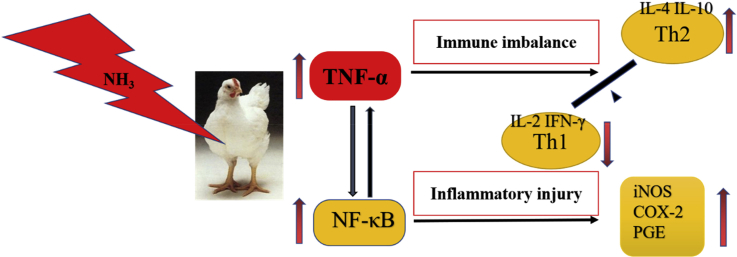
Graphical abstract
Key words: ammonia, chicken spleen, Th1/Th2 immune balance, inflammation
Abstract
Ammonia is a hazardous environmental pollutant that can be harmful to animal health. In this study, we aimed to evaluate the effect of ammonia exposure on broiler chicken spleens. We randomly divided one hundred twenty 1-day-old broiler chickens into 3 groups and raised them with exposure to different ammonia concentrations (low, middle, and high); at 42 D of age, the chicken spleens were extracted. We observed histopathologic changes in spleen tissues by microscopy and measured the expression of Th1/Th2 secreted cytokines (interleukin [IL]-1β, IL-2, IL-4, IL-6, IL-10, interferon-γ [IFN-γ], tumor necrosis factor-α) by RT-PCR. We also measured the expression of nuclear receptor-κB (NF-κB) pathway–related genes (cyclooxygenase-2 [COX-2], nitric oxide synthase [iNOS], and prostaglandin synthetase [PGE]) in spleens by RT-PCR and Western blot analysis. Histopathologic observations indicated that the spleen tissues were seriously injured in the high ammonia concentration group. There was abnormal cytokine expression, including increased IL-4, IL-6, and IFN-γ and decreased IL-2, which indicated an imbalance in the Th1/Th2 response. The proinflammatory factors such as NF-κB, COX-2, iNOS, and PGE were upregulated in the high ammonia group. In conclusion, this study illustrated that ammonia exposure led to a Th1/Th2 immune imbalance and triggered the NF-κB pathway, causing inflammatory damage to the spleen.
Introduction
Ammonia (NH3), which is a colorless irritant gas, has been considered to be one of the main pollutants in the atmosphere that usually comes from the treatment of livestock waste and volatile synthetic fertilizers (Bonyadi et al., 2016), as well as petroleum smelting (Marta et al., 2010). Ammonia is an alkaline gas; therefore, it can neutralize acidic substances in the atmosphere to produce ammonium salts that can reduce atmospheric visibility. Ammonia can increase PM2.5 in the atmosphere, which effects the balance of regional ecosystems, and high atmospheric NH3 is one of the main factors that can effect human and animal health (Bonyadi et al., 2016, Lu et al., 2017). Ammonia stimulation can generate reactive oxygen species (Kosenko et al., 2003), which leads to oxidative stress, damage to cell membrane integrity, reduced enzyme activity, and pathologic injury or even cell death (Drose and Brandt, 2008, Liang et al., 2016). At the same time, NH3 can also effect the immune response and increase inflammatory cytokine expression (Qi et al., 2017).
It has been reported that an elevated Th1 immune response occurs in the early stages of infection, which can lead to immune-mediated neuronal damage, whereas in the later stages, the Th2 immune response helps to repair damaged nerves and recovery from Campylobacter jejuni–associated Guillain-Barré syndrome (Nyati et al., 2012). As we known, the ratio of Th1/Th2 maintains the balance between cellular and humoral immune responses. Many studies have shown that inflammation and diseases follow a Th1/Th2 imbalance, including pneumonia (Zhao et al., 2016), pyelonephritis, and systemic lupus erythematosus (Huang et al., 2011, Talaat et al., 2015). A recent study suggested that the treatment of asthma by regulating the balance of Th1/Th2 in humans was effective (Wang et al., 2016). We know the cytokines that are associated with T helper cells; the biomarkers secreted by Th1 cells are interferon-γ (IFN-γ), interlenkin (IL)-2 and tumor necrosis factor-α (TNF-α), whereas the biomarkers secreted by Th2 cells are IL-4, IL-6, and IL-10, with all of these cytokines being closely related to the balance in Th1/Th2 (Sun et al., 2018). Additionally, nuclear receptor-κB (NF-κB) is a key regulatory factor in many inflammatory diseases; NF-κB activates proinflammatory cytokines such as TNF-α, IL-6, as well as Th2 cytokines including IL-4 and interleukin-13 (Chi et al., 2017). Together, NF-κB and TNF-α coregulate the vast network of immune and inflammatory responses (Tak and Firestein, 2001). Chang's research further illustrated that the potential mechanism of nano-NiO-induced lung lesions was related to NF-κB activation and a Th1/Th2 imbalance (Chang et al., 2016).
There are limited data available on the negative effects of NH3-induced immune imbalance and inflammation. In this study, we used chickens as research subjects and established a model of different concentrations of NH3 exposure. To explore the impact of an NH3 concentration gradient on chicken spleens, we measured the expression of immune-related genes (IL-2, IFN-γ, IL-4, IL-6, IL-1β, and IL-10), NF-κB, TNF-α, nitric oxide synthase (iNOS), cyclooxygenase-2(COX-2), and prostaglandin synthetase (PGE) in spleens under different concentrations of NH3 by quantitative real-time PCR and Western blot analysis. This article will provide a research basis for NH3-induced inflammatory damage and NH3 toxicology research.
Materials and methods
Experimental Animals and Ammonia Concentration
Our experiment was normative and was approved by the Institutional Animal Care and Use Committee of the Agricultural University with the approved protocol number SRM-08. Briefly, 120 broiler chickens (1-day-old) were used and purchased from the Weiwei Company in Harbin, China. The one hundred twenty 1-day-old broilers were randomly divided into 3 treatment groups. Each treatment group was placed in a separate, environmentally controlled chamber with O2, CO2, NH3, temperature, and humidity sensors, which monitored gas concentration continuously. Each group had 4 replicates with 10 birds per replicate. Ammonia gas was fed into the room continuously for 24 h. The chambers were maintained at 5, 10, and 20 ppm NH3 gas from 0 to 3 wk. After 22 D, the NH3 concentration in all chambers was adjusted to 5, 15, and 45 ppm, respectively, and then maintained at these levels out to 6 wk. The environmental parameters of each environmental control room, such as temperature, humidity, wind speed, and illumination, were adjusted once a wk according to the requirements of the ages of the broilers. Aside from the NH3 gas, the ambient parameters of each environmental control room were basically the same as the environmental parameters. The supply, access, and concentration controls of the NH3 in each room control chamber were controlled by NH3 cylinders, safety valves, and flow meters, respectively (Shi et al., 2019). The basal diet was prepared according to the nutrient requirements of the broilers (NRC, 1994). The broilers were free to consume food and water when fed, and we ensured that the temperature, relative humidity, and light time of the 3 treatment groups were consistent. After 42 D, all broilers were euthanized with sodium pentobarbital, and the spleen tissues from each chicken were collected and immediately frozen in liquid nitrogen and stored at −80°C.
Histologic Observation of Spleen Tissue
After treatment with NH3, spleen tissue sections were stained with hematoxylin and eosin. The method used was consistent with our previous studies (Chen et al., 2017).
Real-Time PCR Analysis
Total RNA were extracted from chicken spleens using the TRIzol reagent (Invitrogen, Carlsbad, CA). Oligo dT primers and Superscript II reverse transcriptase (Invitrogen) were used to make complementary DNA from 200 ng of total RNA. β-actin was used as internal control. The primer sequences for IL-1β, IL-2, IL-4, IL-6, IL-10, IFN-γ, NF-κB, COX-2, iNOS, PGE, TNF-α, and β-actin are described in Table 1. The relative expression levels of mRNAs were evaluated by the 2−ΔΔCt method (Jin et al., 2018).
Table 1.
Genes primers used in the real-time quantitative PCR.
| Target genes | Primer sequence (5′-3′) | Target genes | Primer sequence (5′-3′) |
|---|---|---|---|
| β-actin | F:ACGTCGCACTGGATTTCGAG | IFN-γ | F:AGCCGCACATCAAACACATA |
| R:TGTCAGCAATGCCAGGGTAC | R:CGCTGGATTCTCAAGTCGTT | ||
| IL-2 | F:TGCAGTGTTACCTGGGAGAA | IL-1β | F:CTCCTCCAGCCAGAAAGTGA |
| R:CGGTGTGATTTAGACCCGTAA | R:GAGCTTGTAGCCCTTGATGC | ||
| IL-4 | F:ACGCCATCAGGAAGGTTGTT | IL-10 | F:AGGAAACCTCTCCCTGGATGTC |
| R:GTGCCCACGCTGTGCTTAC | R:CGCTGTCACCGCTTCTTCA | ||
| TNF-α | F:AGATGGGAAGGGAATGAACC | COX-2 | F:TGTCCTTTCACTGCTTTCCAT |
| R:ACTGGGCGGTCATAGAACAG | R:TTCCATTGCTGTGTTTGAGGT | ||
| iNOS | F:CCTGGAGGTCCTGGAAGAGT | PGE | F:GTTCCTGTCATTCGCCTTCTAC |
| R:CCTGGGTTTCAGAAGTGGC | R:CGCATCCTCTGGGTTAGCA | ||
| NF-κB | F:TCAACGCAGGACCTAAAGACAT | IL-6 | F:TGCAGTGTTACCTGGGAGAA |
| R:GCAGATAGCCAAGTTCAGGATG | R:CGGTGTGATTTAGACCCGTAA |
Abbreviations: COX-2, cyclooxygenase-2; iNOS, nitric oxide synthase; NF-κB, nuclear receptor-κB; PGE, prostaglandin synthetase; TNF-α, tumor necrosis factor-α.
Western Blot Analysis
First, chicken spleen proteins were subjected to SDS-polyacrylamide gel electrophoresis. The separated proteins were then transferred to nitrocellulose membranes using a tank transfer for 2 h at 200 mA in Tris-glycine buffer containing 20% methanol. The membranes were blocked with 5% skim milk for 24 h and incubated overnight with diluted primary chicken antibodies against iNOS (1:1500, polyclonal antibody prepared by our laboratory), COX-2, NF-κB, and TNF-α (1:500, Santa Cruz Biotechnology, Santa Cruz, CA). To verify equal loading of samples, the membrane was incubated with a monoclonal β-actin antibody (1:1000, Santa Cruz Biotechnology), followed by an horseradish peroxidase–conjugated secondary antibody. The bound primary antibodies were detected with a horseradish peroxidase–conjugated secondary antibody against mouse IgG (1:1500, Santa Cruz Biotechnology). The signal was measured by enhanced chemiluminescence detection reagents (Applygen Technologies Inc., Beijing, China). The protein bands were visualized using a Champ Chemi imaging system (Beijing Sage Creation Science Co. Ltd., Beijing, China). The relative abundances of the proteins were expressed as the ratios of the optical densities of each protein to that of β-actin.
Statistical Analysis
We analyzed the data using GraphPad Prism software (Graph Pad Software Inc., version 5.0, San Diego, CA). In each group, the data used were the means ± the standard deviation, and they were analyzed using a one-way analysis of variance with Tukey's correction. Statistically significant differences (P < 0.05) between the data samples are represented by different lowercase letters.
Results
Effect of NH3 Exposure on Morphological Changes in the Spleen
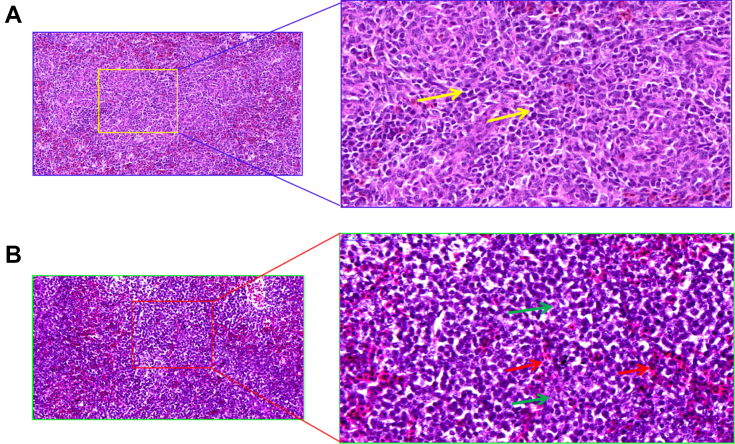
The histology results of the spleen tissues from the chickens are shown on Figure 1. The histology results of the spleen tissues are shown in Figure 1 as the center of cellular and humoral immunity, spleen containing amount of lymphocytes and macrophages, so that we evaluated the effect of NH3 exposure on the spleen. As shown in Figure 1, in the control group, spleen tissue showed clear boundaries between red and white pulps. The white pulp contained abundant lymphocytes and macrophages, and the cell structure was intact as the yellow arrow indicated (Figure 1A). However, In the high NH3 group, the border between the red and white pulp was blurred, trabecular swelling or disappeared, destroyed cell structural (green arrow) (Figure 1B), lymphocytes and macrophages infiltrated, and neutrophils increased (red arrow). The results suggested that NH3 exposure would induce lesion in the pathologic structure of chicken spleen and abnormalities in lymphocytes and macrophages.
Figure 1.
(A) Represents low ammonia (NH3) stimulation, the clearly bounds of white pulps and red pulps, pictures were captured at 400 × , the yellow frame was amplified at 800 × , and it shows the integrated cell structure, and yellow arrows indicated the lymphocytes. (B) Represents the high concentration NH3 exposure, and the picture was captured at 400 × , red arrow was amplified at 800 ×, and it shows the indistinct bounds of pulps and the trabecular swelling, lymphocytes and macrophages infiltrated, and the red arrow indicated neutrophils, and the green arrow indicated destroyed cell structural.
Effect of NH3 Exposure on the mRNA Expression of Th1/Th2-Related Genes in Spleen Tissues
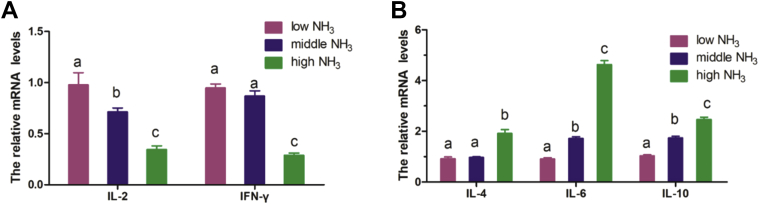
To explore the effects of NH3 exposure on the Th1/Th2 balance in chicken spleens, we measured the mRNA expression of Th1/Th2-related genes by RT-PCR. The results are showed in Figure 2. The mRNA expression of IFN-γ was downregulated in the high NH3 exposure group compared with the low and middle NH3 exposure groups, and IL-2 was expressed significantly among the 3 groups (Figure 2A). The mRNA expression levels of IL-6 and IL-10 were obviously upregulated in the high NH3 and middle NH3 exposure groups when compared with the low NH3 group. However, there was no significant difference in IL-4 expression between the middle NH3 and low NH3 groups, with an increase only in the high NH3 exposure group (Figure 2B). These results indicated a Th1/Th2 imbalance.
Figure 2.
(A) Shows the decrease genes of IL-2 and IFN-γ expression. (B) Indicated the increasing genes of IL-4, IL-6, and IL-10 expression. Each value represents the mean ± SD (n = 10/group). In each histogram, the bars sharing different small letters represent statistically significantly differences between the groups (P < 0.05); the bars with a common letter are not significantly different (P > 0.05).
Effect of NH3 Exposure on the mRNA and Protein Expression of NF-κB, COX-2, iNOS, and TNF-α in Spleen Tissues
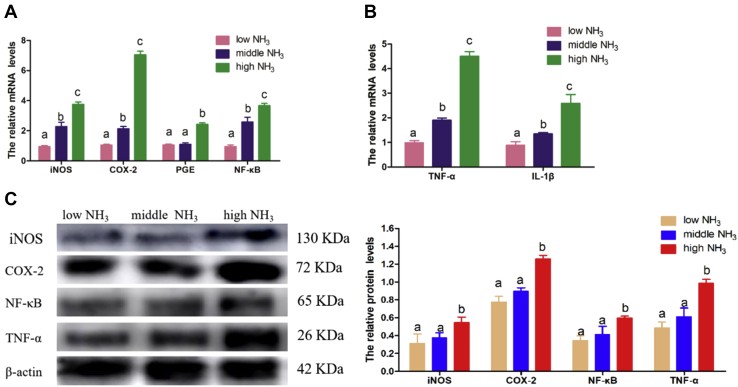
To investigate the role of the NF-κB pathway, we measured the expression of NF-κB and NF-κB pathway–related genes (iNOS, COX-2, and PGE). The results are shown in Figure 3. We found that the mRNA expression of NF-κB, iNOS, and COX-2 were all heightened in an NH3 concentration–dependent manner (Figure 3A). The middle and high NH3 concentrations both had increased PGE mRNA levels, but there was no difference between them when compared. The activation of NF-κB is always accompanied by the proinflammatory factors TNF-α and IL-1β. Therefore, we examined the expression of TNF-α and IL-1β (Figure 3B). The results indicated that TNF-α and IL-1β mRNA levels were increased in the high and middle NH3 stimulation groups, and TNF-α was also different at the protein level (Figure 3C). These results suggested the activation of NF-κB and proinflammatory factors.
Figure 3.
(A) Represents NF-κB and downstream genes expression at mRNA level, NF-κB, iNOS, and COX-2 expressed differently among the 3 groups, PGE was heightened when NH3 stimulation was high; however, there was no difference between middle and low groups. (B) It is the proinflammation factors expression of TNF-α and IL-1β. (C) Represents the protein level of iNOS, COX-2, NF-κB, and TNF-α, and β-actin was selected as internal reference. Bars that do not share the same letters are significantly different (P < 0.05) from each other. Grayscale images are then analyzed by using ImageJ. Bars represent the mean ± SD, n = 5. Abbreviations: COX-2, cyclooxygenase-2; iNOS, nitric oxide synthase; NF-κB, nuclear receptor-κB; PGE, prostaglandin synthetase; TNF-α, tumor necrosis factor-α.
Discussion
Ammonia is a type of harmful gas. Many studies have reported that NH3 has detrimental effects on ecosystems, human health, animal health, and the environment. The precursor of secondary particulate matter (PM2.5) is NH3, which is beneficial for producing PM2.5 (Erisman and Schaap, 2004, Behera, 2012). When NH3 is combined with the oxides of N and S, it produces these particles, resulting in damage to the body and health (Nesta et al., 2015, Zhang et al., 2018). In this study, we found that prolonged exposure to high concentrations of NH3 induced increased cell bleeding and changes to the structural boundaries of spleen cells in chicken. This was followed by a Th1/Th2 imbalance, abnormal expression of related cytokines, and increased expression of proinflammatory genes.
Maintaining the Th1/Th2 balance plays an important role in regulating inflammation. When the Th1/Th2 balance breaks down, the cytokines secreted by Th1/Th2 cells will be abnormally expressed, which always triggers the progression of the inflammation (Hao et al., 2017). Cytokines play an important role in the defense against stimulation or infection. Thanks to the work of many researchers, we know that cytokines have multiple functions, including in cell metabolism, hematopoietic cell proliferation, and differentiation. We found that in inflammatory damage, cytokines including IFN-γ and IL-4/6 were essential (Altan-Bonnet and Mukherjee, 2019). Li et al. also demonstrated that increased IL-4 and decreased IFN-γ expression in the mouse respiratory tract (stimulated by ovalbumin or sulfur dioxide) caused a Th1/Th2 imbalance and led to inflammation (Li et al., 2014). Additionally, the downregulated expression of IFN-γ induced the production of inflammatory factors by traditional T cells, which may further aggravate chronic liver inflammatory responses (Weng et al., 2017). Previous studies also revealed that NH3 exposure regulated the immune response and altered the gut microbial community in crucian carp (Qi et al., 2017). Our results showed that the mRNA expression of IL-4, IL-6, and IL-10 increased and the mRNA expression of IFN-γ decreased under high NH3 exposure, which indicated that NH3 causes an imbalance of Th1/Th2 in the immune system.
TNF-α is a multifunctional cytokine that regulates immune and inflammatory responses and which is also considered an inflammatory marker (Sun et al., 2017). TNF-α always promotes inflammation, even causing necroptosis in chick myocardial cells, as well as noticeably participating in the NF-κB inflammatory pathway (Crusz and Balkwill, 2015, Yang et al., 2017). Similarly, our study showed increased expression of TNF-α under high NH3 exposure compared with the other 2 groups. Activated NF-κB enters the nucleus to induce the production of inflammatory cytokines, such as TNF-α and IL-6, and leads to inflammatory injuries that are usually accompanied by activation of the downstream enzymatic genes COX-2 and iNOS (Yang et al., 2010, Crusz and Balkwill, 2015). These enzymes can form a rich and complex network of inflammatory responses. In addition, Wang et al. confirmed that hydrogen sulfide could induce Th1/Th2 imbalance, trigger NF-κB channels, and aggravate the LPS-induced chicken pneumonia response (Wang et al., 2018). Herein, previous research led us to conclude that the activation of the NF-κB pathway may regulate inflammatory injury after poisonous gas stimulation. Therefore, we examined the expression levels of genes in the NF-κB pathway and found that genes associated with the NF-κB pathway were highly expressed. At the same time, more proinflammatory factors were activated (such as TNF-α and IL-1β, which were highly expressed), which in turn regulates the activation of NF-κB, further aggravating the inflammatory damage (Li et al., 2014, Cao et al., 2016). In this study, the cytokines IL-1β, IL-4, IL-6, and IL-10 were upregulated, INF-γ was downregulated, and the downstream NF-κB pathway genes COX-2, iNOS, and PGE were activated in the high NH3 exposed group, resulting in an imbalance between proinflammatory and anti-inflammatory cytokines in chicken spleens.
In conclusion, this study demonstrated that high concentrations of NH3 exposure can induced morphologic inflammation injuries in broiler chicken spleens. In addition, high concentrations of NH3 exposure caused a Th1/Th2 immune imbalance and activated the NF-κB pathway, which promoted inflammation in the chicken spleens. We hope that these results will provide a theoretical basis for studying the toxicity of NH3.
Acknowledgments
Xu and Li conceived and designed the experiments. Fuqing Zhao, Wei Wang, and Jianping Qu performed the experiments. Fuqing Zhao analyzed the data and wrote the article. Xu assisted in critically revising the manuscript. The authors thank the China Agriculture Research System, Grant/Award Number: CARS-41-17.
The National Key Research and Development Program of China provided funding for these experiments.
Conflict of Interest Statement: No conflicts of interest exist in this manuscript, and the manuscript was read and approved for publication by all authors.
Contributor Information
Shu Li, Email: lishu@neau.edu.cn.
Shiwen Xu, Email: shiwenxu@neau.edu.cn.
References
- Altan-Bonnet G., Mukherjee R. Cytokine-mediated communication: a quantitative appraisal of immune complexity. Nat. Rev. Immunol. 2019;19:205–217. doi: 10.1038/s41577-019-0131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera S.N. Transformation of atmospheric ammonia and acid gases into components of PM2.5: an environmental chamber study. Environ. Sci. Pollut. Res. 2012;19:1187–1197. doi: 10.1007/s11356-011-0635-9. [DOI] [PubMed] [Google Scholar]
- Bonyadi Z., Ehrampoush M.H., Ghaneian M.T., Mokhtari M., Sadeghi A. Cardiovascular, respiratory, and total mortality attributed to PM 2.5 in Mashhad, Iran. Environ. Monit. Assess. 2016;188:570. doi: 10.1007/s10661-016-5574-y. [DOI] [PubMed] [Google Scholar]
- Cao H., Gao F., Xia B., Zhang M., Liao Y., Yang Z., Hu G., Zhang C. Alterations in trace element levels and mRNA expression of Hsps and inflammatory cytokines in livers of duck exposed to molybdenum or/and cadmium. Ecotoxicol. Environ. Saf. 2016;125:93–101. doi: 10.1016/j.ecoenv.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Chang X., Zhu A., Liu F., Zou L., Su L., Li S., Sun Y. Role of NF-ΰB activation and Th1/Th2 imbalance in pulmonary toxicity induced by nano NiO. Environ. Toxicol. 2016;32:1354–1362. doi: 10.1002/tox.22329. [DOI] [PubMed] [Google Scholar]
- Chen M.,H., Li X., Fan R., Cao C., Yao H., Xu S. Selenium antagonizes cadmium-induced apoptosis in chicken spleen but not involving Nrf2-regulated antioxidant response. Ecotoxicol. Environ. Saf. 2017;145:503–510. doi: 10.1016/j.ecoenv.2017.08.001. [DOI] [PubMed] [Google Scholar]
- Chi Q.,R., Liu T., Sun Z., Tan S., Li S., Li S. Involvement of mitochondrial pathway in environmental metal pollutant lead-induced apoptosis of chicken liver: perspectives from oxidative stress and energy metabolism. Environ. Sci. Pollut. Res. 2017;24:28121–28131. doi: 10.1007/s11356-017-0411-6. [DOI] [PubMed] [Google Scholar]
- Crusz S.M., Balkwill F.R. Inflammation and cancer: advances and new agents. Nat. Rev. Clin. Oncol. 2015;12:584–596. doi: 10.1038/nrclinonc.2015.105. [DOI] [PubMed] [Google Scholar]
- Drose S., Brandt U. The mechanism of mitochondrial superoxide production by the cytochrome bc1 complex. J. Biol. Chem. 2008;283:21649–21654. doi: 10.1074/jbc.M803236200. [DOI] [PubMed] [Google Scholar]
- Erisman J.W., Schaap M. The need for ammonia abatement with respect to secondary PM reductions in Europe. Environ. Pollut. 2004;129:159–163. doi: 10.1016/j.envpol.2003.08.042. [DOI] [PubMed] [Google Scholar]
- Hao Y., Li Y., Li H., Lyu M., Zhang D., Fu R., Guan Y., Wang S., Sun B., Dou X. Increased plasma sCXCL16 levels may have a relationship with Th1/Th2 imbalance in primary immune thrombocytopenia. Cytokine. 2017;99:124–131. doi: 10.1016/j.cyto.2017.08.024. [DOI] [PubMed] [Google Scholar]
- Huang D.L., Xu Z.C., Dang X.Q., Zeng X.Q., He X.J., Yi Z.W., He Q.N. [Relationship between renal Th1/Th2 ratio and renal microvascular injury in children with Henoch-Sch-nlein purpura nephritis] Zhongguo Dang Dai Er Ke Za Zhi. 2011;13:273–277. [PubMed] [Google Scholar]
- Jin X., Jia T., Liu R., Xu S. The antagonistic effect of selenium on cadmium-induced apoptosis via PPAR-gamma/PI3K/Akt pathway in chicken pancreas. J. Hazard. Mater. 2018;357:355–362. doi: 10.1016/j.jhazmat.2018.06.003. [DOI] [PubMed] [Google Scholar]
- Kosenko E., Venediktova N., Kaminsky Y., Montoliu C., Felipo V. Sources of oxygen radicals in brain in acute ammonia intoxication in vivo. Brain Res. 2003;981:193–200. doi: 10.1016/s0006-8993(03)03035-x. [DOI] [PubMed] [Google Scholar]
- Li R., Kou X., Tian J., Meng Z., Cai Z., Cheng F., Dong C. Effect of sulfur dioxide on inflammatory and immune regulation in asthmatic rats. Chemosphere. 2014;112:296–304. doi: 10.1016/j.chemosphere.2014.04.065. [DOI] [PubMed] [Google Scholar]
- Liang Z., Liu R., Zhao D., Wang L., Sun M., Wang M., Song L. Ammonia exposure induces oxidative stress, endoplasmic reticulum stress and apoptosis in hepatopancreas of pacific white shrimp ( Litopenaeus vannamei ) Fish. Shellfish Immunol. 2016;54:523–528. doi: 10.1016/j.fsi.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Lu M., Bai J., Xu B., Sun Q.Y., Wei F.X., Tang X.F., Zhang H.F., Li J., Wang G.L., Yin Q.Q. Effect of alpha-lipoic acid on relieving ammonia stress and hepatic proteomic analyses of broilers. Poult. Sci. 2017;96:88–97. doi: 10.3382/ps/pew285. [DOI] [PubMed] [Google Scholar]
- Marta S., Magdalena Z., Jan A. Stimulation of natriuretic peptide receptor C attenuates accumulation of reactive oxygen species and nitric oxide synthesis in ammonia-treated astrocytes. J. Neurochem. 2010;115:1068–1076. doi: 10.1111/j.1471-4159.2010.07008.x. [DOI] [PubMed] [Google Scholar]
- National Research Council . Press; Washington, DC: 1994. Nutrient Requirements of Poultry. 9th rev. ed. Natl. Acad. [Google Scholar]
- Nesta B.S., Yoshinori I., Osei A., Nakayama S.M.M., Yared Beyene Y., Elvis B., Hazuki M., Mayumi I. Levels, potential sources and human health risk of polycyclic aromatic hydrocarbons (PAHs) in particulate matter (PM(10)) in Kumasi, Ghana. Environ. Sci. Pollut. Res. 2015;22:9658–9667. doi: 10.1007/s11356-014-4022-1. [DOI] [PubMed] [Google Scholar]
- Nyati K.K., Prasad K.N., Kharwar N.K., Soni P., Husain N., Agrawal V., Jain A.K. Immunopathology and Th1/Th2 immune response of Campylobacter jejuni-induced paralysis resembling Guillain-Barre syndrome in chicken. Med. Microbiol. Immunol. 2012;201:177–187. doi: 10.1007/s00430-011-0220-3. [DOI] [PubMed] [Google Scholar]
- Qi X.Z., Xue M.Y., Yang S.B., Zha J.W., Wang G.X., Ling F. Ammonia exposure alters the expression of immune-related and antioxidant enzymes-related genes and the gut microbial community of crucian carp (Carassius auratus) Fish. Shellfish Immunol. 2017;70:485. doi: 10.1016/j.fsi.2017.09.043. [DOI] [PubMed] [Google Scholar]
- Shi Q., Wang W., Chen M., Zhang H., Xu S. Ammonia induces Treg/Th1 imbalance with triggered NF-kappaB pathway leading to chicken respiratory inflammation response. Sci. Total. Environ. 2019;659:354–362. doi: 10.1016/j.scitotenv.2018.12.375. [DOI] [PubMed] [Google Scholar]
- Sun Z., Liu C., Pan T., Yao H.D., Li S. Selenium accelerates chicken dendritic cells differentiation and affects selenoproteins expression. Develop. Comp. Immunol. 2017;77:30. doi: 10.1016/j.dci.2017.07.018. [DOI] [PubMed] [Google Scholar]
- Sun Z., Xu Z., Wang D., Yao H.D., Li S. Selenium deficiency inhibits differentiation and immune function and imbalances the Th1/Th2 of dendritic cells. Metallomics. 2018;10:759–767. doi: 10.1039/c8mt00039e. [DOI] [PubMed] [Google Scholar]
- Tak P.P., Firestein G.S. NF-kappaB: a key role in inflammatory diseases. J. Clin. Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talaat R.M., Mohamed S.F., Bassyouni I.H., Raouf A.A. Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: Correlation with disease activity. Cytokine. 2015;72:146–153. doi: 10.1016/j.cyto.2014.12.027. [DOI] [PubMed] [Google Scholar]
- Wang J., Zhao Q., Wang G., Yang C., Xu Y., Li Y., Yang P. Circulating levels of Th1 and Th2 chemokines in patients with ankylosing spondylitis. Cytokine. 2016;81:10–14. doi: 10.1016/j.cyto.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Wang W., Chen M., Jin X., Li X., Yang Z., Lin H., Xu S. H2S induces Th1/Th2 imbalance with triggered NF-kappaB pathway to exacerbate LPS-induce chicken pneumonia response. Chemosphere. 2018;208:241–246. doi: 10.1016/j.chemosphere.2018.05.152. [DOI] [PubMed] [Google Scholar]
- Weng X., He Y., Visvabharathy L., Liao C.M., Tan X., Balakumar A., Wang C.R. Crosstalk between type II NKT cells and T cells leads to spontaneous chronic inflammatory liver disease. J. Hepatol. 2017;67:791–800. doi: 10.1016/j.jhep.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Cao C., Jie Y., Liu T., Xin G.L., Zhang Z., Xu S. miR-200a-5p regulates myocardial necroptosis induced by Se deficiency via targeting RNF11. Redox Biol. 2017;15:159–169. doi: 10.1016/j.redox.2017.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.R., Jiang Y.B., Yin Q.Q., Liang H.D., She R.P. Chicken intestine defensins activated murine peripheral blood mononuclear cells through the TLR4-NF-κB pathway. Veter. Immunol. Immunopath. 2010;133:59–65. doi: 10.1016/j.vetimm.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Zhang H., Li H., Liu L., Zhang Y., Zhang X., Li Z. The potential role of malonic acid in the atmospheric sulfuric acid - ammonia clusters formation. Chemosphere. 2018;203:26. doi: 10.1016/j.chemosphere.2018.03.154. [DOI] [PubMed] [Google Scholar]
- Zhao J.L., Wang X., Wang Y.S. Relationships between Th1/Th2 cytokine profiles and chest radiographic manifestations in childhood Mycoplasma pneumoniae pneumonia. Ther. Clin. Risk Manag. 2016;12:1683–1692. doi: 10.2147/TCRM.S121928. [DOI] [PMC free article] [PubMed] [Google Scholar]