Abstract
Salmonella is one of the main foodborne pathogens that affect humans and farm animals. The Salmonella genus comprises a group of food-transmitted pathogens that cause highly prevalent foodborne diseases throughout the world. The aim of this study was to appraise the viability of Salmonella Typhimurium biofilm under water treatment at room temperature on different surfaces, specifically stainless steel (SS), plastic (PLA), rubber (RB), and eggshell (ES). After 35 D, the reduction of biofilm on SS, PLA, RB, and ES was 3.35, 3.57, 3.22, and 2.55 log CFU/coupon without water treatment and 4.31, 4.49, 3.50, and 1.49 log CFU/coupon with water treatment, respectively. The dR value (time required to reduce bacterial biofilm by 99% via Weibull modeling) of S. Typhimurium without and with water treatment was the lowest on PLA (176.86 and 112.17 h, respectively) and the highest on ES (485.37 and 2,436.52 h, respectively). The viability of the S. Typhimurium on ES and the 3 food-contact surfaces was monitored for 5 wk (35 D). The results of this study provide valuable information for the control of S. Typhimurium on different surfaces in the food industry, which could reduce the risk to consumers.
Key words: biofilm, eggshell, S. Typhimurium, survival ability, Weibull model
Introduction
The bacteria on surfaces in food-processing environments are a potential source of cross-contamination and can lead to food spoilage or transmission of disease, through scratched or unclean food-contact surfaces in processing lines (Nidaullah et al., 2017; Coradini et al., 2019). Owing to condensation, these surfaces are favorable sites for bacteria to grow in static biofilms (Petridis et al., 2019). Biofilm do not possess a uniform structure, and bacterial species as well as several extrinsic factors (temperature, flow conditions, pH, presence of salts, nutrients, and and so on) play a major role in influencing biofilm formation and the degree of attachment (Jain and Chen, 2007; Hannig et al., 2018). Biofilm formation is strongly influenced by the food-contact surfaces (Jeon et al., 2018; Mizan et al., 2018).
In a biofilm, cells can initiate attachment on food products and food-contact surfaces (Jeon et al., 2018; Mizan et al., 2018), which provide a potential transmission route for foodborne pathogens (Hald et al., 2016). In the food industry, water cleaning is vital to control and remove biofilms (Liu et al., 2016; Esbelin et al., 2018). Nevertheless, poor or ineffective cleaning processes can increase the risk of foodborne outbreaks leading to public health concerns. In addition, the food industry may incur economic losses due to product recalls as well as legal and customer claims (Davey et al., 2013). Biofilms are involved in over 80% of all microbial diseases according to the US National Health Institute and the Centers for Disease Control and Prevention (Khatoon et al., 2018).
It has been inferred that biofilms formed on surfaces at various food-processing locations are a crucial source of Salmonella contamination of food (Khieu et al., 2013; Lamas et al., 2018). One of the most extensively used materials in machinery and food-contact surfaces in the food industry is stainless steel (SS). Schlisselberg and Yaron (2013) described how various treatments of SS could affect the biofilms formation of S. Typhimurium. The crucial problem of biofilms on food industry surfaces is their transfer to food and resulting contamination of foodstuffs. In this point of view, Wang et al. (2015) evaluated the transfer of Salmonella biofilms formed on the food-contact surface of SS to foodstuff (meat). Salmonella spp. can contaminate fresh produce during any stage, from farm to table, through cross-contamination by washing with water, handling by workers, and contact with food surfaces (Kroupitski et al., 2009). Salmonella biofilm on eggshell (ES) can cause cross-contamination of Salmonella (Carrasco et al., 2012; Pande et al., 2016) onto other food-contact surfaces on egg-processing lines and can finally lead to food contamination (Carrasco et al., 2012).
More than 70% of human salmonellosis cases in the United States have been attributed to the consumption of contaminated chicken, turkey, and eggs with 175, 133, and 45 illness outbreaks involving 1,003, 358, and 11 people, respectively (CDC, 2019). Egg products associated with S. Typhimurium outbreaks have been frequently reported in Australia (Group, 2012; Kirk et al., 2014). Numerous egg-related human S. Typhimurium outbreaks have garnered significant interest from the general public, public health authorities, and egg industry (Chousalkar et al., 2017).
The major materials used for food-contact surfaces are known to be Teflon and nitrile butyl rubber, SS, glass, rubber (RB), and polyurethane (Chia et al., 2009; Fink et al., 2017). In the present study, the viability of S. Typhimurium biofilm was evaluated when stored under room temperature for a long period (35 D) on ES and 3 different surfaces (SS, plastic [PLA], and RB) treated with and without water. Indeed, the ability of pathogenic bacteria, including S. Typhimurium, to form biofilms on various food-contact surfaces and under different conditions for different periods has been investigated in several research studies (Lamas et al., 2016). In a review, Mizan et al. (2015) stated that Salmonella spp. biofilms self-gather and form flat or mushroom-shaped 3D structures on SS. Other studies have examined the viability of Salmonella spp. on polypropylene surfaces (Iibuchi et al., 2010), Salmonella spp. on ES surfaces at different temperatures (Park et al., 2015b), and specifically, S. Typhimurium on ES (McAuley et al., 2015).
In spite of the boundless appliance for forecasting mathematical models of survival and growth and the significant amount of scientific literature on the modeling of biofilm survival ability (Giertsen et al., 2011; Dimakopoulou-Papazoglou et al., 2016), no attempt has been made to study the effects of environmental factors during biofilm formation to establish and apply mathematical models. Therefore, the aim of this study was to establish and appraise predictive mathematical models for the effect of water treatment on the viability of S. Typhimurium biofilm on SS, PLA, RB, and ES.
The fitting and performance of linear and modified Weibull models were assessed to estimate the survival behavior of Salmonella spp. on SS, PLA, RB, and ES. Survival modeling of S. Typhimurium on table egg during storage at different temperatures (Pasquali et al., 2016) as well as the modeling of biofilm formation by Salmonella enterica ser. Newport as a function of pH and water activity (Dimakopoulou-Papazoglou et al., 2016) have been reported.
Many investigations have been carried out on food-contact surfaces concerning viability and biofilm attachment ability. However, there is still a need to explore the survival time and mathematical modeling of S. Typhimurium biofilm (the major cause of human bacterial gastroenteritis) on the 3 types of food-contact surface (SS, PLA, and RB), especially focusing on egg processing line materials and cookware utensils. Thus, the purpose of this study was to appraise the viability of S. Typhimurium on SS, PLA, RB, and ES surfaces.
Materials and methods
Bacterial Strains and Growth Conditions
S. Typhimurium ATCC14028 was used in this study to evaluate the viability of S. Typhimurium on food-contact surfaces (SS, PLA, RB) and ES with and without water treatment. Bacterial stock culture was maintained at −70°C in Tryptic Soy Broth (TSB; Difco Laboratories Detroit, MI) supplemented with 15% (vol/vol) glycerol (Fisher Scientific, Itasca, IL). The strain was consecutively subcultured twice aerobically at 37°C for 24 h in TSB. Cultured cells were centrifuged at 11,000 × g at 4°C for 10 min and washed twice with sterile phosphate buffered saline (PBS; pH 7.2). The pellets were resuspended in peptone water (PW; Oxoid, Basingstoke, Hampshire, England). The bacterial cell suspension was diluted in 0.1% PW to yield the final cell concentration (105–106 CFU/mL) for inoculation to make biofilm on coupons. By plating on xylose lysine deoxycholate agar (Difco Laboratories) plates and incubating at 37°C for 24 h, microbial numbers were determined.
Preparation of SS/PLA/RB/ES Coupons, Inoculation, and Biofilm Formation
SS (SUS 304 2B; Posco Co., Ltd., Pohang, Korea), PLA (egg packaging; Join Co., Ltd., Eumseong, Korea), and RB (Komax Industrial Co., Ltd., Goyang-ro, Korea) were selected as delegate surfaces used in the food industry. In this study, we used SS, PLA, and RB coupons (2 cm × 2 cm × 0.1 cm) that were processed as earlier addressed (Sadekuzzaman et al. 2018; Hossain et al., 2020). ES coupons were processed as previously described by Park et al. (2018). Briefly, eggs were collected from a local grocery store in Anseong-Si, South Korea (Eggs with a remaining shelf life of at least 40 D were selected and stored at 4°C until use.). Each egg was gently broken, and the ES was cut into 2 cm × 2 cm × 0.1 cm coupons using a sterilized knife. Immediately after, the ES was soaked into 70% alcohol for 10 min, washed 3 times with sterilized deionized water, and then treated with UV in a laminar flow biosafety hood for 15 min on each side to remove background flora before inoculation. The bacterial cell suspension was diluted at 1:50 into 50-mL Falcon tubes with each coupon completely submerged in 10-mL TSB. The 50-mL Falcon tubes were incubated without shaking to form biofilms on the coupons at 37°C for 24 h.
With or Without Water Treatment, Storage and Biofilm Detachment
After incubation of biofilm formation, half of the S. Typhimurium biofilms on SS, PLA, RB, and ES coupons were washed under running sterile water with swirling of 10 s (3 times) and stored at room temperature in a humidity chamber (relative humidity 50%; V8111H-150; Vision Scientific Co., Ltd., Gyeonggi-do, Korea). The remaining half of each sample was stored at room temperature in a humidity chamber (relative humidity 50%) without water treatment except for an initial wash process for removing planktonic cells. The viability of the S. Typhimurium on ES and the 3 food-contact surfaces was monitored for 5 wk (35 D). Biofilm cells detachment was done according to the study by Jahid et al. (2014) with minor modifications. After incubation, each coupon expect ES was shifted in a small petri dish (55 mm × 12 mm) containing 2 mL of 0.1% PW and agitated by holding the SS, PLA, and RB coupons on the petri dish using sterile tweezers at the same time to rotate clockwise and anticlockwise. Agitation was always performed by the same person to ensure the same amount of pressure was applied to all the coupons. The suspension was then transferred to a test tube and ultrasonicated for 2 min in a sonicator (380 W, 37 kHz, Elmasonic P; Elma Schmidbauer, GmbH, Singen, Germany) to disperse the biofilm population. Each sample of the ES coupon was vortexed with 10 glass beads and 10-mL PW in a 50-mL Falcon tube for 1 min. In PW, the dispersed biofilm cells were serially diluted for cells counting by plating on xylose lysine deoxycholate agar and incubated for 24 h at 37°C.
The Modified Weibull Model
The modified Weibull model (a two-parameter nonlinear model) can be expressed as
| (1) |
where Nt is the concentration of biofilm (CFU/coupon) after exposure time t, N0 is the initial concentration of biofilm (CFU/coupon), t is the exposure time, and b and n are the scale (characteristic time) and shape parameters as a behavior index, respectively (van Boekel, 2002). To reduce the first log cycle, the value of b represents the required time to reduce the bacterial biofilm population, while that of n indicates the shape of the survival curve (n = 1 corresponds to a linear survival curve, while n > 1 and n < 1 correspond to downward and upward concavity, respectively). For the calculation of dR (analogous to the traditional D value) from the Weibull parameters, the following equation is used (Buzrul and Alpas, 2007):
| (2) |
where dR is the time required to reduce the bacterial biofilm by 99%. GraphPad Prism version 5.0 for Windows (GraphPad Software, San Diego, CA) was used to appraise the inactivation kinetics for nonlinear regression which was fitted by the modified Weibull method.
Field Emission Scanning Electron Microscopy
Field emission scanning electron microscopy images of the S. Typhimurium biofilms on SS, PLA, RB, and ES coupons were obtained according to previously reported procedures (Mizan et al., 2018; Hossain et al., 2020) with some modifications. The adhered cells on coupons were fixed for 24 h with 2% glutaraldehyde (Sigma Aldrich, St. Louis, MO) in PBS and then washed 3 times with PBS. The fixed cells were serially treated with ethanol (50, 60, 70, 80, and 90% for 15 min, respectively, and 100% 2 times for 15 min each) and with 33, 50, 66, and 100% hexamethyldisilazane (Sigma Aldrich) in ethanol and successively dehydrated for 15 min each to observe the dehydrated samples which were coated with platinum and visualized on field emission scanning electron microscopy (FE-SEM; Carl Zeiss, Oberkochen, Germany) with an accelerating voltage of 5 kV and 5 mm working distance.
Statistical Analysis
Data were analyzed via one-way analysis of variance (Duncan's test) using SAS version 9.2 (SAS Institute Inc., Cary, NC). Three independent trials were used in all experiments repeated 3 times. Data were considered to be statistically significant at P < 0.05.
Results and discussion
Viability of S. Typhimurium biofilm on Food-Contact Surfaces and ES With and Without Water Treatment Stored Under Room Temperature
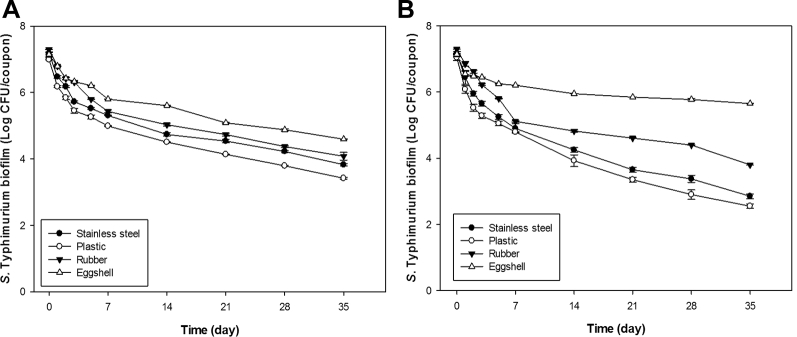
The viability of S. Typhimurium biofilm was measured at predetermined times (0, 1, 2, 3, 5, 7, 14, 21, 28, and 35 D), after inoculation on SS, PLA, RB, and ES surfaces. The amounts of S. Typhimurium biofilm detected from the surfaces with and without water treatment significantly decreased over time (P < 0.05; Figure 1).
Figure 1.
The viability of Salmonella Typhimurium biofilm formation on different food-contact surfaces on different time intervals without (A) and with (B) water treatment at room temperature.
The viability of S. Typhimurium biofilm on food-contact surfaces without and with water treatment at room temperature is shown in Figures 1A and 1B, respectively. The reduction of S. Typhimurium biofilm after 35 D on food-contact surfaces without water treatment (Figure 1A) was the highest on PLA (3.57 log10 CFU/coupon) and the lowest on ES (2.55 log10 CFU/coupon). For SS and RB, the corresponding values were 3.35 and 3.22 log10 CFU/coupon, respectively. The amounts of S. Typhimurium biofilm detected on the surfaces of ES and the 3 food-contact surfaces significantly decreased over time (P < 0.05). From these data, S. Typhimurium biofilm survived for the shortest period on PLA among the 3 food contact surfaces, and the viability was maintained for the longest period on ES among all the surfaces. The characteristics of the food-contact surfaces could be important for bacteria attachment and viability (Stepanović et al., 2004). The bacterial biofilm viability on different food-contact surfaces depends on various factors, including temperature, humidity, and nutrient availability for bacteria (Lamas et al., 2018). According to Brankatschk et al. (2014), Salmonella uses its genetic marker to effectively attach to surfaces and finally colonize.
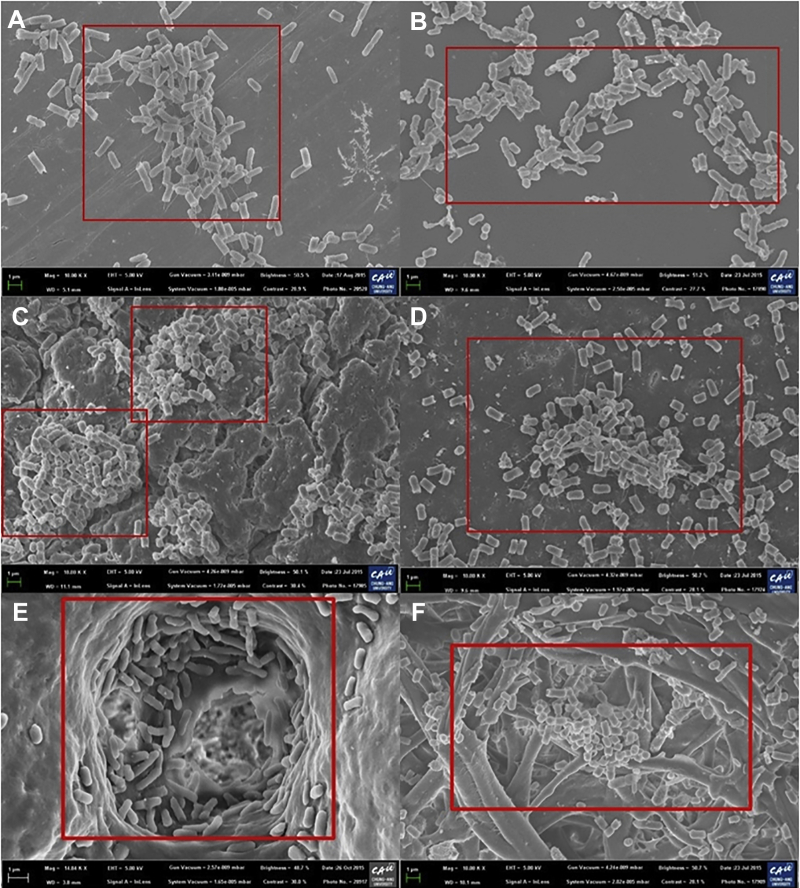
The viability of the S. Typhimurium biofilms is rated from the highest to the lowest as ES > RB > SS > PLA (Figure 1A), due to the properties of the surfaces. According to our FE-SEM study, the surface roughness is rated from highest to lowest as RB > ES > SS > PLA. FE-SEM images of the S. Typhimurium biofilm formation on the study surfaces are depicted in Figure 2. FE-SEM images reveal that S. Typhimurium colonized and consequently formed compact or dense biofilms on the surfaces. The adhesion forces for Streptococcus spp. on composite resins with different surface roughness values were evaluated by Mei et al. (2011), who confirmed that they increased with increasing surface roughness. It was also reported that the surface roughness of a polyester urethane conveyor belt had a significant influence on the biofilm-forming ability of Listeria monocytogenes (Stepanović et al., 2004). For this reason, S. Typhimurium could make a stronger biofilm on RB than other surfaces (Ronner and Wong, 1993). However, the viability was higher on ES than on RB (Figures 1A, 1B). ES has a surface roughness and porosity similar to those of wood, which allow the gas and water exchange necessary for the developing chick embryo but also microbial ingress and contamination of the egg contents. Thus, S. Typhimurium (1 μm) can enter through an ES pore (15–65 μm) and make a strong biofilm (Ghaneian et al., 2011; Abramian and El-Rassy, 2012). According to our FE-SEM study, we observed pore on ES (Figures 2E, 2F).
Figure 2.
FESEM images of S. Typhimurium biofilm formation without water treatment on different food-contact surfaces. SS (A); PLA (B); RB (C, D); and ES (E, F). Red box indicates the biofilm formation ability on different surfaces.
After 35 D, the reduction of the S. Typhimurium biofilms on food-contact surfaces with water treatment was the highest on PLA (4.49 log10 CFU/coupon) and the lowest on ES (1.49 log10 CFU/coupon) (Figure 1B). The reduction values for SS and RB were 4.31 and 3.50 log10 CFU/coupon, respectively. The amounts of S. Typhimurium biofilm detected on the 3 surfaces (SS, PLA, and RB) decreased significantly over time (P < 0.05), but they decreased significantly less (P < 0.05) on the surface of ES than on the other surfaces. The order of viability of the biofilms on the washed surfaces was the same as that on an unwashed surface, with the S. Typhimurium biofilm on ES being the highest (Kim et al., 2016; Jung et al., 2018). This difference could have been due to ES absorbing water through its pore during the 3 times washing step. Although the ES was dried and stored for 24 h in a humidity chamber before washing, its interior region could have remained wet and thus absorbed most of the biofilm. This may explain the high observed viability of the S. Typhimurium biofilm on the ES surface (Kim et al., 2016). Similarly, it has been suggested that wood encourages biofilm formation because of its porosity and absorbency, which can entrap organic material and bacteria (Adetunji and Isola, 2011; Al-kafaween et al., 2019). Moreover, moisture retention on the ES could also account for the greater viability of S. Typhimurium at low temperatures because the eggs were stored while still wet (Rizk et al., 1966).
Overall, it was found that the viability of S. Typhimurium biofilm (rated from highest to lowest: ES > RB > SS > PLA) showed several sharp decreases after 1, 2, 3, 5, and 7 D, but after 7 D, the decrease in the rate of viability was slight (Figure 1). It was also determined that the biofilms on the 3 food-contact surfaces (except for ES) were more viable when not washed with water, although the difference in the amount of S. Typhimurium biofilm was not significant (P > 0.05). The viability of S. Typhimurium biofilm on washed ES was significantly higher in value than that on unwashed ES (Wolf-Hall and Nganje, 2017), and the amount of S. Typhimurium biofilm was significantly different (P < 0.05). This could have been due to ES absorbing water via its pores during the washing step (Pande et al., 2016).
After 35 D with and without water treatment on all food-contact surfaces, the results of the present study show that the viability of S. Typhimurium biofilms was maintained. McAuley et al. (2015) found that S. Typhimurium biofilm was attached early to the ES surface after exposure for only 20 min but was not maintained after 2 wk. It was also recently reported that the viability of S. enterica on ES surfaces was maintained after 3 wk (Park et al., 2015a). The results of these 2 studies are contradictory because S. Typhimurium cells attached to an ES surface had weaker adhesive power than S. enterica biofilm (exposure for 24 h). The results of the study by Park et al. (2015a) showed that the validity curve for S. enterica on ES was similar to that for S. Typhimurium biofilm in the present study. Consequently, the viability of S. Typhimurium on food-contact surfaces is variable according to the biofilm state and biofilm formation ability.
Weibull Modeling to Obtain Survival dR Value Against S. Typhimurium Biofilms on the Food-Contact Surfaces
In food-contact surfaces, the viability data of S. Typhimurium biofilm were fitted with the Weibull model for nonlinear microbial survival. The values of the Weibull model parameters (b, n, dR, and R2) are reported in Table 1. The survival curves were a good fit for this model by using R2 to estimate the goodness of fit of the model, whereas the values were over 0.96. Microbial inactivation has commonly been modeled using a first-order kinetics process (Whiting et al., 1996; Pankaj et al., 2013) fitted for comparison with the Weibull model. Moreover, many researchers have revealed that the Weibull model might have a better fit than first-order models for kinetic viability of bacteria and viruses, such as mixed-culture biofilms of S. Typhimurium (Jahid et al., 2015), reduction of Cladosporium cladosporioides and Penicillium citrinum (Park and Ha, 2015), and survival of norovirus (Kim et al., 2014) and the hepatitis A virus (Bae et al., 2014). Thus, for its simplicity and flexibility, the Weibull model is used extensively (Chen and Hoover, 2004; Chen, 2007; Muñoz-Cuevas et al., 2013).
Table 1.
Weibull modeling parameters for Salmonella Typhimurium survivability on the food-contact surfaces.
| Water treatment | Parameters | Surface types |
|||
|---|---|---|---|---|---|
| Stainless steel | Plastic | Rubber | Egg shell | ||
| Without | b ± SE | 3.36 ± 1.10 | 2.88 ± 0.67 | 6.44 ± 2.24 | 18.6 ± 4.72 |
| n ± SE | 0.37 ± 0.02 | 0.37 ± 0.01 | 0.42 ± 0.03 | 0.47 ± 0.03 | |
| dR ± SE | 204.04 ± 23.90a,B,C | 176.86 ± 14.50a,C | 241.33 ± 30.23a,B | 485.37 ± 31.14b,A | |
| R2 | 0.99 | 0.99 | 0.98 | 0.98 | |
| With | b ± SE | 3.54 ± 0.72 | 2.67 ± 0.65 | 5.71 ± 3.43 | 9.62 ± 2.28 |
| n ± SE | 0.42 ± 0.01 | 0.41 ± 0.02 | 0.42 ± 0.05 | 0.28 ± 0.01 | |
| dR ± SE | 133.28 ± 10.94b,B,C | 112.17 ± 11.44b,C | 216.75 ± 55.05a,B | 2,436.52 ± 35.81a,A | |
| R2 | 0.99 | 0.99 | 0.96 | 0.99 | |
Values are mean ± SE.
b = scale parameter; n = shape parameter (concave upward survival curve if n < 1, concave downward if n > 1, and linear if n = 1); dR = time (h) required to reduce the bacterial biofilm by 99%; R2 = correlation coefficient (a higher R2 value indicates a better fit to the data). Means in the same column with superscript lowercase letters are significantly different via Duncan's multiple range test (P < 0.05). Means in the same row with superscript uppercase letters are significantly different via Duncan's multiple range test (P < 0.05).
The Weibull model, which has 2 parameters (b and n), can be affected by external conditions (temperature, pH, the presence of a preservative, and so on) (Peleg and Cole, 2000; Mattick et al., 2001). The Weibull model (without water treatment) was used to predict survival curves and calculate the dR values. In this study period, the R2 values from the linear model and the Weibull model were 0.85 to 0.90 (data not shown) and 0.98 to 0.99, respectively, whereas the Weibull model was a better fit to the data than the linear model. The Weibull parameters (b and n) represent the required time to reduce the bacterial biofilm by 1 log10 by calculating the dR value. The calculated dR values were 204.04, 176.86, 241.33, and 485.37 h for SS, PLA, RB, and ES, respectively. The dR values for S. Typhimurium biofilm without water treatment rated from significantly highest to lowest (P < 0.05) were in the order of ES > RB > SS > PLA (Table 1). The Weibull model (with water treatment) was used to predict survival curves and calculate the dR values. The R2 values were 0.75 to 0.89 (data not shown) and 0.96 to 0.99, respectively, from the linear model and the Weibull model, indicating the better fit of the data to the Weibull model than to the linear model. The dR value which was calculated from the Weibull parameters (b and n) represents the required time to reduce the bacterial biofilm by 1 log10. The calculated dR values were 133.28, 112.17, 216.75, and 2,436.52 h for SS, PLA, RB, and ES, respectively. The dR values for S. Typhimurium biofilm with water treatment rated from significantly highest to lowest (P < 0.05) were in the order of ES > RB > SS > PLA (Table 1).
Overall, it was found that the dR values for S. Typhimurium biofilm rated from highest to lowest were in the order of ES > RB > SS > PLA. The dR values of S. Typhimurium biofilm on unwashed surfaces (RB, SS, and PLA) except for ES were significantly higher than those on washed surfaces (P < 0.05). However, the dR values of S. Typhimurium biofilm on washed ES were higher than those on unwashed ES. The amounts of S. Typhimurium biofilm on the unwashed and washed surfaces of ES were significantly different (P < 0.05). From this result, it can be determined that S. Typhimurium biofilm inactivation on the surface of PLA requires less time than on the other surfaces. The S. Typhimurium biofilm inactivation on the surface of ES was estimated to take a longer time than on the other surfaces. Consequently, the results indicate that the Weibull modeling arrived at the same result as the experiments.
In summary, the viability of S. Typhimurium biofilm on food-contact surfaces without and with water treatment was investigated. The reduction of S. Typhimurium biofilm without and with water treatment was highest on PLA (3.57 and 4.49 log10 CFU/coupon, respectively) and lowest on ES (2.55 and 1.49 log10 CFU/coupon, respectively). The order of reduction value (from highest to lowest) was PLA > SS > RB > ES. After 35 D treated with and without water, the viability of S. Typhimurium biofilm was maintained on all food-contact surfaces.
Weibull modeling was conducted to obtain survival dR values for S. Typhimurium biofilms on the food-contact surfaces without and with water treatment. The dR values of S. Typhimurium biofilm without and with water treatment were lowest on PLA (176.86 and 112.17 h, respectively) and highest on ES (485.37 and 2,436.52 h, respectively). Overall, the dR values of S. Typhimurium biofilm from highest to lowest were in the order of ES > RB > SS > PLA (Hingston et al., 2013). It was also found that the dR values of S. Typhimurium biofilm on the unwashed RB, SS, and PLA (not ES) were less than those on the washed surfaces. The result of this study suggests that washing ES every day does not reduce S. Typhimurium biofilm viability significantly.
Conclusion
The viability of S. Typhimurium on food-contact surfaces varies according to the biofilm state and formation ability. The viability of S. Typhimurium biofilm over time depends on the surface roughness and porosity. The results of this study show that S. Typhimurium biofilm formed during food formulation and processing can survive persistently in factories and cookware; thus, ensuring a safe environment is very crucial during these procedures. Reducing S. Typhimurium biofilm contamination during food processing will reduce the occurrence of associated diseases. The findings in this study provide valuable information for the control of S. Typhimurium on different food-contact surfaces in the food industry to prevent foodborne disease. However, the experimental scope of the study is limited to only one strain of Salmonella. Further studies are urged to extend the application of this study in food quality and safety regulations.
Acknowledgement
This research was supported by the Chung-Ang University Graduate Research Scholarship in 2018 and National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2020R1G1A1011977).
Conflict of Interest: No potential conflict of interest was reported by the authors.
References
- Abramian L., El-Rassy H. Adsorption kinetics and thermodynamics of azo-dye orange II onto highly porous titania aerogel. Chem. Eng. J. 2012;209:682. [Google Scholar]
- Adetunji V.O., Isola T.O. Crystal violet binding assay for assessment of biofilm formation by Listeria monocytogenes and Listeria spp on wood, steel and glass surfaces. Glob. Vet. 2011;6:6–10. [Google Scholar]
- Al-kafaween M.A., Hilmi A.B.M., Jaffar N., Al-Jamal H.A.N., Zahri M.K. Determination of optimum incubation time for formation of Pseudomonas aeruginosa and Streptococcus pyogenes biofilms in microtiter plate. Bull. Natl. Res. Cent. 2019;43:100. [Google Scholar]
- Bae S.C., Park S.Y., Kim A.N., Oh M.H., Ha S.D. Survival of hepatitis A virus on various food-contact surfaces during 28 days of storage at room temperature. Food Res. Int. 2014;64:849–854. doi: 10.1016/j.foodres.2014.08.023. [DOI] [PubMed] [Google Scholar]
- Brankatschk K., Kamber T., Duffy B., Pothier J., Smits T.H.M. Transcriptional profile of Salmonella enterica subsp. enterica serovar Weltevreden during alfalfa sprout colonization. Microb. Biotechnol. 2014;7:528–544. doi: 10.1111/1751-7915.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzrul S., Alpas H. Modeling inactivation kinetics of food borne pathogens at a constant temperature. Lwt-food Sci. Technol. 2007;40:632–637. [Google Scholar]
- Carrasco E., Morales-Rueda A., García-Gimeno R.M. Cross-contamination and recontamination by Salmonella in foods: a review. Food Res. Int. 2012;45:545–556. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Salmonella, outbreaks of Salmonella infections linked to backyard poultry. 2019. www.cdc.gov/Salmonella/backyardpoultry-05-19/index.html
- Chen H. Use of linear, Weibull, and log-logistic functions to model pressure inactivation of seven foodborne pathogens in milk. Food Microbiol. 2007;24:197–204. doi: 10.1016/j.fm.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Chen H., Hoover D.G. Use of Weibull model to describe and predict pressure inactivation of Listeria monocytogenes Scott A in whole milk. Innov. Food Sci. Emerg. 2004;5:269–276. [Google Scholar]
- Chia T., Goulter R., McMeekin T., Dykes G., Fegan N. Attachment of different Salmonella serovars to materials commonly used in a poultry processing plant. Food Microbiol. 2009;26:853–859. doi: 10.1016/j.fm.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Chousalkar K.K., Sexton M., McWhorter A., Hewson K., Martin G., Shadbolt C., Goldsmith P. Salmonella typhimurium in the Australian egg industry: Multidisciplinary approach to addressing the public health challenge and future directions. Crit. Rev. Food Sci. Nutr. 2017;57:2706–2711. doi: 10.1080/10408398.2015.1113928. [DOI] [PubMed] [Google Scholar]
- Coradini M.G.L., Maia D.S.V., Iglesias M.A., Haubert L., Lopes G.V., da Silva D.A.L., Nero L.A., da Silva W.P. Occurrence and characterization of Listeria monocytogenes from beef jerky processing line. J. Food Sci. Technol. 2019;56:436–442. doi: 10.1007/s13197-018-3505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey K., Chandrakash S., O’Neill B. A new risk analysis of Clean-In-Place milk processing. Food Control. 2013;29:248–253. [Google Scholar]
- Dimakopoulou-Papazoglou D., Lianou A., Koutsoumanis K.P. Modelling biofilm formation of Salmonella enterica ser. Newport as a function of pH and water activity. Food Microbiol. 2016;53:76–81. doi: 10.1016/j.fm.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Esbelin J., Santos T., Hebraud M. Desiccation: an environmental and food industry stress that bacteria commonly face. Food Microbiol. 2018;69:82–88. doi: 10.1016/j.fm.2017.07.017. [DOI] [PubMed] [Google Scholar]
- Fink R., Okanovič D., Dražič G., Abram A., Oder M., Jevšnik M., Bohinc K. Bacterial adhesion capacity on food service contact surfaces. Int. J. Environ. Heal Res. 2017;27:169–178. doi: 10.1080/09603123.2017.1310188. [DOI] [PubMed] [Google Scholar]
- Ghaneian M.T., Ehrampoush M.H., Ghanizadeh G.H., Momtaz M. Study of eggshell performance as a natural sorbent for the removal of reactive red 198 dye from aqueous solution. Spring. 2011;10:70–81. [Google Scholar]
- Giertsen E., Arthur R., Guggenheim B. Effects of xylitol on survival of mutans streptococci in mixed-six-species in vitro biofilms modelling supragingival plaque. Caries Res. 2011;45:31–39. doi: 10.1159/000322646. [DOI] [PubMed] [Google Scholar]
- Group O.W. Monitoring the incidence and causes of diseases potentially transmitted by food in Australia: Annual report of the OzFoodNet network, 2010. Commun. Dis. Intell. Q. Rep. 2012;36:E213. [PubMed] [Google Scholar]
- Hald T., Aspinall W., Devleesschauwer B., Cooke R., Corrigan T., Havelaar A.H., Gibb H.J., Torgerson P.R., Kirk M.D., Angulo F.J., Lake R.J., Speybroeck N., Hoffmann S. World health Organization estimates of the relative Contributions of food to the Burden of disease due to selected foodborne Hazards: a structured Expert Elicitation. PLoS One. 2016;11:e0145839. doi: 10.1371/journal.pone.0145839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannig C., Helbig R., Hilsenbeck J., Werner C., Hannig M. Impact of the springtail's cuticle nanotopography on bioadhesion and biofilm formation in vitro and in the oral cavity. Roy. Soc. Open Sci. 2018;5:171742. doi: 10.1098/rsos.171742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingston P.A., Stea E.C., Knøchel S., Hansen T. Role of initial contamination levels, biofilm maturity and presence of salt and fat on desiccation survival of Listeria monocytogenes on stainless steel surfaces. Food Microbiol. 2013;36:46–56. doi: 10.1016/j.fm.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Hossain M.I., Mizan M.F.R., Ashrafudoulla M., Nahar S., Joo H.J., Jahid I.K., Park S.H., Kim K.-S., Ha S.D. Inhibitory effects of probiotic potential lactic acid bacteria isolated from kimchi against Listeria monocytogenes biofilm on lettuce, stainless-steel surfaces, and MBEC™ biofilm device. LWT. 2020;118:108864. [Google Scholar]
- Iibuchi R., Hara-Kudo Y., Hasegawa A., Kumagai S. Survival of Salmonella on a polypropylene surface under dry conditions in relation to biofilm-formation capability. J. Food Prot. 2010;73:1506–1510. doi: 10.4315/0362-028x-73.8.1506. [DOI] [PubMed] [Google Scholar]
- Jahid I.K., Han N.R., Srey S., Ha S.D. Competitive interactions inside mixed-culture biofilms of Salmonella Typhimurium and cultivable indigenous microorganisms on lettuce enhance microbial resistance of their sessile cells to ultraviolet C (UV-C) irradiation. Food Res. Int. 2014;55:445–454. [Google Scholar]
- Jahid I.K., Han N., Zhang C.-Y., Ha S.-D. Mixed culture biofilms of Salmonella Typhimurium and cultivable indigenous microorganisms on lettuce show enhanced resistance of their sessile cells to cold oxygen plasma. Food Microbiol. 2015;46:383–394. doi: 10.1016/j.fm.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Jain S., Chen J. Attachment and biofilm formation by various serotypes of Salmonella as influenced by cellulose production and thin aggregative fimbriae biosynthesis. J. Food Prot. 2007;70:2473–2479. doi: 10.4315/0362-028x-70.11.2473. [DOI] [PubMed] [Google Scholar]
- Jeon H.R., Kwon M.J., Yoon K.S. Control of Listeria innocua biofilms on food contact surfaces with slightly acidic electrolyzed water and the risk of biofilm cells transfer to duck meat. J. Food Prot. 2018;81:582–592. doi: 10.4315/0362-028X.JFP-17-373. [DOI] [PubMed] [Google Scholar]
- Jung S.-J., Park S.Y., Ha S.D. Synergistic effect of X-ray irradiation and sodium hypochlorite against Salmonella enterica serovar Typhimurium biofilms on quail eggshells. Food Res. Int. 2018;107:496–502. doi: 10.1016/j.foodres.2018.02.063. [DOI] [PubMed] [Google Scholar]
- Khatoon Z., McTiernan C.D., Suuronen E.J., Mah T.F., Alarcon E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon. 2018;4:e01067. doi: 10.1016/j.heliyon.2018.e01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khieu V., Srey S., Schar F., Muth S., Marti H., Odermatt P. Strongyloides stercoralis is a cause of abdominal pain, diarrhea and urticaria in rural Cambodia. BMC Res. Notes. 2013;6:200. doi: 10.1186/1756-0500-6-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A.-N., Park S.Y., Bae S.-C., Oh M.-H., Ha S.D. Survival of norovirus surrogate on various food-contact surfaces. Food Environ. Virol. 2014;6:182–188. doi: 10.1007/s12560-014-9154-4. [DOI] [PubMed] [Google Scholar]
- Kim M., Park S.Y., Ha S.D. Synergistic effect of a combination of ultraviolet–C irradiation and sodium hypochlorite to reduce Listeria monocytogenes biofilms on stainless steel and eggshell surfaces. Food Control. 2016;70:103–109. [Google Scholar]
- Kirk M., Ford L., Glass K., Hall G. Foodborne illness, Australia, circa 2000 and circa 2010. Emerg. Inf. Dis. 2014;20:1857. doi: 10.3201/eid2011.131315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroupitski Y., Pinto R., Brandl M.T., Belausov E., Sela S. Interactions of Salmonella enterica with lettuce leaves. J. Appl. Microbiol. 2009;106:1876–1885. doi: 10.1111/j.1365-2672.2009.04152.x. [DOI] [PubMed] [Google Scholar]
- Lamas A., Fernandez-No I.C., Miranda J.M., Vázquez B., Cepeda A., Franco C.M. Biofilm formation and morphotypes of Salmonella enterica subsp. arizonae differs from those of other Salmonella enterica subspecies in isolates from poultry houses. J. Food Prot. 2016;79:1127–1134. doi: 10.4315/0362-028X.JFP-15-568. [DOI] [PubMed] [Google Scholar]
- Lamas A., Regal P., Vázquez B., Miranda J.M., Cepeda A., Franco C.M. Salmonella and Campylobacter biofilm formation: a comparative assessment from farm to fork. J. Sci. Food Agric. 2018;98:4014–4032. doi: 10.1002/jsfa.8945. [DOI] [PubMed] [Google Scholar]
- Liu S., Gunawan C., Barraud N., Rice S.A., Harry E.J., Amal R. Understanding, monitoring, and controlling biofilm growth in drinking water distribution systems. Environ. Sci. Technol. 2016;50:8954–8976. doi: 10.1021/acs.est.6b00835. [DOI] [PubMed] [Google Scholar]
- Mattick K., Legan J., Humphrey T., Peleg M. Calculating Salmonella survival during non-isothermal heat inactivation. J. Food Prot. 2001;64:606–613. doi: 10.4315/0362-028x-64.5.606. [DOI] [PubMed] [Google Scholar]
- McAuley C.M., Duffy L.L., Subasinghe N., Hogg G., Coventry J., Fegan N. Salmonella Typhimurium and Salmonella Sofia: growth in and persistence on eggs under production and retail conditions. Biomed. Res. Int. 2015;2015:914987. doi: 10.1155/2015/914987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L., Busscher H.J., van der Mei H.C., Ren Y. Influence of surface roughness on streptococcal adhesion forces to composite resins. Dent. Mater. 2011;27:770–778. doi: 10.1016/j.dental.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Mizan M.F.R., Ashrafudoulla M., Sadekuzzaman M., Kang I., Ha S.D. Effects of NaCl, glucose, and their combinations on biofilm formation on black tiger shrimp (Penaeus monodon) surfaces by Vibrio parahaemolyticus. Food Control. 2018;89:203–209. [Google Scholar]
- Mizan M.F.R., Jahid I.K., Ha S.D. Microbial biofilms in seafood: a food-hygiene challenge. Food Microbiol. 2015;49:41–55. doi: 10.1016/j.fm.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Muñoz-Cuevas M., Guevara L., Aznar A., Martínez A., Periago P.M., Fernández P.S. Characterisation of the resistance and the growth variability of Listeria monocytogenes after high hydrostatic pressure treatments. Food Control. 2013;29:409–415. [Google Scholar]
- Nidaullah H., Abirami N., Shamila-Syuhada A.K., Chuah L.O., Nurul H., Tan T.P., Abidin F.W.Z., Rusul G. Prevalence of Salmonella in poultry processing environments in wet markets in Penang and Perlis, Malaysia. Vet. World. 2017;10:286–292. doi: 10.14202/vetworld.2017.286-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande V.V., McWhorter A.R., Chousalkar K.K. Salmonella enterica isolates from layer farm environments are able to form biofilm on eggshell surfaces. Biofouling. 2016;32:699–710. doi: 10.1080/08927014.2016.1191068. [DOI] [PubMed] [Google Scholar]
- Pankaj S., Misra N., Cullen P. Kinetics of tomato peroxidase inactivation by atmospheric pressure cold plasma based on dielectric barrier discharge. Innov. Food Sci. Emerg. 2013;19:153–157. [Google Scholar]
- Park S., Choi S., Kim H., Kim Y., Kim B.S., Beuchat L.R., Ryu J.H. Fate of mesophilic aerobic bacteria and Salmonella enterica on the surface of eggs as affected by chicken feces, storage temperature, and relative humidity. Food Microbiol. 2015;48:200–205. doi: 10.1016/j.fm.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Park S., Oh Y., Jo B., Lee J., Lee S., Jeong J. Study of formation factor of biofilm on aluminum surface and removal efficiency of biofilm by antimicrobials. Korean Chem. Eng. Res. 2015;53:730–739. [Google Scholar]
- Park S.Y., Ha S.D. Application of cold oxygen plasma for the reduction of Cladosporium cladosporioides and Penicillium citrinum on the surface of dried filefish (Stephanolepis cirrhifer) fillets. Int. J. Food Sci. Technol. 2015;50:966–973. [Google Scholar]
- Park S.Y., Jung S.J., Ha S.D. Synergistic effects of combined X-ray and aqueous chlorine dioxide treatments against Salmonella Typhimurium biofilm on quail egg shells. LWT. 2018;92:54–60. [Google Scholar]
- Pasquali F., Klein G., Reich F., Manfreda G., Valero A. Modelling survival behaviour of Salmonella enterica ser. Enteritidis, Typhimurium and Tennessee on table eggs during storage at different temperatures. Food Control. 2016;59:314–319. [Google Scholar]
- Peleg M., Cole M. Estimating the survival of Clostridium botulinum spores during heat treatments. J. Food Prot. 2000;63:190–195. doi: 10.4315/0362-028x-63.2.190. [DOI] [PubMed] [Google Scholar]
- Petridis X., Busanello F.H., So M.V.R., Dijkstra R.J.B., Sharma P.K., van der Sluis L.W.M. Chemical efficacy of several NaOCl concentrations on biofilms of different architecture: new insights on NaOCl working mechanisms. Int. Endod. J. 2019;52:1773–1788. doi: 10.1111/iej.13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk S., Ayres J., Kraft A. Effect of holding condition on the development of Salmonellae in artificially inoculated hens’ eggs. Poult. Sci. 1966;45:825–829. doi: 10.3382/ps.0450825. [DOI] [PubMed] [Google Scholar]
- Ronner A.B., Wong A.C.L. Biofilm development and sanitizer inactivation of Listeria monocytogenes and Salmonella Typhimurium on stainless-steel and Buna-n rubber. J. Food Prot. 1993;56:750–758. doi: 10.4315/0362-028X-56.9.750. [DOI] [PubMed] [Google Scholar]
- Sadekuzzaman M., Mizan M.F.R., Kim H.S., Yang S., Ha S.D. Activity of thyme and tea tree essential oils against selected foodborne pathogens in biofilms on abiotic surfaces. LWT. 2018;89:134–139. [Google Scholar]
- Schlisselberg D.B., Yaron S. The effects of stainless steel finish on Salmonella Typhimurium attachment, biofilm formation and sensitivity to chlorine. Food Microbiol. 2013;35:65–72. doi: 10.1016/j.fm.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Stepanović S., Ćirković I., Ranin L., vabić-Vlahović M.S. Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Lett. Appl. Microbiol. 2004;38:428–432. doi: 10.1111/j.1472-765X.2004.01513.x. [DOI] [PubMed] [Google Scholar]
- van Boekel M.A. On the use of the Weibull model to describe thermal inactivation of microbial vegetative cells. Int. J. Food Microbiol. 2002;74:139–159. doi: 10.1016/s0168-1605(01)00742-5. [DOI] [PubMed] [Google Scholar]
- Wang H., Zhang X., Zhang Q., Ye K., Xu X., Zhou G. Comparison of microbial transfer rates from Salmonella spp. biofilm growth on stainless steel to selected processed and raw meat. Food Control. 2015;50:574–580. [Google Scholar]
- Whiting R., Sackitey S., Calderone S., Morely K., Phillips J. Model for the survival of Staphylococcus aureus in nongrowth environments. Int. J. Food Microbiol. 1996;31:231–243. doi: 10.1016/0168-1605(96)01002-1. [DOI] [PubMed] [Google Scholar]
- Wolf-Hall C., Nganje W. CABI; 2017. Microbial Food Safety: A Food Systems Approach. [Google Scholar]