Abstract
In this study, a strain of Trichosporon was isolated from white pseudomembranes and ulcers formed on mucous membranes of pigeon bursas and was identified through gene sequencing. Bacteriostatic actions of Acorus gramineus, Sophora flavescens, Polygonum hydropiper, and Chinese herbal mixture on this species were explored in vitro, and the minimum inhibitory concentration of herbal medicines against Trichosporon was determined through microdilution method. Therapeutic effects of herbal medicines on chickens infected by Trichosporon were studied, whose results showed that minimum inhibitory concentration of A. gramineus was 32 μg/μL, that of S. flavescens was 2 μg/μL, that of P. hydropiper was 120 μg/μL, and that of Chinese herbal mixture was 36 μg/μL. Antibacterial effects of S. flavescens were the best. In accordance with animal experiments, therapeutic effects of Chinese herbal medicines on infected chickens were better than those of fluconazole. The mortality rate of the Chinese herbal medicine treatment group was 33.33%, that of the fluconazole treatment group was 46.67%, and that of the Chinese medicine protection group was 23.33%. The longer the time of Chinese medicine treatments was, the better the treatment effects would be. Glutamic oxaloacetylase values of the serum and liver in the Chinese herbal medicine treatment group were both significantly lower than those of the nontreatment group. From the results, it can be seen that A. gramineus, S. flavescens, P. hydropiper, and Chinese herbal mixture have certain inhibitory effects on Trichosporon spp. Chinese herbal medicine protections in advance could reduce Trichosporon infections.
Key words: Trichosporon, chicken, Chinese herbal medicine, protection and treatment
Introduction
Trichosporon spp. was first discovered by Beigel among patients with mild hair infections. Currently, more than 50 different subspecies and around 16 different strains can cause human diseases (Colombo et al., 2011; Górz and Boroń, 2016). They are highly pathogenic and widely distributed, which are found in air, soil, dolphins, ants, beetles, birds, cattle, and sheep (Cafarchia et al., 2006; Gugnani et al., 2007; Pagnocca et al., 2010; Delavenne et al., 2011; Gujjari et al., 2011; Ueda et al., 2017). In recent years, Trichosporon has been detected in the cloaca of healthy domestic pigeons, feces, pet parrots, and gastrointestinal tracts of dogs (Brilhante et al., 2010; Bryan et al., 2014; Medina et al., 2017), which can infect humans with trichosporum by polluting their living environments. Studies have found that Trichosporon in the cloaca of migratory birds can cause dermal or systemic diseases for animals and humans. Migratory birds are potential reservoirs of pathogens and play an important role in the ecology, circulation, and spread of pathogens. Migrations of birds have certain effects on horizontal and vertical spread of pathogens in different species and regions (Cafarchia et al., 2006; Foti et al., 2011).
Although reports on Trichosporon infections have been increasing, relatively fewer reports have been made on virulence factors, pathogenic processes, and antifungal resistance mechanisms of trichosporum. Studies have shown that trichosporum has certain resistances to first-line antifungal drugs, such as amphotericin B (AMB), fluconazole, voriconazole and micafungin, and the number of resistant strains is increasing (Biegańska et al., 2018), meanwhile the drug resistance is significantly enhanced. Aiming at resistances of trichosporum, researchers have begun to explore new antifungal drugs and their synergists. Studies of Oliveira et al. have shown that bullfrog oil obtained from adipose tissues of amphibious wood frogs could significantly enhance antifungal activities of AMB and reduce its toxicity (Oliveira et al., 2018). Ludwig et al. developed through the use of nanotechnologies that similar results could also be achieved by chitosan-coated AMB-containing polylactide-glycolide nanoparticles (Ludwig et al., 2018). Rattlesnake amine extracted from snake venom has significant affinities and antifungal activities on negatively charged membranes (Mas et al., 2017). Antimicrobial peptides are also considered a promising solution to microbial resistances (Wimley and Hristova, 2011). Owing to the adverse effects of synthetic compounds, people are more and more interested in research on natural products, which gradually leads to a boom in those on traditional Chinese medicines. Traditional Chinese medicines have a variety of complex components, different modes of actions, small dosages, and low toxicities, which have been used in antifungal therapies. Studies found that Acorus gramineus was good in alleviating the onset of Alzheimer's diseases, lowering blood lipids, promoting gastrointestinal contractions and peristalses, inhibiting asthmas, as well as strengthening body immunities, meanwhile, they are also antitumor, antidepression, antithrombosis, antiarteriosclerosis, and antibacterial (Zhang and Shen, 2018; Zhang and Guo, 2019). Sophora flavescens has a certain effect on killing Staphylococcus aureus, Escherichia coli, Tetracoccus, Bacillus subtilis,and so on, which is mixed in salicylic acid, Coptis, honeysuckle, and other drugs to have certain effects on treatments of fungal hand-foot moss (Zhang and Shen, 2018). Polygonum hydropiper has the strongest antibacterial effect, which can inhibit growths of various bacteria such as E. coli and Staphylococcus (Wang et al., 2017). There are also other Chinese herbal medicines such as rhizoma coptidis, Houttuynia cordata, magnolia obovate, baicaline, Anemone, and so on, which were used in antifungal therapies (Shang, 2012; Shi et al., 2014; Wu, 2017).
In this study, we isolated a strain of Trichosporon from clinically diseased pigeons of Baoding, Hebei Province. To find sensitive drugs, 3 herbal medicines, namely S. flavescens, A. grandiflora, and P. hydropiper were selected to explore therapeutic effects on isolated trichosporum both in vitro and in vivo.
Materials and methods
This study was approved by the Experimental Animal Ethics Committee of Hebei Agricultural University.
Preparation of Chinese Herbal Medicines
S. flavescens, A. gramineus, and P. hydropiper were purchased from the Anguo Oriental Medicine City (Hebei, China). All of the 3 Chinese herbal medicines weighed 50 g, and Chinese herbal mixture weighed 60 g as per the ratio of S. flavescens: A. gramineus: P. hydropiper = 1: 1: 1. First, they were crushed into powder, to which ethanol aqueous solution with a concentration of 60% was added as per a ratio of 1:30 (Yi et al., 2006; Tang et al., 2011; Fang et al., 2016; Han et al., 2018; Xiang et al., 2019). After immersed in ethanol with a concentration of 60% at room temperature for 12 h, Chinese herbal medicines were sonicated in an ultrasonic instrument for 3 times at 40°C, each of which took 15 min. The medicine solution extracted through ultrasound was filtered in a vacuum suction filter bottle; then, ethanol with a concentration of 60% was added to the medicine residue and sonicated again at 40°C twice for 15 min per time; then, the 2 filtered solutions were mixed and centrifuged at 3,000 r/min for 10 min. The supernatant was taken out and concentrated to 1.5 g/mL through a rotary evaporator at 80°C. The evaporated liquid was then stored at 4°C.
Isolation of Trichosporon spp.
The Trichosporon spp. was isolated from clinically diseased pigeons of Baoding, Hebei Province. Diseased and dead pigeons were dissected, and white ulcers and rug-like lesions were observed in their sacs. The exudates were taken from the lesions on a glass slide, on which 1 drop of KOH solution with the concentration of 10 g/L was added, and it was fixed under a microscope after heat fixation to see whether there were spores or hypha.
A sterile cotton test paper was dipped into mucous membranes in the sacs of diseased pigeons, which was diluted with normal saline and applied to sabouraud dextrose agar (SDA) medium and then incubated at 37°C for 48 h to observe morphologic characteristics of the colonies; single colonies were picked and streaked continually, which were cultured until a pure culture was obtained. Gram staining was performed on single colonies, and shapes of mycelia and spores were observed through a microscope. Strains were frozen in glycerol tubes and stored in SDA at 37°C for later use.
Molecular Biological Identification
The total DNA of the bacterial solution was extracted following operation procedures of the fungal genomic DNA extraction kit (Courtesy of OMEGR). Fungal universal internal transcribed spacer (ITS) sequence primers (ITS1 5′-TCCGTAGGTGAACCTGCGG-3 ′) and (ITS4 3′-TCCTCCGCTTATTGATATGC-5′) were used to amplify ITS-1, 5.8S rRNA and ITS-2 in a final volume of 50 μL, including 5.0 μL of 10 × Taq buffer, 4.0 μL of dNTP, 2 μL of of ITS1 and ITS4, 0.5 μL of rTaq, 2 μL of template DNA, and 34.5 μL of ddH2O. They were first predenatured at 94°C for 3 min, then entered 35 cycles, denatured at 94°C for 30 s, annealed at 57°C for 30 s, extended at 72°C for 1 min, and finally stored at 4°C. The PCR product obtained was electrophoresed on agarose gel with a concentration of 1% and purified using a QIAick PCR purification kit (Company of QIAGEN). The purified PCR product was sequenced by an applied biosystems 3730xl sequencer, whose results were processed through a Web-based blasting program and the Basic Local Alignment Search Tool (http://www.ncbi.nlm.nih.gov/BLAST); the data were compared with those in the NCBI/GenBank database, and MEGA7.0 was used to build an evolutionary tree.
Preventions and Treatments of Chinese Herbal Medicines on Infected Chickens
In the animal test, 150 5-day-old healthy Roman gray chickens were provided by Dingnong Corporation of Hebei (Baoding, China) and approved by the Animal Ethics Committee of Hebei Agricultural University, which were raised, managed,and dissected as per the national legislations on animal welfare protections. They were randomly divided into 5 groups: control group, Chinese herbal medicine protection group, Chinese herbal medicine treatment group, fluconazole treatment group, and nontreatment group. There were 30 animals in each group with 3 repeating groups. The chickens were raised for 3 D on the same condition before the experiment began; then, mycotic infections and treatments were conducted.
Physiological saline was used to dilute 1.5 g/mL of Chinese herbal mixture to a concentration of 1 g/mL, and bacteria suspension with the concentration of 1 × 107 CFU/mL was prepared with physiological saline for use. First, chickens in the Chinese herbal medicine protection group were fed with Chinese herbal mixtures as per the BW of 1 mL/kg for 5 D with intragastric administration. Then, except for the control group, the other groups were consecutively fed with 0.5 mL of bacterial suspension for 7 D, during which chickens in the Chinese herbal medicine protection group were continually fed with herbal mixture. After 7 D, 9 chickens were randomly selected from each group and killed through carotid artery bleeding to inspect bacterial infections through pathologic autopsies and bacteria isolations based on histology. The blood and liver were collected for transaminase measurements. Funguses collected from the crops were cultured. In the follow-up experiment, chickens in the Chinese herbal medicine protection group and the Chinese medicine treatment group were all fed with Chinese herbal mixture as per the BW of 1 mL/kg for 7 D, and those in the fluconazole treatment group was fed as per the fluconazole drug instruction manual provided by Guangdong P.D. Pharmaceutical Co., Ltd. At the end of the study, all animals were dead, pathologic examinations and sample collections were conducted the same as aforementioned.
Susceptibility Tests of Chinese Herbal Medicine In Vitro
The minimum inhibitory concentration (MIC) of Chinese herbal medicines was determined based on M27-E4 (Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: approved standard – Fourth Edition) standard compiled by the Clinical and Laboratory Standards Institute. Chinese herbal medicines (1.5 g/mL) were diluted to 1,024 μg/μL, 512 μg/μL, 256 μg/μL, 128 μg/μL, 64 μg/μL, 32 μg/μL, 16 μg/μL, 8 μg/μL, and 4 μg/μL with RPMI1640 medium through double-dilution method. A total volume of 180 μL of RPMI1640 medium, including Chinese herbal medicines, was taken in a 96-well plate, 2 repetitions were set for each concentration. Positive and negative controls were added to 180 μL of RPMI1640 medium without Chinese herbal medicines. Activated colonies were taken from SDA medium and diluted to bacterial suspension with a concentration of 5 × 103 CFU/mL in RPMI 1640 medium. All test wells were added with 20 μL of bacteria suspension expect for the negative control wells, which were added with 20 μL of RPMI1640 medium; then, the 96-well plate was placed in an incubator at 37°C for 48 h, and the optical density (OD)600 value of each well was measured using a microplate reader. Compared with negative controls, the drug concentration with an OD value that was higher than 80% was MIC80. This experiment was repeated 3 times.
Based on the aformentioned test results, through the same test method, the MIC80 was further diluted with A. gramineus, S. flavescens, P. hydropiper, and Chinese herbal mixture. Each test was repeated 3 times.
Drug Sensitivity Test on Each Herbal Medicine
Chinese herbal medicines with a concentration of 1.5 g/mL were added to SDA medium to make Chinese herbal medicine medium plates with concentrations of 125 μg/μL and 64 μg/μL. The same method was used to prepare ethanol medium plate with a concentration of 60% as control. Normal saline was used to prepare bacterial suspension with a concentration of 10 CFU/mL. A total volume of 100 μL of bacteria suspension was evenly spread on the SDA medium plates, including Chinese herbal medicines with different concentrations, and the SDA medium without Chinese herbal medicines was taken as a control, which were cultured at 37°C. Colony growths were observed at 24 h, 48 h, 72 h, 96 h, 120 h, and 144 h after culturing, and diameters of the colonies were measured.
Preparation of medium plates continued as per the aforementioned test results with concentrations of 32, 16, 8, 4, and 2, meanwhile S. flavescens with a concentration of 1 μg/μL as well as medium plates of A. gramineus with concentrations of 32, 16, and 8 μg/μl were prepared. Through the same culture method, colony growths were observed, and colony diameters were measured.
Statistical Analyses
All the results were expressed as means ± SEM. Statistical analyses were performed through SPSS software package V11.5 (SPSS Inc., Chicago, IL). All data were analyzed through 1-way ANOVA to determine differences among the groups. In this study, the groups were considered to be significantly different if P < 0.05 and extremely significant if P < 0.01.
Results
Morphology of the Pathogenic Fungus
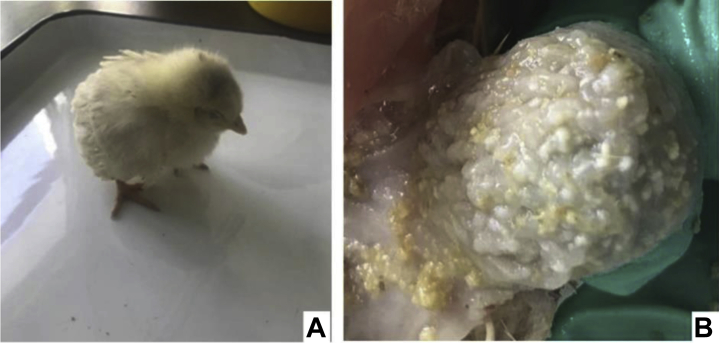
Pathogenic funguses mainly grow in the esophagus and crops of pigeons, and white pseudo membranes and ulcers were found on the mucosa of crops (Figure 1A). Pathogenic funguses were cultured on an SDA medium for 48 h, forming white, moist and centrally raised folds, and mycelium-divergent growth-type colonies with a diameter of 8–11 mm (Figure 1B). Gram staining results were positive. Blue-purple cylindrical and barrel-shaped arthrospores were seen through a microscope, and long hyphae were broken into irregular distributions. No attachment cell, aspirator, or adhesion pad was seen (Figure 1C).
Figure 1.
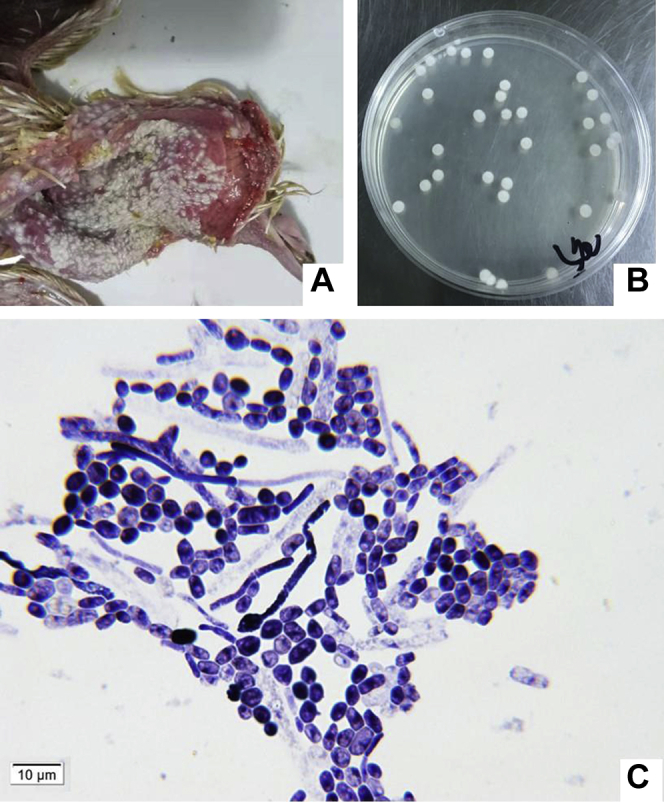
The pathogenic fungus from the crop of a diseased pigeon. (A) The crop of a diseased pigeon. (B) Colonies of pathogenic fungus on SDA medium. (C) Morphology of the pathogenic fungus Gram staining. Abbreviation: SDA, sabouraud dextrose agar.
Molecular Identification
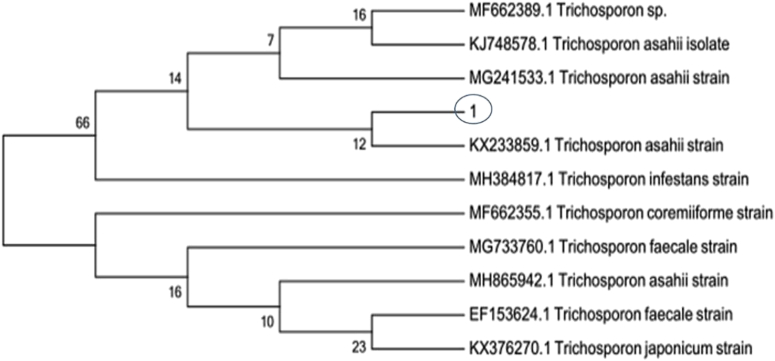
Target genes of common ITS sequence of the funguses were amplified by PCR, and sequencing results of purified products compared with strains whose serial numbers in the Basic Local Alignment Search Tool Library on NCBI website were MF6623899.1, KJ748578.1, MG241533.1, KX233859.1, MH384817.1, MF662355.1, and MG733760.1. MH865942.1, EF153624.1, and KX376270.1 could reach a homology of 100%. Software MEGA7.0 was used for homology analysis, and the evolution tree is shown in Figure 2. It was shown by the homology analysis that isolated strains belonged to Trichosporon genus, and growth morphologies as well as spore characteristics of the colonies also conformed to characteristics of trichosporum.
Figure 2.
Phylogenetic tree of strains. “1” was the isolated strain from the crop of a diseased pigeon.
Prevention Function of Chinese Herbal Medicine on Infected Chickens
After 7 D of infections, chickens in the Chinese herbal medicine protection group still had appetite and a better mental state. Chickens in the rest of infected groups showed mental depression, loss of appetite, and loose feathers (Figure 3A). With weight losses as infections increased, some of them died on the fifth day. At the end of infections, it was shown by pathologic anatomies that white pseudo membrane ulcers appeared in the craws of chickens in the infected group, and hyperplasia and hypertrophy were shown in the stratified flat epithelium (Figure 3B). Pathogenic funguses from diseased parts were collected for isolation and culture, through which a pure culture of Trichosporon was obtained.
Figure 3.
Chicken infected by trichosporum for 7 D. (A) A chicken from the trichosporum-infected group; (B) the crop of a chicken from panel A.
After chickens were infected by trichosporum for 7 D, the content of glutamic oxalacetic transaminase (GOT) in blood of chickens in the Chinese herbal medicine protective group was lower than that of the infected group (P < 0.05), which was similar to that of the control group (Table 1), especially GOT in the liver of chickens in the Chinese herbal medicine protective group was extremely lower than that of the infected group (P < 0.01). For glutamate pyruvic transaminase in the blood and liver, there was no significant difference between the Chinese herbal medicine protective group and the infected group (P > 0.05). Results showed that protections of traditional Chinese medicines had positive effects on the liver of Trichosporon-infected chickens.
Table 1.
GOT and GPT of the blood and liver after chicken infected by trichosporum for 7 D.
| Groups | GOT of the blood (U/L) | GOT of the liver (U/g) | GPT of the blood (U/L) | GPT of the liver (U/g) |
|---|---|---|---|---|
| Control group | 69.535 ± 4.122a | 67.233 ± 4.336A,a | 12.575 ± 1.704a | 29.005 ± 1.566a |
| Infected group | 81.51 ± 1.739b | 124.503 ± 3.487B | 21.845 ± 2.328b | 34.272 ± 2.996a |
| Protective group | 63.635 ± 1.874b | 85.855 ± 0.884A,b | 21.013 ± 2.216b | 31.933 ± 3.531a |
a,b,cValues significantly different among different groups.
A,BValues extremely significant among different groups.
Abbreviations: GOT, glutamic oxalacetic transaminase; GPT, glutamate pyruvic transaminase.
Treatment Effect of Chinese Herbal Medicines
After trial chickens were treated with drugs for 7 D, their feed intakes were slightly increased except for the those in the nontreatment group, but the number of dead chickens increased. The mortality rate of chickens in the Chinese herbal medicine protective group and that of the Chinese medicine treatment group were significantly lower than that of the fluconazole treatment group and the nontreatment group, but were higher than that of the control group (Table 2). Glutamic oxalacetic transaminase in the blood of chickens in the herbal protective group, herbal treatment group, and fluconazole treatment group was lower than that of the nontreatment group (P < 0.05), and GOT in the liver of chickens in the herbal protective group and herbal treatment group was extremely lower than that of the fluconazole treatment group and nontreatment group (P < 0.01). There was no difference in glutamate pyruvic transaminase in the blood and liver among the herbal protective group, herbal treatment group, and fluconazole treatment group (P > 0.05) (Table 3).
Table 2.
Mortality rate of each animal group.
| Group | Control | Protective (herb) | Treatment (herb) | Treatment (fluconazole) | No treatment |
|---|---|---|---|---|---|
| Death rate | 3.33% | 23.33% | 33.33% | 46.67% | 50.00% |
Death rate = death count/trial count.
Table 3.
GOT and GPT of blood and liver after chicken treatment for 7 D.
| Groups | GOT of the blood (U/L) | GOT of the liver (U/g) | GPT of the blood (U/L) | GPT of the liver (U/g) |
|---|---|---|---|---|
| Control group | 65.635 ± 4.122a | 62.132 ± 4.336A,a | 15.425 ± 1.704a | 28.125 ± 1.524a |
| Herb protection | 74.057 ± 3.593b | 72.51 ± 5.976A,b | 13.62 ± 1.337a | 20.734 ± 3.282b |
| Herb treatment | 73.216 ± 8.606b | 82.158 ± 9.271A,c | 12.303 ± 2.224a | 19.899 ± 3.433b |
| Fluconazole | 74.420 ± 6.007b | 117.811 ± 10.134B | 19.963 ± 2.255a | 21.613 ± 3.593b |
| No treatment | 85.166 ± 12.386c | 127.16 ± 7.277B | 21.432 ± 3.854a | 30.728 ± 3.117a |
a,b,cValues significantly different among different groups.
A,BValues extremely significant among different groups.
Abbreviations: GOT, glutamic oxalacetic transaminase; GPT, glutamate pyruvic transaminase.
Susceptibility of Chinese Herbal Medicines on Trichosporon
Measurement results of Chinese medicines on Trichosporon MIC80 showed that growth speed of Trichosporon gradually decreased with increase in the concentration of Chinese herbal medicines, and the value of OD600 decreased. When the concentration of A. gramineus was higher than 32 μg/μL, that of S. flavescens was higher than 2 μg/μL, that of P. hydropiper was higher than 120 μg/μL, and that of Chinese herbal mixture was higher than 36 μg/μL, the value of OD600 decreased by more than 80% compared with that of the negative control well (Table 4). Ethanol with a concentration of 60% at 128 μg/μL had an inhibitory effect on Trichosporon. Test results showed that inhibitory effects of S. flavescens on Trichosporon were the strongest among the 3 traditional Chinese herbal medicines. The effect of MIC80 of fluconazole (1 μg/μL) was far better than 3 Chinese herbal medicines in vitro.
Table 4.
MIC80 of different groups (n = 3).
| Groups | Control (alcohol) | Acorus | Sophora | Polygonum | Mixture (herb) | Fluconazole |
|---|---|---|---|---|---|---|
| MIC80 (μg/μL) | 128 | 32 | 2 | 120 | 36 | 1 |
MIC80 means comparing with the negative control, the lowest drug concentration with an OD value declining more than 80%.
Abbreviation: MIC, minimum inhibitory concentration.
Inhibitory Effect of Chinese Herbal Medicines on the Growth of Trichosporon
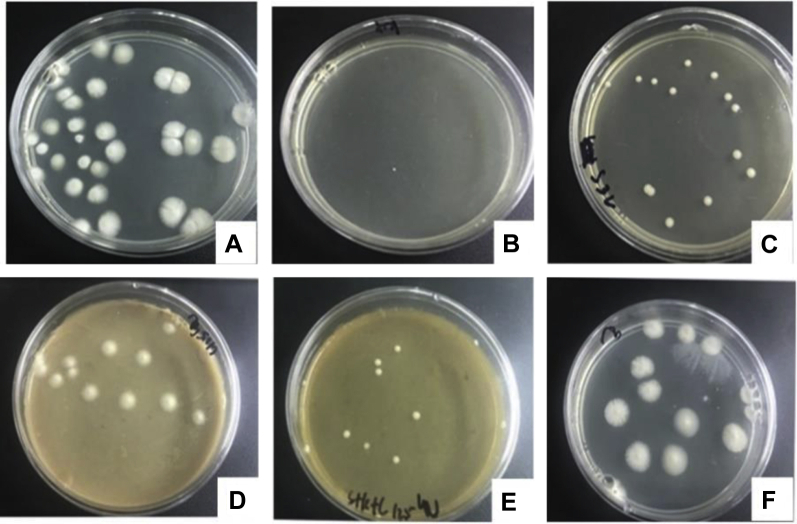
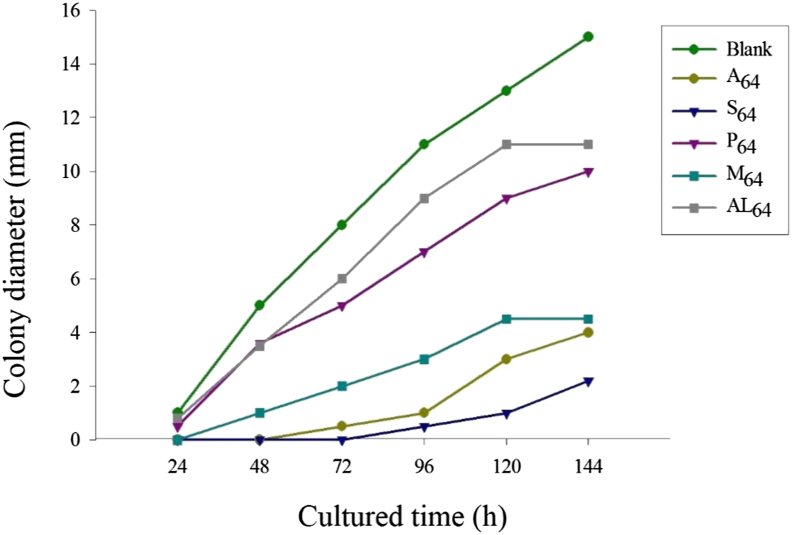
Based on the aforementiioned results, by adding drugs with different concentrations to the solid medium, a drug-sensitive medium was used to observe colony growths at 24 h, 48 h, 72 h, 96 h, 120 h, and 144 h. Cultured for 48 h, colony growths on solid medium are shown in Figure 4. CFU on the solid medium were invisible when the concentration of S. flavescens increased to 4 μg/μL and that of A. gramineus increased to 64 μg/μL. The value of CFU was 15 when the concentration of P. hydropiper increased to 64 μg/μL, and it was 7 when the mixture of the 3 herbal medicines was of the same concentration. Alcohol had weak inhibitory effects on the growths of Trichosporon (Table 5). The colony diameter increased as culture time increased, and results of different drugs with a concentration of 64 μg/μL on the growths of Trichosporon are shown in Figure 5. In the control group, the diameter of colonies reached 15 mm in 144 h, whereas it was only 3 mm in the S. flavescens group, the other Chinese herbal medicines had certain inhibitory effects on growths of Trichosporon. From the results, S. flavescens had the greatest inhibitory effects on growths of Trichosporon colonies, followed by A. gramineus, herbal mixture, and P. hydropiper.
Figure 4.
Colony growth on the solid medium with different herbal medicine for 48 h. (A) Blank control (solid medium with no herbal medicine); (B) S4 (solid medium with Sophora flavescens in 4 μg/μL); (C) A32 (solid medium with Acorus gramineus in 32 μg/μL); (D) P125 (solid medium with Polygonum hydropiper in 125 μg/μL); (E) M125 (solid medium with Chinese herbal mixture in 125 μg/μL); (F) AL125 (solid medium with alcohol in 125 μg/μL).
Table 5.
Chinese herbal medicine on the colony growth in different concentration (n = 3).
| Concentration (μg/μL) | Colony No. (48 h) | Colony No. (72 h) | Colony No. (96 h) |
|---|---|---|---|
| Blank | 32.0 ± 1.7 | ||
| A8 | 30.3 ± 2.3 | ||
| A16 | 14.7 ± 1.5∗ | ||
| A32 | 12.7 ± 0.9∗ | ||
| A64 | No | 2.7 ± 0.3∗∗ | |
| A125 | No | No | No |
| S2 | 16.3 ± 1.2∗ | ||
| S4 | No | 1.0 ± 0.6∗∗ | |
| S8 | No | No | No |
| S16 | No | No | No |
| S32 | No | No | No |
| P64 | 15.0 ± 1.2∗ | ||
| P125 | 11.0 ± 0.6∗ | ||
| M64 | 7.3 ± 0.9∗∗ | ||
| M125 | 6.0 ± 0.6∗∗ | ||
| AL64 | 25.7 ± 1.8 | ||
| AL125 | 13.3 ± 1.2∗ |
∗Statistical significance (P < 0.05).
∗∗P < 0.01.
Abbreviations: A, Acorus gramineus; AL, alcohol; M, Chinese Herbal Mixture; P, Polygonum hydropiper; S, Sophora flavescens.
Figure 5.
Diameter of colony on the solid medium with different herbal medicine cultured different time. Abbreviations: A, Acorus gramineus; AL, alcohol; M, Chinese Herbal Mixture; P, Polygonum hydropiper; S, Sophora flavescens.
Discussion
Fungal infections were often found in poultry farming. The funguses we isolated from a pigeon were identified through gene sequencing, and it was shown by a homology analysis that it belonged to Trichosporon spp. In an animal test, chickens were fed with Trichosporon in which typical pathologic changes arose among chickens, so Trichosporon spp. could spread in poultries. Although studies have found that Trichosporon in the cloaca of migratory birds could cause skin or systemic diseases for animals and humans (Sun., 2015), whether the Trichosporon isolated from pigeons could infect mammals and its pathogenicity on humans were unclear.
Comparing different extraction methods, better extraction results could be achieved through alcohol extraction (Fang et al., 2016; Han et al., 2018). Active ingredients of 3 Chinese herbal medicines and herbal mixture were extracted through ultrasonic alcohol extraction method, which was used for both vivo and vitro tests.
From the animal test, ethanol extract in the mixture of 3 Chinese herbal medicines had certain protections on chickens fed with herbal mixture before Trichosporon infections, and treatment effects of Chinese herbal medicines were better than that of the fluconazole treatment group. The reason was probably that in the invasive processes of Trichosporon, trichosporum was adhered to implanted devices in the form of spores, and biofilms were formed to escape medical sterilizations, as this ability promoted resistance to antibiotics and immune responses of hosts (JosBurgo et al., 2018). Once a disseminated and systemic deep infection is caused in the body, treatments would be difficult, and the mortality rate would be high, which was why mortality rate of the Chinese herbal medicine treatment group and fluconazole treatment group were higher than that of the Chinese herbal medicine protective group, that is, protective effects could improve animal survival rate. Otherwise, Chinese medicines had better therapeutic effects on chickens. It was possible that the side effects of traditional Chinese medicines were lower than that of western medicines, which made the mortality rate of the Chinese medicine treatment group significantly lower than that of the western medicine treatment group.
Results of Chinese medicines on Trichosporon MIC80 showed that inhibitory effects of S. flavescens (MIC80 = 2 μg/μL) were the strongest among the 3 Chinese herbal medicines. Antibacterial concentration of Chinese herbal mixture was among that of the 3, which might be related to the proportion of mixed extraction of the 3 Chinese herbal medicines. The MIC80 of fluconazole was 1 μg/μL, whereas its treatment effects on infected chickens were lower than that of Chinese herbal mixture. It was possible because of its side effects on chickens and its resistance to Trichosporon.
Results of in vitro drug-susceptibility tests showed that when the concentration of A. gramineus was higher than 32 μg/μL, it would have a significant inhibitory effect on trichosporum and could significantly inhibit growths of colonies in a solid sensitive medium. S. flavescens had the strongest anti-trichosporum effects, concentration of MIC80 was only 2 μg/μL, which significantly inhibited the growth of trichosporum. It could only reach 6 mm, which was significantly lower than that of the other traditional Chinese medicine groups, and basically no colony grew on the drug-sensitive medium with a concentration that was higher than 2 μg/μL. However, it was found in this study that concentration of MIC80 of P. hydropiper was only 120 μg/μL for Trichosporon, which was significantly lower than that of the other 2 traditional Chinese medicine groups, and its inhibitory effects on colony diameter in drug-sensitive medium were not significant. However, it had effects of strengthening the spleen, dampening, dehumidifying and stagnation, stopping dysenteries and pains, as well as detoxifying and removing blood stasis, and it might also promote works of other tissues and organs of animals and achieve better antibacterial effects than in vitro. Concentration of MIC80 in Chinese herbal mixture was 36 μg/μL, which was an intermediate value of the 3 traditional Chinese medicines, and its inhibitions of colony growths and diameters in drug-sensitive medium were also among the 3. The results might be related to proportions of the 3 traditional Chinese medicines or interactions of the components during extractions. A. gramineus had a strong sedative effect on the nervous system, matrine could sterilize and kill viruses, detoxify, dehumidify, and dilute water; P. hydropiper coordinate with the 3 Chinese medicines to react in animals. Taking the in vitro bacteriostatic effects of the drugs into account, a treatment method of traditional Chinese medicine mixture was adopted. Feeding traditional Chinese medicine mixture in advance could protect the animals. The mortality rate was significantly lower than that of other experimental groups. Results of the traditional Chinese medicine treatment group were significantly better than those of the western medicine groups. In the treatment group, it could be seen that the toxicity of traditional Chinese medicines were lower than that of western medicines; research results showed that the longer the use of traditional Chinese medicines was, the better the treatment effects would be.
Although traditional Chinese medicines play a role in treatments of infections caused by Trichosporon, processes and main active ingredients of the traditional Chinese medicines are not clear and need further studies.
Conclusions
A. gramineus, S. flavescens, P. hydropiper, and their Chinese herbal medicine mixture all had certain effects on inhibiting growths of trichosporum. S. flavescens had the strongest effects in vitro. Advance protections of traditional Chinese medicines could reduce infections of Trichosporon, and with increases in the use time of traditional Chinese medicines, treatment effects would be better.
Acknowledgments
This project was supported by Fund for Scientific Research and Development of Agricultural University of Hebei, Project No: JY2018005.
Conflict of Interest: The authors declare there is no conflict of interest regarding publication of this manuscript.
References
- Biegańska M.J., Rzewuska M., Dąbrowska I., Dworecka-Kaszak B., Malewska-Biel B., Ostrzeszewicz M. Mixed infection of Respiratory tract in a dog caused by Rhodotorula mucilaginosa and trichosporon jirovecii: a Case report. Mycopathologia. 2018;183:637–644. doi: 10.1007/s11046-017-0227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilhante R.S.N., Castelo-Branco D.S.C.M., Soares G.D.P., Astete-Medrano D.J., Monteiro A.J., Cordeiro R.A., Sidrim J.J.C., Rocha M.F.G. Characterization of the gastrointestinal yeast microbiota of cockatiels : a potential hazard to human health. J. Med. Microbiol. 2010;59:718–723. doi: 10.1099/jmm.0.017426-0. [DOI] [PubMed] [Google Scholar]
- Bryan L.K., Porter B.F., Wickes B.L., Spaulding K.A., Kerwin S.C., Lawhon S.D. Meningoencephalitis in a dog due to trichosporon montevideense. J. Comp. Pathol. 2014;151:157–161. doi: 10.1016/j.jcpa.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Cafarchia C., Camarda A., Romito D., Campolo M., Quaglia N.C., Tullio D., Otranto D. Occurrence of yeasts in cloacae of migratory birds. Mycopathologia. 2006;161:229–234. doi: 10.1007/s11046-005-0194-z. [DOI] [PubMed] [Google Scholar]
- Colombo A.L., Padovan A.C.B., Chaves G.M. Current knowledge of trichosporon spp and trichosporonosis. Clin. Microbiol. Rev. 2011;24:682–700. doi: 10.1128/CMR.00003-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delavenne E., Mounier J., Asmani K., Jany J.-L., Barbier G., Blay G.L. Fungal diversity in cow, goat and Ewe milk. Int. J. Food Microbiol. 2011;151:247–251. doi: 10.1016/j.ijfoodmicro.2011.08.029. [DOI] [PubMed] [Google Scholar]
- Fang K., Chen C., Gao R., Wang S., Zhang Y., Zhang Q., Yan B. Ultrasonic extraction of total flavones from sophora flavescentis l. Shandong J. Tradit Chin Med. 2016;35:989–991. (in Chinese) [Google Scholar]
- Foti M., Rinaldo D., Guercio A., Giacopello C., Aleo A., Leo F.D., Fisichella V., Mammina C. Pathogenic microorganisms carried by migratory birds passing through the territory of the island of Ustica, Sicily. Avian Pathol. 2011;40:405–409. doi: 10.1080/03079457.2011.588940. [DOI] [PubMed] [Google Scholar]
- Górz A., Boroń P. The yeast fungus trichosporon lactis found as an Epizoic Colonizer of Dung beetle Exoskeletons. Microb. Ecol. 2016;71:422–427. doi: 10.1007/s00248-015-0674-8. [DOI] [PubMed] [Google Scholar]
- Gugnani H.C., Paliwal-Joshi A., Rahman H., Padhye A.A., Singh T.S.K., Das T.K., Khanal B., Bajaj R., Rao S., Chukhani R. Occurrence of pathogenic fungi in soil of burrows of rats and of other sites in bamboo plantations in India and Nepal. Mycoses. 2007;50:507–511. doi: 10.1111/j.1439-0507.2007.01402.x. [DOI] [PubMed] [Google Scholar]
- Gujjari P., Suh S., Lee C., Zhou J. Trichosporon xylopini sp. nov., a hemicellulose-degrading yeast isolated from the wood-inhabiting beetle Xylopinus saperdioides. Int. J Syst Evol Micr. 2011;61:2538–2542. doi: 10.1099/ijs.0.028860-0. [DOI] [PubMed] [Google Scholar]
- Han G., Gao Z., Gao E. Progress in extraction and purification of alkaloids from sophora flavescens, China. Mod. Food Sci. Technol. 2018;5:163–166. [Google Scholar]
- JosBurgo F., Mengelle D.E., Abraham A., Kremer G., Autorino C.M. Periprosthetic fungal infection of a hip caused by Trichosporon inkin. Arthroplasty Today. 2018;4:24–26. doi: 10.1016/j.artd.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig D.B., De Camargo L.E.A., Khalil N.M., Auler M.E., Mainardes R.M. Antifungal activity of chitosan-coated Poly(lactic-co-glycolic) acid nanoparticles containing amphotericin B. Mycopathologia. 2018;183:659–668. doi: 10.1007/s11046-018-0253-x. [DOI] [PubMed] [Google Scholar]
- Mas C.D., Pinheiro D.A., Campeiro J.D., Mattei B., Oliveira V., Oliveira E.B., Miranda A., Perez K.R., Hayashi M.A.F. Biophysical and biological properties of small linear peptides derived from crotamine, a cationic antimicrobial/antitumoral toxin with cell penetrating and cargo delivery abilities. Biochim. Biophys. Acta. 2017;1859:2340–2349. doi: 10.1016/j.bbamem.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Medina I.R., Fuentes L.R., Arteaga M.B., Valcrcel F.R., Arbelo F.A., Castillo D.P.D., Surez S.D., Quintana O.F., Gutirrez B.V., Sergent F.S., Acosta-Hernndez B. Pigeons and their droppings as reservoirs of Candida and other zoonotic yeasts. Rev. Iberoam Micol. 2017;34:211–214. doi: 10.1016/j.riam.2017.03.001. [DOI] [PubMed] [Google Scholar]
- Oliveira W., Amaral-Machado L., Alencar E., Marcelino H., Genre J., Silva-Rocha W., Gondim A., Chaves G., Fernandes-Pedrosa M., Egito E. Getting the Jump on the Development of bullfrog oil Microemulsions: a Nanocarrier for amphotericin B Intended for antifungal treatment. AAPS PharmSciTech. 2018;19:2585–2597. doi: 10.1208/s12249-018-1093-1. [DOI] [PubMed] [Google Scholar]
- Pagnocca F.C., Legaspe M.F.C., Rodrigues A., Ruivo C.C.C., Nagamoto N.S., Bacci M., Forti L.C. Yeasts isolated from a fungus-growing ant nest, including the description of Trichosporon Chiarellii Sp. Nov., an anamorphic basidiomycetous yeast. Int. J. Syst. Evol. Microbiol. 2010;60:1454–1459. doi: 10.1099/ijs.0.015727-0. [DOI] [PubMed] [Google Scholar]
- Shang Q. Study on the effect and mechanism of houttuynia sodium against candida albicans. Master. Fujian Uni Tradit Chin Med. 2012;3:15–21. (in Chinese) [Google Scholar]
- Shi G., Shao Q., Wang T., Wang C. New progress in the antibacterial action of scutellaria baicalensis and its active components. China J. Chin Mater. Med. 2014;39:3713–3718. (in Chinese) [PubMed] [Google Scholar]
- Sun J. Shandong Agr Uni; 2015. In Vitro Screening of Traditional Chinese Medicine against Avian Trichomoniasis of Pigeon Origin and its Mechanism of Action. Master. (in Chinese) [Google Scholar]
- Tang J., Chen S., Liu R., Xu X., Yu X. Ultrasonic extraction of total flavonoids from polygonum polygonum l. Lishizhen Med. Mater. Med. Res. 2011;22:1387–1388. (in Chinese) [Google Scholar]
- Ueda K., Nakamura I., Itano E., Takemura K., Nakazato Y., Sano A. Trichosporon asteroides isolated from Cutaneous lesions of a Suspected Case of 'paracoccidioidomycosis ceti' in a Bottlenose dolphin. Mycopathologia. 2017;182:937–946. doi: 10.1007/s11046-017-0147-3. [DOI] [PubMed] [Google Scholar]
- Wang Y., Yue T., Wang Z., Wang Q., Kuang H. GC-MS analysis and antibacterial effect of volatile. Oil. Chem. Eng. 2017;31:26–29. (in Chinese) [Google Scholar]
- Wimley W.C., Hristova K. Antimicrobial peptides: Successes, Challenges and Unanswered Questions. J. Membr. Biol. 2011;239:27–34. doi: 10.1007/s00232-011-9343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. Study on the antifungal activity screening and mechanism of traditional Chinese medicine. Master. Sec Milit Med. Uni. 2017;22:38–65. (in Chinese) [Google Scholar]
- Xiang Y., He L., He W., Gao B., Yuan M., Luo S., Xu Z., Hong X. Screening and optimization of extraction technology of total flavonoids from polygonum polygonum l. Chin J. Vet. Drug. 2019;53:40–47. (in Chinese) [Google Scholar]
- Yi J., Li X., Su J., Tan W. Preparation of volatile oil of acorus gramineus β- cyclodextrin inclusion by ultrasonic method. Lishizhen Med. Mater. Med. Res. 2006;10:381–383. (in Chinese) [Google Scholar]
- Zhang M., Shen Y. Research progress on antibacterial pharmacological effects of matrine alkaloids. Anti-Infection Pharm. 2018;15:369–374. (in Chinese) [Google Scholar]
- Zhang X., Guo H. Research progress on pharmacological effects of Iris calamus. Chin J. Tradit Med. Sci. Technol. 2019;26:320–321. (in Chinese) [Google Scholar]