Abstract
Prebiotics, probiotics, and synbiotics, delivered in ovo influence the colonization and development of the peripheral immune system in poultry. This study aimed to investigate the influence of the host genotype (broiler chickens [Ross 308] and old native Polish breed Green-legged Partridgelike [GP] chickens) on the number of B and T cells in the spleen and cecal tonsils (CT). The solution of a bioactive compound was injected in ovo on day 12 of egg incubation: prebiotics (galactooligosaccharides [GOS]), probiotics (Lactococcus lactis subsp. cremoris IBB477), and synbiotics (GOS + L. lactis). The samples were collected on day 7, day 21, and day 42 after hatching (n = 8). The number of Bu-1+ (B) cells, CD4+ cells, and CD8+ cells in the spleen and CT was estimated using immunohistochemistry. The number of germinal centers (GC) was determined in the spleen. In broilers, probiotics increased (P < 0.05) the number of CD4+ cells in the CT on day 7. On day 21, prebiotics raised (P < 0.01) the number of cells involved in cellular immunity in the CT (CD4+ and CD8+ cells) and spleen (CD8+ cells). On day 42, it was synbiotics that stimulated the colonization of both the CT and spleen by B cells, but colonization of the spleen only by CD4+ and CD8+ cells. In GP chickens, synbiotics enforced the cellular immunity (CD4+ or CD8+ cells) in the spleen at all time points. Synbiotics also stimulated the GC appearance on day 21 and day 42. In GP chickens, the influence of bioactive compounds on colonization of the CT was very limited. In broilers, we determined pronounced and age-dependent effects of prebiotics and synbiotics on the number of B and T cells in both the CT and spleen. In GP chickens, the most potent compound was synbiotics, which stimulated cellular immunity in the spleen but not in the CT. However, given the long-term effects on adaptive immune cells, synbiotics were the most potent compounds in both chicken genotypes.
Key words: Green-legged Partridgelike, spleen, cecal tonsil, morphology, immune system
Introduction
The immune responses vary between different types of chickens. There is a body of evidence suggesting that genetic selection toward performance traits influenced the immune system in chickens. For example, light-weight broiler chickens have high and long-lasting anti-SRBC titers after primary immunization as compared with heavy-weight broilers (Miller et al., 1992). The comparison between broilers and layers indicated that broilers mount strong short-term humoral responses, whereas layers mount long-term humoral responses in combination with strong cellular responses (Koenen et al., 2002).
Aside from genetics, also the environmental component can shape immune system development, especially at early stages of development. One of the most critical factors in immune system maturation is interaction with the healthy microbiome (reviewed by Broom and Kogut (2018)). The gut microbiome includes the total genetic information provided by a community of commensal, symbiotic, or pathogenic microorganisms (microbiota) inhabiting the mucosa and lumen of the gastrointestinal tract (GIT) (Wei et al., 2013). It can be modulated by prebiotics or probiotics (Ballou et al., 2016). Prebiotics are nonfermentable polysaccharides (e.g., inulin, fructooligosaccharides, oligofructose, galactooligosaccharides [GOS], mannooligosaccharides), which modulate the microbiome of the host's GIT (Charalampopoulos and Rastall, 2009). They selectively stimulate beneficial microorganisms, improve colonic microbiota composition, and have to be resistant to digestion in the upper segments of the GIT. Probiotics are microorganisms, including bacteria (e.g., Lactobacillus, Bifidobacterium, Bacillus, or Enterococcus), yeast (e.g., Saccharomyces boulardii or Saccharomyces cerevisiae), or other fungal species (e.g., Aspergillus oryzae or Candida pintolepsii). They should be nonpathogenic to the host organism, be resistant to low pH, and have a high concentration of bile acids.
In poultry practice, bioactive compounds (prebiotics and probiotics alone or combined into synbiotics) can be delivered to the host in different time points: on day 12 of egg incubation (in ovo stimulation), around days 17-18 of egg incubation (in ovo feeding), and in food/water during the posthatching period. When using synbiotics for in ovo stimulation on day 12 of egg incubation, prebiotic and probiotic components are not available to the host at the same time. Prebiotics, owing to the relatively small size, can penetrate the outer and inner shell membranes and stimulate development of the innate microflora in the embryonic guts (Płowiec, 2018). Probiotics are available only after the mechanical breakage of the inner shell membrane by the chick's beak at the beginning of hatching (day 19) (Płowiec, 2018). However, they might act as pioneer colonizers, which augment the development of complex microbiota by modifying the intestinal environment (Pedroso et al., 2016). The advantages and disadvantages of in ovo stimulation with prebiotics and synbiotics were recently reviewed by Siwek et al. (2018). Previous studies indicated that selected prebiotics or synbiotics delivered in ovo on day 12 of egg incubation influence the gut microbiota (Bednarczyk et al., 2011), the GIT structure (Bogucka et al., 2017), immune responses (Sławińska et al., 2014b; Płowiec et al., 2015), as well as immune system status and development (Sławinska et al., 2014a; Madej et al., 2015; Madej and Bednarczyk, 2016; Slawinska et al., 2016; Stefaniak et al., 2019).
Intestinal microflora after in ovo stimulation is potent enough for competitive exclusion and is a key factor in the development and regulation of innate and adaptive immunity (Clavijo and Flórez, 2018). This is especially important at hatching, when gut-associated lymphoid tissue (GALT) is immature and requires early stimulation. Otherwise, it will impair the health status and performance of the animal (Yegani and Korver, 2008). Communication between intestinal microbiota and the immune system of the host is mediated by the receptors located in the GALT. They recognize and bind the bacterial ligands, called microbe-associated molecular patterns (Brisbin et al., 2008; Kogut and Swaggerty, 2012). This mechanism allows for subtle recognition between commensals and pathogens, reacting either by tolerance or immune responses. Thanks to such distinction, beneficial microbiota is allowed stable growth, whereas pathogenic microbiota is challenged with instant and local immune responses of the host (Kogut and Swaggerty, 2012).
Genetics of the host has influence on the development of the intestinal microflora, as reviewed by Kers et al. (2018). In this study, we analyzed the effects of in ovo stimulation in contrasting genotypes, that is, in Ross broiler chickens and in Green-legged Partridgelike (GP) chickens. Ross broiler chickens are fast-growing crossbreeds, developed as meat-type chickens. Green-legged Partridgelike chickens are an old native Polish breed that was developed as a dual-purpose chicken before the advent of commercial stocks (Siwek et al., 2013). Green-legged Partridgelike chickens resemble rather layer-type chicken owing to the low body weight and slim body conformation. The breed characteristics include partridgelike plumage and the reseda green color of the shanks. The genetic structure reveals its admixture between East Asian and European gene pools (Siwek et al., 2013). Green-legged Partridgelike chickens also constitute an excellent model for immunological studies owing to their sturdiness, tolerance to severe climatic conditions, and adaptation for pasture raising (Krawczyk, 2009).
Based on the aforementioned information, the authors hypothesized that the genotype and bioactive compound delivered in ovo may affect colonization of lymphatic organs with lymphocytes. Hence, the aim of this study was to examine how the genotype (broilers vs. GP chickens) influences the structure and cellular composition of peripheral immune system organs, after in ovo administration of prebiotics, probiotics, and synbiotics on day 12 of egg incubation.
Materials and methods
Animal Trials
Two animal trials were carried out using 2 distinct chicken genotypes, that is, broiler chicken (Ross 308) and GP chickens, which is a dual-purpose breed. Both experiments were designed in the same way. The experimental factor was a bioactive compound injected in ovo: prebiotics, probiotics, or synbiotics. The bioactive compounds were delivered into the air cell on day 12 of egg incubation by manual injection. The procedure of in ovo delivery of the bioactive compounds is described in the following section. After hatching, the birds were distributed into replicate pens (4 pens/group, 12 animals/pen). Rearing lasted 42 D and was performed under uniform environmental conditions, in accordance with the recommendations for each line. The animals were fed with diets respective to their age and genotype (Table 1). The chickens were housed on deep litter. Fresh, good-quality water and commercial feeds were available ad libitum. Sampling was carried out on the following days: day 7 (d7), day 21 (d21), and day 42 (d42). At each time point, 2 individuals from each pen (n = 8) were sacrificed by cervical dislocation, and the samples of the spleen and cecal tonsils (CT) were taken. The experiments were conducted at an experimental farm of Wroclaw University of Environmental and Life Sciences (Wroclaw, Poland) with the consent of the Local Ethics Committee for Animal Experiments (Bydgoszcz, Poland) (study approval reference number: 16/2014).
Table 1.
Chemical composition of commercial feeds used for chicken broilers and Green-legged Partridgelike chickens.
| Items | Broilers |
Green-legged Partridgelike |
||||
|---|---|---|---|---|---|---|
| Starter (days 1–10) | Grower I (days 11–21) | Grower II (days 22–33) | Finisher (days 34–42) | Starter (days 1–28) | Grower (days 19–42) | |
| MEN (MJ/kg) | 12.50 | 12.95 | 13.35 | 13.41 | 11.9 | 11.7 |
| Crude protein (g/kg) | 220 | 200 | 190 | 184 | 200 | 185 |
| Crude fiber (g/kg) | 28.00 | 30.0 | 31.0 | 32.0 | 34.0 | 35.0 |
| Lysine (g/kg) | 13.8 | 12.5 | 11.3 | 10.5 | 11.0 | 10.0 |
| Methionine + cystine (g/kg) | 10.3 | 9.5 | 8.8 | 8.2 | 8.2 | 7.2 |
| Threonine (g/kg) | 9.2 | 8.3 | 7.6 | 7.2 | 7.6 | 7.0 |
| Tryptophan [g/kg] | 2.2 | 2.0 | 1.9 | 1.9 | 2.1 | 2.0 |
In Ovo Treatment
Fertilized broiler and GP chicken eggs (n = 150) were incubated under standard conditions. In ovo delivery of bioactive compounds into the air cell was carried out on day 12 of egg incubation. All bioactive compounds were prepared as aqueous solutions (physiological saline was a solvent), and the injection volume was 0.2 mL. Eggs were candled before in ovo injection to discard unfertilized or nonviable embryos. Prebiotics (PRE group) contained GOS at a concentration of 3.5 mg/egg (trade name: Bi2tos, Clasado Biosciences Ltd., Jersey, UK). Probiotics (PRO group) belonged to a strain Lactococcus lactis subsp. cremoris IBB477 (Institute of Biochemistry and Biophysics, Polish Academy of Sciences, Warsaw, Poland) and were delivered at a dose of 1 × 105 cfu/egg. Growth conditions of PRO have been reported in the study by Sławińska et al. (2014b). Synbiotics (SYN group) were composed of PRE (GOS, 3.5 mg/egg) and PRO (L. lactis subsp. cremoris IBB477, 1 × 105 cfu/egg). Control eggs (C group) were mock injected with sterile physiological saline. The in ovo injection procedure was performed in 3 steps; first, the hole was punctured (needle diameter = 0.9 mm); second, the 0.2-mL solution was injected (needle diameter = 0.45 mm); and third, the hole was sealed with a liquid glue. Eggs were returned to an incubator directly after in ovo injection.
Histological Examination
Eight chickens from each treatment group (C, PRE, PRO, and SYN) were randomly selected on d7, d21, and d42 after hatching. Cecal tonsil and spleen samples were collected and bisected into 2 subsamples. The first subsample was fixed in 4% phosphate-buffered paraformaldehyde (pH 7.4) for 1 h, washed in 0.1 M phosphate buffer, and infiltrated with buffered 30% sucrose. This was then frozen in a cryostat (Leica CM1850, Leica Microsystems GmbH, Wetzlar, Germany) and cut into 10-μm serial sections, air-dried overnight, and frozen. The second subsample was fixed in 4% buffered formaldehyde (pH 7.4) and routinely processed in paraffin. Sections (5-μm thick) of each tissue were stained with hematoxylin according to Mayer (Roth GmbH, Karlsruhe, Germany) and eosin (POCH S.A., Gliwice, Poland). The slices were examined and photographed under a light microscope (Nikon Eclipse 80i; Nikon, Melville, NY) using a video camera.
Immunohistochemical Staining
Initially quenching of endogenous peroxidase in 3% hydrogen peroxide solution followed by blocking of nonspecific binding by preincubation with Antibody Diluent with a Background Reducing Component (Dako, Glostrup, Denmark) for 20 min was performed. Then, the serial sections were stained with monoclonal mouse antichicken antibodies (SouthernBiotech, Birmingham, AL) directed against antigens, Bu-1 (clone AV20, 1:500), CD4 (clone CT-4, 1:200), CD8α (clone CT-8, 1:200), and PBS, which was used as a control, and were incubated at room temperature for 1 h. The visualization of the antigens was performed using EnVision Systems (Dako) with the 3,3′-diaminobenzidine chromogen, according to the manufacturer's instructions.
Morphometry
Morphometric analysis of the area occupied by the antigen-positive (brown-colored) cells was performed on an area of 0.29 mm2 (magnification of 200×) using an NIS-Elements AR 2.30 (Nikon, Melville, NY) program, and the results were expressed as a percentage of the field of view. The germinal center (GC) area in the spleen was calculated on an area of 1.16 mm2 (magnification of 100×) and also expressed as a percentage of the field of view. Any false positive artifacts were in each case eliminated by a histologist. In the CT, the fields of view were always selected starting from the lamina propria mucosae in the direction of the lumen of the organ.
Statistical Analysis
The morphometric data were analyzed using Statistica 13.1 software (StatSoft Polska Sp. z o.o., Krakow, Poland). The significance of differences was assessed using one-way ANOVA with post hoc Tukey tests for data accordant with the normal distribution or the Kruskal–Wallis test for ranks (one-way ANOVA on ranks) for data not accordant with the normal distribution. A value of P <0.05 was considered significant.
Results
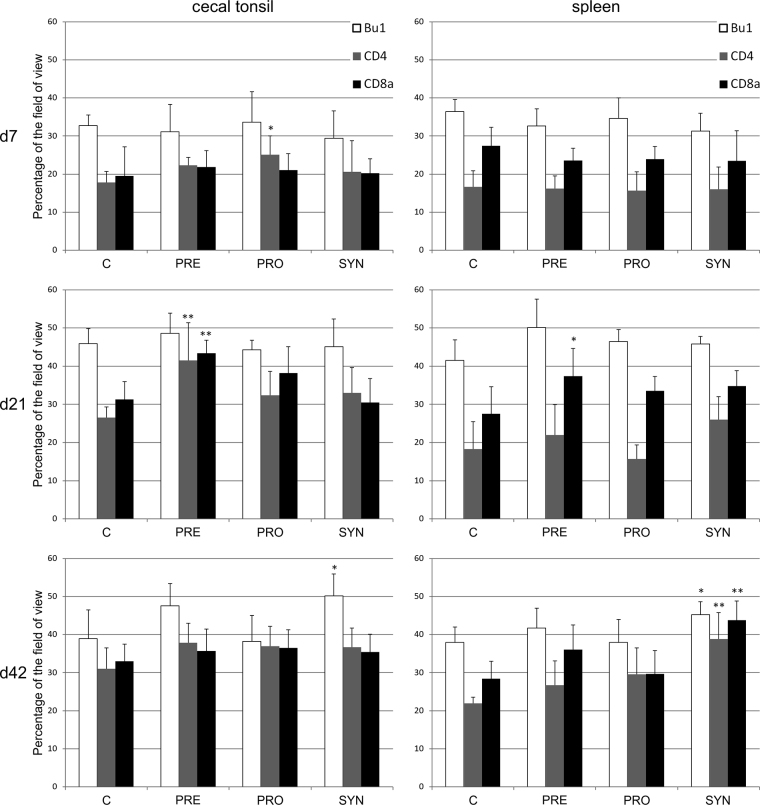
The lymphocyte colonization of the CT and spleen was different in broilers and GP chickens. In broilers, on d7, the number of CD4+ lymphocytes in the CT was higher in the PRO group than in the control group (P < 0.05) (Figure 1). The similar pattern (but statistically not significant) was observed in the PRE group. On d21, prebiotics significantly increased the number of CD4+ and CD8+ cells in the CT (P < 0.05) and CD8+ cells in the spleen (P < 0.05). On d42, synbiotics increased the number of Bu-1+ cells in the CT (P < 0.05), but in the spleen, they increased the number of B cells (P < 0.05) as well as Th and cytotoxic cells (P < 0.01).
Figure 1.
The area occupied by the antigen-positive cells (mean ± SD, n = 8) in the cecal tonsil and spleen of 7-day-old, 21-day-old, and 42-day-old broilers. C: control, PRE: prebiotics (galactooligosaccharides [GOS]), PRO: probiotics (L. lactis subps. cremoris), SYN: synbiotics (GOS + L. lactis subsp. cremoris). Significant difference compared with the control (∗P < 0.05; ∗∗P < 0.01). Abbreviations: d7, day 7; d21, day 21; d42, day 42.
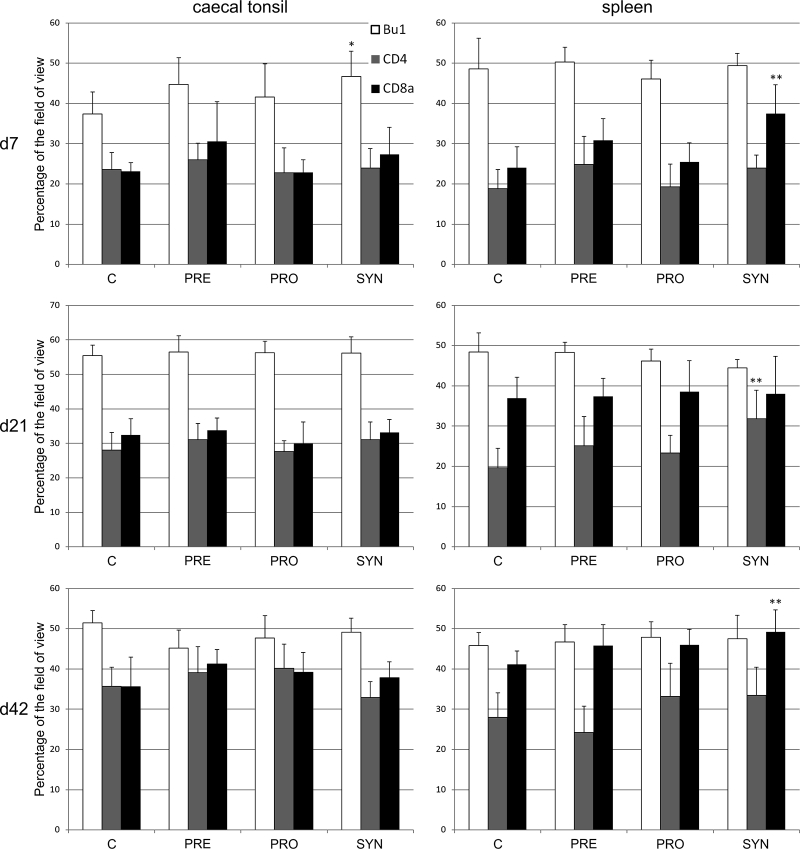
In GP chickens, the most potent factor that stimulated the number of immune cells in the spleen was synbiotics (Figure 2). On d7 and d42, in the SYN group, the number of CD8+ cells increased, but on d21, the number of CD4+ cells increased (P < 0.01). As a result, on d21, the CD4-to-CD8 ratio in the SYN group showed tendency (P < 0.1) to be higher than that in the C group (SYN = 0.84 ± SD 0.27 vs. C = 0.53 ± SD 0.13). In the CT, the influence of applied bioactive compounds was not very strong. In these organs, synbiotics stimulated transitorily the Bu-1+ cell number on d7 only. Prebiotics and probiotics alone did not influence the number of B or T cells in the CT or in the spleen at all time points studied.
Figure 2.
The area occupied by the antigen-positive cells (mean ± SD, n = 8) in the cecal tonsil and spleen of 7-day-old, 21-day-old, and 42-day-old Green-legged Partridgelike chickens. CON: control, PRO: probiotics (L. lactis subps. cremoris), PRE: prebiotics (galactooligosaccharides [GOS]), SYN: synbiotics (GOS + L. lactis subsp. cremoris). Significant difference compared with the control (∗P < 0.05; ∗∗P < 0.01). Abbreviations: d7, day 7; d21, day 21; d42, day 42.
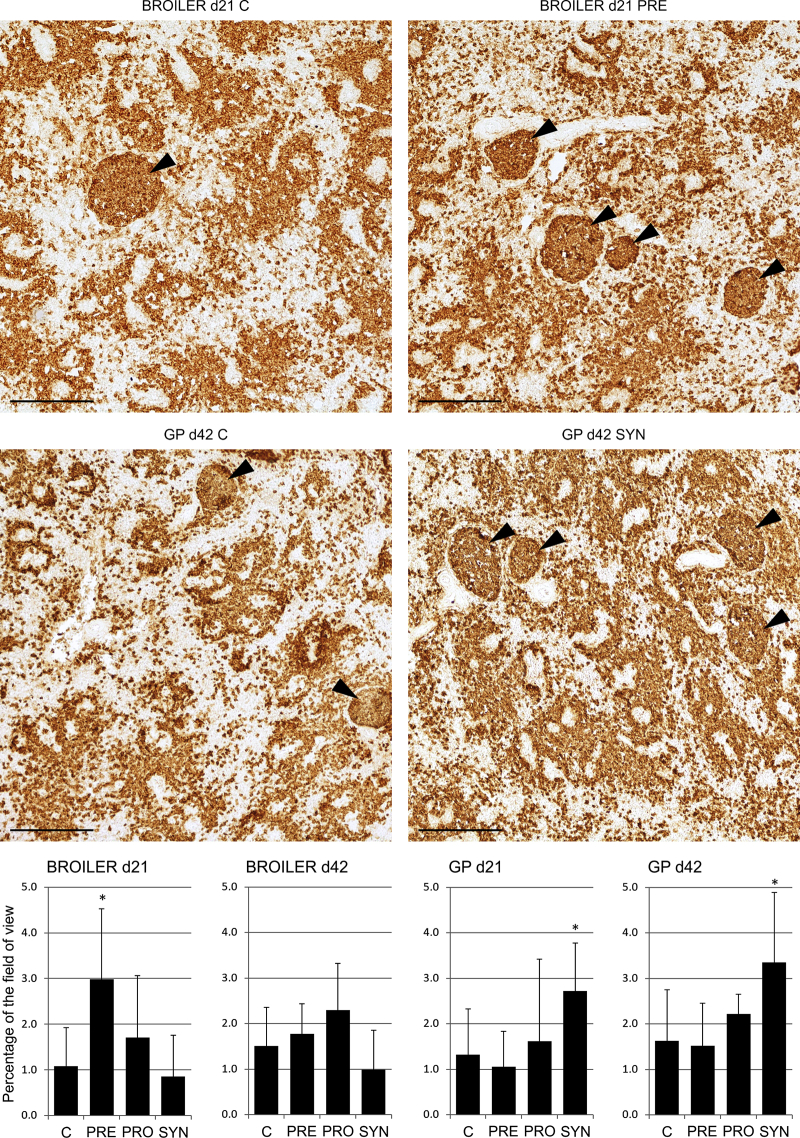
In both chicken lines, in all experimental groups, the number of Bu-1+, CD4+, and CD8+ cells was never significantly lower than in the control group; on the contrary, the values were higher. In both lines of chickens, the GC were formed in the spleen on d21 and d42 in all groups. In broilers, prebiotics significantly increased the GC formation in the spleen on d21 (P < 0.05) (Figure 3). However, in GP chickens, synbiotics stimulated the GC formation on d21 and d42 (P < 0.05). Surprisingly, the percentage of B cells in the field of view did not correlate (Spearman's rank correlation coefficient r < 0.15) with the area of GC (measured on Bu-1–stained slides).
Figure 3.
Selected examples of germinal center morphology in the spleen of 21-day-old and 42-day-old broilers and Green-legged Partridgelike (GP) chickens. Germinal center (Bu-1+ cells): ar, 200×, scale bar: 200 μm. C: control, PRE: prebiotics (galactooligosaccharides [GOS]), PRO: probiotics (L. lactis subps. cremoris), SYN: synbiotics (GOS + L. lactis subsp. cremoris). Mean areas occupied by all germinal centers in the field of view are presented in the charts. Significant difference compared with the control (∗P < 0.05). Abbreviations: d21, day 21; d42, day 42.
To find how the genotype influences the number of immune cells, the populations of Bu1+, CD4+, and CD8+ cells between broiler and GP chicken control groups were compared (Table 2). The number of Bu1+ cells was significantly higher in GP chickens than that in broilers in both organs and in all time points studied. In addition, the number of CD8+ cells was also higher in the spleen in GP chicken groups on d21 and d42.
Table 2.
Influence of the genotype on Bu-1+, CD4+, and CD8α + cell populations in the control groups of broiler (BR) and Green-legged Partridgelike (GP) chickens.
| Cell population | Cecal tonsil |
Spleen |
||
|---|---|---|---|---|
| BR | GP | BR | GP | |
| Day 7 | ||||
| Bu1 | 32.78 ± 2.78∗ | 37.41 ± 5.48∗ | 36.38 ± 3.21∗ | 48.60 ± 7.64∗ |
| CD4 | 17.86 ± 2.86∗ | 23.62 ± 4.19∗ | 16.63 ± 4.21 | 18.82 ± 4.81 |
| CD8α | 19.48 ± 7.69 | 23.10 ± 2.23 | 27.46 ± 4.80 | 23.98 ± 5.20 |
| Day 21 | ||||
| Bu1 | 45.86 ± 3.98∗∗ | 55.46 ± 2.97∗∗ | 41.55 ± 5.29∗ | 48.41 ± 4.73∗ |
| CD4 | 26.58 ± 2.75 | 28.05 ± 5.09 | 18.31 ± 7.14 | 19.64 ± 4.81 |
| CD8α | 31.28 ± 4.64 | 32.43 ± 4.67 | 27.49 ± 7.12∗ | 36.97 ± 5.12∗ |
| Day 42 | ||||
| Bu1 | 38.98 ± 7.51∗∗ | 51.44 ± 3.09∗∗ | 37.94 ± 4.05∗∗ | 45.78 ± 3.25∗∗ |
| CD4 | 31.02 ± 5.53 | 35.64 ± 4.80 | 21.96 ± 1.63 | 27.94 ± 6.13 |
| CD8α | 32.97 ± 4.49 | 35.61 ± 7.31 | 28.42 ± 4.60∗∗ | 41.14 ± 3.30∗∗ |
∗P < 0.05; ∗∗P < 0.01: significant difference between the groups.
Discussion
Our previous studies indicated that in ovo administration of prebiotics (inulin and GOS) and synbiotics (inulin + L. lactis subsp. lactis IBB SL1 and GOS + L. lactis subsp. cremoris IBB SC1) on day 12 of egg incubation modulate the central and peripheral lymphatic organ development in broilers (Madej et al., 2015; Madej and Bednarczyk, 2016). As a result of in ovo stimulation, GALT development (defined by B-and T-cell colonization) on d7 and d21 after hatching was enhanced. The effects were more pronounced after in ovo treatment with synbiotics rather than with prebiotics. In the spleen, the increase of the GC area was determined in synbiotic-treated groups, but the number of B cells was not counted.
The results of the present 2 animal trials confirmed that bioactive compounds (prebiotics, probiotics, and synbiotics) delivered in ovo influence the colonization and development of the peripheral immune system in poultry. The most potent immunostimulation was exerted by GOS (prebiotics) and GOS + L. lactis subsp. cremoris (synbiotics). However, the main factor determining the structure and cellular composition of immune system organs was the host genotype. In broilers, the influence of the selected bioactive compound depended on age. Initially (d7), probiotics increased the number of Th (CD4+) cells in the CT. Then (d21), prebiotics strongly raised the number of cells involved in cellular immunity in the CT and spleen and stimulated GC formation and development in the spleen. However, at the end of the experiment (d42), it was synbiotics that stimulated the colonization of both organs by B cells (humoral immunity) and the spleen by CD4+ and CD8+ cells (cellular immunity). In the second experiment, performed on GP chickens, synbiotics enforced the cellular immunity (CD4+ or CD8+ cells) in the spleen at all time points and stimulated the GC appearance on d21 and d42 after hatching. Noticeable increase of the CD4-to-CD8 ratio (d21) in the spleen after synbiotic treatment suggests that it promoted helper activity. Surprisingly, in GP chickens, the influence of selected bioactive compounds on the CT was very limited.
Germinal centers are a part of the white pulp of the spleen, where B cells proliferate after antigenic stimulation. In this study, the general number of B cells in the spleen did not depend on the GC area. It seems that in spleen, the number of B cells indicates the degree of colonization and potential to develop of humoral immune response. On the other hand, the area of GC reflects the intensity of humoral responses to the antigen that emerged in the host's body currently or in the recent past. In the present study, the increased area of spleen GC was observed in GP chickens treated with synbiotics. It suggests that this formulation can enforce the systemic immune response against environmental antigens. These findings are in accordance with those of Slawinska et al. (2014a), who indicated that some synbiotics could significantly upregulate the gene expression of IL-4 (Th2) and IL-6 (proinflammatory) in the spleen, which in turn can stimulate B-cell proliferation and synthesis of immunoglobulin.
A study conducted by Koenen et al. (2002) indicated that various types of poultry develop immune responses that differ in intensity and even engage different groups of cells. In our study, the higher number of B cells in the CT as well as B and cytotoxic cells in the spleen suggests that the immune system of GP chickens is better prepared for possible response against antigens than that of broilers. Because the differences were found in both GALT and the spleen, it can be concluded that the immune system of GP chickens can efficiently respond to gut- and blood-derived antigens, respectively. The higher number of cells that are responsible for both humoral and cellular responses observed in GP chickens is in agreement with that found in the study of Koenen et al. (2002), in which layer-type chickens, in contrast to broilers, developed long-term humoral responses with a strong cellular response.
Heavy-weight broilers and light-weight GP chickens differ significantly in body weight and composition. The number of B cells in the CT and spleen from GP chicken control groups was higher than that from broilers at the same age. These results confirm, on the morphological level, the previous observations concerning a negative relationship between body weight and antibody titer that was described in the same (Miller et al., 1992) or different types of chickens (Koenen et al., 2002). Taking into account the relatively high number of B cells in the GP chicken control group, it can be concluded that synbiotics were not potent enough to increase the general number of these cells in the spleen, but they stimulated the GC formation. On the other hand, in broilers, synbiotics increased the B cell number in the spleen on d42. Therefore it seems that in GP chickens, in ovo stimulation with synbiotics induces qualitative rather than quantitative changes of the humoral immune system in contrast to broilers.
In conclusion, it is difficult to clearly determine which of the bioactive compounds (or their combinations) gives the best effects in terms of immune system stimulation in poultry. In broilers, prebiotics and synbiotics increased the number of adaptive immune cells (T and B lymphocytes) both in the CT and spleen. In GP chickens, the most potent substance was synbiotics, which stimulated cellular immunity in the spleen but not in CT. However, given the long-term effect, synbiotics were the most potent in both tested lines of chickens on the last day of the experiment. In relation to broilers, d42 posthatching coincides with the time of slaughter; hence, the good condition of the immune system indicating the health of the animal is an additional advantage of poultry meat delivered to the market.
Acknowledgments
This study was financed by grant no. UMO-2013/11/B/NZ9/00783 from the National Science Centre (Poland). The publication of the article was financed under the Leading Research Groups support project from the subsidy increased for the period 2020–2025 in the amount of 2% of the subsidy referred to Art. 387 (3) of the Law of 20 July 2018 on Higher Education and Science, obtained in 2019.
Conflict of Interest Statement: The authors declare that they have no conflicts of interests.
References
- Ballou A.L., Ali R.A., Mendoza M.A., Ellis J.C., Hassan H.M., Croom W.J., Koci M.D. Development of the chick microbiome: how early exposure influences future microbial diversity. Front. Vet. Sci. 2016;3:2. doi: 10.3389/fvets.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarczyk M., Urbanowski M., Gulewicz P., Kasperczyk K., Maiorano G., Szwaczkowski T. Field and in vitro study on prebiotic effect of raffinose family oligosaccharides in chickens. Bull Vet. Inst. Pulawy. 2011;55:465–469. [Google Scholar]
- Bogucka J., Dankowiakowska A., Elminowska-Wenda G., Sobolewska A., Jankowski J., Szpinda M., Bednarczyk M. Performance and small intestine morphology and ultrastructure of male broilers injected in ovo with bioactive substances. Ann. Anim. Sci. 2017;17:179–195. [Google Scholar]
- Brisbin J.T., Gong J., Sharif S. Interactions between commensal bacteria and the gut-associated immune system of the chicken. Anim. Health Res. Rev. 2008;9:101–110. doi: 10.1017/S146625230800145X. [DOI] [PubMed] [Google Scholar]
- Broom L.J., Kogut M.H. The role of the gut microbiome in shaping the immune system of chickens. Vet. Immunol. Immunopathol. 2018;204:44–51. doi: 10.1016/j.vetimm.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Charalampopoulos D., Rastall R. Springer-Verlag; New York, NY: 2009. Prebiotics and Probiotics Science and Technology; pp. 31–50. [Google Scholar]
- Clavijo V., Flórez M.J.V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: a review. Poult. Sci. 2018;97:1006–1021. doi: 10.3382/ps/pex359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kers J.G., Velkers F.C., Fischer E.A.J., Hermes G.D.A., Stegeman J.A., Smidt H. Host and environmental factors affecting the intestinal microbiota in chickens. Front. Microbiol. 2018;9:235. doi: 10.3389/fmicb.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen M.E., Boonstra-Blom A.G., Jeurissen S.H.M. Immunological differences between layer- and broiler-type chickens. Vet. Immunol. Immunopathol. 2002;89:47–56. doi: 10.1016/s0165-2427(02)00169-1. [DOI] [PubMed] [Google Scholar]
- Kogut M.H., Swaggerty C.L. Effects of prebiotics and probiotics on the host immune response. In: Callaway T.R., Ricke S.C., editors. Direct-Fed Microbials and Prebiotics for Animals: Science and Mechanisms of Action. Springer-Verlag; New York, NY: 2012. pp. 61–72. [Google Scholar]
- Krawczyk J. Quality of eggs from Polish native Greenleg Partridge chicken-hens maintained in organic vs. backyard production systems. Anim. Sci. Pap. Rep. 2009;27:227–236. [Google Scholar]
- Madej J.P., Bednarczyk M. Effect of in ovo-delivered prebiotics and synbiotics on the morphology and specific immune cell composition in the gut-associated lymphoid tissue. Poult. Sci. 2016;95:19–29. doi: 10.3382/ps/pev291. [DOI] [PubMed] [Google Scholar]
- Madej J.P., Stefaniak T., Bednarczyk M. Effect of in ovo-delivered prebiotics and synbiotics on lymphoid-organs’ morphology in chickens. Poult. Sci. 2015;94:1209–1219. doi: 10.3382/ps/pev076. [DOI] [PubMed] [Google Scholar]
- Miller L.L., Siegel P.B., Dunnington E.A. Inheritance of antibody response to sheep erythrocytes in lines of chickens divergently selected for fifty-six-day body weight and their crosses. Poult. Sci. 1992;71:47–52. doi: 10.3382/ps.0710047. [DOI] [PubMed] [Google Scholar]
- Pedroso A.A., Batal A.B., Lee M.D. Effect of in ovo administration of an adult-derived microbiota on establishment of the intestinal microbiome in chickens. Am. J. Vet. Res. 2016;77:514–526. doi: 10.2460/ajvr.77.5.514. [DOI] [PubMed] [Google Scholar]
- Płowiec A. UTP Bydgoszcz; Poland: 2018. Impact of Prebiotics and Synbiotics Delivered in Ovo on Modulation of the Gene Expression in Chicken. PhD Diss. [Google Scholar]
- Płowiec A., Sławinska A., Siwek M.Z., Bednarczyk M.F. Effect of in ovo administration of inulin and lactococcus lactis on immune-related gene expression in broiler chickens. Am. J. Vet. Res. 2015;76:975–982. doi: 10.2460/ajvr.76.11.975. [DOI] [PubMed] [Google Scholar]
- Siwek M., Slawinska A., Stadnicka K., Bogucka J., Dunislawska A., Bednarczyk M. Prebiotics and synbiotics - in ovo delivery for improved lifespan condition in chicken. BMC Vet. Res. 2018;14:402. doi: 10.1186/s12917-018-1738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwek M., Wragg D., Sławińska A., Malek M., Hanotte O., Mwacharo J.M. Insights into the genetic history of Green-legged Partridgelike fowl: MtDNA and genome-wide SNP analysis. Anim. Genet. 2013;44:522–532. doi: 10.1111/age.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawinska A., Plowiec A., Siwek M., Jaroszewski M., Bednarczyk M. Long-term transcriptomic effects of prebiotics and synbiotics delivered in ovo in broiler chickens. PLoS One. 2016;11:e0168899. doi: 10.1371/journal.pone.0168899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sławinska A., Siwek M.Z., Bednarczyk M.F. Effects of synbiotics injected in ovo on regulation of immune-related gene expression in adult chickens. Am. J. Vet. Res. 2014;75:997–1003. doi: 10.2460/ajvr.75.11.997. [DOI] [PubMed] [Google Scholar]
- Sławińska A., Siwek M., Zylińska J., Bardowski J., Brzezińska J., Gulewicz K.A., Nowak M., Urbanowski M., Płowiec A., Bednarczyk M. Influence of synbiotics delivered in ovo on immune organs development and structure. Folia Biol. (Krakow) 2014;62:277–285. doi: 10.3409/fb62_3.277. [DOI] [PubMed] [Google Scholar]
- Stefaniak T., Madej J.P., Graczyk S., Siwek M., Łukaszewicz E., Kowalczyk A., Sieńczyk M., Bednarczyk M. Selected prebiotics and synbiotics administered in ovo can modify innate immunity in chicken broilers. BMC Vet. Res. 2019;15:105. doi: 10.1186/s12917-019-1850-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S., Morrison M., Yu Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. 2013;92:671–683. doi: 10.3382/ps.2012-02822. [DOI] [PubMed] [Google Scholar]
- Yegani M., Korver D.R. Factors affecting intestinal health in poultry. Poult. Sci. 2008;87:2052–2063. doi: 10.3382/ps.2008-00091. [DOI] [PMC free article] [PubMed] [Google Scholar]