Abstract
An isothiocyanato-functionalized phthalocyanine (Pc) was synthesized in good yield from the corresponding amine-substituted Pc. This Pc reacted with ethanolamine, biotin hydrazine, and biotin diethylamine under mild conditions (room temperature in DMF or DMSO in the presence of TEA) to produce the corresponding thiourea products in 60–75% yields. All Pcs showed intense Q absorptions in DMF around 677 nm, emissions centered at 683 nm, and fluorescence quantum yields in the range 0.18–0.27. The Pcs were phototoxic to human carcinoma HEp2 cells (IC50 ~ 7 at 1.5 J/cm2) and localized in multiple organelles, including the lysosomes, Golgi and ER. One biotin-Pc conjugate was injected via tail vein into nude mice bearing HT-29 tumors and demonstrated selective localization in the tumor tissue.
Keywords: phthalocyanine, isothiocyanate, biotin, photosensitizer, PDT
INTRODUCTION
Phthalocyanines (Pcs) are efficient photosensitizers (ps) for the treatment of cancers by photodynamic therapy (PDT) [1]. PDT is a minimally invasive treatment that uses light, a ps and molecular oxygen to selective destroy malignant tissues via the generation of singlet oxygen (1O2) and reactive oxygen species (ROS). Photosens, a silicon(IV)Pc, and CGP55847, a zinc(II)Pc, are used clinically for the treatment of breast, gastrointestinal, and squamous cell carcinoma of the upper aerodigestive tract [2–4]. Pcs are an excellent choice as ps due to their strong absorbance in the near-infrared spectral region (> 670 nm) where light penetrates deeply through the skin, their high chemical and photo-stability, in addition to being excellent singlet oxygen generators [5–7]. However, Pcs generally have poor solubility in water and most organic solvents, which limits their purification and applications. Strategies used to address the poor solubility of Pcs include the introduction of bulky or water-solubilizing substituents, and the use of isomeric mixtures of compounds [4, 8–13]. Since the use of isomeric Pc mixtures can lead to reproducibility challenges, synthesis of isomerically pure compounds is a more desirable approach. In particular, isomerically pure Pcs bearing versatile functional groups can be used as templates to produce functionalized Pcs via, for example, esterification, substitution and acylation reactions [11–19]. The Pc templates can be conjugated to hydrophilic and cell-targeting moieties, yielding amphiphilic compounds with enhanced solubility, serum life, and specificity towards receptors overexpressed on cancer cells [11, 14, 20–22]. This strategy is particularly useful when the required precursors bearing targeting moieties are not readily available or are unstable under cyclization conditions [23–24]. The conjugation of tumor-cell-targeting moieties to Pc templates has been reported to improve the tumor specificity and uptake of Pc macrocycles [5, 9, 11, 20, 25–28]. However, some targeting agents, particularly those of high molecular weight, may trigger an immunogenic response [29]. Therefore, small tumor-targeting substituents such as low molecular weight PEG groups, peptides, and vitamins, including folic acid, riboflavin, vitamin B-12 and biotin, have been investigated as targeting moieties and have been shown to increase tumor selectivity and uptake into cancer cells [12, 25, 29–34]. Several cancer cell lines overexpress folate receptors, vitamin B-12 receptors and biotin receptors. Studies conducted on leukemia, ovarian, colon, mastocytoma, lung, renal, and breast cancer cell lines showed enhanced uptake of biotin conjugates compared to folate and vitamin B-12 conjugates [29]. Meerovich et al. reported that a biotin-containing Photosens showed higher photodynamic efficacy compared with the parent drug [35]. Photosens, however, is a mixture of isomers. Herein, we report the synthesis of an isomerically pure biotin-containing Pc, via reaction on a Pc template bearing an amine reactive isothiocyanato group. The conjugation of porphyrins, chlorins, bacteriochlorins and BODIPYs to various targeting molecules via an isothiocyanate functional group under mild conditions generally yields the targeted conjugates in short reaction times and high yields [36–41]. Duan et al. [11], Lopez-Duarte et al. [17], and Hammer et al. [16], have reported the conjugation of isothiocyanate-functionalized Pcs to monoclonal antibodies, aluminosilicate Zeolite L and oligonucleotides, respectively. We report the synthesis of a regioisomerically pure isothiocyanate-functionalized Pc and its conjugation to biotin hydrazine and biotin diethylamine [42]. The spectroscopic and biological properties of the conjugates were evaluated and compared to those of the unconjugated Pc.
RESULTS AND DISCUSSION
Synthesis
Scheme 1 shows the synthetic route to isothiocyanate-functionalized Pc 4. The precursor, 3-N-Boc-aminophenol phthalonitrile 1, was prepared according to our previously reported procedure [43]. Initial attempts to prepare the Boc-protected Pc 2 using various phthalonitrile ratios ranging from 1:3 to 1:9 led to formation of mixtures of Pc isomers. Such low ratios of phthalonitriles in cross condensation reactions are known to lead to mixtures of regioisomeric Pcs due to the interactions between the different phthalonitriles leading to the formation of adj- and opp-A2B2, A3B and AB3-type Pcs, in addition to the A4 and B4 Pcs in a single reaction vessel. These isomers are difficult to separate chromatographically due to their similar Rf values. However, using a large excess of one phthalonitrile (A) favors the formation of only A3B and A4 Pcs [18–19]. Therefore, the targeted A3B-type Pc 2 was synthesized using a 30-fold excess of unsubstituted phthalonitrile, and easily separated from the single byproduct A4 Pc due to the low solubility of the latter Pc. The condensation occurred in the presence of zinc(II) acetate and a catalytic amount of 1,5-diazabicyclo[4.3.0]non-5-ene (DBN) as base in dimethylethanolamine(DMAE) at 140 °C for 1 h. Pc 2 was isolated in 12% yield after separation from the unsubstituted A4 Pc by filtration, followed by re-crystallization. Pc 2 bearing a Boc-protected aminophenoxy α-substituent shows increased solubility compared with the unsubstituted A4 Pc and decreased tendency for aggregate formation.
Scheme 1.
Synthetic route to Pc-NCS 4
Pc 2 was quantitatively deprotected using trifluoroacetic acid (TFA) in dichloromethane at 0 °C to give Pc 3. The amine group on Pc 3 was converted to the isothiocyanate functional group in Pc 4 using 1,1′-thicarbonyldi-2(1H)-pyridone (TDP) in anhydrous DMF, as shown in Scheme 1, using a procedure similar to that previously reported [36, 42]. Pc 4 was obtained in 90% yield. The isothiocyanate functional group is highly reactive toward amines, producing the corresponding thioamide addition products under mild conditions in high yields.
Pc 4 reacted with ethanolamine in the presence of triethylamine (TEA) as base, at room temperature for 30 min, to produce hydroxyl Pc 5 in 66% yield, as shown in Scheme 2. Biotin ethylenediamine was synthesized by activation of the carboxylate group with EDCI and HOBt in DMSO, followed by reaction with N-Boc ethylenediamine in a 1:1 ratio, as previously reported [44]. Deprotection of the Boc group using TFA in dichloromethane for 2 h at 0 °C gave biotin ethylenediamine. Optimal reaction conditions for the conjugation of Pc 4 with biotin hydrazine and biotin ethylenediamine were found to be a 1:1 reaction ratio using TEA as the base, in DMSO over 2 h, to give Pc 6 and Pc 7 in 60–75% yields, as shown in Scheme 2.
Scheme 2.
Reactions of Pc-NCS 4
The structures of all Pcs were characterized by 1H-and 13C-NMR spectroscopy and by MALDI-TOF mass spectrometry (see Supporting information).
Spectroscopic properties
The spectroscopic properties for Pcs 4, 5, 6 and 7 in DMF are summarized in Table 1 and their absorption spectra are shown in Fig. 1. The absorption spectra of the Pcs in DMF displayed a characteristic Q band (π–π* transition) between 674 and 678 nm and a Soret band between 300 and 400 nm. The spectra followed the Beer-Lambert law at concentrations between 2 and 10 μL (see Supporting information). The fluorescence emissions appeared between 682 and 684 nm, and the fluorescence quantum yields ranged from 0.18–0.27. The biotin–Pc conjugates displayed lower fluorescence quantum yields than Pcs 4 and 5, maybe due to the higher flexibility of the conjugates which tend to increase non-radiative decay to the ground state.
Table 1.
Spectroscopic properties for Pcs 4, 5, 6, and 7 in DMF at room temperature
| Pc | Absorption (λmax, nm) | Emissiona (λmax, nm) | Log ε (nm) | Φfb |
|---|---|---|---|---|
| 4 | 677 | 682 | 5.00 | 0.26 |
| 5 | 678 | 684 | 4.95 | 0.27 |
| 6 | 674 | 682 | 4.85 | 0.18 |
| 7 | 678 | 684 | 4.80 | 0.19 |
Excitation at 635 nm;
calculated using Zn (Φf = 0.17) as the Ref. [45]
Fig. 1.
Absorption spectra of Pcs 4 (red), 5 (purple), 6 (pink), and 7 (blue) at 4 μM concentration in DMF at room temperature
Cellular studies
The cellular properties of Pcs 4, 5, 6 and 7, including their time-dependent cellular uptake, cytotoxicity and intracellular localization, were investigated in human carcinoma HEp2 cells, and the results are summarized in Table 2, Figs 2–6, and the Supporting information. For uptake studies, the HEp2 cells were incubated with 10 μM solutions of each Pc and the extent of uptake determined after 1, 2, 4, 8 and 24 h (Fig. 2). The isothiocyanate-Pc 4 showed lower uptake at all time points investigated compared to the hydroxy-Pc 5 and biotin conjugates 6 and 7. On the other hand, Pc 5 showed the highest uptake of all Pcs at all time points, and after 24 h accumulated within cells 8 times more than Pc 4. This might be due to its lower molecular weight and favorable amphiphilicity. The two biotin-containing Pcs 6 and 7 showed similar cellular uptake, and after 24 h were found within cells 4 and 5 times more than Pc 4, respectively.
Table 2.
Cytotoxicity (CTB assay, light dose ~ 1.5 J/cm2) and comparative uptake for Pcs in HEp2 cells
| Pc | Dark toxicity IC50 (μM) | Phototoxicity IC50 (μM) | Uptake ratio at 24 ha |
|---|---|---|---|
| 4 | >200 | 70 | 1 |
| 5 | >200 | 7 | 8 |
| 6 | >200 | 9 | 4 |
| 7 | >200 | 6.5 | 5 |
Relative to 4 (Pc/Pc 4).
Fig. 2.
Time-dependent uptake of Pcs 4 (red), 5 (purple), 6 (pink), and 7 (blue) at 10 μM by human carcinoma HEp2 cells
Fig. 6.
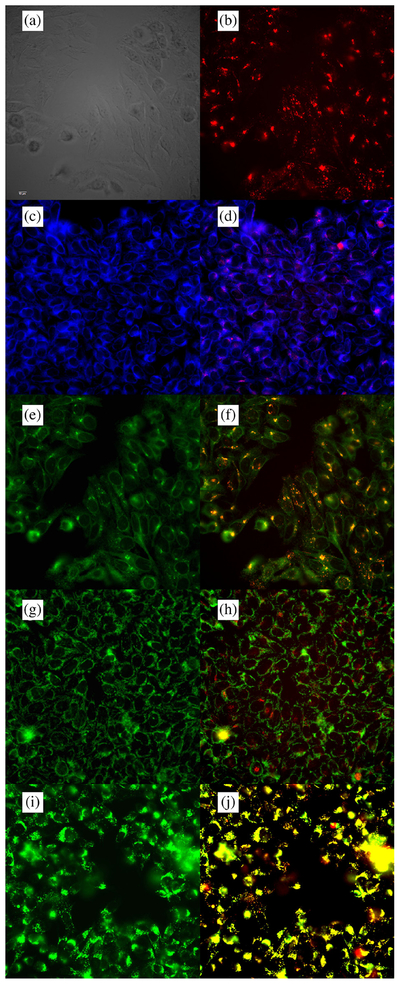
Subcellular localization of Pc 7 in HEp2 cells at 10 μM for 6 h. (a) Phase contrast (b) Overlay and fluorescence of Pc 7 (c) ER tracker Blue/White fluorescence (e) MitoTrack green fluorescence (g) BODIPY ceramide (i) LysoSensor green fluorescence (d, f, h, j) overlays of tracers with Pc 7 fluorescence. Scale bar: 10 μm
The organelle-specific fluorescence probes BODIPY Ceramide (Golgi), Lyso-Sensor Green (lysosomes), Mito-Tracker Green (Mitochondria), and ER Tracker Blue/White (ER) (Invitrogen) were used in co-localization experiments. The red color indicates the Pc, the purple/pink and orange/yellow colors indicate co-localization of the Pc and organelle probes in Figs 3–6. All Pcs localized in the ER, Golgi apparatus, and lysosomes (Table 3). In addition, to a small extent Pc 5 was also found in the mitochondria. The biotinylated Pcs 6 and 7 showed similar localization in the lysosomes and the Golgi apparatus, with Pc 7 appearing to have enhanced localization in the ER compared with Pc 6.
Fig. 3.
Subcellular localization of Pc 4 in HEp2 cells at 10 μM for 6 h. (a) Phase contrast (b) Overlay and fluorescence of Pc 4 (c) ER tracker Blue/White fluorescence (e) MitoTrack green fluorescence (g) BODIPY ceramide (i) LysoSensor green fluorescence (d, f, h, j) overlays of tracers with Pc 4 fluorescence. Scale bar: 10 μm
Table 3.
Major (+++) and Minor (+) subcellular sites of localization in HEp2 Cells
| Pc | ER | Golgi | Mitochondria | Lysosomes |
|---|---|---|---|---|
| 4 | ++ | ++ | − | +++ |
| 5 | ++ | +++ | + | +++ |
| 6 | + | +++ | − | +++ |
| 7 | +++ | +++ | − | +++ |
The dark toxicity and phototoxicity of the Pcs were investigated using Promega’s Cell Titer Blue viability assay. None of the Pcs was toxic in the dark up to 200 μM concentration. However, upon exposure to a low light dose (~1.5 J/cm2), all Pcs were phototoxic, particularly Pcs 5–7, with determined IC50 values (50% inhibition of cell proliferation based on dose-response curves) of 70, 7, 9 and 6.5 μM for Pcs 4, 5, 6 and 7, respectively. Since all Pcs showed similar intracellular distributions, this result may be due to the lower cellular uptake of Pc 4 into cells. The most phototoxic Pc was biotin conjugated Pc 7, probably due to its increased localization in the ER compared with all other Pcs.
In vivo studies
The biotin-Pc conjugate 6 was selected based on the in vitro studies, for preliminary investigation in nude mice bearing human HT-29 colorectal tumors. In this experiment, Pc 6 was injected via tail vein into mice with subcutaneous tumors of approximately 5 mm in diameter. Post-injection, the mice were anesthetized and imaged using an excitation wavelength of 640 nm and an emission wavelength of 710 nm, at various time intervals for up to 120 h. As shown in Fig. 7a, systemic fluorescence of Pc 6 was observed in a representative mouse as early as 3 h post-injection, and selective localization was seen within tumors by 6 h after administration. Detection persisted through 48 h at the tumor site but declined after 72 and 120 h. However, non-specific background expression was also detected in the peritoneal region at each time point. After the final imaging scan, the mice were euthanized, and the organ and tumor tissues were harvested. Fluorescence analysis of tumor tissue from a representative mouse showed high levels of localization of Pc 6, as well as high levels in the kidneys, peritoneal fat, and uterus (Fig. 7b). Lower levels of Pc 6 were also detected in pancreas, liver, lungs, and intestines, while there was no detectable Pc 6 in either heart or spleen.
Fig. 7.
(a) Fluorescence images (excitation 640 nm and emission 710 nm) of a nude mouse bearing subcutaneous HT-29 tumor implants at 0, 3, 6, 24, 48, 72, and 120 h following i.v administration of Pc 6. The tumor position is circled. (b) Fluorescence images (excitation 640 nm and emission 710 nm) of organ and tumor tissues harvested from the mouse after the final imaging time point
EXPERIMENTAL
Synthesis
General.
All reagents and solvents were purchased from commercial sources and used directly without purification. Analytical thin-layer chromatography (TLC) was performed using plastic backed TLC plates 254 (precoated, 200 μm) from Sorbent Technologies to monitor the reactions. Silica gel 60 (230 × 400 mesh) was used for column chromatography. NMR spectra were obtained on an AV-III-400-NanoBay Bruker spectrometer (400 MH for 1H, 100 MHz for 13C). Chemical shifts are reported in δ (ppm) relative to DMF-d7 8.03 ppm (1H), 163.15 ppm (13C); THF-d8 3.58 ppm (1H), 67.57 ppm (13C); DMSO-d6 2.5 ppm (1H), 39.51 ppm (13C). An α-Cyano-4-hydroxycinnamic acid matrix was used for MALDI-TOF mass spectra measurements on a Bruker ProFlex III spectrometer. Melting points were measured using a Barnstead Electrothermal Mel-Temp 1101D Capillary Melting Point Apparatus. Phthalonitrile 1 was synthesized as previously reported.43
Pc 2.
A mixture of 3-(4-N-Boc-aminophenol) phthalo nitrile (0.03 g, 8.95 × 10−2 mmol), phthalonitrile (0.34 g, 2.68 mmol), and zinc(II) acetate (1.52 g, 0.69 mmol) dissolved in DMAE (6.0 mL) was heated at 140 °C with two drops of DBN under argon for 1 h. The solvent was removed under reduced pressure and the crude mixture was filtered using acetone. The product was further purified by silica gel column chromatography column to give Pc 2, a blue solid eluted using dichloromethane/methanol (95:5). Pc 2 was dried under vacuum for 2 days to give a blue solid in 12% yield. M.p.: 210 °C. 1H NMR (THF-d8, 400 MHz): 7.8–7.75 (m, 2H, Ar-H), 7.63–7.61 (m, 2H, Ar-H), 7.19–7.14 (m, 2H, Ar-H), 6.97–6.92 (m, 6H, Ar-H), 6.82–69 (m, 6H, Ar-H), 2.06 (C (CH3)3). 13C NMR (DMF-d7, 100 MHz): 212.43 (C=O), 208.9, 168.8, 167.3, 155.0, 153.8, 153.5, 153.4, 149.8, 138.9, 137.2, 136.6, 135.5, 129.2, 122.6, 122.4, 120.6, 119.8, 118.6, 117.0, 79.3, 55.7, 27.9 (C (CH3)3). MS (MALDI- TOF) m/z 783.230 [M]+, calcd for C43H29N9O3Zn, 783.168.
Pc 3.
The Boc group of Pc 2 was deprotected using TFA:DCM (1:1) at 0 °C for 4 h. The solvents were removed under reduced pressure and the resulting residue was dissolved in dichloromethane/methanol (9:1). A few drops of 2N NaOH were added to neutralize TFA and the precipitate removed by filtration. The product was dried under vacuum for 2 h to give Pc 3 as a blue solid in quantitative yield. M.p.: 250 °C. 1H NMR (DMF-d7, 400 MHz): 9.5 (s, 2H, Ar-H), 8.3 (s, 2H, Ar-H), 7.9–7.5 (M, 14H, Ar-H), 6.9–6.8 (M, 4H, Ar-H). 13C NMR (DMF-d7, 100 MHz): 153.4, 139.1, 138.7, 138.7, 130.0, 128.8, 122.4, 122.2, 120.9, 117.1, 116.4, 115.2. (Ar-C, CN). MS (MALDI- TOF) m/z [M + H]+ 684.172, calcd for C38H22N9OZn, 684.124.
Pc 4.
Pc 3 (50 mg, 0.73 μmol) was dissolved in 100 μL anhydrous DMF. 1, 1′-Thiocarbonyldi-2,2′-pyridone (16.9 mg, 0.73 μmol) dissolved in DMF was added to the Pc solution and the final mixture stirred at room temperature for 6 h. The solvent was removed under reduced pressure and the resulting residue was purified on silica gel preparative TLC plates using dichloromethane for elution, to give Pc 4 in 47.76 mg, 90% yield. M.p.: 180 °C. 1H NMR (DMF-d7, 400 MHz): 8.17 (s, 2H, Ar-H), 7.51–7.46 (m, 9H, Ar-H), 6.38 (d, J = 2.8 Hz, 4H, Ar-H), 6.21 (t, J = 2.8 Hz, 4H, Ar-H). 13C NMR (DMF-d7, 100 MHz): 149.9, 149.6, 149.3, 141.0, 136.0, 135.8, 135.5, 130.0, 123.8, 123.6, 123.3, 122.9, 120.6, 104.9, 93.6. MS (MALDI- TOF) m/z 726.0803 [M + H]+, calcd for C39H20N9OSZn, 726.062.
Pc 5.
Pc 4 (5 mg, 6.896 μmol) was dissolved in 50 μL DMF. Triethylamine (0.523 mg, 5.17 μmol) and ethanolamine (0.316 mg, 5.171 μmol) in 100 μL DMF were added to the Pc 4 solution and the final mixture was stirred for 0.5 h at room temperature. The solvent was removed under reduced pressure, and the crude product purified by column chromatography using dichloromethane/methanol (9:1) for elution to give Pc 5 as a blue solid (2.7 mg, 66.41%). M.p.: 220 °C. 1H (DMF-d7, 400 MHz): 8.3 (m, 4H, Ar-H), 7.5 (m, 6H, Ar-H), 6.3 (s, 4H, Ar-H), 6.2 (s, 4H, Ar-H), 3.3 (m, 5H). 13C NMR (DMF-d7, 100 MHz): 161.3, 141.0, 135.7, 120.4, 104.8, 70.4, 60.5, 42.1, 40.5. MS (MALDI- TOF) m/z 787.038 [M + H]+, calcd for C41H27N10O2SZn, 787.133.
Pc 6.
Pc 4 (5 mg, 6.90 μmol) was dissolved in 100 μL of DMSO. Biotin hydrazine (1.98 mg, 6.90 μmol) and triethylamine (28.85 μL, 207 mmol) in 50 μL DMSO were added to the Pc 4 solution. The final mixture was stirred for 2 h at room temperature. The crude product was precipitated out of solution by the addition of diethyl ether. The residue was re-dissolved in dichloromethane/methanol (9:1) and washed once with water. The organic phase was dried over anhydrous Na2SO4 to give Pc 6 as a blue compound (4.74 mg, 70%). M.p.: 190 °C. 1H NMR (DMSO-d6): 9.5–9.45 (6H, Ar-H), 8.3–8.3 (m, 6H, Ar-H), 7.8 (d, 1H, Ar-H), 7.66 (m, 2H, Ar-H), 7.5–7.4 (m, 5H, Ar-H), 6.3 (m, 2H, Ar-H), 6.2–6.1 (m, 2H, Ar-H), 4.1 (br, 2H), 3.6 (2H), 3.3 (3H), 3.1–3.0 (1H), 1.6 (1H), 1.3 (5H), 0.9 (1H). 13C NMR (DMSO-d6, 100 MHz): 149.9, 149.6, 149.3, 147.2, 141.0, 138.8, 136.0, 135.6, 135.7, 135.5, 130.0, 123.8, 123.6, 123.3, 122.8, 120.6, 115.2, 111.0, 110.1, 104.9, 93.6, 90.6, 90.3, 55.0, 49.1, 40.4, 20.0. MS (MALDI- TOF) m/z 1006.190 [M + Na]+, calcd for C49H37N13O3S2Zn, 1006.177.
Pc 7.
Pc 4 (10 mg, 0.14 μmol) was dissolved in 100 μL of DMF. Biotin ethylenediamine (7.89 mg, 0.28 μmol) was dissolved in 200 μL DMSO and triethylamine (41.87 mg, 0.41 mmol) was added onto this before adding this mixture to the Pc 4 solution. The solvent was removed. The crude in dichloromethane/methanol 9:1 was purified on silica gel TLC preparative plates to give title Pc 7 (16.74 mg, 60%). M.p.: 170 °C. 1H NMR (DMSO-d6): 9.3–9.1 (m, 5H, Ar-H), 8.2–8.1 (m, 7H, Ar-H), 7.6–7.5 (m, 3H, Ar-H), 7.2–7.0 (m, 1H, Ar-H), 3.8–3.7 (m, 5H), 3.5 (m, 2H), 3 (m, 2H), 2.4 (m, 1H), 2.3–2.1 (m, 4H), 1.8–1.5 (m, 7H), 1.5 (b, 2H), 1 (m, 1H) 13C NMR (DMSO-d6, 100 MHz): 162.6, 162.3, 162.0, 149.9, 149.6, 123.9, 123.6, 123.4, 55.0, 35.4, 35.2, 35.0, 34.8, 34.6, 34.4, 34.2, 30.3, 30.1, 29.9, 29.7, 29.5, 29.3, 29.1. MS (MALDI-TOF) m/z 1050.097 [M]+, calcd for C51H41N13O3S2ZnK, 1050.182.
Spectroscopic properties
All studies were performed in peptide-sequencing grade DMF solutions. All absorption spectra were measured on a UV-vis Perkin Elmer Lambda 35 spectrophotometer. All experiments were carried out within 3 h of solution preparation at room temperature (23–25 °C) using a 10 mm path length spectrophotometric cell. Emission spectra were recorded on a Fluorolog®-HORIBA JOBINVYON (Model LFI-3751). The fluorescence quantum yields (Φf) were determined via a secondary standard method using ZnPc (Φf = 0.17) as the Ref. [45].
Cell studies
The HEp2 cells were obtained from ATCC and maintained in a 75 cm2 flask (Chemglass) with medium (DMEM:Advanced, 1:1) containing 10% FBS and 1% antibiotic (Life Technologies). The Pc solutions were prepared by dissolving each Pc in 100% DMSO at a concentration of 32 mM (stock solution).
Dark cytotoxicity.
The HEp2 cells were placed in a Costar 96-well plate (15,000 cells/well). Each plate was treated with Pc concentrations of 200, 100, 50, 25, 12.5, and 0 μM for 0–24 h incubation at 37 °C. To end treatment, excess Pc was removed by washing cells with PBS and replaced with media containing 20% Cell Titer Blue (Promega). The cells were incubated for an additional 4 h at 37 °C, and measured fluorescently at 570/615 nm using a FluoStar Optima micro-plate reader. Dark toxicity is expressed in terms of percent survival of cells.
Phototoxicity.
The Pc concentrations of 100, 50, 25, 12.5, 6.25, 3.125, and 0 μM were used for the phototoxicity experiments. HEp2 cells were placed in 96 well plates as described above, and treated with the Pc solutions for 24 h at 37 °C. After this treatment the loading media was removed. The cells were washed with a PBS buffer, and then refilled with fresh media. The cells were exposed to a 600 W halogen lamp light source filtered with a water filter and a beam turning mirror (Newport) for 20 min. The total light dose was approximately 1.5 J/cm2. After being exposed to light, the cells were returned to the incubator for 24 h. After 24 h incubation, the medium was removed and replaced with media containing 20% Cell Titer Blue. The cells were incubated for an additional 4 h. The viable cells were measured fluorescently at 570/615 nm using a FluoStar Optima micro-plate reader. Phototoxicity is expressed in terms of percent survival of cells.
Time-dependent cellular uptake.
The HEp2 cells were plated in a 96-well plate as described above. The cells were treated by adding 100 μL of 10 μM Pc solution at different time periods of 0, 1, 2, 4, 8, and 24 h. The cells were washed with PBS, and solubilized by adding 100 μL 0.25% Triton X-100 in PBS per well. The cells were quantified by CyQuant Cell Proliferation Assay (Life Technologies). The compound and cell number were determined using a FluoStar Optima microplate reader. Cellular uptake is expressed in terms of nM Pc concentration per cell.
In vivo studies
For the in vivo studies, athymic nu/nu mice (Charles River Laboratories, Wilmington MA) were purchased at six weeks of age and quarantined for one week. Subsequently, the human colorectal adenocarcinoma HT-29 cell line (ATCC; Manassas, VA) was implanted subcutaneously in the lower right flank. Initially, HT-29 cells were cultured in McCoy’s 5A medium containing 10% fetal bovine serum (Atlanta Biologicals; Flowery Branch, GA) and incubated at 37 °C and 5% CO2 under humidified conditions to approximately 75% confluence. The cells were then harvested with a 0.25% (w/v) trypsin −0.53 mM EDTA solution, concentrated via centrifugation, and resuspended in Dulbecco’s PBS before injection. Each of the mice was injected with 5 × 106 cells in 0.1 mL PBS. Tumors were allowed to develop until approximately 5 mm in diameter, after which mice were subjected to lateral tail vein injection of the Pc conjugate resuspended at 2 mM in Dulbecco’s PBS containing 10% DMSO and 5% Kolliphor EL (Sigma-Aldrich, St. Louis, MO). After injection, the mice were observed for any acute adverse responses and then anesthetized using 3% isoflurane for imaging. Fluorescence imaging was performed at 0, 3, 6, 24, 48, 72, and 120 h after injection using a Spectral AMI optical imaging system (Spectral Instruments Imaging; Tucson, AZ) set at an excitation 640 nm and emission 710 nm. All experimental protocols involving live animals were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of LSU.
CONCLUSIONS
We describe the synthesis of an isomerically pure A3B-type Pc functionalized with an isothiocyanate group, Pc 4, in 11% overall yield. The strategy involves the condensation of a Boc-protected amino phthalonitrile with a large excess (30-fold) of phthalonitrile, followed by deprotection with TFA and reaction with TDP. The highly reactive isothiocyanate functional group readily reacts with primary amines under mild conditions and in high yields. Using this strategy, three new Pcs were prepared bearing ethanolamine, biotin hydrazine or biotin ethylenediamine, in 60–75% yields. These amphiphilic conjugates showed enhanced solubility and uptake into human carcinoma HEp2 cells. In DMF solutions the Pcs show absorption and emission spectra typical of Pc macrocycles, and fluorescence quantum yields in the range 0.18–0.27. All Pcs were non-toxic in the dark up to 200 μM concentrations, but displayed significant phototoxicity, with IC50 values in the order of 7 μM using 1.5 J/cm2 light dose. The biotin-Pc 6 was further investigated in a mouse model bearing a HT-29 tumor. This conjugate was found to selectively localize within tumors as soon as 6 h after i.v. administration via the tail vein. Our results demonstrate that the isomerically pure isothiocyanate-functionalized Pc 4 is an excellent template for the synthesis of tumor cell-targeted amphiphilic Pc analogs in good overall yields and under mild conditions.
Supplementary Material
Fig. 4.
Subcellular localization of Pc 5 in HEp2 cells at 10 μM for 6 h. (a) Phase contrast (b) Overlay and fluorescence of Pc 5 (c) ER tracker Blue/White fluorescence (e) MitoTrack green fluorescence (g) BODIPY ceramide (i) LysoSensor green fluorescence (d, f, h, j) overlays of tracers with Pc 5 fluorescence. Scale bar: 10 μm
Fig. 5.
Subcellular localization of Pc 6 in HEp2 cells at 10 μM for 6 h. (a) Phase contrast (b) Overlay and fluorescence of Pc 6 (c) ER tracker Blue/White fluorescence (e) MitoTrack green fluorescence (g) BODIPY ceramide (i) LysoSensor green fluorescence (d, f, h, j) overlays of tracers with Pc 6 fluorescence. Scale bar: 10 μm
Acknowledgments
This research was funded by the National Institutes of Health, grant number R01 CA179902.
Footnotes
Supporting information
Absorption spectra, dark toxicity and phototoxicity (Figs S1–S6) are given in the supplementary material. This material is available free of charge via the Internet at http://www.worldscinet.com/jpp/jpp.shtml.
REFERENCES
- 1.Sharma D, Huijser A, Savolainen J, Steen G and Herek JL. Faraday Discuss. 2013; 163: 433–445. [DOI] [PubMed] [Google Scholar]
- 2.Vakoulovskaya EG, Shental VV, Oumnova LV and Vorozhcsov GN. Proceedings of the SPIE 2003; 4952: 149–151. [Google Scholar]
- 3.Baron ED, Malbasa CL, Santo-Domingo D, Fu P, Miller JD, Hanneman KK, Hsia AH, Oleinick NL, Colussi VC and Cooper KD. Lasers Surg. Med 2010; 42: 728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sekkat N, van den Bergh H, Nyokong T and Lange N. Molecules 2011; 17: 98–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weissleder R and Ntziachristos V. Nat. Med 2003; 9: 123–128. [DOI] [PubMed] [Google Scholar]
- 6.Ntziachristos V, Bremer C and Weissleder R. Eur. Rad 2003; 13: 195–208. [DOI] [PubMed] [Google Scholar]
- 7.Luo S, Zhang E, Su Y, Cheng T and Shi C. Biomaterials 2011; 32: 7127–7138. [DOI] [PubMed] [Google Scholar]
- 8.Ongarora BG, Hu X, Verberne-Sutton SD, Garno JC and Vicente MGH. Theranostics 2012; 2: 850–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vrouenraets MB, Visser GW, Stigter M, Oppelaar H, Snow GB and van Dongen GA. Cancer Res 2001; 61: 1970–1975. [PubMed] [Google Scholar]
- 10.Vrouenraets MB, Visser GW, Stigter M, Oppelaar H, Snow GB and van Dongen GA. Int. J. Cancer 2002; 98: 793–798. [DOI] [PubMed] [Google Scholar]
- 11.Duan W, Smith K, Savoie H, Greenman J and Boyle RW. Org. Biomol. Chem 2005; 3: 2384–2386. [DOI] [PubMed] [Google Scholar]
- 12.Ke MR, Yeung SL, Fong WP, Ng DK and Lo PC. Chem. —Eur. J 2012; 18: 4225–4233. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez ME, Zhang P, Azizuddin K, Delos Santos GB, Chiu SM, Xue LY, Berlin JC, Peng X, Wu H, Lam M, Nieminen AL, Kenney ME and Oleinick NL. Photochem. Photobiol 2009; 85: 1189–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ongarora BG, Zhou Z, Okoth EA, Kolesnichenko I, Smith KM and Vicente MGH. J. Porphyrins Phthalocyanines 2014; 18: 1021–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Göksel M. Bioorg. Med. Chem 2016; 24: 4152–4164. [DOI] [PubMed] [Google Scholar]
- 16.Hammer RP, Owens CV, Hwang SH, Sayes CM and Soper SA. Bioconjugate Chem 2002; 13: 1244–1252. [DOI] [PubMed] [Google Scholar]
- 17.López-Duarte I, Dieu LQ, Dolamic I, Martínez-Díaz MV, Torres T, Calzaferri G and Brühwiler D. Chem. —Eur. J 2011; 17: 1855–1862. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Fronczek FR and Vicente MGH. Tetrahedron Lett 2008; 49: 4828–4830. [Google Scholar]
- 19.Zorlu Y, Dumoulin F, Bouchu D, Ahsen V and Lafont D. Tetrahedron Lett 2010; 51: 6615–6618. [Google Scholar]
- 20.Vrouenraets MB, Visser GWM, Stewart FA, Stigter M, Oppelaar H, Postmus PE, Snow GB and van Dongen GAMS. Cancer Res 1999; 59: 1505–1513. [PubMed] [Google Scholar]
- 21.Carcenac M, Larroque C, Langlois R, van Lier JE, Artus JC and Pelegrin A. Photochem. Photobiol 1999; 70: 930–936. [PubMed] [Google Scholar]
- 22.Sehgal I, Li H, Ongarora BG, Devillier D and Vicente MGH. J. Porphyrins Phthalocyanines 2013; 17: 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leznoff CC and Sosa-Sanchez JL. Chem. Commun 2004; 0: 338–339. [DOI] [PubMed] [Google Scholar]
- 24.Li XY and Ng DKP. Tetrahedron Lett 2001; 42: 305–309. [Google Scholar]
- 25.Josefsen LB and Boyle RW. Br. J. Pharmacol 2008; 154: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hellebust A and Richards-Kortum R. Nanomedicine (London) 2012; 7: 429–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carcenac M, Dorvillius M, Garambois V, Glaussel F, Larroque C, Langlois R, Hynes NE, van Lier JE and Pèlegrin A. Br. J. Cancer 2001; 85; 1787–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moret F and Reddi E. J. Porphyrins Phthalocyanines 2017; 21: 239–256. [Google Scholar]
- 29.Russell-Jones G, McTavish K, McEwan J, Rice J and Nowotnik D. J. Inorg. Biochem 2004; 98: 1625–1633. [DOI] [PubMed] [Google Scholar]
- 30.Tripodo G, Mandracchia D, Collina S, Rui M and Rossi D. Med. Chem 2014; 8, 1–4. [Google Scholar]
- 31.Sibrian-Vazquez M, Ortiz J, Nesterova IV, Fernández-Lázaro F, Sastre-Santos A, Soper SA and Vicente MGH. Bioconjugate Chem 2007; 18: 410–420. [DOI] [PubMed] [Google Scholar]
- 32.Chen S, Zhao X, Chen J, Chen J, Kuznetsova L, Wong SS and Ojima I. Bioconjugate Chem 2010; 21: 979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Kim CY, Lee S, Lee D, Chung HM, Kim G, Heo SH, Kim C, Hong KS and Yoon J. J. Am. Chem. Soc 2017; 139: 10880–10886. [DOI] [PubMed] [Google Scholar]
- 34.Stallivieri A, Baros F, Jetpisbayeva G, Myrzakhmetov B and Frochot C. Curr. Med. Chem 2015; 22: 3185–3207. [DOI] [PubMed] [Google Scholar]
- 35.Meerovich IG, Jerdeva V, Derkacheva VM, Meerovich GA, Lukyanets EA, Kogan EA and Savitsky AP. J. Photochem. Photobiol. B 2005; 80: 57–64. [DOI] [PubMed] [Google Scholar]
- 36.Sutton JM, Clarke OJ, Fernandez N and Boyle RW. Bioconjugate Chem 2002; 13: 249–263. [DOI] [PubMed] [Google Scholar]
- 37.Hudson R, Carcenac M, Smith K, Madden L, Clarke OJ, Pèlegrin A, Greenman J and Boyle RW. Br. J. Cancer 2005; 92: 1442–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sibrian-Vazquez M, Jensen TJ, Fronczek FR, Hammer RP and Vicente MGH. Bioconjugate Chem 2005; 16: 852–863. [DOI] [PubMed] [Google Scholar]
- 39.Clarke OJ and Boyle RW. Chem. Commun 1999; 0: 2231–2232. [Google Scholar]
- 40.Soini AE, Yashunsky DV, Meltola NJ and Ponomarev GV. Luminescence 2003; 18: 182–192. [DOI] [PubMed] [Google Scholar]
- 41.Malatesti N, Smith K, Savoie H, Greenman J and Boyle RW. Int. J. Oncol 2006; 28: 1561–1569. [PubMed] [Google Scholar]
- 42.Kim S and Yi KY. J. Org. Chem 1986; 51: 2613–2615. [Google Scholar]
- 43.Ongarora BG, Hu X, Li H, Fronczek FR and Vicente MGH. MedChemComm 2012; 3: 179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaudhary PM, Murthy RV, Yadav R and Kikkeri R. Chem. Commun 2015; 51: 8112–8115. [DOI] [PubMed] [Google Scholar]
- 45.Zorlu Y, Dumoulin F, Durmuş M and Ahsen V. Tetrahedron 2010; 66: 3248–3258. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.