Abstract
Reproductive failure associated with heat stress is a well-known phenomenon in poultry. High temperatures also induce various metabolic disturbances in many animals. Because the liver plays a central role in metabolism, the present study aimed to clarify the relationship between liver and reproduction in Japanese quails exposed to high temperatures. In the consecutive 20-D experimental period, quails were treated with 25°C (control) or 34°C (heat) from 12:00 to 16:00. Eggs were collected for hatching. On completion of the experimental period, quails were humanely euthanized for hormone analyses (e.g., serum and ovarian follicles). Serum metabolites were analyzed using GC/MS. Liver and ovary samples were collected for mRNA levels, histomorphology, and metabolic analysis. Ovary and oviduct weights significantly decreased after daily heat exposure. The number and weight of hierarchical follicles also decreased. Consequently, egg weight decreased. Although there was no difference in fertilization rate, chick birth weight significantly decreased in the heated group. Corticosterone and 17β-estradiol in the serum significantly increased in the heated group. Yolk corticosterone and 17β-estradiol concentration and content were higher in the heated group. Ovary sterologenic enzymes gene P450scc and estrogen receptor expression level increased. The FSH receptor decreased in heat-stressed quails. MetaboAnalyst analysis indicated that high temperature affects propanoate metabolism, beta-alanine metabolism, aspartate metabolism, and histidine metabolism. Triglyceride and cholesterol levels in the liver increased in the heated group. The heated group also had an increased mRNA expression of AGPAT5, apoptosis gene caspase3, and the immunocytokine genes IL-6 and TLR4. However, NF-κB gene expression decreased. These results suggest that high temperatures affect lipid metabolism and apoptosis and cause inflammation in the liver. High temperature induced ovarian dysfunction, which resulted in the decline of hierarchical follicle number and weight, egg weight, and chick birth weight. The increased level of 17β-estradiol suggests liver damage. Protecting liver function from damage may assist quails cope in summer.
Key words: heat stress, Japanese quail, serum metabolites, liver, ovary
Introduction
High temperature is associated with reproduction failure and has become a major challenge for domestic animals and wildlife. For example, in response to global climate change, diverse array of avian taxa are now nesting considerably earlier (<10 D) in both the United States and Britain (Butler, 2003). Declines in bird population abundance are greater in areas where the mean temperature has increased rapidly (Spooner et al., 2018). In the poultry industry, heat stress can reduce the number of eggs laid (Kirunda and Scheideler, 2001). Heat stress can also decrease ovarian weight (Rozenboim et al., 2007) and hierarchical follicle number (Mashaly et al., 2004; Star et al., 2007).
In addition to affecting reproduction, numerous studies have pointed out that physiological and biochemical characteristics change during heat stress. For example, heat stress increases apical glucose transport in the chicken jejunum (Garriga et al., 2006), and acute heat stress induces superoxide radical production in the chicken skeletal muscle (Mujahid et al., 2005). Heat stress increases the vasoconstrictor responses to 5-HT by a mechanism that involves extracellular Ca2+ influx through calcium channels (Siddegowda et al., 2007) and reduces hepatic mitochondrial respiration, reactive oxygen species production, and lipid peroxidation in broiler chickens (Yang et al., 2010). Heat stress also affects the gene expression of brain-gut neuropeptides in broiler chickens (Gallus gallus domesticus) (Lei et al., 2013).
The liver plays a central role in metabolism. The liver regulates glycogen storage and the decomposition of red blood cells. The liver is also an accessory digestive gland that produces bile, an alkaline compound, which aids the breakdown of fat. In sexually mature birds, elevated levels of estrogen indirectly regulate the rapid growth of oocytes by potentiating the synthesis of yolk precursor proteins in the liver (Schneider et al., 1998). It is reasonable to hypothesize that the liver will respond to high temperatures due to its central role in maintaining the overall metabolism of an organism.
The Japanese quail (Coturnix japonica) is considered an ideal biological and experimental specimen due to its rapid development. Quails reach sexual maturity at 6–7 wk of age. Female quails have a unique reproductive system, completing follicular development, ovulation, and fertilization continuously. There are many prehierarchical ovarian follicles, which are categorized by follicle diameter size. Prehierarchical follicles consist of small white follicles (SWFs), large white follicles (LWFs), and small yellow follicles (SYFs). According to the volume, hierarchical follicles are divided into F1, F2, and F6 (Liu and Zhang, 2008; Onagbesan et al., 2009).
In the present study, Japanese quails were used to investigate reproduction performance under heat stress. Serum metabolism and liver damage were investigated. The relationship between liver damage and reproduction in female Japanese quail exposed to heat stress was investigated.
Materials and methods
Animals and Experimental Design
Female Japanese quails (laying strain) were hatched and raised in a breeding colony in the Laboratory of Veterinary Physiology at the Tokyo University of Agriculture and Technology. Quails were feed ad libitum a commercial quail food (Quail Cosmos, Aichi, Japan) and supplied unlimited water. The quails were housed in metal cages in a controlled environment (light from 05:00 to 19:00; temperature, 23 ± 2°C; humidity, 50 ± 10%; air exchanged 20 times hourly).
At the age of 20 wk, 12 healthy female quails (with normal laying rates) were selected and randomly divided into a control and heated group. Both groups were put into a Biomulti incubator (LP-80CCFL-6CTAR, Japan). The Biomulti incubators were set with the same environmental conditions as their original cages. The heated group were exposed to 34°C for 4 h per day (12:00–16:00) for 20 consecutive days. Healthy male quails were introduced to each female from 17:00 to next day 11:00 every day during the experiment. We recorded the number of eggs and their weight daily. On the final day (day 20) of the experiment, quails were killed by decapitation after the heat treatment. The ovaries were dissected, and the 3 largest follicles of each follicular hierarchy (F1 > F2 > F3) were collected. All follicles were weighed and stored at −20°C. Weights were collected of the body, ovary, oviduct, and liver. A sample of liver tissue was fixed in 4% paraformaldehyde for 24 h and then transferred through a graded series of ethanol and xylene before being embedded in paraffin wax. Liver tissue and the ovary was collected and stored at −80°C for RT-PCR, triglyceride, and cholesterol measurements.
The ethical committee of animal experimentation of the Tokyo University of Agriculture and Technology permitted all experimental protocols.
Egg Incubation
Eggs collected during the experimental period were counted, weighed, and then stored at +15°C every day at 10:00 am. The eggs were incubated in a humidified egg incubator (Showa Furanki, Co., Ltd., Japan). Eggs were candled to assess fertilization on the 14th D of incubation. Hatched and non-hatched eggs were recorded and newborn chicks were weighed.
Hormone Measurement
Sample Preparation
Blood was collected from carotid artery within 1 h after last treatment and centrifuged at 3,500 rpm for 30 min at 4°C. Serum was separated and stored at −20°C until assayed for corticosterone and 17β-estradiol.
Small amounts of yolk (100 mg) were sampled from the inside of the ovarian follicles. Each yolk sample was diluted in 1 mL of deionized water and homogenized.
Radioimmunoassay
Serum and yolk concentrations of corticosterone were determined using a double-antibody RIA system with 125I-labeled radioligands as described previously (Taya, 1985). The antiserum against 17β-estradiol was sheep anti-17β-estradiol (GDN244), kindly provided by Dr. GD Niswender, Animal Reproduction and Biotechnology Laboratory (Colorado State University, Fort Collins, CO). The antiserum against corticosterone was goat anti-corticosterone (Qasimi et al., 2017).
RNA Extraction, Reverse Transcription (RT), and Quantitative PCR
Total RNA was extracted from the liver and ovary using an ISOGEN reagent kit (Nippon Gene, Tokyo, Japan) according to the manufacturer's instructions. The concentration and purity of the isolated total RNA were determined spectrophotometrically at 260 and 280 nm using a Nanodrop spectrophotometer (Thermo Fisher Scientific, Wilmington). Total RNA (1 μg) was reverse-transcribed to cDNA with an Omniscript Reverse Transcription kit (Takara, Tokyo, Japan) with Oligo-dT primers (Takara, Tokyo, Japan) according to the manufacturer's protocol.
Target genes and the housekeeping gene (GAPDH and β-actin) were quantified by real-time PCR using an ABI 7500 Fast and a commercial kit (SYBR Premix Ex Taq II, Takara, Tokyo, Japan). The specificity of the PCR product was verified by a melting curve and agarose gel electrophoresis. The relative concentration of mRNA was calculated using the 2−ΔΔCt method (Schmittgen and Livak, 2008). Ct values from the control samples were used as calibrators. The gene-specific primers were designed based on chicken mRNA sequences using Primer Version 5.0 (Table 1).
Table 1.
Primers used for real-time PCR analysis.
| Genes | Primer sequences | Size of PCR product (bp) | Accession no. |
|---|---|---|---|
| P450scc | F: 5′-ACGCTGGTGCAGGTTGGTCTC-3′ | 154 | XM_015872775.1 |
| R: 5′-GCTCAGTCCCTTGAAGTGCTT-3′ | |||
| 3β-HSD | F: 5′-CTGGGGAAACAGCAACAGCAG-3′ | 175 | XM_015874122.1 |
| R: 5′-TTATTTTGGTTCTGGGGATGA-3′ | |||
| P450arom | F: 5′-GAATTCTTCCCAAAACCGAATGAG-3′ | 162 | XM_015872888.1 |
| R: 5′-GCACCGTCTCAGAAGAGTCACCAG-3′ | |||
| 17β-HSD | F: 5′-TCTTGGTGTGGGAATGTCAA-3′ | 187 | XM_015849994.1 |
| R: 5′-CCGGAATAGAAGGAACACCA-3′ | |||
| ESR1 | F: 5′-GCCTGGCAGGATTTCACTCT-3′ | 154 | XM_017010382.2 |
| R: 5′-GCTTCCCTCATCCCAAAGCT-3′ | |||
| FSHR | F: 5′-CGTTCATGGGCCTGAGTTCT-3′ | 179 | XM_015856889.1 |
| R: 5′-CAAAACAACAGGCCCGATGG-3′ | |||
| LHR | F: 5′-CAGTCAACTCCTGCGCAAAC-3′ | 195 | NM_137366.4 |
| R: 5′-CCTGTGAGGCTTTACTGGGG-3′ | |||
| IL-1β | F: 5′-ACCCGCTTCATCTTCTACCG-3′ | 173 | AB559570.2 |
| R: 5′-TAGCTTGTAGGTGGCGATGT-3′ | |||
| IL-6 | F: 5′-GGTGATAAATCCCGATGAAGT-3′ | 142 | XM_015853677.1 |
| R: 5′-TCTCCATAAACGAAGTAAAGTCTC-3′ | |||
| NF-κΒ | F: 5′-TCAACGCAGGACCTAAAGACAT-3′ | 165 | NM_001279309.1 |
| R: 5′-GCAGATAGCCAAGTTCAGGATG-3′ | |||
| TLR4 | F: 5′-ATGTCCTCTTGCCATCCCAA-3′ | 192 | XM_015878841.1 |
| R: 5′-TCTCCCCTTTCTGCAGAGTG-3′ | |||
| IL-18 | F: 5′-AGCGTCCAGGTAGAAGATAA-3′ | 187 | XM_015883845.1 |
| R: 5′-AATATGATGTTACTTTCACCAGGA-3′ | |||
| IL-12β | F: 5′-TGTCTCACCTGCTATTTGCCTTAC-3′ | 158 | XM_015876070.1 |
| R: 5′-CATACACATTCTCTCTAAGTTTCCACTGT-3′ | |||
| Caspase3 | F: 5′-TGGCGATGAAGGACTCTTCT-3′ | 197 | XM_017312543.1 |
| R: 5′-CTGGTCCACTGTCTGCTTCA-3′ | |||
| AGPAT5 | F: 5′-TGCTGTTTCTAGGTGGCCTG-3′ | 184 | XM_015859300.1 |
| R: 5′-ACCCACAGGCAGCCAATTAA-3′ | |||
| GAPDH | F: 5′-TGATGCTCCCATGTTCGTGA-3′ | 149 | XM_015873412.1 |
| R: 5′-TAAGACCCTCCACGATGCC-3′ | |||
| β-actin | F: 5′-TGAACCCCAAAGCCAACAG-3′ | 201 | NM_001124235.1 |
| R: 5′-CCACAGGACTCCATACCCAAG-3′ |
Non-targeted Analysis of Low Molecular Weight Metabolites in the Serum
Serum metabolomics analysis was performed using GC/MS as described previously (Takemoto et al., 2017) with some modifications. A sample of 50 μL of serum was mixed with 5 μL of 1 mg/mL 2-isopropylmalic acid (Sigma-Aldrich, Tokyo, Japan) in distilled water as an internal standard, and 250 μL of a methanol–chloroform–water (2.5:1:1) mixture. Then, the samples were lyophilized, and 40 μL of 20 mg/mL methoxyamine hydrochloride (Sigma-Aldrich) was dissolved in pyridine for oximation. After mixing, the samples were shaken for 90 min at 30°C. Next, 20 μL of N-methyl N-trimethylsilyl-trifluoroacetamide (GL Science, Tokyo, Japan) was added for trimethylsilylation. The mixture was incubated at 37°C for 45 min. The sample was subjected to GC/MS (GCMS QP2010-Ultra; Shimadzu, Kyoto, Japan). The Shimadzu Smart Metabolites Database (Shimadzu) was used to identify metabolites. Samples were normalized by a pooled sample from the control group. A metabolic pathway analysis was performed using MetaboAnalyst (Xia and Wishart, 2011). Metabolites that significantly differed between the 2 groups were subjected to an enrichment analysis (http://www.metaboanalyst.ca/faces/upload/EnrichUploadView.xhtml).
Histomorphology
Paraffin-embedded samples were serially sectioned (5 μm thick) onto 3-aminopropyl-triethoxysilane (APES)-coated slides and stained with hematoxylin–eosin (HE). The stained sections were observed and photographed under a light microscope.
Triglyceride and Cholesterol in the Liver
Sample Preparation
One hundred milligrams of tissue was weighed and placed into a centrifuge tube containing 0.2 mL saline. The sample was homogenized, placed on ice, and then 0.8 mL chloroform-methanol mixture (1:1) was added. The sample was mixed well before incubating at 4°C for 18 h. The samples were centrifuged at 3,000 rpm for 10 min at 4°C. There were 3 clearly separated layers, the upper layer was water phase, the middle layer was tissue, and the bottom layer was the lipid phase. The lipid phase was gently collected and stored at 4°C for measuring triglyceride and cholesterol.
Triglyceride and Cholesterol Measurements
Serum and liver triglyceride levels were detected using a LabAssay Triglyceride kit (Wako, Osaka, Japan) according to the manufacturer's instructions. Cholesterol was detected using the LabAssay Cholesterol kit (Wako, Osaka, Japan) according to the manufacturer's instructions.
Statistical Analysis
All data are presented as mean ± standard error of the mean (SEM). Data from control and or heat group were analyzed by a t-test and P < 0.05 was considered to be statistically significant and designated “∗”; and P < 0.01 was designated “∗∗.” Statistical analysis was performed using GraphPad Prism software (GraphPad Software, San Diego, CA).
Results
Body and Organ Weight
Heat stress did not significantly affect the female body weight (Table 2). However, ovary and oviduct weights significantly decreased in heat-challenged quails (P < 0.05) (Table 2).
Table 2.
Body weight and tissues weight in the control group and heat challenge group.
| Control | Heat | P-value | Sig. | |
|---|---|---|---|---|
| BW/g | 145.1 ± 1.8 | 147.7 ± 4.4 | 0.2865 | N.S |
| Ovary/g | 2.98 ± 0.26 | 2.34 ± 0.106 | 0.0307 | 1 |
| Oviduct/g | 2.70 ± 0.09 | 2.32 ± 0.20 | 0.0395 | 1 |
| Liver/g | 3.05 ± 0.09 | 3.19 ± 0.11 | 0.1779 | N.S |
| Spleen/g | 0.086 ± 0.010 | 0.079 ± 0.012 | 0.3265 | N.S |
Values are expressed as mean ± SEM. Number of quails in each group is 6.
N.S. means no significant difference (P > 0.05, T test).
Significantly different from the values at control (P < 0.05, T test).
Ovarian Follicles and Egg Weights
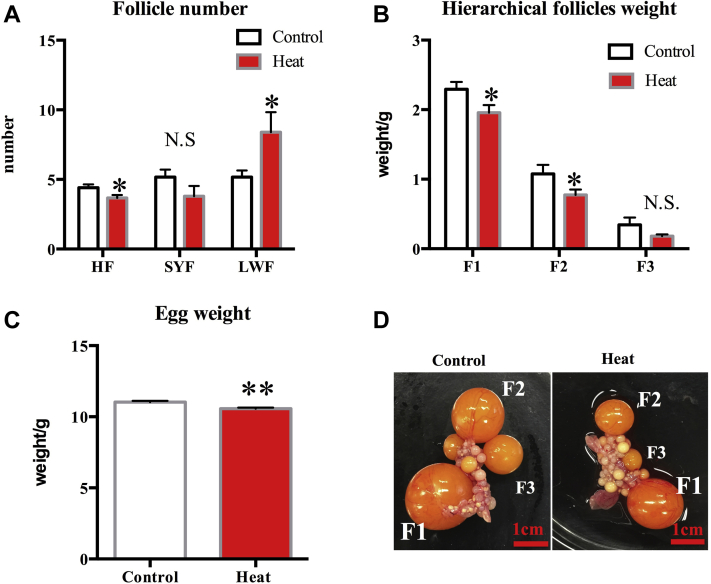
Hierarchical follicle (HF) number and weight (F1 and F2) significantly decreased in the heated group when compared with the control group (P < 0.05) (Figures 1A, 1B). In addition, egg weight also decreased in the heat-challenged quails (P < 0.01) (Figure 1C). Ovaries varied between the control and heat-stressed females (see Figure 1D). The hierarchical follicle number and size decreased in the ovary of the heated group (Figure 1D). However, the number of prehierarchical follicles (LWF) increased in the heated ovary (P < 0.05) (Figure 1A).
Figure 1.
Ovarian follicle number (A) and weight (B) in the control and heat challenge group. Egg weight in the control and heat challenge group (C). Representative photo of an ovary of control (D, left) and heat group (D, right). Hierarchical follicle (HF), small yellow follicle (SYF), large white follicle (LWF). F1, F2, F3 indicate hierarchical follicles (F1 > F2 > F3), bar = 1 cm. Each bar represents the mean ± SEM. ∗Significantly different from the values at control (P < 0.05, T test). ∗∗Significantly different from the values at control (P < 0.01, T test). N.S. means no significant difference (P > 0.05, T test).
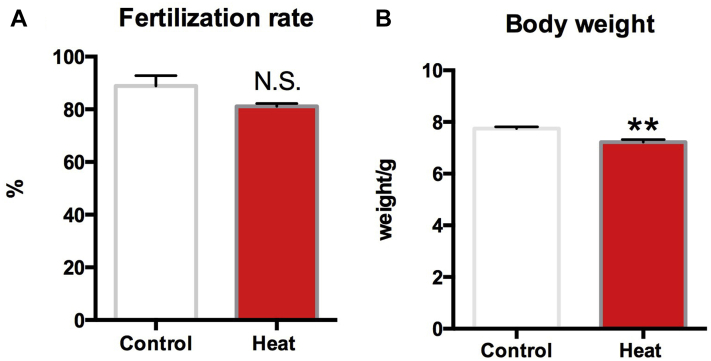
Fertilization Rate and Birth Weight
The egg fertilization rate decreased slightly in the heated group when compared with the control group, but the difference was not significant (Figure 2A). After hatching, the body weight of newborn chicks was significantly lower in the heat-stressed group when compared with the control group (P < 0.01) (Figure 2B).
Figure 2.
Egg fertilization rate (A) and newborn chicks body weight (B) in the control and the heat challenge group. Each bar represents the mean ± SEM. ∗∗Significantly different from the values at control (P < 0.01, T test). N.S. means no significant difference (P > 0.05, T test).
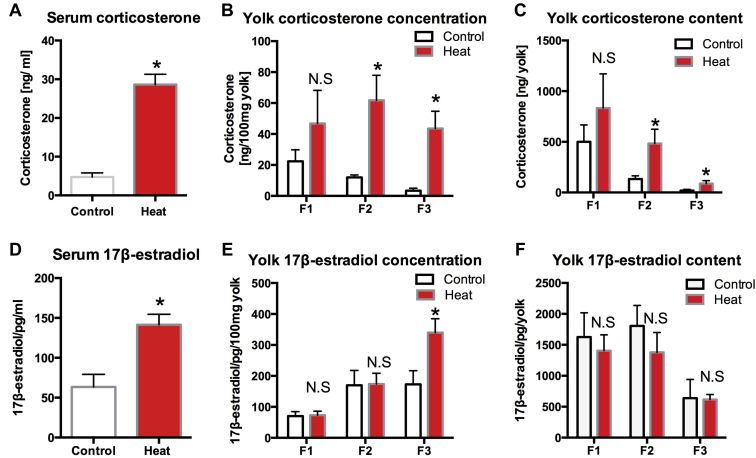
Serum and Follicular Yolk Corticosterone and 17β-Estradiol Levels
The serum corticosterone level was significantly higher in the heated group (P < 0.05) (Figure 3A). The concentration (Figure 3B) and content (Figure 3C) of yolk corticosterone was significantly influenced by heat stress. Quails subjected to heat stress possessed significantly higher corticosterone concentrations in the hierarchical follicles (P < 0.05) (F2, F3).
Figure 3.
Serum corticosterone (A) and 17β-estradiol (D) in the control and the heat challenge group. Follicular yolk corticosterone and 17β-estradiol concentration (B, E) and contents (C, F). F1, F2, F3 indicate hierarchical follicles (F1 > F2 > F3). Each bar represents the mean ± SEM. ∗Significantly different from the values at control (P < 0.05, T test). N.S. means no significant difference (P > 0.05, T test).
Serum 17β-estradiol levels were significantly higher in the heated group when compared with the control group (P < 0.05) (Figure 3D). The concentration (Figure 3E) of yolk 17β-estradiol in F3 was significantly higher in the heated group (P < 0.05). The 17β-estradiol content (Figure 3F) was not significantly different (P > 0.05).
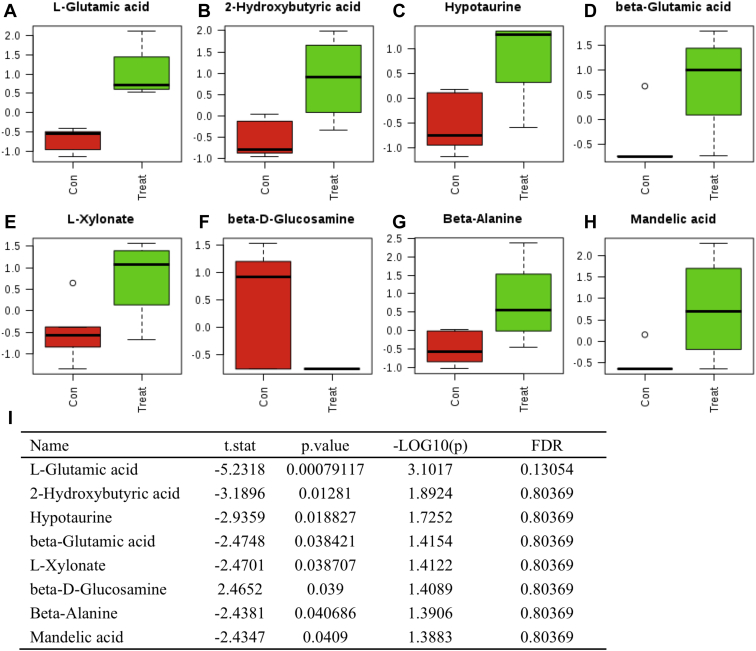
Metabolites and Metabolic Pathways Affected by Heat
A total of 165 metabolites were identified in the serum when a non-target analysis was performed. There were significant differences between the control and heated group in the concentration of 8 metabolites (Figure 4). The 8 metabolites were in a significantly higher concentration in the heat-stressed group; they were L-glutamic acid, 2-hydroxybutyric acid, hypotaurine, beta-glutamic acid, L-xylose, beta-d-glucosamine, beta-alanine, and mandelic acid.
Figure 4.
Eight serum metabolites significantly changed in the control (Con) compared with the heat (Treat) group. (A) L-glutamic acid, (B) 2-hydroxybutyric acid, (C) hypotaurine, (D) beta-glutamic acid, (E) L-xylonate, (F) beta-D-glucosamine, (G) beta-alanine, (H) mandelic acid). (I) t. stat and P value of each metabolites (P < 0.05, T test).
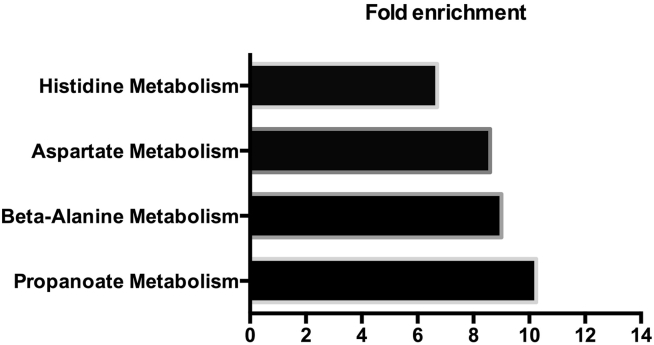
MetaboAnalyst analysis indicated that propanoate metabolism, beta-alanine metabolism, aspartate metabolism, and histidine metabolism were affected by heat stress (Figure 5).
Figure 5.
Metabolic pathway affected in the serum of quails after the heat challenge. Significantly different serum metabolites content between the control group and heat-challenged group is subjected to the enrichment analysis of MetaboAnalyst. Metabolic pathway significantly affected by the high temperature and their fold-enrichment are shown.
Effect of Heat Exposure on the Ovary
Ovary Sterologenic Enzyme Gene Expression
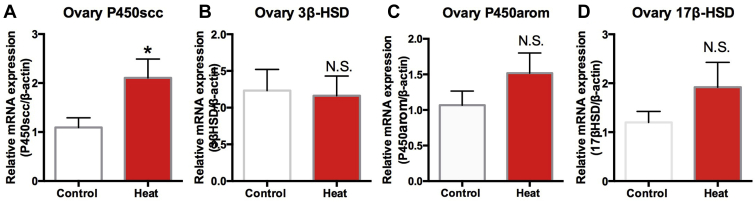
Ovary P450scc significantly increased in the heat group when compared with the control group (P < 0.05) (Figure 6A). Ovary 3β-HSD (Figure 6B), P450arom (Figure 6C), and 17β-HSD (Figure 6D) were not significantly different between the 2 groups (P > 0.05).
Figure 6.
Expression of sterologenic enzymes genes in ovary of control and heat groups. Each mRNA was normalized to the β-actin mRNA expression level in the same preparation, and the mean of each experimental control was assigned a value of 1.0. Ovary sterologenic enzymes genes P450scc (A), 3β-HSD (B), P450arom (C), and 17β-HSD (D). The values shown are the mean ± SEM of 6 animals per group. ∗Significantly different from the values at control (P < 0.05, T test). N.S. means no significant difference (P > 0.05, T test).
Ovarian Receptor Expression of Estrogen, FSH, and LH
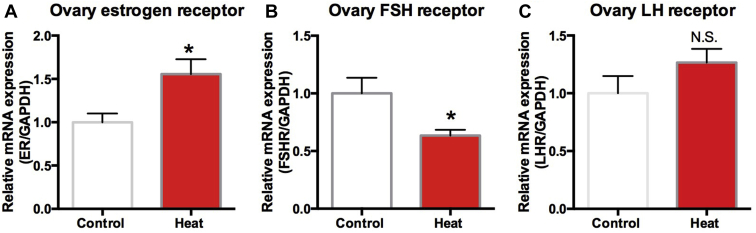
Ovarian estrogen receptor α mRNA level significantly increased in the heated group (P < 0.01) (Figure 7A). Conversely, ovarian FSH receptor expression significantly decreased in the heated group (P < 0.05) (Figure 7B). There was no significant difference in LH receptor gene expression between the control and the heated groups (P > 0.05) (Figure 7C).
Figure 7.
Expression of ovarian receptor mRNA expression of estrogen (A), FSH (B), and LH (C) in the control and the heat group. Each mRNA was normalized to the GAPDH mRNA expression level in the same preparation, and the mean of each experimental control was assigned as value of 1.0. The values shown are the mean ± SEM of 6 animals per group. ∗Significantly different from the values at control (P < 0.05, T test). N.S. means no significant difference (P > 0.05, T test).
Effect of Heat Stress on the Liver
Histomorphology Analysis of the Liver
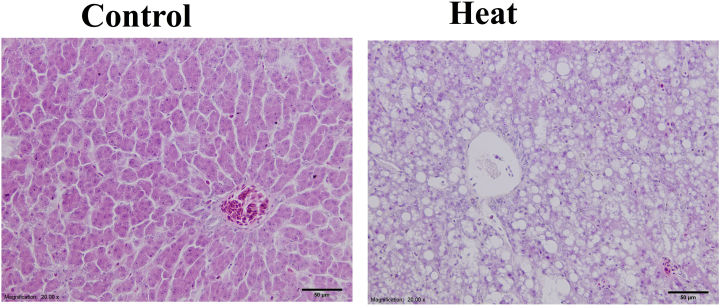
To investigate the effects of heat stress on the liver structure, we examined morphological changes in quail livers. Typical morphological changes in lipid steatosis were detected in the livers of the heated group (Figure 8, image on the right). This is the histologic appearance of hepatic macrovesicular steatosis (fatty change). Lipid accumulates in hepatocytes as vacuoles. The vacuoles have a clear appearance with HE staining.
Figure 8.
Hematoxylin–eosin (HE) staining of sections derived from Japanese quail liver tissues in the control group (left) and the heat group (right). Bar = 50 μm.
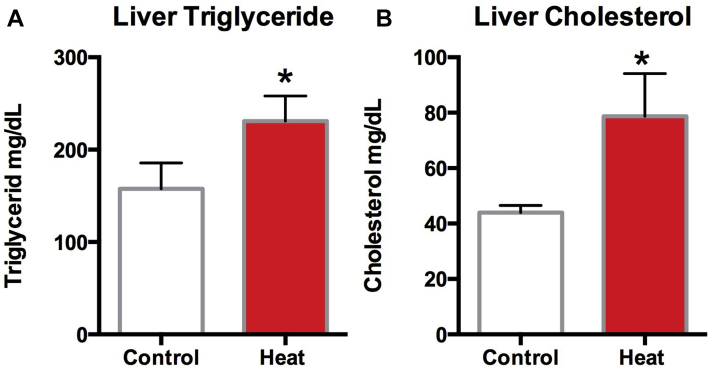
Liver Triglyceride and Cholesterol Levels
Liver triglyceride and cholesterol levels were measured, both were found to be significantly higher in the liver of heat-stressed female quails (P < 0.05) (Figures 9A, 9B).
Figure 9.
Liver triglyceride and cholesterol levels of the control and the experimental group. Liver was homogenized and made with the final concentration of 10%, and then measured by assay kit. Liver triglyceride (A) and liver cholesterol (B). Each bar represents the mean ± SEM. ∗Significantly different from the values at control (P < 0.05, T test).
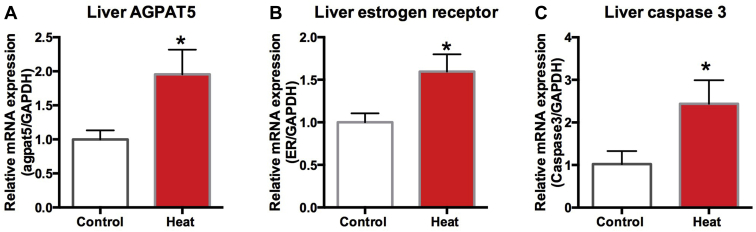
AGPAT5, Estrogen Receptor, and Caspase3 Gene Expression in the Liver
The liver lipid metabolism gene AGPAT5 (Figure 10A), estrogen receptor (Figure 10B), and caspase3 (Figure 10C) were significantly increased in the heat-treated quails (P < 0.05).
Figure 10.
Expression of 1-acylglycerol-3-phosphate O-acyltransferase 5 (AGPAT5) (A), estrogen receptor (B), and caspase3 gene expression in liver of the control and the experimental group. Each mRNA was normalized to the GAPDH mRNA expression level in the same preparation, and the mean of the control was assigned as value of 1.0. The values shown are the mean ± SEM. ∗Significantly different from the values at control (P < 0.05, T test).
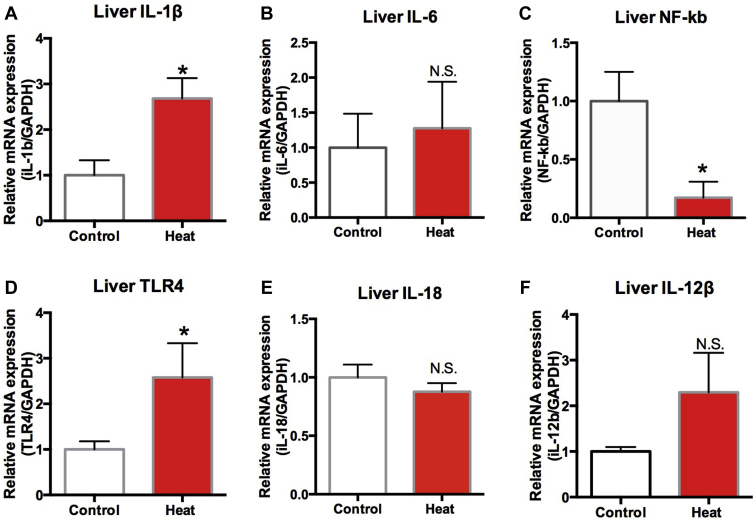
Immunocytokines Gene Expression in the Liver
Several immunocytokine expression levels differed between the 2 groups. IL-1β and TLR4 mRNA levels were significantly higher in the heated group (P < 0.05) (IL-1β is illustrated in Figure 11A and TLR4 is illustrated in Figure 1D). However, NF-κb (Figure 11C) mRNA levels were significantly decreased in the heated group (P < 0.05).
Figure 11.
Expression of lipid metabolic gene expression in liver of the control and the experimental groups. Each mRNA was normalized to the GAPDH mRNA expression level in the same preparation, and the mean of each experimental control was assigned as value of 1.0. (A) liver IL-1β, (B) liver IL-6, (C) liver NF-κb, (D) TLR4, (E) IL-18, (F) IL-12β. The values shown are the mean ± SEM. ∗Significantly different from the values at control (P < 0.05, T test). N.S. means no significant difference (P > 0.05, T test).
Discussion
High ambient temperatures influenced Japanese quail reproduction. Liver damage induced by the heat aggravated this situation. In the present study, decreased ovary and oviduct weight were observed in quails exposed to the high temperature (Table 2). Hierarchical follicles number and weight decreased with heat stress (Figures 1A, 1B). Previous data indicated that decreased egg weight was due to a decrease in yolk weight. The decrease in egg weight (Figure 1C) could result from smaller hierarchical follicles. Rozenboim et al. (2007) found heat exposure caused significant hyperthermia and reduced egg production, egg weight, ovarian weight, and the number of large follicles, which is confirmed by our results. High temperature induces infertility in farm animals and represents a major source of economic loss to the livestock sector. Fertilization rate slightly decreased in the heated group when compared with the control group (Figure 2A). Sahin et al. pointed out that the decrease in animal fertility was caused by an elevated body temperature that influences ovarian functions, including estrous behavior, oocyte health, and embryonic development (Sahin and Kucuk, 2003).
Chick hatch weight significantly decreased when laying females were heat stressed (Figure 2B). Embryo development is based on avian egg nutrients; body weight at hatching was positively correlated with egg weight (Sklan et al., 2003; Ulmer-Franco et al., 2010). The decreased egg weight should be responsible for the lighter body weight at hatching. In addition, the different egg composition in heat-stressed females might suggest heat stress has an effect on embryo development.
According to our results, serum corticosterone and 17β-estradiol increased after heat stress. Follicular yolk corticosterone and 17β-estradiol showed the same tendency. Corticosterone was found at levels 2 to 3 times higher in the heated group (Figure 3). Maternal corticosterone in birds can be transferred to the developing embryo via the egg (Saino et al., 2005). Prenatal corticosterone exposure is known to have both short- and long-term consequences (Love and Williams, 2008), such as a decreased hatch weight (Janczak et al., 2006) and compromised immunity (Rubolini et al., 2005). Our pervious study found that a 10-D heat challenge decreased serum 17β-estradiol (data available upon request). However, the present study indicated a 20-D heat challenge increased 17β-estradiol (Figure 3D). There are many reports that heat stress induced apoptosis in granulosa cells (GCs) and 17β-estradiol secretion decrease (Rozenboim et al., 2007; Li et al., 2016). However, serum 17β-estradiol increased in the present study (Figure 3D). Prehierarchical follicles number increased in the heated ovary. Conversely, the hierarchical follicles number and size as well as the oviduct weight showed an overall decline in the heated quails.
To further our understanding, a non-targeted analysis of low molecular weight metabolites in the serum was conducted. The present study explored metabolite content in the serum of quail under a 20-D heat challenge. Metabolites are intermediates or products of different metabolic pathways. Therefore, their concentrations could be influenced by innate genetic predisposition, environmental exposures or stimuli, as well as interactions between the 2. Unlike genomics, metabolome represents the organisms' conditions at any given time and thus is able to capture the dynamic physiological condition corresponding to the behavioral and clinical outcomes of interests (Chen et al., 2015). A MetaboAnalyst analysis revealed that (1) L-glutamic acid, 2-hydroxybutyric acid, hypotaurine, beta-glutamic acid, L-xylonate, beta-D-glucosamine, beta-alanine, and mandelic acid were increased by the heat challenge, and (2) propanoate metabolism, D-glutamine and D-glutamate metabolism, taurine, and hypotaurine metabolism were affected. Because the liver plays an important role in the increase of metabolite content, an increase in liver triglyceride and cholesterol levels, as well as lipid metabolic gene expression suggested that the heat challenge caused a disturbance in some metabolic pathways (such as lipid metabolism in the liver).
Steatosis occurred in livers of the heat-challenged quails (Figure 8B), with the increased liver triglyceride and cholesterol. To support high energetic demands for reproduction, female mammals display plasticity in many physiological processes, such as the lipid transport system. Abnormal propanoate metabolism indicates lipid metabolic abnormality (Yang et al., 2009). In avian species, lipids (especially triglyceride) are stored in adipocytes, hepatocytes, and growing oocytes (Hermier, 1997). When hepatic lipogenesis exceed the capacity of very low-density lipoprotein (VLDL) secretion, triglycerides accumulated in the liver, causing a steatosis (Hermier, 1997). AGAPT5 increased in the liver after heat stress. Acylglycerophosphate acyltransferases/lysophosphatidic acid acyltransferases (AGPAT) are categorized as a homologous family of enzymes that catalyze the formation of phosphatidic acid (PtdOH). Parks et al. (2015) conducted antisense oligonucleotides (ASOs) to silence AGPAT5 expression in the liver and adipose tissue and identify AGPAT5 as a gene associated with plasma insulin levels and insulin resistance. An increased AGAPT5 level was observed (Figure 10A), and liver triglyceride and cholesterol increased in the heated group (Figures 9A, 9B). This result suggests steatosis in the heated quails. Consequently, the liver showed apoptosis (Figure 10C) together with inflammation (Figure 11).
NF-κB activation in the liver could have both antiapoptotic and proliferative effects. Mice deficient in the p65 subunit of NF-κB die during gestation from hepatocyte apoptosis (Beg et al., 1995). Excessive or sustained activation of TLR4 induces an inflammatory response.
In the present study, a decrease in hierarchical follicle weight (Figure 1B) and quail birth weight (Figure 2B) can be induced by steatosis in the liver. Egg production causes major changes in the metabolism of lipids to meet the demands of yolk formation (Richards et al., 2003). Liver damage induces lipid metabolism dysfunction, which is associated with vitellogenesis and further development of the embryo. Embryo development is based on avian egg nutrients causing different egg sizes and compositions (Tůmová et al., 2009). Body weight at hatching was positively correlated with egg weight (Ulmer-Franco et al., 2010).
17β-estradiol was dramatically higher in the heated group (Figure 3D). However, the ovary and oviduct were lighter than those of the control group (Table 2). This indicated that increased 17β-estradiol did not show a beneficial effect on reproduction. Estrogen receptor gene expression increased in the ovary (Figure 6A) and liver (Figure 9C). This outcome could represent that the FSH level was decreased by increased estrogen, according to negative feedback in the hypothalamic-pituitary-ovarian axis. The decreased FSH receptor gene expression supported our hypothesis. A follicle in each ovulatory cycle selected into the hierarchical follicle from a growing small white follicle is dependent on FSH (Johnson, 2014). The decreased number of hierarchical follicles (Figure 1A) in the present study may be due to decreased FSH.
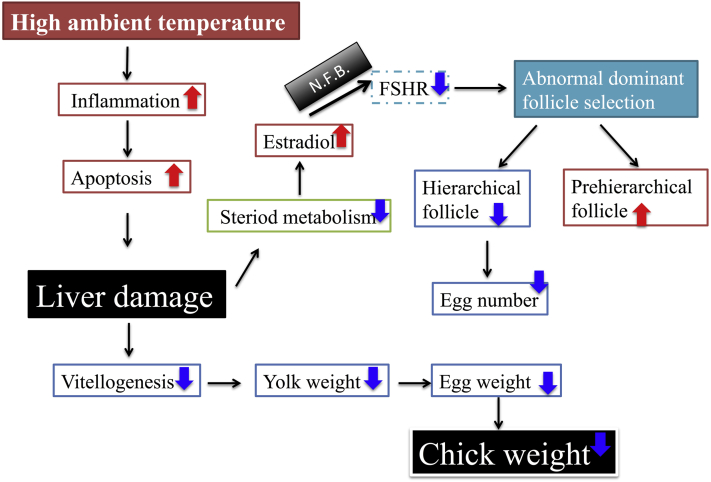
Figure 12 shows a possible mechanism underlying the effect of high ambient temperature on female quail reproduction. Liver damage and abnormal lipid metabolism induced by heat stress affect steroid metabolism and vitellogenesis. Consequently, egg weight and egg number decreased. Protection of the liver under high temperatures could be one solution for coping with high summer temperatures.
Figure 12.
Schema showing the relation between liver damage and reproduction in female Japanese quail exposed to high ambient temperature. N.F.B. means negative feedback, and FSHR means follicle-stimulating hormone receptor.
Acknowledgments
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Beg A.A., William C.S., Bronson R.T., Ghosh S., Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- Butler C.J. The disproportionate effect of global warming on the arrival dates of short-distance migratory birds in North America. IBIS. 2003;145:484–495. [Google Scholar]
- Chen H.H., Tseng Y.J., Wang S.Y., Tsai Y.S., Chang C.S., Kuo T.C., Yao W.J., Shieh C.C., Wu C.H., Kuo P.H. The metabolome profiling and pathway analysis in metabolic healthy and abnormal obesity. Int. J. Obes. 2015;39:1241. doi: 10.1038/ijo.2015.65. [DOI] [PubMed] [Google Scholar]
- Garriga C., Hunter R.R., Amat C., Planas J.M., Mitchell M.A., Moretó M. Heat stress increases apical glucose transport in the chicken jejunum. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R195–R201. doi: 10.1152/ajpregu.00393.2005. [DOI] [PubMed] [Google Scholar]
- Hermier D. Lipoprotein metabolism and fattening in poultry. J. Nutr. 1997;127:805S–808S. doi: 10.1093/jn/127.5.805S. [DOI] [PubMed] [Google Scholar]
- Janczak A., Braastad B., Bakken M. Behavioural effects of embryonic exposure to corticosterone in chickens. Appl. Anim. Behav. Sci. 2006;96:69–82. [Google Scholar]
- Johnson A.L. The avian ovary and follicle development: some comparative and practical insights. Turk. J. Vet. Anim. Sci. 2014;38:660–669. [Google Scholar]
- Kirunda D., Scheideler S. The efficacy of vitamin E (DL-α-tocopheryl acetate) supplementation in hen diets to alleviate egg quality deterioration associated with high temperature exposure. Poult. Sci. 2001;80:1378–1383. doi: 10.1093/ps/80.9.1378. [DOI] [PubMed] [Google Scholar]
- Lei L., Hepeng L., Xianlei L., Hongchao J., Hai L., Sheikhahmadi A., Yufeng W., Zhigang S. Effects of acute heat stress on gene expression of brain–gut neuropeptides in broiler chickens (Gallus gallus domesticus) J. Anim. Sci. 2013;91:5194–5201. doi: 10.2527/jas.2013-6538. [DOI] [PubMed] [Google Scholar]
- Li L., Wu J., Luo M., Sun Y., Wang G. The effect of heat stress on gene expression, synthesis of steroids, and apoptosis in bovine granulosa cells. Cell Stress Chaperones. 2016;21:467–475. doi: 10.1007/s12192-016-0673-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Zhang C. Effects of daidzein on messenger ribonucleic acid expression of gonadotropin receptors in chicken ovarian follicles. Poult. Sci. 2008;87:541–545. doi: 10.3382/ps.2007-00274. [DOI] [PubMed] [Google Scholar]
- Love O.P., Williams T.D. The adaptive value of stress-induced phenotypes: effects of maternally derived corticosterone on sex-biased investment, cost of reproduction, and maternal fitness. Am. Nat. 2008;172:E135–E149. doi: 10.1086/590959. [DOI] [PubMed] [Google Scholar]
- Mashaly M., Hendricks G., 3rd, Kalama M., Gehad A., Abbas A., Patterson P. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult. Sci. 2004;83:889–894. doi: 10.1093/ps/83.6.889. [DOI] [PubMed] [Google Scholar]
- Mujahid A., Yoshiki Y., Akiba Y., Toyomizu M. Superoxide radical production in chicken skeletal muscle induced by acute heat stress. Poult. Sci. 2005;84:307–314. doi: 10.1093/ps/84.2.307. [DOI] [PubMed] [Google Scholar]
- Onagbesan O., Bruggeman V., Decuypere E. Intra-ovarian growth factors regulating ovarian function in avian species: a review. Anim. Reprod. Sci. 2009;111:121–140. doi: 10.1016/j.anireprosci.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Parks B.W., Sallam T., Mehrabian M., Psychogios N., Hui S.T., Norheim F., Castellani L.W., Rau C.D., Pan C., Phun J. Genetic architecture of insulin resistance in the mouse. Cell Metab. 2015;21:334–346. doi: 10.1016/j.cmet.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qasimi M.I., Nagaoka K., Watanabe G. The effects of phytosterols on the sexual behavior and reproductive function in the Japanese quail (Coturnix coturnix japonica) Poult. Sci. 2017;96:3436–3444. doi: 10.3382/ps/pex161. [DOI] [PubMed] [Google Scholar]
- Richards M.P., Poch S.M., Coon C.N., Rosebrough R.W., Ashwell C.M., McMurtry J.P. Feed restriction significantly alters lipogenic gene expression in broiler breeder chickens. J. Nutr. 2003;133:707–715. doi: 10.1093/jn/133.3.707. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Tako E., Gal-Garber O., Proudman J., Uni Z. The effect of heat stress on ovarian function of laying hens. Poult. Sci. 2007;86:1760–1765. doi: 10.1093/ps/86.8.1760. [DOI] [PubMed] [Google Scholar]
- Rubolini D., Romano M., Boncoraglio G., Ferrari R.P., Martinelli R., Galeotti P., Fasola M., Saino N. Effects of elevated egg corticosterone levels on behavior, growth, and immunity of yellow-legged gull (Larus michahellis) chicks. Horm. Behav. 2005;47:592–605. doi: 10.1016/j.yhbeh.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Sahin K., Kucuk O. Zinc supplementation alleviates heat stress in laying Japanese quail. J. Nutr. 2003;133:2808–2811. doi: 10.1093/jn/133.9.2808. [DOI] [PubMed] [Google Scholar]
- Saino N., Romano M., Ferrari R.P., Martinelli R., Møller A.P. Stressed mothers lay eggs with high corticosterone levels which produce low-quality offspring. J. Exp. Zool. A Comp. Exp. Biol. 2005;303:998–1006. doi: 10.1002/jez.a.224. [DOI] [PubMed] [Google Scholar]
- Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C T method. Nat. Protoc. 2008;3:1101. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schneider W., Osanger A., Waclawek M., Nimpf J. Oocyte growth in the chicken: receptors and more. Biol. Chem. 1998;379:965–971. [PubMed] [Google Scholar]
- Siddegowda Y.K.B., Leo M.D.M., Kumar D., Hooda O.K., Prakash V.R., Mishra S.K. Influence of heat stress on the reactivity of isolated chicken carotid artery to vasoactive agents. Exp. Physiol. 2007;92:1077–1086. doi: 10.1113/expphysiol.2007.038844. [DOI] [PubMed] [Google Scholar]
- Sklan D., Heifetz S., Halevy O. Heavier chicks at hatch improves marketing body weight by enhancing skeletal muscle growth. Poult. Sci. 2003;82:1778–1786. doi: 10.1093/ps/82.11.1778. [DOI] [PubMed] [Google Scholar]
- Spooner F.E., Pearson R.G., Freeman R. Rapid warming is associated with population decline among terrestrial birds and mammals globally. Global Change Biol. 2018;24 doi: 10.1111/gcb.14361. [DOI] [PubMed] [Google Scholar]
- Star L., Nieuwland M., Kemp B., Parmentier H. Effect of single or combined climatic and hygienic stress on natural and specific humoral immune competence in four layer lines. Poult. Sci. 2007;86:1894–1903. doi: 10.1093/ps/86.9.1894. [DOI] [PubMed] [Google Scholar]
- Takemoto S., Tomonaga S., Funaba M., Matsui T. Effect of long-distance transportation on serum metabolic profiles of steer calves. Anim. Sci. J. 2017;88:1970–1978. doi: 10.1111/asj.12870. [DOI] [PubMed] [Google Scholar]
- Taya K. Radioimmunoassay for progesterone, testosterone and estradiol17β usingˆ< 125> I-iodohistamine radiolligands. Jpn. J. Anim. Reprod. 1985;31:186–197. [Google Scholar]
- Tůmová E., Skřivan M., Englmaierová M., Zita L. The effect of genotype, housing system and egg collection time on egg quality in egg type hens. Czech J. Anim. Sci. 2009;54:17–23. [Google Scholar]
- Ulmer-Franco A., Fasenko G., O’Dea Christopher E. Hatching egg characteristics, chick quality, and broiler performance at 2 breeder flock ages and from 3 egg weights. Poult. Sci. 2010;89:2735–2742. doi: 10.3382/ps.2009-00403. [DOI] [PubMed] [Google Scholar]
- Xia J., Wishart D.S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 2011;6:743. doi: 10.1038/nprot.2011.319. [DOI] [PubMed] [Google Scholar]
- Yang L., Tan G.Y., Fu Y.Q., Feng J.H., Zhang M.H. Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010;151:204–208. doi: 10.1016/j.cbpc.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Yang X., Deignan J.L., Qi H., Zhu J., Qian S., Zhong J., Torosyan G., Majid S., Falkard B., Kleinhanz R.R. Validation of candidate causal genes for obesity that affect shared metabolic pathways and networks. Nat. Genet. 2009;41:415. doi: 10.1038/ng.325. [DOI] [PMC free article] [PubMed] [Google Scholar]