Abstract
Marine microalgae (MA) has received wide attention as a promising source of omega-3 long-chain polyunsaturated fatty acids (n-3 LC-PUFA) enrichment in animal products to improve the health status and wellbeing of the consumers. This study evaluated dynamic changes in n-3 LC-PUFA, color, and functional properties as well as atherogenic and thrombogenic health lipid indices of egg yolk from hens fed graded levels (0, 0.5, and 1.0%) of docosahexaenoic acid (DHA)–rich MA (Aurantiochytrium sp.) during a 56-D feeding period. Egg freshness parameters and yolk lipid oxidative stability were also measured after 0, 14, and 28 D of refrigerated storage. The hen performance and egg quality (except for yolk color) were not affected (P > 0.05) by MA supplementation. Docosahexaenoic acid contents in yolk from hens fed 1.0% MA increased quadratically with feeding time with a plateau at about 30 D (P < 0.05). Afterward, the DHA content leveled off to a constant value (946.3 mg/100 g yolk) with the n-6/n-3 ratio at 3.5: 1. Dietary inclusion of 1.0% of MA also significantly decreased the atherogenic and thrombogenic indices of yolk lipid (P < 0.05). Because the microalgal carotenoids incorporated into egg yolk, the L∗ value of yolk from hens fed MA decreased whereas a∗ value increased (P < 0.05), corresponding to yolk Roche color scores. As expected, there were no significant changes in yolk functional properties (e.g., viscosity and emulsifying activity) related to DHA enrichment (P > 0.05). Microalgal carotenoids enrichment also helped attenuate fatty acid oxidation of the DHA-enriched yolk and increase their lipid oxidative stability. In conclusion, dietary supplementation with up to 1.0% of MA significantly increased DHA contents with more health-promoting n-6/n-3 ratio and atherogenic and thrombogenic indices, as well as more intense yolk color within consumers' acceptability, and the feeding strategy had a minimal impact on yolk physical and functional properties or oxidative stability during subsequent refrigerated storage.
Key words: microalgae, DHA enrichment, functional properties, oxidative stability, laying hen
Introduction
Long-chain omega-3 polyunsaturated fatty acids (n-3 LC-PUFA), and especially docosahexaenoic acid (DHA, C22:6 n-3) are generally accepted to have the potential to prevent several cardiovascular diseases, as well as to promote brain development (Balk and Lichtenstein, 2017). The DHA mainly exists in marine organism, which is difficult to uptake sufficient amount from foods especially for those who have limited access to marine food or do not consume them often. Therefore, the daily recommended intake of n-3 LC-PUFA, more particularly DHA, is rarely met by the majority of consumers worldwide (Lemahieu et al., 2015a). Hen's eggs are very good food product to enrich with DHA, which could provide a health-enhancing alternative food source independently or as an ingredient in processed foods. Traditionally, DHA-enriched eggs are obtained by supplementing fish oil to the hen diets because the fatty acid (FA) profile in yolk is readily modified through alterations of dietary FA (Lemahieu et al., 2014). However, the off-flavors detected in eggs with fish oil supplemented were hard to accept for human consumption. Even when deodorized or microencapsulated fish oil is used, negative effects on the sensory parameters of eggs are still observed (Gonzalez-Esquerra and Leeson, 2000). Additionally, the potential of pollutants also restrict the wide application of fish oil into hen diets.
Heterotrophic or autotrophic microalgae (MA) as the primary natural producers of n-3 LC-PUFA are promising sources to replace fish oil for n-3 LC-PUFA enrichment in animal products (Matos, 2017). A vital difference in fish oil and MA for supplementation of laying hen diets with n-3 LC-PUFA is that MA contain significant amounts of carotenoids (Lemahieu et al., 2014). As the microalgal DHA gets incorporated into egg yolk, carotenoids are simultaneously transferred into the yolk, which may act as antioxidants to preserve the unstable PUFA and thus increase yolk lipid stability. Incorporation of DHA and carotenoids from MA into egg yolk is a gradual process. Knowledge of the dynamic alterations of DHA and carotenoid enrichment is of vital importance to understand the inclusion time and levels of MA supplemented to hen diets, so as to obtain a compromise between DHA content stated on egg's labels and the cost of MA supplementation. However, previous studies focused primarily on the changes in DHA deposition and yolk color at the end of the feeding trial but rarely reported dynamic alterations of the DHA concentration, n-6/n-3 ratio, and yolk color.
Egg yolk is essential for the food industry because of its excellent emulsifying and rheological properties determined by its specific composition, inter alia phospholipid, and proteins (Buxmann et al., 2010). Dietary MA supplementation resulted in changes of yolk PUFA and other chemical compositions, which may also affect their functional properties (Aro et al., 2011). The n-3 PUFA-enriched eggs produced from flaxseed supplementation exhibited less foaming capabilities of whole egg than the conventional eggs, indicating that the functional properties of eggs may be associated with their FA composition (Aro et al., 2011). By studying how DHA enrichment affects FA profile and subsequent egg yolk rheology and stability, we may make better sense of yolk emulsion properties and how these properties are altered by yolk lipid modifications. However, the rheology and stability of DHA-enriched yolk produced from MA supplementation have not been reported.
In addition, lipids in yolk with more PUFA are more susceptible to oxidation, especially during drying or storage (Matumoto-Pintro et al., 2017). Feeding flaxseed or fish oil in hen diets has been found to increase the thiobarbituric acid reactive substances (TBARS) and peroxide values in yolk (Ao et al., 2015). Likewise, the freshness and quality characteristics of n-3 PUFA enriched eggs are prone to deteriorate over time, more particularly in poor storage conditions (Herber and Van Elswyk, 1996). In contrast to previous studies on linseed or fish oils, no significant increases in yolk lipid peroxidation were observed when fed MA presumably because of the microalgal carotenoids acting as antioxidants to increase lipid stability (Pangestuti and Kim, 2011; Ao et al., 2015).
Thraustochytrids Aurantiochytrium sp. are heterotrophic MA with the highest cell densities and DHA productivities (Aasen et al., 2016). In this study, graded levels of a commercial dehydrated MA Aurantiochytrium sp. were included to the diets of laying hens. The objectives of this trial were to (1) determine the dynamic alterations of DHA incorporation, n-6/n-3 ratio and yolk color; (2) compare the functional properties of fresh DHA-enriched yolk with the control; and (3) measure the egg freshness characteristics and yolk oxidative stability during refrigerated storage. We hypothesized that such nutrient enrichment would result in DHA-enriched yolk but with minimum alterations in other physical and functional properties and lipid oxidation stability of egg yolk.
Materials and methods
Animals and Diet Formulation
The experimental procedures were in accordance with the Chinese Guidelines for Animal Welfare and approved by the Institutional Animal Care and Use Committee of Zhejiang University (Hangzhou, China). A total of 360 healthy hens (HY-Line Brown, 26-wk-old) with similar body weight and performance were used in the 10-wk feeding trial. Birds were maintained in an environmentally controlled room with a constant temperature of 23 ± 3°C and a relative humidity of 60 to 70%. Birds were housed in a 2-tier battery cage system with 3 birds per cage (45 × 45 × 50 cm, providing 675 cm2 per bird) under a 16:8-h light-dark cycle. Hens had free access to feed and water. After 14 D adaption to the new environmental conditions and the commercially available standard diet, birds were randomly assigned into 3 dietary treatments with 8 replicates of 15 birds each. Each replicate consisted of 5 adjacent cages with 3 birds per cage. Treatments were consisted of feeding a corn–soybean meal standard diet alone (control) or provided with 0.5 or 1.0% MA (Aurantiochytrium sp., Alltech Inc., New York, NY). The commercially available standard diet formulated to meet the NRC (1994) requirements. All diets were formulated to be isocaloric and isonitrogenous containing 2.75 Mcal/kg ME and 16.80% CP, respectively. The formulation and nutrient levels of the experimental diets are shown in Table 1. The supplemented MA (Aurantiochytrium sp.) contained 64.85% crude fat, 18.45% DHA, and 12.10% CP (analyzed values). The analyzed major FA content of MA (Aurantiochytrium sp.) and the experimental diets are listed in Table 2. All experimental diets were prepared fresh weekly and stored in a cool area until the feed was utilized.
Table 1.
Ingredients and nutrient levels of experimental diets for laying hens (air-dry basis).
| Item | Control | 0.5%MA | 1.0%MA |
|---|---|---|---|
| Ingredients, % | |||
| Corn | 59.70 | 59.55 | 59.40 |
| Soybean meal | 26.50 | 26.40 | 26.30 |
| Soybean oil | 2.00 | 1.75 | 1.50 |
| Microalgae | 0 | 0.50 | 1.00 |
| Limestone | 8.50 | 8.50 | 8.50 |
| CaHPO4 | 1.00 | 1.00 | 1.00 |
| Salts | 0.30 | 0.30 | 0.30 |
| DL-Methionine | 0.20 | 0.20 | 0.20 |
| Lysine-HCl | 0.05 | 0.05 | 0.05 |
| Premix1 | 1.75 | 1.75 | 1.75 |
| Nutrient levels2 | |||
| ME, Mcal/kg3 | 2.75 | 2.75 | 2.75 |
| Crude protein, % | 16.80 (16.84) | 16.80 (16.82) | 16.80 (16.83) |
| Crude fat, % | 4.65 (4.64) | 4.67 (4.66) | 4.68 (4.65) |
| Ca, % | 3.66 (3.63) | 3.66 (3.62) | 3.66 (3.64) |
| Total phosphorus, % | 0.62 (0.65) | 0.62 (0.65) | 0.62 (0.64) |
| Available phosphorus, % | 0.35 | 0.35 | 0.35 |
| Lysine, % | 0.91 | 0.91 | 0.91 |
| Methionine, % | 0.45 | 0.45 | 0.45 |
| Cysteine + Methionine, % | 0.77 | 0.77 | 0.77 |
Abbreviation: MA, microalgae.
The premix provided the following per kilogram of diet: VA, 6,250 IU; VD3, 3,125 IU; VE, 15 IU; VK, 2 mg; thiamine, 1 mg; riboflavin, 8.5 mg; calcium pantothenate, 50 mg; niacin, 32.5 mg; pyridoxine, 8 mg; folate, 5 mg; VB, 125 mg; choline, 500 mg; Fe, 60 mg; Cu, 8 mg; Mn, 65 mg; Zn, 60 mg; Se, 0.3 mg; and I, 1 mg.
The values in parentheses indicated the analyzed value. Others are calculated values.
The ME values were estimated from Chinese feed database provided with tables of feed composition and nutritive values in China (28th edition, 2017).The ME of Aurantiochytrium sp. was 6,057 kcal/kg calculated with the formula: ME (kcal/kg) = 53 + 38 [CP (%) + 2.25 × crude fat (%)] (Carpenter and Clegg, 1956).
Table 2.
Major fatty acid profile in microalgae (Aurantiochytrium sp.) and the experimental diets.1
| Fatty acid content (mg/g) | Microalgae | Control | 0.5%MA | 1.0% MA |
|---|---|---|---|---|
| C16:0 Palmitic acid | 356.50 | 6.72 | 8.03 | 9.26 |
| C18:0 Stearic acid | 12.08 | 1.56 | 1.59 | 1.62 |
| C18:1 n-9 Oleic acid | 2.05 | 8.20 | 8.56 | 8.85 |
| C18:2 n-6 Linoleic acid | 0.76 | 16.05 | 15.97 | 15.59 |
| C18:3 n-3 α-linolenic acid | 10.52 | 1.00 | 1.05 | 1.04 |
| C20:5n-3 Eicosapentaenoic acid | 1.50 | 0.02 | 0.03 | 0.03 |
| C22:6 n-3 Docosahexaenoic acid | 184.5 | 0.02 | 0.94 | 1.85 |
Abbreviation: MA, microalgae.
Values are the means of 4 analyses per sample.
Laying Performance
Egg production and mass were monitored daily, and feed disappearance was recorded weekly on a replicate basis (8 replicates per dietary treatment) to calculate the laying rate, average daily egg mass, average daily feed intake, and feed conversion ratio (feed/egg: g/g).
Egg Sample Collection and Preparation
Eggs collected every 3 D for the first 36 D and every 6 D for the remaining days were used for DHA content, FA profile, and yolk color analysis. For sample preparation, the yolks were separated from the albumen and then rolled on a filter paper (Whatman) to remove traces of albumen. After egg yolk color determination, 5 yolks from each replicate were mixed, and the pooled yolk samples were then stored at −20°C until analyses. In addition, total carotenoids and cholesterol contents and functional properties of the obtained fresh samples on 56 D of the supplementation period were also determined. Another batch of eggs were collected, labeled, and then stored at 4°C for 0, 14, and 28 D for determination of egg freshness–related characteristics and yolk oxidative stability.
Egg Quality Parameters Measurement
The egg quality parameters were determined by a digital egg tester DET6000 (Kyoto, Japan) on 5 eggs per replicate, and the measurements taken included whole egg weight (g), albumen height (mm), Haugh unit (HU), and eggshell strength (kg/cm2). Then, the yolk and albumen were individually weighed, and the ratios of albumen, yolk, and eggshell to egg weight were calculated. Yolk index (YI) was calculated as yolk height divided by yolk diameter. Eggshell thickness (mm) was measured (without shell membrane) with an egg shell thickness gauge (Robotmation Co. Ltd., Tokyo, Japan) at 3 points (air cell, equator, and sharp end). The color changes of egg yolk were determined by a Minolta Chroma Meter CR-300 (Konica Minolta Optics, Inc., Osaka, Japan) in a CIELAB space (L∗, lightness; a∗, redness; and b∗, yellowness). Meanwhile, the yolk color changes were also scored visually by comparison with a Roche yolk color fan (range 1-15, DSM, Basel, Switzerland).
Determination of DHA Content and Fatty Acid Profile
Fatty acids were extracted from the pooled yolk with chloroform/methanol (2:1, v/v) by the modified method of Folch et al. (1957). The fatty acid methyl esters prepared and quantified according to the method of Hansen et al. (2015) with an internal standard (C11: 0, Nu-Chek-Prep Inc., Elysian, MN) were added to allow quantification of DHA content. Separation and quantification of these fatty acid methyl esters were performed by gas chromatography with an Agilent 6890A(Hewlett-Packard, Palo Alto, CA) following the protocol as we previously described (Liu et al., 2019). Fatty acid profile was determined on 8 replicates per treatment, with each replicate consisting of a pool of 5 egg yolks. The DHA values were reported as mg/100 g yolk, and FA profiles were reported as percentage of the total FA.
Health Lipid Indices of Egg Yolk
Fatty acids in yolk are grouped in classes based on the double bonds as saturated FA (SFA), unsaturated FA (UFA), monounsaturated FA (MUFA), and PUFA. The health profile of DHA-enriched yolk was measured considering the n-6/n-3 PUFA, MUFA/SFA, UFA/SFA, and PUFA/SFA ratios. The saturation index (S/P), atherogenic index (AI), and thrombogenic index (TI) of yolk lipid were calculated as follows (Omri et al., 2019): S/P = (C14:0 + C16:0 + C18:0)/(MUFA + PUFA); AI = (4 × C14:0 + C16:0 + C18:0)/(MUFA + PUFA); and TI = (C14:0 + C16:0 + C18:0)/[(MUFA + n-6 PUFA)/2 + 3 × n-3 PUFA + n-3: n-6].
Total Carotenoid and Cholesterol Quantification
The contents of total carotenoids in yolk collected on 56 D of the trial were analyzed by the method of Islam and Schweigert (2015). Yolk total carotenoid contents equivalent to ug ß-carotene per g sample was calculated by using the extinction coefficients E1cm1% = 2,550. The cholesterol contents were measured by an enzymatic spectrophotometric method as described by Petrović et al. (2012) with a commercial reagent kit (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China). Eight replicates of 5 pooled yolk samples per treatment were determined.
Raw Yolk Viscosity and Emulsification Properties Measurement
Fresh yolk viscosity was assayed according to the method of Walker et al. (2012) by a TA Instruments DHR2 Rheometer (TA Instruments, New Castle, DE) with parallel geometry sensor (40 mm diameter, 1 mm gap). Two viscosity tests of yolk samples were measured at 25°C. The first test involved changing the shear rate linearly from 0.10 s−1 to 60.00 s−1 for 30 s, and the data were fitted to the Eq: τ = κ × (γ/γo)n, where τ is the shear stress (Pa), κ is the consistency index (Pa), γ is the shear rate (s−1), γo is the constant shear rate (s−1), and n is the flow index. The second test conducted at a constant shear rate at 60.00 s−1 for 60 s to measure the apparent viscosity, which can be calculated by the equipment software. The emulsion activity (EA) and emulsion stability (ES) of fresh yolk were measured as described by Gouda et al. (2017). Eight replicates of 5 pooled yolks per treatment were determined.
TBARS Measurement
The TBARS concentrations of egg yolk were measured after 0, 14, and 28 D of refrigerated storage using the method described by Goliomytis et al. (2014). Quantification of TBARS values was conducted by determining the absorbance at 540 nm using a UV/Vis spectrophotometer (Hitachi UV-3100, Tokyo, Japan) according to the standard curve of malondialdehyde obtained from the hydrolysis of 1,1,3,3-tetramethoxypropane. The TBARS values were expressed as mg malondialdehyde/kg yolk.
Statistical Analysis
Data were subjected to the General Linear Model procedure of SPSS 20.0 software (SPSS, Inc., Chicago, IL) with each replicate as an experimental unit. One-way ANOVA was used to compare laying performance, egg quality parameters, FA composition, total cholesterol and carotenoid contents, and functional properties of yolk. Two-way ANOVA was conducted to compare egg freshness parameters and lipid oxidative stability during refrigerated storage. Statistical models included the effects of dietary treatments, refrigerated storage days, and the interactions between these 2 factors. Significant differences at 5% among treatment means were evaluated using Tukey's multiple range test.
Results and discussion
Laying Performance and Egg Quality Characteristics
Compared with the control group, laying rate, average daily egg mass, average daily feed intake, and feed conversion ratio were not affected (P > 0.05) by different levels of MA (Aurantiochytrium sp.) supplementation (Table 3), which was consistent with previous studies supplemented with different species of MA to laying hens, such as DHA-enriched heterotrophic MA Schizochytrium limacinum (Ao et al., 2015) and EPA-containing autotrophic MA Nannochloropsis sp. (Bruneel et al., 2013; Wu et al., 2019). However, Park et al. (2015) found that dietary supplementation with 1.0% Schizochytrium sp. showed positive effects on hen production. On the contrary, Herber and Van Elswyk (1996) observed feeding 4.8% heterotrophic MA to hens decreased laying performance compared with the blank control. These different effects of MA on hen performance might be attributed to the differences in composition of MA, the MA inclusion levels, feed formulation, and hen's age and strain. No significant differences (P > 0.05) were observed in albumen percentage, yolk percentage, shell percentage, albumen height, HU, eggshell strength, or eggshell thickness among treatments (Table 4). Results from similar trials on laying hens (Moran et al. 2019; Yonke and Cherian, 2019) agreed that DHA-enriched MA supplementation did not affect the egg quality parameters (except for yolk color), indicating the suitability of MA as a feed ingredient.
Table 3.
Production performance of laying hens fed graded levels of microalgae (Aurantiochytrium sp.) over 56-D feeding period.1
| Item | Control | 0.5%MA | 1.0 MA% | SEM | P-value |
|---|---|---|---|---|---|
| LR, % | 92.75 | 93.87 | 93.98 | 0.43 | 0.101 |
| ADFI, g/hen/D | 111.84 | 111.21 | 110.66 | 1.31 | 0.346 |
| ADEM, g/hen/D | 56.00 | 56.46 | 56.80 | 0.48 | 0.502 |
| FCR, g/g | 2.00 | 1.97 | 1.95 | 0.02 | 0.328 |
Abbreviations: ADEM, average daily egg mass; ADFI, average daily feed intake; FCR, feed conversion ratio (average daily feed intake: average daily egg mass); LR, laying rate; MA, microalgae.
Values are means of 8 replicates of 15 hens each.
Table 4.
Egg quality parameters of laying hens fed graded levels of microalgae (Aurantiochytrium sp.) for 56 D.1
| Item | Control | 0.5%MA | 1.0 MA% | SEM | P-value |
|---|---|---|---|---|---|
| Albumen percentage, % | 64.12 | 64.18 | 64.41 | 0.45 | 0.895 |
| Yolk percentage, % | 24.59 | 24.42 | 24.43 | 0.28 | 0.884 |
| Shell percentage, % | 11.28 | 11.40 | 11.17 | 0.28 | 0.838 |
| Albumen height, mm | 8.61 | 8.59 | 8.34 | 0.16 | 0.461 |
| Haugh unit | 92.60 | 92.54 | 91.23 | 0.83 | 0.415 |
| Eggshell strength, kg/cm2 | 4.42 | 4.40 | 4.50 | 0.21 | 0.947 |
| Eggshell thickness, mm | 0.38 | 0.38 | 0.39 | 0.01 | 0.925 |
Abbreviation: MA, microalgae.
Values are the means of 8 replicates of 5 eggs each.
Dynamics of DHA Enrichment and n-6/n-3 Ratio Alterations in Yolk
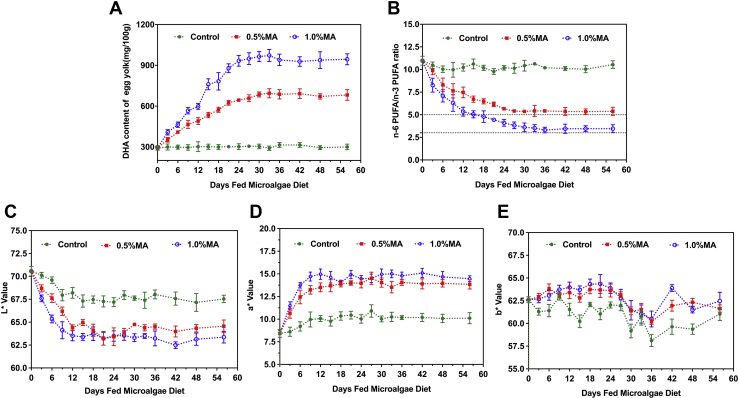
The dynamics of DHA accumulation in fresh yolk from hens fed graded levels of MA over 56-D feeding period are shown in Figure 1A. The DHA contents of control eggs remained constant (on average 300.8 mg DHA/100 g yolk) throughout the feeding trial. As would be expected, microalgal DHA supplementation to hen diets consequently produced dose-dependent enrichments of DHA in yolks (P < 0.05), which was in good agreement with the findings of Manor et al. (2019) with a defatted Nannochloropsis oceanica MA. Docosahexaenoic acid contents in yolk from hens fed 1.0% MA reached the peak (949.3 mg DHA/100 g yolk) first after 27 D, whereas DHA contents in yolk reached the peak (694.9 mg DHA/100 g yolk) of hens fed 0.5% MA after 33 D, after which DHA level reached a plateau (683.1 and 946.3 mg DHA/100 g yolk, for 0.5 and 1.0% MA supplementation, respectively) that was sustained throughout the experiment. Between day 0 and the day at which reaching the highest concentration, linear increases of the enrichment were observed with the extension of feeding time, supporting previous studies (Lemahieu et al., 2015b; Keegan et al., 2019). These results indicated that around 30 D of MA inclusion appeared to be sufficient to stabilize the incorporation of DHA in egg yolk. Similar results were reported by Hargis et al. (1991) and Keegan et al. (2019), who also observed the minimum time was 4 wk to allow yolk DHA enrichment reaching the plateau. However, other studies showed a maximum n-3 PUFA incorporation was observed after 2 or 3 wk of Phaeodactylum tricornutum, Isochrysis galbana, and Chlorella fusca (Lemahieu et al., 2014) or Nannochloropsis sp. (Kim et al., 2016; Wu et al., 2019) supplementation. This discrepancy may be because of the differences in microalgal species and hen's age and strain. After reaching the plateaus, DHA contents in egg yolk from hens fed 1.0% MA were approximately 2-fold increase over the control eggs. In addition, compared with 0.5% MA supplementation, doubling the inclusion levels of MA in hen diet did not result in double DHA content in yolk (384.7 vs. 648.6 mg DHA/100 g yolk). From the results, we can conclude that the MA supplemental levels influence the DHA incorporation efficiency, a decreasing conversion efficiency of DHA enrichment in the yolk with the increasing inclusion levels, supporting the previous findings (Bruneel et al., 2013; Keegan et al., 2019; Feng et al., 2020).
Figure 1.
Dynamics of DHA enrichment (A), n-6/n-3 ratio (B), L∗ value (C), a∗ value (D), and b∗ value (E) of fresh egg yolk from laying hens fed graded levels of microalgae (Aurantiochytrium sp.) over 56-D feeding period. Data points are treatment means and standard error bars (n = 8). Abbreviations: DHA, docosahexaenoic acid; MA, microalgae.
The n-6/n-3 PUFA ratio is a vital parameter in terms of human health because of their different metabolic effects (Lemahieu et al., 2014). Dramatic alterations in n-6/n-3 ratio were also determined and are presented in Figure 1B. The n-6/n-3 ratio of control eggs did not significantly change (P > 0.05) during the experiment and that was in good accordance with a previous study (Petrović et al., 2012). Yolk from hens fed 0.5 and 1.0% MA diets exhibited constant reductions in n-6/n-3 ratio until reaching stabilization in the 30th D of the supplementation period. This trend corresponded to the results obtained in yolk DHA enrichment in Figure 1A. After reaching the plateaus, the n-6/n-3 ratios decreased from the usual value in control yolk (10.8: 1) to the values below 5: 1, which was in good agreement with the results with flaxseed or fish oil supplementation (Petrović et al., 2012; Kralik et al., 2015). As expected, the largest decrease was obtained by 1.0% MA supplementation, and the ultimate ratio stabilized at 3.5: 1, which is in agreement with the recommended ratio for human health purposed (Simopoulos, 2008).
Total Cholesterol Contents and Fatty Acid Composition in Yolk
Owing to the risk of cholesterol for cardiovascular disease, more and more researchers have tried to reduce its amount in egg yolk (Viveros et al., 2007). No remarkable differences were observed in total cholesterol content of yolk across the treatments (Table 5, P > 0.05). These results were in accordance with the findings of Ebeid et al. (2008) and Wu et al. (2019), who also found that dietary n-3 PUFA was not able to reduce the cholesterol contents in yolk. This is presumably because of the physiological control mechanisms that ultimately leads to the production cessation when the cholesterol content is inadequate for embryo survival (Milinsk et al., 2003). Fatty acid profile in yolk at the end (day 56) of the feeding period is shown in Table 5. Yolk FA composition was significantly affected by graded levels of MA supplementation (P < 0.05). Compared with the control, yolks from hens supplemented with 0.5 and 1.0% MA significantly increased DHA (C22:6 n-3) contents, whereas remarkably decreased yolk FA C18:1 n-9, C18:3 n-6, C20:1 n-9, C20:3 n-6, and C20:4 n-6 contents (P < 0.05). The reduction in n-6 PUFA concentrations (especially C20:3 n-6 and C20:4 n-6 contents) caused by MA supplementation were attributed to the competition of substrates and biosynthesis enzymes between the n-3 and n-6 PUFA synthesis pathways (Jia et al., 2008). Consequently, total n-3 PUFA levels increased as the inclusion levels of MA increased (P < 0.05). Concomitant with the reductions in MUFA content, total PUFA was remarkably increased (P < 0.05), especially in yolk from hens fed 1.0% MA. Similar results were observed by Petrović et al. (2012) and Baeza et al. (2015).
Table 5.
Fatty acid composition of pooled egg yolk from laying hens fed graded levels of microalgae (Aurantiochytrium sp.) for 56 D.1
| Item | Control | 0.5%MA | 1.0%MA | SEM | P-value |
|---|---|---|---|---|---|
| Cholesterol, mg/g yolk | 13.33 | 12.99 | 12.51 | 0.282 | 0.222 |
| Fatty acids, % of total fat | |||||
| C14:0 Myristic acid | 0.35 | 0.34 | 0.341 | 0.01 | 0.731 |
| C16:0 Palmitic acid | 25.14 | 24.54 | 24.07 | 0.42 | 0.194 |
| C16:1 n-7 Palmitoleic acid | 4.71 | 5.10 | 4.82 | 0.22 | 0.531 |
| C18:0 Stearic acid | 8.97 | 8.65 | 9.10 | 0.25 | 0.424 |
| C18:1 n-9 Oleic acid | 40.84a | 39.30b | 38.70b | 0.26 | 0.004 |
| C18:2 n-6 Linoleic acid | 14.57 | 15.81 | 15.70 | 0.38 | 0.054 |
| C18:3 n-6 γ-Linolenic acid | 0.18a | 0.13b | 0.15b | 0.01 | 0.001 |
| C18:3 n-3 α-Linolenic acid | 0.38 | 0.41 | 0.46 | 0.02 | 0.101 |
| C20:0 Arachidic acid | 0.09 | 0.08 | 0.09 | 0.01 | 0.162 |
| C20:1 n-9 Gadoleic acid | 0.44a | 0.34b | 0.31b | 0.01 | <0.001 |
| C20:2 n-6 Eicosadienoic acid | 0.13 | 0.13 | 0.12 | 0.01 | 0.333 |
| C20:3 n-6 Eicosatrienoic acid | 0.23a | 0.13b | 0.14b | 0.01 | 0.001 |
| C20:4 n-6 Arachidonic acid | 2.74a | 1.96b | 1.60c | 0.11 | <0.001 |
| C20:5 n-3 Eicosapentaenoic acid | 0.05 | 0.05 | 0.06 | 0.01 | 0.856 |
| C22:6 n-3 Docosahexaenoic acid | 1.21c | 3.10b | 4.50a | 0.08 | <0.001 |
| SFA | 34.54 | 33.60 | 33.59 | 0.41 | 0.192 |
| MUFA | 45.99a | 44.73b | 43.83b | 0.33 | 0.001 |
| PUFA | 19.35b | 21.61a | 22.60a | 0.37 | 0.008 |
| Total n-6 PUFA2 | 17.84 | 18.16 | 17.71 | 0.37 | 0.673 |
| Total n-3 PUFA3 | 1.65c | 3.57b | 5.00a | 0.06 | <0.001 |
Abbreviations: MA, microalgae; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids.
Means (8 replicates of 5 pooled yolks) with different superscripts within the same row differs significantly (P < 0.05).
Total n-6 PUFA = C18:2 n-6 + C18:3 n-6 + C20:2 n-6+ C20:3 n-6 + C20:4 n-6.
Total n-3 PUFA = C18:3 n-3 + C20:5 n-3 + C22:6 n-3.
Health Lipid Indices of Egg Yolk
The consumption of FA has a direct influence on the stimulation or preclusion of atherosclerosis and coronary thrombosis because of their effects on blood cholesterol and low-density lipoprotein cholesterol contents (Ulbricht and Southgate, 1991). The health lipid indices, including n-6/n-3, PUFA/SFA, MUFA/SFA, and UFA/SFA ratios, as well as AI and TI are vital parameters for evaluating the healthiness of lipid for human consumption. The health indices of yolk lipid are summarized in Table 6. It was observed that dietary supplementation with 1.0% MA remarkably increased the PUFA/SFA and MUFA/SFA ratios (P < 0.05), whereas significantly decreased the n-6/n-3 PUFA ratio, AI, and TI (P < 0.05). Generally, dietary PUFA/SFA ratio above 0.45 and n-6/n-3 PUFA ratio below 4.0 are required to prevent coronary heart disease, because balanced dietary PUFA/SFA and n-6/n-3 ratios are vital in regulating blood cholesterol (Simopoulos, 2008). In this study, the PUFA/SFA ratios (0.67) of yolk form hens fed 1.0%MA were higher than the recommended values, whereas the n-6/n-3 ratios (3.52) were within the recommended levels, indicating the FA in the obtained yolk have a protective action against coronary heart diseases (Simopoulos, 2008) and can be recommended as a healthy PUFA source.
Table 6.
Health-related indices of pooled egg yolk from laying hens fed graded levels of microalgae (Aurantiochytrium sp.) for 56 D.1
| Item | Control | 0.5%MA | 1.0%MA | SEM | P-value |
|---|---|---|---|---|---|
| n-6/n-3 PUFA ratio | 10.92a | 5.10b | 3.53c | 0.33 | <0.001 |
| PUFA/SFA ratio | 0.56b | 0.64a | 0.67a | 0.02 | <0.001 |
| MUFA/SFA ratio | 1.33 | 1.33 | 1.31 | 0.02 | 0.626 |
| UFA/SFA ratio | 1.90 | 1.98 | 1.98 | 0.04 | 0.179 |
| Saturation index (S/P) | 0.53 | 0.51 | 0.51 | 0.01 | 0.170 |
| Atherogenic index (AI) | 0.41a | 0.39a,b | 0.37b | 0.01 | 0.008 |
| Thrombogenic index (TI) | 0.73a | 0.71a | 0.69b | 0.01 | 0.003 |
Abbreviations: MA, microalgae; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids; UFA, unsaturated fatty acid.
Means (8 replicates of 5 pooled yolks) with different superscripts within the same row differs significantly (P < 0.05).
Atherogenic index and TI are vital parameters indicating the potential for stimulating platelet aggregation, which defined as the relationships between proatherogenic FA and antiatherogenic FA and relationships between prothrombogenetic FA and antithrombogenetic FA, respectively (Wołoszyn et al., 2020). From the perspectives of human health, the AI and TI recommended for human consumption are less than 0.5 and 1.0, respectively (Wołoszyn et al., 2020). In this study, the yolks showed AI and TI of 0.37–0.41 and 0.69–0.73, which were within the recommended ranges. The lowest AI and TI was observed in yolks from hens fed 1.0%MA, which is very desirable for human health because of the great protective potential for coronary artery disease.
Yolk Color Enrichment and Total Carotenoid Contents
As shown in Figure 1C–E, not only an enrichment of DHA but also significant color changes in yolk were observed with MA supplementation (P < 0.05). Remarkable yolk color changes were observed in the first 6 D of feeding MA diets as compared with the control, and the values reached a plateau after 12 D, after which it was sustained. Similar results were reported by Bruneel et al. (2013). Before reaching the plateau, L∗ values decreased, whereas a∗ values increased drastically with the extended feeding time and increasing MA inclusion levels, corresponding to the decreased lightness and increased redness of the egg yolk. Remarkable alterations in L∗ and a∗ values may be perceived differently by eye, so we therefore scored the yolk color using Roche values as well. Table 7 summarizes the color values of the yolk obtained at the end of the supplementation period. In general, MA supplementation to hens significantly increased the Roche value of yolk (P < 0.05). Similar results were obtained by supplementing Schizochytrium sp. or other species of MA supplementation (Herber and Van Elswyk, 1998; Lemahieu et al., 2014; Ao et al., 2015). The total carotenoid contents in yolk, after 56 D feeding, were determined and are shown in Table 7. Significant increases were observed in total carotenoid contents in DHA-enriched yolk as compared with the control, regardless of the inclusion levels (P < 0.05). Based on the results of carotenoid contents and other studies with Aurantiochytrium sp. MA, the color shift by graded levels of MA supplementation can presumably be explained by the incorporation of microalgal carotenoids into yolk, specifically canthaxanthin and β-carotene (Herber and Van Elswyk, 1998). It should be noted that the incorporation of certain carotenoids may result in a decreased consumers' acceptability. In the present study, egg yolk scores ranged from 7 to 10, which were within consumers' acceptability, indicating that supplementation with up to 1.0% MA did not result in unacceptable yolk color (Lemahieu et al., 2014).
Table 7.
Yolk color and carotenoid enrichment in egg yolk of laying hens fed graded levels of microalgae (Aurantiochytrium sp.) for 56 D.1
| Item | Control | 0.5%MA | 1.0 MA% | SEM | P-value |
|---|---|---|---|---|---|
| Yolk color | |||||
| Roche value 2 | 7.50b | 8.63a | 9.38a | 0.34 | 0.003 |
| CIELAB value | |||||
| L∗ | 67.08a | 64.57b | 63.00b | 0.57 | <0.001 |
| a∗ | 10.11b | 13.84a | 14.46a | 0.21 | <0.001 |
| b∗ | 61.03 | 61.64 | 62.48 | 0.53 | 0.129 |
| Yolk total carotenoids | 27.63b | 30.54a | 31.93a | 0.718 | 0.003 |
Abbreviation: MA, microalgae.
Means (8 replicates of 5 eggs each) with different superscripts within the same row differs significantly (P < 0.05).
The Roche value was scored according to Roche yolk color fan (range 1-15, DSM, Basel, Switzerland).
Yolk Functional Properties
Dietary DHA supplementation resulted in changes of PUFA and other chemical composition in yolk, which may give rise to changes in technological characteristics of yolk. Therefore, the viscosity and emulsification properties of DHA-enriched yolk were measured. As shown in Table 8, no significant differences were observed in the apparent viscosity, consistency index, or flow index of the raw yolk across the treatments (P > 0.05). In addition, the flow index in this study is between 0 and 1, illustrating a shear thinning profile. These results were in accordance with the findings of Walker et al. (2012), who reported that yolk nutrient enrichment with MA did not significantly affect their viscosity. However, other studies have shown that the apparent viscosity of yolk was associated with FA compositions, especially n-3 PUFA composition and the n-6/n-3 ratio (Shinn et al., 2015). Understanding these different changes in viscosity will require further compositional and microstructural studies of the yolk. There were no significant differences in EA or ES of fresh egg yolk among treatments (P > 0.05), indicating that DHA enrichment has not changed the emulsification properties of egg yolk. Similar results were also reported (Leskanich and Noble, 1997). However, other study had reported n-3 PUFA incorporation with fish or flaxseed oils increased the whole egg EA but decreased its ES (Aro et al., 2011). Our study mainly focused on FA composition alterations and their effects on the yolk functional properties. Further studies are needed to investigate the potential alterations in protein type, proportion, and functionality as caused by nutrient enrichment.
Table 8.
Functional properties (viscosity and emulsification properties) of fresh yolk from laying hens fed graded levels of microalgae (Aurantiochytrium sp.) for 56 D.1
| Item | Control | 0.5%MA | 1.0%MA | SEM | P-value |
|---|---|---|---|---|---|
| Viscosity, Pa·s | 2.03 | 2.09 | 2.12 | 0.12 | 0.368 |
| Consistency index, Pa | 4.53 | 4.88 | 5.39 | 0.37 | 0.324 |
| Flow index | 0.57 | 0.57 | 0.57 | 0.01 | 0.982 |
| Emulsion activity, Abs | 0.49 | 0.50 | 0.51 | 0.01 | 0.588 |
| Emulsion stability, min | 88.79 | 91.19 | 91.81 | 1.77 | 0.462 |
Abbreviation: MA, microalgae.
Values are the means of 8 replicates of 5 pooled yolks.
Egg Freshness Parameters and Oxidative Stability During Storage
Albumen height, HU, and YI are acceptable measures of shell egg freshness parameters (Adeniyi et al., 2016). Lipid oxidation stability of the yolk was evaluated based on the TBARS value. Egg freshness parameters and lipid oxidation (TBARS) measured after 0, 14, and 28 D of refrigerated storage are shown in Table 9. There were no significant differences in albumen height, HU, YI, or TBARS values across the treatments (P > 0.05), indicating that dietary supplementation of up to 1.0% MA did not significantly affect egg physical quality and lipid oxidative stability. This supported the previous study of Ao et al. (2015). Nevertheless, other studies with flaxseed or fish oil supplementation to hens observed increased TBARS values in fresh yolk (Hayat et al., 2010; King et al., 2012). An important difference regarding the inclusion of fish or linseed oil and MA for n-3 PUFA enrichment is that MA contain lots of carotenoids (Pangestuti and Kim, 2011). As mentioned above, the total carotenoids increased in DHA-enriched yolk, which were associated with the matrix of the yolk lipids, helping attenuate oxidation of FA. This can presumably explain non-significant effects of DHA enrichment on lipid oxidative stability of yolk in this study. In addition, albumen height, HU, and YI values showed a significant reduction, whereas TBARS value showed an increase with the extension of storage time up to 28 D (P < 0.05), in agreement with previous studies (Mohiti-Asli et al., 2008; Li et al., 2017). No significant differences were observed in the internal qualities and oxidative stability of the eggs influenced by the interactions between storage days and dietary treatment (P > 0.05). These results validated our hypothesis that there were minimal changes in the quality properties and lipid oxidative stability of eggs up to 1.0% of MA supplementation for DHA enrichment.
Table 9.
Egg freshness parameters and yolk lipid oxidation (TBARS) during refrigerated storage at 4°C for 0, 14, and 28 D.1
| Item | Diet (D) |
SEM | Storage days (SD) |
SEM |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0.5%MA | 1.0%MA | 0 | 14 | 28 | D | SD | D∗SD | |||
| Albumen height, mm | 7.43 | 7.54 | 7.40 | 0.137 | 8.37a | 7.37b | 6.33c | 0.137 | 0.732 | <0.001 | 0.875 |
| Haugh unit | 84.62 | 83.99 | 83.47 | 0.632 | 92.01a | 82.79b | 77.28c | 0.632 | 0.443 | <0.001 | 0.938 |
| Yolk index | 49.82 | 49.73 | 49.63 | 0.378 | 51.34a | 49.38b | 48.45b | 0.378 | 0.941 | <0.001 | 0.996 |
| TBARS, mg MDA/kg yolk | 0.134 | 0.134 | 0.136 | 0.003 | 0.102c | 0.132b | 0.170a | 0.003 | 0.900 | <0.001 | 0.932 |
Abbreviations: D, diet; MA, microalgae; MDA, malondialdehyde; SD, storage days; TBARS, Thiobarbituric acid reactive substances.
Means (8 replicates of 3 eggs each for egg freshness parameters analysis; 8 replicates of 3 pooled yolks each for TBARS analysis) with different superscripts within the same row of storage days differs significantly (P < 0.05).
In conclusion, the present study demonstrated that yolk DHA was enhanced and peaked at about 30th D of MA supplementation for laying hens without adverse effects on performance and egg quality. This feeding strategy has been evaluated to achieve DHA-enriched eggs with more health-promoting n-6/n-3 ratio as well as lower atherogenic and thrombogenic indices. Dietary supplementation with up to 1.0% MA had a minimal impact on physical and functional properties (e.g., YI, viscosity, and emulsification capacity) of yolk. Except for DHA, an enrichment of carotenoids, transferred from the microalgal biomass to yolk, resulted in declined L∗ (lightness) values and increased a∗ (redness) values, corresponding with an increased yolk Roche score. These microalgal carotenoids enrichment also helped attenuate FA oxidation of the DHA-enriched yolk and increase their lipid oxidative stability during subsequent refrigerated storage. These extra benefits of MA need further investigations.
Acknowledgments
This work is financially supported by the open project program of State Key Laboratory of Food Science and Technology, Jiangnan University (No. SKLF-KF-201711). The authors are grateful to Alltech to provide the experimental materials (Aurantiochytrium sp.) and to Rebecca Delles (Alltech, Nicholasville, KY) for her help in revision of the manuscript.
Conflict of Interest Statement: The authors declare no conflict of interest.
References
- Aasen I.M., Ertesvåg H., Heggeset T.M.B., Liu B., Brautaset T., Vadstein O., Ellingsen T.E. Thraustochytrids as production organisms for docosahexaenoic acid (DHA), squalene, and carotenoids. Appl. Microbiol. Biot. 2016;100:4309–4321. doi: 10.1007/s00253-016-7498-4. [DOI] [PubMed] [Google Scholar]
- Adeniyi P., Obatolu V., Farinde E. Comparative evaluation of cholesterol content and storage quality of chicken and quail eggs. World J. Nutr. Health. 2016;4:5–9. [Google Scholar]
- Ao T., Macalintal L., Paul M., Pescatore A., Cantor A., Ford M., Timmons B., Dawson K. Effects of supplementing microalgae in laying hen diets on productive performance, fatty-acid profile, and oxidative stability of eggs. J. Appl. Poult. Res. 2015;2:394–400. [Google Scholar]
- Aro H., Rokka T., Valaja J., Hiidenhovi J., Huopalahti R., Ryhänen E.L. Functional and sensory properties of hen eggs with modified fatty acid compositions. Food Funct. 2011;2:671–677. doi: 10.1039/c1fo10132c. [DOI] [PubMed] [Google Scholar]
- Baeza E., Chartrin P., Lessire M., Meteau K., Chesneau G., Guillevic M., Mourot J. Is it possible to increase the n-3 fatty acid content of eggs without affecting their technological and/or sensorial quality and the laying performance of hens? Br. Poult. Sci. 2015;56:748–754. doi: 10.1080/00071668.2015.1113500. [DOI] [PubMed] [Google Scholar]
- Balk E.M., Lichtenstein A.H. Omega-3 fatty acids and cardiovascular disease: Summary of the 2016 agency of healthcare research and quality evidence review. Nutrients. 2017;9:865–877. doi: 10.3390/nu9080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxmann W., Bindrich U., Heinz V., Knorr D., Franke K. Influencing emulsifying properties of egg yolk by enzymatic modification by phospholipase D from streptomyces chromofuscus: Part 1: technological properties of incubated egg yolk. Colloid Surf. B. 2010;76:186–191. doi: 10.1016/j.colsurfb.2009.10.032. [DOI] [PubMed] [Google Scholar]
- Bruneel C., Lemahieu C., Fraeye I., Ryckebosch E., Muylaert K., Buyse J., Foubert I. Impact of microalgal feed supplementation on omega-3 fatty acid enrichment of hen eggs. J. Funct. Foods. 2013;5:897–904. [Google Scholar]
- Carpenter K.J., Clegg K.M. The metabolizable energy of poultry feeding stuffs in relation to their chemical composition. J. Sci. Food Agric. 1956;7:45–51. [Google Scholar]
- Ebeid T., Eid Y., Saleh A., El-Hamid H.A. Ovarian follicular development, lipid peroxidation, antioxidative status and immune response in laying hens fed fish oil-supplemented diets to produce n-3-enriched eggs. Animal. 2008;2:84–91. doi: 10.1017/S1751731107000882. [DOI] [PubMed] [Google Scholar]
- Feng J., Long S., Zhang H.J., Wu S.G., Qi G.H., Wang J. Comparative effects of dietary microalgae oil and fish oil on fatty acid composition and sensory quality of table eggs. Poult. Sci. 2020;99:1734–1743. doi: 10.1016/j.psj.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J., Lees M., Sloane Stanley G. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Goliomytis M., Orfanou H., Petrou E., Charismiadou M., Simitzis P., Deligeorgis S. Effect of hesperidin dietary supplementation on hen performance, egg quality and yolk oxidative stability. Br. Poult. Sci. 2014;55:98–104. doi: 10.1080/00071668.2013.870328. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Esquerra R., Leeson S. Effect of feeding hens regular or deodorized menhaden oil on production parameters, yolk fatty acid profile, and sensory quality of eggs. Poult. Sci. 2000;79:1597–1602. doi: 10.1093/ps/79.11.1597. [DOI] [PubMed] [Google Scholar]
- Gouda M., Zhang S., Liu Y., Sheng L., Ma M. Effects of four natural antioxidant phenyl terpenes on emulsifying and rheological properties of egg yolk. Lwt-food Sci. Technol. 2017;83:59–67. [Google Scholar]
- Hansen H., Wang T., Dolde D., Xin H., Prusa K. Supplementation of laying-hen feed with annatto tocotrienols and impact of α-tocopherol on tocotrienol transfer to egg yolk. J. Agr. Food Chem. 2015;63:2537–2544. doi: 10.1021/jf505536u. [DOI] [PubMed] [Google Scholar]
- Hargis P., Van Elswyk M., Hargis B. Dietary modification of yolk lipid with menhaden oil. Poult. Sci. 1991;70:874–883. doi: 10.3382/ps.0700874. [DOI] [PubMed] [Google Scholar]
- Hayat Z., Cherian G., Pasha T.N., Khattak F.M., Jabbar M.A. Oxidative stability and lipid components of egg from flax-fed hens: effect of dietary antioxidant and storage. Poult. Sci. 2010;89:1285–1292. doi: 10.3382/ps.2009-00256. [DOI] [PubMed] [Google Scholar]
- Herber S., Van Elswyk M. Dietary marine algae promotes efficient deposition of n-3 fatty acids for the production of enriched shell eggs. Poult. Sci. 1996;75:1501–1507. doi: 10.3382/ps.0751501. [DOI] [PubMed] [Google Scholar]
- Herber S., Van Elswyk M. Dietary marine algae maintains egg consumer acceptability while enhancing yolk color. Poult. Sci. 1998;77:493–496. doi: 10.1093/ps/77.3.493. [DOI] [PubMed] [Google Scholar]
- Islam K., Schweigert F.J. Comparison of three spectrophotometric methods for analysis of egg yolk carotenoids. Food Chem. 2015;172:233–237. doi: 10.1016/j.foodchem.2014.09.045. [DOI] [PubMed] [Google Scholar]
- Jia W., Slominski B., Guenter W., Humphreys A., Jones O. The effect of enzyme supplementation on egg production parameters and omega-3 fatty acid deposition in laying hens fed flaxseed and canola seed. Poult. Sci. 2008;87:2005–2014. doi: 10.3382/ps.2007-00474. [DOI] [PubMed] [Google Scholar]
- Keegan J.D., Currie D., Knox A., Moran C.A. Heterotrophic Aurantiochytrium sp. supplementation to layer diets sustainably increases the omega-3 concentration of eggs. Br. Poult. Sci. 2019;60:570–578. doi: 10.1080/00071668.2019.1622079. [DOI] [PubMed] [Google Scholar]
- Kim J., Barcus M., Magnuson A., Tao L., Lei X.G. Supplemental defatted microalgae affects egg and tissue fatty acid composition differently in laying hens fed diets containing corn and flaxseed oil. J. Appl. Poult. Res. 2016;25:528–538. [Google Scholar]
- King E.J., Hugo A., de Witt F.H., van der Merwe H.J., Fair M.D. Effects of dietary fat source on fatty acid profile and lipid oxidation of eggs. S. Afr. J. Anim. Sci. 2012;42:503–506. [Google Scholar]
- Kralik G., Kralik Z., Grčević M., Kralik I., Hanžek D. The effect of feeding laying hen conventional and omega-3 enriched diet on fatty acid profiles in egg yolk lipids. J. Agr. Sci. Tech.-Iran. 2015;5:506–511. [Google Scholar]
- Lemahieu C., Bruneel C., Ryckebosch E., Muylaert K., Buyse J., Foubert I. Impact of different omega-3 polyunsaturated fatty acid (n-3 PUFA) sources (flaxseed, Isochrysis galbana, fish oil and DHA Gold) on n-3 LC-PUFA enrichment (efficiency) in the egg yolk. J. Funct. Foods. 2015;19:821–827. [Google Scholar]
- Lemahieu C., Bruneel C., Termote-Verhalle R., Muylaert K., Foubert I., Buyse J. Dynamics of omega-3 long chain polyunsaturated fatty acid incorporation in egg yolk by autotrophic microalgal supplementation. Eur. J. Lipid Sci. Tech. 2015;117:1391–1397. [Google Scholar]
- Lemahieu C., Bruneel C., Termote-Verhalle R., Muylaert K., Buyse J., Foubert I. Effect of different microalgal n-3 PUFA supplementation doses on yolk color and n-3 LC-PUFA enrichment in the egg. Algal Res. 2014;6:119–123. [Google Scholar]
- Leskanich C., Noble R. Manipulation of the n-3 polyunsaturated fatty acid composition of avian eggs and meat. World Poult. Sci. J. 1997;53:155–183. [Google Scholar]
- Li W., Zhang X., Jia Y., Liu X. Quality changes of n-3 PUFAs enriched and conventional eggs under different home storage conditions with wireless sensor network. Appl. Sci. 2017;7:1151–1166. [Google Scholar]
- Liu B., Jiang J., Lin G., Yu D.Y., Xiong Y.L. Upregulation of antioxidant enzymes by organic mineral co-factors to improve oxidative stability and quality attributes of muscle from laying hens. Food Res. Int. 2019;125:108575. doi: 10.1016/j.foodres.2019.108575. [DOI] [PubMed] [Google Scholar]
- Manor M.L., Derksen T.J., Magnuson A.D., Raza F., Lei X.G. Inclusion of dietary defatted microalgae dose-dependently enriches ω-3 fatty acids in egg yolk and tissues of laying hens. J. Nutr. 2019;149:942–950. doi: 10.1093/jn/nxz032. [DOI] [PubMed] [Google Scholar]
- Matos Â.P. The impact of microalgae in food science and technology. J. Am. Oil Chem. Soc. 2017;94:1333–1350. [Google Scholar]
- Matumoto-Pintro P.T., Murakami A.E., Vital A.C.P., Croge C., da Silva D.F., Ospina-Roja I.C., Guerra A.F.Q.G. Effects of storage time and temperature on lipid oxidation of egg powders enriched with natural antioxidants. Food Chem. 2017;228:463–468. doi: 10.1016/j.foodchem.2017.02.044. [DOI] [PubMed] [Google Scholar]
- Milinsk M., Murakami A., Gomes S., Matsushita M., De Souza N. Fatty acid profile of egg yolk lipids from hens fed diets rich in n-3 fatty acids. Food Chem. 2003;83:287–292. [Google Scholar]
- Mohiti-Asli M., Shariatmadari F., Lotfollahian H., Mazuji M.T. Effects of supplementing layer hen diets with selenium and vitamin E on egg quality, lipid oxidation and fatty acid composition during storage. Can. J. Anim. Sci. 2008;88:475–483. [Google Scholar]
- Moran C.A., Morlacchini M., Keegan J.D., Fusconi G. Increasing the omega-3 content of hen's eggs through dietary supplementation with Aurantiochytrium limacinum microalgae: effect of inclusion rate on the temporal pattern of docosahexaenoic acid enrichment, efficiency of transfer, and egg characteristics. J. Appl. Poult. Res. 2019;28:329–338. [Google Scholar]
- NRC. 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Omri B., Chalghoumi R., Izzo L., Ritieni A., Lucarini M., Durazzo A., Abdouli H., Santini A. Effect of dietary incorporation of linseed alone or together with tomato-red pepper mix on laying hens’ egg yolk fatty acids profile and health lipid indexes. Nutrients. 2019;11:813–825. doi: 10.3390/nu11040813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangestuti R., Kim S.K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods. 2011;3:255–266. [Google Scholar]
- Park J., Upadhaya S.D., Kim I.H. Effect of dietary marine microalgae (Schizochytrium) powder on egg production, blood lipid profiles, egg quality, and fatty acid composition of egg yolk in layers. Asian Austral. J. Anim. 2015;28:391–397. doi: 10.5713/ajas.14.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrović M., Gačić M., Karačić V., Gottstein Ž., Mazija H., Medić H. Enrichment of eggs in n-3 polyunsaturated fatty acids by feeding hens with different amount of linseed oil in diet. Food Chem. 2012;135:1563–1568. doi: 10.1016/j.foodchem.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Shinn S.E., Gilley A.D., Proctor A., Anthony N.B. Effect of trans, trans CLA egg enrichment from CLA-rich soy oil on yolk fatty acid composition, viscosity and physical properties. J. Agr. Food Chem. 2015;63:2506–2513. doi: 10.1021/jf504759w. [DOI] [PubMed] [Google Scholar]
- Simopoulos A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008;233:674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- Ulbricht T.L.V., Southgate D.A.T. Coronary heart disease: Seven dietary factors. Lancet. 1991;338:985–992. doi: 10.1016/0140-6736(91)91846-m. [DOI] [PubMed] [Google Scholar]
- Viveros A., Centeno C., Arija I., Brenes A. Cholesterol-lowering effects of dietary lupin (Lupinus albus var multolupa) in chicken diets. Poult. Sci. 2007;86:2631–2638. doi: 10.3382/ps.2007-00128. [DOI] [PubMed] [Google Scholar]
- Walker L.A., Wang T., Xin H., Dolde D. Supplementation of laying-hen feed with palm tocos and algae astaxanthin for egg yolk nutrient enrichment. J. Agr. Food Chem. 2012;60:1989–1999. doi: 10.1021/jf204763f. [DOI] [PubMed] [Google Scholar]
- Wołoszyn J., Haraf G., Okruszek A., Wereńska M., Goluch Z., Teleszko M. Fatty acid profiles and health lipid indices in the breast muscles of local Polish goose varieties. Poult. Sci. 2020;99:1216–1224. doi: 10.1016/j.psj.2019.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.B., Li L., Wen Z.G., Yan H.J., Yang P.L., Tang J., Xie M., Hou S.S. Dual functions of eicosapentaenoic acid-rich microalgae: enrichment of yolk with n-3 polyunsaturated fatty acids and partial replacement for soybean meal in diet of laying hens. Poult. Sci. 2019;98:350–357. doi: 10.3382/ps/pey372. [DOI] [PubMed] [Google Scholar]
- Yonke J.A., Cherian G. Choline supplementation alters egg production performance and hepatic oxidative status of laying hens fed high-docosahexaenoic acid microalgae. Poult. Sci. 2019;98:5661–5668. doi: 10.3382/ps/pez339. [DOI] [PubMed] [Google Scholar]