Abstract
This study aimed to investigate the effects of different acute high ambient temperatures on redox status in liver of broilers. A total of 144 35-day-old Arbor Acres broilers were randomly divided into 4 groups with 6 replicates of 6 birds each and subsequently distributed in different environment chambers for acute heat stress. The temperature of 4 environment chambers were set to 26°C (control), 29°C, 32°C, 35°C for 6 h, respectively. Various indicators were tested to evaluate hepatic redox status. Then, the hallmarks of hepatocellular antioxidant and apoptosis were measured by qRT-PCR and Western Blot. The results showed that with the ambient temperature increase (i) the content of hydrogen peroxide (H2O2) and protein carbonyl (PC) in the liver of broilers increased significantly (P < 0.05), but the content of malondialdehyde (MDA) and 8-hydroxyguanosine (8-OHdG) was not affected; (ii) the activity of catalase (CAT) and glutathione reductase (GR) increased significantly (P < 0.05). Similarly, the superoxide dismutase (SOD) had an increasing tendency (P = 0.07), and the content of the reduced glutathione (GSH) was also significantly increased (P < 0.05) under high temperature; (iii) the heat shock protein (HSP70), nuclear factor erythroid-2-related factor 2 (Nrf2), and other antioxidant gene (HO-1, NQO1, GCLc, GST, SOD1, SOD2, CAT, Prx3) were upregulated in broilers liver. Moreover, the protein level of HSP70, Nrf2, and Prx3 were also upregulated; (iv) high temperature upregulated the antiapoptotic gene expression (BCL-2); however, the proapoptotic genes (BAK1, caspase-3, and caspase-9) did not change significantly; meanwhile, there was no significant changes in the protein level of caspase-3 and caspase-9. The results of this study indicated that 35-day-old Arbor Acres broilers have a certain tolerance to oxidative stress induced by high ambient temperature. Six hours of acute heat stress–activated Nrf2 signaling pathway. Meanwhile, the expression of related antioxidant genes and proteins is upregulated, consequently resulted in increased antioxidant enzymes activity and GSH. These effects enable the body to scavenge large amounts of reactive oxygen species produced by high temperature and prevent the occurrence of apoptosis.
Key words: acute heat stress, redox status, antioxidant, apoptosis, broiler
Introduction
High ambient temperature as a stressor of poultry has been investigated extensively for many years. On account of lacking sweat gland, abundant feathers and fast metabolic rates, broilers are very susceptible to heat stress (Sahin et al., 2009; Xie et al., 2015). The heat stress adversely affects the physiological function of broilers, causing disturbance of metabolic, depression of immunity, and impairment in endocrine (Sahin et al., 2012; Lara and Rostagno, 2013). In addition, heat stress results in the generation of reactive oxygen species (ROS) and imbalance of the redox status (Yang et al., 2010; Zhang et al., 2015). Recently, the involvement of heat stress as an inducer of oxidative stress has been acknowledged (Akbarian et al., 2016). When the ROS is excessive, liver is a major organ attacked (Sha et al., 2015). Owing to its special chemical characteristics, ROS can initiate biomacromolecules peroxidation, resulting in protein modification, lipid peroxidation, and nucleic acid damage (Galicia-Moreno and Gutiérrez-Reyes, 2014). Oxidative stress results in mitochondrial permeability transition, the destruction of mitochondrial membrane structure, and the releasing of apoptotic effectors, which further leads to apoptosis.
However, these ROS are not necessarily a threat to the body under physiological conditions (Mittler, 2002). Cells have developed a sophisticated antioxidant defense system that enable to counteract ROS to a certain degree (Jaeschke et al., 2012), especially when the broilers suffer from acute high ambient temperatures within a short time (Altan et al., 2003; Yang et al., 2010). The general antioxidant system contains enzymatic antioxidants and small molecules. Furthermore, nuclear factor erythroid-2-related factor 2 (Nrf2) is a key transcription factor that regulates the cellular antioxidant system response against ROS. Under normal conditions, Kelch-like ECH associated protein-1 subjects Nrf2 to rapid ubiquitination and degradation and thus suppresses the transcriptional activity of Nrf2 under unstressed conditions. Signals from ROS, which dissociates Nrf2 form Kelch-like ECH associated protein-1 and Nrf2, are thus activated. The activated Nrf2 transfers into the nucleus and interacts with antioxidant response element, promoting antioxidant enzymes and the expression of antioxidant genes and proteins (Zhang et al., 2013). Moreover, the enhanced antioxidant signaling regulated protects mitochondria from oxidative damages and maintains the mitochondrial redox state (Sies et al., 2017), which prevents more serious damage to the body.
Previous studies have confirmed that various feed supplements, such as curcumin, lycopene, and some microelement can alleviate oxidative damage caused by chronic heat stress in broilers by modulating the expression of Nrf2 (Sahin et al., 2016, 2017; Zhang et al., 2018). However, under the condition of acute heat stress, limited information is available about the antioxidant potential and related regulatory mechanism of broilers. Therefore, the objectives of this study were to investigate the effects of 6 h acute heat exposure on hepatic redox status of Arbor Acres broilers at 35 D old to evaluates the antioxidant mechanism of Nrf2 signaling pathway in broilers and to explore the situation of apoptosis under this special physiological state.
Materials and methods
Ethics Statement
All animal experimental procedures were performed according to Regulations for the Administration of Affairs Concerning Experimental Animals of the State Council of the People's Republic of China and authorized by Animal Welfare Committee of Institutes of Animal Sciences, Chinese Academy of Agricultural Sciences (IASCAAS).
Animals and Experimental Design
Broiler chicks (Arbor Acres) at 1 D of age were obtained from Beijing Huadu Poultry Breeding Co., Ltd. (Beijing, China) and reared in environment-controlled chambers, following the procedures described in the Arbor Acres Broilers Husbandry Manual. All diets (Table 1) were formulated to meet the nutrient requirement for broilers suggested by National Research Council (NRC, 1994). During the entire experimental period, the broilers had free access to feed and water, and the light regimen was 24 h. At 27 D of age, a total of 144 chickens with similar body weights (1,135 ± 33.4 g) were selected and randomly divided into 4 groups with 6 replicates of 6 birds each. Subsequently, broilers were still reared in the environment-controlled chambers and housed at 26°C (relative humidity, 60%) for 7 D. After the adaptation period, at 35 D of age, the temperature of the temperatures were adjusted to 26°C, 29°C, 32°C, 35°C (relative humidity, 60%) within 1 h and maintained for 6 h, respectively. Six birds in each group were randomly selected for sample collection after 6 h.
Table 1.
Composition and nutrient levels of the basal diet (as-fed basis).
| Ingredients | Amount (%) | Calculated nutrient levels | Amount (%) |
|---|---|---|---|
| Corn | 56.51 | ME (MJ/Kg)2 | 12.73 |
| Soybean meal | 35.52 | CP | 20.07 |
| Soybean oil | 4.50 | Ca | 0.90 |
| Salt | 0.30 | Available phosphorus | 0.40 |
| Limestone | 1.00 | Lysine | 1.00 |
| Dicalcium phosphate | 1.78 | Methionine | 0.42 |
| DL-methionine | 0.11 | Methionine + cysteine | 0.78 |
| Premix1 | 0.28 | ||
| Total | 100.00 |
Premix provided per kilogram of diet: retinyl acetate for vitamin A,10,000 IU; cholecalciferol for vitamin D3, 3,400 IU; DL-α-tocopheryl acetate for vitamin E, 16 IU; vitamin K3, 2.0 mg; vitamin B1, 2.0 mg; vitamin B2, 6.4 mg; vitamin B6, 2.0 mg; vitamin B12, 0.012 mg; pantothenic acid calcium, 10 mg; nicotinic acid, 26 mg; folic acid, 1 mg; choline chloride, 500 mg; biotin, 0. 1 mg; Zn (ZnSO4·7H2O), 40 mg; Fe (FeSO4·7H2O), 80 mg; Cu (CuSO4·5H2O), 8 mg, Mn (MnSO4·H2O), 80 mg; I (KI), 0.35 mg; Se (Na2SeO3), 0.15 mg.
ME = metabolizable energy.
Sample Collection
The broilers were sacrificed by cervical dislocation and exsanguinated to get liver tissues. The liver samples were harvested and immediately frozen in liquid nitrogen and stored at −80°C until subsequent analysis.
Redox Indicators Measurement
The collected hepatic samples were used to detect the level of oxidative damage biomarkers and antioxidant capacity. The content of hydrogen peroxide (H2O2), malondialdehyde (MDA), protein carbonyl (PC), reduced glutathione (GSH), and the activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), and glutathione reductase (GR) in liver were measured using the commercial kits (Beijing Solarbio Science and Technology Co., Ltd., Beijing, China). The hepatic 8-hydroxyguanosine (8-OHdG) was determined by ELISA kit, according to the manufacturer's instructions (MEIMIAN, Jiangsu Feiya Biological Technology Co. Ltd., Jiangsu, China).
Analysis of mRNA Levels by Real-Time Quantitative PCR
The mRNA expression level of related antioxidant genes on Nrf2 signaling pathway, and some genes involved in apoptosis were detected by the real-time quantitative PCR. Total RNA from hepatic tissues was extracted using the Qiagen Kit (RNeasy Mini Kit, cat. nos. 74,104, Qiagen., Beijing, China) according to the manufacturer's instructions. cDNA was transcribed via reverse transcription kits (PrimeScript RT reagent Kit with gDNA Eraser, RR047 A, Takara., Beijing, China). The forward (F) and reverse (R) primers for representative genes were given in Table 2, which were obtained from the NCBI database and synthesized by Shanghai Sangon Biotechnology Company (Shanghai, China). Real-time RT-PCR reactions was carried out using the SYBR green method (TB Green Premix Ex Taq,RR420 A, Takara) on the CFX96 Touch Real-Time PCR Detection System (BIO-RAD, Hercules, CA) according to the following steps: 30 s at 95°C, 40 cycles of denaturation at 95°C for 5 s, and annealing and extension at 60°C for 30 s. The relative fold change was performed using the 2-ΔΔCt method with β-actin as an internal control (Livak and Schmittgen, 2001).
Table 2.
Specific gene primers used for real-time quantitative PCR.
| Gene | Accession no. | Primers sequences |
|---|---|---|
| HSP70 | NM_001006685.1 | F:GGGGCACTTTTGATGTGT |
| R:CCAGCAATGTCACGCTT | ||
| Nrf2 | NM_205117.1 | F:GGCCTTGTCCTTTGATGA |
| R:GGGTGGCTGAGTTTGATTA | ||
| HO-1 | NM_205344.1 | F:GGCAAGAAGCATCCAGAG |
| R:GTGAAGAAAGCCAACCCTT | ||
| NQO1 | NM_001277619.1 | F:GCTGGGAAGTCACCATCTC |
| R:GACAAAGCACTCGGGGTT | ||
| Prx1 | NM_001277724.2 | F:CTGTCACCTTGCCTGGAT |
| R:ATAGTGCCCAAACCACCTT | ||
| Prx3 | XM_426543.5 | F:GGATTTCACCTTTGTGTGC |
| R:TGGAACTCATTCGCTTTGT | ||
| CAT | NM_001031215.2 | F:TTACGGAGGTAGAACAGATGG |
| R:TGTCAGGATACGCAAAGAGA | ||
| SOD1 | NM_205064.1 | F:AGGGAGGAGTGGCAGAAG |
| R:GCAGTGTGGTCCGGTAAG | ||
| SOD2 | NM_204211.1 | F:GGGGAGCCTAAAGGAGAA |
| R:AAGTTTGCGAAGGAACCA | ||
| GCLC | XM_419910.5 | F:ATGATGGAGAAGCAGCAAA |
| R:GCCTGGAATGTTACCTGAA | ||
| GCLM | NM_001007953.1 | F:TGTGCTGCTGCTTAACCATTCCG |
| R:GCAACGCGCACATTGTTCTAGC | ||
| GST | NM_001001777.1 | F:CGTCCAACCAGCAGATAAA |
| R:TCCGTGGTCCTTCAAAAC | ||
| BCL-2 | NM_205339.2 | F:GAGTTCGGCGGCGTGATGTG |
| R:TTCAGGTACTCGGTCATCCAGGTG | ||
| BAK1 | NM_001030920.1 | F:CAGCAGCGAGGCAGCAGAAC |
| R:AACAGCACAGAGCGGACACAAC | ||
| Caspase3 | NM_204725.1 | F:AACACGCCAGGAAACTTG |
| R:TATTCTGCCACTCTGCGAT | ||
| Caspase9 | XM_424580.6 | F:AACTTGCCGACGTTCCA |
| R:GGCTCGTCCTCATTCCC | ||
| β-actin | NM_205518.1 | F:CTCTATCCTGGCCTCCCT |
| R:GGGTGTGGGTGTTGGTAA |
Abbreviations: BAK1, BCL2 antagonist/killer 1; BCL-2, BCL2, apoptosis regulator; Caspase3, caspase 3; Caspase9, caspase 9; CAT, catalase; GCLc, glutamate-cysteine ligase catalytic subunit; GCLm, glutamate-cysteine ligase modifier subunit; GST, glutathione S-transferase alpha 3; HO-1, heme oxygenase 1; HSP70, heat shock 70 kDa protein 2; Nrf2, nuclear factor, erythroid 2 like 2; NQO1, NAD(P)H quinone dehydrogenase 1; Prx1, peroxiredoxin 1; Prx3, peroxiredoxin 3; SOD1, superoxide dismutase 1; SOD2, superoxide dismutase 2.
Western Blot Analysis for Hsp70, Nrf2, Prx3, and Caspase-3
For Western blot, total protein was extracted from frozen hepatic tissues with cell lysis buffer for Western (Beyotime Biotechnology, Jiangsu, China) which supplemented with phenylmethylsulfonyl fluoride and protease and phosphatase inhibitor cocktail. Protein concentration was determined using the pierce BCA Protein Assay Kit (Thermo Scientific 23,225, Beijing, China). The proteins were separated by 10% SDS-PAGE gel electrophoresis, then transferred to an polyvinylidene fluoride membrane. The membrane was washed in tris buffered saline containing tween and blocked in 5% nonfat milk for 2 h at room temperature. After that, the blots were incubated overnight at 4°C with the primary antibodies: β-actin (ab8227, Abcam, Cambridge, UK), HSP70 (ab69412, Abcam), Nrf2 (ab31163, Abcam), Prx3 (ab73349, Abcam), and Caspase-3 (ab90437, Abcam). The membranes were then incubated with the corresponding secondary antibody (ab6721, Abcam) for 40 min at room temperature. After further washed with tris buffered saline containing tween, blots were incubated in commercial chemiluminescent reagents for protein detection. The intensity of the bands on the blots was measured by ImageJ software (National Institutes of Health). The relative expression of targeted protein was normalized in relation to β-actin as the internal protein.
Statistical Analysis
Statistical analyses were carried out using the SAS version 9.4 software (SAS Institute, Cary, NC). All data were subjected to using one-way ANOVA followed by Duncan's multiple comparison tests. The results are expressed as the mean ± SD of measurements on tissues from 6 replicate broilers at each group setting P < 0.05 as a criterion of statistical significance.
Results
Acute Heat Stress Disturbed the Redox Balance in Liver
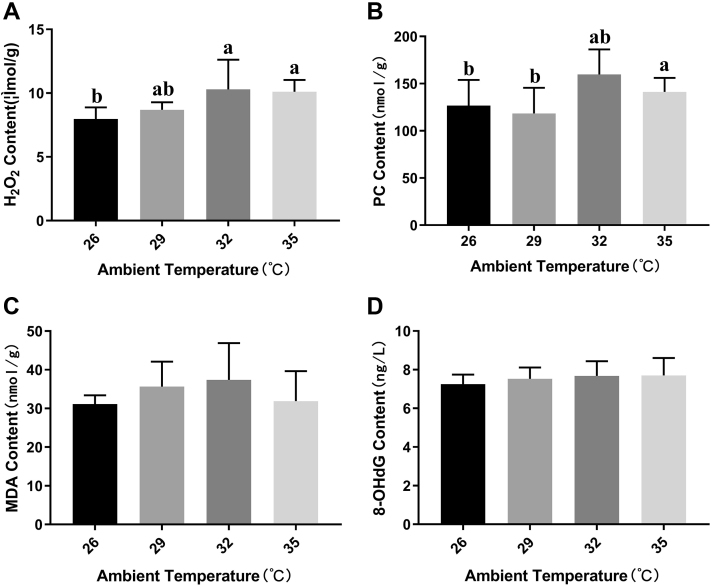
Acute heat stress had significant effect on the content of H2O2 (Figure 1A) and PC (Figure 1B) in the liver of broilers, both of which had the most significant changes in the broilers liver under the group 32°C. However, acute heat stress had no significant effect on the content of MDA (Figure 1C) and 8-OHdG (Figure 1D) in broilers liver. With the increasing of ambient temperature, the content of MDA in broilers liver of each group did not change significantly. Although the content of 8-OHdG showed an increasing trend, the difference between the groups was also not significant neither.
Figure 1.
Effects of acute heat stress at different ambient temperature on the level of oxidative damage biomarkers. Broilers hepatic hydrogen peroxide (H2O2), protein carbonyl (PC), malonaldehyde (MDA), and 8-hydroxyguanosine (8-OGdG) level changes after 6 h acute heat exposure is, respectively, shown in panels A, B, C, and D. Values are mean ± SD of 6 replications. Data points with different letters are significantly different (one-way ANOVA) at the level of P < 0.05 by Duncan's multiple comparison test.
Acute Heat Stress Caused Increased Activity of Antioxidant Enzymes in the Liver
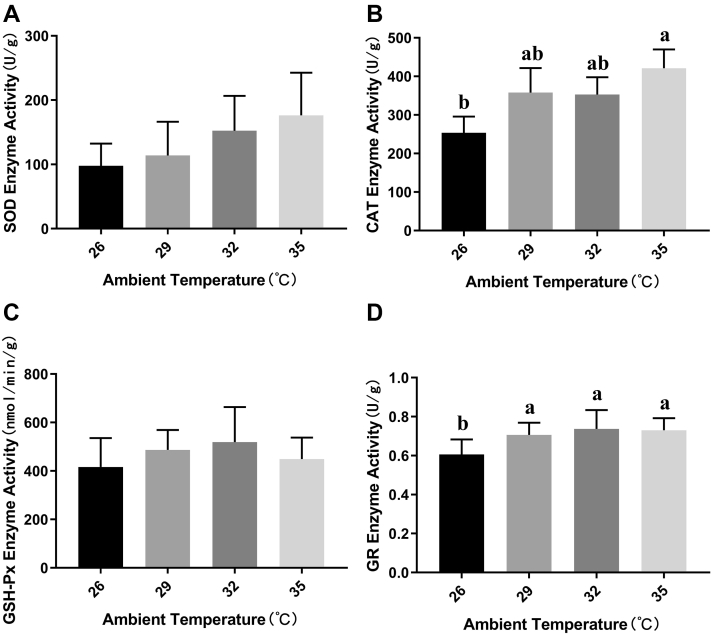
The activity of SOD, CAT, GSH-Px, and GR in the liver of broilers was shown in the Figure 2. The results showed that GSH-Px activity (Figure 2C) was not affected by acute heat stress. There were significant differences in the activities of CAT (Figure 2B) and GR (Figure 2D) in broilers liver between the control and the groups exposed to high ambient temperature. Although it did not enhanced linearly with increasing ambient temperature, the activity of these 2 enzymes was significantly increased under high ambient temperature. In addition, GR was more sensitive to acute heat stress, and when the temperature exceeded 29°C, the activity of GR in broilers liver could increase significantly. Meanwhile, although the activity of SOD did not change significantly (Figure 2A), it also tended to gradually increase with temperature increasing (P = 0.07).
Figure 2.
Effects of acute heat stress at different ambient temperature on the activities of antioxidant enzymes. Broilers hepatic superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), and glutathione reductase (GR) activity changes after 6 h acute heat exposure is, respectively, shown in panels A, B, C, and D. Values are mean ± SD of 6 replications. Data points with different letters are significantly different (one-way ANOVA) at the level of P < 0.05 by Duncan's multiple comparison test.
Nrf2 Signaling Pathway Alleviates Acute Heat Stress Induced Oxidative Damage
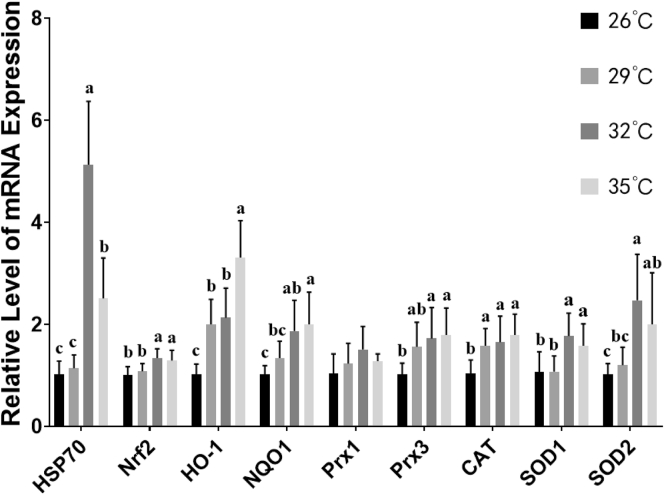
The changes of the Nrf2 signaling pathway and other antioxidant genes in broilers liver after acute heat stress was shown in Figure 3. The hepatic Nrf2 mRNA expressions were significantly upregulated in the heat exposure group, although it did not enhanced linearly with increasing ambient temperature. The mRNA expression of the downstream antioxidant genes regulated by Nrf2 were elevated obviously. Compared with the control group, the HO-1 level was upregulated more than 3.3-fold in the 35°C group, SOD2 level was upregulated more than 2.4-fold in the 32°C group, as well the NQO1 was nearly 2-fold in the 35°C group. In addition, the highest elevated level of CAT, SOD1, and Prx3 were similar, which were approximately 1.7-fold. In addition, the mRNA expression of molecular chaperone HSP70 were extremely significantly upregulated in the heat exposure groups (P < 0.01).
Figure 3.
Effects of acute heat stress at different ambient temperature on the nuclear factor erythroid-2-related factor 2 (Nrf2) signaling pathway and other antioxidant genes expression in broilers liver. Values are mean ± SD of 6 replications. Data points with different letters are significantly different (one-way ANOVA) at the level of P < 0.05 by Duncan's multiple comparison test. Abbreviations: HSP70, heat shock 70 kDa protein 2; HO-1, heme oxygenase 1; NQO1, NAD(P)H quinone dehydrogenase 1; Prx1, peroxiredoxin 1; Prx3, peroxiredoxin 3; CAT, catalase; SOD1, superoxide dismutase 1; SOD2, superoxide dismutase 2.
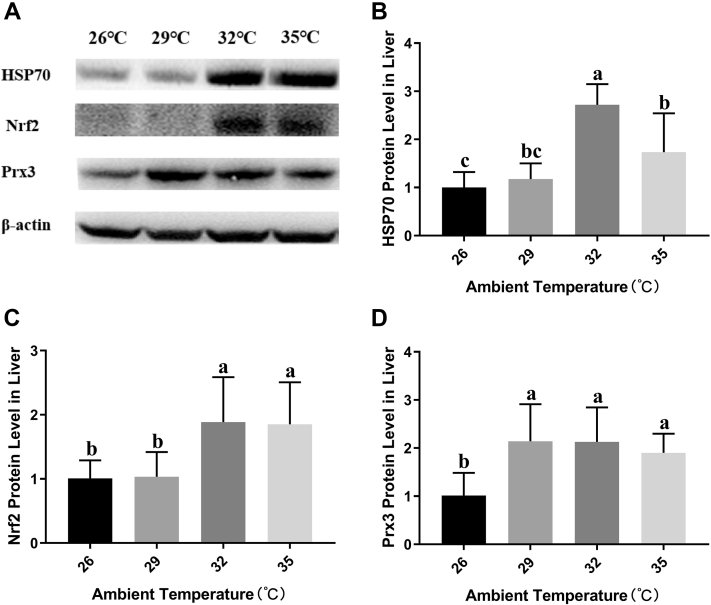
The results of Western Blot were shown in Figure 4A. Correspond with the results of RT-PCR, in comparison with the control group, the hepatic protein expression of HSP70 (Figure 4B) was extremely significantly upregulated by 1.18-, 2.72-, 1.73-fold in broilers exposed to acute heat stress (P < 0.01), respectively. Nrf2 (Figure 4C) protein level in the 29°C group was similar to the control group, but in the 32°C group and 35°C group was upregulated more than 1.8-fold. Additionally, the protein expression of Prx3 (Figure 4D) was significantly upregulated when the temperature was higher.
Figure 4.
Effects of acute heat stress at different ambient temperature on the HSP70, Nrf2, and Prx3 protein expression in broilers liver. The Western blot images of hepatic HSP70, Nrf2, and Prx3 protein expression is shown in panel A. The relative expression of HSP70, Nrf2 and Prx3 protein is, respectively, shown in panels B, C and D. Values are mean ± SD of 6 replications. Data points with different letters are significantly different (one-way ANOVA) at the level of P < 0.05 by Duncan's multiple comparison test. Abbreviations: HSP70, heat shock 70 kDa protein 2; Prx3, peroxiredoxin 3; Nrf2, nuclear factor erythroid-2-related factor 2.
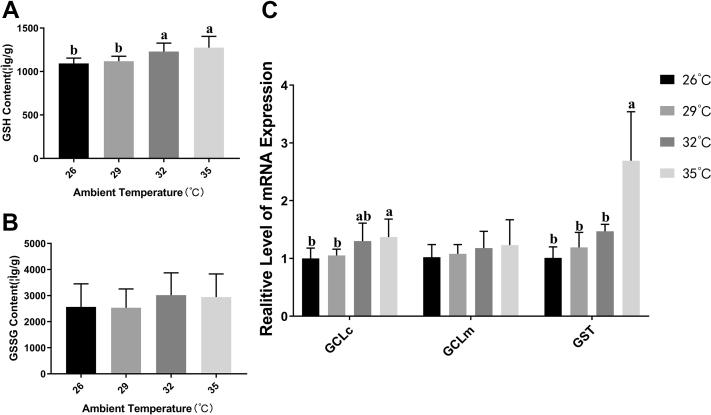
GSH Increased in the Liver of Broilers Exposed to Acute Heat Stress
As were shown in the Figure 5, the content of GSH in broilers liver was gradually increased with the ambient temperature increase, but the content of GSSG had no significant changes under different ambient temperature. Similarly, The gene related to GSH synthesis GCLc mRNA expression in liver was enhanced by 5, 30, and 37%, respectively, in 29°C, 32°C, and 35°C group in comparison with the control group, but GCLm showed no statistical difference in each group. In addition, the relative expression of GST was also significantly increased with the ambient temperature increasing, which was more than 2.5-fold in the 35°C group.
Figure 5.
Effects of acute heat stress at different ambient temperature on the content of glutathione (GSH),oxidized glutathione(GSSG), and other related genes expression in broilers liver. The hepatic content of GSH in broilers is shown in panel A. The hepatic content of GSSG in broilers is shown in panel B. The expression of related mRNA is shown in panel C. Values are mean ± SD of 6 replications. Data points with different letters are significantly different (one-way ANOVA) at the level of P < 0.05 by Duncan's multiple comparison test.
Acute Heat Stress Increased mRNA Expression of Bcl-2
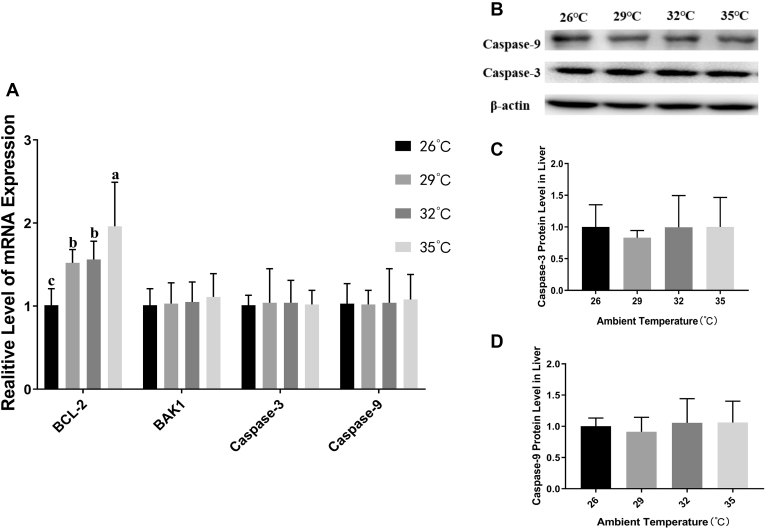
Liver apoptosis–related genes mRNA and Caspase-3 protein expression results was revealed in Figure 6. With the ambient temperature increasing, hepatic antiapoptotic factor BCL-2 mRNA levels were extremely significantly upregulated by 1.52-fold, 1.56-fold, 1.96-fold in the heat exposure group, respectively. The mRNA expression of BAK1 which antagonized to BCL-2 did not change significantly. Similarly, the apoptosis effectors caspase-3 and caspase-9 had no significant differences at mRNA level. Also, there was no significant difference in caspase-3 and caspase-9 at the protein level.
Figure 6.
Effects of acute heat stress at different ambient temperature on the apoptosis related genes and protein level in broilers liver. The mRNA relative expression level of hepatic apoptosis is shown in panel A. The Western blot images of hepatic Caspase-3 and Caspase-9 protein expression is shown in panel B. The relative expression of Caspase-3 and Caspase-9 is, respectively, shown in panels C and D. Values are mean ± SD of 6 replications. Data points with different letters are significantly different (one-way ANOVA) at the level of P < 0.05 by Duncan's multiple comparison test.
Discussion
It is well known that the upper critical temperature of broilers was 26°C (Meltzer, 1983; Diarra and Tabuaciri, 2014). When the ambient temperature is higher, heat stress is likely to occur in broilers. Generally, there are 3 stages in the reaction to a stressor when the animals receive environmental stimulation: alarm, resistance, and exhaustion (Selye, 1950; Mcewen, 2005). The duration of acute heat stress is usually very short, with regard to redox status in the animal has the following physiological characteristics: at the initial stage, the antioxidant defense system in the body starts to work, scavenging a large amount of ROS produced by high temperature and protecting tissues and organs against surplus ROS formation (Anu et al., 2014). In the present study, the hepatic content of H2O2 in broilers increased significantly at high ambient temperature, indicating that 6 h acute heat exposure could cause the increase of ROS in the body of broilers. One of the negative effect of ROS is the modification of several biological macromolecules, leading to lipid peroxidation and oxidative damage to protein and nuclear acid (Trevisan et al., 2001; Orrenius et al., 2007). In this study, the content of lipid, protein, and DNA oxidation biomarkers MDA, PC, 8-OHdG in the liver of broiler chickens was detected. The results showed that the content of MDA and 8-OHdG was not significantly affected by temperature. Only the content of PC was significantly changed after acute heat stress, and this probably because proteins are among the most abundant cellular components vulnerable to oxidation (Anjum et al., 2015). These results indicate that, under the conditions of this experiment, the maximum temperature of 35°C, 6 h acute heat exposure, could result in increased ROS production, but might not cause serious oxidative injury to the liver of broilers.
Numerous studies have reported that, when exposed to acute high ambient temperature, the activity of various antioxidant enzymes in the tissues and organs of broilers and quails usually tended to elevate (Lin et al., 2006; Tan et al., 2010; Vesco et al., 2016). Similar results were found in our research, as the first line of defense during oxidative stress; although the activities did not exactly increase with temperature strictly, there were significant difference in the activity of CAT and GR in liver between the control and the groups exposed to high ambient temperature. In addition, the activity of SOD also tended to increase. The enzymatic antioxidants have effective protective effects against oxidative attack (He et al., 2017), and when slight disturbance occurs in the redox balance, the activity of various antioxidant enzymes increases and exerts beneficial effects on decomposing ROS. This may be the reason why the content of MDA in broilers was not significantly changed in the current study. Meanwhile, the hepatic mRNA and protein level of HSP70 in broilers was significantly upregulated when the ambient temperature increased. As a molecular chaperone, this heat shock protein is highly conserved protein which get activated by high temperature and plays a role in cytoprotective function to improve the adaptability of broilers under high temperature condition (Miao et al., 2006; Yang et al., 2017).
The Nrf2 signaling pathway is the major regulator of cytoprotective responses to the stress caused by ROS (Levonen, 2012). With the increasing of ROS, Nrf2 binds together with small Maf proteins to the antioxidant response element and induce expression of its target antioxidant genes (Kansanen et al., 2013). Previous study about mice has reported heat exposure increased Nrf2 mRNA and protein level (Li et al., 2018). Jin et al. (2016) found that when the bovine mammary epithelial cells were exposed to 42.5°C for 1 h, the Nrf2 signaling could be activated by acute heat stress, indicating a possible self-defense mechanism of the bovine mammary epithelial cells to respond to acute heat stress (Jin et al., 2016). In addition, Li et al. (2014) reported that 8-wk-old mice were exposed to a single scrotal heat treatment with 42°C for 25 min, which caused increased expression of Nrf2 mRNA and translocation of Nrf2 protein into interstitial cell nuclei accompanied (Li et al., 2014). However, there is little information about Nrf2 in chickens exposed to acute high temperature. In the present study, compared with the control group, we discovered that Nrf2 was also upregulated at the level of mRNA and protein in broilers liver exposed to acute high temperature. Newly synthesized Nrf2 protein could increase the expression of its target genes such as HO-1 and NQO1 which belong to cellular detoxifying enzymes with great help for antioxidant system. mRNA expression of both of them in the liver was gradually upregulated with increasing temperature. The upregulated mRNA level of CAT, SOD1, and SOD2 in the broilers was further detected, which was consistent with the change of antioxidant enzyme activities. Moreover, Prxs family play an important role as a housekeeping gene to protect against oxidative damage, and Prx3 is one of the efficient peroxidase used in the scavenging of H2O2 and other ROS (Han et al., 2005). Previous study reported that Prx1 and Prx3 protein abundance increased in response to acute heat stress in the small yellow follicles of layer-type chickens (Cheng et al., 2018). Similar results were found in the present study that hepatic mRNA and protein level of Prx3 was significantly upregulated in the broilers exposed to acute heat stress. The present data revealed that Nrf2 was activated by the changes in the redox state which caused by acute heat stress and able to protect liver from oxidative damage by increasing expression of various protective molecules.
Another important role of Nrf2 is to regulate the biosynthesis of GSH. Glutathione is considered as the most abundant molecule among endogenous antioxidants, which scavenge ROS either directly or indirectly. Moreover, it can also exert the function of scavenging ROS by revitalizing other antioxidants (Espinosa-Diez et al., 2015). For instance, GST could conjugate with GSH, exerting detoxification of xenobiotics and electrophiles (Surai et al., 2019). The rate limiting step of GSH synthesized is carried out by glutamate cysteine ligase (GCL), which is a heterodimeric enzyme composed by a heavy subunit GCLc and a smaller one GCLm (Harris et al., 2015). In the present study, with increasing in ambient temperature, the mRNA expression of GCLc and GST was enhanced significantly, indicating more GSH was synthesized in the liver of broilers at the same time. As mentioned above, the activity of GR was also significantly elevated, and GR is the enzyme critical for the reduction of GSSG back to GSH and acceleration for GSH cycle (Couto et al., 2016). These findings suggest that the synthesis and utilization of GSH in the broilers exposed to acute heat stress were improved, especially when the ambient temperature was higher than 32°C, which contributed to maintaining redox status in the liver.
The results of our research demonstrated that acute heat stress can lead to increased ROS while Nrf2 was activated. Thus, we continued to focus on investigating the expression of apoptosis-related molecules in the liver under this special physiological condition. Reactive oxygen species and the redox state of a cell play a vital role in regulating apoptosis (Curtin et al., 2002). Protein family caspases and BCL-2 were found to be intimately related to apoptosis caused by ROS. The presence of BCL-2 could rescue cells from cell death and controlled by Nrf2 (Niture and Jaiswal, 2012), but BAK1 is the proapoptosis members. Furthermore, caspases activation was suggested as an important criterion of cell death (Kroemer et al., 2005). In the present study, the mRNA expression of BCL-2 was significantly upregulated to inhibit apoptosis with the increasing ambient temperature. However, the other 3 detected genes did not change significantly. In addition, the level of caspase-3 and caspase-9 protein had no significant difference either. Based on these results, the antioxidant defense system and the antiapoptotic genes of broilers play a vital role in preventing apoptosis induced by increased ROS after 6 h acute heat exposure with the highest temperature being 35°C.
In conclusion, broilers have a certain tolerant to oxidative stress induced by high temperatures. When the ambient temperature exceeded the thermoneutral zone in a short time, the antioxidant defense system in the body would play a role in scavenging excess ROS. Specifically, Nrf2 signaling pathway starts activation, and the expression of various antioxidant genes and proteins was upregulated. Meanwhile, the activity of antioxidant enzymes and synthesis of GSH were increased. These physiological changes work together to maintain an appropriate redox status in the body under high ambient temperature condition.
Acknowledgments
This study was supported by the National Key R&D Program of China (2016YFD0500501).
Conflict of Interest Statement: The authors did not provide any conflict of interest statement.
References
- Akbarian A., Michiels J., Degroote J., Majdeddin M., Golian A., De Smet S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016;7:37. doi: 10.1186/s40104-016-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altan Ö., Pabuçcuoğlu A., Altan A., Konyalioğlu S., Bayraktar H. Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. Br. Poult. Sci. 2003;44:545–550. doi: 10.1080/00071660310001618334. [DOI] [PubMed] [Google Scholar]
- Anjum N.A., Sofo A., Scopa A., Roychoudhury A., Gill S.S., Iqbal M., Lukatkin A.S., Pereira E., Duarte A.C., Ahmad I. Lipids and proteins—major targets of oxidative modifications in abiotic stressed plants. Environ. Sci. Pollut. Res. Int. 2015;22:4099–4121. doi: 10.1007/s11356-014-3917-1. [DOI] [PubMed] [Google Scholar]
- Anu R., Amit K., Vivek S., Brijesh Y., Ruchi T., Sandip C., Kuldeep D. Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed. Res. Int. 2014;2014 doi: 10.1155/2014/761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C.Y., Tu W.L., Chen C.J., Chan H.L., Chen C.F., Chen H.H., Tang P.C., Lee Y.P., Chen S.E., Huang S.Y. Functional genomics study of acute heat stress response in the small yellow follicles of layer-type chickens. Sci. Rep. 2018;8:1320. doi: 10.1038/s41598-017-18335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto N., Wood J., Barber J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016;95:27–42. doi: 10.1016/j.freeradbiomed.2016.02.028. [DOI] [PubMed] [Google Scholar]
- Curtin J.F., Donovan M., Cotter T.G. Regulation and measurement of oxidative stress in apoptosis. J. Immunol. Methods. 2002;265:49–72. doi: 10.1016/s0022-1759(02)00070-4. [DOI] [PubMed] [Google Scholar]
- Diarra S.S., Tabuaciri P. Feeding Management of poultry in high environmental temperatures. Int. J. Poult. Sci. 2014;13:657–661. [Google Scholar]
- Espinosa-Diez C., Miguel V., Mennerich D., Kietzmann T., Sánchez-Pérez P., Cadenas S., Lamas S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015;6:183–197. doi: 10.1016/j.redox.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiciamoreno M., Gutiérrezreyes G. The role of oxidative stress in the development of alcoholic liver disease. Rev. Gastroenterol. Mex. 2014;79:135–144. doi: 10.1016/j.rgmx.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Han J.Y., Song K.D., Shin J.H., Han B.K., Park T.S., Park H.J., Kim J.K., Lillehoj H.S., Lim J.M., Kim H. Identification and characterization of the peroxiredoxin gene family in chickens. Poult. Sci. 2005;84:1432–1438. doi: 10.1093/ps/84.9.1432. [DOI] [PubMed] [Google Scholar]
- Harris I.S., Treloar A.E., Satoshi I., Masato S., Chiara G., Kim Chung L., Ka Yi Y., Dirk B., Knobbe-Thomsen C.B., Cox M.A. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell. 2015;27:211–222. doi: 10.1016/j.ccell.2014.11.019. [DOI] [PubMed] [Google Scholar]
- He L., He T., Farrar S., Ji L., Liu T., Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 2017;44:532–553. doi: 10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- Jaeschke H., McGill M.R., Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: Lessons learned from acetaminophen hepatotoxicity. Drug Metab. Rev. 2012;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X.L., Wang K., Liu L., Liu H.Y., Zhao F.Q., Liu J.X. Nuclear factor-like factor 2-antioxidant response element signaling activation by tert-butylhydroquinone attenuates acute heat stress in bovine mammary epithelial cells. J. Dairy Sci. 2016;99:9094–9103. doi: 10.3168/jds.2016-11031. [DOI] [PubMed] [Google Scholar]
- Kansanen E., Kuosmanen S.M., Leinonen H., Levonen A.L. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol. 2013;1:45–49. doi: 10.1016/j.redox.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G., Galluzzi L., Vandenabeele P., Abrams J., Alnemri E.S., Baehrecke E.H., Blagosklonny M.V., El-Deiry W.S., Golstein P., Green D.R. Classification of cell death: recommendations of the Nomenclature Committee on cell death. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara L., Rostagno M. Impact of heat stress on poultry production. Animals. 2013;3:356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levonen A.L. Activation of stress signaling pathways by oxidized and Nitrated lipids. Free Radic. Biol. Med. 2012;52:973–982. doi: 10.1016/j.freeradbiomed.2011.11.038. [DOI] [PubMed] [Google Scholar]
- Li Y., Cao Y., Wang F., Li C.M. Scrotal heat induced the Nrf2-driven antioxidant response during oxidative stress and apoptosis in the mouse testis. Acta Histochem. 2014;116:883–890. doi: 10.1016/j.acthis.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Li Z., Li Y., Zhou X., Dai P., Li C. Autophagy involved in the activation of the Nrf2-antioxidant system in testes of heat-exposed mice. J. Therm. Biol. 2018;71:142–152. doi: 10.1016/j.jtherbio.2017.11.006. [DOI] [PubMed] [Google Scholar]
- Lin H., Decuypere E., Buyse J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 2006;144:11–17. doi: 10.1016/j.cbpa.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔ C T method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mcewen B.S. Stressed or stressed out: what is the difference? J. Psychiatry Neurosci. 2005;30:315–318. [PMC free article] [PubMed] [Google Scholar]
- Meltzer A. Thermoneutral zone and resting metabolic rate of broilers. Br. Poult. Sci. 1983;24:471–476. doi: 10.1080/00071668308416763. [DOI] [PubMed] [Google Scholar]
- Yang M., Tan H., Yang Q.L., Wang F., Yao H.L., Wei Q.Y., Tanguay R.M., Wu T.C. Association of hsp70 polymorphisms with risk of noise-induced hearing loss in Chinese automobile workers. Cell Stress Chaperones. 2006;11:233–239. doi: 10.1379/CSC-192R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Niture S.K., Jaiswal A.K. Nrf2 protein up-regulates antiapoptotic protein Bcl-2 and prevents cellular apoptosis. J. Biol. Chem. 2012;287:9873. doi: 10.1074/jbc.M111.312694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S., Gogvadze V., Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu. Rev. Pharmacol. Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- Sahin K., Orhan C., Tuzcu M., Sahin N., Hayirli A., Bilgili S., Kucuk O. Lycopene activates antioxidant enzymes and nuclear transcription factor systems in heat-stressed broilers. Poult. Sci. 2016;95:1088–1095. doi: 10.3382/ps/pew012. [DOI] [PubMed] [Google Scholar]
- Sahin K., Orhan C., Tuzcu Z., Tuzcu M., Sahin N. Curcumin ameloriates heat stress via inhibition of oxidative stress and modulation of Nrf2/HO-1 pathway in quail. Food Chem. Toxicol. 2012;50:4035–4041. doi: 10.1016/j.fct.2012.08.029. [DOI] [PubMed] [Google Scholar]
- Sahin K., Sahin N., Kucuk O., Hayirli A., Prasad A.S. Role of dietary zinc in heat-stressed poultry: a review. Poult. Sci. 2009;88:2176–2183. doi: 10.3382/ps.2008-00560. [DOI] [PubMed] [Google Scholar]
- Sahin N., Hayirli A., Orhan C., Tuzcu M., Akdemir F., Komorowski J.R., Sahin K. Effects of the supplemental chromium form on performance and oxidative stress in broilers exposed to heat stress. Poult. Sci. 2017;96:4317–4324. doi: 10.3382/ps/pex249. [DOI] [PubMed] [Google Scholar]
- Selye H. Stress and the general adaptation syndrome. Br. Med. J. 1950;1:1383–1392. doi: 10.1136/bmj.1.4667.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha L., Hor-Yue T., Ning W., Zhang-Jin Z., Lixing L., Chi-Woon W., Yibin F. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015;16:26087–26124. doi: 10.3390/ijms161125942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H., Berndt C., Jones D.P. Oxidative stress. Annu. Rev. Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- Surai P.F., Kochish I.I., Fisinin V.I., Kidd M.T. Antioxidant defence systems and oxidative stress in poultry biology: an update. Antioxidants. 2019;8:235. doi: 10.3390/antiox8070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan G.Y., Yang L., Fu Y.Q., Feng J.H., Zhang M.H. Effects of different acute high ambient temperatures on function of hepatic mitochondrial respiration, antioxidative enzymes, and oxidative injury in broiler chickens. Poult. Sci. 2010;89:115–122. doi: 10.3382/ps.2009-00318. [DOI] [PubMed] [Google Scholar]
- Trevisan M., Browne R., Ram M., Muti P., Freudenheim J., Carosella A.M., Armstrong D. Correlates of markers of oxidative status in the general population. Am. J. Epidemiol. 2001;154:348–356. doi: 10.1093/aje/154.4.348. [DOI] [PubMed] [Google Scholar]
- Vesco A., Del P., Gasparino E., Grieser D.O., Zancanela V., Gasparin F.R.S., Constantin J., Oliveira Neto A.R. Effects of methionine supplementation on the redox state of acute heat stress-exposed quails. J. Anim. Physiol. Anim. Nutr. 2016;101:806–815. doi: 10.2527/jas.2013-6829. [DOI] [PubMed] [Google Scholar]
- Xie J.J., Tang L., Lu L., Zhang L.Y., Lin X., Liu H.-C., Odle J., Luo X.G. Effects of acute and chronic heat stress on plasma metabolites, hormones and oxidant status in restrictedly fed broiler breeders. Poult. Sci. 2015;94:1635–1644. doi: 10.3382/ps/pev105. [DOI] [PubMed] [Google Scholar]
- Yang F.L., Lee C.C., Subeq Y.M., Lee C.J., Ke C.Y., Lee R.P. Heat adaptation from regular hot water immersion decreases proinflammatory responses, HSP70 expression, and physical heat stress. J. Therm. Biol. 2017;69:95–103. doi: 10.1016/j.jtherbio.2017.06.012. [DOI] [PubMed] [Google Scholar]
- Yang L., Tan G.Y., Fu Y.Q., Feng J.H., Zhang M.,H. Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2010;151:204–208. doi: 10.1016/j.cbpc.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Zhang J.F., Bai K.W., Su W.P., Wang A.A., Zhang L.L., Huang K.H., Wang T. Curcumin attenuates heat-stress-induced oxidant damage by simultaneous activation of GSH-related antioxidant enzymes and Nrf2-mediated phase II detoxifying enzyme systems in broiler chickens. Poult. Sci. 2018;97:1209–1219. doi: 10.3382/ps/pex408. [DOI] [PubMed] [Google Scholar]
- Zhang Y.K., Wu K.C., Klaassen C.D. Genetic activation of Nrf2 protects against Fasting-induced oxidative stress in livers of mice. PLoS One. 2013;8:e59122. doi: 10.1371/journal.pone.0059122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.F., Hu Z.P., Lu C.H., Yang M.X., Zhang L.L., Wang T. Dietary curcumin supplementation protects against heat-stress-impaired growth performance of broilers possibly through a mitochondrial pathway. J. Anim. Sci. 2015;93:1656–1665. doi: 10.2527/jas.2014-8244. [DOI] [PubMed] [Google Scholar]