Abstract
Coccidiosis is one of the most prevalent diseases seen in the poultry industry leading to excessive economic losses. The aim of this study was to investigate the effect of butyric acid glycerol esters (BE) on the ileal and cecal microbiota in birds challenged with Eimeria maxima (EM). Ross 708 male broilers were fed a diet supplemented with 0 (control) or 0.25% BE from day 1. On day 21, half of the birds were infected with 103 EM oocysts. For determing microbiota, ileal and cecal contents and epithelial scrapings were collected at 7 and 10 D postinfection (PI). Alpha diversity of bacterial communities was mostly affected (P < 0.05) by time PI and EM infection. The richness of luminal bacterial populations in the ileum and ceca was affected (P < 0.05) by addition of BE and by time PI × EM × BE interaction, respectively. In the ileal and cecal luminal and mucosal bacterial communities, permutational multivariate analysis of variance (PERMANOVA, unweighted UniFrac) showed significant (P < 0.05) differences because of time PI and interaction between time PI, EM, and BE. Significant (P < 0.05) differences in taxonomic composition at the family level were observed in microbiota of luminal and mucosal populations of the ileum and ceca owing to time PI, EM, BE, and their interactions. The bacterial community present in the cecal lumen was characterized by the lowest number of differential bacteria, whereas the cecal mucosal community was characterized by the highest number of differentially abundant bacteria. In conclusion, our results show that EM infection and time PI has the biggest impact on microbial diversity in the chicken gut. The presence of BE in the diet had a limited effect on gut microbiota.
Key words: 16S, microbiota, butyrate, gastrointestinal tract, chicken
Introduction
Coccidiosis is one of the most endemic enteric diseases in poultry production resulting in more than $3 billion in annual losses worldwide because of weight loss and poor feed use (Blake and Tomley, 2014; Chapman, 2014). It is caused by a protozoal parasite of the genus Eimeria, and severity of infection depends on species, magnitude, and site of infection (Chapman, 2014). Eimeria tenella, Eimeria maxima, and Eimeria acervulina are the most important species in terms of global disease burden and economic impact (Blake and Tomley, 2014). Coccidiosis may result in a limited enteritis (fluid loss and malabsorption), inflammation of the intestinal wall with hemorrhaging and epithelial cell sloughing, and complete villi destruction leading to impaired growth and feed utilization and death of infected birds (Blake and Tomley, 2014; Chapman, 2014). Moreover, coccidiosis increases intestinal colonization of other bacterial pathogens such as Clostridium perfringens and Salmonella (Wu et al., 2014). Conventional strategies to treat coccidiosis rely on combinations of husbandry, chemoprophylaxis, and live vaccines. In the era of increasing bacterial antibiotic resistance, use of natural alternatives such as fats, antioxidants, essential oils, herbal extracts, prebiotics, and probiotics have shown some promise in ameliorating the negative effects of coccidiosis.
Butyrate, a short-chain fatty acid, is the product of fermentation of nonstarch polysaccharides and unabsorbed starch by anaerobic bacteria (Liu et al., 2017). In chickens, butyrate has been implicated in inducing expression of antimicrobial host defense peptides (Sunkara et al., 2011) and modulating the expression and release of anti-inflammatory and proinflammatory cytokines (Zhang et al., 2011). In addition, butyrate has been shown to have immunomodulatory activity by reducing bacterial colonization, modulating immunity, and suppressing inflammation, as well as improving growth performance in chickens (Smulikowska et al., 2009). Moreover, it has been previously shown that butyrate has a positive effect on coccidia infection by sustaining broiler health and production, lowering severity of infection, and depressing oocyst production (Ali et al., 2014).
Little is known of the effect of E. maxima on the microbiota in the small intestine and ceca. Therefore, the present study addresses the effect of E. maxima infection on chicken gut microbiome as well as the role of butyric acid glycerol esters on the ileal and cecal microbiota in healthy chickens and chickens challenged with E. maxima.
Material and methods
Animals and Experimental Protocols and Tissue Sampling
All animal care procedures were approved by the USDA-Agricultural Research Service Institutional Animal Care and Use Committee. Ninety-six 1-day old male broiler chicks (Ross 708) were obtained from a local hatchery (Longenecker's Hatchery, Elizabethtown, PA) and placed into 1 m2 wire pens (25 birds per pen). Nineteen-day-old birds were moved into 24 battery cages (Petersime finisher units; Petersime, Gettysburg, OH), with 4 birds placed per cage (pen). All birds had full access to a commercial type corn–soybean meal–based diet from day 1 to the end of the experiment. In addition, the diet was supplemented with 0 (control, C) or 0.25% (w/w) of butyric acid glycerol esters (BE; ProPhorce SR 130; Perstorp, Waspik, Netherlands). At 21 D of age, half of the birds (48 birds, total) in each feeding group (C and BE) were infected with 103 E. maxima (EM) oocysts (laboratory strain APU-1) (Fetterer and Barfield, 2003) per bird by oral gavage in a volume of 1 mL (EM and BE + EM). Remaining half of the birds were sham infected with water (C and BE).
Birds from all treatment groups (C, EM, BE, and BE + EM) were sacrificed by cervical dislocation 7 or 10 D postinfection (PI) (n = 3 for each treatment group). At each sampling point, the distal part of the ileum (from Meckel's diverticulum to ileocecal junction) and ceca were dissected from 1 bird per pen for collection of ileal content (IlC) and cecal content (CeC) as well as their epithelial scrapings (ileal scraping [IlS] and cecal scraping [CeS]) to collect luminal and mucosal bacterial populations, respectively. Isolated specimens were snap frozen in liquid nitrogen and stored at −80°C until bacterial DNA isolation.
DNA Isolation, Library Preparation, and Analysis
DNA was extracted from each of the samples using a DNeasy PowerSoil kit (Qiagen, Valencia, CA) and a QIAcube instrument (Qiagen) as per the manufacturer's protocol. DNA concentration and quality were assessed by NanoDrop (TermoFisher Scientific, Inc. Waltham, MA) and TapeStation System (Agilent Technologies, Santa Clara, CA), respectively. The 16S rRNA gene amplicon libraries were generated using the workflow and chemistry supplied by Illumina (Illumina, Inc., San Diego, CA) and PCR primers described by Daquigan et al. (2016) that target the V1-V3 variable region of the 16S gene. The pooled DNA library was diluted to a final concentration of 4 pM and mixed with PhiX (Illumina, Inc., 4 nmol) control (20% v/v) and pair-end 2 × 300 bp sequenced using the Illumina MiSeq platform and a MiSeq Reagent Kit v3 (Illumina, Inc). The 16S rRNA gene sequences determined in this study were deposited in the NCBI Sequence Read Archive database (SRA accession # PRJNA556819).
Quantitative Insight Into Microbial Ecology software package 2 (version 2017.12.0, http://qiime2.org) was used to perform quality control and analysis of the sequence reads. Greengenes v13_8 database (http://greengenes.secongenome.com) was used for taxonomic composition.
Statistical Analyses
Microbiome composition data were obtained by normalization of total number of reads in each sample (relative abundance) and were analyzed using three-way ANOVA by the GLM of the Statistical Analysis System software v9.4 (SAS Institute, Cary, NC). Main effects were analyzed only when 2-way analysis was not significant (P > 0.05). Significance was set to P < 0.05.
Results and discussion
In the era of increasing antibiotic resistance owing to use of antibiotics in food production animals, the development of natural alternatives to growth promoters and antibiotics is attractive to the producer as well as the consumer. Butyrate is one of the feed additives that can be used as an alternative to antibiotic growth promoters, as a factor to ameliorate negative effects of some diseases such as coccidiosis, and as a modulator of microbiota of the gastrointestinal tract. In this article, we describe the effect of butyrate addition to the feed on diversity of the intestinal microbiota in healthy and coccidia-challenged broiler chickens.
Microbial Diversity
Among the 4 microbiota populations, IlC was the most affected, whereas IlS was the least affected, with respect to alpha diversity (Table 1). The IlC had higher (P < 0.05) Shannon and Evenness index on 7 D PI in comparison with 10 D PI, and the richness index was significantly lower in birds receiving BE in the diet. The number of operational taxonomic unit (OTU) in the IlS of EM-infected birds was significantly higher than that in uninfected birds. The richness of the bacterial community of CeC was significantly higher at 7 D PI than at 10 D PI regardless of whether they were infected with EM or treated with BE. In addition, for CeC richness, there was a significant interaction between time PI, infection, and butyrate addition in feed. In CeS microbiota, the number of OTU and evenness were affected (P < 0.05) by time, with birds at 7 D PI characterized by the lower (P < 0.05) number of OTU and higher evenness in comparison with day 10 PI (Table 1). Changes in microbiota due to time and due to infection were expected. It has been shown that the chicken luminal and mucosal bacterial populations undergo substantial changes over time (Awad et al., 2016). Moreover, Eimeria infection could result in large shifts in microbiota composition and diversity (Wu et al., 2014). Conversely, Leung et al. (2019) showed that mixed Eimeria species challenge in birds had no effect on alpha diversity. It has been reported previously that dietary butyrate glycerides modulate intestinal microbiota (Yang et al., 2018). In our experiment, only the IlC bacterial population was affected by presence of butyrate in the diet. The lack of changes in alpha diversity because of presence of BE is in accordance with the study by Bortoluzzi et al. (2017). Damaged intestines can lead to the shift in the gut microbiota owing to a combination of changes in the immune response and the increased presence of undigested nutrients in the distal end of the intestine, that is, in the ceca (Leung et al., 2019). In this study, a significant effect of infection in the ileum and time PI on CeC and CeS alpha diversity with interaction of all 3 factors (time PI, EM, and BE) affecting CeC richness was observed.
Table 1.
Effect of time postinfection (PI), infection with Eimeria maxima (EM) or butyric acid glycerol esters (BE) addition in the diet on alpha diversity indices and beta diversity (PERMANOVA) in ileal content (IlC) and cecal content (CeC) and scrapings (ileal scraping [IlS] and cecal scraping [CeS]).
| Analysis | Pr = F |
|||
|---|---|---|---|---|
| ILC | IlS | CeC | CeS | |
| Observed OTU | ||||
| Time PI | 0.056 | 1.00 | 0.112 | 0.043 |
| EM | 0.139 | 0.049 | 0.583 | 0.507 |
| BE | 0.324 | 0.298 | 0.751 | 0.248 |
| Time PI × EM × BE | 0.161 | 0.170 | 0.614 | 0.204 |
| Shannon diversity index | ||||
| Time PI | 0.049 | 0.386 | 0.273 | 0.356 |
| EM | 0.139 | 0.453 | 0.729 | 0.166 |
| BE | 0.666 | 0.356 | 0.773 | 0.326 |
| Time PI × EM × BE | 0.134 | 0.513 | 0.737 | 0.486 |
| Faith's Phylogenetic Diversity (Richness) index | ||||
| Time PI | 0.065 | 0.133 | <0.001 | 0.094 |
| EM | 0.758 | 0.094 | 0.686 | 0.204 |
| BE | 0.042 | 0.453 | 0.729 | 0.225 |
| Time PI × EM × BE | 0.169 | 0.244 | 0.017 | 0.170 |
| Pielou's Evenness index | ||||
| Time PI | 0.019 | 0.299 | 0.729 | 0.043 |
| EM | 0.295 | 0.729 | 0.773 | 0.065 |
| BE | 0.951 | 0.686 | 0.488 | 1.000 |
| Time PI × EM × BE | 0.103 | 0.675 | 0.700 | 0.210 |
| PERMANOVA analysis (unweighted UniFrac) | ||||
| Time PI | 0.040 | 0.001 | 0.001 | 0.001 |
| EM | 0.191 | 0.670 | 0.472 | 0.553 |
| BE | 0.140 | 0.827 | 0.502 | 0.404 |
| Time PI × EM × BE | 0.194 | 0.001 | 0.001 | 0.001 |
Abbreviations: BE, birds receiving 0.25% butyric acid glycerol esters in a diet; BE + EM, birds receiving 0.25% BE in a diet and infected with EM, 7 and 10 day PI; C, control birds; EM, birds infected with Eimeria maxima (EM); OTU, operational taxonomic unit; PERMANOVA, permutational multivariate analysis of variance; PI, postinfection.
Previously published data show that highly diverse microbial populations are characterized by a stronger homeostasis of intestinal microbiota, improved gut health, and resistance to pathogens and were associated with greater activation of immune cells and inflammatory processes (Leung et al., 2019). Permutational multivariate analysis of variance based on unweighted distances was used to determine the similarities between pairs of microbial communities. Time PI as well as interaction between EM and BE were significant in IlS and both cecal bacterial populations, whereas the ILC population was only affected by time PI (Table 1). Although the ileum and cecum play different physiological functions, with the ileum being responsible for nutrient absorption and cecum being the main site of microbial fermentation, the bacterial populations residing in them responded to infection and presence of BE in a similar way. The lack of observed effect of BE on the cecal bacterial population could be related to stability of cecal microbiota owing to higher diversity resulting in stronger resilience (Choi et al., 2018). In accordance with our results, Stanley et al. (2014) reported significant changes in beta diversity of the cecal microbial population during mixed Eimeria model infection. However, in contrast to our results, Bortoluzzi et al. (2017) showed that unweighted and weighted UniFrac analysis revealed differences in beta diversity associated with the presence of butyrate in the diet.
Taxonomic Composition of Bacterial Community
At the phylum level, normal chicken microbiota (C) was dominated by Firmicutes and Proteobacteria in IlC and CeC, whereas IlS and CeS where characterized by predominance of Firmicutes, Proteobacteria, and unclassified bacteria (UNB, data not shown). Similar results were observed by Choi et al. (2014) and Yang et al. (2018), whereas Wei et al. (2013) have reported that Proteobacteria is the main bacterial phyla present in the chicken gut. These variations in taxonomic composition could be explained by type of diet and age of the chicken. In addition, we cannot exclude that the E. maxima infection affected the level of less predominant phyla in the chicken gut.
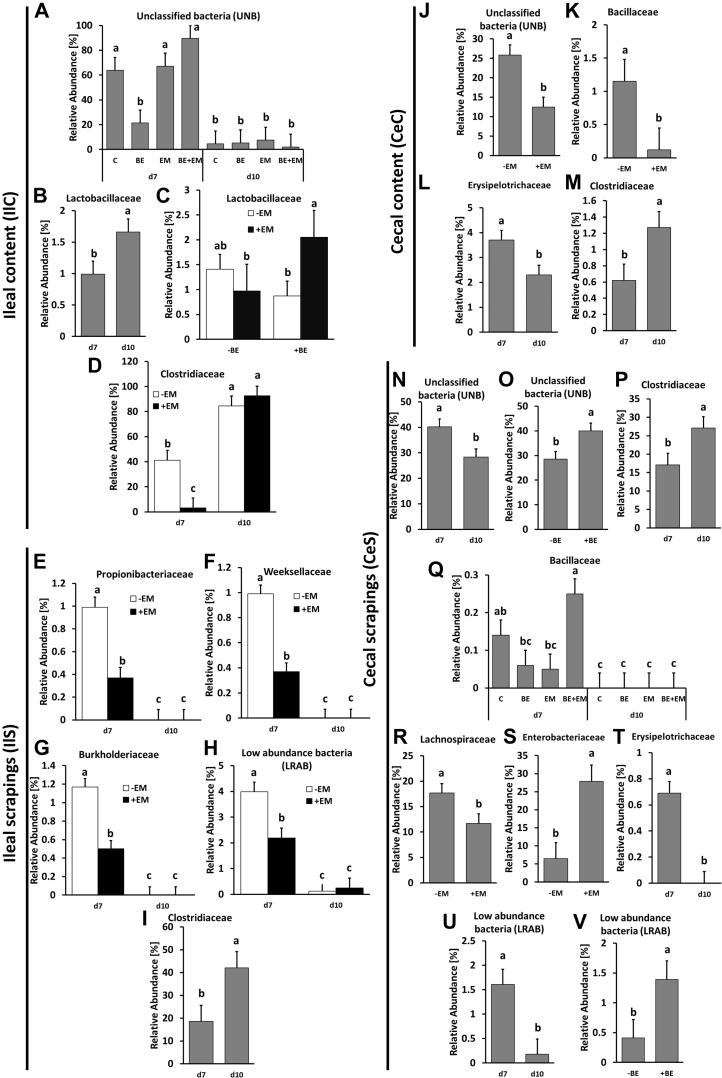
Significant changes in the bacterial level were observed at the family level in all 4 microbiota populations. Moreover, all 4 microbial populations responded differently to infection and BE, with CeC being the least affected population and CeS the most affected bacterial population for taxonomic composition at the family level. The only significant (P < 0.05) changes in microbiota families are presented in Figure 1. Abundance of UNB was affected (P < 0.05) by time PI, interaction between EM and BE, with C, EM, and BE + EM birds been characterized by the highest abundance of these bacteria in comparison with BE and day 10 PI groups in IlC (Figure 1A), whereas in CeC, the UNB level was affected by EM infection with significantly lower bacterial abundance in infected birds (Figure 1J). In CeS, UNB were affected by time PI with a significantly (P < 0.05) higher level at 7 D PI (Figure 1N). In CeS, UNB were also affected by presence of BE in feed, with birds receiving BE having a higher (P < 0.05) abundance of UNB (Figure 1O). The level of UNB could be related to the 16S primers used to generate the data. Our unpublished data suggest that the choice of 16S rRNA primers and database selection have effect on detection of bacterial taxonomic composition.
Figure 1.
The effect of time post infection (PI, 7 vs. 10 D), presence of butyric acid glycerol esters in diet (+BE vs. −BE), infection with Eimeria maxima (+EM vs. −EM), and their interactions on relative bacteria abundance in chicken ileal content (IlC) (A–D), ileal scrapings (IlS) (E–I), cecal content (CeC) (J–M), and cecal scrapings (CeS) (N–V). When 3-factor interaction was nonsignificant (P > 0.05), 2-factor interaction was considered followed by evaluation of main factors. Only statistically (P < 0.05) significant data are shown. Abbreviations: BE, birds receiving 0.25% butyric acid glycerol esters (BE) in a diet; BE + EM, birds receiving 0.25% BE in a diet and infected with EM, 7 and 10 D PI; C, control birds; EM, birds infected with Eimeria maxima (EM); PI, postinfection. Different letters denote statistically significant (P < 0.05) differences between treatment groups.
The level of Lactobacillaceae significantly (P < 0.05) increased on day 10 in comparison with 7 D PI (Figure 1B) and was significantly (P < 0.05) higher in infected birds that were receiving BE than in not-infected birds that were receiving BE, or birds infected but not supplemented with BE (Figure 1C). Lactobacillaceae has been shown to be dominant in microbiota of the ileum and is responsible for the sanitary status of the gut and microbiota balance (Pineda-Quiroga et al., 2018). Lactobacillaceae can also stimulate butyric acid–producing bacteria and was shown to produce effective bacteriocins against some pathogenic bacteria and other pathogens (Pineda-Quiroga et al., 2018). Interestingly, in our study, chickens infected with EM that were also receiving butyrate had elevated abundance of Lactobacillaceae. It has been shown that bacteriocins produced by Lactobacillaceae are active against Eimeria, which may indicate a positive effect of BE on the ileal microbiota.
In our study, Clostridiaceae abundance in IlC was affected (P < 0.05) by time as well as interaction between time and infection, with birds at day 10 PI being characterized by a significantly higher abundance of this family than those at 7 D PI, regardless of infection (Figure 1D). Similarly, in IlS, its abundance was also higher on day 10 PI than on day 7 PI (Figure 1I). In CeC and CeS, increase (P < 0.05) in abundance of Clostridiaceae was observed at day 10 PI. Clostridiaceae is a highly diverse family that includes genera important to nutrient digestibility but also those that are considered pathogenic (Yang et al., 2018).
In comparison with EM-infected birds at 7 D PI in IlS, we found that the presence of Propionibacteriaceae (Figure 1E), Weeksellaceae (Figure 1F), and Burkholderiaceae (Figure 1G) was significantly higher at 7 D PI than at 10 D PI, as well as in noninfected birds. In early-culture–based studies, Propionibacteriaceae were shown by to be a part of the chicken microbiota and to contribute in birds to modulation of microbiota, early development of epithelial cells, mucus production if supplied as probiotic, and led to increased protection against entrance of pathogens (Martinez et al., 2016). Conversely, Weeksellaceae spp. are considered to be a source of potential pathogens, and their presence in the microbiota has been shown to be modified by probiotics containing Lactobacillus (Gioacchini et al., 2018). In this study, a decrease in the abundance of Weeksellaceae may be a result of increase in Lactobacillaceae. Burkholderiaceae belongs to the phylum Proteobacteria, and most of its members are considered as opportunistic human pathogens (Hoger et al., 2016). Currently, the specific role of Burkholderiacea in the chicken microbiota has not been defined.
In CeC, Bacillaceae were affected (P < 0.05) by EM infection with significantly lower abundance in infected birds (Figure 1K), whereas in CeS, their abundance was affected (P < 0.05) by BE × EM × PI interaction (Figure 1Q). The BE + EM group had the highest (P < 0.05) abundance of Bacillaceae, which was higher than the BE and EM groups, but not than C group at 7 D PI, and to all treatment groups at 10 D PI. Members of the Bacillaceae, Bacillus spp., are often used as a source of probiotics in poultry production because of their antimicrobial and immune-stimulatory properties (Mongkolthanaruk, 2012). Increase in Bacillaceae after BE treatment during infection with EM suggests positive effect of BE on the microbiota.
In the CeC and CeS, Erysipelotrichaceae were affected (P < 0.05) by time PI with lower level at 10 D PI than 7 D P. Lachnospiraceae are known to break down complex plant-derived carbohydrates and resistant starches to make them available for fermentation and high energy metabolism (Pineda-Quiroga et al., 2018). In our study, abundance of Lachnospiraceae was affected by EM infection with a decreased level of Lachnospiraceae in infected birds (Figure 1R). Lachnospiraceae are responsible for butyrate production (Li et al., 2017), and an increase in short-chain fatty acids has been shown to have a negative effect on Enterobacteriaceae population (Smulikowska et al., 2009). Indeed, in our study, an increased (P < 0.05) level of Enterobacteriaceae was observed in CeS of infected birds (Figure 1S).
Finally, the level of low-abundance bacteria was affected (P < 0.05) by the interaction between PI time and EM infection in IlS (Figure 1H) and by time PI and presence of BE in diet in CeS (Figures 1U, 1V). It is interesting that the low-abundance bacterial families were mostly affected by experimental treatments in mucosal microbiota in both, the ileum and cecum. The increase in low-abundance bacteria due to presence of BE, which was observed in this experiment, would suggest a beneficial effect of supplying BE to the diet because increased microbial diversity is associated with improved gut health. Overall, the low number of changes observed in taxonomic composition in cecal luminal microbiota suggests that the cecal population is resistant to changes, possibly because of higher microbial diversity. Similar results were reported in the study by Bortoluzzi et al., 2017, which showed that the most abundant members of cecal microbiota were not affected by dietary treatment. In addition to the effect of time PI, EM infection also decreased the population of these bacteria in IlS. Similar results were observed by Wu et al. (2014).
In conclusion, we have shown that addition of BE into the chicken diet increased the abundance of Bacilli and Lactobacillaceae as well as increasing the diversity of the bacterial populations in birds infected with E. maxima. Moreover, our results indicate that EM infection and time PI produced the largest impact on the balance of gut microbiota in chickens. Further studies are required to fully understand the mechanisms by which butyrate elicits positive effects on broiler performance during Eimeria infection.
Acknowledgments
The work was funded by in house USDA-ARS CRIS # 8042-31000-108-00D.
Conflict of Interest Statement: The authors declare no conflict of interest.
References
- Ali A.M., Seddiek Sh A., Khater H.F. Effect of butyrate, clopidol and their combination on the performance of broilers infected with Eimeria maxima. Br. Poult. Sci. 2014;55:474–482. doi: 10.1080/00071668.2014.920488. [DOI] [PubMed] [Google Scholar]
- Awad W.A., Mann E., Dzieciol M., Hess C., Schmitz-Esser S., Wagner M., Hess M. Age-related differences in the luminal and mucosa-associated gut microbiome of broiler chickens and shifts associated with Campylobacter jejuni infection. Front Cell Infect. Microbiol. 2016;6:154. doi: 10.3389/fcimb.2016.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D.P., Tomley F.M. Securing poultry production from the ever-present Eimeria challenge. Trends Parasitol. 2014;30:12–19. doi: 10.1016/j.pt.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Bortoluzzi C., Pedroso A.A., Mallo J.J., Puyalto M., Kim W.K., Applegate T.J. Sodium butyrate improved performance while modulating the cecal microbiota and regulating the expression of intestinal immune-related genes of broiler chickens. Poult. Sci. 2017;96:3981–3993. doi: 10.3382/ps/pex218. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. Milestones in avian coccidiosis research: a review. Poult. Sci. 2014;93:501–511. doi: 10.3382/ps.2013-03634. [DOI] [PubMed] [Google Scholar]
- Choi J.H., Kim G.B., Cha C.J. Spatial heterogeneity and stability of bacterial community in the gastrointestinal tracts of broiler chickens. Poult. Sci. 2014;93:1942–1950. doi: 10.3382/ps.2014-03974. [DOI] [PubMed] [Google Scholar]
- Choi J.H., Lee K., Kim D.W., Kil D.Y., Kim G.B., Cha C.J. Influence of dietary avilamycin on ileal and cecal microbiota in broiler chickens. Poult. Sci. 2018;97:970–979. doi: 10.3382/ps/pex360. [DOI] [PubMed] [Google Scholar]
- Daquigan N., Grim C.J., White J.R., Hanes D.E., Jarvis K.G. Early Recovery of Salmonella from food using a 6-Hour non-selective Pre-enrichment and Reformulation of Tetrathionate Broth. Front Microbiol. 2016;7:2103. doi: 10.3389/fmicb.2016.02103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetterer R.H., Barfield R.C. Characterization of a developmentally regulated oocyst protein from Eimeria tenella. J. Parasitol. 2003;89:553–564. doi: 10.1645/GE-3159. [DOI] [PubMed] [Google Scholar]
- Gioacchini G., Ciani E., Pessina A., Cecchini C., Silvi S., Rodiles A., Merrifield D.L., Olivotto I., Carnevali O. Correction to: effects of Lactogen 13, a New probiotic Preparation, on gut microbiota and Endocrine Signals Controlling growth and Appetite of Oreochromis niloticus Juveniles. Microb. Ecol. 2018;76:1063–1074. doi: 10.1007/s00248-018-1177-1. [DOI] [PubMed] [Google Scholar]
- Hoger A.C., Mayo M., Price E.P., Theobald V., Harrington G., Machunter B., Choy J.L., Currie B.J., Kaestli M. The melioidosis agent Burkholderia pseudomallei and related opportunistic pathogens detected in faecal matter of wildlife and livestock in northern Australia. Epidemiol. Infect. 2016;144:1924–1932. doi: 10.1017/S0950268816000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung H., Yitbarek A., Snyder R., Patterson R., Barta J.R., Karrow N., Kiarie E. Responses of broiler chickens to Eimeria challenge when fed a nucleotide-rich yeast extract. Poult. Sci. 2019;98:1622–1633. doi: 10.3382/ps/pey533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Wang W., Liu D., Guo Y. Effects of Lactobacillus acidophilus on gut microbiota composition in broilers challenged with Clostridium perfringens. PLoS One. 2017;12:e0188634. doi: 10.1371/journal.pone.0188634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.D., Bayir H.O., Cosby D.E., Cox N.A., Williams S.M., Fowler J. Evaluation of encapsulated sodium butyrate on growth performance, energy digestibility, gut development, and Salmonella colonization in broilers. Poult. Sci. 2017;96:3638–3644. doi: 10.3382/ps/pex174. [DOI] [PubMed] [Google Scholar]
- Martinez E.A., Babot J.D., Lorenzo-Pisarello M.J., Apella M.C., Chaia A.P. Feed supplementation with avian Propionibacterium acidipropionici contributes to mucosa development in early stages of rearing broiler chickens. Benef. Microbes. 2016;7:687–698. doi: 10.3920/BM2016.0077. [DOI] [PubMed] [Google Scholar]
- Mongkolthanaruk W. Classification of Bacillus beneficial substances related to plants, humans and animals. J. Microbiol. Biotechnol. 2012;22:1597–1604. doi: 10.4014/jmb.1204.04013. [DOI] [PubMed] [Google Scholar]
- Pineda-Quiroga C., Camarinha-Silva A., Borda-Molina D., Atxaerandio R., Ruiz R., Garcia-Rodriguez A. Feeding broilers with dry whey powder and whey protein concentrate affected productive performance, ileal digestibility of nutrients and cecal microbiota community. Animal. 2018;12:692–700. doi: 10.1017/S1751731117002208. [DOI] [PubMed] [Google Scholar]
- Smulikowska S., Czerwinski J., Mieczkowska A., Jankowiak J. The effect of fat-coated organic acid salts and a feed enzyme on growth performance, nutrient utilization, microflora activity, and morphology of the small intestine in broiler chickens. J. Anim. Feed Sci. 2009;18:478–489. [Google Scholar]
- Stanley D., Wu S.B., Rodgers N., Swick R.A., Moore R.J. Differential responses of cecal microbiota to fishmeal, Eimeria and Clostridium perfringens in a necrotic enteritis challenge model in chickens. PLoS One. 2014;9:e104739. doi: 10.1371/journal.pone.0104739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkara L.T., Achanta M., Schreiber N.B., Bommineni Y.R., Dai G., Jiang W., Lamont S., Lillehoj H.S., Beker A., Teeter R.G., Zhang G. Butyrate enhances disease resistance of chickens by inducing antimicrobial host defense peptide gene expression. PLoS One. 2011;6:e27225. doi: 10.1371/journal.pone.0027225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S., Morrison M., Yu Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. 2013;92:671–683. doi: 10.3382/ps.2012-02822. [DOI] [PubMed] [Google Scholar]
- Wu S.B., Stanley D., Rodgers N., Swick R.A., Moore R.J. Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens. Vet. Microbiol. 2014;169:188–197. doi: 10.1016/j.vetmic.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Yang X., Yin F., Yang Y., Lepp D., Yu H., Ruan Z., Yang C., Yin Y., Hou Y., Leeson S., Gong J. Dietary butyrate glycerides modulate intestinal microbiota composition and serum metabolites in broilers. Sci. Rep. 2018;8:4940. doi: 10.1038/s41598-018-22565-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.H., Jiang Y., Zhu Q.F., Gao F., Dai S.F., Chen J., Zhou G.H. Sodium butyrate maintains growth performance by regulating the immune response in broiler chickens. Br. Poult. Sci. 2011;52:292–301. doi: 10.1080/00071668.2011.578121. [DOI] [PubMed] [Google Scholar]