Abstract
Background and purpose
Magnetic Resonance (MR)-only planning has been implemented clinically for radiotherapy of prostate cancer. However, fewer studies exist regarding the overall success rate of MR-only workflows. We report on successes and challenges of implementing MR-only workflows for prostate.
Materials and methods
A total of 585 patients with prostate cancer underwent an MR-only simulation and planning between 06/2016 – 06/2018. MR simulation included images for contouring, synthetic-CT generation and fiducial identification. Workflow interruptions occurred that required a backup CT, a re-simulation or an update to our current quality assurance (QA) process. The challenges were prospectively evaluated and classified into synthetic-CT generation, motion/artifacts in the MRs, fiducial QA and bowel preparation guidelines.
Results
MR-only simulation was successful in 544 (93.2%) patients. In seventeen patients (2.9%), reconstruction of synthetic-CT failed due to patient size, femur angulation, or failure to determine the body contour. Twenty-four patients (4.1%) underwent a repeat/backup CT scan because of artifacts on the MR such as image blur due to patient motion or biopsy/surgical artifacts that hampered identification of the implanted fiducial markers. In patients requiring large coverage due to nodal involvement, inhomogeneity artifacts were resolved by using a two-stack acquisition and adaptive inhomogeneity correction. Bowel preparation guidelines were modified to address frequent rectum/gas issues due to longer MR scan time.
Conclusions
MR-only simulation has been successfully implemented for a majority of patients in the clinic. However, MR-CT or CT-only pathway may still be needed for patients where MR-only solution fails or patients with MR contraindications.
Keywords: MR-only simulation, Prostate cancer, MRCAT, Synthetic CT
1. Introduction
Multiple studies have shown the superiority of Magnetic Resonance Imaging (MRI) for target and normal tissue segmentation as well as to reduce interobserver variability for contouring compared with computed tomography (CT) [1], [2]. Although incorporating MRI decreases over-segmentation of structures as compared with CT-based segmentation, a combined CT + MRI method is challenging due to errors introduced by mis-registration of the image sets and the changes to the shape and location of the soft tissues e.g., bladder, rectum, and seminal vesicles, that are inherent when acquiring multiple image sets [3], [4], [5]. An MRI-only workflow can potentially eliminate the use of an additional CT scan and help in reducing systematic registration uncertainty [6]. Use of hybrid technologies such as MR-linear accelerator devices (MR-linac) and MR-Positron emission tomography (MR-PET) as well as integration of multiparametric MRI provides further argument for an MR-only workflow for prostate [7], [8], [9], [10], [11], [12]. Other reported advantages of MR-only include cost advantage in terms of scanner and personnel resources as well as lower imaging doses by eliminating as additional CT scan [13], [14], [15]. However, more evidence is needed to confirm the overall cost-benefit of implementing this new technology in the clinic in terms of quality assurance (QA), personnel training, synthetic CT generation as well as clinical outcomes.
A recent review article on the clinical implementation of pelvic MRI-only planning concluded that MR-only planning has been implemented clinically for prostate cancer treatments [16]. Many studies have reported on the validation of synthetic CT generation using commercial or in-house software in terms of dosimetric and positional accuracy [17], [18], [19], [20], [21], [22]. However, fewer studies have reported on the details of clinical workflows and implementation as well as challenges encountered in implementing these workflows. Some groups have prospectively assessed the feasibility of MR-only workflow with a limited number of patients, with a backup CT and investigated acceptance criteria for dosimetric accuracy as well as fiducial identification [23], [24], [25]. Christiansen et al reported on the verification of an end-to-end MR-only workflow using one patient. They reported failure of synthetic CT during their validation step and attributed to the ‘sanity check’ of the commercial algorithm [24]. Kerkmeijer et al have reported on the clinical implementation of MR-only workflow after validation on 14 prostate patients. However, the study is focused more on the validation of MR-only components rather than the workflow details [25]. Tenhunen et al have reported a success rate of 92% in their MR-only workflow based on 400 patients. 8% of patients requiring CT were due to image artifacts due to hip prosthesis, uncertainties in gold marker visualization or body contour reaching out of FOV [13]. The group also presented early clinical data between an MR only cohort and dual-modality cohort and found that early PSA response in the MRI-only planned group showed similar treatment results compared with the CT planned group and with an equal toxicity level which is very encouraging.
Our group previously reported on the details on MR-only clinical workflow implementation for prostate cancer [26]. In this study we aim to present successes and challenges with MR-only planning for prostate using a larger patient cohort and the integration of this new technology and workflow within a high-volume clinical practice, highlighting the technical challenges, workflow process and solutions.
2. Materials and methods
2.1. Patient statistics
A total of 585 prostate cancer patients were simulated with MR-only planning between June 2016 and June 2018. The study was conducted under MSKCC IRB approved retrospective protocol number 16–682. Out of these, 318 patients were treated with hypofractionated regimens (800 cGy × 5 alone, 500 cGy × 5 combined with brachytherapy, or 600 cGy × 5 for residual disease) while the remaining patients were treated using a moderate hypofractionation (270 cGy × 26) or a standard fractionation (180 cGy × 40) prescription. The clinical parameters of the patient population are included in Table 1 in supplementary documents.
2.2. MR-only workflow
All prostate patients receiving external beam radiation therapy (EBRT) were scheduled to undergo MR-only simulation in the supine position. A thermoplastic immobilization mold extending from the abdomen to mid-thigh indexed to the table was made in CT suite to allow more efficient use of our MR simulation resource. At the same time, a set of orthogonal CT scout images were acquired to perform fiducial QA on the MRs as described later. The CT scout images were also used to assess patient size, MR-only contraindications (eg implant) and LDR brachytherapy seeds. All patients were instructed to follow a full bladder (1 cup/45 min) and empty rectum protocol for simulation. A Foley catheter for definition of urethra was used for MR simulation for patients undergoing stereotactic body radiation therapy (SBRT) as well as patients receiving external beam radiotherapy boost after brachytherapy. Three gold fiducial markers of 3 mm length and 1.2 mm diameter were implanted into the prostate under ultrasound guidance roughly two weeks prior to simulation.
The MR acquisition included images for contouring (2D T2w axial, coronal, sagittal), synthetic-CT generation using a dual echo 3D fast field echo (FFE)-based mDixon and fiducial identification using a 3D balanced FFE-based as previously described [26]. As part of an IRB-approved study involving identification of a dominant intraprostatic lesion (DIL), a subset of SBRT patients underwent diffusion-weighted imaging (DWI) as part of their MR simulation procedure. A prescription of 800 cGy x5 with a boost of 900 cGy x5 to the DIL was planned for these patients. Imaging QA was performed for each patient by the technologist following each series to allow for re-acquisition if artifacts or issues occurred and documented as part of MR QA questionnaire. Qualitative QA of the fiducial markers was also performed at the same time. Physics team was notified immediately, and the patients sent for a CT scan if the issues could not be resolved by reacquisition. Synthetic- CT (Syn-CT) was generated at the console using MRCAT (MR for Calculating Attenuation) commercial software that uses a single mDixon based MR sequence along with a constrained shape model to estimate body contour as well as segment bone structures [27]. Post-processing of the mDixon images results in classification of five different tissue types – air, fat, water, soft/spongy bone and hard/cortical bone. Electron densities are assigned to each tissue type and synthetic CT is generated. Dosimetric evaluation and clinical workflow implementations using MRCAT have already been described by our group and others [17], [22], [25], [28], [29]. Automatic failure detection modes, also called ‘sanity checks’, such as presence of hip prosthesis, large patient separation and deviation from the bone model boundary conditions are built into the MRCAT algorithm to detect problems with tissue-type classification and prevent MRCAT syn-CT generation.
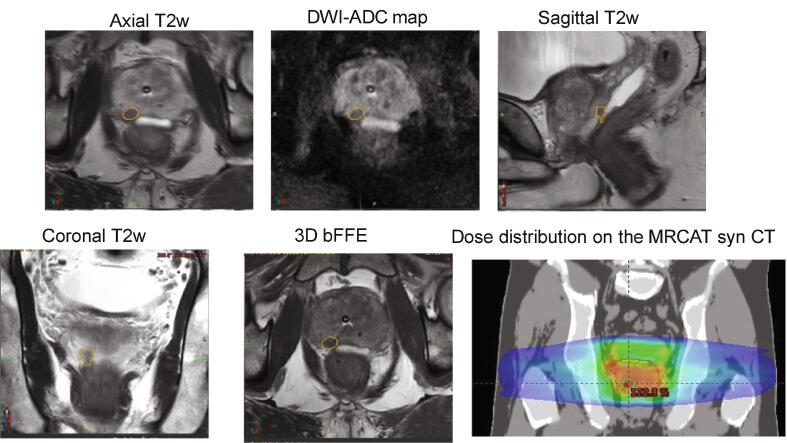
MR images including syn-CTs were used for contouring and aligned with respect to the fiducials using automated workflows generated in MIMTM (MIM Software, Inc.). Additional workflows for automatically generating planner structures were also created. An MR-only workflow in MIMTM was also used to support delineation of DIL using multiparametric MRIs such as apparent diffusion coefficient (ADC) maps derived from DWI as shown in Fig. 1. Dominant lesions were contoured on the axial T2w MRI with reference to DWI image and ADC maps by expert radiologists. Part of the contouring workflow included fiducial QA by the planners. To ensure accurate identification and localization of the prostate fiducials on the MRs, the fiducial locations on MR were compared to those from orthogonal CT scouts acquired during the immobilization mold-making step in the CT suite. Patient setup for treatment was achieved by matching the MRCAT DRRs/MRs with the orthogonal KV radiographs/CBCT based on either fiducial ROIs (for patients with intact prostate) or bones (for patients with prostate bed). Further details of our clinical workflows are described in our previous publication [26].
Fig. 1.
MR-only workflow used to contour and plan dominant intraprostatic lesion (DIL). Anatomical (axial, coronal, sagittal T2 w MRI,3D bFFE goldseed) and apparent diffusion coefficient (ADC) maps derived from diffusion-weighted MRI are used to delineate dominant lesion. A Rx of 800x5 cGy with a boost of 900x5 cGy is planned.
Workflow interruptions and challenges occurred for a few prostate cases during MR-only simulation, treatment planning and treatment localization/delivery that required a backup CT, a re-simulation or an update to our current QA process respectively. All the challenges were prospectively reported. The issues encountered ultimately led to changes in our workflow, the vendor’s software upgrades, or both and are described. The challenges were classified into simulation and treatment planning related as: (i) challenges with synthetic CT generation (ii) motion/artifacts in the MRs (iii) bowel preparation guideline and (iv) fiducial QA
3. Results
MR-only workflow worked seamlessly for 544 patients (93.2%). Overall, physicians found the MR soft-tissue contrast excellent and the MR-only workflow convenient and efficient for image visualization and segmentation. Contours and dose distributions are now routinely visualized and reviewed on the T2 axial MR images during peer-reviewed chart rounds (supplementary Fig. 1). MR-only workflow failed for the remaining 41 patients. Challenges that occurred for the remaining patients differed in severity.
3.1. Simulation-related challenges
3.1.1. Synthetic CT generation
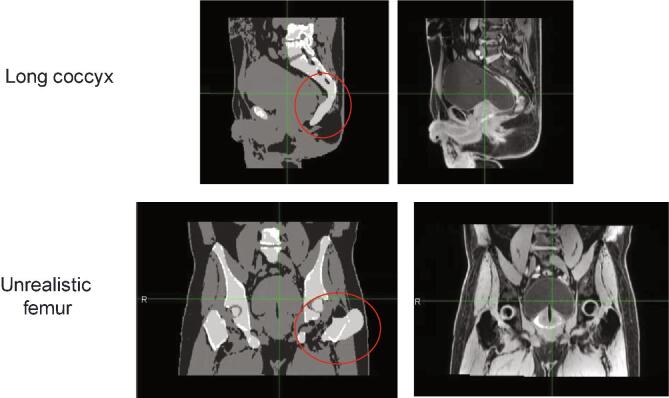
MRCAT reconstruction of the Syn-CT failed at the MR console during MR simulation for Seventeen patients (2.9%). The reconstruction failure was attributed to the software’s “sanity check” ability that prevented erroneous synthetic CT generation. The exact cause was later determined to be due to large patient size, femur angulation, motion-blurred MRCAT source MR images, the presence of pelvic bony metastases, or failure to determine the body contour. Other Syn-CT challenges included erroneous coccyx reconstructions on a few patients and unrealistic femur reconstruction on two patients (Fig. 2). The failures were determined at the acquisition stage by trained personnel and allowed patients to convert to a dual modality CT + MR simulation pathway
Fig. 2.
Elongated coccyx (top row) and unrealistic femur reconstructions (bottom row) during MRCAT synthetic CT generation.
3.1.2. MR image quality
Twenty-four patients (4.1%) underwent a repeat/backup CT scan and a combined CT + MR planning process because of artifacts on the MR such as blurry images due to patient motion or biopsy artifacts that limited identification of implanted fiducial markers. Details about these artifacts are described below. These artifacts were identified at the MR acquisition stage during which trained MR technologists perform an image quality assessment QA and document it.
3.1.2.1. Motion artifacts
Supplementary Fig. 2 shows an example of motion artifact due to the bulk patient motion seen on the axial 2D T2w MRI sequence used for contouring target and normal structures as well as the 3D bFFE sequence that precluded the identification of fiducials. The patient motion was due to discomfort from a foley catheter or involuntary bowel motion that resulted in motion artifact on the MR images. Patients were sent for a backup CT scan if repeat acquisition did not resolve motion artifact. Sixteen patients underwent a backup CT because of motion.
3.1.2.2. Biopsy/blood artifacts
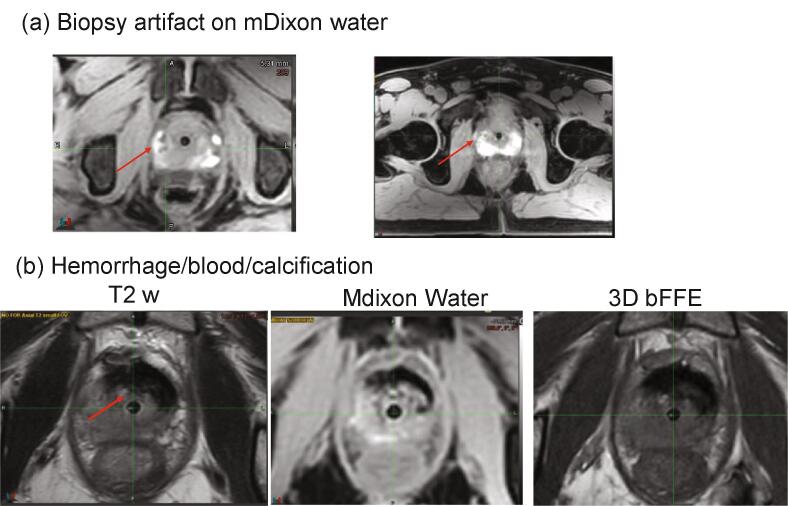
Intraprostatic biopsy often results in bleeding which can lead to signal hyperintensity on T1w images and hypointensity on T2w MR images. In five cases, artifacts due to blurry MRCAT source image, hemorrhage and calcification precluded identification of the fiducial markers (Fig. 3a, 3b) and resulted in patient re-simulation as this was not appreciated during the image acquisition QA step and eventually determined during treatment planning stage. As a result, MR technologists were educated to (a) identify biopsy/blood artifacts, (b) inform the physicists and physicians and document when artifacts were observed and (c) immediately send the patient for a CT scan for a combined CT-MR planning process.
Fig. 3.
(a) Biopsy artifact (signal hyperintensity indicated by red arrow) on the MRCAT source mDixon water MR image. (b) Hemorrhage/blood/calcification artifacts on T2w, mDixon water and 3D bFFE. The artifact appears as a dark signal on mDixon water image. One possible explanation for areas with low signal on T1 and T2 is the presence hemosiderin.
3.1.2.3. Surgical clip artifacts
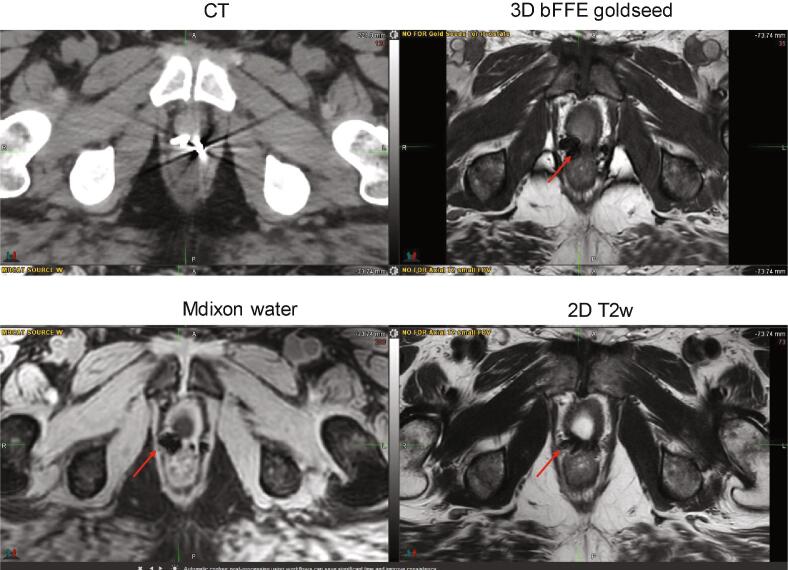
The presence of surgical clips after prostatectomy resulted in signal voids which confound the detection of metallic fiducial markers. Three patients receiving treatment for residual/recurrent disease after prostatectomy had a fiducial marker placed in the region of residual disease to facilitate image guidance during treatment delivery. During planning stage it was determined that the fiducial markers could not be distinguished from surgical clips (Fig. 4), introducing the potential for inaccurate patient setup and treatment delivery. These patients returned for either a simulation CT scan or an image-only session on the linear accelerator during which a CBCT scan was acquired and used for fiducial localization. As a result, physicians were educated to clearly document post prostatectomy recurrent cases in the prescription or simulation order form and upfront order a small field-of-view backup CT scan at the time of the MR simulation/mold appointment for these patients.
Fig. 4.
CT and MR images for a patient with an implanted fiducial who underwent irradiation for residual disease after prostatectomy. The patient required a backup CT to distinguish the fiducial from surgical clips. The implanted markers appear as a very large dark signal on 3D bFFE, mDixon water as well as T2 w MRI sequence.
3.1.2.4. Inhomogeneity artifacts
In cases where a large inferior-superior region was included for coverage of pelvic lymph nodes, a single-series, two-stack T2w 2D axial MRI acquisition was performed. However, anterior signal loss in some cases was noted which was likely due to dielectric effects on the 3 T MR scanner. The issue was resolved by acquiring the two stacks separately in two different series with individualized pre-scan optimization including adaptive RF inhomogeneity correction (supplementary Fig. 3). This strategy also provided the benefit that only one stack need be repeated if involuntary motion occurred during the acquisition, resulting in time-savings.
3.1.3. Bowel prep issues
Rectal content and gas issues were more challenging for MR-only simulation compared to CT-only simulation due to the longer MR scan time. Our institutional policy is empty rectum and full bladder for all prostate patients undergoing EBRT. Even though a rectal catheter was inserted as needed to remove gas prior to the start of MR simulation, new gas would move inferiorly during the 20–25 min simulation and affect the relevant sequences (supplementary Fig. 4). For some patients, the rectal catheter was used multiple times which often prolonged MR simulation time. Therefore, an assessment for bowel/rectum filling was done upfront during MR-only simulation, a rectal catheter was used if needed and proceeded with scans that can be acquired up to 25 min later. MR technologists were instructed to be alert to changes in rectal gas occurring between sequences. A rectal catheter, however, was not helpful for situations where the rectum is filled with stool instead of gas. Patients were then prescribed an enema while they were onsite and an MR-only simulation was attempted again after a few hours.
3.2. Treatment planning challenges
3.2.1. Fiducial mismatch on orthogonal scout QA image
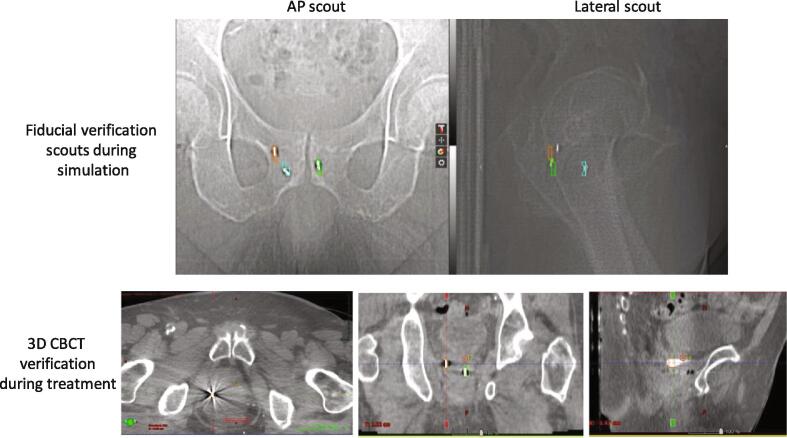
For four patients, it was difficult to verify the fiducial locations on MR using the fiducial QA orthogonal scout pair obtained in the CT. This was typically due to the presence of a large amount of rectal gas during the immobilization mold making step that was subsequently removed or released prior to the acquisition of the MR images. Differences in the amount and location of rectal gas between the acquisition of the scout and MR images may result in deformation or rotation of the prostate leading to inaccuracies or an inability to match the fiducials on the scouts and the MR (Fig. 5). Although additional effort was required, the fiducials were identified and delineated on the MR sequences and were found to match acceptably well during the daily IGRT treatment (Fig. 5).
Fig. 5.
(Top row) Differences between fiducial locations identified on MR (segmented structures) and those on the CT fiducial verification scout images due to changes in rectal gas between imaging sessions. (Bottom Row) Orthogonal scout mismatch vs CBCT match. The segmented structures on the CBCT were derived from the MR images.
4. Discussion
In this study, we described several technical issues and workflow challenges that occurred during the initial period of clinical implementation of MR-only simulation and planning in the clinic and the resulting modifications made to further improve our clinical workflow. Our data showed that MR-only pathway works successfully for the vast majority of prostate patients undergoing external beam radiotherapy.
Our success rate of ~93% is comparable to Tenhuenen et al although our challenges were related to both workflow as well as image artifacts [13]. The challenges investigated in this study were reported prospectively, and changes were brought to the clinical guidelines as they were reported. Additional personnel training was implemented to identify, anticipate and mitigate errors at the acquisition stage. Image QA was performed following each series during MR simulation to allow for re-acquisition if artifacts (such as biopsy or motion) or issues (with synthetic CT generation) occurred and documented as part of MR QA questionnaire. The patients were sent for a CT scan if the issues could not be resolved by reacquisition. Guidelines were also updated and communicated to the group as to which patients will likely require a backup or repeat CT scan. For example, prostate bed recurrence with implanted fiducial marker and multiple surgical clips also get a small FOV backup CT. Physicians were educated to clearly document post prostatectomy recurrent cases in the prescription or simulation order form and upfront order a small field-of-view backup CT scan at the time of the MR simulation/mold appointment for these patients.
One of the major workflow interruptions was due to the failure of synthetic CT reconstruction. Syn-CTs failed during the sanity check process that prevented the software from erroneously generating the syn-CT. The software does not report the exact cause and is a black box in that respect. The issues related to unrealistic femur reconstruction however, still passed the vendor’s sanity check program as the orientation and angulation of the patient’s femoral heads differed significantly from the mean model value in the segmentation. Even the segmentation of elongated coccyx was due to coccyx contrast being much darker on the mDixon images compared to the background tissue. The cause for syn-CT failures and discrepancies were determined upon sharing the dataset to the vendor which was helpful as that lead to the improvement of the syn-CT model in subsequent software release. This shows that clearly defined criteria as input data for sanity check as well as ongoing QA are necessary for successful implementation and generation of syn-CTs for MR-only planning. Kim et al have also shown in their failure mode and effects analysis that the greatest source of risk in an MR-only workflow is the synthetic CT generation process [30].
The second significant challenge in our MR-only workflow was patient motion including bulk motion due to claustrophobia, discomfort from a foley catheter or involuntary bowel motion that resulted in motion artifact on the MR images. The MR images are typically reacquired if motion artifact is observed during the image QA process. Roughly 10% of our cases require repeat acquisition of at least one MR sequence. All patients undergoing SBRT or a combined external beam/brachytherapy regimen currently receive a Foley catheter at our institution. We have been investigating MR imaging-based methods for urethra visualization so that the Foley catheter can be eliminated [31]. In addition to foley catheter, poor bowel preparation was also a challenge for MR-only simulation. Fast scan times during CT simulation can often compensate for inadequate preparation since rectal gas changes occur slowly compared to the scan time. In comparison, moving gas during MR-only simulation can severely compromise image quality and can lead to significant changes to the position of normal tissues and the prostate between sequences. Overcoming this limitation required strict patient adherence to a bowel and bladder regimen and more importantly, technical improvements to the MR simulation process to speed up image acquisition, ideally to approach that of CT simulation. Over time, bowel prep guidelines for simulation were modified in our clinic to incorporate two Fleet enemas. Patients were asked to do the enema preparation 3 h before simulation and, when possible, a second one upon arrival for their simulation appointment. For some patients, however, this regimen was difficult and achieving an empty rectum was quite challenging. Another concern for rectum and bowel filling at the time of simulation is reproducibility of the bowel, rectum, and prostate at the time of treatment. If the technologists observed a small amount of gas but determined that the bowel and rectal walls were not significantly distorted, (e.g. the structures have a uniform diameter that is within a specific range) they proceeded with the MR simulation, avoiding the use of multiple rectal catheters. This also avoided further exacerbation of the problem by extending the time the patient was undergoing simulation.
The last significant challenge in our MR-only workflow has been consistent visibility of fiducials on all MR series including axial 2D T2w, 3D bFFE and MRCAT source MR. Because patient motion can occur between imaging series during a 25-minute MR-only simulation, a workflow in MIM was developed that allows evaluation and fusion of all MR series based on the implanted fiducial markers. Successful use of such an approach required that markers be visible on all the different MRs. It should also be noted that the implanted fiducial markers are not inherently visible on the Syn-CT to be used as a reference for image-guided setup and treatment. Therefore, correct identification and delineation of fiducials on the MRs and subsequent translation of the fiducial segmentations to the synthetic CT was crucial for patient setup. To ensure accurate fiducial localization, we evaluated several fiducial markers over the time period of this study and ultimately changed from 1.2x3 mm to 0.9x5 mm to 1.2x5 mm to allow clear visualization on all the relevant MR sequences (Supplementary Fig. 5). In future, robust acquisition methods also need to be developed that can facilitate the visualization with much ease allowing MR technicians to obtain immediate feedback regarding the quality and localization of the fiducial markers [32].
Finally, even though MR-only workflow offers advantages including reducing systematic uncertainty by potentially eliminating a CT scan, the increased complexity of transitioning from a dual-modality workflow to an MR-only workflow in the clinic may lead to increased cost and resources in terms of overall QA. In addition, CT + MR or CT-only pathway may still be needed for patients where MR-only solution fails or patients with MR contraindications. A successful MR-only pathway is dependent on acquiring high quality MR images usable for synthetic generation, fiducial identification as well as contouring tumor and OARs. Additional system and workflow QA, and personnel training need to be employed to identify, anticipate and mitigate errors at the acquisition stage. Further studies are also needed that can provide evidence of the improved patient outcomes or treatment pathway improvement in moving from a dual modality workflow to an MR-only planning.
In conclusion, MR-only simulation and planning has been successfully implemented in the clinic for majority of patients undergoing prostate radiotherapy. However, MR-CT or CT-only pathway may still be needed for patients where MR-only solution fails or patients with MR contraindications. The time required for MR-only simulation remains a concern and we and others are exploring the use of new methods for Syn-CT generation as well as new image sequences and compressed sense methods to further speed up acquisition time for MR-only simulation.
5. Ethics approval and consent to participate
The study was conducted under MSKCC IRB approved retrospective protocol number 16–682.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was partially supported by the NIH/NCI Cancer Center Support Grant/Core Grant (P30 CA008748).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.phro.2020.09.009.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Roach M., 3rd, Faillace-Akazawa P., Malfatti C., Holland J., Hricak H. Prostate volumes defined by magnetic resonance imaging and computerized tomographic scans for three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 1996;35:1011–1018. doi: 10.1016/0360-3016(96)00232-5. [DOI] [PubMed] [Google Scholar]
- 2.Rasch C., Barillot I., Remeijer P., Touw A., van Herk M., Lebesque J.V. Definition of the prostate in CT and MRI: a multi-observer study. Int J Radiat Oncol Biol Phys. 1999;43:57–66. doi: 10.1016/s0360-3016(98)00351-4. [DOI] [PubMed] [Google Scholar]
- 3.Hanvey S., Sadozye A.H., McJury M., Glegg M., Foster J. The influence of MRI scan position on image registration accuracy, target delineation and calculated dose in prostatic radiotherapy. Br J Radiol. 2012;85:e1256–e1262. doi: 10.1259/bjr/26802977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gill S., Dang K., Fox C., Bressel M., Kron T., Bergen N. Seminal vesicle intrafraction motion analysed with cinematic magnetic resonance imaging. Radiat Oncol. 2014;9:174. doi: 10.1186/1748-717X-9-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mak D., Gill S., Paul R., Stillie A., Haworth A., Kron T. Seminal vesicle interfraction displacement and margins in image guided radiotherapy for prostate cancer. Radiat Oncol. 2012;7:139. doi: 10.1186/1748-717X-7-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonsson J., Nyholm T., Soderkvist K. The rationale for MR-only treatment planning for external radiotherapy. Clin Transl Radiat Oncol. 2019;18:60–65. doi: 10.1016/j.ctro.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pathmanathan A.U., Van As N., Kerkmeijer L.G., Christodouleas J., Lawton C., Vesprini D. MRI-guided adaptive radiotherapy: a “game chanager” for prostate radiotherapy. Int J Radiat Oncol Biol Phys. 2017;100:361–373. doi: 10.1016/j.ijrobp.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 8.Ranta I., Teuho J., Linden J., Klen R., Teras M., Kapanen M. Assessment of MRI-Based Attenuation Correction for MRI-Only Radiotherapy Treatment Planning of the Brain. Diagnostics (Basel) 2020;10 doi: 10.3390/diagnostics10050299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tetar S.U., Bruynzeel A.M.E., Lagerwaard F.J., Slotman B.J., Bohoudi O., Palacios M.A. Clinical implementation of magnetic resonance imaging guided adaptive radiotherapy for localized prostate cancer. Phys Imag Radiat Oncol. 2019;9:69–76. doi: 10.1016/j.phro.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.den Hartogh M.D., de Boer H.C.J., de Groot-van Breugel E.N., van der Voort van Zyp J.R.N., Hes J., van der Heide U.A. Planning feasibility of extremely hypofractionated prostate radiotherapy on a 1.5T magnetic resonance imaging guided linear accelerator. Phys Imag Radiat Oncol. 2019;11:16–20. doi: 10.1016/j.phro.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsson L.E., Johansson M., Zackrisson B., Blomqvist L.K. Basic concepts and applications of functional magnetic resonance imaging for radiotherapy of prostate cancer. Phys Imag Radiat Oncol. 2019;9:50–57. doi: 10.1016/j.phro.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grosu A.-L., van der Heide U.A. Imaging for radiation treatment planning and monitoring in prostate Cancer: Precision, personalization, individualization of therapy. Phys Imag Radiat Oncol. 2019;11:61–62. doi: 10.1016/j.phro.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tenhunen M., Korhonen J., Kapanen M., Seppälä T., Koivula L., Collan J. MRI-only based radiation therapy of prostate cancer: workflow and early clinical experience. Acta Oncol. 2018;57:902–907. doi: 10.1080/0284186X.2018.1445284. [DOI] [PubMed] [Google Scholar]
- 14.Elguindi S., Zelefsky M.J., Jiang J., Veeraraghavan H., Deasy J.O., Hunt M.A. Deep learning-based auto-segmentation of targets and organs-at-risk for magnetic resonance imaging only planning of prostate radiotherapy. Phys Imag Radiat Oncol. 2019;12:80–86. doi: 10.1016/j.phro.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuisma A., Ranta I., Keyriläinen J., Suilamo S., Wright P., Pesola M. Validation of automated magnetic resonance image segmentation for radiation therapy planning in prostate cancer. Phys Imag Radiat Oncol. 2020;13:14–20. doi: 10.1016/j.phro.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bird D., Henry A.M., Sebag-Montefiore D., Buckley D.L., Al-Qaisieh B., Speight R. A Systematic Review of the Clinical Implementation of Pelvic Magnetic Resonance Imaging-Only Planning for External Beam Radiation Therapy. Int J Radiat Oncol Biol Phys. 2019;105:479–492. doi: 10.1016/j.ijrobp.2019.06.2530. [DOI] [PubMed] [Google Scholar]
- 17.Kemppainen R., Suilamo S., Tuokkola T., Lindholm P., Deppe M.H., Keyrilainen J. Magnetic resonance-only simulation and dose calculation in external beam radiation therapy: a feasibility study for pelvic cancers. Acta Oncol. 2017;56:792–798. doi: 10.1080/0284186X.2017.1293290. [DOI] [PubMed] [Google Scholar]
- 18.Kemppainen R., Suilamo S., Ranta I., Pesola M., Halkola A., Eufemio A. Assessment of dosimetric and positioning accuracy of a magnetic resonance imaging-only solution for external beam radiotherapy of pelvic anatomy. Phys Imag Radiat Oncol. 2019;11:1–8. doi: 10.1016/j.phro.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim J., Glide-Hurst C., Doemer A., Wen N., Movsas B., Chetty I.J. Implementation of a novel algorithm for generating synthetic CT images from magnetic resonance imaging data sets for prostate cancer radiation therapy. Int J Radiat Oncol Biol Phys. 2015;91:39–47. doi: 10.1016/j.ijrobp.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Korhonen J., Kapanen M., Keyrilainen J., Seppala T., Tenhunen M. A dual model HU conversion from MRI intensity values within and outside of bone segment for MRI-based radiotherapy treatment planning of prostate cancer. Med Phys. 2014;41 doi: 10.1118/1.4842575. [DOI] [PubMed] [Google Scholar]
- 21.Persson E., Gustafsson C., Nordstrom F., Sohlin M., Gunnlaugsson A., Petruson K. MR-OPERA: A Multicenter/Multivendor Validation of Magnetic Resonance Imaging-Only Prostate Treatment Planning Using Synthetic Computed Tomography Images. Int J Radiat Oncol Biol Phys. 2017;99:692–700. doi: 10.1016/j.ijrobp.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Tyagi N., Fontenla S., Zhang J., Cloutier M., Kadbi M., Mechalakos J. Dosimetric and workflow evaluation of first commercial synthetic CT software for clinical use in pelvis. Phys Med Biol. 2017;62:2961–2975. doi: 10.1088/1361-6560/aa5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Persson E., Jamtheim Gustafsson C., Ambolt P., Engelholm S., Ceberg S., Back S. MR-PROTECT: Clinical feasibility of a prostate MRI-only radiotherapy treatment workflow and investigation of acceptance criteria. Radiat Oncol. 2020;15:77. doi: 10.1186/s13014-020-01513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christiansen R.L., Jensen H.R., Brink C. Magnetic resonance only workflow and validation of dose calculations for radiotherapy of prostate cancer. Acta Oncol. 2017;56:787–791. doi: 10.1080/0284186X.2017.1290275. [DOI] [PubMed] [Google Scholar]
- 25.Kerkmeijer L.G.W., Maspero M., Meijer G.J. van der Voort van Zyp JRN, de Boer HCJ, van den Berg CAT. Magnetic Resonance Imaging only Workflow for Radiotherapy Simulation and Planning in Prostate Cancer. Clin Oncol (R Coll Radiol) 2018;30:692–701. doi: 10.1016/j.clon.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Tyagi N., Fontenla S., Zelefsky M., Chong-Ton M., Ostergren K., Shah N. Clinical workflow for MR-only simulation and planning in prostate. Radiat Oncol. 2017;12:119. doi: 10.1186/s13014-017-0854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helle M., Schadewaldt N., Frantzen-Steneker M., Schulz H., Stehning C., Heide U. Evaluation of DIXON based soft tissue and bone classification in the pelvis for MR-only-based radiation therapy planning. Proc Intl Soc Mag Reson Med. 2014;22:4238. [Google Scholar]
- 28.Maspero M., van den Berg C.A.T., Landry G., Belka C., Parodi K., Seevinck P.R. Feasibility of MR-only proton dose calculations for prostate cancer radiotherapy using a commercial pseudo-CT generation method. Phys Med Biol. 2017;21:9159–9176. doi: 10.1088/1361-6560/aa9677. [DOI] [PubMed] [Google Scholar]
- 29.Maspero M., Seevinck P.R., Schubert G., Hoesl M.A., van Asselen B., Viergever M.A. Quantification of confounding factors in MRI-based dose calculations as applied to prostate IMRT. Phys Med Biol. 2017;62:948–965. doi: 10.1088/1361-6560/aa4fe7. [DOI] [PubMed] [Google Scholar]
- 30.Kim J., Miller B., Siddiqui M.S., Movsas B., Glide-Hurst C. FMEA of MR-Only Treatment Planning in the Pelvis. Adv Radiat Oncol. 2019;4:168–176. doi: 10.1016/j.adro.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zakian K.L., Wibmer A., Vargas H.A., Alberts E., Kadbi M., Mychalczak B. Comparison of Motion-Insensitive T2-Weighted MRI Pulse Sequences for Visualization of the Prostatic Urethra During MR Simulation. Pract Radiat Oncol. 2019;9:e534–e540. doi: 10.1016/j.prro.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shcherbakova Y., Bartels L.W., Mandija S., Beld E., Seevinck P.R., van der Voort van Zyp J.R.N. Visualization of gold fiducial markers in the prostate using phase-cycled bSSFP imaging for MRI-only radiotherapy. Phys Med Biol. 2019;64:185001. doi: 10.1088/1361-6560/ab35c3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.