis of all samples was carried out using High Capacity cDNA Reverse Transcription Kit (ThermoFisher Scientific, Burlington, ON, Canada). The protocol used was 1x buffer, 4 mmol deoxyribonucleotide triphosphates, 1x random primers, 1x RNase inhibitor, and 2 U multiscribe reverse transcriptase. (The volumes of the reagents are those recommended by the manufacturer.) The RT conditions were 1 cycle 25°C for 10 min, one cycle at 37°C for 120 min, and 1 cycle 85°C for 5 min. The cDNA was stored at −20°C for later use. All samples were processed for real-time PCR amplification of β-actin, VP2 IBDV, VDR, TLR3, TLR21, MDA5, MyD88, TRIF, IRF-7, INF-α, INF-β, OAS, PKR, viperin, IL1β, IL12, and IL6. For the amplification of these genes, the SsoAdvanced Universal SYBR Green Supermix PCR mix (BioRad, Mississauga, ON, Canada) was used following the manufacturer's recommendations.
Statistical Analysis
The Proc Mixed Procedure of SAS (SAS institute, Cary, NC) was used to analyze CT values for all genes based on one level of Vit D, 2 levels of MOI, and 6 time points (0, 3, 6, 12, 24, and 36 h). Levels of expression for all genes were calculated relative to β-actin, and gene expression was presented as fold changes relative to the control gene. Gene expression fold change, standard error, and statistical significance were calculated using REST 2009 (Qiagen, Valencia, CA) (Pfaffl, 2001) where all data were considered significantly different at P < 0.05.
Results
Effect of Vit D Supplementation on Viral Replication
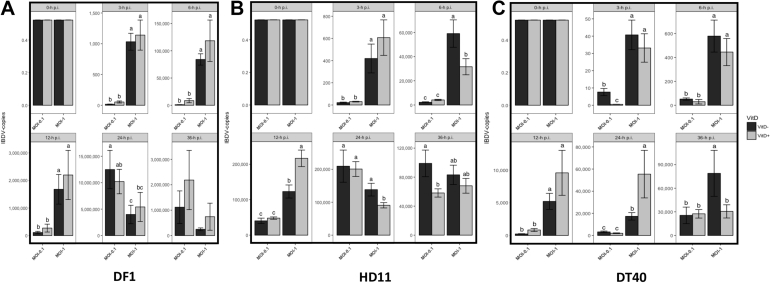
Viral load, determined as the amount of viral RNA, was higher post 0 h.p.i. for DF-1 cells followed by HD-11 and DT-40. The presence of viral RNA in the 3 cell types at MOI 1 was detected after 3 h.p.i. and gradually increased at 6 h.p.i. until reaching a peak at 12 h.p.i. (Figures 1A–1C). After 12 h.p.i., viral load increased in MOI of 0.1, particularly in HD-11 and DF-1 cells, with the latter showing the highest levels (>106 copies) (Figures 1A, 1B). It is interesting to note that in HD-11 cells at MOI of 1 and at 24 h.p.i and MOI 0.1 at 36 h.p.i, viral load was higher in cells that were not supplemented with Vit D (P < 0.05) (Figure 1B). The maximum viral load for both HD-11 and DT-40 cells was 2 × 105 copies (Figures 1B and 1C).
Figure 1.
Dynamics of IBDV infection in DF-1 (A), HD11 (B), and DT40 (C) cell lines and the effect of vitamin D supplementation on the replicative capacity of the virus infection at 0, 3, 6, 12, 24, and 36 h.p.i.
Innate Response in Cells Infected With IBDV and Supplemented With Vitamn D
DF1 cells
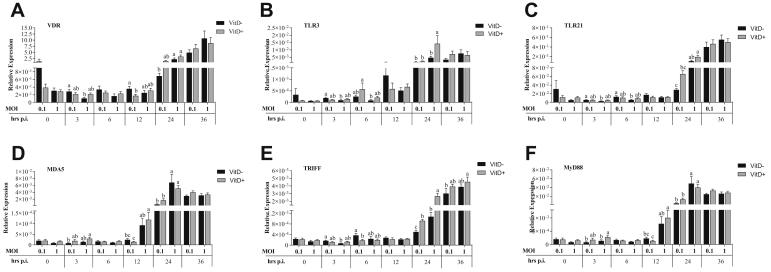
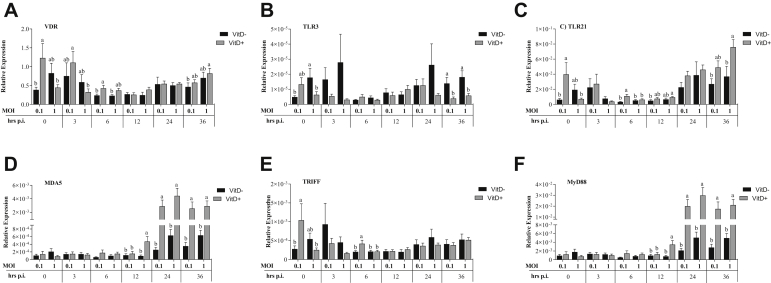
At 3 h.p.i., MOI 1 without Vit D resulted in a significantly lower expression of VDR, TLR3, TLR21, and TRIFF than MOI of 0.1 (P < 0.031). Significantly higher expressions of MDA5 and MyD88 were observed at MOI of 1 with Vit D than at MOI of 0.1 without Vit D (Figure 2). Expression of TLR3 was significantly higher in MOI of 0.1 with Vit D than in MOI of 1 without Vit D (P = 0.023) while expression of TLR21 was significantly higher in MOI of 0.1 without Vit D than in MOI of 1 without Vit D (P = 0.012). At MOI of 0.1, expression of TRIFF was significantly higher without Vit D than with Vit D (P = 0.035). Expression of VDR was significantly higher in MOI of 0.1 without Vit D than with Vit D (P = 0.039). Expressions of MDA5 and MyD88 followed the same pattern where their expression was significantly higher in MOI of 1 with and without Vit D than in MOI of 0.1 with Vit D (P < 0.029), while only MOI of 1 with Vit D was significantly higher than MOI of 0.1 without Vit D (P = 0.009). At 24 h.p.i., expression of VDR was significantly higher at MOI of 1 with and without Vit D than at MOI of 0.1 without Vit D (P < 0.020), and TLR3 was significantly higher in MOI of 1 with Vit D than all treatments (P < 0.022) (Figure 2). Expression of TLR21 was significantly higher at MOI of 1 with Vit D than at MOI of 0.1 with and without Vit D (P < 0.05) and at MOI of 1 than at MOI of 0.1 without Vit D (P < 0.05). Expressions of MDA5 and MyD88 were significantly higher in MOI of 1 than in MOI of 0.1 with and without Vit D (P < 0.045). Expression of TRIFF was significantly higher in MOI of 1 with and without Vit D than in MOI of 0.1 without Vit D (P < 0.001) and at MOI of 0.1 with Vit D than without Vit D (P = 0.034). At 36 h.p.i., a significantly higher TRIFF expression was observed in MOI of 1 with Vit D than in MOI of 0.1 without Vit D (P = 0.044) (Figure 2).
Figure 2.
Relative expression of vitamin D receptor (VDR) (A), TLR-3 (B), TLR-21 (C) and MDA5 (D) of receptors, TRIFF (E) and MyD88 (F) of signal transduction adaptors in DF-1 cells supplemented with vitamin D and infected with IBDV a MOI 0.1 and 1 at times 0, 3, 6, 12, 24, and 36 h.p.i.
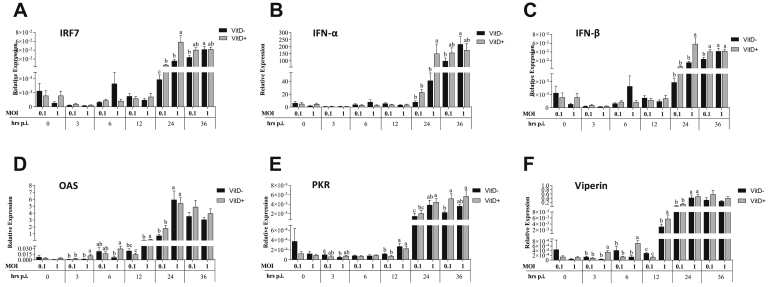
The expressions of OAS and viperin were significantly higher at 3 h.p.i. in MOI of 1 with Vit D than in other treatments (P < 0.005), while PKR expression was significantly higher in MOI of 0.1 than in MOI of 1 without Vit D (P = 0.019). Expression of OAS was significantly higher in MOI of 1 with Vit D than without Vit D (P = 0.016), and viperin expression was significantly higher in MOI of 1 with Vit D than in all treatments (P < 0.048). At 12 h.p.i., a significantly higher expression of OAS was observed in MOI of 1 with or without Vit D than in MOI of 0.1 with Vit D (P < 0.004) and in MOI of 1 with Vit D than in MOI of 0.1 without Vit D (P < 0.001). Expressions of PKR and viperin were significantly higher in MOI of 1 than in MOI of 0.1 with or without Vit D (P < 0.002) and in MOI of 1 with Vit D than without Vit D (P = 0.003) (Figure 3). At 24 h.p.i., significantly higher expressions of IRF-7, IFN-α, and IFN-β were observed in MOI of 1 with Vit D than in all treatments (P < 0.045), while a significantly higher IRF-7 expression was observed in MOI of 1 without Vit D and MOI of 0.1 with Vit D than in MOI of 0.1 without Vit D (P < 0.028). A significantly higher expression of OAS and viperin was observed in MOI of 1 than in MOI of 0.1 with or without Vit D (P < 0.011) (Figure 3). Expression of PKR was significantly higher in MOI of 1 with Vit D than in MOI of 1 with or without Vit D (P < 0.033) and in MOI of 1 than in MOI of 0.1 without Vit D (P = 0.009). At 36 h.p.i., there was a significantly higher expression of IRF-7 and IFN-α in MOI of 1 than in MOI of 0.1 without Vit D (P < 0.048). A significantly higher IFN-β expression was observed in MOI of 1 with and without Vit D and MOI of 0.1 with Vit D than in MOI of 0.1 without Vit D (P < 0.028). A significantly higher PKR expression was observed in MOI of 0.1 and 1 with Vit D than in MOI of 0.1 without Vit D (P < 0.016) (Figure 3).
Figure 3.
Relative expression of interferons regulator factor IRF-7 (A), and interferons type I INF-α (B), INF-β (C), and related interferon antiviral genes OAS (D), PKR (E), and viperin (F) genes in DF-1 cells supplemented with vitamin D and infected with IBDV a MOI 0.1 and 1 at times 0, 3, 6, 12, 24, and 36 h.p.i.
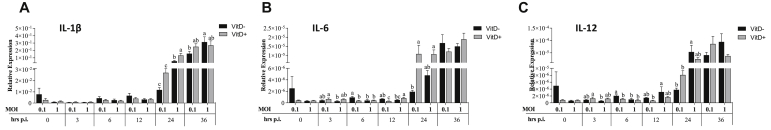
At 3 h.p.i., significantly higher expression of IL-12 and IL-6 was observed in MOI of 0.1 with Vit D than in MOI of 1 without Vit D (P < 0.038) (Figure 4). At 6 h.p.i., significantly higher expression of IL-12 and IL-6 was observed in the MOI of 0.1 without Vit D than in all treatments (P < 0.016). At 12 h.p.i., a significantly higher IL-12 expression was observed in the MOI of 1 without Vit D than in MOI of 0.1 with Vit D (P = 0.028). A significantly higher IL-6 expression was observed in MOI of 1 with Vit D and MOI of 0.1 without Vit D than in MOI of 0.1 with Vit D (P < 0.015). At 24 h.p.i., significantly higher IL-1β was observed in the MOI of 1 than in MOI of 0.1 with or without Vit D (P < 0.001), and in MOI of 1 with Vit D than without Vit D (P < 0.001) (Figure 4). A significantly higher IL-12 was observed in MOI of 1 without Vit D than in MOI of 0.1 with or without Vit D (P < 0.017). A significantly higher IL-6 expression was observed in both MOI of 1 and 0.1 with Vit D than in MOI of 0.1 without Vit D (P < 0.026). At 36 h.p.i., a significantly higher IL-1β expression was observed in MOI of 1 than in MOI of 0.1 without Vit D (P = 0.044) (Figure 4).
Figure 4.
Relative expression of cytokines associated with proinflammation IL-1β (A), IL-6 (B), and IL-12 (C) proinflamatory genes in DF-1 cells supplemented with vitamin D and infected with IBDV a MOI 0.1 and 1 at times 0, 3, 6, 12, 24, and 36 h.p.i.
HD11 cells
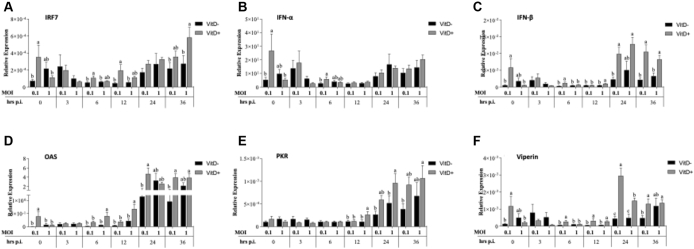
Expression of VDR, TLR21, and TRIFF at 0 h was significantly higher in MOI 0.1 with Vit D than in MOI of 0.1 without Vit D and MOI of 1 with Vit D (P < 0.029) while that of TLR3 was significantly higher in MOI of 1 without Vit D than in MOI of 0.1 without Vit D and MOI of 1 with Vit D (P < 0.049) (Figure 5). At 3 h.p.i., a significantly higher VDR expression was observed in MOI of 0.1 with Vit D than in MOI of 1 with Vit D (P = 0.037). At 6 h.p.i., a significantly higher VDR expression in MOI of 0.1 with Vit D than in MOI of 0.1 and MOI of 1 with Vit D was observed (P < 0.015) while a significantly higher TLR21 and TRIFF expression was observed compared to all treatments (P < 0.032). At 12 h.p.i., a significantly higher TLR21 expression in MOI of 1 with Vit D than in MOI of 0.1 without Vit D and a significantly higher MDA5 and MyD88 expression in MOI of 1 with Vit D than that in all treatments were observed (P < 0.033) (Figure 5). At 24 h.p.i., significantly higher MDA5 and MyD88 expression was observed in both MOIs with Vit D than without Vit D (P < 0.036). At 36 h.p.i., significantly higher VDR expression in MOI of 1 with Vit D than in MOI of 0.1 without Vit D and a significant TLR3 expression in both MOIs without Vit D compared to with Vit D were observed (P < 0.048). The expression of TLR21 was significantly higher in MOI of 1 with Vit D than in MOI of 0.1 and MOI of 1 both without Vit D (P < 0.011). Significantly higher MDA5 and MyD88 was observed in both MOIs with Vit D than in MOIs without Vit D (P < 0.0406) (Figure 5).
Figure 5.
Relative expression of vitamin D receptor (VDR) (A), TLR-3 (B), TLR-21 (C) and MDA5 (D) of receptors, TRIFF (E) and MyD88 (F) of signal transduction adaptors in HD-11 cells supplemented with vitamin D and infected with IBDV a MOI 0.1 and 1 at times 0, 3, 6, 12, 24, and 36 h.p.i.
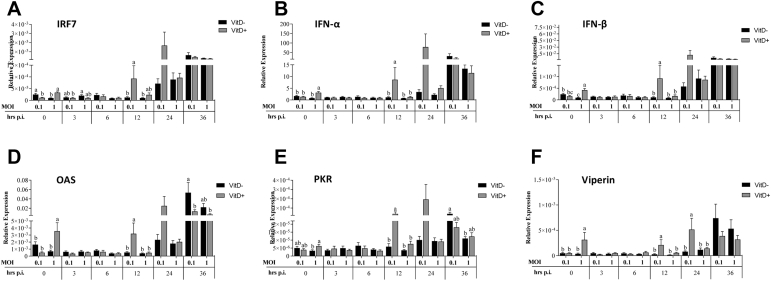
The expression of IRF-7, IFN-α, IFN-β, and viperin at 0 h was significantly higher in MOI of 0.1 with Vit D than in MOI of 0.1 without Vit D and in MOI of 1 with Vit D (P < 0.044). The expression of OAS was significantly higher in MOI of 0.1 with Vit D than that in all treatments (P < 0.024) (Figure 6). At 6 h.p.i., a significantly higher IRF-7 expression was observed in MOI of 0.1 with Vit D than in MOI of 0.1 and MOI of 1 both without Vit D (P < 0.026), while expression of IFN-α was significantly higher in MOI of 0.1 with Vit D than in MOI of 0.1 without Vit D (P = 0.023). The expression of IFN-β and viperin was significantly higher in MOI of 0.1 with Vit D than that in all treatments (P < 0.041). The expression of OAS was significantly higher in MOI of 1 with Vit D than that in all treatments (P < 0.027). At 12 h.p.i., a significantly higher IRF-7 expression was observed in MOI of 0.1 with Vit D than without Vit D (P < 0.010), while a significantly higher IFN-α was observed in MOI of 0.1 with Vit D than without Vit D (P < 0.05). Higher IFN-β, OAS, and PKR expression were observed in MOI of 1 with Vit D than those in all treatments (P < 0.033). Furthermore, a significantly higher viperin expression was observed in both MOIs with Vit D than without Vit D (P < 0.043) (Figure 6). At 24 h.p.i., no significant difference in TRF7 and IFN-α expression was observed among treatments, while a significantly higher IFN-β was observed in both MOIs with Vit D than in MOI of 0.1 without Vit D (P < 0.011), and a significantly higher OAS expression was observed in MOI of 0.1 with Vit D than without Vit D (P < 0.023). A significantly higher expression of PKR was found with MOI of 1 with Vit D than with both MOIs without Vit D (P < 0.037). Furthermore, a significantly higher viperin expression was observed in both MOIs with Vit D than in both MOIs without Vit D (P < 0.001), as well as a significantly higher expression level in MOI of 0.1 with Vit D than in MOI of 1 with Vit D (P = 0.009). At 36 h.p.i., expression of IRF-7 was significantly higher in MOI of 1 with Vit D than in both MOIs without Vit D (P < 0.023), while no significant difference in IFN-α expression was observed among treatments (P > 0.05), and a significantly higher IFN-β expression was observed in both MOIs with Vit D than without Vit D (P < 0.013). A significant OAS and viperin expression were found in both MOIs with Vit D than in MOI of 0.1 without Vit D (P < 0.046), while a significant PKR expression was observed in MOI of 1 with Vit D than in MOI of 0.1 without Vit D (P = 0.043) (Figure 6).
Figure 6.
Relative expression of interferons regulator factor IRF-7 (A), and interferons type I INF-α (B), INF-β (C) and related interferon antiviral genes OAS (D), PKR (E), and viperin (F) genes in HD-11 cells supplemented with vitamin D and infected with IBDV a MOI 0.1 and 1 at times 0, 3, 6, 12, 24, and 36 h.p.i.
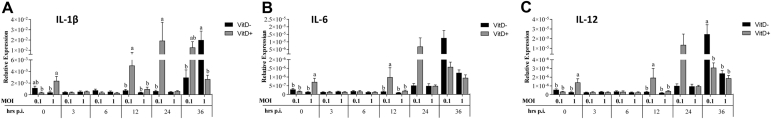
Significantly higher expression of IL-1β and IL-12 was observed at 0 h.p.i. in MOI of 0.1 with Vit D than in MOI of 0.1 without Vit D and MOI of 1 with Vit D (P < 0.048), while a significantly higher IL-6 expression was observed in MOI of 0.1 with Vit D than that in all treatments (P < 0.041) (Figure 7). At 3 h.p.i., significantly higher expression of IL-1β was found with MOI of 0.1 without Vit D than with MOI of 1 with Vit D (P = 0.028), while a significantly higher IL-12 expression was observed in MOI of 0.1 without Vit D than that in all treatments (P < 0.024). Significantly higher IL-6 expression in MOI of 0.1 with Vit D was observed than that in all treatments (P < 0.015). At 6 h.p.i., a significantly higher IL-1β in MOI of 0.1 with Vit D was found than in MOI of 1 with Vit D (P = 0.022). A significantly higher IL-12 expression in MOI of 0.1 with Vit D than in both MOIs without Vit D (P < 0.016) and a significantly higher IL-6 in MOI of 0.1 with Vit D than that in all treatments (P < 0.041) were observed. At 24 h.p.i., a significantly higher IL-1β was observed in both MOIs with Vit D than in MOI of 0.1 without Vit D (P < 0.018). A significantly higher IL-6 expression was observed in MOI of 1 without Vit D than in MOI of 1 with Vit D (P = 0.048). At 36 h.p.i., a significantly higher IL-1β was observed in MOI of 0.1 with Vit D than in both MOIs without Vit D (P < 0.003), while a significantly higher IL-12 expression was observed in MOI of 1 with Vit D than in both MOIs without Vit D (P < 0.024) (Figure 7).
Figure 7.
Relative expression of cytokines associated with proinflammation IL-1β (A), IL-6 (B), and IL-12(C) proinflamatory genes in HD-11 cells supplemented with vitamin D and infected with IBDV a MOI 0.1 and 1 at times 0, 3, 6, 12, 24, and 36 h.p.i.
DT40 cells
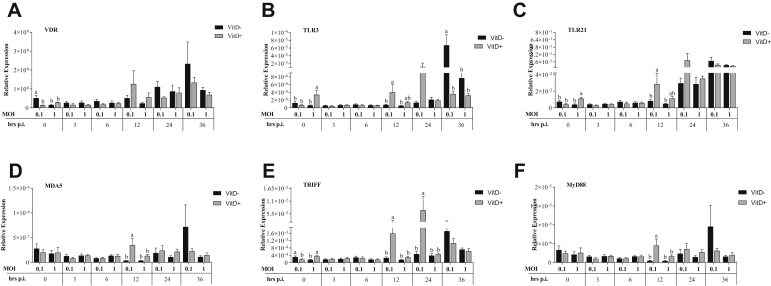
Expression of VDR was significantly higher in MOI of 0.1 without Vit D than that in all treatments at 0 h (P < 0.0196). At 0 h, significantly higher TLR3 and TLR21 expressions were observed in MOI of 1 with Vit D than those in all treatments (P < 0.042). Both MOI 0.1 without Vit D and MOI of 1 with Vit D showed a significantly higher TRIFF expression than both MOI of 0.1 with Vit D and MOI of 1 without Vit D (P < 0.008) (Figure 8). At 12 h.p.i., significantly higher expression of TLR3, TLR21, MDA5, TRIFF, and MyD88 in MOI of 0.1 with Vit D was observed than that in all treatments (P < 0.041), except for TLR3 where no significant difference was observed compared to MOI of 1 with Vit D (P = 0.877). At 24 h.p.i., significantly higher expression of TRIFF was observed in MOI of 0.1 with Vit D than that in all treatments (P < 0.045). At 36 h.p.i., a significantly higher TLR3 expression was observed in MOI of 0.1 without Vit D than that in all treatments (P < 0.004) (Figure 8).
Figure 8.
Relative expression of vitamin D receptor (VDR) (A), TLR-3 (B), TLR-21 (C) and MDA5 (D) of receptors, TRIFF (E) and MyD88 (F) of signal transduction adaptors in DT-40 cells supplemented with vitamin D and infected with IBDV a MOI 0.1 and 1 at times 0, 3, 6, 12, 24, and 36 h.p.i.
The expressions of IRF-7, IFN-α, IFN-β, OAS, and viperin were significantly higher in MOI of 1 with Vit D than those in all treatments at 0 h (P < 0.042), while the expression of PKR was only significantly higher compared to MOI of 1 without Vit D (P = 0.026). Furthermore, expression of IFN-β was significantly higher in MOI of 0.1 than in MOI of 1 without Vit D (P < 0.05) (Figure 8). The expression of IRF-7 was significantly higher in MOI of 1 without Vit D than in MOI of 0.1 with Vit D (P < 0.019). At 12 h.p.i., significantly higher expressions of IFN-α, IFN-β, OAS, PKR, and viperin in MOI of 0.1 with Vit D than those in all treatments (P < 0.049) and a significantly higher expression of IRF-7 than that in all treatments (P < 0.05), except for MOI of 1 with Vit D (P > 0.05), were observed. At 24 h.p.i., expression of viperin was significantly higher in MOI of 0.1 with Vit D than that in all treatments (P < 0.025). At 36 h.p.i., a significantly higher OAS expression was observed in MOI of 0.1 without Vit D than in MOI of 0.1 and MOI of 1 with Vit D (P < 0.022). Furthermore, a significantly higher PKR expression was observed in MOI of 0.1 without Vit D than in MOI of 1 without Vit D (P = 0.044) (Figure 8).
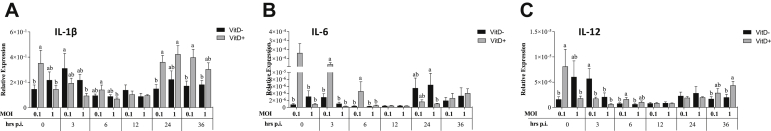
At 0 h, a significantly higher IL-1β in MOI of 1 with Vit D than in MOI of 0.1 with Vit D and MOI of 1 without Vit D (P < 0.003) and a significantly higher IL-12 and IL-6 in MOI of 1 without Vit D than those in all treatments were observed (P < 0.012) (Figure 9). At 12 h.p.i., significantly higher IL1-β, IL-12, and IL-6 were observed in MOI of 0.1 with Vit D than those in all treatments (P < 0.043). At 36 h.p.i, expression of IL-1β was significantly higher in MOI of 1 without Vit D than in MOI of 0.1 without Vit D and MOI of 1 with Vit D (P < 0.030). Furthermore, a significantly higher IL-12 was observed in MOI of 0.1 without Vit D than that in all treatments (P < 0.004) (Figure 10).
Figure 9.
Relative expression of interferons regulator factor IRF-7 (A), and interferons type I INF-α (B), INF-β (C) and related interferon antiviral genes OAS (D), PKR (E), and viperin (F) genes in DT-40 cells supplemented with vitamin D and infected with IBDV a MOI 0.1 and 1 at times 0, 3, 6, 12, 24, and 36 h.p.i.
Figure 10.
Relative expression of cytokines associated with proinflammation IL-1β (A), IL-6 (B), and IL-12(C) proinflamatory genes in DT-40 cells supplemented with vitamin D and infected with IBDV a MOI 0.1 and 1 at times 0, 3, 6, 12, 24, and 36 h.p.i.
Discussion
IBDV preferentially targets IgM + B cells found in the gut-associated lymphoid organs and the BF inducing host cell apoptosis, while it also targets monocyte-macrophage lineage in a persistent manner (Rasoli et al., 2015). Dietary Vit D has been shown to be a potent immunomodulator even though this is not fully understood in chickens. Furthermore, the main focus of research in this area has been mainly in response to bacterial infections in poultry, and limited information exists regarding viral infections. Therefore, the present study evaluated the innate immune response profile of cells susceptible to IBDV with and without Vit D supplementation. Of the 3 cells tested, the main benefit of Vit D in the innate immune modulation was observed mainly in HD-11. Tests in the promonocytic cell lines IN24 and LSCC-NP1 showed high susceptibility to the virus, demonstrating the importance of this type of cells in the immune response against the virus (Inoue et al., 1992). When the virus infects a cell, surveys both at the endosomal and at cytoplasmic levels recognize it. A good recognition (in quantity and quality) will be reflected in a good effector response, that is, in the production of effector proteins for virus control.
The expression of TLR-21 was higher in the BF and the spleen of adult chickens (Brownlie et al., 2009). This receptor has been identified in chickens and turkeys (Roach et al., 2005), and initially it was given the homologous function of the mammalian TLR-9, that is, to recognize bacterial genomic DNA (Brownlie et al., 2009), and as a sensor for DNA virus infection by exerting an effective antiviral immune response by producing type I IFN (Lund et al., 2003; Krug et al., 2004; Bussey et al., 2019). Following sequentially the beneficial effect of Vit D supplementation on HD-11 cells, it was initially found that the avian endosomal sensor TLR-21 was significantly upregulated after 12 h.p.i. independent of the MOI. Little is known about the expression of this endosomal receptor in chicken macrophages, specifically after infection with IBDV. As a dsRNA, IBDV may be recognized by TLR-21 with the same efficiency as viruses with dsDNA genome as was shown in previous studies. Tests with the Marek's disease virus showed that the use of TLR-21 agonists modulated antivirus immunity including cytokine responses in Marek's disease virus–infected chickens (Parvizi et al., 2014). Furthermore, the administration of encapsulated TLR-21 ligands resulted in diminished tumor incidence by upto 60% compared to chickens without the ligands, and this may be associated with the upregulation of both IL-1β and IL-18 (Bavananthasivam et al., 2018). It is important to note that upregulation of receptors, signaling pathways, and effector proteins in the immune response may not always be beneficial and depends on the pathogen and disease. In the case of autoimmune diseases, overexpression would produce deleterious effects, and in some cell types, it can induce or favor the generation of tumors. In the present study, we found an upregulation of TLR-21 with Vit D supplementation. This effect is particularly beneficial as in this case, where it was more extensive in macrophages, as they play an important role in the immune response. There are no studies in birds determining the immunomodulatory effect of Vit D on the regulation of TLR-21 expression; the closest comparison would be with the homologous TLR-9 of mammals. In this scenario, there are studies that show that Vit D supplementation subexpresses TLR-9 during the initial stages of infection (12 h.p.i.) with herpes simplex virus-1 in Hela cells and also acts directly as an antiviral compound (Kumar et al., 2018). In our study, upregulation of TLR-21 occurred after 12 h.p.i., although the authors do not explain what happens after this time, if there was downregulation of TLR-21 above 12 h.p.i. in Hela cells, it may be beneficial in the sense that their proliferative activity would be diminished.
IBDV, similar to other RNA viruses, is also detected at the cytoplasmic level. For this purpose, the expression of MDA-5 was particularly evaluated. At this point, a significant upregulation was found in the HD-11 cells starting at 12 h.p.i. when it was supplemented with Vit D and independent of the MOI. MDA5 and RIG-I are members of an evolutionary conserved family and lead to the activation of the interferon system (Pichlmair et al., 2006). The present study is the first one to determine the immunomodulatory effect of Vit D on MDA-5, which might be important to control IBDV. It is important to point out that Vit D supplementation in macrophages contributes to the upregulation of the receptor genes that recognize vRNA-IBDV both at the endosomal and cytoplasmic levels, which is important because it helps to survey the virus at 2 different sites during the pathogenesis. The importance of MDA-5 in viral infections has already been reported as in the case of influenza A viruses where MDA-5 is the primary cytosolic virus sensor in chicken cells including HD-11 cells (Liniger et al., 2012).
The signaling of the endosomal and cytoplasmic sensors leads to the activation of several pathways and the expression of antiviral effector genes. The activation of the TLR-21 leads to the activation of the MyD88 pathway. In our study, this pathway was also significantly upregulated at 24 and 36 h.p.i. with Vit D and independent of the MOI in HD-11 cells, indicating that upregulation of TLR-21 also leads to upregulation of MyD-88. This pathway in birds has been reported in bacterial infections (Kogut et al., 2012). The activation of cytoplasmic receptors MDA-5 and Nucleotide-binding oligomerization domain-like receptors by effect of Vit D supplementation has been described in respiratory type viruses (Greiller and Martineau, 2015) and particularly as an immunomodulator targeting various immune cells, including monocytes, macrophages, dendritic cells, and T- and B-lymphocytes (Baeke et al., 2010). The upregulation of MDA-5 with Vit D in the present study may be beneficial to the immune response of birds to IBDV. Studies show that the virus VP3 protein blocks MDA-5 binding to viral genomic dsRNA in vitro and in vivo leading to a significant inhibition of INF-β expression (Ye et al., 2014). Another important signaling pathway, IRF-7, where we found significant differences in expression at 0, 6, 12, and 36 h.p.i. in HD-11 cells favored by Vit D supplementation, can be associated with the activation pathway through MDA-5. Upregulation of MDA-5 has been reported to increase IRF-7–dependent pathways leading to the activation of IFN-β and Mx promoters and inhibit replication of IBDV in DT40 cells (Ouyang et al., 2018).
The pathways described previously lead to the expression of cytokines that can be grouped into proinflammatory and antiviral responses. In the first case, a significant upregulation of IL-1β was observed in Vit D–supplemented HD-11 cells independent of MOI. This may be associated with the upregulation of IRF-7, which marks a very well-determined pathway. The importance of this route is given by the fact an upregulation of IL-1β will lead to higher level of inflammasomes, which, in the case of macrophages, is determinant for their activity as effector cells in inflammatory responses. The same pattern was found for IL-6 in macrophages where a significant upregulation at 0, 3, and 6 h.p.i. with supplementation of Vit D was observed. The effect exerted by IBDV on the regulation of IL-1β and IL-6 has been studied with inconclusive observations, possibly due to variation in the strains used. Some studies have shown that birds inoculated with the classic virulent strain IBDV-IM led to an upregulation of INF-γ and IL-1β (Rautenschlein et al., 2007; Raj et al., 2011) and IL-6 (Aricibasi et al., 2010), while others have shown reduced bursal IL-1β expression and serum IL-6 concentration with vvIBDV inoculation (Gallardo et al., 2014; Tan et al., 2015). In our case, supplementation with Vit D led to an upregulation of both IL-1β and IL-6. This could favor the response against IBDV, as proposed in studies with other immunomodulatory supplements such as L-arginine where increased serum IL-6 level in IBDV-inoculated groups was observed suggesting that dietary arginine may have a potential regulatory effect on IBDV-induced immunosuppression in an IL-6–dependent manner (Tan et al., 2015).
With regard to cytokines involved in antiviral responses, INF-α and INF-β key regulators of responses leading to the activation of antiviral proteins (AVPs) that are responsible for blocking viral replication. In the present study, in HD-11 cells, the expression of INF-β was favored with Vit D supplementation with MOI 0.1 and 1 at 24 and 36 h.p.i. The aforementioned statement confirms that the activation uprelated pathways up to this point result in an upregulation of INF-β. This has a very important value since this interferon ensures not only a greater control of the cell but also, by its paracrine capacity, the protection of neighboring cells against IBDV. The viral protein VP4 has protease activity and acts as an essential viral component to suppress type I interferon expressions via binding to glucocorticoid-induced leucine zipper protein (Li et al., 2013) that inhibits the activation of nuclear factor kappa enhancer binding protein. This led to the proposal that suppression of type I interferon expressions via VP4 binding to glucocorticoid-induced leucine zipper protein to be one of the mechanisms of IBDV evasion of host immune response (Qin and Zheng, 2017). In our study, supplementation with Vit D in vitro led to overexpression of type 1 interferons, particularly INF-β in macrophages, which is of great benefit for the control of the infection.
The activation of INF-β was found to be fundamentally reflected on the expression of PKR and OAS AVPs in macrophages. In an inactive form, PKR is localized in the nucleus and, upon activation, mediated through viral dsRNA recognition, oxidative stress, growth factors, cytokines, and cellular proteins such as PKR-associated activator, or the stimulation of TLRs, phosphorylates the eukaryotic initiation factor 2. This action impairs the guanine nucleotide exchange reaction inhibiting translation of mRNA in infected cells (Munir and Berg, 2013). In our study, a significant upregulation was found in HD-11 cells supplemented with Vit D from 12 h.p.i. Different viruses, including influenza virus, herpes simplex virus type I, and hepatitis C virus, encode for inhibitory factors to inhibit PKR actions, while this kinase can still surpass and exert antiviral activities (Santhakumar et al., 2017). In IBDV, its direct or indirect effect on PKR is not precisely known, but supplementation with Vit D affects its expression, increasing the capacity of macrophages to control the infection. In the case of OAS, upregulation was also favored by Vit D supplementation, particularly at 0.1 MOI. The existence of 2 alleles of the 2′-5′-OAS gene in chickens has been identified (Yamamoto et al., 1998), and its expression has been revealed to be age-dependent (Tatsumi et al., 2000). In vitro studies using the same cell line (HD-11) found that infection with IBDV leads to a dose-dependent upregulation of chicken OAS (Lee et al., 2015). It is clear that Vit D acts as an immunomodulator to benefit the production of OAS, contributing to the control of the infection.
The other 2 cell types studied, DF-1 and DT-40, did not have a profile of pathway activation by Vit D supplementation as marked as with macrophages. The virus survey seemed to be favored by Vit D supplementation through TLR-3, while TLR-21 was involved in macrophages. In the subsequent signaling, supplementation with Vit D favored a significant upregulation of TRIF and IRF-7 in DT-40 cells, particularly with 0.1 MOI at 12 and 24 h.p.i. This may be associated with an activation pathway of TLR-3, TRIF, and IRF-7 that is dependent on the MOI (0.1) in lymphocytes. In reference to the proinflammatory cytokines, a significant upregulation of IL-1β and IL-6 was found in DT-40 cells at 12, 24, and 36 h.p.i. by supplementation with Vit D and with dependence on the MOI. The expression of INFs in lymphocytes was also favored by Vit D supplementation. Similar to macrophages, an upregulation of INF-β was found, but it was dependent on the MOI (0.1); the difference was manifested with the INF-α. Supplementation with Vit D significantly favored upregulation of INF-α with 0.1 MOI at 0, 12, and 24 h.p.i. Regarding AVPs, it was found that for both fibroblasts and B lymphocytes, supplementation with Vit D affects the upregulation of PKR and OAS, being dependent on the MOI for DT-40 and independent for DF-1. This would indicate that Vit D supplementation in B lymphocyte activates signaling pathways, while in fibroblasts, it directly affects the upregulation of AVPs.
This study contributes to the understanding of the activation pathways of innate immunity induced by IBDV in chickens and the benefit of Vit D supplementation as an immunomodulator in vitro. We propose the study of these results in vivo to validate the beneficial effect of Vit D in the control of the infection caused by IBDV.
Acknowledgments
The authors would like to thank Beatrice Despress for her technical assistance and support in this research. This research was funded by Natural Sciences and Engineering Research Council of Canada (NSERC) and the Canadian Poultry Research Council (CPRC).
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Abdul R., Murgia M.V., Rodriguez-Palacios A., Lee C.-W., Saif Y.M. Persistence and tissue distribution of infectious bursal disease virus in experimentally infected SPF and commercial broiler chickens. Avian Dis. 2013;57:759–766. doi: 10.1637/10448-110812-Reg.1. [DOI] [PubMed] [Google Scholar]
- Alkie T.N., St Paul M., Barjesteh N., Brisbin J., Sharif S. Expression profiles of antiviral response genes in chicken bursal cells stimulated with Toll-like receptor ligands. Vet. Immunol. Immunopathol. 2015;163:157–163. doi: 10.1016/j.vetimm.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Aricibasi M., Jung A., Heller E.D., Rautenschlein S. Differences in genetic background influence the induction of innate and acquired immune responses in chickens depending on the virulence of the infecting infectious bursal disease virus (IBDV) strain. Vet. Immunol. Immunopathol. 2010;135:79–92. doi: 10.1016/j.vetimm.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Baeke F., Takiishi T., Korf H., Gysemans C., Mathieu C. Vitamin D: modulator of the immune system. Curr. Opin. Pharmacol. 2010;10:482–496. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Bahar-Shany K., Ravid A., Koren R. Upregulation of MMP-9 production by TNFalpha in keratinocytes and its attenuation by vitamin D. J. Cell Physiol. 2010;222:729–737. doi: 10.1002/jcp.22004. [DOI] [PubMed] [Google Scholar]
- Bavananthasivam J., Alkie T.N., Astill J., Abdul-Careem M.F., Wootton S.K., Behboudi S., Yitbarek A., Sharif S. In ovo administration of Toll-like receptor ligands encapsulated in PLGA nanoparticles impede tumor development in chickens infected with Marek’s disease virus. Vaccine. 2018;36:4070–4076. doi: 10.1016/j.vaccine.2018.05.091. [DOI] [PubMed] [Google Scholar]
- Brownlie R., Zhu J., Allan B., Mutwiri G.K., Babiuk L.A., Potter A., Griebel P. Chicken TLR21 acts as a functional homologue to mammalian TLR9 in the recognition of CpG oligodeoxynucleotides. Mol. Immunol. 2009;46:3163–3170. doi: 10.1016/j.molimm.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Bussey K.A., Murthy S., Reimer E., Chan B., Hatesuer B., Schughart K., Glaunsinger B., Adler H., Brinkmann M.M. Endosomal toll-like receptors 7 and 9 Cooperate in detection of murine Gammaherpesvirus 68 infection. J. Virol. 2019;93 doi: 10.1128/JVI.01173-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo R.A., Carrasco-Medanic R., Zhou H., Lyu S., Wang Y., Woolcock P.R., Hoerr F.J. Effects of challenge with very virulent infectious bursal disease virus reassortants in commercial chickens. Avian Dis. 2014;58:579–586. doi: 10.1637/10844-040914-Reg.1. [DOI] [PubMed] [Google Scholar]
- Greiller C.L., Martineau A.R. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. 2015;7:4240–4270. doi: 10.3390/nu7064240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansdottir S., Monick M.M., Hinde S.L., Lovan N., Look D.C., Hunninghake G.W. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J. Immunol. 2008;181:7090–7099. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Chen Y., Kang S., Chen G., Wei P. Differential regulation of chTLR3 by infectious bursal disease viruses with different virulence in vitro and in vivo. Viral Immunol. 2017;30:490–499. doi: 10.1089/vim.2016.0134. [DOI] [PubMed] [Google Scholar]
- Helming L., Böse J., Ehrchen J., Schiebe S., Frahm T., Geffers R., Probst-Kepper M., Balling R., Lengeling A. 1alpha,25-Dihydroxyvitamin D3 is a potent suppressor of interferon gamma-mediated macrophage activation. Blood. 2005;106:4351–4358. doi: 10.1182/blood-2005-03-1029. [DOI] [PubMed] [Google Scholar]
- Inoue M., Yamamoto H., Matuo K., Hihara H. Susceptibility of chicken monocytic cell lines to infectious bursal disease virus. J. Vet. Med. Sci. 1992;54:575–577. doi: 10.1292/jvms.54.575. [DOI] [PubMed] [Google Scholar]
- Jackwood D.J. Advances in vaccine research against economically important viral diseases of food animals: infectious bursal disease virus. Vet. Microbiol. 2017;206:121–125. doi: 10.1016/j.vetmic.2016.11.022. [DOI] [PubMed] [Google Scholar]
- Jadhav N.J., Gokhale S., Seervi M., Patil P.S., Alagarasu K. Immunomodulatory effect of 1, 25 dihydroxy vitamin D3 on the expression of RNA sensing pattern recognition receptor genes and cytokine response in dengue virus infected U937-DC-SIGN cells and THP-1 macrophages. Int. Immunopharmacol. 2018;62:237–243. doi: 10.1016/j.intimp.2018.07.019. [DOI] [PubMed] [Google Scholar]
- Khatri M., Palmquist J.M., Cha R.M., Sharma J.M. Infection and activation of bursal macrophages by virulent infectious bursal disease virus. Virus Res. 2005;113:44–50. doi: 10.1016/j.virusres.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Kogut M.H., Chiang H.-I., Swaggerty C.L., Pevzner I.Y., Zhou H. Gene expression Analysis of toll-like receptor pathways in Heterophils from genetic chicken lines that differ in their susceptibility to Salmonella enteritidis. Front. Genet. 2012;3:121. doi: 10.3389/fgene.2012.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug A., Luker G.D., Barchet W., Leib D.A., Akira S., Colonna M. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood. 2004;103:1433–1437. doi: 10.1182/blood-2003-08-2674. [DOI] [PubMed] [Google Scholar]
- Kumar A., Singh M.P., Kumar R.S., Ratho R.K. 25-Hydroxyvitamin D3 and 1,25 Dihydroxyvitamin D3 as an antiviral and immunomodulator against herpes simplex virus-1 infection in HeLa cells. Viral Immunol. 2018;31:589–593. doi: 10.1089/vim.2018.0026. [DOI] [PubMed] [Google Scholar]
- Lee C.-C., Wu C.C., Lin T.L. Role of chicken melanoma differentiation-associated gene 5 in induction and activation of innate and adaptive immune responses to infectious bursal disease virus in cultured macrophages. Arch. Virol. 2015;160:3021–3035. doi: 10.1007/s00705-015-2612-y. [DOI] [PubMed] [Google Scholar]
- Li Z., Wang Y., Li X., Li X., Cao H., Zheng S.J. Critical roles of glucocorticoid-induced leucine zipper in infectious bursal disease virus (IBDV)-induced suppression of type I Interferon expression and enhancement of IBDV growth in host cells via interaction with VP4. J. Virol. 2013;87:1221–1231. doi: 10.1128/JVI.02421-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liniger M., Summerfield A., Ruggli N. MDA5 can be exploited as efficacious genetic adjuvant for DNA vaccination against lethal H5N1 influenza virus infection in chickens. PLoS One. 2012;7:e49952. doi: 10.1371/journal.pone.0049952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J., Sato A., Akira S., Medzhitov R., Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munir M., Berg M. The multiple faces of proteinkinase R in antiviral defense. Virulence. 2013;4:85–89. doi: 10.4161/viru.23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W., Qian J., Pan Q.-X., Wang J.-Y., Xia X.-X., Wang X.-L., Zhu Y.-M., Wang Y.-S. gga-miR-142-5p attenuates IRF7 signaling and promotes replication of IBDV by directly targeting the chMDA5’s 3' untranslated region. Vet. Microbiol. 2018;221:74–80. doi: 10.1016/j.vetmic.2018.05.018. [DOI] [PubMed] [Google Scholar]
- Parvizi P., Abdul-Careem M.F., Mallick A.I., Haq K., Haghighi H.R., Orouji S., Heidari M., Behboudi S., Sharif S. The effects of administration of ligands for Toll-like receptor 4 and 21 against Marek’s disease in chickens. Vaccine. 2014;32:1932–1938. doi: 10.1016/j.vaccine.2014.01.082. [DOI] [PubMed] [Google Scholar]
- Pender M.P. CD8+ T-cell deficiency, Epstein-Barr virus infection, vitamin D deficiency, and steps to Autoimmunity: a Unifying Hypothesis. Autoimmune Dis. 2012. 2012:189096. doi: 10.1155/2012/189096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichlmair A., Schulz O., Tan C.P., Näslund T.I., Liljeström P., Weber F., Reis e Sousa C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5’-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- Qin Y., Zheng S.J. Infectious bursal disease virus-host interactions: Multifunctional viral proteins that Perform multiple and differing Jobs. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj G.D., Rajanathan T.M.C., Kumanan K., Elankumaran S. Changes in the cytokine and toll-like receptor gene expression following infection of Indigenous and commercial chickens with infectious bursal disease virus. Indian J. Virol. 2011;22:146–151. doi: 10.1007/s13337-011-0053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasoli M., Yeap S.K., Tan S.W., Roohani K., Kristeen-Teo Y.W., Alitheen N.B., Rahaman Y.A., Aini I., Bejo M.H., Kaiser P., Omar A.R. Differential modulation of immune response and cytokine profiles in the bursae and spleen of chickens infected with very virulent infectious bursal disease virus. BMC Vet. Res. 2015;11:75. doi: 10.1186/s12917-015-0377-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautenschlein S., Alkie T.N. Infectious bursal disease virus in poultry: current status and future prospects. VMRR. 2016;9 doi: 10.2147/VMRR.S68905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautenschlein S., von Samson-Himmelstjerna G., Haase C. A comparison of immune responses to infection with virulent infectious bursal disease virus (IBDV) between specific-pathogen-free chickens infected at 12 and 28 days of age. Vet. Immunol. Immunopathol. 2007;115:251–260. doi: 10.1016/j.vetimm.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Roach J.C., Glusman G., Rowen L., Kaur A., Purcell M.K., Smith K.D., Hood L.E., Aderem A. The evolution of vertebrate Toll-like receptors. Proc. Natl. Acad. Sci. USA. 2005;102:9577–9582. doi: 10.1073/pnas.0502272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Lecompte J.C., Yitbarek A., Cuperus T., Echeverry H., van Dijk A. The immunomodulatory effect of vitamin D in chickens is dose-dependent and influenced by calcium and phosphorus levels. Poult. Sci. 2016;95:2547–2556. doi: 10.3382/ps/pew186. [DOI] [PubMed] [Google Scholar]
- Santhakumar D., Rubbenstroth D., Martinez-Sobrido L., Munir M. Avian interferons and their antiviral effectors. Front. Immunol. 2017;8:49. doi: 10.3389/fimmu.2017.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojadoost B., Behboudi S., Villanueva A.I., Brisbin J.T., Ashkar A.A., Sharif S. Vitamin D3 modulates the function of chicken macrophages. Res. Vet. Sci. 2015;100:45–51. doi: 10.1016/j.rvsc.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Tan J.-Z., Guo Y.-M., Applegate T.J., Du E.-C., Zhao X. Dietary L-arginine modulates immunosuppression in broilers inoculated with an intermediate strain of infectious bursa disease virus. J. Sci. Food Agric. 2015;95:126–135. doi: 10.1002/jsfa.6692. [DOI] [PubMed] [Google Scholar]
- Tatsumi R., Hamada K., Sekiya S., Wakamatsu M., Namikawa T., Mizutani M., Sokawa Y. 2’,5'-oligoadenylate synthetase gene in chicken: gene structure, distribution of alleles and their expression. Biochim. Biophys. Acta. 2000;1494:263–268. doi: 10.1016/s0167-4781(00)00174-3. [DOI] [PubMed] [Google Scholar]
- Teymoori-Rad M., Shokri F., Salimi V., Marashi S.M. The interplay between vitamin D and viral infections. Rev. Med. Virol. 2019:e2032. doi: 10.1002/rmv.2032. [DOI] [PubMed] [Google Scholar]
- Yamamoto A., Iwata A., Koh Y., Kawai S., Murayama S., Hamada K., Maekawa S., Ueda S., Sokawa Y. Two types of chicken 2’,5'-oligoadenylate synthetase mRNA derived from alleles at a single locus. Biochim. Biophys. Acta. 1998;1395:181–191. doi: 10.1016/s0167-4781(97)00148-6. [DOI] [PubMed] [Google Scholar]
- Ye C., Jia L., Sun Y., Hu B., Wang L., Lu X., Zhou J. Inhibition of antiviral innate immunity by birnavirus VP3 protein via blockage of viral double-stranded RNA binding to the host cytoplasmic RNA detector MDA5. J. Virol. 2014;88:11154–11165. doi: 10.1128/JVI.01115-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zofková I., Kancheva R.L. The effect of 1,25(OH)2 vitamin D3 on CD4+/CD8+ subsets of T lymphocytes in postmenopausal women. Life Sci. 1997;61:147–152. doi: 10.1016/s0024-3205(97)00369-x. [DOI] [PubMed] [Google Scholar]