Abstract
A total of 960 male Cobb 500 broilers were used in a growth performance study to explore the effect of coccidial vaccination and/or coccidial challenge on blood biochemistry and veterinary postmortem metrics. Day-old chicks were randomly divided into one of the 4 experimental treatments. Treatments were arranged in a 2 × 2 factorial arrangement, with the factors being without or with vaccination (administered on day 1) or coccidial challenge (oral gavage on day 7). Growth performance was monitored on a weekly basis. Blood sample collection, as well as full veterinary necropsies, were carried out on days 6, 8, 13, 20, 27, and 34. Birds that did not receive the vaccination but were challenged with coccidiosis had higher feed conversion ratio, lower body weights, and higher mortality than the other experimental groups, and this effect was particularly evident from day 13 to day 20. Birds challenged with coccidiosis had lower plasma sodium and total carotenoid concentrations and higher potassium and globulin concentrations than nonchallenged birds. Significant interactions between age and experimental treatment for these blood parameters were observed, particularly on day 13. The necropsy results confirmed the effectiveness of the challenge and vaccination treatments, wherein birds that were challenged had higher coccidiosis scores on day 13 and day 27 than birds that were not challenged. These results demonstrate the potential for plasma sodium, potassium, total protein, total carbon dioxide, globulin, and carotenoid analysis for early diagnosis of coccidiosis in growing broiler chickens. Further work is necessary to establish whether the changes in blood biochemistry observed in the present study are transferable to alternative flocks of chicken and whether early diagnosis and intervention may mitigate performance losses associated with this disease.
Key words: coccidiosis, broiler, blood, vaccination, performance
Introduction
In poultry, coccidiosis is caused by parasites from various species of the protozoan Eimeria, with species of notable interest being Eimeria acervulina, Eimeria maxima, and Eimeria tenella (Rose and Long, 1980). The various species of Eimeria vary in prevalence, pathogenicity, and orientation in the intestinal tract but all parasitize the epithelium resulting in inflammation, degradation of the mucosa, and, in severe cases, systemic effects such as sepsis, blood loss, and even death (Conway and McKenzie, 2007). Economic losses associated with coccidiosis are considerable and have been estimated to be as much as $0.05 per bird (Williams, 1999), although this will vary considerably with changes in feed cost and chicken meat prices. These losses are associated partially with the direct cost of coccidiosis control measures (approximately 20% of the total) but mostly with increases in feed conversion ratio (FCR), reductions in egg production, or meat yield (Vermeulen et al., 2001) and compounded by an increase in the severity of the symptoms of subsequent infections such as necrotic enteritis (Rodgers et al., 2015).
Coccidiosis control measures vary but include the use of in-feed prophylactic medication, management strategies to reduce infection pressure, and vaccination programs (Vermeulen et al., 2001; Dalloul and Lillehoj, 2006). Nutritional intervention, including the use of feed enzymes such as exogenous protease (Peek et al., 2009), dietary protein concentration (Lee et al., 2011), probiotics (Ritzi et al., 2016), various botanicals and antioxidants (Naidoo et al., 2008; Abbas et al., 2012), and a variety of other factors such as immunostimulants, betaine, and the mechanical structure of the feed (Langhout, 1999) have been demonstrated to be partially successful in controlling the symptoms of coccidiosis.
The emergence of resistant strains of Eimeria, with concomitant reductions in the efficacy of many currently used coccidiostats and changes in the tolerance of the use of medication in livestock production by consumers (Stringfellow et al., 2011), has resulted in an increased avoidance of anticoccidial chemotherapeutics. Alternative coccidiosis control measures are being explored. It is likely that a multifactorial approach that may include vaccination programs, management/biosecurity practices, and adjacent nutritional intervention to reduce oocyst shedding or improve the resilience of the host animal will be required. However, although there is a substantial body of research to explore strategies to optimize the efficacy of vaccination, nutrition, and management programs, there is less attention on biomarkers for early diagnosis of coccidiosis. It was therefore the aim of the present study to systematically explore the effect of both coccidial vaccination and challenge on performance and blood biochemical parameters in broiler chickens. The goal of the work was ultimately to provide objective metrics of coccidial infection that may be used to build a diagnostic algorithm to enable early diagnosis and treatment of this disease.
Materials and methods
Ethics
All animal work was approved by the Institutional Animal Care and Use Committee of North Carolina State University.
Facilities and Rearing
A total of 960 Cobb 500 male broiler chicks were obtained from the resident broiler breeder flock (North Carolina State University, Raleigh, NC) and randomly allocated to 48 pens (20 chicks per pen). The experiment comprised 2 factors, being without or with coccidial vaccination (day 1, Merck B-52 Coccivac; Merck & Co, Kenilworth, NJ) and without or with a coccidial challenge (day 7; 10 times the dose of the same vaccine used on day 1 delivered via oral gavage), generating a total of 4 experimental treatments. The Merck B-52 coccidial vaccine contains live oocysts from E. acervulina, E. maxima, E. maxima MFP, Eimeria mivati, and E. tenella. A common starter (day 1–14) and grower (day 15–35) diet was fed to all pens (Table 1) on an ad libitum basis. Water was also available ad libitum. Temperature was set at 95°F for the first 12 h and was subsequently reduced by 1°F per day until 70 °F was reached. At this point, this temperature was maintained for the remainder of the experiment. The photoperiod was 23 h of light for the first 6 D and was gradually reduced to 16 h by the end of the experiment. Strict biosecurity protocols were adopted to ensure that personnel did not cross-contaminate pens. These protocols covered pen allocation, day-to-day observations, feeding, sampling, and weighing birds.
Table 1.
Composition (%) and calculated nutrient provision (%, unless otherwise stated) of the starter and grower diets.
| Ingredients | Starter4 | Grower5 |
|---|---|---|
| Corn | 57.65 | 63.90 |
| Soybean meal (48% CP) | 32.02 | 23.89 |
| Poultry by-product meal | 5.00 | 5.86 |
| Poultry fat | 2.00 | 2.51 |
| Dicalcium phosphate (18.5% P) | 1.24 | 1.16 |
| Limestone | 0.61 | 0.82 |
| Salt | 0.50 | 0.50 |
| Choline chloride (60%) | 0.20 | 0.20 |
| Vitamin premix1 | 0.05 | 0.05 |
| Mineral premix2 | 0.20 | 0.20 |
| Selenium premix3 | 0.05 | 0.05 |
| DL-Methionine | 0.23 | 0.17 |
| L-Lysine | 0.14 | 0.20 |
| L-Threonine | 0.11 | 0.09 |
| Total | 100.00 | 100.00 |
| Calculated nutrient content | ||
| Crude protein | 23.00 | 20.00 |
| Calcium | 0.90 | 0.90 |
| Available phosphorus | 0.45 | 0.45 |
| Potassium | 0.88 | 0.93 |
| Total lysine | 1.31 | 1.14 |
| Total methionine | 0.59 | 0.49 |
| Total threonine | 0.88 | 0.76 |
| Total methionine + cysteine | 0.95 | 0.81 |
| Sodium | 0.22 | 0.22 |
| Metabolizable energy (kcal/g) | 2,935 | 3,050 |
Vitamin premix supplied the following per kg of diet: 13,200 IU of vitamin A, 4,000 IU of vitamin D3, 33 IU of vitamin E, 0.02 mg of vitamin B12, 0.13 mg of biotin, 2 mg of menadione (K3), 2 mg of thiamine, 6.6 mg of riboflavin, 11 mg of d-pantothenic acid, 4 mg of vitamin B6, 55 mg of niacin, and 1.1 mg of folic acid.
Mineral premix supplied the following per kg of diet: manganese, 120 mg; zinc, 120 mg; iron, 80 mg; copper, 10 mg; iodine, 2.5 mg; and cobalt, 1 mg.
Selenium premix provided 0.2 mg of Se (as Na2SeO3) per kg of diet.
Starter diet was fed to 14 D of age.
Grower diet was fed from 15 to 35 D of age.
Live Performance and Blood Indicators
Bird weight (BW) and FCR per pen was recorded on day 7, 14, 21, 28, and 35. Mortality was monitored daily, and the weights of any dead birds were used to correct FCR values. On day 6, 8, 13, 20, 27, and 34, one bird per pen was randomly selected, and 4 to 5 mL of blood was collected from each bird and placed in heparinized (0.5 mL), EDTA (1 mL), and nonadditive (3 mL) blood collection tubes. On day 7 and 14, blood was collected from the heart; whereas on day 14, 21, 28, and 35, blood was collected from the right jugular vein. After blood collection, all birds were euthanized, and postmortem evaluation carried out. Veterinary postmortem analysis was specifically oriented toward metrics that were known to be associated with coccidiosis, specifically gross acervulina (GAc), gross tenella (GTn), gross micro max (GMx), and micro max (mMx) scores. These observations have a score from 0 to 4, with 0 being “absent” and 4 being “severe” as described by Conway and McKenzie (2007).
Heparinized blood (approximately 0.2 mL) was analyzed in the i-Stat Alinity v handheld blood analyzer fitted with a Chem8+ cartridge (Abbott Point of Care Inc., Princeton, NJ), which measures hematocrit (HCT), ionized calcium (Ca), glucose (GLU), chloride (Cl), sodium (Na), potassium (K), total carbon dioxide (TCO2) and anion gap (AnGap) levels.
A total of 0.1 mL was analyzed in the Vetscan VS2 Chemistry Analyzer (Abaxis, Inc, Union City, CA) using the Avian/Reptilina Profile Plus cartridge (Abbott Point of Care Inc., Princeton, NJ). This resulted in analysis of aspartate aminotransferase (AST), creatine kinase (CK), uric acid (UA), GLU, Ca, phosphorus (P), total protein (TP), albumin (ALB), albumin/globulin (GLOB), K (Kv), and Na (Nav) levels.
Precisely, 1.0 mL of EDTA blood was mixed with 0.20 mL of the cellular fixant (Transfix; MBL International, Woburn, MA), stored, and shipped on wet ice to Cayman Analytical Laboratories (Ann Arbor, MI) for heterophil (HET)-to-lymphocyte (LYM) ratio analysis by flow cytometry as described by Lentfer et al., (2015) and Bílková et al., 2017.
Whole blood (3 mL) was spun at 1,000 g for 15 min, and the serum was removed and stored on wet ice. A total of 0.40 mL of serum was used for analysis of total carotenoid (CAR) content using the iCheck carotene photometer device and test kit (BioAnalyt GmbH; Potsdam, Germany) as described by Kawashima et al., 2010. The remaining serum was frozen on dry ice and shipped to Cayman Analytical Laboratories (Ann Arbor, MI) for thiobarbituric acid analysis (Wills, 1966), which measures malondialdehyde (MDA) content in the blood.
Statistical Analysis
Data were analyzed using JMP version 12.2.0 (SAS Institute, Cary, NC), with model terms including vaccination (+/−), challenge (+/−) (in the case of weight gain, FCR, and mortality), and age (6, 8, 13, 20, 27 and 34 D) (for blood biochemical measurements). Significance was set at P < 0.05. All data were included with the exception of 2 outliers (one for plasma Na and one for AST), which had values higher than 3 standard deviations from the treatment mean values and were thus excluded from the analysis.
Results
Bird Performance
The effect of coccidial vaccination and/or challenge on the average body weight or FCR of broiler chickens is presented in Tables 2 and 3. There was no effect of treatment on average bird body weight or FCR on day 1 or day 7. However, from day 14 and for the remainder of the experiment, the birds that received the coccidial challenge without vaccination had lower body weight and higher FCR than any of the alternative treatment groups, resulting in a significant vaccination × challenge interaction. Overall mortality (day 1–35, 19.1%) was also higher in challenged birds without vaccination than in the birds from the other treatments, resulting in a significant vaccination × challenge interaction for mortality.
Table 2.
Effect of coccidial challenge and vaccination on the body weight (BW) of broiler chickens from day 1 to day 351.
| Vaccine | Challenge | BW, day 1, kg/b | BW, day 7, kg/b | BW, day 14, kg/b | BW, day 21, kg/b | BW, day 28, kg/b | BW, day 35, kg/b |
|---|---|---|---|---|---|---|---|
| Treatment effects | |||||||
| − | + | 0.048 | 0.220 | 0.441 | 0.895 | 1.46 | 2.24 |
| − | − | 0.048 | 0.222 | 0.586 | 1.130 | 1.77 | 2.37 |
| + | + | 0.048 | 0.222 | 0.481 | 1.004 | 1.67 | 2.35 |
| + | − | 0.048 | 0.222 | 0.563 | 1.033 | 1.71 | 2.38 |
| P < | NS | NS | 0.001 | 0.001 | 0.05 | 0.001 | |
| SEM | 0.001 | 0.002 | 0.008 | 0.019 | 0.069 | 0.020 | |
| Interaction terms | |||||||
| Vaccine × challenge | NS | NS | 0.001 | 0.001 | 0.05 | 0.05 | |
NS: not statistically significant (P > 0.05).
Table 3.
Effect of coccidial challenge and vaccination on the feed conversion ratio (FCR; g:g) and mortality (%) of broiler chickens from day 1 to day 351.
| Vaccine | Challenge | FCR, day 1–7 | FCR, day 1–14 | FCR, day 1–21 | FCR, day 1–28 | FCR, day 1–35 | Mortality, day 1–35, % |
|---|---|---|---|---|---|---|---|
| Treatment effects | |||||||
| − | + | 1.07 | 1.51 | 1.57 | 1.58 | 1.63 | 19.1 |
| − | − | 1.08 | 1.23 | 1.31 | 1.42 | 1.51 | 2.4 |
| + | + | 1.05 | 1.38 | 1.41 | 1.48 | 1.55 | 2.4 |
| + | − | 1.06 | 1.24 | 1.40 | 1.49 | 1.56 | 8.9 |
| P < | NS | 0.001 | 0.001 | 0.001 | 0.001 | 0.05 | |
| SEM | 0.011 | 0.030 | 0.022 | 0.017 | 0.011 | 0.06 | |
| Interaction terms | |||||||
| Vaccine × challenge | NS | 0.05 | 0.001 | 0.001 | 0.001 | 0.05 | |
NS: not statistically significant (P > 0.05).
Blood Biochemistry
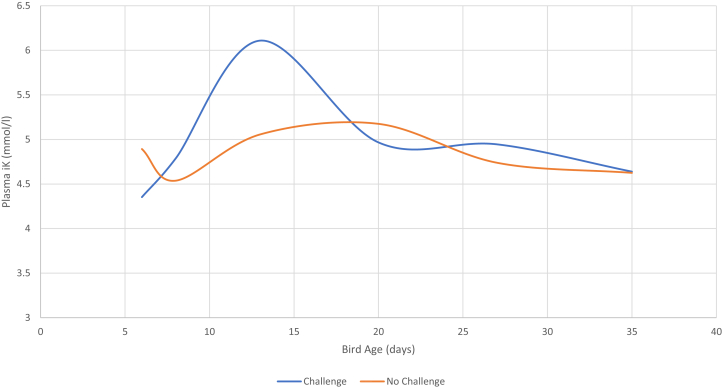
The effect of coccidial vaccination and challenge on plasma Ca, P, Na, K, Cl, and AnGap from day 6 to day 34 is presented in Table 4. There was no effect (P > 0.05) of coccidial vaccination or challenge on plasma Ca (either measured by VetScan or iStat), P, Cl, Na (VetScan), or K (VetScan) content. However, birds that received the coccidial challenge had lower plasma Na (iStat) content on day 13 than those that did not receive the challenge, resulting in a challenge × age interaction (P < 0.001). In contrast, birds that received the coccidial challenge had higher plasma K (iStat) content on day 13 than those that did not, resulting in a challenge × age interaction (P < 0.001; Figure 1). On day 13, the plasma AnGap level was lower in birds that received the challenge than those that did not, resulting in a significant challenge × age interaction. There was a significant linear and/or quadratic increase in plasma Ca, Na, and K (both iStat and VetScan) content and a concomitant decrease in plasma P and AnGap levels with increasing bird age. There was no effect (P > 0.05) of bird age on plasma Cl content.
Table 4.
Interaction between coccidial vaccination, coccidial challenge, and bird age on blood calcium (Ca), phosphorus (P), potassium (K), chloride (Cl) and anion gap levels of broiler chickens from day 6 to day 341.
| Treatment | Vaccine | Challenge | Age | vCa, mmol/l | iCa, mmol/l | vP, mmol/l | vNa, mmol/l | iNa, mmol/l | vK, mmol/l | iK, mmol/l | iCl, mmol/l | iAnGap, mmol/l |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Main effects | ||||||||||||
| − | 10.3 | 1.2 | 9.8 | 147.3 | 143.8 | 6.7 | 4.9 | 108.2 | 19.6 | |||
| + | 10.4 | 1.2 | 10.0 | 147.7 | 144.4 | 6.4 | 4.9 | 108.5 | 19.7 | |||
| P < | NS | NS | NS | NS | NS | NS | NS | NS | NS | |||
| − | 10.3 | 1.2 | 9.8 | 147.4 | 144.0 | 6.5 | 5.0 | 108.6 | 19.5 | |||
| + | 10.3 | 1.3 | 10.1 | 147.5 | 144.3 | 6.5 | 4.8 | 108.1 | 19.8 | |||
| P < | NS | NS | NS | NS | NS | NS | NS | NS | NS | |||
| 6 | 9.5 | 1.2 | 12.3 | 145.9 | 143.5 | 5.3 | 4.6 | 108.0 | 20.5 | |||
| 8 | 9.3 | 1.1 | 11.3 | 147.9 | 144.7 | 5.3 | 4.7 | 109.3 | 20.7 | |||
| 13 | 10.2 | 1.3 | 9.8 | 147.1 | 142.5 | 6.6 | 5.6 | 108.7 | 18.4 | |||
| 20 | 10.7 | 1.3 | 9.2 | 147.6 | 144.1 | 6.4 | 5.1 | 108.8 | 19.9 | |||
| 27 | 11.4 | 1.3 | 8.6 | 147.9 | 144.5 | 7.6 | 4.8 | 107.0 | 19.1 | |||
| 34 | 11.0 | 1.3 | 7.9 | 148.5 | 145.3 | 7.9 | 4.6 | 108.2 | 18.9 | |||
| P < | 0.001 | 0.001 | 0.001 | 0.01 | 0.001 | 0.001 | 0.001 | NS | 0.01 | |||
| L < | 0.001 | 0.001 | 0.001 | 0.01 | 0.05 | 0.001 | NS | NS | 0.01 | |||
| Q < | 0.001 | 0.001 | 0.001 | 0.01 | 0.01 | 0.01 | 0.01 | NS | 0.01 | |||
| SEM | 0.47 | 0.06 | 0.86 | 0.92 | 0.85 | 0.49 | 0.28 | 1.27 | 1.04 | |||
| Interaction terms | ||||||||||||
| Vaccine × challenge | NS | NS | NS | NS | NS | NS | NS | NS | NS | |||
| Vaccine × age | NS | NS | NS | NS | NS | NS | NS | NS | NS | |||
| Challenge × age | NS | NS | NS | NS | 0.001 | NS | 0.001 | NS | 0.05 | |||
| Vaccine × challenge × age | NS | NS | NS | NS | NS | NS | NS | NS | NS | |||
Superscripts for column headers refer to either VetScan ‘v’ or iStat ‘i’ devices that were used.
L: linear P value; Q: quadratic P value; NS: not statistically significant (P > 0.05).
Figure 1.
Effect of age and a mixed-species coccidial challenge (introduced on day 7) on plasma potassium (K) concentration in male Cobb 500 broiler chickens. A significant age × challenge interaction was observed, which was generated by the transient increase around day 13.
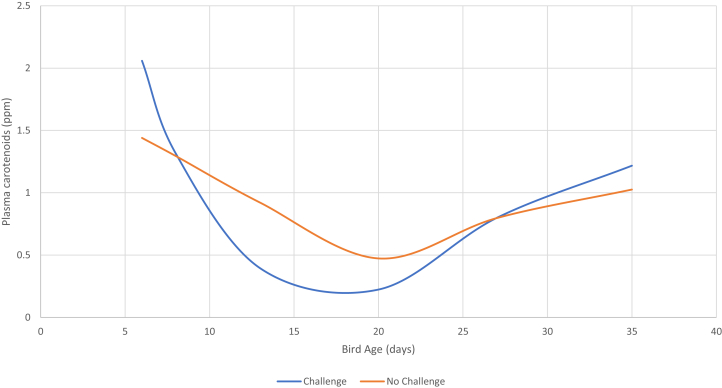
The effect of experimental treatment on plasma AST, CK, BA, UA, TP, GLOB, ALB, and CAR levels is presented in Table 5. There was no effect (P > 0.05) of coccidial vaccination or challenge on plasma CK, BA, UA, or ALB levels. Birds that received the coccidial vaccination had a lower (P < 0.05) AST concentration than those that did not receive. Birds that received the coccidial challenge had elevated plasma TP and GLOB concentrations on day 13 compared with birds that were not challenged, resulting in a significant challenge × age interaction. Both vaccination and coccidial challenge reduced plasma CAR concentration, and this was exaggerated from day 13 to day 20. However, birds that were not vaccinated or challenged had higher plasma CAR concentrations, notably at the same age, resulting in a significant vaccination × challenge × age interaction. There was a significant linear and/or quadratic increase in plasma AST, CK, TP, ALB, and GLOB levels with increasing bird age. In contrast, plasma concentrations of UA and CAR decreased (P < 0.05) with increasing bird age. In the case of BA, there was a significant quadratic correlation with bird age wherein plasma BA concentration was higher on day 6, reduced on day 27, and then increased on day 35.
Table 5.
Interaction between coccidial vaccination, coccidial challenge, and bird age (days) on blood aspartate aminotransferase (AST), creatine kinase (CK), bile acid (BA), uric acid (UA), total protein (TP), albumin (ALB), globulin (GLOB), and total carotenoid (CAR) levels of broiler chickens from day 6 to day 341.
| Treatment | Vaccine | Challenge | Age | AST, U/l | CK, U/l | BA, μmol/l | UA, mg/dl | TP, g/l | ALB, g/l | GLOB, g/l | CAR, ppm |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Main effects | |||||||||||
| − | 237.3 | 3,022.6 | 4.1 | 6.4 | 2.7 | 2.1 | 0.6 | 1.08 | |||
| + | 208.3 | 3,196.2 | 3.9 | 6.9 | 2.7 | 2.0 | 0.6 | 0.91 | |||
| P < | 0.05 | NS | NS | NS | NS | NS | NS | 0.001 | |||
| − | 218.8 | 2,976.3 | 3.7 | 6.6 | 2.7 | 2.0 | 0.6 | 1.01 | |||
| + | 226.8 | 3,242.5 | 4.4 | 6.8 | 2.7 | 2.1 | 0.6 | 0.98 | |||
| P < | NS | NS | NS | NS | NS | NS | NS | NS | |||
| 6 | 271.7 | 2,612.7 | 9.4 | 7.8 | 2.3 | 1.8 | 0.4 | 1.71 | |||
| 8 | 156.3 | 1,523.1 | 3.4 | 8.0 | 2.3 | 1.9 | 0.3 | 1.33 | |||
| 13 | 182.5 | 2,257.7 | 1.6 | 7.1 | 2.6 | 2.0 | 0.6 | 0.65 | |||
| 20 | 177.5 | 3,243.3 | 1.2 | 5.9 | 2.7 | 2.0 | 0.8 | 0.35 | |||
| 27 | 214.9 | 5,504.2 | 1.9 | 4.8 | 3.0 | 2.3 | 0.7 | 0.79 | |||
| 34 | 333.8 | 3,515.2 | 6.5 | 6.4 | 3.1 | 2.4 | 0.7 | 1.12 | |||
| P < | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | |||
| L < | NS | NS | NS | 0.01 | 0.001 | 0.001 | 0.01 | 0.05 | |||
| Q < | 0.01 | 0.01 | 0.001 | 0.01 | 0.05 | 0.05 | 0.01 | 0.001 | |||
| SEM | 35.89 | 720.90 | 1.73 | 0.78 | 0.12 | 1.68 | 0.07 | 0.101 | |||
| Interaction terms | |||||||||||
| Vaccine × challenge | NS | NS | NS | NS | NS | NS | NS | 0.01 | |||
| Vaccine × age | NS | NS | NS | NS | NS | NS | NS | 0.01 | |||
| Challenge × age | NS | NS | NS | NS | 0.05 | NS | 0.001 | 0.001 | |||
| Vaccine × challenge∗age | NS | NS | NS | NS | NS | NS | NS | 0.001 | |||
L: linear P value; Q: quadratic P value; NS: not statistically significant (P > 0.05).
The effect of coccidial vaccination and/or challenge on plasma GLU, TCO2, HCT, MDA, HET, and LYM levels and HET:LYM ratio is presented in Table 6. There was no effect (P > 0.05) of coccidial vaccination or challenge on plasma GLU, HCT, MDA, HET, and LYM levels or HET:LYM ratio. Birds that received the coccidial challenge had lower plasma TCO2 concentration on day 13 but higher concentration on day 35 than those that did not, resulting in a challenge × age interaction (P < 0.05). Plasma GLU, TCO2, HCT, HET, and LYM levels and HET:LYM ratio increased linearly with increasing bird age. In contrast, plasma MDA concentration decreased (P < 0.001) with increasing bird age.
Table 6.
Interaction between coccidial vaccination, coccidial challenge, and bird age (day) on blood glucose (Glu), total carbon dioxide (TCO2), hematocrit (HCT), TBARS (malondialdehyde [MDA]), heterophil (HET), and lymphocyte (LYM) levels and the HET:LYM ratio of broiler chickens from day 6 to day 341.
| Treatment | Vaccine | Challenge | Age | vGlu, mg/dl | iGlu, mg/dl | TCO2, mmol/l | HCT, % | MDA, μM | HET | LYM | HET:LYM |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Main effects | |||||||||||
| − | 222.4 | 223.2 | 22.1 | 19.0 | 1.00 | 2,249.2 | 5,008.4 | 0.44 | |||
| + | 217.6 | 215.2 | 22.1 | 18.4 | 0.99 | 2,412.1 | 5,522.3 | 0.45 | |||
| P < | NS | NS | NS | NS | NS | NS | NS | NS | |||
| − | 221.7 | 222.4 | 21.9 | 18.9 | 0.99 | 2,432.0 | 5,445.0 | 0.44 | |||
| + | 218.4 | 216.0 | 22.3 | 18.5 | 0.99 | 2,229.3 | 5,085.8 | 0.45 | |||
| P < | NS | NS | NS | NS | NS | NS | NS | NS | |||
| 6 | 215.6 | 212.4 | 20.7 | 15.1 | 1.47 | 417.7 | 1,997.2 | 0.24 | |||
| 8 | 201.2 | 202.6 | 20.3 | 16.5 | 1.14 | 583.7 | 2,161.3 | 0.25 | |||
| 13 | 223.2 | 227.6 | 22.1 | 18.9 | 1.22 | 4,461.1 | 4,580.3 | 0.99 | |||
| 20 | 218.8 | 217.4 | 21.5 | 19.6 | 0.94 | 2,436.2 | 8,541.5 | 0.30 | |||
| 27 | 233.6 | 230.2 | 24.1 | 21.7 | 0.78 | 2,355.0 | 8,106.6 | 0.30 | |||
| 34 | 227.8 | 225.0 | 24.0 | 20.5 | 0.42 | 3,730.3 | 6,205.4 | 0.60 | |||
| P < | 0.05 | 0.05 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | |||
| L < | 0.05 | 0.05 | 0.001 | 0.001 | 0.001 | 0.01 | 0.001 | 0.01 | |||
| Q < | 0.05 | 0.05 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | |||
| SEM | 12.94 | 13.83 | 1.26 | 1.33 | 0.166 | 668.73 | 722.50 | 0.102 | |||
| Interaction terms | |||||||||||
| Vaccine × challenge | NS | NS | NS | NS | NS | NS | NS | NS | |||
| Vaccine × age | NS | NS | NS | NS | NS | NS | NS | NS | |||
| Challenge × age | NS | NS | 0.05 | NS | NS | NS | NS | NS | |||
| Vaccine × challenge∗age | NS | NS | NS | NS | NS | NS | NS | NS | |||
Superscripts for column headers refer to either VetScan ‘v’ or iStat ‘i’ devices that were used.
L: linear P value; Q: quadratic P value; NS: not statistically significant (P > 0.05).
Coccidiosis-Associated Intestinal Lesions
The effect of coccidial challenge and vaccination on veterinary postmortem metrics is described in Table 7. There was no effect (P > 0.05) of experimental treatment on GTn or GMx scores. Birds that were not vaccinated and challenged had higher GAc scores on day 13 than was the case for alternative treatment groups, resulting in a significant challenge × vaccination × age interaction. Birds that were challenged had higher mMx scores on day 13 and day 27 than birds that were not challenged, resulting in a significant challenge × age interaction. Furthermore, on day 13 and day 27, birds that were not vaccinated had higher mMx scores than those that were vaccinated, resulting in a significant vaccination × age interaction. There was a significant quadratic effect of age on GAc, GTn, GMx, and mMx scores, with consistently higher scores from day 13 to day 27 than from day 6 to day 8 or from day 27 to day 34.
Table 7.
Effect of coccidial vaccination or challenge on the veterinary necropsy results from day 6 to day 341.
| Treatment | Vaccine | Challenge | Age | GAc | GTn | GMx | mMx |
|---|---|---|---|---|---|---|---|
| Main Effects | |||||||
| − | 0.81 | 0.08 | 0.28 | 0.28 | |||
| + | 0.89 | 0.07 | 0.31 | 0.10 | |||
| P < | NS | NS | NS | 0.01 | |||
| − | 0.87 | 0.12 | 0.27 | 0.31 | |||
| + | 0.83 | 0.03 | 0.32 | 0.08 | |||
| P < | NS | 0.05 | NS | 0.001 | |||
| 6 | 0.02 | 0.00 | 0.01 | 0.01 | |||
| 8 | 0.23 | 0.02 | 0.02 | 0.02 | |||
| 13 | 1.58 | 0.23 | 0.44 | 0.48 | |||
| 20 | 1.96 | 0.19 | 0.93 | 0.02 | |||
| 27 | 0.73 | 0.00 | 0.21 | 0.67 | |||
| 34 | 0.60 | 0.00 | 0.17 | 0.02 | |||
| P < | 0.001 | 0.01 | 0.001 | 0.001 | |||
| L < | 0.05 | 0.05 | 0.05 | NS | |||
| Q < | 0.001 | 0.01 | 0.001 | 0.001 | |||
| SEM | 0.279 | 0.105 | 0.178 | 0.153 | |||
| Interaction terms | |||||||
| Vaccine × challenge | NS | NS | NS | NS | |||
| Vaccine × age | NS | NS | NS | 0.01 | |||
| Challenge × age | NS | NS | NS | 0.001 | |||
| Vaccine × challenge∗age | 0.05 | NS | NS | NS | |||
Abbreviations: GAc, gross acervulina; GTn, gross tenella (score 0–4); GMx, gross micro max (score 0–4); mMx, micro max (score 0–4)
L: linear P value; Q: quadratic P value; NS: not statistically significant (P > 0.05).
Discussion
The performance of the control population in the present study was in line with breeder guidelines (Tables 2 and 3; day 35: BW of 2.37 kg; FCR of 1.51, and mortality of 2.4%). Administering a coccidial vaccination, without the subsequent challenge, increased FCR without influence on BW, but was highly effective in mitigating the negative consequences of coccidial challenge on broiler performance and on veterinary necropsy scores (Table 7). Coccidial challenge, in the absence of vaccination, resulted in a significant increase in FCR (1.63 vs. 1.51) and a reduction in terminal BW (2.24 kg vs. 2.37 kg). The compromised performance of birds that received the coccidial vaccination has been shown previously. For example, Wang et al. (2019) noted that coccidial vaccination reduced the body weight gain of broiler chickens. Similarly, and in agreement with the present study, Lee et al. (2011) noted that coccidial vaccination decreased BW and increased FCR in broiler chickens but was successful in reducing the severity of the effects of coccidial challenge on bird growth. Reduced BW in the starter phase in response to coccidial vaccination was also reported by Da Silva et al. (2009), but these effects did not persist in the grower phase of the experiment. It appears likely that the deterioration in growth, especially during the grower phase, associated with coccidial vaccination is associated with repartitioning of nutrients from lean gain to mount an immune response to the vaccine. Although this is clearly undesirable, it is necessary to generate protective immunity against coccidiosis, and often, these performance losses are transient, with mitigation by compensatory growth over the life of the bird relative to nonvaccinated healthy birds (Lee et al., 2011). Regardless, the poorer performance of the vaccinated birds in the present study and the effectiveness of the vaccine to counter the negative effects of coccidial challenge is in line with previous observations.
Instructively, the effect of broiler genetics, age, and gender on blood biochemistry has recently been reported (Livingston et al., 2020). Although there are some minor differences with the values presented herein, the blood metabolite concentrations for male Cobb 500 (the same gender and genetics used in the present work) are in agreement with those presented in the following paragraphs. This observation brings confidence that the effect of vaccination and challenge on blood biochemistry observed in the present study was not associated with normal fluctuation associated with bird age, gender, or genetics, but represents a genuine deviation from baseline values. The statistically confirmed separation from the control group in the present study provides further important reinforcement of the validity of these blood biochemical changes.
Changes in blood mineral composition in relation to coccidiosis infection have been reported previously. For example, Turk (1986) observed a decrease in plasma Ca and Na content and an increase in plasma K content in response to acute coccidial infection in broiler chickens, although these responses may vary and depend on the species of Eimeria involved. The results for Na and K content are in agreement with the results presented in Table 4, wherein there was a significant increase in the concentration of K and a reduction in Na content in the blood on day 13 in response to the coccidial challenge. The appearance of K in the blood in response to the coccidial challenge, which is illustrated in Figure 1, may be associated with cellular damage in the intestinal lining (Turk, 1986) or with water loss or a change of the hydration state of the bird if absorption in the caudal gut was compromised. The reducing effect of coccidial challenge on AnGap levels on day 13 in the present work is interpreted to be associated with the changes in blood Na and K content ratios. However, it is also possible that these changes are associated with hypoalbuminemia in response to inflammation caused by the coccidiosis infection, in a similar way that has been previously reported for mycotoxins (Maurice et al., 1983). Although there was not a significant effect of coccidial challenge on plasma ALB levels in the present work, there was a numerical decrease on day 13, which is coherent with this hypothesis. Further work on the effect of coccidial infection on inflammation and blood peptide composition would be needed to clarify these observations.
The reduction in plasma Ca content noted by Turk (1986) was not repeated in the present work, which may be associated with differences in the Eimeria species used to challenge the birds in the 2 studies. Turk (1986) used a variety of species and noted that only high inoculation concentrations of E. acervulina (in the proximal small intestine) were associated with reductions in plasma Ca concentration. This species-dependent effect on plasma mineral composition offers potential for more accurate diagnosis of coccidial infection at a species level in the future and indeed may extend to several alternative blood biomarkers.
In the present study, there was a significant increase in plasma GLOB and TP levels in response to the coccidial challenge on day 13. Increase of TP levels in the blood is commonly observed in cases of dehydration or volume contraction secondary to fluid loss. Hyperglobulinemia is associated with immune response to infection and has been previously noted in broilers challenged with a Clostridium perfringens vaccine (Saleh et al., 2011). It is possible that similar effects were responsible for the marked elevation in plasma GLOB concentrations in the starter phase of the present experiment. The effect of both vaccination and coccidial challenge on plasma carotenoid concentration was marked (Table 5; Figure 2). Previously, Bletner et al. (1966) and Ruff et al. (1974) observed a significant reduction in blood carotenoid concentrations in response to coccidial infection in chickens. The mechanism for this reduction may be associated with both a reduction in absorptive capacity of the intestine and the destruction of these compounds by generation of reactive oxygen species intermediates during the Eimeria challenge (Allen, 1997). The fact that Bletner et al. (1966) observed significant reductions in plasma carotenoid concentrations without any change in bird performance during a mild coccidial challenge is evidence for mechanisms that extend beyond malabsorption. Plasma carotenoid concentration may be a useful biomarker for both mild and severe coccidial challenge.
Figure 2.
Effect of age and a mixed-species coccidial challenge (introduced on day 7) on plasma carotenoid concentration in male Cobb 500 broiler chickens. A significant age × challenge interaction was observed, which was generated by the transient decrease from day 13 to day 20.
In the present work, there was a significant reduction in plasma AST levels associated with vaccination but no effect of coccidial challenge (Table 5). Adamu et al. (2013) noted changes in various enzymes in the serum of chickens infected with coccidiosis such as reductions in AST levels, which are aligned with the present results. Arczewska-Włosek et al., 2018 observed an interaction between dietary crude protein concentration and coccidial vaccination for plasma AST levels, whereby AST activity was decreased by vaccination only when offered simultaneously with a diet high in crude protein. Alternative studies (Patra et al., 2010; Abd El-Maksoud et al., 2014) noted an increase in AST levels in response to coccidiosis, so there are conflicting reports in the literature on this point. Thus, for AST to be a useful biomarker for coccidial challenge, it may be necessary to also consider other factors such as diet composition to set context for relative changes in the AST level.
There was no effect of coccidial vaccination or challenge on plasma Glu, HCT, HET, LYM, or MDA levels or the HET:LYM ratio (Table 5), although these blood metrics were influenced by the age of the bird. Arczewska-Włosek et al., 2018 also noted no change in HCT or HET levels in response to coccidial vaccination but observed a reduction in plasma Glu, MDA, and LYM content, although, similar to the effects on AST mentioned previously, there were complex interactions between dietary crude protein concentration and coccidial vaccination that make these biochemical changes difficult to contextualize across studies. In an early study, Natt and Herrick (1956) observed a reduction in the HCT value in response to cecal coccidiosis in chickens. Similarly, Turk (1986) observed a decrease in hemoglobin and erythrocyte concentrations and HCT levels during acute coccidial infection in chickens. Da Silva et al. (2009) also noted a significant reduction in the HCT level in response to coccidial vaccination. The reason why HCT did not respond to either the vaccination or challenge in the present study is not clear, but it may be related to the specific mix of Eimeria species used in this study.
Conclusions
A persistent negative impact of coccidial challenge and a transient negative influence of coccidial vaccination were noted in growing broiler chickens fed with a corn/soy-based diet, and confirmation of infection was noted on postmortem inspection. Several blood biochemical characteristics proved to be plastic in response to these challenges, especially in the starter phase (specifically around day 13). These blood markers included plasma Na, K, AnGap, carotenoids, TCO2, globulin, and TP levels. These data offer the potential for a biochemical “fingerprint” of coccidiosis to be generated and used to support macroscopic veterinary inspection to achieve objective early diagnosis. Further research is needed to assess how consistently these blood metrics are influenced by the Eimeira challenge and whether specific Eimeira species may influence blood biochemistry in uniquely identifiable patterns. Finally, an appreciation for changes in blood biochemistry in response to coccidiosis challenge may allow nutritionists an opportunity to intervene at a dietary level to improve flock resilience, welfare, and performance.
Acknowledgments
This work was financially supported by DSM Nutritional Products, Kaiseraugst, Switzerland.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Abbas R.Z., Colwell D.D., Gilleard J. Botanicals: an alternative approach for the control of avian coccidiosis. Worlds Poult. Sci. J. 2012;68:203–215. [Google Scholar]
- Abd El-Maksoud H.A., Afaf D.A.M., El-Badry M.A. Biochemical effect of coccidia infestation in laying hen. Vet. Med. J. 2014;26:127–133. [Google Scholar]
- Adamu M., Boonkaewwan C., Gongruttananun N., Vongpakorn M. Hematological, biochemical and histopathological changes caused by coccidiosis in chickens. Kasetsart J. Nat. Sci. 2013;47:238–246. [Google Scholar]
- Allen P.C. Production of free radical species during Eimeria maxima infections of chickens. Poult. Sci. 1997;76:814–821. doi: 10.1093/ps/76.6.814. [DOI] [PubMed] [Google Scholar]
- Arczewska-Włosek A., Świątkiewicz S., Ognik K., Józefiak D. Effect of dietary crude protein level and Supplemental Herbal Extract Blend on selected blood Variables in broiler chickens vaccinated against coccidiosis. Animals. 2018;8:1–13. doi: 10.3390/ani8110208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bílková B., Bainová Z., Janda J., Vinkler M. Different breeds, different blood: Cytometric analysis of whole blood cellular composition in chicken breeds. Vet. Immunol. Immunopathol. 2017;188:71–77. doi: 10.1016/j.vetimm.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Bletner J.K., Mitchell R.P., Jr., Tugwell R.L. The effect of Eimeria maxima on broiler pigmentation. Poult. Sci. 1966;45:689–694. doi: 10.3382/ps.0450689. [DOI] [PubMed] [Google Scholar]
- Conway D.P., McKenzie M.E. Poultry Coccidiosis: Diagnostic and Testing Procedures. 3rd ed. Blackwell Publishing Professional; Ames, IA: 2007. pp. 7–20. [Google Scholar]
- Da Silva I.C.M., Leal Ribeiro A.M., Wageck Canal C., Pinheiro C.C., de Moraes Vieira M., Goncalves T.A., Alves Pereira R., Lacerda L. Broiler chicken responses to immunological stimuli as mediated by different levels of vitamin E in the diet. J. Appl. Poult. Res. 2009;18:752–760. [Google Scholar]
- Dalloul R.A., Lillehoj H.S. Poultry coccidiosis: recent advancements in control measures and vaccine development. Exp. Rev. Vaccin. 2006;5:143–163. doi: 10.1586/14760584.5.1.143. [DOI] [PubMed] [Google Scholar]
- Kawashima C., Nagashima S., Sawada K., Schweigert F.J., Miyamoto A., Kida K. Effect of ß-carotene supply during Close-up dry Period on the Onset of first Postpartum Luteal activity in. Dairy Cows. 2010;287:282–287. doi: 10.1111/j.1439-0531.2009.01558.x. [DOI] [PubMed] [Google Scholar]
- Langhout P.J. The role of nutrition on coccidial infections. In: va der Sluis W., editor. World Poultry. Elsevier; Amsterdam: 1999. pp. 29–30. [Google Scholar]
- Lee J.T., Eckert N.H., Ameiss K.A., Stevens S.M., Anderson P.N., Anderson S.M., Barri A., McElroy A.P., Danforth H.D., Caldwell D.J. The effect of dietary protein level on performance characteristics of coccidiosis vaccinated and nonvaccinated broilers following mixed-species Eimeria challenge. Poult. Sci. 2011;90:1916–1925. doi: 10.3382/ps.2011-01362. [DOI] [PubMed] [Google Scholar]
- Lentfer T.L., Pendl H., Fröhlich E.K.F., Von Borell E., Pendl H., Fröhlich E.K.F. H/L ratio as a measurement of stress in laying hens – methodology and reliability. Br. Poult. Sci. 2015;56:157–163. doi: 10.1080/00071668.2015.1008993. [DOI] [PubMed] [Google Scholar]
- Livingston M.L., Cowieson A.J., Crespo R., Hoang V., Nogal B., Browning M., Livingston K.A. Effect of broiler genetics, age, and gender on performance and blood biochemistry. Heliyon. 2020;6:e04400. doi: 10.1016/j.heliyon.2020.e04400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice D.V., Bodine A.B., Rehrer N.J. Metabolic effects of low aflatoxin B1 levels on broiler chicks. Appl. Environ. Microbiol. 1983;45:980–984. doi: 10.1128/aem.45.3.980-984.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo V., McGaw L.J., Bisschop S.P.R., Duncan N., Eloff J.N. The value of plant extracts with antioxidant activity in attenuating coccidiosis in broiler chickens. Vet. Parasitol. 2008;153:214–219. doi: 10.1016/j.vetpar.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Natt M.P., Herrick C.A. THe effect of cecal coccidiosis on the blood cells of the domestic fowl: 1. A comparison of the changes in the erythrocyte count resulting from hemorrhage in infected and mechanically bled birds. The use of the hematocrit value as an index of the severity of the hemorrhage resulting from the infection. Poult. Sci. 1956;35:1100–1106. [Google Scholar]
- Patra G., Ali M.A., Chanu K.V., Jonathan L., Joy L.K., Prava M., Ravindran R., Das G., Devi L.I. PCR based diagnosis of Eimeria tenella infection in broiler chickens. Int. J. Poult. Sci. 2010;9:813–818. [Google Scholar]
- Peek H.W., van der Klis J.D., Vermeulen B., Landman W.J.M. Dietary protease can alleviate the negative effects of a coccidiosis infection on production performance in broiler chickens. Anim. Feed Sci. Tehnnol. 2009;150:151–159. [Google Scholar]
- Ritzi M., Abdelrahman W., van-Heerden K., Mohnl M., Barrett N.W., Dalloul R.A. Combination of probiotics and coccidiosis vaccine enhances protection against an Eimeria challenge. Vet. Res. 2016;47:1–8. doi: 10.1186/s13567-016-0397-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers N.J., Swick R.A., Geier M.S., Moore R.J., Choct M., Wu S.-.B. A multifactorial analysis of the extent to which Eimeria and fishmeal predispose broiler chickens to necrotic enteritis. Avian Dis. 2015;59:38–45. doi: 10.1637/10774-011614-reg.1. [DOI] [PubMed] [Google Scholar]
- Rose M.E., Long P.L. Vaccination against coccidiosis in chickens. In: Taylor A.E.R., Muller R., editors. Vaccination against Parasites. Blackwell Scientific; Oxford, UK: 1980. pp. 57–74. [Google Scholar]
- Ruff M.D., Reid W.M., Johnson J.K. Lowered blood carotenoid levels in chickens infected with coccidia. Poult. Sci. 1974;53:1801–1809. doi: 10.3382/ps.0531801. [DOI] [PubMed] [Google Scholar]
- Saleh N., Fathalla S.I., Nabil R., Mosaad A.A. Clinicopathological and immunological studies on toxoids vaccine as a successful alternative in controlling clostridial infection in broilers. Anaerobe. 2011;17:426–430. doi: 10.1016/j.anaerobe.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Stringfellow K., Caldwell D., Lee J., Mohnl M., Beltran R., Schatzmayr G., Fitzcoy S., Broussard C., Farnell M. Evaluation of probiotic administration on the immune response of coccidiosis-vaccinated broilers. Poult. Sci. 2011;90:1652–1658. doi: 10.3382/ps.2010-01026. [DOI] [PubMed] [Google Scholar]
- Turk D.E. Macroelements in the circulation of coccidiosis-infected chicks. Poult. Sci. 1986;65:462–468. doi: 10.3382/ps.0650462. [DOI] [PubMed] [Google Scholar]
- Vermeulen A.N., Schaap D.C., Schetters T.h.P.M. Control of coccidiosis in chickens by vaccination. Vet. Parasitol. 2001;100:13–20. doi: 10.1016/s0304-4017(01)00479-4. [DOI] [PubMed] [Google Scholar]
- Wang X., Peebles E.D., Kiess A.S., Wamsley K.G.S., Zhai W. Effects of coccidial vaccination and dietary antimicrobial alternatives on the growth performance, internal organ development, and intestinal morphology of Eimeria-challenged male broilers. Poult. Sci. 2019;98:2054–2065. doi: 10.3382/ps/pey552. [DOI] [PubMed] [Google Scholar]
- Williams R.B. A compartmentalized model for the estimation of the cost of coccidiosis to the worlds chicken production industry. Int. J. Parasitol. 1999;29:1209–1229. doi: 10.1016/s0020-7519(99)00086-7. [DOI] [PubMed] [Google Scholar]
- Wills E.D. Mechanisms of lipid peroxide formation in animal tissues. Biochem. J. 1966;99:667–676. doi: 10.1042/bj0990667. [DOI] [PMC free article] [PubMed] [Google Scholar]