Abstract
It was hypothesized that dietary guanidinoacetic acid (GAA), the precursor of creatine (Cr), would be beneficial to heat-stressed finisher broilers owing to improved cellular energy status and arginine sparing effects. A total of 720 one-day-old male Ross 308 broilers were allocated to 3 treatments, 0 (control), 0.6, or 1.2 g/kg of GAA added to complete corn–soybean meal diets, and were fed for 39 D, with 12 replicates (20 birds each) per treatment. A chronic cyclic heat stress model (at a temperature of 34°C and 50 to 60% relative humidity for 7 h daily) was applied in the finisher phase (day 25–39). Samples were taken on day 26 and 39 to determine thrombocyte, white blood cell, corticosterone, protein and amino acid levels in blood and Cr, phosphocreatine (PCr), and adenosine triphosphate levels in the breast muscle. Meat quality was assessed on day 40 after overnight fasting. Guanidinoacetic acid at a dose of 1.2 g/kg decreased feed-to-gain ratio compared with the control in the grower phase (1.32 vs. 1.35, respectively; P <0.05). In the finisher period, the supplementation of 1.2 g/kg of GAA reduced feed intake compared with the control (–3.3%, P <0.05), whereas both GAA supplementation levels improved feed efficiency markedly (1.76, 1.66, and 1.67 for 0 [control], 0.6, and 1.2 g/kg of GAA, respectively, P <0.05). Mortality outcomes highlight that GAA feeding improved survival during heat stress, supported by lower panting frequency (linear effect, P <0.05). Plasma arginine was higher with increase in dietary GAA concentration on day 26 (+18.3 and + 30.8% for 0.6 and 1.2 g/kg of GAA, respectively; P <0.05). This suggests enhanced availability of arginine for other metabolic purposes than de novo GAA formation. In the breast muscle, PCr (day 39, P <0.05), free Cr (day 39, P <0.05), total Cr (both days, P <0.05), and PCr-to-adenosine triphosphate ratio (day 39, P <0.05) levels were increased with higher GAA content in diet. Guanidinoacetic acid supplementation improved feed conversion and survival during chronic cyclic heat stress, which may be associated with enhanced breast muscle energy status and arginine sparing effect.
Key words: guanidinoacetic acid, broiler, heat stress, creatine, arginine
Introduction
Heat exposure affects poultry production on a worldwide basis and has a significant impact on well-being and production. Furthermore, broiler production is growing globally, with highest rates in (sub)tropical regions, and concomitantly, the impact of climate change worldwide increases the likelihood of heat stress (HS) in poultry production (Mottet and Tempio, 2016). The existence of feathers on the body, absence of sweat glands, and high metabolic rate of modern strains make broilers very susceptible to high temperatures. Heat stress occurs when the amount of heat produced by an animal surpasses the animal's capacity to dissipate the heat to its surrounding environment (Lara and Rostagno, 2013). The physiological consequences of HS are numerous, and a number of mitigation strategies have been proposed (Lin et al., 2006; Renaudeau et al., 2012). Dietary solutions have been generally proposed as being effective and relatively cheap (Renaudeau et al., 2012).
One of the primary consequences of heat discomfort is the reduction of feed intake. In practice, nutritionists may formulate more energy-dense and higher fat diets in summer periods to counteract the reduced intake and to lower body heat increment (Renaudeau et al., 2012). Next, it was shown that during acute HS, the cellular energy demand increases (Yang et al., 2010) and that during chronic HS, mitochondrial adenosine triphosphate (ATP) generation is reduced (Azad et al., 2010). In addition, HS induces higher utilization of muscle energy reserves in the form of glycogen (Gonzalez-Esquerra and Leeson, 2006). On this note, it could be perceived that enhancing the cellular creatine (Cr)–phosphocreatine (PCr) energy shuttle system might offer benefits for the broiler subjected to HS. Indeed, this system functions as a backup to the adenosine diphosphate–ATP cycle to store and mobilize energy when required on short notice (Wyss and Kaddurah-Daouk, 2000; Curt et al., 2015). The Cr–PCr system occurs in all cells, but primarily, it is confined to cells that have high but variable energy demands, particularly muscle cells (e.g., breast and heart) or macrophages. In the body, Cr is synthesized via a two-step pathway: L-arginine (Arg) and glycine are condensed to guanidinoacetic acid (GAA) and L-ornithine in the kidney and pancreas by virtue of L-arginine:glycine amidinotransferase, and subsequently, GAA is methylated at the amidino group by S-adenosylmethionine to form Cr in a reaction that is catalyzed by the enzyme S-adenosyl-L-methionine:N-guanidinoacetate methyltransferase (Wyss and Kaddurah-Daouk, 2000; Curt et al., 2015). This second reaction occurs in the liver. Importantly, it has been repeatedly shown in broilers that dietary GAA is efficiently converted to Cr and increases Cr loading in the muscle and the PCr-to-ATP ratio, a marker of cellular energy status, resulting in improved performances in broilers (Khajali et al., 2020). Furthermore, Chamruspollert et al. (2004) reported that higher temperatures slowed Arg metabolism, negatively affecting Cr synthesis pathways, which was evidenced by reduced Cr and creatinine levels in excreta.
Another primary physiological response during HS is the increased blood flow to the body surface or upper respiratory tract to dissipate internal body heat (Yahav et al., 1997). Therefore, the blood flow to some visceral organs is significantly reduced. In this respect, Arg plays a pivotal role as it is the nitrogenous precursor for the endogenous synthesis of nitric oxide by nitric oxide synthase. Nitric oxide is a potent vasodilator that directly relaxes vascular smooth muscle and modulates or inhibits the production and release of vasoconstrictors such as serotonin. Higher Arg bioavailability might thus be beneficial for heat-stressed birds, as it has been demonstrated in Pekin ducks (Zhu et al., 2014). Furthermore, conclusively, it was demonstrated that dietary supplemented GAA is able to spare Arg in broilers (Dilger et al., 2013; DeGroot et al., 2018) owing to the fact that less endogenous GAA is synthesized. Consequently, more Arg would be available for its protein and other nonprotein functions, such as a precursor for nitric oxide and polyamines. Regarding the latter, L-ornithine is the precursor of putrescine, which in turn is converted to the polyamines spermidine and spermine by merging with recurrent adenopropyl groups from S-adenosylmethionine. These biogenic amines are important for cell division, protein synthesis, and tissue growth and play a key role in gut function.
It is thus appealing to investigate the potential of dietary GAA to ameliorate the detrimental effects of HS in broilers (Amiri et al., 2019). It was hypothesized that supplementation with GAA would be beneficial to heat-stressed finishing broilers because of 1) improved energy status owing to pivotal role of Cr in energy homeostasis and 2) improved Arg metabolism owing to Arg sparing effects. These hypotheses were tested in heat-stressed finishing broilers in a model of chronic cyclic HS (Akbarian et al., 2014).
Materials and methods
Animals, Housing, and Diets
The experiment was carried out according to the guidelines of the Ethics Committee of The Faculty of Veterinary Medicine, Ghent University (Belgium), for the humane care and use of animals in research (reference: EC2016/49). A total of 720 one-day-old male Ross 308 broilers (Broeierij Vervaeke-Belavi, Tielt, Belgium) were allocated to 3 dietary treatments with 12 pen replicates of 20 birds each (density of 14.7 birds/m2) such that the average initial pen weights were not different among all treatments. Before arrival, the solid floor was covered with fresh wood shavings (1.5 kg/m2). The light schedule was 23 h of light and 1 h of darkness and 18 h of light and 6 h of darkness (18 h of light from 4:00 am to 10:00 pm) during day 0–7 and beyond, respectively. The room temperature was 34°C at the setting and linearly decreased to 22°C by day 25. During the first 5 D of trial, additional infrared lamp heating (one per pen) was used. From day 25 onward (finisher phase), a specific temperature and moisture regime was introduced to induce chronic cyclic HS (Supplementary Figure 1). The basal temperature was 22°C. Between 8:00 and 9:00 am, the temperature was gradually increased to 34°C, and this high temperature was then maintained for 7 h (until 4:00 pm). After that, the temperature was decreased to the basal level, again taking 1 h. Air humidity was kept between 50 and 60% during the HS episodes by nebulization of water. The broilers were vaccinated at 1 D of age against Newcastle disease and infectious bronchitis at the hatchery facilities. At 18 D of age, the vaccination against Newcastle disease was repeated with Nobilis ND Clone 30 (MSD Animal Health, Boxmeer, the Netherlands) by spraying.
The dietary treatments were as follows: 0 (control), 0.6, or 1.2 g/kg of GAA (CreAMINO, GAA, feed grade >96.0%; made available by Evonik Nutrition & Care GmbH, Hanau-Wolfgang, Germany, at the time of the study), which were added to corn–soybean meal–based diets (Table 1). The birds were fed with a starter (day 0–10), grower (day 10–25), and finisher (day 25–39) diet. The strategy of high-energy (high-fat) diet formulation was applied. Starter feed was pelleted on a 2.5 × 20 mm die, whereas grower and finisher diets were pelleted on a 3.0 × 35 mm die. Basal diets were formulated according to Ross 308 guidelines (Aviagen, 2014) for energy, digestible amino acids, Ca, and P and according to National Research Council (1994), Centraal Veevoederbureau (2016), and Ross 308 guidelines (Aviagen, 2014) for other nutrients. Choline levels in starter and grower diet were set slightly higher (approximately 30%) than those recommended by Ross 308 guidelines (Aviagen, 2014) to ensure remethylation pathways and improve cysteine availability for glutathione synthesis in the HS finisher period. Proximate and amino acid composition of major ingredients was determined to serve diet formulation. Experimental diets were analyzed for proximate and amino acid composition and GAA, Cr, creatinine, and choline content, as described by Michiels et al. (2012) and DeGroot et al. (2018). Feeds and water were given ad libitum throughout the trial until day 39. On day 39, the birds were fasted overnight. Pen live weight and feed leftovers were recorded at day 0, 10, 25, and 39. Weight gain, feed intake, feed-to-gain ratio (F:G; adjusted for mortality and calculated as total feed intake divided by total gain including the weight of lost birds per pen), and mortality were measured on day 10, 25, and 39. The European Production Efficiency Factor was calculated as follows: viability day 0–39 (%) × BW at day 39 (kg) × 100]/[age (D) × F:G day 0–39].
Table 1.
Ingredient and analyzed composition of corn–soybean–based basal diets for the starter (day 0–10), grower (day 10–25), and finisher (day 25–39) phase (as-is basis).1
| Dietary treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item |
Starter |
Grower |
Finisher |
||||||
| Supplemental GAA, g/kg | 0.0 | 0.6 | 1.2 | 0.0 | 0.6 | 1.2 | 0.0 | 0.6 | 1.2 |
| Ingredients, % | |||||||||
| Corn | 55.96 | 55.96 | 55.96 | 57.48 | 57.48 | 57.48 | 60.92 | 60.92 | 60.92 |
| Soybean meal | 27.91 | 27.91 | 27.91 | 27.36 | 27.36 | 27.36 | 20.78 | 20.78 | 20.78 |
| Corn gluten meal | 3.50 | 3.50 | 3.50 | ||||||
| Full fat toasted soybeans | 8.00 | 8.00 | 8.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Animal fat | 1.11 | 1.11 | 1.11 | 5.24 | 5.24 | 5.24 | 5.36 | 5.36 | 5.36 |
| Soybean oil | 2.00 | 2.00 | 2.00 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Dicalcium phosphate | 1.66 | 1.66 | 1.66 | 1.30 | 1.30 | 1.30 | 1.00 | 1.00 | 1.00 |
| Limestone | 1.05 | 1.05 | 1.05 | 1.08 | 1.08 | 1.08 | 1.10 | 1.10 | 1.10 |
| DL-Methionine | 0.40 | 0.40 | 0.40 | 0.36 | 0.36 | 0.36 | 0.27 | 0.27 | 0.27 |
| L-Lysine HCl | 0.32 | 0.32 | 0.32 | 0.24 | 0.24 | 0.24 | 0.26 | 0.26 | 0.26 |
| Sodium chloride | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Sodium bicarbonate | 0.26 | 0.26 | 0.26 | 0.25 | 0.25 | 0.25 | 0.40 | 0.40 | 0.40 |
| L-Threonine | 0.19 | 0.19 | 0.19 | 0.14 | 0.14 | 0.14 | 0.10 | 0.10 | 0.10 |
| L-Valine | 0.09 | 0.09 | 0.09 | 0.05 | 0.05 | 0.05 | |||
| L-Arginine | 0.06 | 0.06 | 0.06 | 0.002 | 0.002 | 0.002 | 0.01 | 0.01 | 0.01 |
| 3-Phytase (500 FTU/kg) | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Coccidiostatic2 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.01 | 0.01 | 0.01 |
| Vitamin and trace element premix3 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Choline chloride 50S | 0.23 | 0.23 | 0.23 | 0.24 | 0.24 | 0.24 | 0.08 | 0.08 | 0.08 |
| GAA (CreAMINO)4 | 0.06 | 0.12 | 0.06 | 0.12 | 0.06 | 0.12 | |||
| Analyzed nutrient composition, or calculated5 | |||||||||
| Dry matter, g/kg | 885 | 888 | 886 | 884 | 885 | 885 | 884 | 884 | 883 |
| Crude ash, g/kg | 57 | 58 | 57 | 53 | 52 | 53 | 46 | 47 | 47 |
| Crude protein, g/kg | 218 | 222 | 225 | 206 | 212 | 211 | 192 | 202 | 198 |
| Ether extract, g/kg | 71 | 72 | 72 | 93 | 92 | 92 | 94 | 93 | 93 |
| ME poultry, kcal/kg5 | 3,000 | 3,000 | 3,000 | 3,120 | 3,120 | 3,120 | 3,220 | 3,220 | 3,220 |
| Calcium, g/kg | 9.5 | 9.7 | 9.6 | 8.6 | 8.5 | 8.7 | 7.4 | 7.4 | 7.5 |
| Phosphorus (total), g/kg | 6.8 | 6.9 | 6.9 | 5.8 | 5.8 | 5.9 | 5.0 | 5.1 | 5.0 |
| Amino acids, g/kg | |||||||||
| Met | 6.8 | 6.8 | 6.9 | 6.4 | 6.1 | 6.3 | 5.7 | 5.6 | 5.8 |
| Cys | 3.4 | 3.4 | 3.4 | 3.3 | 3.2 | 3.3 | 3.2 | 3.2 | 3.1 |
| Digestible Met + Cys5 | 9.5 | 9.5 | 9.5 | 8.7 | 8.7 | 8.7 | 8.0 | 8.0 | 8.5 |
| Lys | 14.3 | 14.2 | 14.4 | 13.2 | 12.8 | 13.3 | 11.5 | 11.6 | 11.7 |
| Digestible Lys5 | 12.8 | 12.8 | 12.8 | 11.5 | 11.5 | 11.5 | 10.2 | 10.2 | 10.2 |
| Thr | 10.2 | 10.0 | 10.2 | 9.3 | 9.1 | 9.3 | 8.3 | 8.3 | 8.2 |
| Digestible Thr5 | 8.6 | 8.6 | 8.6 | 7.7 | 7.7 | 7.7 | 6.8 | 6.8 | 6.8 |
| Arg | 15.2 | 15.0 | 15.0 | 13.8 | 13.6 | 13.8 | 12.1 | 12.2 | 11.9 |
| Digestible Arg5 | 13.4 | 13.4 | 13.4 | 12.3 | 12.3 | 12.3 | 10.9 | 10.9 | 10.9 |
| Val | 11.3 | 11.1 | 11.2 | 10.3 | 10.2 | 10.3 | 9.1 | 9.3 | 9.1 |
| Digestible Val5 | 9.6 | 9.6 | 9.6 | 8.7 | 8.7 | 8.7 | 7.9 | 7.9 | 7.9 |
| Glyequivalents | 17.1 | 16.7 | 16.7 | 15.9 | 15.8 | 16.1 | 14.6 | 14.9 | 14.6 |
| GAA, mg/kg | <1 | 512 | 1,269 | <1 | 581 | 1,200 | <1 | 597 | 1,179 |
| Cr, mg/kg | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Creatinine, mg/kg | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Choline, mg/kg | 2,210 | 2,360 | 2,020 | 2,040 | 2,280 | 2,260 | 1,400 | 1,440 | 1,540 |
Abbreviations: GAA, guanidinoacetic acid; Cr, creatine.
Diet formulation was based on analyzed values for corn, soybean meal (48% CP), full fat soybeans, and corn gluten meal (61% CP) provided by Evonik and matrix values for other ingredients provided by DSM Nutritional Products, Belgium (updated matrix by January 2016) (Deinze, Belgium).
Coccidiostatic: salinomycin in starter and grower diet and narasin in finisher diet.
Vitamin and trace element premix providing per kg of diet: vitamin A (retinyl acetate), 10,000 IU; vitamin D3 (cholecalciferol), 2,500 IU; vitamin E (dl-α-tocopherol acetate), 50 mg; vitamin K3 (menadione), 1.5 mg; vitamin B1 (thiamin), 2.0 mg; vitamin B2 (riboflavin), 7.5 mg; niacin, 35 mg; d-pantothenic acid, 12 mg; vitamin B6 (pyridoxine HCl), 3.5 mg; vitamin B12 (cyanocobalamin), 20 μg; folic acid, 1.0 mg; biotin, 0.2 mg; choline chloride, 460 mg; Fe (FeSO4.H2O), 80 mg; Cu (CuSO4.5H2O), 12 mg; Zn (ZnO), 60 mg; Mn (MnO), 85 mg; I (Ca(IO3)2), 0.8 mg; Co (Co2CO3(OH)2), 0.77 mg; and Se (Na2O3Se), 0.15 mg.
Added on top of basal diet.
Calculated value.
Measurements and Sampling During Acute and Chronic HS
Panting frequency was measured on day 27 and 38 during the HS protocol (starting >4 h after inducing HS on that respective day) for two chickens at random per pen, based on video recordings. Panting frequency was determined as the number of breaths per minute. On day 26 (1 D of HS; acute HS) and day 39 (14 D of HS; chronic HS), one bird per pen with a weight close to the average weight of the pen was selected. Sampling started >4 h after inducing HS on that day. Rectal temperature was determined immediately using a digital thermometer inserted to a minimum depth of 3 cm in the cloaca. Then, the birds were placed in a dark box for 10 min in the room, followed by induction of euthanasia with 40 mg of pentobarbiturate per kg BW intramuscularly (left breast). Blood was taken by puncture from the heart using an 80-mm 22-G needle and collected in two K2EDTA tubes and one plain tube. One K2EDTA tube was used for fixation of blood by the addition of TransFix reagent (Caltag Medsystems Ltd., Buckingham, UK), pending analysis for thrombocytes and white blood cell differentials. The unfixed K2EDTA blood sample was used for harvesting plasma, followed by storage at –80°C, pending analysis of blood protein and amino acids. Serum was stored at –20°C and used for corticosterone quantification. Immediately after euthanasia, the skin was removed from the breast, and a 2-cm flat piece of the right breast (pectoralis major, middle of the right breast in length, and 2 cm away from the median, at a depth of 0.5 cm) was transferred to liquid N2. Snap-frozen tissue samples were broken, collected in precooled cryovials, and submerged in liquid N2 and stored at –80°C, pending analysis of energy metabolites.
Sampling for Meat Quality
On day 39, the remaining chickens were fasted overnight. Then, on day 40, one bird per pen with a weight close to the average weight of the pen was euthanized by electrical stunning, followed by exsanguination. pH of the right breast muscle was measured upon slaughter. Eviscerated carcasses were transferred to the chilling room (4°C) immediately after slaughter. pH of the right breast muscle was measured again 24 h after postmortem, together with color measurements (Michiels et al., 2012, 2014). Finally, the breast muscle was excised and vacuum stored at −20°C, pending measurements of thawing loss (Michiels et al., 2012) and lipid oxidative stability during simulated retail display. Thaw loss was the proportionate weight loss of a sample before frozen vacuum storage at −20°C and after overnight thawing at 4°C. Regarding oxidative stability, defined subsamples of the pectoralis major that had been frozen and thawed were wrapped in oxygen-permeable polyethylene film and displayed under fluorescent light (1,000 lux) for 7 D at 4°C. Lipid oxidation was assessed by measuring thiobarbituric acid reactive substances using the distillation method and was expressed as micrograms of malondialdehyde per gram of meat (Michiels et al., 2014).
Blood Analyses
Thrombocytes and white blood cell differentials were measured in fixated blood samples as outlined by Seliger et al. (2012). This automated analysis of chicken blood is based on flow cytometry using an anti-CD45 monoclonal antibody in combination with selected subset-specific markers. Serum corticosterone concentration, as a marker of stress, was also analyzed as outlined by Dehnhart et al. (2003).
Protein and Amino Acids in Plasma and Energy Metabolites in the Breast Muscle
Guanidinoacetic acid, homocysteine, and the entire amino acid profile in plasma were determined by means of liquid chromatography coupled to tandem mass spectrometry (HPLC pump, Agilent, Waldbronn, Germany; HTC PAL autosampler, CTC Analytics, Zwingen, Switzerland; 4000QTRAP mass spectrometer, AB Sciex, Framingham, MA). Sample preparation included a simple protein precipitation and addition of suitable internal standards (stable isotope–labeled) before analysis. Albumin was determined via the photometric color test at 570 and 660 nm, whereas total protein was determined via the biuret reaction photometrically at 540 and 660 nm. Uric acid was determined via an enzymatic color test (uricase/phenol/4-aminophenazone method) at 660 and 800 nm. Albumin, uric acid, and total protein levels were assayed using an AU 5800 Beckman Coulter (Beckman Coulter GmbH, Krefeld, Germany).
Adenosine triphosphate, PCr, and free Cr levels in the breast muscle were determined as described by DeGroot et al. (2018; 2019) in muscle biopsies.
Statistical Analysis
Statistical analytical techniques appropriate to a completely randomized design were used (SAS Enterprise Guide 7, SAS Institute Inc., Cary, NC). The experimental unit for all performance variables was pen. For physiological variables, one bird per pen was taken, and similarly, a pen was considered the experimental unit. Data were checked for normality using the Kolmogorov–Smirnov and Shapiro–Wilk tests and for homogeneity of variances using Levene's test. Parameters were analyzed using a one-way ANOVA model or the nonparametric Kruskal–Wallis test, the latter only for mortality data, with GAA supplementation being the fixed effect. Linear and quadratic contrasts were included as well. The post hoc Tukey test was used to compare means in case the one-way ANOVA model was significant. The level of P <0.05 was considered significant; 0.05 < P < 0.10 was considered a trend. Furthermore, referring to mortality, survival curves were constructed using the Kaplan–Meier representation and statistically analyzed using the log-rank test for trend (GraphPad Prism 5.00, San Diego).
Results
Diets, Bird Performance, Breast Meat Quality, and Rectal Temperature
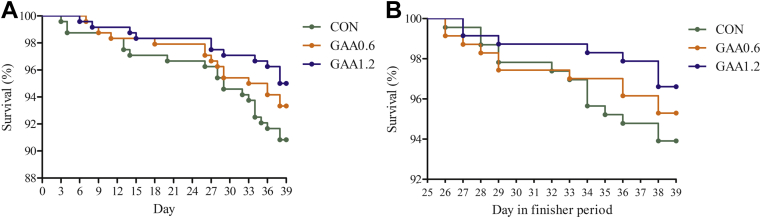
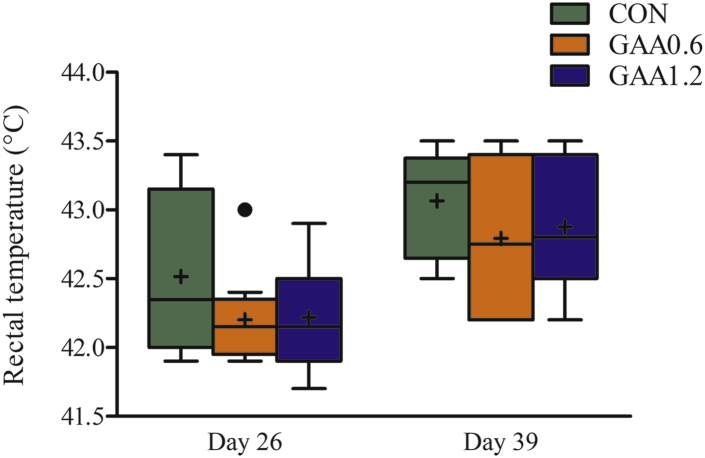
Until day 25 (starter and grower phases), when the birds were raised under standard conditions, BW was not affected by GAA application; however, feeding 1.2 g/kg of GAA decreased F:G compared with the control (1.30 vs. 1.27, P <0.05, and linear effect, P <0.05), in part caused by a linear reduction in feed intake (P <0.05) (Table 2). In the finisher period, when HS was prevailing, 0.6 and 1.2 g/kg of GAA reduced feed intake (linear effect, P <0.05) by 1.1 and 3.3%, respectively, associated with large improvements of F:G compared with the control (1.76, 1.66, and 1.67 for 0 [control], 0.6, and 1.2 g/kg of GAA, respectively, P <0.05). This resulted in a better F:G for the entire 39-D period when feeding GAA (P <0.05). In the finisher period, mortality was 6.1% in the control treatment owing to implemented HS. In all rearing phases, absolute values for mortality were lowest with graded supplemental GAA, but this did not reach statistical significance. Although Figure 1 demonstrates that time-dependent survival of birds tended to be altered by treatment (P = 0.071), in particular it shows that toward the end of the finisher period, mortality increased sharply for the control birds while survival remained higher for GAA-fed broilers. In line with this, the European Production Efficiency Factor linearly increased with graded dietary GAA (P <0.05), and this value was improved by feeding 1.2 g/kg of GAA compared with the control (P <0.05) (Table 2). Panting frequency was determined during the periods of HS on day 27 and 38. A linear effect indicated that panting frequency was lowered in GAA-supplemented broilers on day 38 compared with the control broilers (P <0.05) (Table 2), and this was also the case for the average of day 27 and 38 (linear effect, P <0.05). Regarding breast meat quality characteristics on day 40, no treatment effects were found (Supplementary Table 1). On day 26 and 39, the birds were sampled at the end of the HS period on the respective day. Of these birds, rectal temperature confirmed HS, whereby hyperthermia was more prone on day 39 (older birds, chronic HS); however, it was not influenced by treatment (Figure 2).
Table 2.
Effect of guanidinoacetic acid (GAA) supplementation on BW, ADG, ADFI, feed-to-gain ratio (F:G), mortality, European Production Efficiency Factor (EPEF), and panting in male broilers subjected to chronic cyclic heat stress in the finisher phase.1
| Item |
Dietary treatment |
Pooled SEM |
P-value |
||||
|---|---|---|---|---|---|---|---|
| Supplemental GAA, g/kg | 0.0 | 0.6 | 1.2 | Model | Linear | Quadratic | |
| Day 0 to 10 (starter) | |||||||
| Initial BW, g | 48.1 | 48.1 | 48.1 | 0.05 | 0.993 | 0.921 | 0.954 |
| Final BW, g | 344 | 339 | 341 | 1.8 | 0.625 | 0.563 | 0.440 |
| ADG, g/D | 29.4 | 29.5 | 29.3 | 0.18 | 0.627 | 0.687 | 0.383 |
| ADFI, g/D | 29.9 | 29.5 | 29.5 | 0.11 | 0.188 | 0.137 | 0.283 |
| F:G | 1.02 | 1.02 | 1.01 | 0.005 | 0.858 | 0.664 | 0.735 |
| Mortality, % | 1.3 | 1.3 | 0.8 | - | 0.855 | - | - |
| Day 10 to 25 (grower) | |||||||
| Final BW, g | 1,555 | 1,540 | 1,547 | 5.3 | 0.500 | 0.520 | 0.326 |
| ADG, g/D | 80.5 | 80.0 | 80.3 | 0.30 | 0.771 | 0.797 | 0.504 |
| ADFI, g/D | 109a | 107a,b | 106b | 0.4 | 0.022 | 0.006 | 0.705 |
| F:G | 1.35a | 1.34a | 1.32b | 0.004 | 0.001 | <0.001 | 0.575 |
| Mortality, % | 2.1 | 0.9 | 0.8 | - | 0.470 | - | - |
| Day 0 to 25 | |||||||
| ADG, g/D | 59.2 | 59.0 | 59.5 | 0.25 | 0.749 | 0.656 | 0.541 |
| ADFI, g/D | 76.8 | 75.8 | 75.3 | 0.27 | 0.067 | 0.025 | 0.580 |
| F:G | 1.30a | 1.29a,b | 1.27b | 0.004 | 0.002 | 0.001 | 0.730 |
| Mortality, % | 3.3 | 2.1 | 1.7 | - | 0.522 | - | - |
| Day 25 to 39 (finisher) | |||||||
| Final BW, g | 3,018 | 3,064 | 3,032 | 15.5 | 0.475 | 0.716 | 0.247 |
| ADG, g/D | 103 | 107 | 105 | 1.1 | 0.228 | 0.449 | 0.123 |
| ADFI, g/D | 180a | 178a,b | 174b | 0.9 | 0.027 | 0.008 | 0.725 |
| F:G | 1.76a | 1.66b | 1.67b | 0.014 | 0.002 | 0.003 | 0.043 |
| Mortality, % | 6.1 | 4.7 | 3.4 | - | 0.375 | - | - |
| Day 0 to 39 | |||||||
| ADG, g/D | 75.3 | 76.7 | 76.4 | 0.40 | 0.340 | 0.299 | 0.298 |
| ADFI, g/D | 111 | 110 | 109 | 0.4 | 0.129 | 0.045 | 0.949 |
| F:G | 1.48a | 1.44b | 1.43b | 0.005 | <0.001 | <0.001 | 0.047 |
| Mortality, % | 9.2 | 6.7 | 5.0 | - | 0.329 | - | - |
| EPEF2 | 476b | 510a,b | 517a | 7.1 | 0.036 | 0.016 | 0.337 |
| Panting, #/min | |||||||
| Day 27 | 185 | 160 | 160 | 6.5 | 0.202 | 0.122 | 0.366 |
| Day 38 | 190 | 185 | 163 | 5.0 | 0.062 | 0.028 | 0.392 |
| Mean (day 27, day 38) | 187 | 173 | 162 | 4.4 | 0.055 | 0.017 | 0.839 |
a,bMeans within the row lacking a common superscript letter differ (P <0.05).
Broilers were fed a corn–soybean starter diet from day 0 to 10, a grower diet from day 10 to 25, and a finisher diet from day 25 to 39. Values are means of 12 replicate pens of 20 chickens.
European Production Efficiency Factor: viability day 0-39 (%) ∗ BW day 39 (kg) ∗ 100/age (D) ∗F:G day 0–39.
Figure 1.
Effect of guanidinoacetic acid (GAA) supplementation on survival curves using the Kaplan–Meier representation for the total period (A; P = 0.071) and for the finisher period (B; P = 0.169) in male broilers subjected to chronic cyclic heat stress. CON, GAA0.6, and GAA1.2 with dietary GAA at a dose of 0, 0.6, and 1.2 g/kg, respectively.
Figure 2.
Effect of guanidinoacetic acid (GAA) supplementation on rectal temperature in male broilers subjected to chronic cyclic heat stress in the finisher phase on day 26 (P = 0.139) and day 39 (P = 0.343). Box and with median, 25th and 75th percentiles, and whiskers extending from the upper and lower edge of the box, which represents the interquartile (IQ) range, to the highest and lowest values that are no more than 1.5 times the IQ range; out values are denoted by circles, values between 1.5 and 3 times the IQ range; + denotes mean. CON, GAA0.6, and GAA1.2 with dietary GAA at a dose of 0, 0.6, and 1.2 g/kg, respectively. Values are from 12 replicate chickens.
Thrombocytes, White Blood Cell Differentials, and Corticosterone in Blood
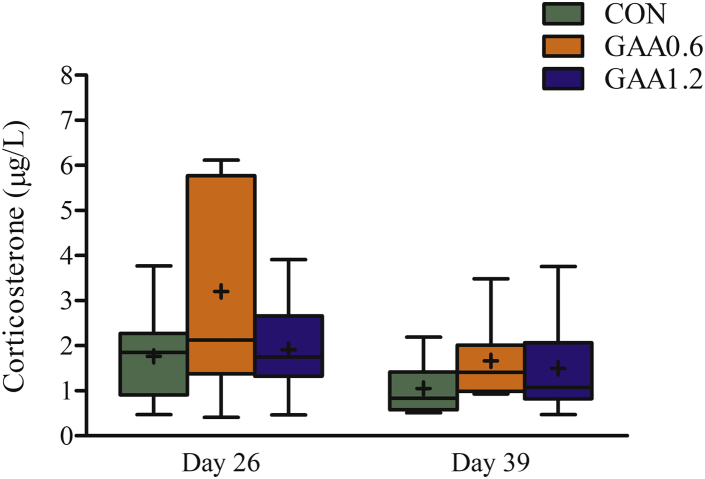
Data on thrombocytes and immune cells in blood were found to be very variable, but multiple effects were seen on day 26 (Table 3). Thrombocytes showed a linear decrease with increase in GAA content in the diet (P < 0.05). Guanidinoacetic acid supplementation linearly decreased the number of lymphocytes on day 26, caused by linear decreases in T cells (both P <0.05). Altogether, the number of leukocytes was substantially reduced by feeding GAA on day 26 (P <0.05). Serum corticosterone concentration was only affected by treatment at day 26, without post hoc differences (P = 0.05) (Figure 3).
Table 3.
Effect of guanidinoacetic acid (GAA) supplementation on thrombocytes and white blood cell differentials in blood of male broilers subjected to chronic cyclic heat stress in the finisher phase at day 26 and 39.1
| Item |
Dietary treatment |
Pooled SEM |
P-value |
||||
|---|---|---|---|---|---|---|---|
| Supplemental GAA, g/kg | 0.0 | 0.6 | 1.2 | Model | Linear | Quadratic | |
| Thrombocytes, #/μL | |||||||
| Day 26 | 72,500 | 56,700 | 51,000 | 4,200 | 0.088 | 0.040 | 0.552 |
| Day 39 | 53,800 | 50,800 | 49,000 | 2,000 | 0.648 | 0.358 | 0.909 |
| Heterophils, #/μL | |||||||
| Day 26 | 9,000 | 7,700 | 6,800 | 490 | 0.215 | 0.089 | 0.866 |
| Day 39 | 12,000 | 11,200 | 8,800 | 800 | 0.235 | 0.104 | 0.666 |
| Monocytes, #/μL | |||||||
| Day 26 | 1,200 | 790 | 430 | 170 | 0.194 | 0.076 | 0.946 |
| Day 39 | 1,620 | 780 | 730 | 190 | 0.100 | 0.052 | 0.336 |
| Lymphocytes, #/μL | |||||||
| Day 26 | 12,200a | 10,000a,b | 8,200b | 560 | 0.009 | 0.003 | 0.857 |
| Day 39 | 10,000 | 10,200 | 8,000 | 500 | 0.126 | 0.090 | 0.268 |
| T cells, #/μL | |||||||
| Day 26 | 10,300a | 8,600a,b | 6,800b | 470 | 0.006 | 0.002 | 0.989 |
| Day 39 | 8,500 | 8,600 | 6,900 | 420 | 0.186 | 0.121 | 0.336 |
| B cells, #/μL | |||||||
| Day 26 | 1,900 | 1,460 | 1,370 | 127 | 0.189 | 0.103 | 0.517 |
| Day 39 | 1,490 | 1,580 | 1,030 | 105 | 0.064 | 0.063 | 0.152 |
| Total leukocytes, #/μL | |||||||
| Day 26 | 22,400a | 18,600a,b | 15,500b | 870 | 0.002 | 0.001 | 0.798 |
| Day 39 | 23,600 | 22,100 | 17,500 | 1,300 | 0.117 | 0.048 | 0.571 |
| Heterophils/lymphocytes | |||||||
| Day 26 | 0.8 | 0.8 | 0.9 | 0.05 | 0.904 | 0.734 | 0.749 |
| Day 39 | 1.2 | 1.1 | 1.1 | 0.07 | 0.703 | 0.476 | 0.652 |
a,bMeans within a row lacking a common superscript letter differ (P <0.05).
Broilers were fed a corn–soybean starter diet from day 0 to 10, a grower diet from day 10 to 25, and a finisher diet from day 25 to 39. Values are means of 12 replicate chickens.
Figure 3.
Effect of guanidinoacetic acid (GAA) supplementation on serum corticosterone in male broilers subjected to chronic cyclic heat stress in the finisher phase on day 26 (P = 0.050) and day 39 (P = 0.192). Box and with median, 25th and 75th percentiles, and whiskers extending from the upper and lower edge of the box, which represents the interquartile (IQ) range, to the highest and lowest values that are no more than 1.5 times the IQ range; + denotes mean. CON, GAA0.6, and GAA1.2 with dietary GAA at a dose of 0, 0.6, and 1.2 g/kg, respectively. Values are from 12 replicate chickens.
Protein and Amino Acids in Plasma
Neither uric acid and albumin nor total protein in plasma was affected by treatment, on either sampling day (Table 4). Among all plasma amino acids and dipeptides shown in Table 4, only Arg, carnosine, and glycine were affected by treatment. Blood Arg content increased linearly with increase in dietary GAA content (+18.3 and +19.9% for 0.6 g/kg of GAA on day 26 and day 39, respectively, both different from the control, and +30.8 and +33.6% for 1.2 g/kg of GAA, only different, P <0.05, from the control at day 26 owing to higher variance on day 39). Carnosine content was reduced by supplementing the diet with GAA on day 39 (P <0.05), with treatment with 0.6 g/kg of GAA showing the lowest value. In addition, for this treatment at day 39, plasma glycine content was elevated compared with the control (P <0.05), whereas GAA at a dose of 1.2 g/kg exhibited an intermediate value, and this resulted in a quadratic effect (P <0.05).
Table 4.
Effect of guanidinoacetic acid (GAA) supplementation on protein and amino acids in plasma of male broilers subjected to chronic cyclic heat stress in the finisher phase at day 26 and 39.1
| Item |
Dietary treatment |
Pooled SEM |
P-value |
||||
|---|---|---|---|---|---|---|---|
| Supplemental GAA, g/kg | 0.0 | 0.6 | 1.2 | Model | Linear | Quadratic | |
| Uric acid, mmol/l | |||||||
| Day 26 | 342 | 320 | 271 | 15.1 | 0.147 | 0.057 | 0.666 |
| Day 39 | 233 | 274 | 251 | 9.2 | 0.184 | 0.409 | 0.100 |
| Albumin, g/l | |||||||
| Day 26 | 11 | 10 | 10 | 0.2 | 0.196 | 0.096 | 0.483 |
| Day 39 | 10 | 10 | 10 | 0.1 | 0.783 | 0.903 | 0.494 |
| Total protein, g/l | |||||||
| Day 26 | 30 | 28 | 28 | 0.5 | 0.315 | 0.217 | 0.376 |
| Day 39 | 28 | 29 | 28 | 0.4 | 0.816 | 0.718 | 0.602 |
| Alanine, μmol/L | |||||||
| Day 26 | 768 | 761 | 692 | 29.5 | 0.521 | 0.304 | 0.626 |
| Day 39 | 452 | 445 | 442 | 15.9 | 0.967 | 0.800 | 0.963 |
| α-Aminobutyric acid μmol/L | |||||||
| Day 26 | 18 | 16 | 18 | 0.8 | 0.657 | 0.987 | 0.363 |
| Day 39 | 14 | 13 | 13 | 0.5 | 0.896 | 0.671 | 0.851 |
| Arginine, μmol/L | |||||||
| Day 26 | 263b | 311a,b | 344a | 12.8 | 0.029 | 0.009 | 0.752 |
| Day 39 | 226 | 271 | 302 | 16.9 | 0.185 | 0.070 | 0.847 |
| Aspargine, μmol/L | |||||||
| Day 26 | 106 | 118 | 98 | 8.1 | 0.592 | 0.628 | 0.357 |
| Day 39 | 46 | 74 | 63 | 6.2 | 0.172 | 0.256 | 0.132 |
| Aspartic acid, μmol/L | |||||||
| Day 26 | 74 | 74 | 68 | 6.2 | 0.912 | 0.727 | 0.808 |
| Day 39 | 91 | 81 | 78 | 3.4 | 0.264 | 0.124 | 0.596 |
| Carnosine, μmol/L | |||||||
| Day 26 | 54 | 44 | 47 | 2.4 | 0.243 | 0.264 | 0.206 |
| Day 39 | 54a | 40b | 46a,b | 1.9 | 0.012 | 0.100 | 0.011 |
| Citrulline, μmol/L | |||||||
| Day 26 | 5.4 | 6.8 | 6.8 | 0.48 | 0.438 | 0.272 | 0.509 |
| Day 39 | 9.1 | 10.9 | 9.4 | 0.51 | 0.293 | 0.759 | 0.127 |
| Cystine, μmol/L | |||||||
| Day 26 | 46 | 49 | 46 | 1.5 | 0.628 | 0.975 | 0.339 |
| Day 39 | 51 | 54 | 51 | 1.5 | 0.430 | 0.897 | 0.200 |
| Glutamine, μmol/L | |||||||
| Day 26 | 883 | 929 | 783 | 33.7 | 0.196 | 0.224 | 0.179 |
| Day 39 | 806 | 806 | 804 | 21.2 | 0.342 | 0.970 | 0.146 |
| Glutamic acid, μmol/L | |||||||
| Day 26 | 187 | 177 | 158 | 6.7 | 0.204 | 0.080 | 0.793 |
| Day 39 | 177 | 176 | 181 | 3.6 | 0.843 | 0.682 | 0.681 |
| Glycine, μmol/L | |||||||
| Day 26 | 384 | 413 | 375 | 12.9 | 0.466 | 0.776 | 0.232 |
| Day 39 | 349b | 411a | 387a,b | 10.2 | 0.039 | 0.113 | 0.041 |
| Histidine, μmol/L | |||||||
| Day 26 | 38 | 34 | 38 | 1.1 | 0.306 | 0.985 | 0.127 |
| Day 39 | 39 | 41 | 43 | 1.5 | 0.581 | 0.301 | 0.967 |
| Hydroxyproline, μmol/L | |||||||
| Day 26 | 145 | 174 | 158 | 6.2 | 0.164 | 0.384 | 0.090 |
| Day 39 | 156 | 137 | 148 | 6.1 | 0.472 | 0.610 | 0.267 |
| Isoleucine, μmol/l | |||||||
| Day 26 | 60 | 55 | 63 | 2.5 | 0.443 | 0.702 | 0.226 |
| Day 39 | 67 | 76 | 66 | 2.9 | 0.343 | 0.958 | 0.147 |
| Leucine, μmol/L | |||||||
| Day 26 | 157 | 151 | 170 | 6.1 | 0.452 | 0.387 | 0.361 |
| Day 39 | 169 | 179 | 152 | 5.4 | 0.133 | 0.195 | 0.120 |
| Lysine, μmol/L | |||||||
| Day 26 | 120 | 90 | 102 | 6.6 | 0.191 | 0.278 | 0.142 |
| Day 39 | 165 | 151 | 141 | 18.9 | 0.595 | 0.314 | 0.921 |
| Methionine, μmol/L | |||||||
| Day 26 | 60 | 65 | 64 | 2.4 | 0.725 | 0.551 | 0.596 |
| Day 39 | 62 | 74 | 67 | 3.0 | 0.286 | 0.471 | 0.159 |
| Ornithine, μmol/L | |||||||
| Day 26 | 22 | 21 | 23 | 1.1 | 0.704 | 0.943 | 0.407 |
| Day 39 | 26 | 33 | 25 | 2.0 | 0.227 | 0.907 | 0.088 |
| Phenylalanine, μmol/L | |||||||
| Day 26 | 96 | 90 | 96 | 2.4 | 0.487 | 0.953 | 0.229 |
| Day 39 | 109 | 119 | 112 | 4.5 | 0.246 | 0.647 | 0.109 |
| Proline, μmol/L | |||||||
| Day 26 | 334 | 336 | 344 | 13.9 | 0.948 | 0.759 | 0.914 |
| Day 39 | 237 | 235 | 234 | 8.7 | 0.989 | 0.882 | 0.977 |
| Serine, μmol/L | |||||||
| Day 26 | 505 | 522 | 434 | 20.9 | 0.194 | 0.166 | 0.239 |
| Day 39 | 412 | 454 | 449 | 11.4 | 0.261 | 0.185 | 0.334 |
| Taurine, μmol/L | |||||||
| Day 26 | 155 | 217 | 185 | 14.9 | 0.240 | 0.407 | 0.141 |
| Day 39 | 105 | 91 | 101 | 8.5 | 0.800 | 0.851 | 0.334 |
| Threonine, μmol/L | |||||||
| Day 26 | 429 | 415 | 393 | 18.3 | 0.721 | 0.928 | 0.425 |
| Day 39 | 339 | 357 | 375 | 22.8 | 0.509 | 0.249 | 0.999 |
| Tryptophan, μmol/L | |||||||
| Day 26 | 67 | 59 | 63 | 1.9 | 0.325 | 0.429 | 0.203 |
| Day 39 | 82 | 88 | 83 | 1.9 | 0.341 | 0.936 | 0.146 |
| Tyrosine, μmol/L | |||||||
| Day 26 | 131 | 155 | 150 | 6.1 | 0.245 | 0.250 | 0.219 |
| Day 39 | 142 | 150 | 153 | 8.9 | 0.871 | 0.614 | 0.893 |
| Valine, μmol/L | |||||||
| Day 26 | 95 | 88 | 102 | 3.1 | 0.197 | 0.352 | 0.122 |
| Day 39 | 103 | 111 | 95 | 4.1 | 0.309 | 0.459 | 0.181 |
a,bMeans within a row lacking a common superscript letter differ (P <0.05).
Broilers were fed a corn–soybean starter diet from day 0 to 10, a grower diet from day 10 to 25, and a finisher diet from day 25 to 39. Values are means of 12 replicate chickens.
Energy Metabolites in the Breast Muscle
In the breast muscle, PCr (day 26, linear effect, P <0.05; day 39, P <0.05), free Cr (day 39, P <0.05), total Cr (both days, P <0.05), and PCr-to-ATP ratio (day 26, linear effect, P <0.05; day 39, P <0.05) levels were increased with higher GAA content in diet (Table 5). Muscle Cr levels appeared to be lower at day 39 compared with day 26, whereas the opposite was seen for PCr and PCr-to-ATP ratio levels.
Table 5.
Effect of guanidinoacetic acid (GAA) supplementation on breast meat creatine–phosphocreatine system of male broilers subjected to chronic cyclic heat stress in the finisher phase at day 26 and 39.1
| Item |
Dietary treatment |
Pooled SEM |
P-value |
||||
|---|---|---|---|---|---|---|---|
| Supplemental GAA, g/kg | 0.0 | 0.6 | 1.2 | Model | Linear | Quadratic | |
| ATP, μmol/g DM | |||||||
| Day 26 | 26 | 27 | 26 | 0.6 | 0.703 | 0.898 | 0.410 |
| Day 39 | 29 | 29 | 28 | 0.5 | 0.472 | 0.387 | 0.388 |
| PCr, μmol/g DM | |||||||
| Day 26 | 49 | 55 | 65 | 3.0 | 0.085 | 0.029 | 0.748 |
| Day 39 | 85b | 95a,b | 99a | 2.2 | 0.016 | 0.005 | 0.532 |
| Free Cr, μmol/g DM | |||||||
| Day 26 | 111 | 128 | 129 | 3.9 | 0.097 | 0.056 | 0.310 |
| Day 39 | 45b | 57a | 61a | 1.9 | 0.001 | <0.001 | 0.245 |
| Total Cr, μmol/g DM | |||||||
| Day 26 | 160b | 183a | 194a | 3.9 | <0.001 | <0.001 | 0.357 |
| Day 39 | 130b | 151a | 160a | 3.4 | <0.001 | <0.001 | 0.274 |
| PCr:ATP | |||||||
| Day 26 | 1.9 | 2.1 | 2.6 | 0.13 | 0.080 | 0.033 | 0.488 |
| Day 39 | 3.0b | 3.3a,b | 3.7a | 0.9 | 0.008 | 0.002 | 0.785 |
a,bMeans within a row lacking a common superscript letter differ (P <0.05).
Abbreviations: ATP, adenosine triphosphate; Cr, creatine; GAA, guanidinoacetic acid; PCr, phosphocreatine.
Broilers were fed a corn–soybean starter diet from day 0 to 10, a grower diet from day 10 to 25, and a finisher diet from day 25 to 39. Values are means of 12 replicate chickens.
Discussion
Guanidinoacetic Acid Maintains Growth Performance and Improves Feed Conversion and Survival in Heat-Stressed Broilers
In agreement with our hypothesis, this study confirmed that dietary supplementation with GAA is beneficial to heat-stressed finishing broilers owing to muscle Cr loading and Arg sparing. Accordingly, prominent and important improvements in feed efficiency, survival, panting frequency, lymphocyte numbers, and muscle energy metabolites in the finisher period were observed. Here, HS was confirmed by exuberant high panting frequencies and rectal temperatures found in broilers during the daily episodes of high temperatures in the finisher period. Panting occurred heavily during episodes of HS and ranged between 160 to 185 #/min and 163 and 190 #/min for day 27 and day 38, respectively. Respiratory frequency of birds under thermoneutral conditions would be between 40 and 80, depending on age and metabolism. Similar to the dramatic increase in rectal temperature, means for rectal temperature exceeded 42.2°C on day 26, and on day 39, means were even higher. To note, rectal temperature of birds under thermoneutral conditions fluctuates between 40.5°C and 41.5°C.
In the present study, no effect of GAA supplementation on BW gain in the starter, grower, or finisher period was seen, which is in contrast to several studies under thermoneutral and to one study under HS conditions. For example, an improvement in growth performance under thermoneutral conditions when GAA was added to vegetable diets was shown by Michiels et al. (2012) in the grower period, whereas Dilger et al. (2013) and DeGroot et al. (2018) reported growth responses when 0.12% of GAA was added to an Arg-deficient basal diet. Under HS conditions, Amiri et al. (2019), who studied the efficacy of GAA supplementation at different crude protein levels, described linearly improved final BW and overall ADG by supplementing GAA. However, the findings of our present study are in agreement with those of the study by Majdeddin et al. (2018). Heat-challenged birds seek homeostasis, and because energy is redirected to restrict body temperature increases by ways such as panting behavior, this leads to less energy available for growth promotion. In addition, energy is lost owing to the occurrence of mitochondrial dysfunction, which causes redox energy dissipation as heat instead of being used for ATP synthesis (Mujahid et al., 2007). Furthermore, the response of heat-stressed broilers is commonly associated with less appetite and lower feed consumption, which is an evident defense mechanism to decrease heat increment and to sustain homeothermy (Lara and Rostagno, 2013). Altogether, lower growth rates are typically found during HS owing to reduced feed intake and feed efficiency; hence, here, we found that GAA had the potential to allow even higher reduction of feed intake while still maintaining BW.
Notably, findings for ADFI showed a reduction in broiler chickens fed with diets containing 1.2 g/kg of GAA during the grower and finisher period, but it corroborates the findings of our previous studies (Majdeddin et al., 2018, 2019). In different studies performed under thermoneutral conditions, inconsistent results have been obtained with regard to the influence of GAA on feed intake. For instance, Ringel et al. (2008), Michiels et al. (2012), EFSA (2016), Córdova-Noboa et al. (2018a), and DeGroot et al. (2018, 2019) reported no effect of GAA on feed intake, whereas Lemme et al. (2010) showed that GAA supplementation reduced feed intake in turkeys. Feed intake reductions were seen in some studies reported in EFSA (2016), at least for the whole rearing period. The effect of supplementing the diet with GAA on ADFI remains debatable and may likely depend on other nutritional factors such as dietary energy, possibly explaining part of the contradictory results seen in the published literature. In our previous article, we related this reduced feed consumption to the hepatic energy status theory (Majdeddin et al., 2018). Essentially, we speculated that GAA supplementation might improve hepatic cellular energy status, reflected by higher ATP-to-adenosine diphosphate ratio and hence reducing appetite, which is congruent to the findings by Ji and Friedman (1999) in rats. Furthermore, it is well known that adenosine monophosphate (AMP)–activated protein kinase is the main sensor of cellular energy status and is activated in response to energy stress to restore energy balance by inhibiting ATP-consuming processes and promoting ATP-generating pathways. Adenosine monophosphate–activated protein kinase is allosterically activated through fluctuations in the AMP-to-ATP ratio in the liver (Corton et al., 1994). Hence, again, GAA feeding might enhance cellular energy in the liver and thus lower AMP-activated protein kinase stimulation, altering energy metabolism. Potentially, GAA may impede upregulation of AMP-activated protein kinase, and more importantly, it may counteract reduced cholesterol 7 alpha-hydroxylase expression. The latter would mean that fat digestion can be improved, which is of utmost importance as HS has repeatedly been shown to reduce digestibility of nutrients (Bonnet et al., 1997). Altogether, it could mean that GAA improves dietary energy utilization, even more under HS conditions. This can be confirmed as strong improvements in F:G for the finisher period were observed in this study. Here, supplementation with GAA improved F:G in the grower and particularly in the finisher period, when HS was prevailing, and overall, as such corroborating the findings of many previous studies (Michiels et al., 2012; EFSA, 2016; Majdeddin et al., 2018, 2019). Feed-to-gain ratio in the finisher period was improved by 10 and 11 points in birds fed with 0.6 and 1.2 g/kg of GAA, respectively. Much in agreement with that, Amiri et al. (2019) showed a 13-point reduction in F:G at a dose of 1.2 g/kg of GAA in the finisher period under HS conditions. However, reports on GAA feeding under thermoneutral conditions gives F:G reductions in the finisher period by 3.6 ± 3.5 and 6.3 ± 4.6 points for 0.6 and 1.2 g/kg of GAA, respectively (Michiels et al., 2012; Mousavi et al., 2013; Heger et al., 2014; EFSA, 2016; Kodambashi Emami et al., 2017; Cordova-Nobova et al., 2018b; Majdeddin et al., 2018, 2019), thus emphasizing the additional benefits when fed to heat-stressed broilers.
Mortality in the finisher period accounted for 6.1, 4.7, and 3.4% in the groups fed with 0, 0.6, or 1.2 g/kg of GAA, respectively, undoubtedly illustrating that HS impacted survival. Survival curves for the finisher period shows that GAA feeding limited mortality mainly toward the end of the study. This corroborates the linear reduction in panting frequency on day 38, and not on day 27. Even so, but only numerically, rectal temperature was reduced by GAA mostly on day 38, by 0.3 to 0.4°C from 43.1°C for control birds. These figures support the idea that heat tolerance was improved by feeding GAA, in particular when HS gets a chronic nature. However, it can also be said that GAA-fed broilers were likely better prepared for the implementation of HS by the previous feeding period, hereby referring to improved feed conversion in the grower period.
Guanidinoacetic Acid Has Minimal Effect on the Birds' Stress Response, but May Spare Arginine
Supplementation of GAA had no effect on heterophils or the heterophil-to-lymphocyte ratio, the latter described as a marker of HS, and is thus in contrast with the findings from DeGroot et al. (2018). These authors reported that supplementation of GAA decreased heterophils as the largest proportion of leukocytes. However, in our study, supplementation of GAA on day 26 decreased total leukocyte counts caused by decrease in lymphocyte counts, the latter in turn because of decreasing T-cell counts. These findings indicate that GAA may alter cell-mediated immune responses, in particular in the acute phase of HS. The full interpretation of these findings warrants further research. If cell-mediated immunity is indeed altered, this could be further investigated by functional studies. In our study, treatment had no (day 39) or just significant (day 26) effect on corticosterone. In fact, corticosterone content in GAA-fed chickens was increased at a dose of 0.6 g/kg of GAA during acute HS. Notably, as illustrated in Figure 3, variation across birds for the group fed with 0.6 g/kg of GAA was exceptionally high. It should also be noted that high levels of corticosterone can only be maintained for short periods, less than 1 h, to cope with the immediate stressor, here the high temperatures. When birds are chronically exposed to high temperatures, concentration of corticosterone may decline after the initial stage (Etches et al., 2008). Responses indicate that GAA feeding has minimal effect on the birds' stress response.
Supplementation of GAA did not affect plasma amino acid or dipeptide concentrations, apart from Arg, glycine, and carnosine concentrations. The Arg sparing effect was demonstrated by higher plasma Arg levels, only significant on day 26. Khajali et al. (2020) concluded that GAA feeding must be able to save Arg for other metabolic functions than Cr synthesis. Higher Arg bioavailability can be truly beneficial for heat-stressed broilers, as has been demonstrated in heat-stressed Pekin ducks (Zhu et al., 2014). Furthermore, our findings indicate that 0.6 g/kg of GAA increased plasma glycine content at day 39. This increase amounted to 15%, and interestingly, serine content was also increased by 10%. Because glycine and serine are interconvertible (Siegert and Rodehustcord, 2019), this suggests a potential Glyequiv sparing effect for this treatment, although it does not appear to be the case on day 26, or for 1.2 g/kg of GAA at day 39. Nonetheless, if feeding GAA evokes lower endogenous GAA synthesis, then similar to the effect on Arg, Glyequiv should be less used for this purpose. However, as glycine and serine are not essential amino acids, these effects may have less impact on birds' metabolism. Anyway, one may argue that in the context of HS, this could lead to higher productivity. Indeed, HS may accentuate some Glyequiv-consuming metabolic processes. Uric acid synthesis is a major consumer of glycine in the bird. It remains to be confirmed whether GAA is able to promote glycine metabolic functions, possibly advantaging broilers under HS. The level of carnosine, a dipeptide formed by histidine and β-alanine, declined by supplementing the diet with GAA on day 39. Carnosine is believed to serve as a free radical scavenger in animal tissues. Manhiani et al. (2011) reported that an increase in carnosine levels in the brain, breast, and thigh of broilers in response to stress could temporarily and may decrease after a certain time. In addition, in horses, Dunnett et al. (2002) found higher plasma carnosine concentrations at 5 to 30 min and lower carnosine concentrations at 120 min after inducing exercise as a stressor. Carnosine values returned to normal values after 1 D. Differences in plasma carnosine content among animals exposed to stress may be accounted for by the difference in species, stress duration time, and intensity. It remains hard to relate GAA and Cr metabolism with carnosine, and, actually, the muscle serves as the major storage site for carnosine, which was not determined here.
Energy Metabolites Are Enhanced in the Breast Muscle by Feeding GAA
It is well established that supplementation of GAA increases concentrations of Cr-related metabolites in the chicken breast muscle (Khajali et al., 2020). Here, supplementing GAA to the diets of birds under HS conditions resulted in dose-related elevations for PCr, free Cr, total Cr, and PCr-to-ATP ratio levels, supposedly substantially contributing to the better feed conversion observed in the finisher phase. These muscle Cr and PCr outcomes are in line with the findings of Lemme et al. (2007), EFSA (2009), Michiels et al. (2012), Majdeddin et al. (2018, 2019), and DeGroot et al. (2018, 2019). Interestingly, all effects on PCr, free Cr, total Cr, and PCr-to-ATP ratio were found to be linear, suggesting that no saturation of Cr storage and metabolites thereof occurred within the GAA dosage range applied. The PCr-to-ATP ratio is believed to be an indicator of the buffering capacity for ATP hydrolysis upon fast energy usage. The effects were largely maintained throughout the 14-D HS finisher period. In addition, the simultaneous increase in the PCr and total Cr concentrations in the muscle underlines that dietary GAA is successfully absorbed and metabolized to Cr and is thus an efficient precursor in terms of Cr incorporation into the muscle. The increases of the PCr-to-ATP ratio in the breast muscle at day 39 were 10 and 23% at 0.6 and 1.2 g/kg of GAA, respectively. It shows an increased potential for energy expenditure in chickens consuming supplemental GAA (DeGroot et al., 2019).
In conclusion, GAA supplementation improved feed conversion ratio and survival, with the largest benefits in the finisher period when birds were subjected to cyclic HS. This was associated with enhanced energy status and an Arg and potentially a glycine sparing effect. It suggests that supplementation of GAA in broilers' diet may be economically profitable under heat-challenged conditions.
Acknowledgments
The study was funded by AlzChem Trostberg GmbH (Trostberg, Germany) and Evonik Nutrition & Care GmbH (Hanau-Wolfgang, Germany). The Department of Veterinary Science, Institute for Animal Physiology, München, Germany (Prof. B. Kaspers), is acknowledged for determination of thrombocytes and white blood cell differentials in blood, as well as Medizinisches Labor Bremen GmbH (Bremen, Germany) for determination of creatine, creatinine, GAA, amino acid, and homocysteine content in plasma and Swiss BioQuant AG (Reinach, Switzerland) for determination of uric acid and total protein content in plasma, corticosterone content in serum, and ATP, Cr, and PCr content in the breast muscle. M.M. was supported by the Ferdowsi University of Mashhad, Iran (grant number: 3/28524).
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
Footnotes
Supplementary data associated with this article can be found in the online version at http://doi.org/10.1016/j.psj.2020.05.023.
Supplementary data
Supplementary Figure 1.
References
- Akbarian A., Michiels J., Golian A., Buyse J., Wang Y., De Smet S. Gene expression of heat shock protein 70 and antioxidant enzymes, oxidative status, and meat oxidative stability of cyclically heat-challenged finishing broilers fed Origanum compactum and Curcuma xanthorrhiza essential oils. Poult. Sci. 2014;93:1930–1941. doi: 10.3382/ps.2014-03896. [DOI] [PubMed] [Google Scholar]
- Amiri M., Ghasemi H.A., Hajkhodadadi I., Farahani A.H.K. Efficacy of guanidinoacetic acid at different dietary crude protein levels on growth performance, stress indicators, antioxidant status, and intestinal morphology in broiler chickens subjected to cyclic heat stress. Anim. Feed Sci. Technol. 2019;254:14. [Google Scholar]
- Aviagen. 2014 Ross 308: broiler nutrition specifications. www.aviagen.com Accessed Dec. 2015.
- Azad M.A.K., Kikusato M., Maekawa T., Shirakawa H., Toyomizu M. Metabolic characteristics and oxidative damage to skeletal muscle in broiler chickens exposed to chronic heat stress. Comp. Biochem. Physiol. Part A. Mol. Integr. Physiol. 2010;155:401–406. doi: 10.1016/j.cbpa.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Bonnet S., Geraert P.A., Lessire M., Carre B., Guillaumin S. Effect of high ambient temperature on feed digestibility in broilers. Poult. Sci. 1997;76:857–863. doi: 10.1093/ps/76.6.857. [DOI] [PubMed] [Google Scholar]
- Centraal Veevoederbureau Tabellenboek Veevoeding Pluimvee. Voedernormen Pluimvee en voederwaarden voedermiddelen voor Pluimvee. CVB-reeks nr. 54, november 2016. Federatie Nederlandse Diervoederketen 2016. 2016:21p. [Google Scholar]
- Chamruspollert M., Pesti G.M., Bakalli R.I. Chick responses to dietary arginine and methionine levels at different environmental temperatures. Br. Poult. Sci. 2004;45:93–100. doi: 10.1080/00071660410001668914. [DOI] [PubMed] [Google Scholar]
- Cordova-Noboa H.A., Oviedo-Rondon E.O., Sarsour A.H., Barnes J., Sapeota D., Lopez D., Gross L., Rademacher-Heilshorn M., Braun U. Effect of guanidinoacetic acid supplementation on live performance, meat quality, pectoral myopathies and blood parameters of male broilers fed corn-based diets with or without poultry by-products. Poult. Sci. 2018;97:2494–2505. doi: 10.3382/ps/pey097. [DOI] [PubMed] [Google Scholar]
- Cordova-Noboa H.A., Oviedo-Rondon E.O., Sarsour A.H., Barnes J., Ferzola P., Rademacher-Heilshorn M., Braun U. Performance, meat quality, and pectoral myopathies of broilers fed either corn or sorghum based diets supplemented with guanidinoacetic acid. Poult. Sci. 2018;97:2479–2493. doi: 10.3382/ps/pey096. [DOI] [PubMed] [Google Scholar]
- Corton J.M., Gillespie J.G., Hardie D.G. Role of the AMP-activated protein-kinase in the cellular stress-response. Curr. Biol. 1994;4:315–324. doi: 10.1016/s0960-9822(00)00070-1. [DOI] [PubMed] [Google Scholar]
- Curt M.J.C., Voicu P.M., Fontaine M., Dessein A.F., Porchet N., Mention-Mulliez K., Dobbelaere D., Soto-Ares G., Cheillan D., Vamecq J. Creatine biosynthesis and transport in health and disease. Biochimie. 2015;119:146–165. doi: 10.1016/j.biochi.2015.10.022. [DOI] [PubMed] [Google Scholar]
- Etches R.J., John T.M., Verrinder Gibbins A.M. Behavioural, physiological neuroendocrine and molecular responses to heat stress. In: Daghir N.J., editor. Poultry Production in Hot Climates. CABI International; Wallingford, Oxfordshire, UK: 2008. pp. 48–79. [Google Scholar]
- DeGroot A.A., Braun U., Dilger R.N. Efficacy of guanidinoacetic acid on growth and muscle energy metabolism in broiler chicks receiving arginine-deficient diets. Poult. Sci. 2018;97:890–900. doi: 10.3382/ps/pex378. [DOI] [PubMed] [Google Scholar]
- DeGroot A.A., Braun U., Dilger R.N. Guanidinoacetic acid is efficacious in improving growth performance and muscle energy homeostasis in broiler chicks fed arginine-deficient or arginine-adequate diets. Poult. Sci. 2019;98:2896–2905. doi: 10.3382/ps/pez036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehnhart M., Schreer A., Krone O., Jewgenow K., Krause M., Grossmann R. Measurement of plasma corticosterone and fecal glucocorticoid metabolites in the chicken (Gallus domesticus), the great carmorant (Phalagrocorax carbo), and the goshawk (Acipiter gentilis) Gen. Comp. Endocrinol. 2003;131:345–352. doi: 10.1016/s0016-6480(03)00033-9. [DOI] [PubMed] [Google Scholar]
- Dilger R.N., Bryant-Angeoni K., Payne R.L., Lemme A., Parsons C.M. Dietary guanidino acetic acid is an efficacious replacement for arginine for young chicks. Poult. Sci. 2013;92:171–177. doi: 10.3382/ps.2012-02425. [DOI] [PubMed] [Google Scholar]
- Dunnett M., Harris R.C., Dunnett C.E., Harris P.A. Plasma carnosine concentration: diurnal variation and effects of age, exercise and muscle damage. Equine Vet. J. 2002;34:283–287. doi: 10.1111/j.2042-3306.2002.tb05434.x. [DOI] [PubMed] [Google Scholar]
- EFSA Safety and efficacy of guanidinoacetic acid as feed additive for chickens for fattening. EFSA J. 2009;7:988. [Google Scholar]
- EFSA Safety and efficacy of guanidinoacetic acid for chickens for fattening, breeder hens and roosters, and pigs. EFSA J. 2016;14:4394. [Google Scholar]
- Gonzalez-Esquerra R., Leeson S. Concentrations of putrescine, spermidine, and spermine in duodenum and pancreas as affected by the ratio of arginine to lysine and source of methionine in broilers under heat stress. Poult. Sci. 2006;85:1398–1408. doi: 10.1093/ps/85.8.1398. [DOI] [PubMed] [Google Scholar]
- Heger J., Zelanka J., Machander V., Hampel D. Effects of Guanidinoacetic acid supplementation to broiler diets with varying energy content. Acta Univ. Agric. Fac. Agron. 2014;62:477–485. [Google Scholar]
- Ji H., Friedman M.I. Compensatory hyperphagia after fasting tracks recovery of liver energy status. Physiol. Behav. 1999;68:181–186. doi: 10.1016/s0031-9384(99)00173-0. [DOI] [PubMed] [Google Scholar]
- Khajali F., Lemme A., Rademacher-Heilshorn M. Guanidinoacetic acid as a feed supplement for poultry. Worlds Poult. Sci. J. 2020:1–22. [Google Scholar]
- Kodambashi Emami N., Golian A., Rhoads D.D., Mesgaran M.D. Interactive effects of temperature and dietary supplementation of arginine or guanidinoacetic acid on nutritional and physiological responses in male broiler chickens. Br. Poult. Sci. 2017;58:87–94. doi: 10.1080/00071668.2016.1257779. [DOI] [PubMed] [Google Scholar]
- Lara L.J., Rostagno M.H. Impact of heat stress on poultry production. Animals. 2013;3:356. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemme A., Gobbi R., Esteve-Garcia E. 2010. Effectiveness of creatine sources on performance of broilers at deficient or adequate methionine supply. In Proceedings of the XIII Eur. Poult. Conf. Poster113. [Google Scholar]
- Lemme A., Ringel J., Sterk A., young J.F. Proc. 6th Eur. Symp. Poult. Nutr. 2007. Supplemental guanidino acetic acid affects energy metabolism of broilers; p. 208. [Google Scholar]
- Lin H., Jiao H.C., Buyse J., Decuypere E. Strategies for preventing heat stress in poultry. Worlds Poult. Sci. J. 2006;62:71–85. [Google Scholar]
- Majdeddin M., Golian A., Kermanshahi H., Michiels J., De Smet S. Effects of methionine and guanidinoacetic acid supplementation on performance and energy metabolites in breast muscle of male broiler chickens fed corn-soybean diets. Br. Poult. Sci. 2019;60:554–563. doi: 10.1080/00071668.2019.1631447. [DOI] [PubMed] [Google Scholar]
- Majdeddin M., Golian A., Kermanshahi H., De Smet S., Michiels J. Guanidinoacetic acid supplementation in broiler chickens fed on corn-soybean diets affects performance in the finisher period and energy metabolites in breast muscle independent of diet nutrient density. Br. Poult. Sci. 2018;59:443–451. doi: 10.1080/00071668.2018.1476678. [DOI] [PubMed] [Google Scholar]
- Manhiani P.S., Northcutt J.K., Han I., Bridges W.C., Scott T.R., Dawson P.L. Effect of stress on carnosine levels in brain, breast, and thigh of broilers. Poult. Sci. 2011;90:2348–2354. doi: 10.3382/ps.2011-01426. [DOI] [PubMed] [Google Scholar]
- Michiels J., Maertens L., Buyse J., Lemme A., Rademacher M., Dierick N.A., De Smet S. Supplementation of guanidinoacetic acid to broiler diets: effects on performance, carcass characteristics, meat quality, and energy metabolism. Poult. Sci. 2012;91:402–412. doi: 10.3382/ps.2011-01585. [DOI] [PubMed] [Google Scholar]
- Michiels J., Tagliabue M.M., Akbarian A., Ovyn A., De Smet S. Oxidative status, meat quality and fatty acid profile of broiler chickens reared under free-range and severely feed-restricted conditions compared with conventional indoor rearing. Avian Biol. Res. 2014;7:74–82. [Google Scholar]
- Mottet A., Tempio G. Proc. XXV Worlds Poult. Conf. 2016, Beijing, China. 2016. Global poultry production: current state and future outlook and challenges; pp. 1–8. [Google Scholar]
- Mousavi S.N., Afsar A., Lotfollahian H. Effects of guanidinoacetic acid supplementation to broiler diets with varying energy contents. J. Appl. Poult. Res. 2013;22:47–54. [Google Scholar]
- Mujahid A., Akiba Y., Toyomizu M. Acute heat stress induces oxidative stress and decreases adaptation in young white leghorn cockerels by downregulation of avian uncoupling protein. Poult. Sci. 2007;86:364–371. doi: 10.1093/ps/86.2.364. [DOI] [PubMed] [Google Scholar]
- National Research Council . Ninth Revised Edition. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Renaudeau D., Collin A., Yahav S., de Basilio V., Gourdine J.L., Collier R.J. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal. 2012;6:707–728. doi: 10.1017/S1751731111002448. [DOI] [PubMed] [Google Scholar]
- Ringel J., Lemme A., Redshaw M., Damme K. The effects of supplemental guanidino acetic acid as a precursor of creatine in vegetable broiler diets on performance and carcass parameters. Poult. Sci. 2008;87:72. [Google Scholar]
- Seliger C., Schaerer B., Kohn M., Pendl H., Weigend S., Kaspers B., Hartle S. A rapid high-precision flow cytometry based technique for total white blood cell counting in chickens. Vet. Immunol. Immunopathol. 2012;145:86–99. doi: 10.1016/j.vetimm.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Siegert W., Rodehutscord M. The relevance of glycine and serine in poultry nutrition: a review. Br. Poult. Sci. 2019;60:579–588. doi: 10.1080/00071668.2019.1622081. [DOI] [PubMed] [Google Scholar]
- Wyss M., Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol. Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- Yahav S., Straschnow A., Plavnik I., Hurwitz S. Blood system response of chickens to changes in environmental temperature. Poult. Sci. 1997;76:627–633. doi: 10.1093/ps/76.4.627. [DOI] [PubMed] [Google Scholar]
- Yang L., Tan G.Y., Fu Y.Q., Feng J.H., Zhang M.H. Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens. Comp. Biochem. Physiol. Part C: Tox. Pharmacol. 2010;151:204–208. doi: 10.1016/j.cbpc.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Zhu W., Jiang W., Wu L.Y. Dietary L-arginine supplement alleviates hepatic heat stress and improves feed conversion ratio of Pekin ducks exposed to high environmental temperature. J. Anim. Physiol. N. 2014;98:1124–1131. doi: 10.1111/jpn.12195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.