Abstract
Defensins are antimicrobial peptides composed of 3 conserved disulfide bridges, a β-sheet, and both hydrophobic and cationic amino acids. In this study, we aimed to demonstrate the immunomodulation role of avian β-defensin 8 (AvBD8) in a chicken macrophage cell line. Chicken AvBD8 stimulated the expression of proinflammatory cytokines (IL-1β, interferon gamma, and IL-12p40) and chemokines (CCL4, CXCL13, and CCL20) in macrophages. Furthermore, by Western blotting and immunocytochemistry, we confirmed that AvBD8 activated the mitogen-activated protein kinase signaling pathway via extracellular regulated kinases 1/2 and p38 signaling molecules. Overall, AvBD8 plays a crucial role in host defense as not only an antimicrobial peptide but also an immunomodulator by activating the mitogen-activated protein kinase signaling pathway and inducing the expression of proinflammatory cytokines and chemokines.
Key words: avian β-defensin 8, chicken, immunomodulation, MAPK signaling pathway
Introduction
Antimicrobial peptides (AMP) are known to play an important role in innate immunity by killing bacteria, viruses, and fungi (Reddy et al., 2004). As a first line of defense, AMP kill the invading bacteria mainly by disrupting the bacterial cell membrane, but they might also interfere with DNA, protein, and cell wall syntheses and protein folding (Nguyen et al., 2011). Because most of the AMP have cationic properties, they can attach to negatively charged components in the bacterial cell membrane, such as lipopolysaccharide and lipoteichoic acid. In addition to their direct killing effect, AMP can modulate the immune response. They inhibit lipopolysaccharide-induced proinflammatory cytokine production, induce proinflammatory cytokine production, and promote wound healing (Scott and Hancock, 2000, Scott et al., 2002; Kim et al., 2017). Owing to their various mechanisms of action, broad-spectrum antimicrobial activities, and immunomodulation effect, AMP have been actively studied as alternative antibiotic and immune therapy agents.
The defensin peptides are cysteine-rich antimicrobial peptides composed of 3 conserved disulfide bridges, a β-sheet, and both hydrophobic and cationic amino acids. Defensins are classified into the following 3 categories based on their structure: α-, β-, and θ-defensins (Yang et al., 2004; Selsted and Ouellette, 2005) and are found in mammals, vertebrates, and old world monkeys, respectively (Martin et al., 1995; Ganz 2003). Defensins exhibit antimicrobial activity against pathogens, including gram-negative and gram-positive bacteria, fungi, and certain enveloped viruses such as the human immunodeficiency virus (Harwig et al., 1994; Thomma et al., 2002; Donovan and Topley, 2003). In addition to the antimicrobial activity, defensins also play a role in immunomodulation. Defensins have monocyte chemotactic activity in human neutrophils (Territo et al., 1989), T lymphocytes (Chertov et al., 1996), and immature dendritic cells (Yang et al., 1999). Especially, β-defensins are well known as an immunomodulator. For example, human β-defensin 3 induces the expression of costimulatory molecules on immune cells (Funderburg et al., 2007). Furthermore, human β-defensin 3 has chemotactic effects in immune cells via chemokine receptors 2 and 6 (Röhrl et al., 2010; Smithrithee et al., 2015). Human β-defensin 3 also shows anti-inflammatory activity by targeting the Toll-like receptor (TLR) signaling pathways (Semple et al., 2011).
Avian species express only β-defensins, named as avian β-defensins (AvBD). To date, 14 AvBD (AvBD1–AvBD14) have been identified in the chicken, turkey, duck, ostrich, and king penguin (Evans et al., 1994, 1995; Yu et al., 2001; Thouzeau et al., 2003; Lynn et al., 2004; Soman et al., 2009). Most of these β-defensins exhibit antimicrobial activity against various pathogens, including gram-negative and gram-positive bacteria, fungi, and viruses (Evans et al., 1994; Yu et al., 2001; Ganz and Lehrer, 2002; Ganz 2003; Higgs et al., 2005; Yacoub et al., 2015). However, details of immune regulation mechanisms of AvBDs are limited. In this study, a chicken macrophage cell line was used because macrophages have important roles in immune system including phagocytosis, antigen-presenting cells, and wound healing (Eming et al., 2007). Therefore, we examined the immunomodulating mechanism of AvBD8 in a chicken macrophage cell line.
Materials and methods
Reagents and Antibodies
Rabbit phospho-p44/42 mitogen-activated protein kinase (MAPK) (Erk1/2) (Thr202/Tyr204, #4370) and rabbit phospho-p38 MAPK (Thr180/Tyr182, #4631) monoclonal antibodies were purchased from Cell Signaling (Danvers, MA). Mouse anti-chicken glyceraldehyde-3-phosphate dehydrogenase antibody was purchased from Thermo Fisher Scientific (AM4300; Waltham, MA). Alexa Fluor 488 goat anti-rabbit IgG (H + L) secondary antibody was purchased from Invitrogen (A-11008, Carlsbad, CA). Anti-rabbit IgG (H + L) horseradish peroxidase (HRP)-conjugated antibody was purchased from Promega (W4011; Madison, WI). Goat anti-mouse IgG HRP-conjugated antibody (A16078) was purchased from Thermo Fisher Scientific. In addition, HRP-conjugated rabbit anti-6-His antibody (A190-114P) was purchased from Bethyl Laboratories, Inc. (Montgomery, TX) and 4′,6-diamidino-2-phe-nylindole was purchased from Invitrogen (Rockford, IL). Radioimmunoprecipitation assay lysis and extraction buffers were purchased from Thermo Fisher Scientific.
Cloning of AvBD8
The primers were designed using DNASTAR (DNASTAR Incorporation, Madison, WI) to amplify the mature sequence of AvBD8 from Gallus gallus AvBD 8 (AvBD8) mRNA sequence (NM_0 01001781.1). The AvBD8 coding sequence was amplified using total RNA derived from the intestinal mucosal layer of White Leghorn chickens, kindly provided by the Animal Biosciences and Biotechnology Laboratory (Beltsville, MD) of the USDA Agricultural Research Service. The PCR product was amplified using the following specific primers: forward, 5′-CGGAATTCAACAACGAGGCACAGTGTG-3′ and reverse, 5′- CCAAGCTTGTCGTACACAGTCCG-3′ (the EcoRI and HindIII restriction enzyme sites are underlined) with the DreamTaq Green PCR Master Mix (2 × ) (Thermo Fisher Scientific). The PCR amplification was carried out under the following conditions: a predenaturation step at 95°C for 5 min, a denaturation step at 95°C for 30 s, an annealing step at 55°C for 30 s, an extension step at 72°C for 30 s for 35 cycles, and a final extension at 72°C for 5 min. The PCR products were purified using the FavorPrep GEL/PCR purification kit (Favorgen, Pingtung, Taiwan), cloned into the pCR2.1-TOPO vector (Invitrogen), and transformed using Escherichia coli TOP10 competent cells (Invitrogen) as per the manufacturer's protocol. Through blue–white screening, positive clones were picked out and cultured overnight in Luria–Bertani broth (with 100 μg/mL ampicillin). Plasmids were extracted using the FavorPrep plasmid DNA extraction mini kit (Favorgen) and sequenced by GenoTech (Daejeon, South Korea). The AvBD8/pCR2.1-TOPO vector was digested with the restriction enzymes EcoRI and HindIII (Thermo Fisher Scientific). The protein expression vector pET32a (Novagen, Madison, WI) was also digested with the same restriction enzymes. The digested fragments were purified by agarose gel electrophoresis using the FavorPrep GEL/PCR purification kit (Favorgen) and ligated using T4 DNA ligase (Invitrogen). The ligated vector and insert were transformed into One Shot BL21 (DE3) chemically competent E. coli (Invitrogen) and sequenced.
Production of Recombinant AvBD8 Protein
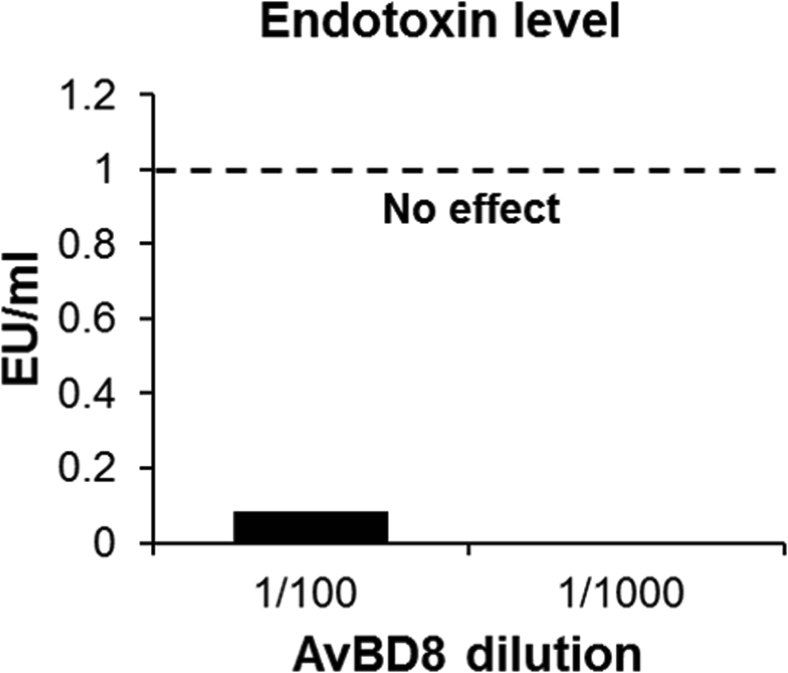
Recombinant AvBD8 protein was produced as previously described for chicken IL-26 (Truong et al., 2016c). Briefly, the positive clones of AvBD8/pET32a were incubated at 37°C overnight in a shaking incubator at 225 rpm in Luria–Bertani broth with 100 μg/mL ampicillin. The bacterial culture was then induced for recombinant protein expression with 1 mM isopropyl-β-D-thiogalactopyranoside (USB Corporation, Cleveland, OH) for 4 h at 37°C and then centrifuged at 5,000 × g for 15 min. The AvBD8 recombinant protein was extracted with the B-PER Bacterial Protein Extraction Reagent (Thermo Fisher Scientific) and purified using HisPur Cobalt Resin (Thermo Fisher Scientific). The recombinant AvBD8 protein was eluted using 250 mM imidazole and analyzed by SDS-PAGE and Western blotting using HRP-conjugated rabbit anti-6-His antibody (Bethyl Laboratories). The purified recombinant protein was dialyzed using SnakeSkin dialysis tubing (Thermo Fisher Scientific) in PBS (pH 7.4) overnight at 4°C with stirring and analyzed by SDS-PAGE and Western blotting. Endotoxins in recombinant AvBD8 were evaluated using the Pierce Chromogenic Endotoxin Quant Kit (Thermo Fisher Scientific) as per the manufacturer's protocol.
Cell Culture and Recombinant Protein Treatment
Chicken macrophage cell line HD11 (Klasing and Peng, 1987) was cultured in complete RPMI 1640 medium (Thermo Fisher Scientific) containing 100 IU/mL penicillin, 100 mg/mL streptomycin, and 10% heat-inactivated fetal bovine serum (Thermo Fisher Scientific) in a humidified 5% CO2 atmosphere at 41°C. The cells (1.0 × 106/well) were incubated in a 12-well plate containing 1 mL of culture medium, treated with 100 ng/mL (final concentration) recombinant AvBD8 protein, and incubated for 0, 0.5, 1, 2, and 4 h in a humidified 5% CO2 atmosphere at 41°C.
Quantitative Real-Time PCR
HD11 cells were washed with ice-cold PBS, and then, total RNA was extracted from the cells using TRIzol reagent (Thermo Fisher Scientific), as per the manufacturer's protocol. Briefly, 0.3 mL of TRIzol reagent was added to each well of a cell culture dish after washing the cells with PBS; then, cell lysates were harvested for RNA extraction. cDNA was synthesized from the total RNA using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) as per the manufacturer's protocol. To analyze the cytokine gene expression, primers were designed using Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) (Table 1) and quantitative real-time PCR was performed using FastStart Essential DNA Green Master (Roche, Indianapolis, IN), as per the manufacturer's instructions, in the LightCycler 96 system (Roche). The chicken glyceraldehyde-3-phosphate dehydrogenase gene was used as the control to normalize RNA quantity. The relative quantification of gene-specific expression was calculated using the 2−ΔΔCt method after normalization with the glyceraldehyde-3-phosphate dehydrogenase gene expression level (Livak and Schmittgen, 2001). All quantitative real-time PCR were performed in triplicate.
Table 1.
Sequence of primers for qRT-PCR analysis of gene expression.
| Primer | F/R | Nucleotide sequence (5′-3′) | Accession No. |
|---|---|---|---|
| GAPDH | F | TGCTGCCCAGAACATCATCC | NM_204305 |
| R | ACGGCAGGTCAGGTCAACAA | ||
| IL-1β | F | TGCCTGCAGAAGAAGCCTCG | NM_204524 |
| R | CTCCGCAGCAGTTTGGTCAT | ||
| IFN-γ | F | AACAACCTTCCTGATGGCGT | NM_205149.1 |
| R | TGAAGAGTTCATTCGCGGCT | ||
| IL-12p40 | F | AGATGCTGGCAACTACACCTG | NM_213571 |
| R | CATTTGCCCATTGGAGTCTAC | ||
| CCL4 | F | CTTCACCTACATCTCCCGGC | NM_001030360 |
| R | CTGTACCCAGTCGTTCTCGG | ||
| CXCL13 | F | GCCTGTGCCTGGTGCTC | NM_001348657.1 |
| R | TGCCCCCTTCCCCTAAC | ||
| CCL20 | F | AGGCAGCGAAGGAGCAC | NM_204438 |
| R | GCAGAGAAGCCAAAATCAAAC | ||
| CD40 | F | GCCTCTGAATGCAACGACAC | NM_204665.2 |
| R | CCAGCGTTGTCCTCACAGAT |
Abbreviation: qRT-PCR, quantitative real-time PCR.
Western Blotting
HD11 cells (5.0 × 106/well) were incubated in a 6-well plate (Thermo Scientific) containing 2 mL of culture medium and stimulated with 100 ng/mL recombinant AvBD8 proteins for 0, 15, 30, 60, and 90 min in a humidified 5% CO2 atmosphere at 41°C. After incubation, the cells were washed with ice-cold PBS, and the proteins were extracted from the cells using radioimmunoprecipitation assay lysis and extraction buffers as per the manufacturer's protocol. Halt Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific) was added to the cell lysate. The cell protein concentration was measured using the Pierce BCA protein assay kit (Thermo Fisher Scientific) as per the manufacturer's protocol. Protein samples were mixed with 4 × sample buffer (200 mM Tris-Cl [pH 6.8], 20% β-mercaptoethanol, 8% SDS, 0.4% bromophenol blue, and 40% glycerol) and heated to 100°C for 5 min. The protein samples (30 μg) were electrophoresed on 12% Tris–glycine SDS polyacrylamide gels. The separated proteins were transferred onto polyvinylidene difluoride membranes (GE Healthcare, Rydalmere, Australia) using the Mini-PROTEAN electrophoresis system (Bio-Rad, Hercules, CA). The membranes were blocked with 5% skim milk (Thermo Fisher Scientific) in PBS (pH 7.4) containing 0.05% Tween-20 (Sigma-Aldrich, MO) (PBST). Antibodies were prepared with 2% skim milk in PBST. The membranes were washed with PBST and treated with anti-rabbit IgG (H + L), HRP-conjugated antibody (Promega). The membranes were then developed using Western Lightning Plus-ECL (Thermo Fisher Scientific) for hyperfilm (GE Healthcare).
Immunocytochemistry
Immunocytochemistry was performed using the Nunc Lab-Tek Chamber Slide (Thermo Fisher Scientific) as previously described (Truong et al., 2016a). Briefly, HD11 cells (4.0 × 104 cells/well) were cultured in a chamber slide for 30 min in a humidified 5% CO2 atmosphere incubator at 41°C in the presence or absence of the recombinant AvBD8 proteins (100 ng/mL). The cells were then fixed with 4% paraformaldehyde in PBS (pH 7.4) for 15 min and then incubated with ice-cold methanol for 10 min at 4°C. After overnight incubation with the anti-rabbit primary antibody at 4°C, the cells were treated with Alexa Fluor488–conjugated secondary antibody for 1 h and then stained with 4′,6-diamidino-2-phe-nylindole for 5 min. Finally, images were captured using the EVOS FLoid Cell Imaging Station (Life Technologies, Carlsbad, CA).
Bioactivity Assay
HD11 cells (2.0 × 104 cells/well) were seeded and cultured in 24-well plates. After culturing overnight, the cells were incubated with the AvBD8 recombinant protein (50, 100, 200, 300, 400, 5,000, and 1,000 ng/mL) for 72 h in a humidified 5% CO2 atmosphere at 41°C to analyze cell proliferation and nitric oxide (NO) production. The NO content was measured using the Griess Reagent System (Promega) and cell proliferation was measured using the Cell Counting Kit-8 (Dojindo Molecular Technologies, Kumamoto, Japan) as per the manufacturer's protocols as previously described (Truong et al., 2016c).
Bioinformatics Analysis
The purified plasmid was sequenced by Genotech (Daejeon, Republic of Korea). To compare the cloned chicken AvBD8 sequence with sequences in GenBank, the data were analyzed using a Nucleotide Basic Local Alignment Search Tool search (http://www.ncbi.nlm.nih.gov/BLAST/). Protein identification was performed using the Expert Protein Analysis System (https://www.expasy.org/) to determine the molecular weight. The protein structure was predicted using RaptorX (http://raptorx.uchicago.edu/) and FirstGlance in Jmol (http://www.bioinformatics.org/firstglance/fgij/).
Statistical Analysis
Data are presented as mean ± SEM of 3 independent experiments. Statistical analyses were performed using IBM SPSS software (SPSS 23.0 for Windows; IBM, Chicago, IL). The results with a P-value of <0.05 were considered statistically significant. Differences between groups were evaluated using Duncan's multiple range test.
Results
Structural Analysis of Chicken AvBD8
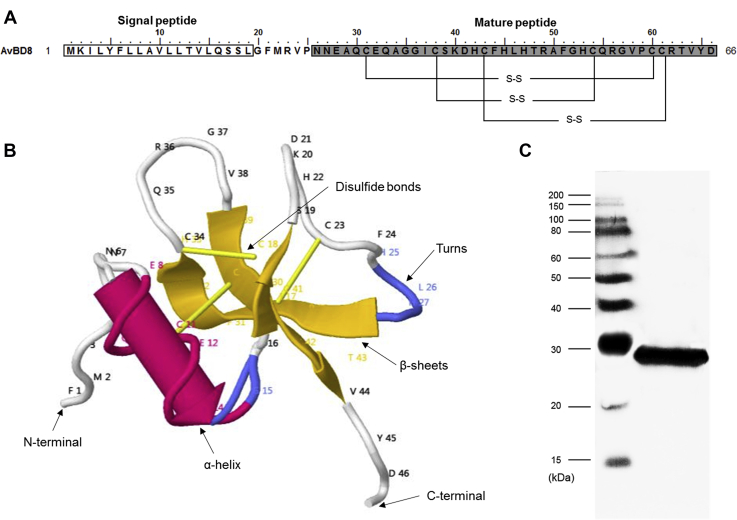
Chicken AvBD8 is composed of a signal peptide (19 aa) and mature peptide (40 aa). It also has 3 disulfide bonds, a typical feature of defensins (Figure 1A). The secondary structure of mature AvBD8 is composed of 1 alpha-helix, 3 beta-sheets, and 3 disulfide bonds (Figure 1B).
Figure 1.
(A) Secondary structure of AvBD8. The white box indicates the signal peptide and gray box indicates the mature peptide. S-S indicates disulfide bonds. (B) The 3-dimensional structure of AvBD8. The letters are abbreviations of amino acids and the numbers beside the abbreviations indicate the position of amino acids in the protein. Alpha helices are shown as red rockets and beta strands are shown as yellow planks; blue lines indicate turns and yellow sticks indicate disulfide bonds. (C) Western blot of recombinant AvBD8 using the anti-6ⅹHistidine antibody. Abbreviation: AvBD8, avian β-defensin 8.
Avian β-Defensin 8 Recombinant Protein Expression
The mature AvBD8 recombinant proteins are produced by cloning mature AvBD8 sequences to pET32a expression vector. After inducing AvBD8 recombinant proteins, target proteins were purified by immobilized metal affinity chromatography methods using the HisPur Cobalt Resin. The purified recombinant proteins were confirmed by Western blotting (Figure 1C). The molecular weight of the mature AvBD8 protein was 4.5 kDa and that of the fusion recombinant protein of pET32a vector was 22 kDa (Trx-Tag, His-tag, thrombin, and S-Tag). Therefore, the target protein molecular weight was around 26.5 kDa.
Avian β-Defensin 8 Stimulates Th1 Cytokine and Chemokine Expression
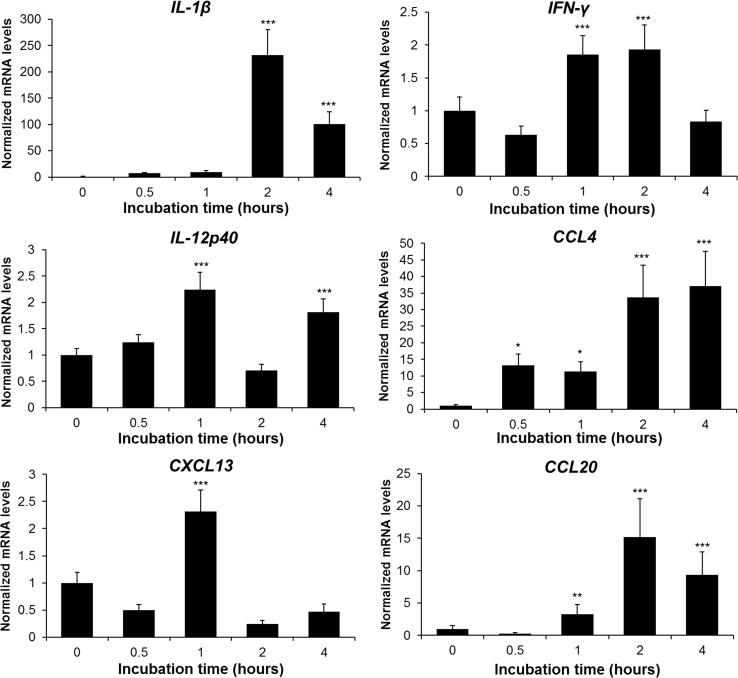
Chicken HD11 cells were stimulated by 100 ng/mL AvBD8 recombinant proteins to examine the role of AvBD8. Th1 cytokine (IL-1β, interferon gamma, and IL-12p40) and chemokine (CCL4, CXCL13, and CCL20) expression was stimulated by AvBD8 (Figure 2). The Th1 cytokine IL-1β was strongly stimulated (231.52-fold), whereas interferon gamma was moderately expressed (1.93-fold) after 2 h of incubation. Furthermore, the expression of IL-12p40 was significantly upregulated by 2.23 times at 1 h. The expression of the chemokine CCL4 was significantly upregulated by 13.26 times at 0.5 h and increased to 37.14 times at 4 h. The expression of CXCL13 was significantly increased by 2.31 times at 1 h. CCL20 was significantly upregulated by 15.20 times at 2 h.
Figure 2.
Effects of AvBD8 on proinflammatory cytokine and chemokine production. HD11 cells were induced by AvBD8 (100 ng/mL) for 0, 0.5, 1, 2, and 4 h and the expression of cytokines and chemokines was measured by qRT-PCR. Expression level was normalized to that of GAPDH. All culture conditions were tested in triplicate. The data are expressed as mean ± SEM and are representative of 3 independent experiments: ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Abbreviations: AvBD8, avian β-defensin 8; IFN, interferon; qRT-PCR, quantitative real-time PCR.
Avian β-Defensin 8 Activates MAPK Signaling Pathway
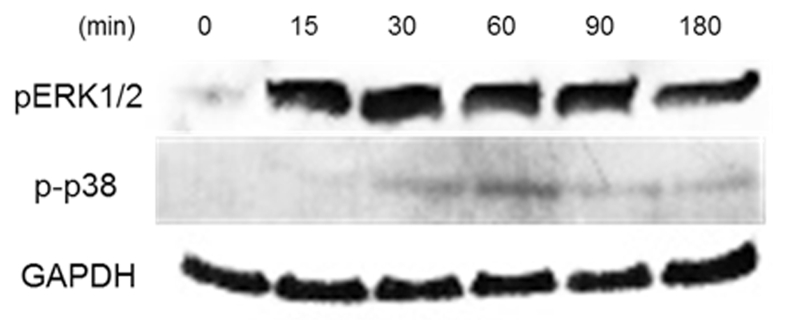
HD11 cells were stimulated with 100 ng/mL recombinant AvBD8 protein for 0, 15, 30, 60, 90, and 120 min to investigate whether AvBD8 is involved in the MAPK pathway. Phosphorylated (Thr202/Tyr204) extracellular regulated kinases 1/2 (ERK1/2) was detected at 15 min after AvBD8 protein treatment, and its level peaked at 30 min and decreased marginally until 120 min (Figure 3). Phosphorylated (Thr180/Tyr182)5 p38 was also detected at 30 min after AvBD8 treatment, and its level peaked at 60 min and then gradually decreased.
Figure 3.
Western blotting of p-ERK1/2 and p-p38. HD11 cells were induced by AvBD8 (100 ng/mL) for 0, 15, 30, 60, 90, and 180 min. Total cell lysates were analyzed by western blotting using antibodies against p-ERK1/2, p-p38, and GAPDH. Abbreviations: AvBD8, avian β-defensin 8; ERK1/2, extracellular regulated kinases 1/2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
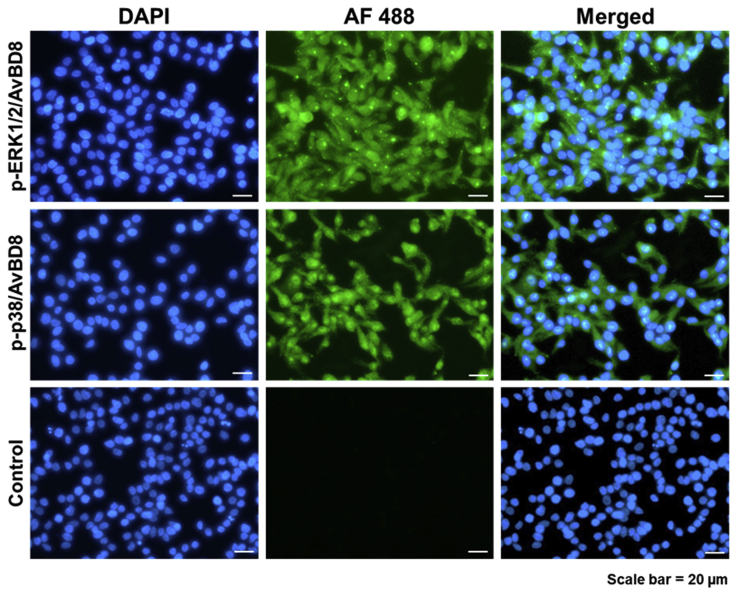
Furthermore, to visualize phosphorylated ERK1/2 and p38, HD11 cells were stimulated with 100 ng/mL AvBD8 protein for 1 h for ICC. The signal of phosphorylated ERK1/2 and p38 was observed in the cytoplasm (green color) after treatment with the recombinant AvBD8 protein (Figure 4). Overall, these results suggested that chicken AvBD8 mediates immune response via the MAPK signaling pathway.
Figure 4.
Immunocytochemistry analysis of p-ERK1/2 and p-p38. HD11 cells were induced by AvBD8 (100 ng/mL) for 1 h and analyzed using immunocytochemistry antibodies against p-ERK1/2 and p-p38. Both untreated and AvBD8-treated cells were incubated with the primary antibodies and Alexa Fluor 488 goat anti-rabbit IgG (H + L) secondary antibody, and then stained with DAPI (blue). Scale bar = 20 μm. Abbreviations: AvBD8, avian β-defensin 8; DAPI, 4′, 6-diamidino-2-phe-nylindole; ERK1/2, extracellular regulated kinases 1/2.
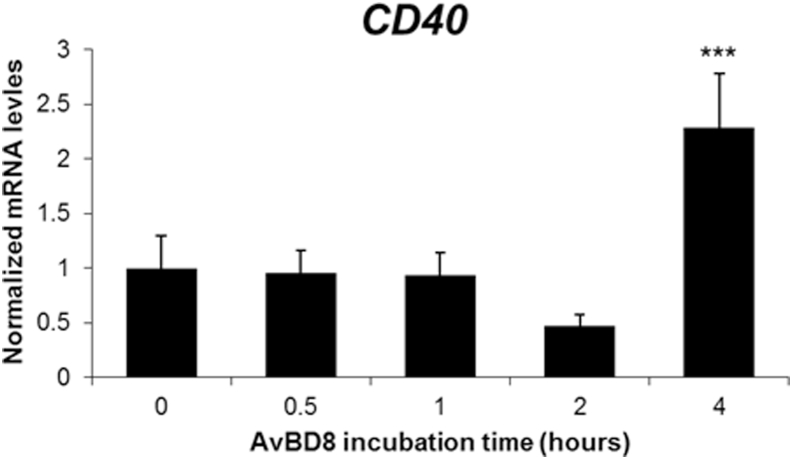
Avian β-Defensin 8 Induces the Expression of Cluster of Fifferentiation 40
Cluster of differentiation 40 (CD40) is a protein that is expressed in antigen-presenting cells. After binding with the CD40 ligand in T cells, it activates antigen-presenting cells and triggers the downstream signals. Avian β-defensin 8 significantly induced the expression of CD40 after 4 h of incubation, with a fold change of 2.28 (Figure 5).
Figure 5.
AvBD8 induced the expression of CD40. HD11 cells were induced with AvBD8 (100 ng/mL) for 0, 0.5, 1, 2, and 4 h and CD40 expression was measured by qRT-PCR. Expression level was normalized to that of GAPDH. All culture conditions were tested in triplicate. The data are expressed as mean ± SEM and are representative of 3 independent experiments: ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Abbreviations: AvBD8, avian β-defensin 8; CD40, cluster of differentiation 40; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; qRT-PCR, quantitative real-time PCR.
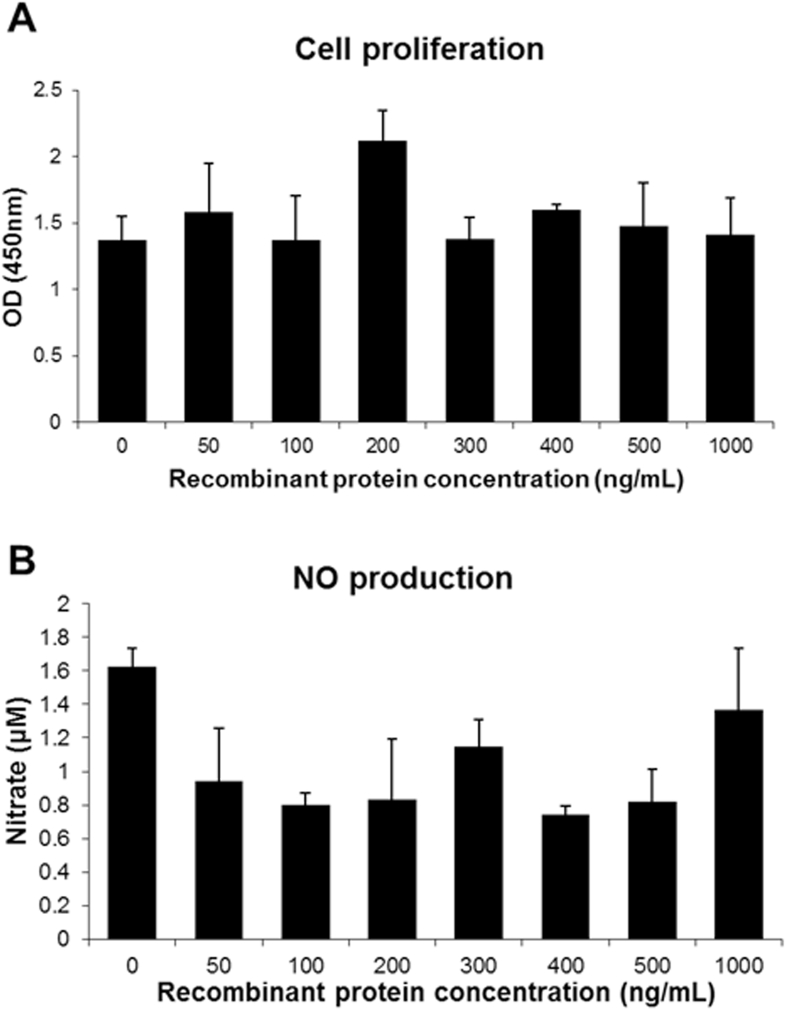
Cell Proliferation and NO Production
Cell proliferation and NO production were measured to determine the effects of AvBD8 in the chicken HD11 cell line (Supplementary Figure 1). The results showed that the proliferation of chicken HD11 cells was neither significantly inhibited nor enhanced. Moreover, NO production was not affected by AvBD8 treatment.
Discussion
In this study, we investigated the immune regulatory mechanism of AvBD8 in a chicken macrophage cell line. Avian β-defensin 8 stimulated proinflammatory cytokines and chemokines expression in a chicken macrophage cell line and activated the MAPK signaling pathway and CD40 expression in chicken macrophage cell line.
α-Defensins are unique to mammals, whereas β-defensins are found ubiquitously in vertebrate species, including avian species (Lynn 2007; Lynn and Bradley, 2007). As an AMP, several AvBD have antimicrobial activities against both gram-positive and gram-negative bacteria (Sugiarto and Yu, 2006; van Dijk et al., 2008). For example, chicken AvBD2, AvBD3, AvBD4, AvBD6, AvBD7, AvBD11, and AvBD12 showed antibacterial effect against E. coli (Lee et al., 2016). Furthermore, duck AvBD2 showed antibacterial effect against Micrococcus lutues, E. coli, and Riemerella anatipestifer (Soman et al., 2009).
Besides antimicrobial activity, AvBD have immunomodulatory activity. Zhao et al. (2016) demonstrated the role of AvBD6 and AvBD12. Avian β-defensin 6 and 12 showed lipopolysaccharide-neutralizing effect and chemotactic effect for chicken macrophages expressing chicken chemokine receptor 2. In addition, AvBD12 induced migration of murine immature dendritic cells. Futhermore, mRNA expression of AvBD2 was upregulated with Newcastle disease virus infection in chicken embryo fibroblasts through p38 MAPK-dependent manner (Liu et al., 2018). Yang et al. (1999) demonstrated that human β-defensin 2 induced chemoattraction of CD4+ memory T cells and immature dendritic cells, by binding to chemokine receptor 6. Moreover, human β-defensin 2, 3, and 4 stimulated the expression of IL-6, IL-10, IP-10, monocyte chemoattractant protein-1, and macrophage inflammatory protein-3α in keratinocytes and induced the phosphorylation of epidermal growth factor receptor, signal transducer and activator of transcription-1 and 3, which are intracellular signaling molecules (Niyonsaba et al., 2007). In this study, AvBD8 upregulated the expression of proinflammatory cytokines (IL-1β, interferon gamma, and IL-12p40) and chemokines (CCL4, CXCL13, and CCL20) (Figure 2). Proinflammatory cytokines initiate the inflammatory response as a host defense mechanism against pathogens mediating the innate immune response. Chemokines induce the migration of immune cells to the site of injury and inflammation (Le et al., 2004). CCL4, known as macrophage inflammatory protein-1β, is a chemoattractant that recruits regulatory T cells to B cells (Bystry et al., 2001), and CXCL13, known as B cell–attracting chemokine 1, regulates the organization of B cells in lymphoid tissues (Ansel et al., 2002). CCL20, known as macrophage inflammatory protein-3, has chemoattractant effects on lymphocytes and dendritic cells. Furthermore, the expression of CCL20 can be stimulated by lipopolysaccharide and proinflammatory cytokines including tumor necrosis factor and interferon gamma (Hieshima et al., 1997; Schutyser et al., 2000). Avian β-defensin 8 regulates the innate immune system of a host infected with pathogens by acting as not only an antimicrobial agent but also an immune-stimulating system via proinflammatory cytokines and chemokines.
The MAPK signaling pathway plays an important role in innate and adaptive immunity and is involved in various cellular functions such as inflammation, cell differentiation, cell proliferation, and cell death (Krishna and Narang, 2008). Extracellular regulated kinases 1/2 and p38 are MAPK that activate various transcription factors and induce the transcription of cytokines and chemokines (Garrington and Johnson, 1999; Ballif and Blenis, 2001; Krishna and Narang, 2008). Bowdish et al. (2004) demonstrated that the human cationic peptide LL-37 activated the MAPK pathway. Kim et al. (2017) demonstrated that chicken NK-lysin–derived cNK-2 stimulated the MAPK pathway and induced the expression of proinflammatory cytokines and chemokines. In chicken, the MAPK pathway–related genes were differentially expressed in necrotic enteritis (NE)–afflicted 2 inbred chicken lines, namely, Marek's disease–resistant line 6.3 and Marek's disease-susceptible line 7.2 (Truong et al., 2017). In the present study, AvBD8 activated the MAPK signaling pathway by phosphorylating ERK1/2 and p38 signaling molecules (Figures 3 and 4). Therefore, we could know that chicken AvBD8 can modulate immune system by activating the MAPK signaling pathway.
Murine β-defensin 2 induce costimulatory molecules CD40, CD80, and CD86 by directly acting on immature dendritic cells as an endogenous ligand for TLR-4 (Biragyn et al., 2002). Furthermore, human β-defensin-3 serves as an activator of antigen-presenting cells by inducing CD40, CD80, and CD86 expression in a TLR-dependent manner and the subsequent activation of MyD88-dependent signaling (Funderburg et al., 2007). It stimulates the production of cytokines, including IL-1α, IL-6, IL-8, CCL18, and tumor necrosis factor-α, by macrophages derived from peripheral blood monocytes (Jin et al., 2010). In macrophages, the binding of CD40L on T cells and CD40 on macrophages results in the activation of antibacterial molecules such as NO and reactive oxygen species and proinflammatory signals including proinflammatory cytokines and chemokines (Suttles and Stout, 2009). In this study, AvBD8 induced the expression of CD40 in macrophages (Figure 5). Furthermore, the expression of inflammatory cytokines and chemokines was stimulated (Figure 2). Because CD40 can bind to the CD40 ligand in TH cells, AvBD8 might act as a bridge between innate and adaptive immunity.
In avian species, there are several studies on the expression of β-defensins after bacterial and viral infections. Hong et al. (2012) demonstrated that genetically disparate 2 commercial chicken lines, Ross and Cobb, afflicted with NE by Eimeria maxima and Clostridium perfringens infection showed differential expression of AvBD. Furthermore, Truong et al. (2016b) reported that 2 Fayoumi chicken lines induced with NE, M15.1 and M15.2, showed differential gene expression pattern of AvBDs. In an in vitro experiment, AvBD8 gene expression was highly upregulated in chicken macrophages and T and B cell lines with lipopolysaccharide treatment (Rengaraj et al., 2018). Therefore, chicken AvBD8 have an important role in immune system when the host was infected with pathogens. In addition, because receptors of AvBD are not yet known, further studies are needed to demonstrate detailed mechanism of AvBDs.
In conclusion, to the best of our knowledge, this is the first study to demonstrate that chicken AvBD8 modulates immune response via activation of the MAPK signaling pathway and induction of proinflammatory cytokines and chemokines in the chicken macrophage cell line. Because AvBD8 regulates immune system when the host is infected with pathogens, it can be applied as a vaccine adjuvant such as NE in the future.
Acknowledgment
This work was supported by a National Research Foundation grant (NRF-2018R1D1A1A09084049) from the Republic of Korea and National Institute of Food and Agriculture grant (#2017-6701526793) from the USA.
Conflict of Interest Statement: The authors have declared that no competing interests exist.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.05.027.
Supplementary data
Supplementary Figure 1.
Supplementary Figure 2.
References
- Ansel K.M., Harris R.B., Cyster J.G. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 2002;16:67–76. doi: 10.1016/s1074-7613(01)00257-6. [DOI] [PubMed] [Google Scholar]
- Ballif B.A., Blenis J. Molecular mechanisms mediating mammalian mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK cell survival signals. Cell Growth Differ. 2001;12:397–408. [PubMed] [Google Scholar]
- Biragyn A., Ruffini P.A., Leifer C.A., Klyushnenkova E., Shakhov A., Chertov O., Shirakawa A.K., Farber J.M., Segal D.M., Oppenheim J.J. Toll-like receptor 4-dependent activation of dendritic cells by β-defensin 2. Science. 2002;298:1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- Bowdish D.M., Davidson D.J., Speert D.P., Hancock R.E. The human cationic peptide LL-37 induces activation of the extracellular signal-regulated kinase and p38 kinase pathways in primary human monocytes. J. Immunol. 2004;172:3758–3765. doi: 10.4049/jimmunol.172.6.3758. [DOI] [PubMed] [Google Scholar]
- Bystry R.S., Aluvihare V., Welch K.A., Kallikourdis M., Betz A.G. B cells and professional APCs recruit regulatory T cells via CCL4. Nat. Immunol. 2001;2:1126–1132. doi: 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- Chertov O., Michiel D.F., Xu L., Wang J.M., Tani K., Murphy W.J., Longo D.L., Taub D.D., Oppenheim J.J. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J. Biol. Chem. 1996;271:2935–2940. doi: 10.1074/jbc.271.6.2935. [DOI] [PubMed] [Google Scholar]
- Donovan K.L., Topley N. What are renal defensins defending? Nephron Exp. Nephrol. 2003;93:e125–e128. doi: 10.1159/000070235. [DOI] [PubMed] [Google Scholar]
- Eming S.A., Krieg T., Davidson J.M. Inflammation in wound repair: molecular and cellular mechanisms. J. Invest. Dermatol. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- Evans E.W., Beach F.G., Moore K.M., Jackwood M.W., Glisson J.R., Harmon B.G. Antimicrobial activity of chicken and Turkey heterophil peptides CHP1, CHP2, THP1, and THP3. Vet. Microbiol. 1995;47:295–303. doi: 10.1016/0378-1135(95)00126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E.W., Beach G.G., Wunderlich J., Harmon B.G. Isolation of antimicrobial peptides from avian heterophils. J. Leukoc. Biol. 1994;56:661–665. doi: 10.1002/jlb.56.5.661. [DOI] [PubMed] [Google Scholar]
- Funderburg N., Lederman M.M., Feng Z., Drage M.G., Jadlowsky J., Harding C.V., Weinberg A., Sieg S.F. Human β-defensin-3 activates professional antigen-presenting cells via Toll-like receptors 1 and 2. Proc. Natl. Acad. Sci. U.S.A. 2007;104:18631–18635. doi: 10.1073/PNAS.0702130104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- Ganz T., Lehrer R. Defensins of vertebrate animals. Curr. Opin. Immunol. 2002;14:96–102. doi: 10.1016/s0952-7915(01)00303-x. [DOI] [PubMed] [Google Scholar]
- Garrington T.P., Johnson G.L. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Immunol. 1999;11:211–218. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- Harwig S.S., Ganz T., Lehrer R.I. Neutrophil defensins: purification, characterization, and antimicrobial testing. Methods Enzymol. 1994;236:160–172. doi: 10.1016/0076-6879(94)36015-4. [DOI] [PubMed] [Google Scholar]
- Hieshima K., Imai T., Opdenakker G., Van Damme J., Kusuda J., Tei H., Sakaki Y., Takatsuki K., Miura R., Yoshie O. Molecular cloning of a novel human CC chemokine liver and activation-regulated chemokine (LARC) expressed in liver Chemotactic activity for lymphocytes and gene localization on chromosome 2. J. Biol. Chem. 1997;272:5846–5853. doi: 10.1074/jbc.272.9.5846. [DOI] [PubMed] [Google Scholar]
- Higgs R., Lynn D.J., Gaines S., McMahon J., Tierney J., James T., Lloyd A.T., Mulcahy G., O’Farrelly C. The synthetic form of a novel chicken β-defensin identified in silico is predominantly active against intestinal pathogens. Immunogenetics. 2005;57:90–98. doi: 10.1007/s00251-005-0777-3. [DOI] [PubMed] [Google Scholar]
- Hong Y., Song W., Lee S., Lillehoj H. Differential gene expression profiles of β-defensins in the crop, intestine, and spleen using a necrotic enteritis model in 2 commercial broiler chicken lines. Poult. Sci. 2012;91:1081–1088. doi: 10.3382/ps.2011-01948. [DOI] [PubMed] [Google Scholar]
- Jin G., Kawsar H.I., Hirsch S.A., Zeng C., Jia X., Feng Z., Ghosh S.K., Zheng Q.Y., Zhou A., McIntyre T.M. An antimicrobial peptide regulates tumor-associated macrophage trafficking via the chemokine receptor CCR2, a model for tumorigenesis. PLoS One. 2010;5:e10993. doi: 10.1371/journal.pone.0010993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W.H., Lillehoj H.S., Min W. Evaluation of the immunomodulatory activity of the chicken NK-Lysin-Derived peptide cNK-2. Sci. Rep. 2017;7 doi: 10.1038/srep45099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasing K.C., Peng R.K. Influence of cell sources, stimulating agents, and incubation conditions on release of interleukin-1 from chicken macrophages. Dev. Comp. Immunol. 1987;11:385–394. doi: 10.1016/0145-305x(87)90082-6. [DOI] [PubMed] [Google Scholar]
- Krishna M., Narang H. The complexity of mitogen-activated protein kinases (MAPKs) made simple. Cell. Mol. Life Sci. 2008;65:3525–3544. doi: 10.1007/s00018-008-8170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Y., Zhou Y., Iribarren P., Wang J. Chemokines and chemokine receptors: their manifold roles in homeostasis and disease. Cell. Mol. Immunol. 2004;1:95–104. [PubMed] [Google Scholar]
- Lee M.O., Jang H.-J., Rengaraj D., Yang S.-Y., Han J.Y., Lamont S.J., Womack J.E. Tissue expression and antibacterial activity of host defense peptides in chicken. BMC Vet. Res. 2016;12:231. doi: 10.1186/s12917-016-0866-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Jiang L., Liu L., Sun L., Zhao W., Chen Y., Qi T., Han Z., Shao Y., Liu S. Induction of avian β-defensin 2 is possibly mediated by the p38 MAPK signal pathway in chicken embryo fibroblasts after Newcastle disease virus infection. Front. Microbiol. 2018;9:751. doi: 10.3389/fmicb.2018.00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lynn D.J. Avian beta-defensin nomenclature: a community proposed update. Immunol. Lett. 2007;110:86–89. doi: 10.1016/j.imlet.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Lynn D.J., Bradley D.G. Discovery of α-defensins in basal mammals. Dev. Comp. Immunol. 2007;31:963–967. doi: 10.1016/j.dci.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Lynn D.J., Higgs R., Gaines S., Tierney J., James T., Lloyd A.T., Fares M.A., Mulcahy G., O’Farrelly C. Bioinformatic discovery and initial characterisation of nine novel antimicrobial peptide genes in the chicken. Immunogenetics. 2004;56:170–177. doi: 10.1007/s00251-004-0675-0. [DOI] [PubMed] [Google Scholar]
- Martin E., Ganz T., Lehrer R.I. Defensins and other endogenous peptide antibiotics of vertebrates. J. Leukoc. Biol. 1995;58:128–136. doi: 10.1002/jlb.58.2.128. [DOI] [PubMed] [Google Scholar]
- Nguyen L.T., Haney E.F., Vogel H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011;29:464–472. doi: 10.1016/j.tibtech.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Niyonsaba F., Ushio H., Nakano N., Ng W., Sayama K., Hashimoto K., Nagaoka I., Okumura K., Ogawa H. Antimicrobial peptides human β-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J. Invest. Dermatol. 2007;127:594–604. doi: 10.1038/sj.jid.5700599. [DOI] [PubMed] [Google Scholar]
- Reddy K., Yedery R., Aranha C. Antimicrobial peptides: premises and promises. Int. J. Antimicrob. Agents. 2004;24:536–547. doi: 10.1016/j.ijantimicag.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Rengaraj D., Truong A.D., Lillehoj H.S., Han J.Y., Hong Y.H. Expression and regulation of avian beta-defensin 8 protein in immune tissues and cell lines of chickens. Asian-australas. J. Anim. Sci. 2018;31:1516. doi: 10.5713/ajas.17.0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhrl J., Yang D., Oppenheim J.J., Hehlgans T. Human β-defensin 2 and 3 and their mouse orthologs induce chemotaxis through interaction with CCR2. J. Immunol. 2010;184:6688–6694. doi: 10.4049/jimmunol.0903984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutyser E., Struyf S., Menten P., Lenaerts J.-P., Conings R., Put W., Wuyts A., Proost P., Van Damme J. Regulated production and molecular diversity of human liver and activation-regulated chemokine/macrophage inflammatory protein-3α from normal and transformed cells. J. Immunol. 2000;165:4470–4477. doi: 10.4049/jimmunol.165.8.4470. [DOI] [PubMed] [Google Scholar]
- Scott M.G., Davidson D.J., Gold M.R., Bowdish D., Hancock R.E. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J. Immunol. 2002;169:3883–3891. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- Scott M.G., Hancock R.E. Cationic antimicrobial peptides and their multifunctional role in the immune system. Crit. Rev. Immunol. 2000;20:407–431. [PubMed] [Google Scholar]
- Selsted M.E., Ouellette A.J. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 2005;6:551. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- Semple F., MacPherson H., Webb S., Cox S.L., Mallin L.J., Tyrrell C., Grimes G.R., Semple C.A., Nix M.A., Millhauser G.L. Human β-defensin 3 affects the activity of pro-inflammatory pathways associated with MyD88 and TRIF. Eur. J. Immunol. 2011;41:3291–3300. doi: 10.1002/eji.201141648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithrithee R., Niyonsaba F., Kiatsurayanon C., Ushio H., Ikeda S., Okumura K., Ogawa H. Human β-defensin-3 increases the expression of interleukin-37 through CCR6 in human keratinocytes. J. Dermatol. Sci. 2015;77:46–53. doi: 10.1016/j.jdermsci.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Soman S.S., Arathy D., Sreekumar E. Discovery of Anas platyrhynchos avian β-defensin 2 (Apl_AvBD2) with antibacterial and chemotactic functions. Mol. Immunol. 2009;46:2029–2038. doi: 10.1016/j.molimm.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Sugiarto H., Yu P.-L. Identification of three novel ostricacins: an update on the phylogenetic perspective of β-defensins. Int. J. Antimicrob. Agents. 2006;27:229–235. doi: 10.1016/j.ijantimicag.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Suttles J., Stout R.D. Macrophage CD40 signaling: a pivotal regulator of disease protection and pathogenesis. Semin. Immunol. 2009;21:257–264. doi: 10.1016/j.smim.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Territo M.C., Ganz T., Selsted M., Lehrer R. Monocyte-chemotactic activity of defensins from human neutrophils. J. Clin. Investig. 1989;84:2017–2020. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma B.P., Cammue B.P., Thevissen K. Plant defensins. Planta. 2002;216:193–202. doi: 10.1007/s00425-002-0902-6. [DOI] [PubMed] [Google Scholar]
- Thouzeau C., Le Maho Y., Froget G., Sabatier L., Le Bohec C., Hoffmann J.A., Bulet P. Spheniscins, avian β-defensins in preserved stomach contents of the king penguin, Aptenodytes patagonicus. J. Biol. Chem. 2003;278:51053–51058. doi: 10.1074/jbc.M306839200. [DOI] [PubMed] [Google Scholar]
- Truong A., Ban J., Park B., Hong Y., Lillehoj H. Characterization and functional analyses of a novel chicken CD8α variant X1 (CD8α1) J. Anim. Sci. 2016;94:2737–2751. doi: 10.2527/jas.2015-0133. [DOI] [PubMed] [Google Scholar]
- Truong A.D., Hong Y., Ban J., Park B., Hoang T.C., Hong Y.H., Lillehoj H.S. Analysis of differentially expressed genes in necrotic enteritis-infected Fayoumi chickens using RNA sequencing. J. Poult. Sci. 2016;54:121–133. doi: 10.2141/jpsa.0160053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong A.D., Park B., Ban J., Hong Y.H. The novel chicken interleukin 26 protein is overexpressed in T cells and induces proinflammatory cytokines. Vet. Res. 2016;47:65. doi: 10.1186/s13567-016-0342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong A.D., Hong Y., Lee J., Lee K., Lillehoj H.S., Hong Y.H. Analysis of MAPK signaling pathway genes in the intestinal mucosal layer of necrotic Eenteritis-afflicted two inbred chicken lines. Korean J. Poult. Sci. 2017;44:199–209. [Google Scholar]
- van Dijk A., Veldhuizen E.J., Haagsman H.P. Avian defensins. Vet. Immunol. Immunopathol. 2008;124:1–18. doi: 10.1016/j.vetimm.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub H.A., Elazzazy A.M., Abuzinadah O.A., Al-Hejin A.M., Mahmoud M.M., Harakeh S.M. Antimicrobial activities of chicken β-defensin (4 and 10) peptides against pathogenic bacteria and fungi. Front. Cell. Infect. Microbiol. 2015;5:36. doi: 10.3389/fcimb.2015.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Biragyn A., Hoover D.M., Lubkowski J., Oppenheim J.J. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu. Rev. Immunol. 2004;22:181–215. doi: 10.1146/annurev.immunol.22.012703.104603. [DOI] [PubMed] [Google Scholar]
- Yang D., Chertov O., Bykovskaia S., Chen Q., Buffo M., Shogan J., Anderson M., Schröder J., Wang J., Howard O. β-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- Yu P.-L., Choudhury S.D., Ahrens K. Purification and characterization of the antimicrobial peptide, ostricacin. Biotechnol. Lett. 2001;23:207–210. [Google Scholar]
- Zhao B.-C., Lin H.-C., Yang D., Ye X., Li Z.-G. Disulfide bridges in defensins. Curr. Top. Med. Chem. 2016;16:206–219. doi: 10.2174/1568026615666150701115911. [DOI] [PubMed] [Google Scholar]