Abstract
This study was conducted to evaluate graded Eimeria challenge on growth performance, apparent ileal digestibility, gastrointestinal permeability, intestinal morphology, gene expression of tight junction protein, and intestinal lesion scores in broiler chickens. There were 5 groups in this study, including a control and 4 different Eimeria treatment doses. A mixed Eimeria spp. solution with 50,000 Eimeria maxima, 50,000 Eimeria tenella, and 250,000 Eimeria acervulina per milliliter was prepared for the high-dose challenge treatment. The 2-fold serial dilution was used to make the medium-high (25,000 E. maxima; 25,000 E. tenella; 125,000 E. acervulina), the medium-low (12,500 E. maxima; 12,500 E. tenella; 62,500 E. acervulina), and the low challenge dose (6,250 E. maxima; 6,250 E. tenella; 31,250 E. acervulina). A total of three hundred sixty 13-day-old male broiler chickens were randomly allocated into 5 treatments with 6 replicated cages. Growth performance was calculated from 0 to 6 D postinfection (DPI). Intestine lesion was scored on 6 DPI. Gastrointestinal permeability was measured on 3, 5, 6, 7, and 9 DPI. The results indicated significant linear reduction in growth performance, intestinal villi height, and ileal nutrient digestibility in response to the increase of Eimeria challenge dose. Furthermore, gene expression of tight junction protein was linearly upregulated by the increasing challenge doses. Significant linear increases of gastrointestinal permeability were found on 5, 6, and 7 DPI (P < 0.01). On 9 DPI, the gastrointestinal permeability was recovered back to normal level in the challenge groups. In conclusion, the higher Eimeria doses birds received, the more severe intestine damage was observed in several gastrointestinal health parameters. The medium-low or medium-high levels of mixed Eimeria oocysts is suggested as an optimum Eimeria-challenge dose to establish a subclinical challenge model for future studies evaluating nutritional strategies. Moreover, it is recommended to measure gastrointestinal permeability on 5 DPI with higher oocysts doses and 6 DPI when using the lower oocysts doses.
Key words: coccidiosis, Eimeria, gastrointestinal permeability, fluorescein isothiocyanate-dextran, nutrient digestibility
Introduction
Coccidiosis is a protozoal disease caused by genus Eimeria. Severe Eimeria infection might lead to poor growth performance and high mortality. It has been estimated that coccidiosis causes an economic loss of 3 billion US dollars annually in the poultry industry (Yun et al., 2000; Chapman, 2014). Vaccination for coccidiosis has been developed and achieved a sustainable control (Chapman et al., 2002; Chapman, 2007, 2014). To establish solid immunity by vaccination, it is necessary for chicken to recycle the parasites by digesting oocysts from litter. The secondary infection could boost the production of antibodies against Eimeria spp. (Chapman, 2000; Chapman et al., 2002). However, vaccination also induces mild coccidiosis, especially during third and fourth life cycles of Eimeria. Subclinical Eimeria infection with light lesion scores should be observed from 14 to 28 D after vaccination (Chapman, 2000). To ameliorate negative outcomes from mild Eimeria infection, nutritional strategies such as supplementing exogenous enzymes could compensate the failure of digestion and improve growth performance of broiler chickens (Parker et al., 2007; Walk et al., 2011; Adedokun and Adeola, 2016; Zhang et al., 2016). Furthermore, altering dietary composition is another potential strategy to adjust nutrient requirement for Eimeria-infected birds. (Takhar and Farrell, 1979; Amerah and Ravindran, 2015; Adedokun and Adeola, 2016).
However, nutritional strategies are not the universal remedy for coccidiosis. Feed additives, such as enzymes, probiotic, and prebiotic, were not designed to inhibit E. spp. Though these approaches cannot replace anticoccidial drugs or vaccination, they are beneficial to improve nutrient digestibility of diets and growth performance under mild infection. To evaluate a nutritional strategy, a subclinical Eimeria-challenged model should be established first. Thus, how challenge dose impact on growth performance and intestine of chicken is important for researcher to select an appropriate dose in the subclinical challenge model. A previous study reported that the increasing inoculation doses of Eimeria resulted in a linear reduction of growth performance and apparent ileal digestibility of amino acids (Rochell et al., 2016). However, more studies are needed to understand the relationship between the severity of Eimeria infection and gastrointestinal health parameters. Thus, the first objective of the study was to evaluate the effects of graded Eimeria-challenge on growth performance and gastrointestinal health in broilers.
The second objective of the study was to evaluate when is the best time point to measure gastrointestinal integrity by fluorescein isothiocyanate dextran (FITC-d). Fluorescein isothiocyanate dextran is a promising tool in assessing gastrointestinal integrity which has been tested in several enteric inflammation models (Kuttappan et al., 2015a, 2015b; Zhang et al., 2016; Latorre et al., 2018; Bortoluzzi et al., 2019). At first, FITC-d has been a marker for evaluating the transcellular transports of arterial endothelial monolayers in a cell chamber system (Mizuno-Yagyu et al., 1987), and this technique was successfully conducted in mice (Furuta et al., 2001; Yan et al., 2009). Recently, the method has been modified for poultry research to evaluate gastrointestinal leakage in different models, including the 24-h feed restriction model, dextran sodium sulfate model, rye-based diet model, and high-fat diet model. (Kuttappan et al., 2015a, 2015b; Vicuna et al., 2015; Baxter et al., 2017). Furthermore, FITC-d was used to evaluate gastrointestinal permeability in coccidiosis and necrotic enteritis models (Zhang et al., 2016; Latorre et al., 2018; Bortoluzzi et al., 2019). It is suggested that parasites cause gastrointestinal leakage at 60 to 72 h postinfection when the second generation of schizont is mature and releases large numbers of merozoites, leading to the tremendous damage in the intestine (Dubey and Jenkins, 2018). However, it is unclear when is the best time point to measure gastrointestinal permeability by using FITC-d as a marker in an Eimeria challenge study. Thus, the second objective of the study was to understand how the dynamic change of intestinal permeability affected by graded challenge dose, as well as to find out the best time for measuring gastrointestinal integrity by FITC-d.
The experiment was conducted to evaluate the effects of increasing Eimeria challenge doses on growth performance, dynamic change of gastrointestinal permeability, ileal digestibility, intestinal morphology, gene expression of tight junction protein, and intestinal lesion scores, as well as to determine the time point for measuring intestinal permeability in an Eimeria-challenge study.
Materials and methods
Experimental Design
The study was conducted at the Poultry Research Center, University of Georgia, Athens, GA. It was approved by the Institutional Animal Care and Use Committee. The completely randomized design was used in the study. A total of three hundred sixty 13-day-old male broiler chickens (males from the Cobb 500 female line) were randomly allocated to 5 treatments with 6 replicates, and 12 birds per cage. The treatments included the control group (Con), the high challenge dose (High), the medium-high challenge dose (Med-high), the medium-low challenge dose (Med-low), and the low challenge dose (Low). Before placement into cages, birds were weighed and gavaged with 1 mL water as sham in the Con or 1 mL Eimeria challenge dose based on the treatments. The numbers of oocysts used in each challenge groups are described in Table 1. The diet for the whole experiment period (day 13 to day 19) was formulated with 0.3% chromium oxide as an indigestible indicator for calculating the apparent ileal digestibility (AID). Feed and water were provided ad libitum, and the environmental temperature program was followed to the recommendation of Cobb Broiler Management Guide.
Table 1.
The Eimeria spp. challenge dose for each treatment in the study.
| Treatments1 | E. maxima | E. tenella | E. acervulina |
|---|---|---|---|
| Con | 0 | 0 | 0 |
| Low | 6,250 | 6,250 | 31,250 |
| Med-low | 12,500 | 12,500 | 62,500 |
| Med-high | 25,000 | 25,000 | 125,000 |
| High | 50,000 | 50,000 | 250,000 |
Unit: oocyst; Con, Control; Low, the low challenge dose; Med-low, the medium-low challenge dose; Med-high, the medium-high challenge dose; High, the high challenge dose.
Growth Performance, Lesion Scores, and Ileal Digesta Collection
The body weight (BW) of birds and feed intake (FI) per cage were recorded on the first day of the experiment and 6 D postinfection (DPI). The body weight gain (BWG) and feed conversion ratio (FCR) were calculated from day 13 to day 19. Any mortalities were removed and recorded to adjust FCR. On 6 DPI, 5 birds per cage were sacrificed by cervical dislocation for sample collection and lesion scoring. The lesion scores were evaluated by the 4-score scale (Johnson and Reid, 1970). The ileal digesta was collected from the distal ileum (the one-third section from the ileo-cecal-colic junction to the Meckel's diverticulum) and kept at -20°C freezer for further processing.
Gastrointestinal Permeability
The fluorescein isothiocyanate dextran (FITC-d; MW 4000; Sigma-Aldrich, Canada) was administrated to evaluate gastrointestinal permeability. The method was modified by previous studies (Baxter et al., 2017; Bortoluzzi et al., 2019). Briefly, at 3, 5, 6, 7, and 9 DPI, 1 bird per cage was gavaged with 1 mL of FITC-d solution (2.2 mg/mL). After 2 h of inoculation, the birds were euthanized, and blood was collected. The blood was kept in a dark container in room temperature for 2 h until clotting and then centrifuged at 1,000 × g for 15 min to separate serum. A standard solution was made by diluting FITC-d with a pool of serum from 10 extra unchallenged birds. The FITC-d levels in the serum samples and standard solution were measured at an excitation wavelength of 485 nm and an emission wavelength of 528 nm by using a microplate reader (Spectramax M5, Molecular Devices, San Jose, CA), respectively.
Ileal Digestible Energy and Apparent Ileal Digestibility of Macrominerals
Oven-dried feed and ileal digesta were ground to measure gross energy and minerals. For analysis of gross energy, the ground samples were measured by a calorimeter (IKA Calorimeter C1, IKA Works Inc., Wilmington, NC). Macromineral levels of feed and ileal digesta were determined by the Soil Laboratory, University of Georgia. The chromic oxide was analyzed according to Dansky and Hill (1952). Briefly, 0.3 g of sample was ashen in a nickel crucible at 600°C overnight to burn out organic materials. Additional 5.8 g of fusion mixture (190 g KNO3 to 100 g Na2CO3) and 5.6 g NaOH was added in the nickel crucible and burned at 600°C for additional 2 h. The fusion mix was dissolved in water, and chromite was oxidized to chromate by H2O2. The concentration of chromate was determined at 400 nm on a spectrophotometer (Spectramax M5, Molecular Devices, San Jose, CA). The ileal digestible energy (IDE) and apparent ileal digestibility (AID) of minerals were calculated according to the following equations.
Intestinal Morphology
Morphometric analyses of the small intestine were performed by the method described by Teng et al. (2017). Three cm long sections from the center of duodenum, jejunum, and ileum were collected from 1 bird per pen, rinsed with phosphate buffer saline, and immediately fixed in 10% formalin. The fixed tissue was embedded in paraffin and cut into 4 μm to be stained by the hematoxylin and eosin method. The slide pictures were captured in 1.6X (duodenum and jejunum) or 5X (ileum) magnification by a light microscope with a camera (Leica DC500 camera, Leica Microsystems Inc., Buffalo Groove, IL). The villi height and crypt depth were measured in 5 favorably oriented and representative villi or crypt per slide using the LAS v4.8 software (Leica Microsystems Inc., Buffalo Groove, IL). The ratio of villi height to crypt depth was calculated from each sample.
Real-Time PCR Analysis
Mucosa was gently scraped down from the jejunum by a microslide and collected into an Eppendorf tube. The samples were frozen with liquid nitrogen immediately and stored in −80°C for further analyses. The total RNA was extracted after homogenization in QiAzol lysis reagents (Qiagen, Valencia, CA) according to the manufacturer's instruction. The RNA quantity and purity were measured by a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, MA). The cDNA was reverse-transcribed by high capacity cDNA synthesis kits (Applied Biosystems, Foster City , CA). Real-time PCR reaction was performed with SYBR Green Master mix with a Step One thermocycler (Applied Biosystem). The cDNA samples were run in duplicate, and the target genes expression were analyzed using the 2−ΔΔCt method (Livak and Schmittgen, 2001). The outliers were removed from the data set if the data point was exceeded ±3 standard deviations from the mean (Su et al., 2014). Primers for housekeeping genes and target genes are listed in Table 2.
Table 2.
List of primers used for qPCR.
| Gene symbol1 | Accession number | Forward primer | Reverse primer |
|---|---|---|---|
| GAPDH2 | NM_204305.1 | CCTCTCTGGCAAAGTCCAAG | GGTCACGCTCCTGGAAGATA |
| Beta-actin2 | NM_205518.1 | CAACACAGTGCTGTCTGGTGGTA | ATCGTACTCCTGCTTGCTGATCC |
| HMBS2 | XM_004947916.3 | GGCTGGGAGAATCGCATAGG | TCCTGCAGGGCAGATACCAT |
| CLDN13 | NM_001013611.2 | TGGAGGATGACCAGGTGAAGA | CGAGCCACTCTGTTGCCATA |
| OCLN3 | XM_025144248.1 | ACGGCAGCACCTACCTCAA | GGCGAAGAAGCAGATGAG |
| ZO23 | XM_025144669.1 | GGCAAATCATTGAGCAGGA | ATTGATGGTGGCTGTAAAGAG |
| JAM23 | XM_025149444.1 | AGCCTCAAATGGGATTGGATT | CATCAACTTGCATTCGCTTCA |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HMBS, hydroxymethylbilane synthase; CLDN1, claudin 1; OCLN, occludin; ZO2, tight junction protein 2; JAM2, junctional adhesion molecule 2.
Housekeeping gene.
Tight junction proteins.
Statistical Analyses
All data were analyzed in the PROC GLM program of SAS software (SAS Institute Inc., Cary, NC). To evaluate the effects of increasing oocysts inoculation doses on responses of each parameter, the linear and quadratic orthogonal polynomial contrasts were used. The Duncan's multiple-range test was used to separate means with significance levels at P ≤ 0.05. The Kruskal-Wallis nonparametric analysis described by Elliott and Hynan (2011) was used for the analysis of intestinal lesion scores. Statistical significance was set at P ≤ 0.05. In addition, the PROC CORR program of SAS software was used to calculate Pearson correlation coefficients among dose, growth performance, and selected parameters measured in the study. Considering the challenge doses were diluted by 2-fold serial dilution from the High group to the Low group, the challenged doses were normalized by calculating the base 2 logarithm of the number of doses in the correlation coefficients analyses.
Results
Growth Performance
Graded levels of challenging oocysts resulted in both linear (P < 0.0001) and quadratic (P < 0.01) reduction in BW, BWG, and FI (Table 3). Moreover, increasing oocysts levels resulted in higher FCR (linear, P < 0.0001). The High group caused severely negative effects on growth performance of broilers, reducing 23.4% of body weight compared with the Con, and even the BW of birds in the Low group decreased from 768 g to 668 g. Overall, the growth performance of broilers was reduced linearly with increasing inoculation doses of the mixed Eimeria oocysts.
Table 3.
Effects of increasing oocysts doses of mixed E. maxima, E. acervulina, and E. tenella on growth performance of broilers (day 13–19).
| Items1 | Con | Low | Med-low | Med-high | High | SEM | Linear | Quadratic |
|---|---|---|---|---|---|---|---|---|
| BW | 768 ± 6a | 668 ± 6b | 644 ± 10b | 605 ± 8c | 588 ± 12c | 12 | <0.0001 | <0.0001 |
| BWG | 370 ± 6a | 270 ± 6b | 243 ± 11c | 205 ± 7d | 189 ± 13d | 12 | <0.0001 | <0.0001 |
| FI | 547 ± 13a | 481 ± 8b | 440 ± 7c | 438 ± 11c | 427 ± 16c | 10 | <0.0001 | 0.0023 |
| FCR | 1.48 ± 0.05a | 1.79 ± 0.03a,b | 1.83 ± 0.08b,c | 2.15 ± 0.09c | 2.32 ± 0.22c | 0.07 | <0.0001 | 0.9787 |
N = 6.
The study evaluated the effects of graded challenge of Eimeria spp. on growth performance and gastrointestinal health of broiler chickens. Birds were challenged with Eimeria spp. on day 13. (Low, 6,250 oocysts of E. maxima, 6,250 oocysts of E. tenella, and 31,250 oocysts of E. acervulina; Med-low, 12,500 oocysts of E. maxima, 12,500 oocysts of E. tenella, and 62,500 oocysts of E. acervulina; Med-high, 25,000 oocysts of E. maxima, 25,000 oocysts of E. tenella, and 125,000 oocysts of E. acervulina; High, 50,000 oocysts of E. maxima, 50,000 oocysts of E. tenella, and 250,000 oocysts of E. acervulina).
BW, body weight (day 19); BWG, body weight gain (day 13–19); FI, feed intake (day 13–19); FCR, feed conversion rate (day13–19); Con, Control; Low, the low challenge dose; Med-low, the medium-low challenge dose; Med-high, the medium-high challenge dose; High, the high challenge dose.
Gastrointestinal Permeability
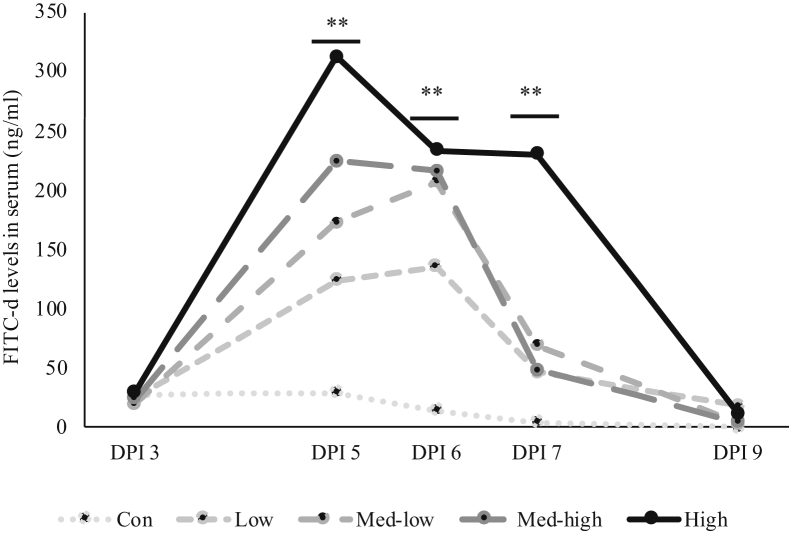
The dynamic change of gastrointestinal permeability from 3, 5, 6, 7, and 9 DPI is shown in Figure 1. The results of gastrointestinal permeability were represented as levels of FITC-d recovered in the serum of birds. Higher concentration of FITC-d in the serum represent the increase of gastrointestinal permeability. On 3 DPI, there was no significant difference among treatments. On 5, 6, and 7 DPI, increasing inoculation levels resulted in higher gastrointestinal leakage (linear, P < 0.01). The most severe gastrointestinal permeability (313 ng/mL) was observed on 5 DPI in the High treatment. Birds challenged in the High group had 230 ng/mL FITC-dextran in the serum, whereas the others showed less than 70 ng/mL on 7 DPI. On 9 DPI, birds challenged with different doses of oocysts had no significant difference on gastrointestinal permeability both linearly and quadratically.
Figure 1.
Effects of increasing oocysts doses of mixed E. maxima, E. acervulina, and E. tenella on dynamic change of gastrointestinal permeability measured by fluorescein isothiocyanate dextran. ∗∗ Significant linear effects on same day postinfection (DPI), P < 0.01. N = 6. The study evaluated the effects of graded challenge of Eimeria spp. on growth performance and gastrointestinal health of broiler chickens. Birds were challenged with Eimeria spp. on d13. (Con, Control; Low, the low challenge dose, 6,250 oocysts of E. maxima, 6,250 oocysts of E. tenella, and 31,250 oocysts of E. acervulina; Med-low, the medium-low challenge dose, 12,500 oocysts of E. maxima, 12,500 oocysts of E. tenella, and 62,500 oocysts of E. acervulina; Med-high, the medium-high challenge dose, 25,000 oocysts of E. maxima, 25,000 oocysts of E. tenella, and 125,000 oocysts of E. acervulina; High, the high challenge dose, 50,000 oocysts of E. maxima, 50,000 oocysts of E. tenella, and 250,000 oocysts of E. acervulina).
Ileal Digestible Energy and Apparent Ileal Digestibility of Macrominerals
Ileal digestible energy was linearly (P < 0.05) and quadratically (P < 0.01) decreased in response to increasing challenge doses (Table 4). The AID of sodium and potassium in the ileum were linearly (P < 0.05) and quadratically (P < 0.05) reduced when birds received higher challenge doses. A linear response (P < 0.05) was observed in the AID of calcium by increasing the challenge doses. However, levels of zinc, magnesium, copper, and phosphorus were not significantly influenced by different levels of Eimeria infection.
Table 4.
Effects of increasing oocysts doses of mixed E. maxima, E. acervulina, and E. tenella on ileal digestible energy (IDE) and apparent ileal digestibility of macrominerals (6 DPI).
| Items1 | Con | Low | Med-low | Med-high | High | SEM | Linear | Quadratic |
|---|---|---|---|---|---|---|---|---|
| IDE2 | 2,830 ± 86a | 2,294 ± 221a,b | 1,807 ± 212b | 1,707 ± 375b | 2,303 ± 176a,b | 123.0 | 0.0410 | 0.0061 |
| Na (%) | -17 ± 10a | -127 ± 52a,b | -281 ± 86b,c | -456 ± 105c | -158 ± 29a,b | 39.84 | 0.0109 | 0.0049 |
| K (%) | 88 ± 1a | 78 ± 3a,b | 67 ± 7b,c | 58 ± 9c | 73 ± 2a,b | 2.92 | 0.0092 | 0.0168 |
| Ca (%) | 49 ± 7b | 36 ± 3c | 54 ± 2a,b | 55 ± 4a,b | 64 ± 4a | 2.49 | 0.0022 | 0.1052 |
| Mg (%) | 14 ± 6 | 18 ± 4 | 18 ± 6 | 22 ± 8 | 31 ± 8 | 2.87 | 0.0754 | 0.5345 |
| Zn (%) | 22 ± 5 | 5 ± 6 | 19 ± 6 | 25 ± 9 | 27 ± 8 | 3.27 | 0.2031 | 0.2270 |
| Cu (%) | -4 ± 11 | -36 ± 16 | -6 ± 7 | -7 ± 13 | -21 ± 10 | 5.47 | 0.9060 | 0.9333 |
| P (%) | 55 ± 4 | 45 ± 3 | 47 ± 5 | 43 ± 7 | 58 ± 3 | 2.24 | 0.8158 | 0.0179 |
N = 6.
The study evaluated the effects of graded challenge of Eimeria spp. on growth performance and gastrointestinal health of broiler chickens. Birds were challenged with Eimeria spp. on day 13. (Low, 6,250 oocysts of E. maxima, 6,250 oocysts of E. tenella, and 31,250 oocysts of E. acervulina; Med-low, 12,500 oocysts of E. maxima, 12,500 oocysts of E. tenella, and 62,500 oocysts of E. acervulina; Med-high, 25,000 oocysts of E. maxima, 25,000 oocysts of E. tenella, and 125,000 oocysts of E. acervulina; High, 50,000 oocysts of E. maxima, 50,000 oocysts of E. tenella, and 250,000 oocysts of E. acervulina).
Abbreviation: DPI, day postinfection
Con, Control; Low, the low challenge dose; Med-low, the medium-low challenge dose; Med-high, the medium-high challenge dose; High, the high challenge dose.
IDE, Ileal digestible energy. Unit: Kcal/kg.
Intestinal Morphology and Gene Expression of Tight Junction Protein
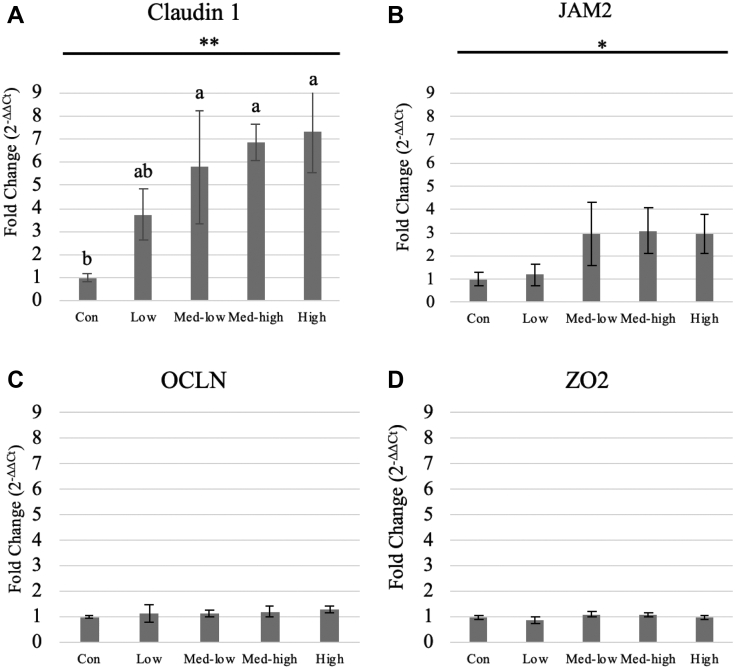
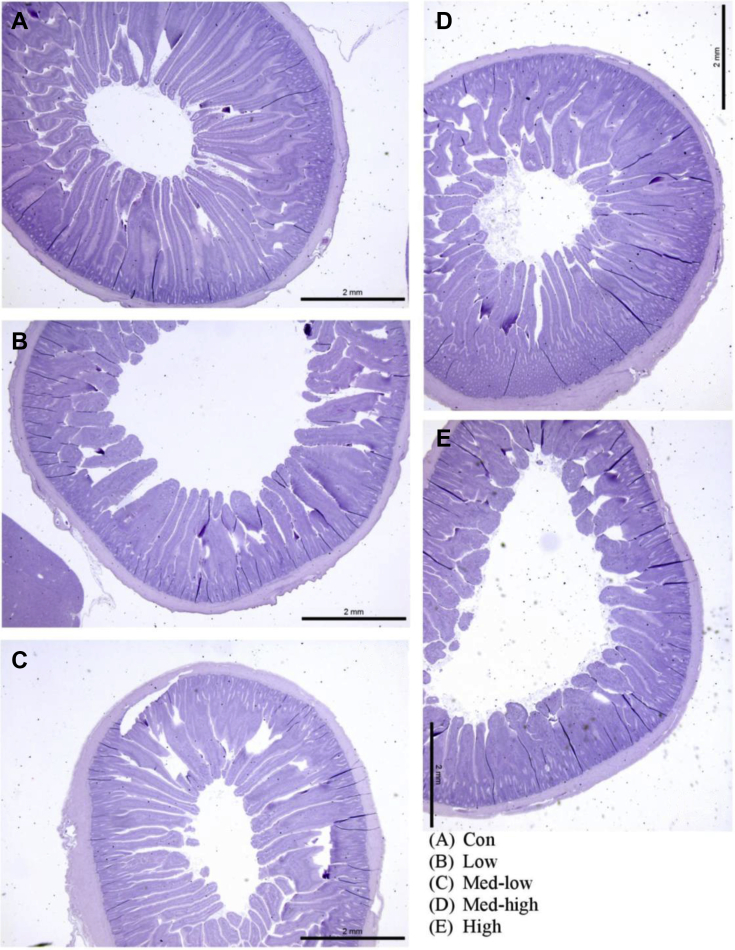
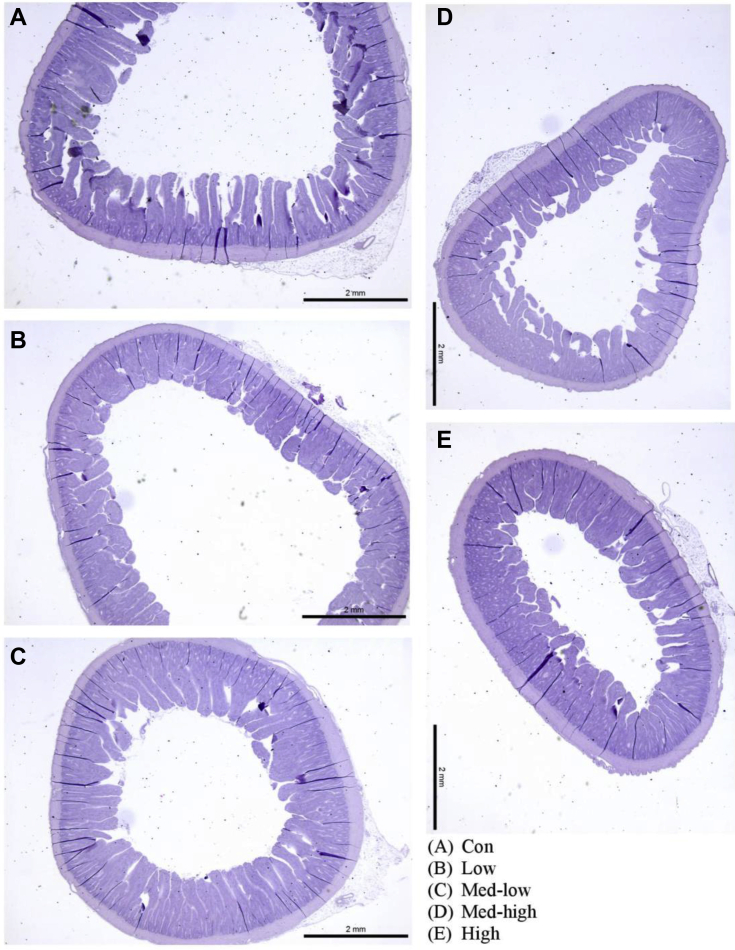
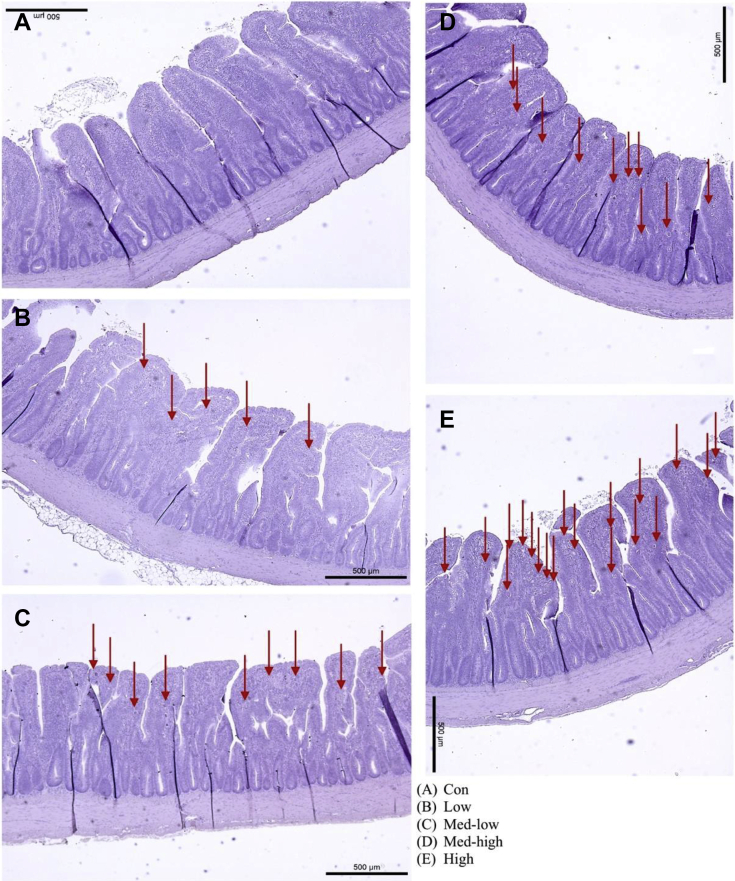
Linear (P < 0.05) responses were observed for villi height, crypt depth, and villi height: crypt depth ratio in the duodenum and jejunum with the increase in inoculation doses (Table 5; Figure 2, Figure 3, Figure 4). In addition, the villi height, crypt depth, and villi height: crypt depth ratio in the jejunum also exhibited a quadratic response to inoculation dose (P < 0.05), and villi height in the ileum was linearly responded to the graded dose (P < 0.05). The Con and Low had larger sizes of transverse section of jejunum than Med-high and High (Figure 3). Moreover, more gametocytes and developing oocysts were present in the ileum of High and Med-high than Med-low, Low, and Con (Figure 4). The increase of Eimeria challenged dose linearly upregulated gene expression of claudin 1 and junctional adhesion molecule 2; however, no significant difference was found for occludin and tight junction protein 2 in the mucosa of the jejunum (Figure 5).
Figure 5.
Effects of increasing oocysts doses of mixed E. maxima, E. acervulina, and E. tenella on gene expression of tight junction proteins in broiler chicken (6 DPI). ∗/∗∗ indicated the significant linear effects (∗, P < 0.05; ∗∗P < 0.01). a, b Treatments with different letters are significantly different (P < 0.05). (A) CLDN1, claudin 1; (B) OCLN, occludin; (C) ZO2, tight junction protein 2; (D) JAM2, junctional adhesion molecule 2. The study evaluated the effects of graded challenge of Eimeria spp. on growth performance and gastrointestinal health of broiler chickens. Birds were challenged with E. spp. on day 13. (Con, control; Low, the low challenge dose, 6,250 oocysts of E. maxima, 6,250 oocysts of E. tenella, and 31,250 oocysts of E. acervulina; Med-low, the medium-low challenge dose, 12,500 oocysts of E. maxima, 12,500 oocysts of E. tenella, and 62,500 oocysts of E. acervulina; Med-high, the medium-high challenge dose, 25,000 oocysts of E. maxima, 25,000 oocysts of E. tenella, and 125,000 oocysts of E. acervulina; High, the high challenge dose, 50,000 oocysts of E. maxima, 50,000 oocysts of E. tenella, and 250,000 oocysts of E. acervulina.) Abbreviations: Con, Control; Low, the low challenge dose; Med-low, the medium-low challenge dose; Med-high, the medium-high challenge dose; High, the high challenge dose; DPI, day postinfection.
Table 5.
Effects of increasing oocysts doses of mixed E. maxima, E. acervulina, and E. tenella on intestinal morphology (6 DPI).
| Items1 | Con | Low | Med-low | Med-high | High | SEM | Linear | Quadratic |
|---|---|---|---|---|---|---|---|---|
| Duodenum | ||||||||
| Villi height | 2,192 ± 77a | 1,876 ± 117a,b | 1,840 ± 133a,b | 1,630 ± 180c | 1,571 ± 241c | 78 | 0.0069 | 0.5760 |
| Crypt depth | 302 ± 7b | 369 ± 25a,b | 431 ± 18a | 406 ± 46a | 405 ± 30a | 14 | 0.0115 | 0.0457 |
| VH: CD2 | 7.30 ± 0.38a | 5.17 ± 0.40b | 4.29 ± 0.28b | 4.16 ± 0.51b | 4.22 ± 1.08b | 0.33 | 0.0009 | 0.0315 |
| Jejunum | ||||||||
| Villi height | 1,153 ± 40a | 902 ± 50b | 786 ± 59b | 744 ± 96b | 833 ± 93b | 40 | 0.0016 | 0.0091 |
| Crypt depth | 283 ± 21b | 374 ± 23a | 370 ± 32a | 443 ± 36a | 378 ± 9a | 14 | 0.0039 | 0.0234 |
| VH: CD | 4.21 ± 0.37a | 2.43 ± 0.11b | 2.25 ± 0.32b | 1.78 ± 0.30b | 2.18 ± 0.20b | 0.20 | <0.0001 | 0.0006 |
| Ileum | ||||||||
| Villi height | 687 ± 45a,b | 788 ± 49a | 670 ± 31a,b | 593 ± 27b | 612 ± 48b | 21 | 0.0130 | 0.4259 |
| Crypt depth | 214 ± 10b | 293 ± 41a | 280 ± 25a,b | 236 ± 10a,b | 252 ± 18a,b | 11 | 0.8174 | 0.0902 |
| VH: CD | 3.22 ± 0.21a | 2.93 ± 0.39a,b | 2.45 ± 0.14b | 2.52 ± 0.10a,b | 2.47 ± 0.22b | 0.11 | 0.1720 | 0.2490 |
Abbreviations: VH: CD, ratio of villi height to crypt depth; DPI, day postinfection.
N = 6, Unit: μm.
The study evaluated the effects of graded challenge doses of Eimeria spp. on growth performance and gastrointestinal health of broiler chickens. Birds were challenged with Eimeria spp. on d13. (Low, 6,250 oocysts of E. maxima, 6,250 oocysts of E. tenella, and 31,250 oocysts of E. acervulina; Med-low, 12,500 oocysts of E. maxima, 12,500 oocysts of E. tenella, and 62,500 oocysts of E. acervulina; Med-high, 25,000 oocysts of E. maxima, 25,000 oocysts of E. tenella, and 125,000 oocysts of E. acervulina; High, 50,000 oocysts of E. maxima, 50,000 oocysts of E. tenella, and 250,000 oocysts of E. acervulina).
Con, Control; Low, the low challenge dose; Med-low, the medium-low challenge dose; Med-high, the medium-high challenge dose; High, the high challenge dose.
Ratio, ratio of villi height to crypt depth.
Figure 2.
Effects of increasing oocysts doses of mixed E. maxima, E. acervulina, and E. tenella on intestinal morphology (duodenum) of broiler chicken (6 DPI). The study evaluated the effects of graded challenge dose of Eimeria spp. on growth performance and gastrointestinal health of broiler chickens. Birds were challenged with E. spp. on day 13. (A) Con, control; (B) Low, the low challenge dose, 6,250 oocysts of E. maxima, 6,250 oocysts of E. tenella, and 31,250 oocysts of E. acervulina; (C) Med-low, the medium-low challenge dose, 12,500 oocysts of E. maxima, 12,500 oocysts of E. tenella, and 62,500 oocysts of E. acervulina; (D) Med-high, the medium-high challenge dose, 25,000 oocysts of E. maxima, 25,000 oocysts of E. tenella, and 125,000 oocysts of E. acervulina; (E) High, the high challenge dose, 50,000 oocysts of E. maxima, 50,000 oocysts of E. tenella, and 250,000 oocysts of E. acervulina. Abbreviation: DPI, day postinfection.
Figure 3.
Effects of increasing oocysts doses of mixed E. maxima, E. acervulina, and E. tenella on intestinal morphology (jejunum) of broiler chicken (6 DPI). The study evaluated the effects of graded challenge of Eimeria spp. on growth performance and gastrointestinal health of broiler chickens. Birds were challenged with E. spp. on d13. (A) Con, control; (B) Low, the low challenge dose, 6,250 oocysts of E. maxima, 6,250 oocysts of E. tenella, and 31,250 oocysts of E. acervulina; (C) Med-low, the medium-low challenge dose, 12,500 oocysts of E. maxima, 12,500 oocysts of E. tenella, and 62,500 oocysts of E. acervulina; (D) Med-high, the medium-high challenge dose, 25,000 oocysts of E. maxima, 25,000 oocysts of E. tenella, and 125,000 oocysts of E. acervulina; (E) High, the high challenge dose, 50,000 oocysts of E. maxima, 50,000 oocysts of E. tenella, and 250,000 oocysts of E. acervulina. Abbreviation: DPI, day postinfection.
Figure 4.
Effects of increasing oocysts doses of mixed E. maxima, E. acervulina, and E. tenella on intestinal morphology (ileum) of broiler chicken (6 DPI). The arrows point out gametocytes or oocysts in the figures. The study evaluated the effects of graded challenge of Eimeria spp. on growth performance and gastrointestinal health of broiler chickens. Birds were challenged with E. spp. on d13. (A) Con, control; (B) Low, the low challenge dose, 6,250 oocysts of E. maxima, 6,250 oocysts of E. tenella, and 31,250 oocysts of E. acervulina; (C) Med-low, the medium-low challenge dose, 12,500 oocysts of E. maxima, 12,500 oocysts of E. tenella, and 62,500 oocysts of E. acervulina; (D) Med-high, the medium-high challenge dose, 25,000 oocysts of E. maxima, 25,000 oocysts of E. tenella, and 125,000 oocysts of E. acervulina; (E) High, the high challenge dose, 50,000 oocysts of E. maxima, 50,000 oocysts of E. tenella, and 250,000 oocysts of E. acervulina.
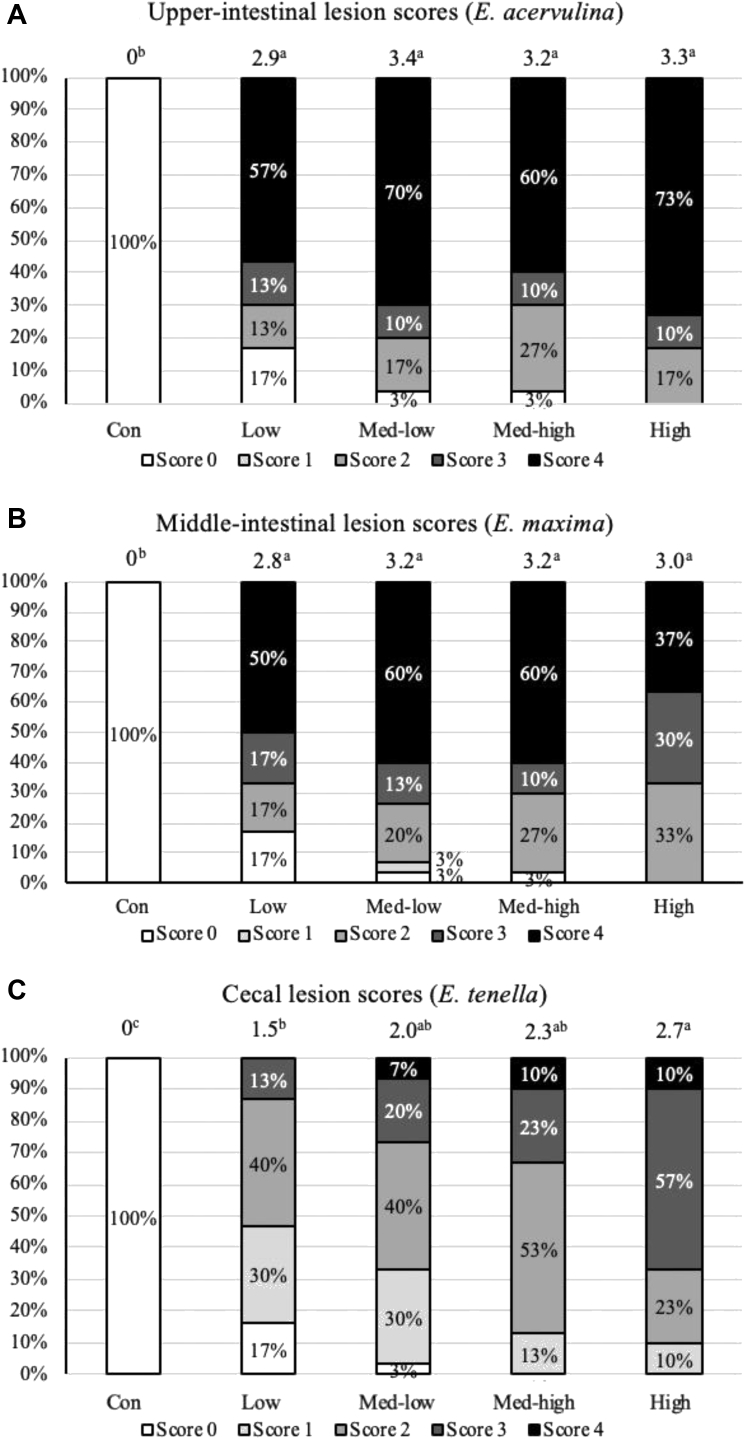
Figure 6.
Effects of increasing oocysts doses of mixed E. maxima, E. acervulina, and E. tenella on lesion scores in the upper intestine, middle intestine, and ceca of broiler chicken (6 DPI). Average scores of each treatment are present at the top of the bar. a, b Treatments with different letters are significantly different (P < 0.05). (A) Upper-intestine; (B) middle-intestine; (C) ceca. The study evaluated the effects of graded challenge of Eimeria spp. on growth performance and gastrointestinal health of broiler chickens. Birds were challenged with E. spp. on day 13. (Con, Control; Low, the low challenge dose, 6,250 oocysts of E. maxima, 6,250 oocysts of E. tenella, and 31,250 oocysts of E. acervulina; Med-low, the medium-low challenge dose, 12,500 oocysts of E. maxima, 12,500 oocysts of E. tenella, and 62,500 oocysts of E. acervulina; Med-high, the medium-high challenge dose, 25,000 oocysts of E. maxima, 25,000 oocysts of E. tenella, and 125,000 oocysts of E. acervulina; High, the high challenge dose, 50,000 oocysts of E. maxima, 50,000 oocysts of E. tenella, and 250,000 oocysts of E. acervulina). Abbreviation: DPI, day postinfection.
Lesion Scores
The results of intestinal lesion scores are shown in Figure 6, presented by the percentage of each score in each treatment. All challenged groups had higher lesion scores than the Con in the upper-intestine, middle-intestine, and ceca. The results indicated that birds in the High group had more severe cecal lesion compared with the Low and Con. Additionally, the Low group showed higher lesion scores than the Con. However, there was no significant difference among the challenged groups in the upper and middle intestine.
Correlation Coefficients Between Challenged Dose and Selected Parameters
Strong linear relationships were found between challenged dose and several parameters, including BW, FI, and villi height to crypt depth ratio in the jejunum (|r | > 0.7; Table 6). Among these parameters, BW exhibited a significant strong negative linear relationship with the challenged dose (r = −0.911). Ileal digestible energy and AID of sodium and potassium were significantly related to each other (IDE and AID of sodium, r = 0.895; IDE and AID of potassium, r = 0.898; AID of sodium and potassium, r = 0.921). Moreover, the correlation coefficients of the challenge dose showed moderated linear relationships with intestinal morphology and apparent digestibility (0.440 < |r | < 0.789).
Table 6.
Correlation coefficients among Eimeria challenged dose and selected gastrointestinal health parameters.
| Items1 | log2 dose | BW | FI | FCR | Permeability | VH: CD duodenum | VH: CD jejunum | VH: CD ileum | IDE | AID Na |
|---|---|---|---|---|---|---|---|---|---|---|
| BW | −0.911 | |||||||||
| (P < 0.0001) | ||||||||||
| FI | −0.823 | 0.810 | ||||||||
| (P < 0.0001) | (P < 0.0001) | |||||||||
| FCR | 0.615 | −0.829 | −0.410 | |||||||
| (P < 0.0001) | (P < 0.0001) | (P = 0.024) | ||||||||
| Permeability | 0.582 | −0.538 | −0.555 | 0.337 | ||||||
| (P < 0.0001) | (P = 0.0004) | (P = 0.003) | (P = 0.086) | |||||||
| VH: CD Duodenum | −0.648 | 0.729 | 0.679 | −0.584 | −0.332 | |||||
| (P < 0.0001) | (P < 0.0001) | (P < 0.0001) | (P = 0.001) | (P = 0.090) | ||||||
| VH: CD Jejunum | -0.789 | 0.761 | 0.695 | −0.513 | −0.459 | 0.654 | ||||
| (P < 0.0001) | (P < 0.0001) | (P < 0.0001) | (P = 0.004) | (P = 0.016) | (P < 0.0001) | |||||
| VH: CD Ileum | −0.440 | 0.488 | 0.501 | −0.372 | −0.140 | 0.544 | 0.381 | |||
| (P = 0.015) | (P = 0.006) | (P = 0.005) | (P = 0.043) | (P = 0.485) | (P = 0.002) | (P = 0.038) | ||||
| IDE | −0.494 | 0.564 | 0.532 | −0.372 | −0.170 | 0.498 | 0.426 | 0.115 | ||
| (P = 0.009) | (P = 0.002) | (P = 0.004) | (P = 0.056) | (P = 0.417) | (P = 0.008) | (P = 0.027) | (P = 0.568) | |||
| AID Na | −0.465 | 0.575 | 0.447 | −0.459 | −0.188 | 0.523 | 0.401 | 0.143 | 0.895 | |
| (P = 0.015) | (P = 0.002) | (P = 0.019) | (P = 0.016) | (P = 0.367) | (P = 0.005) | (P = 0.038) | (P = 0.477) | (P < 0.0001) | ||
| AID K | −0.501 | 0.641 | 0.521 | −0.523 | −0.275 | 0.533 | 0.465 | 0.164 | 0.898 | 0.921 |
| (P = 0.008) | (P < 0.0001) | (P = 0.005) | (P = 0.005) | (P = 0.183) | (P = 0.004) | (P = 0.014) | (P = 0.414) | (P < 0.0001) | (P < 0.0001) |
The study evaluated the effects of graded challenge of Eimeria spp. on growth performance and gastrointestinal health of broiler chickens. Birds were challenged with Eimeria spp. on day 13. (Low, 6,250 oocysts of E. maxima, 6,250 oocysts of E. tenella, and 31,250 oocysts of E. acervulina; Med-low, 12,500 oocysts of E. maxima, 12,500 oocysts of E. tenella, and 62,500 oocysts of E. acervulina; Med-high, 25,000 oocysts of E. maxima, 25,000 oocysts of E. tenella, and 125,000 oocysts of E. acervulina; High, 50,000 oocysts of E. maxima, 50,000 oocysts of E. tenella, and 250,000 oocysts of E. acervulina).
Log2 Dose, numbers of challenged dose were transformed to Log2Dose; BW, body weight; BWG, body weight gain; FI, feed intake; FCR, feed conversion rate; Permeability, Gastrointestinal permeability at 6 D postinfection; VH: CD, ratio of villi height to crypt depth; IDE, ileal digestible energy; AID, apparent ileal digestibility.
Discussion
Severity of Eimeria infection linearly regulated growth performance in the present study which is in agreement with previous reports (Conway et al., 1993; Zhu et al., 2000). The High treatment significantly increased FCR from 1.48 to 2.32 and reduced 49% of BWG compared with the Con. Feed intake decreased linearly and quadratically by 22% in the High treatment. Both BW and FI presented strong negative relationship with the challenge dose (BW, r = −0.911; FI, r = −0.823). A previous study reported that the decrease in FI was the main effects contributing to the reduction in BWG (Kipper et al., 2013), which partially agrees with the results presenting a high correlation coefficients value between BW and FI (r = 0.810).
The dynamic change of intestinal permeability is related to the lifecycle of Eimeria. Upon sporozoites entered in intestinal cells, it will transform into trophozoite in 12 to 48 h (McDougald, 1998). This is a stage prepared for asexual multiple division, reproducing numerous merozoites in the schizonts. When schizonts become mature on 3 DPI, they rapture and release those merozoites in the intestine (McDougald, 1998). Gastrointestinal leakage was slight and could not be measured by FITC-dextran at this time point. However, most of released merozoites could penetrate other epithelial cells and cause tremendous damage on the intestine (Dubey and Jenkins, 2018). Once merozoites enter in the cells, they will repeat the process of development from trophozoite to schizogonous stages (McDougald, 1998). The second generation of merozoites could further penetrate the epithelial cells again. The large number of merozoites severely impair the intestine of chicken. In the current study, the Eimeria infection significantly increases gastrointestinal leakage on 5, 6, and 7 DPI. Moreover, gastrointestinal permeability was linearly increased in response to the graded inoculation dose. The increasing doses could enhance gastrointestinal leakage because more sporozoites in the beginning of infection could reproduce more schizont and merozoites in the following stages. Interestingly, the High and the Med-high groups reached the peak of gastrointestinal permeability on 5 DPI, whereas the Med-low and Low reached on 6 DPI. Crowding effect might be responsible to this finding. The crowding effect occurs when there are large numbers of oocysts occupying the intestine, which leads to a self-inhibition of the reproductive potential and reduction of oocysts shedding (Williams, 1973, 2001). When the birds challenged with fewer oocysts, there might be more space available for E. spp. going through third or fourth asexual cycles, turning out a late peak of gastrointestinal permeability on 6 DPI instead of 5 DPI. Even though the High treatment lead to an earlier peak of gastrointestinal permeability, it still caused the highest FITC-d level among all treatments during the whole infection period. Because the High treatment gave rise to the most severe intestinal impairment, birds needed more time to turn over the intestinal lining cells and had the delay in FITC-d recovery on 7 DPI. If the birds could survive from the severe infection between 5 to 7 DPI, the gastrointestinal permeability would reduce back to normal level on 9 DPI no matter what challenge dose that birds were received.
The results of intestinal lesion scores were also associated with gastrointestinal permeability. The serum FITC-d levels on 6 DPI were very similar among the Med-low, Med-high, and High groups (206, 217, and 234 ng/mL). On the other hand, there was no significant difference among the challenge treatments in the upper and middle-intestinal lesion scores. Thus, based on the results of gastrointestinal permeability, it is speculated a linearly increase of lesion scores in the upper and middle intestine might be found on 5 DPI instead of 6 DPI.
The intestinal epithelium plays an important role as one of many lines of defense in protecting gastrointestinal tract from pathogens (Awad et al., 2017). Those epithelial cells are linked together by junctional complex consisting of the tight junctions, adherens junctions, gap junction, and desmosomes. The tight junction proteins are the most apical transmembrane structure of the intestinal junctional complex, blocking the paracellular pathway between epithelial cells and regulating intestinal permeability (Ulluwishewa et al., 2011). Claudin, occludin, and JAM family are 3 crucial transmembrane proteins building up the main structure of tight junction, whereas plaque proteins, including ZO family proteins, act as adaptors connecting claudin, occludin, and JAM proteins to F-actin in the epithelial cells (Aijaz et al., 2006). In the present study, the gene expression of tight junction proteins was linearly upregulated by graded challenge, suggesting that abnormal changes of claudins and JAM protein were triggered in the stage of acute inflammation (Xu et al., 2016).
The protozoa can impair intestinal cells in the duodenum and reduce endogenous enzymes secretion such as sucrase, isomaltase and sucrase from the intestinal brush border (Su et al., 2014). Without sufficient enzymes to digest carbohydrates and protein, IDE significantly decreased with linear and quadratic responses to the increasing oocysts inoculation in the present study. Similarly, previous studies also reported that coccidia challenge reduced IDE and AID of amino acids (Amerah and Ravindran, 2015; Rochell et al., 2016). Moreover, the lower values of AID of sodium and potassium indicated that there were higher levels of nonabsorbed sodium and potassium in the ileal digesta of Eimeria-challenged birds. Correlation coefficients in Table 5 showed a significant positive relationship between IDE and AID of sodium and potassium, suggesting that the reduction of digestibility is highly related to the electrolyte imbalance in the intestine.
There are several possible reasons for the linear increase of sodium and potassium in the ileal digesta during Eimeria infection. First, the osmotic force exerted by indigestible nutrients pulls sodium ions from the epithelium to the lumen, which increases levels of sodium and potassium in the intestine (Field, 2003). Second, infected birds could not absorb sodium as efficient as nonchallenge birds, because sodium-dependent co-transporters and brush border exchangers might not function properly (Su et al., 2014). The sodium-dependent co-transporters moved sodium along with free amino acids, glucose, and galactose from intestinal lumen to cells (Souba and Pacitti, 1992; Thorsen et al., 2014). If there were few free amino acids or hexose in the intestine, sodium will not be moved by the co-transporters efficiently (Thorsen et al., 2014). Furthermore, a previous study has confirmed that Eimeria infection downregulated gene expression of nutrient transporters, including sodium-dependent amino acid transporters, glucose transporter 2, and amino acid transporters (Su et al., 2014). Apart from co-transporters, brush border exchangers, such as sodium/hydrogen or sodium/potassium-ATPase pumps, which maintain sodium ions balance (Kiela and Ghishan, 2016) might be influenced by Eimeria infection. Coccidiosis causes oxidative stress in the intestinal cells, which inhibits mitochondrial creatine kinase, damages intestinal energetic homeostasis and results in depletion of adenosine triphosphate (ATP) (Galli et al., 2019). Without sufficient ATP, sodium/potassium-ATPase is not capable to move sodium ions across the cell membrane and generates the electrical potential difference for active transportation (Goff, 2015; Adedokun et al., 2016). Third, the inflammatory responses to Eimeria challenge could secrete inflammatory signals, such as NF-κB which stimulates calcium ions signaling, triggers chloride ion secretion, and inhibits sodium absorption in the intestine (Thiagarajah et al., 2015). The inflammatory responses might be associated with the findings that AID of sodium and calcium decreased in the challenge groups. The osmotic forces, ATP deficiency, sodium-dependent co-transporters and brush border exchangers, as well as the inflammatory responses might be contributed to the linear reduction of IDE, AID of sodium and potassium, but the main mechanism causing electrolyte imbalance in the ileal digesta of Eimeria-infected birds remains to be identified in future studies.
In the present study, intestinal morphology showed damaged villi in the challenged birds, especially in the Med-high and High groups (Figure 2, Figure 3, Figure 4). The High treatment reduced villi height in both duodenum and jejunum by 20% compared with the Con. The lower rate of villi height: crypt depth indicates that infected birds have to spend more energy and nutrients accelerating intestinal epithelial cell turnover to expel parasites from the intestine (Cliffe et al., 2005; Clevers, 2013). Moreover, the decreasing villi height is also responsible to the reduction of digestibility in challenged birds as well. The correlation coefficient has showed a moderate relationship between IDE and ratio of villi height to crypt depth in the duodenum (r = 0.5). On the other hand, acute inflammation caused by Eimeria stimulates the proliferation of stem cells at the crypt base which increases intestinal villi height (Sun et al., 2016). A previous study reported that intestinal villi height was increased by low challenge doses (2.5 x 103 and 7 x 103 oocysts) (Sakkas et al., 2018). It is consistent to the current result that Low treatment had numerically longer villi height in the ileum compared with the Con.
Even though there were significant linear effects on nutrient digestibility and intestinal morphology in response to increasing inoculation oocysts doses, it should be noted that the High was not the worst group according to results of several gastrointestinal health parameters. Broilers in the High treatment showed numerically increasing villi height, IDE, AID of sodium and potassium, and numerically lower lesion in the middle-intestine compared with the Med-high. Similarly, a previous study also reported that higher AID of amino acids was observed in the 1 × 106 oocysts-challenged birds compared with the 5 × 105 oocysts dose (Rochell et al., 2016). The crowding effects mentioned above might be potential explanation (Williams, 2001). It suggested that birds challenged with 256,000 oocysts of Eimeria maxima might produce fewer oocysts than birds challenged with 65,000 oocysts. Furthermore, the reproductive potential of other Eimeria spp. was reduced in response to the increase of oocysts inoculation (Williams, 2001). On the other hand, a cross-species crowding effect may exist when E. maxima and Eimeria acervulina compete for available space in the jejunum (Conway and McKenzie, 2007). It is unclear if there is any crowding effect between E. maxima and Eimeria acervulina, but a previous study reported that mixed infection of Eimeria praecox and E. maxima reduced lesion scores and BWG compared with the single challenge of E. praecox or E. maxima (Jenkins et al., 2008), suggesting that a cross-species crowding effect may occur when more than 2 Eimeria spp. occupy the same section of the intestine.
The present study shows how graded challenge of Eimeria impacted on growth performance and intestine health of broiler chickens. However, the challenge dose is not the only factor accounted for severity of infection, but also the virulence of Eimeria strain, viability of oocysts, time, and temperature in storage of oocysts. Thus, fresh passaged Eimeria oocysts are ideal to be used when a study is targeting a specific challenge level. Moreover, the current study reveals the relationship between challenge dose and gastrointestinal parameters, as well as growth performance of chickens. It provides useful information to establish clinical or subclinical challenge models for future studies.
In conclusion, growth performance, apparent ileal digestibility, and other gastrointestinal health parameters showed linear responses to the challenge dose. The gastrointestinal permeability from 3 to 9 DPI provided a whole picture on the dynamic change of intestinal leakage during Eimeria infection. Considering that the peak of intestinal leakage was influenced by different levels of challenge dose, it is recommended to measure gastrointestinal permeability on 5 DPI with higher oocysts doses and 6 DPI when using the lower oocysts doses. Overall, the Med-low and Med-high would be proper doses for future nutrition studies because intestinal health parameters are highly correlated to each other without potential complications such as crowding effect.
Acknowledgments
This study was financed in part by a cooperative agreement 58-6040-8-034 from United States Department of Agriculture-Agricultural Research Service. Special thanks to all members in Dr. Kim's research group and Dr. Jundi LIU.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement
References
- Adedokun S.A., Adeola O. The response in jejunal and ileal nutrient and energy digestibility and the expression of markers of intestinal inflammation in broiler chickens to coccidial vaccine challenge and phytase supplementation. Can. J. Anim. Sci. 2016;97:258–267. [Google Scholar]
- Amerah A.M., Ravindran V. Effect of coccidia challenge and natural betaine supplementation on performance, nutrient utilization, and intestinal lesion scores of broiler chickens fed suboptimal level of dietary methionine. Poult. Sci. 2015;94:673–680. doi: 10.3382/ps/pev022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aijaz S., Balda M.S., Matter K. Tight junctions: molecular architecture and function. Int. Rev. Cytol. Suppl. 2006;248:261–298. doi: 10.1016/S0074-7696(06)48005-0. [DOI] [PubMed] [Google Scholar]
- Awad W.A., Hess C., Hess M. Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins. 2017;9:60. doi: 10.3390/toxins9020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter M.F.A., Merino-Guzman R., Latorre J.D., Mahaffey B.D., Yang Y., Teague K.D., Graham L.E., Wolfenden A.D., Hernandez-Velasco X., Bielke L.R., Hargis B.M., Tellez G. Optimizing fluorescein isothiocyanate dextran measurement as a biomarker in a 24-h feed restriction model to induce gut permeability in broiler chickens. Front. Vet. Sci. 2017;4:56. doi: 10.3389/fvets.2017.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoluzzi C., Lumpkins B., Mathis G.F., Franca M., King W.D., Graugnard D.E., Dawson K.A., Applegate T.J. Zinc source modulates intestinal inflammation and intestinal integrity of broiler chickens challenged with coccidia and Clostridium perfringens. Poult. Sci. 2019;98:2211–2219. doi: 10.3382/ps/pey587. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. Practical use of vaccines for the control of coccidiosis in the chicken. World Poult. Sci. J. 2000;56:7–20. [Google Scholar]
- Chapman H.D. Practical use of vaccines for the control of coccidiosis in the chicken. World Poult. Sci. J. 2007;56:7–20. [Google Scholar]
- Chapman H.D. Milestones in avian coccidiosis research: a review. Poult. Sci. 2014;93:501–511. doi: 10.3382/ps.2013-03634. [DOI] [PubMed] [Google Scholar]
- Chapman H.D., Cherry T.E., Danforth H.D., Richards G., Shirley M.W., Williams R.B. Sustainable coccidiosis control in poultry production: the role of live vaccines. Int. J. Parasitol. 2002;32:617–629. doi: 10.1016/s0020-7519(01)00362-9. [DOI] [PubMed] [Google Scholar]
- Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–284. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Cliffe L.J., Humphreys N.E., Lane T.E., Potten C.S., Booth C., Grencis R.K. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science. 2005;308:1463–1465. doi: 10.1126/science.1108661. [DOI] [PubMed] [Google Scholar]
- Conway D.P., McKenzie E. Blackwell Publishing Professional; Ames, Iowa, United States: 2007. Poultry Coccidiosis: Diagnostic and Testing Procedures. [Google Scholar]
- Conway D.P., Sasai K., Gaafar S.M., Smothers C.D. Effects of different levels of oocyst inocula of Eimeria acervulina, E. tenella, and E. maxima on plasma constituents, packed cell volume, lesion scores, and performance in chickens. Avian Dis. 1993;37:118–123. [PubMed] [Google Scholar]
- Dansky L.M., Hill F.W. Application of the chromic oxide indicator method to balance studies with growing chickens. J. Nutr. 1952;47:449–459. doi: 10.1093/jn/47.3.449. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Jenkins M.C. Re-evaluation of the life cycle of Eimeria maxima Tyzzer, 1929 in chickens (Gallus domesticus) Parasitology. 2018;145:1051–1058. doi: 10.1017/S0031182017002153. [DOI] [PubMed] [Google Scholar]
- Elliott A.C., Hynan L.S. A SAS((R)) macro implementation of a multiple comparison post hoc test for a Kruskal-Wallis analysis. Comput. Meth. Prog. Bio. 2011;102:75–80. doi: 10.1016/j.cmpb.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Field M. Intestinal ion transport and the pathophysiology of diarrhea. J. Clin. Invest. 2003;111:931–943. doi: 10.1172/JCI18326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta G.T., Turner J.R., Taylor C.T., Hershberg R.M., Comerford K., Narravula S., Podolsky D.K., Colgan S.P. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J. Exp. Med. 2001;193:1027–1034. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli G.M., Baldissera M.D., Griss L.G., Souza C.F., Fortuoso B.F., Boiago M.M., Gris A., Mendes R.E., Stefani L.M., da Silva A.S. Intestinal injury caused by Eimeria spp. impairs the phosphotransfer network and gain weight in experimentally infected chicken chicks. Parasitol. Res. 2019;118:1573–1579. doi: 10.1007/s00436-019-06221-0. [DOI] [PubMed] [Google Scholar]
- Goff J.P. Digestion and absorption of nutrients. In: Reece W.O., Erickson H.H., Goff J.P., Uemura E.E., editors. Duke’s Physiology of Domestic Animals 13th Rev. John Wiley & Sons Inc.; Hoboken, NJ: 2015. pp. 502–521. [Google Scholar]
- Jenkins M., Allen P., Wilkins G., Klopp S., Miska K. Eimeria praecox infection ameliorates effects of Eimeria maxima infection in chickens. Vet. Parasitol. 2008;155:10–14. doi: 10.1016/j.vetpar.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Johnson J., Reid W.M. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970;28:30–36. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Kiela P.R., Ghishan F.K. Physiology of intestinal absorption and secretion. Best Pract. Res. Clin. Gastroenterol. 2016;30:145–159. doi: 10.1016/j.bpg.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipper M., Andretta I., Lehnen C.R., Lovatto P.A., Monteiro S.G. Meta-analysis of the performance variation in broilers experimentally challenged by Eimeria spp. Vet. Parasitol. 2013;196:77–84. doi: 10.1016/j.vetpar.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Berghman L.R., Vicuna E.A., Latorre J.D., Menconi A., Wolchok J.D., Wolfenden A.D., Faulkner O.B., Tellez G.I., Hargis B.M., Bielke L.R. Poultry enteric inflammation model with dextran sodium sulfate mediated chemical induction and feed restriction in broilers. Poult. Sci. 2015;94:1220–1226. doi: 10.3382/ps/pev114. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Vicuna E.A., Latorre J.D., Wolfenden A.D., Tellez G.I., Hargis B.M., Bielke L.R. Evaluation of gastrointestinal leakage in multiple enteric inflammation models in chickens. Front. Vet. Sci. 2015;2:66. doi: 10.3389/fvets.2015.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre J.D., Adhikari B., Park S.H., Teague K.D., Graham L.E., Mahaffey B.D., Baxter M.F.A., Hernandez-Velasco X., Kwon Y.M., Ricke S.C., Bielke L.R., Hargis B.M., Tellez G. Evaluation of the epithelial barrier function and ileal microbiome in an established necrotic enteritis challenge model in broiler chickens. Front. Vet. Sci. 2018;5:199. doi: 10.3389/fvets.2018.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McDougald L.R. Intestinal protozoa important to poultry. Poult. Sci. 1998;77:1156–1158. doi: 10.1093/ps/77.8.1156. [DOI] [PubMed] [Google Scholar]
- Mizuno-Yagyu Y., Hashida R., Mineo C., Ikegami S., Ohkuma S., Takano T. Effect of PGI2 on transcellular transport of fluorescein dextran through an arterial endothelial monolayer. Biochem. Pharmacol. 1987;36:3809–3813. doi: 10.1016/0006-2952(87)90442-4. [DOI] [PubMed] [Google Scholar]
- Parker J., Oviedo-Rondon E.O., Clack B.A., Clemente-Hernandez S., Osborne J., Remus J.C., Kettunen H., Makivuokko H., Pierson E.M. Enzymes as feed additive to aid in responses against Eimeria species in coccidia-vaccinated broilers fed corn-soybean meal diets with different protein levels. Poult. Sci. 2007;86:643–653. doi: 10.1093/ps/86.4.643. [DOI] [PubMed] [Google Scholar]
- Rochell S.J., Parsons C.M., Dilger R.N. Effects of Eimeria acervulina infection severity on growth performance, apparent ileal amino acid digestibility, and plasma concentrations of amino acids, carotenoids, and alpha1-acid glycoprotein in broilers. Poult. Sci. 2016;95:1573–1581. doi: 10.3382/ps/pew035. [DOI] [PubMed] [Google Scholar]
- Sakkas P., Oikeh I., Blake D.P., Nolan M.J., Bailey R.A., Oxley A., Rychlik I., Lietz G., Kyriazakis I. Does selection for growth rate in broilers affect their resistance and tolerance to Eimeria maxima? Vet. Parasitol. 2018;258:88–98. doi: 10.1016/j.vetpar.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souba W.W., Pacitti A.J. How amino acids get into cells: mechanisms, models, menus, and mediators. J. Parenter. Enteral. Nutr. 1992;16:569–578. doi: 10.1177/0148607192016006569. [DOI] [PubMed] [Google Scholar]
- Su S., Miska K.B., Fetterer R.H., Jenkins M.C., Wong E.A. Expression of digestive enzymes and nutrient transporters in Eimeria acervulina-challenged layers and broilers. Poult. Sci. 2014;93:1217–1226. doi: 10.3382/ps.2013-03807. [DOI] [PubMed] [Google Scholar]
- Sun L.L., Dong H.B., Zhang Z.C., Liu J., Hu Y., Ni Y.D., Grossmann R., Zhao R.Q. Activation of epithelial proliferation induced by Eimeria acervulina infection in the duodenum may be associated with cholesterol metabolism. Oncotarget. 2016;7:27627–27640. doi: 10.18632/oncotarget.8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takhar B.S., Farrell D.J. Energy and nitrogen metabolism of chickens infected with either Eimeria acervulina or Eimeria tenella. Br. Poult. Sci. 1979;20:197–211. doi: 10.1080/00071667908416569. [DOI] [PubMed] [Google Scholar]
- Teng P.Y., Chang C.L., Huang C.M., Chang S.C., Lee T.T. Effects of solid-state fermented wheat bran by Bacillus amyloliquefaciens and Saccharomyces cerevisiae on growth performance and intestinal microbiota in broiler chickens. Ital. J. Anim. Sci. 2017;16:552–562. [Google Scholar]
- Thiagarajah J.R., Donowitz M., Verkman A.S. Secretory diarrhoea: mechanisms and emerging therapies. Nat. Rev. Gastroenterol. Hepatol. 2015;12:446–457. doi: 10.1038/nrgastro.2015.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsen K., Drengstig T., Ruoff P. Transepithelial glucose transport and Na+/K+ homeostasis in enterocytes: an integrative model. Am. J. Physiol. Cell Physiol. 2014;307:320–337. doi: 10.1152/ajpcell.00068.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulluwishewa D., Anderson R.C., McNabb W.C., Moughan P.J., Wells J.M., Roy N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011;141:769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- Vicuna E.A., Kuttappan V.A., Tellez G., Hernandez-Velasco X., Seeber-Galarza R., Latorre J.D., Faulkner O.B., Wolfenden A.D., Hargis B.M., Bielke L.R. Dose titration of FITC-D for optimal measurement of enteric inflammation in broiler chicks. Poult. Sci. 2015;94:1353–1359. doi: 10.3382/ps/pev111. [DOI] [PubMed] [Google Scholar]
- Walk C.L., Cowieson A.J., Remus J.C., Novak C.L., McElroy A.P. Effects of dietary enzymes on performance and intestinal goblet cell number of broilers exposed to a live coccidia oocyst vaccine. Poult. Sci. 2011;90:91–98. doi: 10.3382/ps.2010-00760. [DOI] [PubMed] [Google Scholar]
- Williams R.B. Effects of different infection rates on the oocyst production of Eimeria acervulina or Eimeria tenella in the chicken. Parasitology. 1973;67:279–288. doi: 10.1017/s0031182000046515. [DOI] [PubMed] [Google Scholar]
- Williams R.B. Quantification of the crowding effect during infections with the seven Eimeria species of the domesticated fowl: its importance for experimental designs and the production of oocyst stocks. Int. J. Parasitol. 2001;31:1056–1069. doi: 10.1016/s0020-7519(01)00235-1. [DOI] [PubMed] [Google Scholar]
- Xu C.M., Li X.M., Qin B.Z., Liu B. Effect of tight junction protein of intestinal epithelium and permeability of colonic mucosa in pathogenesis of injured colonic barrier during chronic recovery stage of rats with inflammatory bowel disease. Asian Pac. J. Trop. Med. 2016;9:148–152. doi: 10.1016/j.apjtm.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Yan Y., Kolachala V., Dalmasso G., Nguyen H., Laroui H., Sitaraman S.V., Merlin D. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS One. 2009;4:e6073. doi: 10.1371/journal.pone.0006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun C.H., Lillehoj H.S., Lillehoj E.P. Intestinal immune responses to coccidiosis. Dev. Comp. Immunol. 2000;24:303–324. doi: 10.1016/s0145-305x(99)00080-4. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Chen X., Eicher S.D., Ajuwon K.M., Applegate T.J. Effect of threonine deficiency on intestinal integrity and immune response to feed withdrawal combined with coccidial vaccine challenge in broiler chicks. Br. J. Nutr. 2016;116:2030–2043. doi: 10.1017/S0007114516003238. [DOI] [PubMed] [Google Scholar]
- Zhu J.J., Lillehoj H.S., Allen P.C., Yun C.H., Pollock D., Sadjadi M., Emara M.G. Analysis of disease resistance-associated parameters in broiler chickens challenged with Eimeria maxima. Poult. Sci. 2000;79:619–625. doi: 10.1093/ps/79.5.619. [DOI] [PubMed] [Google Scholar]