Abstract
Adaptive genetic variations have direct influences on the fitness traits of the animal. The major histocompatibility complex B (MHC-B) region is responsible for adaptive and innate immune responses in chickens. In native Korean chicken breeds, no information on serologically defined B haplotypes is available. We investigated the MHC-B diversity in 5 restored lines of Korean native chicken and Ogye chicken breeds using a recently described MHC-B single-nucleotide polymorphism (SNP) panel and the MHC-linked LEI0258 variable number of tandem repeat marker. High SNP haplotype diversity was observed in Korean native chicken breeds with an average of 9.7 MHC-B SNP haplotypes per line. The total number of haplotypes ranged from 6 to 12 per line, and population-specific haplotypes ranged from 3 to 4. A total of 41 BSNP haplotypes, including 26 novel population-specific haplotypes and 15 common haplotypes, were reported over all populations. The 15 common haplotypes included 7 novel and 8 previously reported standard haplotypes. Selection and breeding evidence supports the observation of common haplotypes between the Korean native chicken and exotic breeds. Similarly, the LEI0258 marker showed allele variation, between 193 bp and 474 bp having 5 to 8 alleles per population. Some of these alleles (193, 249, 309, and 443 bp) were shared and more frequently observed. Comparison between SNP haplotypes and LEI0258 allele sizes for the same samples showed that some LEI0258 allele sizes correspond to more than one BSNP haplotype. The use of the MHC-B SNP panel greatly enhances the identification of MHC diversity compared with the sole use of the LEI0258 marker in native chicken populations.
Key words: MHC diversity, SNP haplotype, Korean native chicken, disease resistance
Introduction
Local chicken breeds are considered to be hardy and more disease resistant than the high-producing strains because of local adaptation and natural selection response to extreme environmental conditions (Zhang et al., 2014; Walugembe et al., 2019). Adequate adaptive variations are required for any species to survive dynamic environment changes (Gebremedhin et al., 2009). Like other species, livestock are no exception as they can respond and thrive under dynamic environmental changes if they have been maintained with adequate adaptive genetic variations (Eckert et al., 2008; Jump and Penuelas, 2005, 2009).
This study focuses on important local chicken populations in Korea. Most of the native Korean chicken breeds were lost during World War II and the Korean War. The few remaining backyard Korean native chickens (KNC) were collected and reared to increase the population size by several researchers and private farmers who were interested in these breeds. To improve these remaining KNC and to supply the rising demand for quality native chicken meat, a nationwide native chicken breed restoration and establishment project was developed from 1992 to 2007, mainly by the National Institute of Animal Science (NIAS) in South Korea. Fifteen years later, 5 distinct KNC lines with distinctive feather color (white [KNCW], black [KNCL], gray–brown [KNCG], red–brown [KNCR], and yellow–brown [KNCY]) were established (Kwak et al., 2014; Seo et al., 2018). Previous genetic analyses using microsatellite markers have shown that these lines are genetically distinct (Suh et al., 2014). Since the 1960s, the backyard poultry production system has rapidly converted to commercial-scale production as a consequence of the rapid increase in per capita meat and egg consumption in Korea (Kim, 1994). Several improved European or US-developed breeds, such as Rhode Island Red (RIR), white and brown Leghorn, White Plymouth Rock, Cornish, and New Hampshire, were imported during the 1960s and used in local production systems to enhance the Korean broiler and layer industry (Sang et al., 1979; Kim, 1994; Kang et al., 1997a,b). Currently, these breeds have been maintained as separate stocks by the NIAS and a private breeding company. More detailed descriptions of these breeds can be found in the study by Seo et al. (2018). Kang et al., 1997a, Kang et al., 1997b reported growth and layer performance of a series of crossbred populations produced by crossing RIR with one of the KNC lines (brown or black). Another study describes a Korean commercial hybrid produced from a three-way cross of a Cornish male line with hybrid female line (produced by a single cross of RIR with one KNC line) by the NIAS (Kang et al., 1998a,b). As the demand for native chicken meat has increased owing to its unique taste and meat quality, the original KNC breeds and commercial breeds have been widely used for diverse crossbreeding since 2000 (Park et al., 2010, 2011; Kang et al., 2010; Lee et al., 2013, 2014). As a result, one of the famous hybrids called “Woorimatdag” produced by the NIAS and “Hanhyup-3-ho” of Hanhyup private company are available on the current market (Choo and Chung, 2014; Seo et al., 2017).
The Korean Ogol fowl (KO), also known as Ogye, is another important native chicken breed for the Korean poultry industry. It is a hypermelanistic breed, with black feathers, skin, beak, comb, shank, and bone. Currently, several farmers are rearing this Ogye chicken, which is bred not for quantity but for its unique characteristics (Sohn et al., 2018). Most farmers believe that this breed has high disease resistance and dynamic environmental adaptation. Hence, understanding the local adaptability and disease resistance in KNC has been important for the conservation and development of these local breeds for sustainable production.
The major histocompatibility complex B (MHC-B) region of the chicken is an important cluster of genes in which the diversity is maintained by balancing selection (Kaufman et al., 1999a,b; Miller and Taylor, 2016; Minias et al., 2018). Genes in this region are functionally linked with host immunity along with other biological and production traits (Lundén et al., 1993; Shiina et al., 2004; Molee et al., 2016). It is well known that MHC-B variation has an impact on specific disease resistance to several, highly pathogenic viral and bacterial diseases in poultry (Shiina et al., 2007; Jin et al., 2010; Miller and Taylor, 2016) as well as internal and external parasites (Owen et al., 2008; Schou et al., 2010). Therefore, identifying MHC-B haplotypes in indigenous chicken and developed breeds is valuable for management and productivity of the poultry industry.
Identification of MHC-B haplotypes in chickens has changed from the initial serology detection method to DNA-based variant detection (Briles and Briles, 1982; Zheng et al., 1999; Iglesias et al., 2003; Fulton et al., 2016a, Fulton et al., 2016b). Generally, MHC-B haplotypes were defined based on the serological information obtained from hemagglutination assays using specifically prepared alloantisera within a given line (Briles, 1962; Fulton et al., 1996, 2001; Miller et al., 2001). However, the identification of serologically defined haplotypes for breeds or crossbreeds in local production systems has not been carried out. The molecular marker–based tools provide a more expedient way to identify the haplotypes in any chicken population, particularly for local breeds, which do not have serological information. DNA-based tools also allow for comparison of MHC haplotypes between multiple breeds and populations (Miller et al., 1988; Landesman et al., 1993; Goto et al., 2002; Maccari et al., 2017).
The variable number of tandem repeat (VNTR) marker LEI0258 is located within the MHC-B region, and variation within this marker has been shown for multiple chicken breeds from multiple sources (Fulton et al., 2006; Chazara et al., 2013; Han et al., 2013; Nikbakht et al., 2013; Kannaki et al., 2017; Mwambene et al., 2019). It is a good indicator of MHC-B variation in chickens owing to its high allele range and allele richness. In addition, it has been shown that LEI0258 alleles are significantly correlated with certain serological B haplotypes (Fulton et al., 2006; Chazara et al., 2008). However, not all alleles are unique to specific MHC-B serological haplotypes as the same allele size has been reported for different serological MHC-B haplotypes (Fulton et al., 2006). Furthermore, the high mutation rate of the VNTR can result in multiple LEI0258 allele sizes being observed for the same serological haplotype (Fulton et al., 2006). In addition, it is difficult to identify novel recombinant haplotypes using a single MHC-B–linked marker.
An alternative approach to overcome these limitations is to examine variation in specific combinations of molecular markers. The use of single-nucleotide polymorphism (SNP) in the MHC- B region for haplotype identification has gained recent attention. A set of SNP in the MHC-B region was selected by the group of researchers to create a SNP panel (Chazara et al., 2010). Later, the SNP panel was modified to a single SNP detection system (KASP), and additional SNP were included to create a new panel with 101 SNP (Fulton et al., 2016a). The KASP chemistry version of the SNP panel was successfully used in different chicken populations to investigate the haplotype diversity, recombination rate, recombination hot spots, and novel haplotypes in the MHC-B region (Fulton et al., 2016a,b, 2017; Nguyen-Phuc et al., 2017; Iglesias et al., 2019). Unique haplotypes in experimental lines, broiler and layer breeds, and serologically MHC-defined lines were identified using the SNP panel and subsequently compared among the breeds to identify similarity relationship and develop a relevant nomenclature system for the SNP-based haplotype analysis. Single-nucleotide polymorphism haplotypes identified in serologically MHC-defined lines were considered standard haplotypes that are comparable and consistent among the different breeds. The objective of this study is to identify the MHC-B haplotypes and diversity using the MHC-B SNP panel in 6 KNC populations. Information for the VNTR marker LEI0258 was obtained from the same samples to investigate the correlations between MHC-B SNP haplotypes and LEI0258 alleles.
Materials and methods
Sample Collection and Genomic DNA Preparation
A total of 379 samples were collected from the 5 KNC lines and the Ogye chicken line maintained by the NIAS, Korea, by following the procedure described in “The Guide for Care and Use of Laboratory Animals” published by the Institutional Animal Care and Use Committee of the NIAS, Korea.
The KNC sample included KNCG (n = 68), KNCR (n = 64), KNCL (n = 63), KNCW (n = 67), KNCY (n = 69), and Ogye (KO, n = 48). Whole blood samples were collected from the brachial vein into the ethylenediaminetetraacetate-coated vacutainer. Whole blood samples were transferred to the FTA Elute micro card (GE Healthcare, Piscataway, NJ) and dried at room temperature before transportation. Genomic DNA (gDNA) was extracted from the dried blood spot from the FTA Elute micro card following the manufacturer's instructions. For some of these lines, gDNA was extracted from whole blood collected in an ethylenediaminetetraacetate-coated vacutainer (Becton Dickinson, Franklin Lakes, NJ) according to the manufacturer's instructions in the PrimePrep gDNA extraction kit (GeNet Bio, Daejeon, South Korea). Extracted DNA was subjected to quality evaluation before genotyping.
Major Histocompatibility Complex B SNP Genotyping, Haplotype Identification, and Nomenclature
Major histocompatibility complex B SNP genotyping was performed using a subset of 90 SNPs in the panel, as described in the study by Fulton et al. (2016a). Samples were genotyped using the single-plex KASP genotyping assay for 90 SNPs of the MHC-B SNP panel that cover the region between 30,189 and 240,933 bp (i.e., between SNP MHCJ06 and MHC178) of the MHC sequence defined by Shiina et al. (2007). Details of SNP and primer information are described in the study by Fulton et al., 2016a.
All haplotypes were identified using the two-step procedure described by Fulton et al. (2016a,b, 2017). First, the birds that were homozygous for all SNP were identified and used to define the initial set of haplotypes. In the second step, the SNP patterns of heterozygous birds were compared with the SNP haplotypes initially identified. If the heterozygous birds' haplotypes could be explained by any of the homozygous haplotypes in the first set, those supported haplotypes were subtracted and the remaining SNP patterns were used to define the additional haplotypes present in the population. This analysis was performed separately within each line. All haplotypes identified in each Korean chicken population were compared with previously identified standard haplotypes of heritage breeds and commercial egg layer elite lines (Fulton et al., 2016a,b). Subsequently, independently identified haplotypes in all 6 populations were compared, and common and line-specific haplotypes were identified. If a haplotype shows equivalence with a standard haplotype for all 90 SNPs, they were named as the standard; otherwise, each of the novel haplotypes was assigned a new name (i.e., BSNP-Kr01, BSNP-Kr02, and so on) and numbered based on the order in which they were identified. LEI0258 allele information for each BSNP haplotype was also incorporated.
Variable Number of Tandem Repeat Marker LEI0258 Analysis
The allele sizes of LEI0258 for all samples were obtained by using the polymerase chain reaction with the primer pair of LEI0258 forward: 5′-CACGCAGCAGAACTTGGTAAGG-3′and reverse: 5′-AGCTGTGCTCAGTCCTCAGTGC-3′ according to the procedure described in the study by Fulton et al. (2006). Polymerase chain reaction fragment sizes were analyzed by capillary electrophoresis using an ABI3730 DNA Analyzer (Applied Biosystems, Foster City, CA). Allele size was determined using GeneMapper 3.4 software (Applied Biosystems, Foster City, CA).
Population genetic statistics including the number of alleles, effective allele size, expected (He) and observed heterozygosity for the VNTR marker were obtained using GenAlex 6.503 software (Peakall and Smouse, 2012).
Results and discussion
Diversity of MHC-B SNP Haplotypes Within and Between KNC Populations
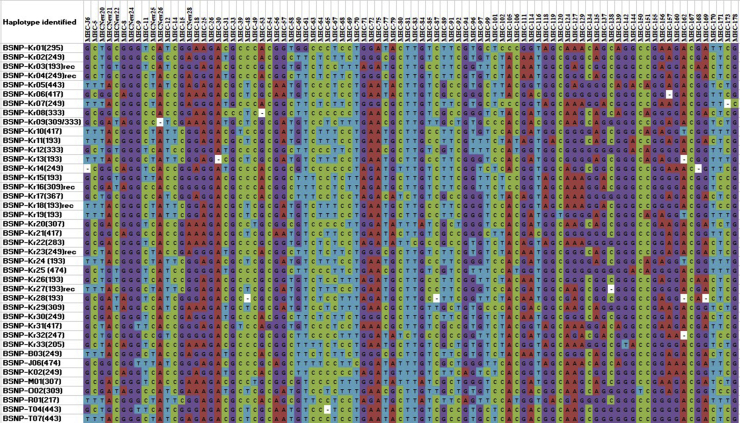
The MHC-B SNP panel provides an excellent alternative tool for mapping MHC diversity in a variety of chicken breeds including 6 KNC populations. The findings of this study are interpreted in the context of MHC variation previously identified in serologically MHC-defined lines and other breeds (Fulton et al., 2016a,b, 2017). Considerably high genetic diversity was observed using the MHC-B SNP panel (Table 1). A total of 41 BSNP haplotypes were found within the 6 Korean chicken lines, with a range from 6 (KO) to 12 (KNCG and KNCY) and an overall average number of 9.7 haplotypes per line. The haplotypes within the Korean chicken populations consist of 33 novel haplotypes and 8 haplotypes previously identified in breeds, as reported by Fulton et al. (2016a) (Table 2, Figure 1). The number of novel unique haplotypes (i.e., population specific) ranged from 3 in KNCW to 7 in KNCR, with a total of 26 novel unique haplotypes identified. Close examination of all BSNP haplotypes revealed that some novel haplotypes are almost identical, differing at only one or 2 SNP. For example, BSNP-Kr06 is identical to BSNP-Kr21 except at one SNP (MHC-65), and BSNP-Kr19 is identical to BSNP-Kr24 except at one SNP (MHC-120) (Figure 1). Haplotypes with single SNP differences were also reported by Fulton et al. (2016b).
Table 1.
The MHC-B SNP haplotype results for Korean native chicken lines.
| Population1 | N2 | Total haplotype3 | Common haplotype4 | Unique haplotype5 | No. recombinant | No. hom | No. het |
|---|---|---|---|---|---|---|---|
| KNCG | 68 | 12 | 8 | 4 | 1 | 10 | 58 |
| KNCL | 63 | 7 | 3 | 4 | 15 | 48 | |
| KNCR | 64 | 11 | 4 | 7 | 2 | 13 | 51 |
| KNCW | 67 | 10 | 7 | 3 | 1 | 21 | 46 |
| KNCY | 69 | 12 | 8 | 4 | 2 | 8 | 61 |
| KO | 48 | 6 | 2 | 4 | 16 | 32 | |
| Total6 | 379 | 41 | 15 | 26 | 6 |
Abbreviations: No. het, number of heterozygous birds for BSNP haplotype; No. hom, number of homozygous birds for BSNP haplotypes; No. recombinant, number of recombinants; KNC, Korean native chicken; MHC-B, major histocompatibility complex B; SNP, single-nucleotide polymorphism.
Population used in this study: KNCG = gray–brown, KNCL = black, KNCR = red–brown; KNCW = white, KNCY = yellow–brown, and KO = Korean Ogye chicken.
The number of samples per population used for haplotype analysis.
Number of haplotypes identified in each population.
The number of common haplotypes per population.
Unique haplotypes observed in each population.
Total number of haplotypes and total common haplotypes without redundancy.
Table 2.
Summary of MHC-B SNP haplotypes and their frequencies in the Korean native chicken lines.
| MHC-B SNP haplotype name1 | Population2 |
Total observations3 | |||||
|---|---|---|---|---|---|---|---|
| KNCG | KNCL | KNCR | KNCW | KNCY | KO | ||
| BSNP-Kr01(295) | 0.103 | 0.051 | 21 | ||||
| BSNP-Kr02(249) | 0.199 | 0.127 | 44 | ||||
| BSNP-Kr03(193)rec | 0.162 | 0.047 | 0.029 | 32 | |||
| BSNP-Kr04(249)rec | 0.007 | 1 | |||||
| BSNP-Kr05(443) | 0.147 | 0.007 | 21 | ||||
| BSNP-Kr06(417) | 0.074 | 0.007 | 11 | ||||
| BSNP-Kr07(249) | 0.074 | 10 | |||||
| BSNP-Kr08(333) | 0.007 | 1 | |||||
| BSNP-Kr09(309/333) | 0.015 | 0.468 | 0.305 | 100 | |||
| BSNP-Kr10(417) | 0.007 | 1 | |||||
| BSNP-Kr11(193) | 0.222 | 28 | |||||
| BSNP-Kr12(333) | 0.143 | 18 | |||||
| BSNP-Kr13(193) | 0.095 | 12 | |||||
| BSNP-Kr14(249) | 0.016 | 2 | |||||
| BSNP-Kr15(193) | 0.211 | 27 | |||||
| BSNP-Kr16(309)rec | 0.008 | 1 | |||||
| BSNP-Kr17(367) | 0.023 | 3 | |||||
| BSNP-Kr18(193)rec | 0.008 | 1 | |||||
| BSNP-Kr19(193) | 0.055 | 7 | |||||
| BSNP-Kr20(193) | 0.141 | 18 | |||||
| BSNP-Kr21(193) | 0.023 | 3 | |||||
| BSNP-Kr22(283) | 0.090 | 12 | |||||
| BSNP-Kr23(249)rec | 0.037 | 5 | |||||
| BSNP-Kr24 (193) | 0.060 | 0.239 | 41 | ||||
| BSNP-Kr25 (474) | 0.007 | 1 | |||||
| BSNP-Kr26(193) | 0.051 | 7 | |||||
| BSNP-Kr27(193)rec | 0.014 | 2 | |||||
| BSNP-Kr28(193) | 0.007 | 1 | |||||
| BSNP-Kr29(309) | 0.007 | 1 | |||||
| BSNP-Kr30(249) | 0.115 | 11 | |||||
| BSNP-Kr31(417) | 0.395 | 38 | |||||
| BSNP-Kr32(247) | 0.052 | 5 | |||||
| BSNP-Kr33(205) | 0.073 | 7 | |||||
| BSNP-B03(249) | 0.140 | 0.040 | 0.063 | 0.052 | 0.232 | 71 | |
| BSNP-J06(474) | 0.559 | 0.065 | 84 | ||||
| BSNP-K02(249) | 0.014 | 0.344 | 35 | ||||
| BSNP-M01(307) | 0.016 | 2 | |||||
| BSNP-O02(309) | 0.181 | 25 | |||||
| BSNP-R01(217) | 0.052 | 7 | |||||
| BSNP-T04(443) | 0.117 | 0.021 | 17 | ||||
| BSNP-T07(443) | 0.066 | 0.109 | 24 | ||||
| 758 | |||||||
Abbreviations: KNC, Korean native chicken; rec, recombinant haplotypes; MHC-B, major histocompatibility complex B; SNP, single-nucleotide polymorphism.
Haplotypes from BSNP-Kr01 to BSNP-Kr31 are novel haplotypes identified in Korean chicken and BSNP-B03 to BSNP-T07 are previously reported standard haplotypes in the study by Fulton et al. (2016a,b). The LEI0258 allele size (bp) is indicated in parentheses.
Population used in this study: KNCG = gray–brown, KNCL = black, KNCR = red–brown, KNCW = white, KNCY = yellow–brown, and KO = Korean Ogye chicken. Haplotype frequencies calculated by each population are given.
Number of observation for each haplotype in the entire data set.
Figure 1.
Visualization of BSNP haplotypes found in the Korean native chicken lines. The haplotype name indicates the corresponding BSNP allele, and the VNTR marker LEI0258 allele sizes (bp) are indicated in parenthesis. Abbreviation: VNTR, variable number of tandem repeat.
The haplotype frequency results revealed that all lines had from 2 to 4 haplotypes at a frequency of more than 0.10 (Table 2), with additional haplotypes at lower frequencies (< 0.10). The 5 KNC lines are being maintained as restored breeds after successful regeneration and establishment project (Kwak et al., 2014; Jin et al., 2017). Initial diverse population sampling in KNC and large population size maintained through advanced breeding practices such as artificial insemination, within-line selection, and within-line breeding may be the possible reasons for having considerable unique genetic diversity at the MHC-B region in 5 KNC lines.
The ability to identify the same haplotypes in different local breeds and the presence of standard haplotypes also found in other breeds provide valuable information on MHC diversity in different breeds. Each MHC-B haplotype found was compared across the Korean chicken populations and with the haplotypes identified in standard chicken breeds described in the study by Fulton et al. (2016a,b). Of the total 15 common haplotypes, there are 7 previously unreported haplotypes that are unique and only shared between 2 to 3 of the KNC lines (Table 2). The remaining 8 common haplotypes within the KNC line were previously identified in several North American and European breeds (Fulton et al., 2016a, 2017). The 8 haplotypes that were identical to haplotypes found in KNC lines, therefore, are named with their previously given standard haplotype names (Table 2). The standard BSNP-B03 haplotype was found in all 5 KNC lines, but not in the KO line. The KNCY population had the highest number of previously described haplotypes; BSNP-B03, BSNP-J06, SNP-K02, BSNP-O02, and BSNP-T07. All other KNC lines except the KNCY population had 2 to 3 of the previously described haplotypes (Table 2). Sharing of the novel BSNP haplotypes across the KNC lines is in agreement with previous studies that found KNC lines have a close genetic relationship by sharing common ancestors at the origin (Seo et al., 2013; Shu et al., 2014; Choi et al., 2015). In the early backyard breeding system, the KNC lines may have interbred despite being separated based on the feather color and body conformation traits (Seo et al., 2013, 2018).
The presence of BSNP haplotypes within the KNC breeds that were previously found in broilers, Barred Plymouth Rock, RIR, White Plymouth Rock, and New Hampshire breeds (Fulton et al., 2016a,b) suggests a possible genetic relationship between KNC and some of these North American breeds. Several studies, using DNA fingerprinting, polymorphic microsatellite markers, and 600k SNP chip data, found evidence of such genetic introgressions into the KNC since 1960. Based on the previous results, KNC lines are closely related to Cornish, New Hampshire, and RIR breeds and genetically more distant from the white Leghorn breed (Lee et al., 1996a, b; Kong et al., 2006; Oh et al., 2008; Suh et al., 2014; Seo et al., 2017; Seo et al., 2018). Seo et al. (2018) further support our observation through their findings of a close relationship between KNC and RIR and Cornish breeds based on the 600K SNP chip data, indicating that before restoration occurred, the RIR and Cornish breeds were likely crossed with the KNC breed.
The Korean Ogye chicken is a unique breed, very distinct from the other KNC purebred lines. A total of 6 haplotypes, including 4 unique and 2 common haplotypes, were found in the Ogye breed. In addition, the Ogye chicken line does not share any haplotypes with 5 KNC lines except for 2 previously reported BSNP standard haplotypes (BSNP-K02 and BSNP-T07) (Table 2) (Fulton et al., 2016a,b). The presence of unique alleles in Ogye chickens provides further evidence that they were different from KNC-restored lines. This result was not unexpected as it is in agreement with a previous diversity study using microsatellite markers that showed a considerable genetic distance between Ogye chickens and other KNC lines (Suh et al., 2014). Further investigation with these unique haplotypes and potential associations with disease resistance of Ogye chickens is warranted.
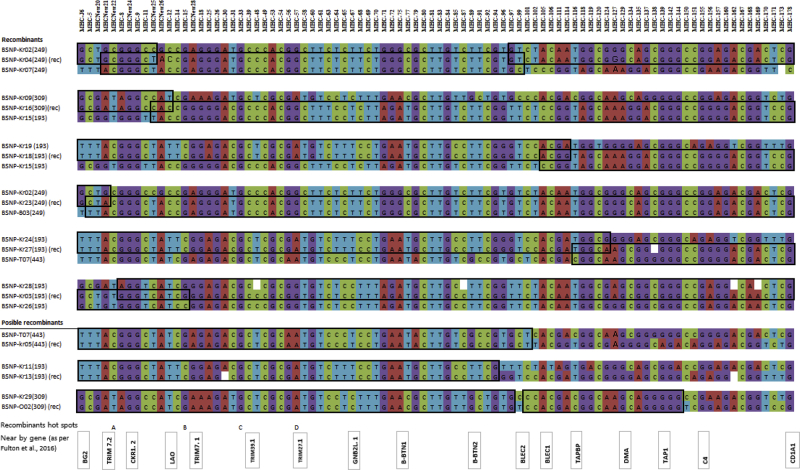
Recombinants were identified when both parental haplotypes were present within the same population. Six recombinant haplotypes were identified using criteria described in the study by Fulton et al., 2016a (Figure 2). Two recombinants were observed in KNCR (BSNP-Kr16(309) and BSNP-Kr18(193)) and in KNCY (BSNP-Kr03(193) and BSNP-Kr27(193)) lines, whereas one recombinant was observed in KNCG (BSNP-Kr04(249)) and in KNCW (BSNP-Kr23(249)) lines. With the exception of BSNP-Kr03(193), the recombinants were found in only one population and at low frequency (< 4%). This is logical, suggesting that new haplotypes arose recently after a recombination event, and may be either retained or lost owing to the drift, unless there is selection pressure to maintain or increase frequency. The haplotype BSNP-Kr03(193) was found in 3 of the populations, with the highest frequency being in the KNCG (16.2%) line. Commonality across multiple populations could be due to shared genetic history, and the high frequency within one of the lines may be due to some selective advantage, a random drift, or a simple founder effect. This novel recombinant haplotype may be worthy of more detailed follow-up for associations with disease resistance. Caution should be applied in defining recombinant haplotypes. It is possible that both parental haplotypes were not identified within a population owing to sampling. Several of the novel haplotypes may be considered as potential recombinants as they appeared to contain 2 or more segments of SNP that were identical to other haplotypes (Figure 2). This may be due to the occurrence of multiple historic recombination events.
Figure 2.
Recombinant haplotypes found in Korean native chicken lines. Crossover regions are indicated as overlapped rectangles. Possible recombinants with one parent haplotype are also indicated. A few genes within the MHC-B region covered by the MHC-B SNP panel are shown. Recombinant hot spots from A to D as per Fulton et al. (2016a) were also indicated to identify the localization of the current crossover region. Abbreviations: MHC-B, major histocompatibility complex B; SNP, single-nucleotide polymorphism.
Close examination of the putative recombinants suggests the presence of 2 putative recombination hot spots (Figure 2). One hot spot is between SNP MHCNew24 and MHC014 (47,000–57,000 bp) (seen in BSNP-Kr03(193), BSNP-Kr04 (249), and BSNPKr16(309)), with a second one between SNP-MHC094 and MHC101 (126,000–138,000 bp) (seen in BSNP-Kr04(249), BSNP-Kr05(443), and BSNP-Kr13(193)). These occur within the putative recombinant hot spots of B and F, as identified by Fulton et al. (2016a).
LEI0258 Allele Diversity and Correlation Between BSNP Haplotypes
Historically, population diversity studies for MHC have been carried out using LEI0258. This study obtained both BSNP haplotypes and LEI0258 allele information for the same samples, providing an opportunity to compare results from 2 commonly used MHC detection methods. To support the haplotype diversity determined using 90 SNP in the MHC-B region in Korean chickens, the number of alleles and population statistics for the MHC-linked VNTR marker LEI0258 were investigated using the same populations (Supplementary Table 1). The LEI0258 marker has shown an allele range from 193 to 474 bp, consistent with allele sizes reported in other chicken populations (Fulton et al., 2006; Chazara et al., 2011, 2013; Han et al., 2013; Mwambene et al., 2019).
Several LEI0258 alleles have identical sizes among the five KNC lines. For example, 193-, 249-, and 309-bp alleles were found in all 5 lines. A total of 5 unique alleles, 2 in KO (205 and 247 bp), 2 in KNCW (217 and 283 bp), and one allele in KNCR (369 bp), were identified. The mean He heterozygosity value estimated for the LEI0258 marker was similar to the estimated He heterozygosity value for BSNP alleles. The observed heterozygosity values for LEI0258 alleles and BSNP alleles ranged between 0.625 (KO) and 0.797 (KNCY) and between 0.667 (KO) and 0.844 (KNCY), respectively (Tables 3 and 4).
Table 3.
Number of alleles and observed and expected heterozygosity for MHC-B SNP haplotypes.
| Population1 | N | Na | Ne | A.R | Ho | He |
|---|---|---|---|---|---|---|
| KNCG | 68 | 12 | 7.81 | 11.03 | 0.838 | 0.872 |
| KNCL | 63 | 7 | 3.39 | 6.89 | 0.762 | 0.705 |
| KNCR | 64 | 11 | 5.75 | 10.72 | 0.797 | 0.826 |
| KNCW | 67 | 10 | 2.91 | 9.15 | 0.687 | 0.657 |
| KNCY | 69 | 12 | 6.21 | 11.20 | 0.884 | 0.839 |
| KO | 48 | 6 | 3.46 | 6.0 | 0.667 | 0.711 |
| Mean | 9.67 | 4.31 | 6.165 | 0.772 | 0.768 |
Abbreviations: A.R, allele richness per population (based on the minimum sample size of 48 diploid individuals); KNC, Korean native chicken; MHC-B, major histocompatibility complex B; N, number of samples per population; Ne, effective allele size (Ne = 1/[1-He]); SNP, single-nucleotide polymorphism.
Observed (Ho) and expected (He) heterozygosity values were calculated considering each MHC-B SNP haplotype as a separate allele.
Population used in the present study: KNCG = gray–brown, KNCL = black, KNCR = red–brown, KNCW = white, KNCY = yellow–brown, and KO = Korean Ogye chicken.
Table 4.
Number of alleles and observed and expected heterozygosity values for the VNTR marker LEI0258.
| Population1 | N | Na | Ne | Ho | He |
|---|---|---|---|---|---|
| KNCG | 68 | 7 | 3.90 | 0.779 | 0.744 |
| KNCL | 63 | 5 | 3.13 | 0.778 | 0.680 |
| KNCR | 64 | 7 | 4.25 | 0.746 | 0.765 |
| KNCW | 67 | 8 | 2.65 | 0.687 | 0.622 |
| KNCY | 69 | 6 | 4.31 | 0.797 | 0.768 |
| KO | 48 | 5 | 2.71 | 0.625 | 0.631 |
| Mean | 6.33 | 3.42 | 0.735 | 0.702 |
Abbreviations: He, expected heterozygosity; Ho, observed heterozygosity; N, number of samples per population; Na, total number of alleles; Ne, number of effective alleles (Ne = 1/[1-He]); KNC, Korean native chicken; VNTR, variable number of tandem repeat.
Population used in the present study: KNCG = gray–brown, KNCL = black, KNCR = red–brown, KNCW = white, KNCY= yellow–brown, and KO = Korean Ogye chicken.
Although LEI0258 has been commonly used in MHC diversity studies, it has limitations including failure to fully distinguish different haplotypes and high mutation rates, thus not providing accurate haplotype diversity, as reported in previous studies. (Fulton et al., 2016a; Iglesias et al., 2019). A total of 14 different alleles for the LEI0258 marker were identified across all populations, whereas the BSNP panel identified a much higher number of 41 different haplotypes. The number of private alleles found for BSNP haplotypes was also higher than that for the LEI0258 marker. One BNSP haplotype (BSNP-Kr09) was found to have 2 possible LEI0258 alleles (309 and 333 bp) that differ in size by 24 bp, suggesting mutation of the R12 repeat within LEI0258 (Table 2). The LEI0258 allele size of 193 bp was found in 10 different BSNP haplotypes, and the 249-bp allele was found in 8 different BSNP haplotypes. These exemplify the underestimation of MHC haplotype diversity that occurs when relying solely on LEI0258. Evidence from several previous studies (Fulton et al., 2016a,b, 2017; Iglesias et al., 2019) and current results suggest that use of the MHC-B SNP panel is more accurate than the single MHC-B–linked marker as a tool for MHC-B haplotype diversity studies.
The LEI0258 marker is a locus with multiple mutation sites, which can result in variable allele sizes in different populations (Chazara et al., 2013; Han et al., 2013). From the diversity perspective, it is indeed attractive, and our results are in agreement with those of the study by Chazara et al. (2011), who concluded that LEI0258 is a good indicator of prediction of MHC heterozygosity. However, from the comparative results presented in Tables 3 and 4, the LEI0258 locus is less informative than MHC-B SNP. Guanxin et al. (2014) have indicated that evolution of LEI0258 might be different from its flanking sequence. Therefore, allele variations in the VNTR marker LEI0258 are not necessarily related to the MHC-B haplotype.
The 5 KNC restored lines used in this study are of commercial importance as they are used as parent populations for creating commercial KNC lines. The genetic variation observed in KNC clearly shows the value of these breeds as reservoirs of MHC gene variation. Understanding the disease resistance of their commercial production crosses is important for commercial production success. Our current results based on the MHC-B SNP panel indicate that KNC lines have maintained high MHC diversity, presumably with high local adaptation and disease resistance. This diversity provides an opportunity for future studies for evaluating the association between specific MHC haplotypes and certain disease resistance, which would allow selection for sustainable breeding for improved disease resistance in KNC lines.
Acknowledgments
This work was supported by the grant number MHC – 2019R1F1A1061067 of the National Research Foundation, Republic of Korea. In addition, the authors greatly acknowledge the Hy-Line International Molecular Genetics Laboratory at Dallas Center, Iowa, for major histocompatibility complex B genotyping.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.05.049
Supplementary data
References
- Briles W.E. Additional blood group systems of chickens. Ann. N.Y. Acad. Sci. 1962;97:173–183. doi: 10.1111/j.1749-6632.1962.tb34633.x. [DOI] [PubMed] [Google Scholar]
- Briles W.E., Briles R.W. Identification of haplotypes of the major histocompatibility complex(B) Immunogenetcs. 1982;15:449–459. doi: 10.1007/BF00345904. [DOI] [PubMed] [Google Scholar]
- Chazara O., Pinard-Van Der Laan M.H., Tixier-Boichard M., Bed’Hom B. Molecular genotype investigation of the Gallus gallus major histocompatibility complex. Dev. Biol. (Basel). 2008;132:271–278. doi: 10.1159/000317171. [DOI] [PubMed] [Google Scholar]
- Chazara O., Fulton J.E., Helle J.M., Chang C.S. and Bed'Hom B., 2010. High-resolution chicken MHC genotyping using a SNP panel. 32nd Conference of the International Society for Animal Genetics, Edinburgh, Scotland, 138 (Abstr).
- Chazara O., Juul-Madsen H., Chang C.S., Tixier-Boichard M., Bed’Hom B. Correlation in chicken between the marker LEI0258 alleles and major histocompatibility complex sequences. BMC Proc. 2011;5:S29. doi: 10.1186/1753-6561-5-S4-S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazara O., Chang C.S., Bruneau N., Benabdeljelil K., Fotsa J.C., Kayang B.B., Loukou N.E., Osei-Amponsah R., Yapi-Gnaore V., Youssao I.A.K., Chen C.F., Pinard-Van Der Laan M.H., Tixier-Boichard M., Bed’Hom B. Diversity and evolution of the highly polymorphic tandem repeat LEI0258 in the chicken MHC-B region. Immunogenetics. 2013;65:447–459. doi: 10.1007/s00251-013-0697-6. [DOI] [PubMed] [Google Scholar]
- Choi N., Seo D.W., Jemaa S.B., Sultana H., Heo K.N., Jo C., Lee J.H. Driscrimination of the commercial Korean native chicken population uisng microsatellite markers. J. Anim. Sci. Technol. 2015;57:1–8. doi: 10.1186/s40781-015-0044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo Y.K., Chung S.H. Brief review on local chicken breeds in korea with respect to growth performance and meat quality. Int. J. Poult. Sci. 2014;13:662–664. [Google Scholar]
- Eckert C.G., Samis K.E., Lougheed S.C. Genetic variation across species’ geographical ranges: the central-marginal hypothesis and beyond. Mol. Ecol. 2008;17:1170–1188. doi: 10.1111/j.1365-294X.2007.03659.x. [DOI] [PubMed] [Google Scholar]
- Fulton J.E., Young E.E., Bacon L.D. Chicken Mhc alloantiserum cross-reactivity analysis by hemagglutination and flow cytometry. Immunogenetics. 1996;43:277–288. [PubMed] [Google Scholar]
- Fulton J.E., Hunt H.D., Bacon L.D. Chicken major histocompatibility complex class I definition using antisera induced by cloned class I sequences. Poult. Sci. 2001;80:1554–1561. doi: 10.1093/ps/80.11.1554. [DOI] [PubMed] [Google Scholar]
- Fulton J.E., Juul-Madsen H.R., Ashwell C.M., McCarron A.M., Arthur J.A., O’Sullivan N.P., Taylor R.L. Molecular genotype identification of the Gallus gallus major histocompatibility complex. Immunogenetics. 2006;58:407–421. doi: 10.1007/s00251-006-0119-0. [DOI] [PubMed] [Google Scholar]
- Fulton J.E., Lund A.R., Mccarron A.M., Pinegar K.N., Korver D.R., Classen H.L., Aggrey S., Utterbach C., Anthony N.B., Berres M.E. MHC variability in heritage breeds of chickens. Poult. Sci. 2016;95:393–399. doi: 10.3382/ps/pev363. [DOI] [PubMed] [Google Scholar]
- Fulton J.E., McCarron A.M., Lund A.R., Pinegar K.N., Wolc A., Chazara O., Bed’Hom B., Berres M., Miller M.M. A high-density SNP panel reveals extensive diversity, frequent recombination and multiple recombination hotspots within the chicken major histocompatibility complex B region between BG2 and CD1A1. Genet. Sel. Evol. 2016;48:1–15. doi: 10.1186/s12711-015-0181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton J.E., Berres M.E., Kantanen J., Honkatukia M. MHC-B variability within the Finnish Landrace chicken conservation program. Poult. Sci. 2017;96:3026–3030. doi: 10.3382/ps/pex102. [DOI] [PubMed] [Google Scholar]
- Gebremedhin B., Ficetola G.F., Naderi S., Rezaei H.R., Maudet C., Rioux D., Luikart G., Flagstad O., Thuiller W., Taberlet P. Frontiers in identifying conservation unit: from neutral markers to adaptive genetic variation. Anim. Conserve. 2009;12:107–109. [Google Scholar]
- Goto R.M., Afanassieff M., Ha J., Iglesias G.M., Ewald S.J., Briles W.E., Miller M.M. Single-strand conformational polymorphism (SSCP) assays for major histocompatibility complex B genotyping in chickens. Poult. Sci. 2002;81:1832–1841. doi: 10.1093/ps/81.12.1832. [DOI] [PubMed] [Google Scholar]
- Guangxin E., Sha R., Zeng S., Wang C., Pan J., Han J. Genetci variability, evedence of potential recombinant event and selection of LEI0258 in chicken. Gene. 2014;537:126–131. doi: 10.1016/j.gene.2013.12.040. [DOI] [PubMed] [Google Scholar]
- Han B., Lian L., Qu L., Zheng J., Yang N. Abundant polymorphisms at the microsatellite locus LEI0258 in indigenous chickens. Poult. Sci. 2013;92:3113–3119. doi: 10.3382/ps.2013-03416. [DOI] [PubMed] [Google Scholar]
- Iglesias G.M., Soria L.A., Goto R.M., Jar A.M., Miquel M.C., Lopez O.J., Miller M.M. Genetic variabilty at the major histocompatibility complex (B and Rfp-Y) IN Camperos broiler chicken. Anim. Genet. 2003;4:88–95. doi: 10.1046/j.1365-2052.2003.00944.x. [DOI] [PubMed] [Google Scholar]
- Iglesias G.M., Canet Z.E., Cantaro H., Miguel M.C., Melo J.E., Miller M.M., Berres M.E., Fulton J.E. MHC-B haplotypes in “Campero-INTA’ chicken synthetic line. Poult. Sci. 2019;98:5281–5286. doi: 10.3382/ps/pez431. [DOI] [PubMed] [Google Scholar]
- Jin Y.C., Wei P., Wei X.X., Zhao Z.Y., Li Y. Marek's disease resistant/susceptible MHC haplotypes in Xiayan chickens identified on the basis of BLB2 PCR-RFLP and BLB2/BF2 sequence analyses. Br. Poult. Sci. 2010;51:530–539. doi: 10.1080/00071668.2010.508489. [DOI] [PubMed] [Google Scholar]
- Jin S., Jayasena D.D., Jo C., Lee J.H. The breeding history and commercial development of the Korean native chicken. World’s Poult. Sci. J. 2017;73:163–174. [Google Scholar]
- Jump A.S., Marchant R., Penuelas J. Environmental change and the option value of genetic diversity. Trends Plant Sci. 2009;14:51–58. doi: 10.1016/j.tplants.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Jump A.S., Penuelas J. Running to stand still: adaptation and the response of plant to rapid climate change. Ecolo. Lette. 2005;8:1010–1020. doi: 10.1111/j.1461-0248.2005.00796.x. [DOI] [PubMed] [Google Scholar]
- Kang B.S., Cheong I.C., Lee S.J., Kim S.H., Ohh B.K., Choi K.S. Estimation of Heterosis for some economic traits in crossbreds between Korean native chicken and Rhode Island Red. (I). Hatching and growing performance in crossbreds between Korean native chicken and Rhode Island Red. Korean J. Poult. Sci. 1997;24:117–126. [Google Scholar]
- Kang B.S., Cheong I.C., Lee S.J., Kim S.H., Ohh B.K., Choi K.S. Estimation of Heterosis for some economic traits in crossbreds between Korean native chicken and Rhode Island Red. (II). laying performance of Korean native chicken and Rhode Island Red crossbred. Korean J. Poult. Sci. 1997;24:117–126. [Google Scholar]
- Kang B.S., Lee S.J., Kim S.H., Kim W.B., Ohh B.K. Study on performance and meat characteristics in Korean native commercial chicken I. Study on performance in Korean native commercial chicken by feeding system. Korean J. Poult. Sci. 1998;25:129–136. [Google Scholar]
- Kang B.S., Lee S.J., Kim S.H., Suh O.S., Na J.C., Jang B.G., Park B.Y., Lee J.M., Ohh B.K. Study on performance and meat characteristics in Korean native commercial chicken - II. Study on meat characteristics in Korean native commercial chicken by feeding system. Korean J. Poult. Sci. 1998;25:137–145. [Google Scholar]
- Kang B.S., Hong E.C., Kim H.K., YU D.J., Park M.N., Seo B.Y., Choo H.J., Na S.H., Seo O.S., Hwangbo J. Hatching and growing performance of three-way crossbreds of Korean native chickens (KNC) Korean J. Poult. Sci. 2010;37:399–404. [Google Scholar]
- Kannaki T.R., Reddy M.R., Raja Ravindra K.S., Chatterjee R.N. Genetic diversity analysis of the major histocompatibility complex (MHC) region in Indian native chicken breeds and pureline chickens using the LEI0258 microsatellite marker. Indian J. Anim. Res. 2017;51:998–1001. [Google Scholar]
- Kaufman J., Jacob J., Shaw J., Walker B., Milne S., Beck S., Salomonsen J. Gene organisation determines evolution of function in the chicken MHC. Immunol. Rev. 1999;167:101–117. doi: 10.1111/j.1600-065x.1999.tb01385.x. [DOI] [PubMed] [Google Scholar]
- Kaufman J., Milne S., Göbel T.W.F., Walker B.A., Jacob J.P., Auffray C., Zoorob R., Beck S. The chicken B locus is a minimal essential major histocompatibility complex. Nature. 1999;401:923–925. doi: 10.1038/44856. [DOI] [PubMed] [Google Scholar]
- Kim C.D. 1994. The Development of Commercial Poultry Production in Korea, Food and Fertilizer Technology Center, for the Asian and Pacific Region, Taipei, Taiwan, 1994.09.01.https://www.fftc.org.tw/en/publications/main/904 Accessed July 2020. [Google Scholar]
- Kong H.S., Oh J.D., Lee J.H., Jo K.J., Sang B.D., Choi C.H., Kim S.D., Lee S.J., Yeon S.H., Jeon G.J., Lee H.K. Genetic variation and relationships of Korean native chickens and foreign breeds using 15 microsatellite markers. Asian-Australasian J. Anim. Sci. 2006;19:1546–1550. [Google Scholar]
- Kwak W., Song K.D., Oh J.D., Heo K.N., Lee J.H., Lee W.K., Yoon S.H., Kim H., Cho S., Lee H.K. Uncovering genomic features and maternal origin of Korean native chicken by whole genome sequencing. PLoS One. 2014;9:e114763. doi: 10.1371/journal.pone.0114763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landesman E., Uni Z., Heller E.D. Designation by restriction fragment length polymorphism of major histocompatibility complex class IV haplotypes in meat-type chickens. Anim. Genet. 1993;24:349–354. doi: 10.1111/j.1365-2052.1993.tb00339.x. [DOI] [PubMed] [Google Scholar]
- Lee H.K., Lee S.J., Hwang K.C., Cheong I.C., Park Y.H., Sohn S.H., Shin Y.S., Ohh B.K., Han J.Y. Analysis of genetic characteristics of Korean native chicken using DNA marker. Korean J. Poult. Sci. 1996;23:177–183. [Google Scholar]
- Lee H.K., Chung H.Y., Chung H.K., Oh H.K., Chun B.S., You C.H., Chun K.J., Kim K.N., Lee K.W., Han J.Y., Chung E.R. Analysis of genetic characteristics by biochemical genetic markers in Korean native chicken. Korean J. Poult. Sci. 1996;23:135–144. [Google Scholar]
- Lee M.J., Kim S.H., Heo K.N., Kim H.K., Choi H.C., Hong E.C., Choo H.J., KIM C.D. The study on productivity of commercial Korea chickens for crossbred Korean native chickens. Korean J. Poult. Sci. 2013;40:291–297. [Google Scholar]
- Lee M.J., Heo K.N., Choi H.C., Hong E.C., Kim C.D. The performance test in crossbreds of Korean native chickens for the establishment of new lines. Korean J. Poult. Sci. 2014;41:39–44. [Google Scholar]
- Lundén A., Edfors-Lilja I., Johansson K., Liljedahl L.E. Associations between major histocompatibility complex genes and production traits in White Leghorns. Poult. Sci. 1993;72:989–999. doi: 10.3382/ps.0720989. [DOI] [PubMed] [Google Scholar]
- Maccari G., Robinson J., Ballingall K., Guethlein L.A., Grimholt U., Kaufman J., Ho C.S., De Groot N.G., Flicek P., Bontrop R.E., Hammond J.A., Marsh S.G.E. IPD-MHC 2.0: an improved inter-species database for the study of the major histocompatibility complex. Nucleic Acids Res. 2017;45:860–864. doi: 10.1093/nar/gkw1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.M., Abplanalp H., Goto R. Genotyping chickens for the B-G subregion of the major histocompatibility complex using restriction fragment length polymorphisms. Immunogenetics. 1988;28:374–379. doi: 10.1007/BF00364237. [DOI] [PubMed] [Google Scholar]
- Miller M.M., Bacon L.D., Hala K. Chicken major histocompatibility complex (MHC) Workshop. In: Schat K.A., editor. Current Progress on Avian Immunology Research. American Association of Avian Pathologists. Cornell University; Ithaca, NY: 2001. pp. 364–373. [Google Scholar]
- Miller M.M., Taylor R.L., Jr. Brief review of the chicken Major Histocompatibility Complex: the genes , their distribution on chromosome 16 , and their contributions to disease resistance distribution on chromosome 16 , and their contributions to disease resistance. Poult. Sci. 2016;95:375–392. doi: 10.3382/ps/pev379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minias P., Pikus E., Whittingham L.A., Dunn P.O. A global analysis of selection at the avian MHC. Evolution. 2018;72:1278–1293. doi: 10.1111/evo.13490. [DOI] [PubMed] [Google Scholar]
- Molee A., Kongroi K., Kuadsantia P., Poompramun C., Likitdecharote B. Association between single nucleotide polymorphisms of the major histocompatibility complex class ii gene and Newcastle disease virus titre and body weight in Leung Hang Khao chickens. Asian-australasian J. Anim. Sci. 2016;29:29–35. doi: 10.5713/ajas.15.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwambene P.L., Kyallo M., Machuka E., Githae D., Pelle R. Genetic diversity of 10 indigenous chicken ecotypes from Southern Highlands of Tanzania based on Major Histocompatibility Complex-linked microsatellite LEI0258 marker typing. Poult. Sci. 2019;98:2734–2746. doi: 10.3382/ps/pez076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Phuc H., Fulton J.E., Berres M.E. Genetic variation of major histocompatibility complex (MHC) in wild red junglefowl (Gallus gallus) Poult. Sci. 2016;95:400–411. doi: 10.3382/ps/pev364. [DOI] [PubMed] [Google Scholar]
- Nikbakht G., Esmailnejad A., Barjesteh N. LEI0258 microsatellite variability in Khorasan, Marandi, and Arian chickens. Biochem. Genet. 2013;51:341–349. doi: 10.1007/s10528-013-9567-z. [DOI] [PubMed] [Google Scholar]
- Oh J.D., Kang B.S., Kim H.K., Park M.N., Chae E.J., Seo O.S., Lee H.K., Jeon G.J., Kong H.S. Genetic relationship between populations and analysis of genetic Structure in the Korean native chicken and the Endemic chicken breeds. Korean J.Poult.Sci. 2008;35:361–366. [Google Scholar]
- Park M.N., Hong E.C., Kang B.S., Kim H.K., Seo B.Y., Choo H.J., Na S.H., Seo O.S., Han J.Y., Hwangbo J. The study on production and performance of crossbred Korean native chickens (KNC) Korean J. Poult. Sci. 2010;37:347–354. [Google Scholar]
- Park M.N., Hong E.C., Kang B.S., Hwangbo J., Kim H.K. Performance and meat quality of three-crossbreed Korean native chickens (KNC) Korean J. Poult. Sci. 2011;38:293–304. [Google Scholar]
- Peakall R., Smouse P.E. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research – an update. Bioinformatics. 2012;28:2537–2539. doi: 10.1093/bioinformatics/bts460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang B.C., Kim D.K., Park S.M., Park S.W., Han S.W. A study on Improvement of Dual breeds stocks. Korean J. Poult. Sci. 1979;6:75–81. [Google Scholar]
- Schou T.W., Labouriau R., Permin A., Christensen J.P., Sorensen P., Cu H.P., Nguyen V.K., Juul-Madsen H.R. MHC haplotype and susceptibility to experimental infections (Salmonella enteritidis, Pasteurella multocida or Ascaridia galli) in a commercial and an indigenous chicken breed. Vet. Immunol. Immunopath. 2010;135:52–63. doi: 10.1016/j.vetimm.2009.10.030. [DOI] [PubMed] [Google Scholar]
- Seo D., Hoque M.R., Choi N.R., Sultana H., Park H.B., Heo K.N., Kang B.S., Lim H.T., Lee S.H., Jo C., Lee J.H. Discrimination of Korean native chicken lines using Fifteen selected microsatellite markers. Asian Australas. J Anim. Sci. 2013;26:316–322. doi: 10.5713/ajas.2012.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D., Lee D.H., Choi N., Sudrajad P., Lee S.H., Lee J.H. Estimation of linkage disequilibrium and analysis of genetic diversity in Korean native chicken lines. PLoS One. 2018;13:e0192063. doi: 10.1371/journal.pone.0192063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J.H., Lee J.H., Kong H.S. Assessment of genetic diversity and phylogenetic relationships of Korean native chicken breeds using microsatellite markers. Asian Australas. J. Anim. Sci. 2017;30:1365–1371. doi: 10.5713/ajas.16.0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiina T., Briles W.E., M Goto R., Hosomichi K., Yanagiya K., Shimizu S., Inoko H., Miller M.M. Extended gene map reveals tripartite motif, C-type lectin, and Ig superfamily type genes within a sub region of the chicken MHC-B affecting infectious disease. J. Immunol. 2007;178:7162–7172. doi: 10.4049/jimmunol.178.11.7162. [DOI] [PubMed] [Google Scholar]
- Shiina T., Shimizu S., Hosomichi K., Kohara S., Watanabe S., Hanzawa K., Beck S., Kulski J.K., Inoko H. Comparative genomic analysis of two avian (quail and chicken) MHC regions. J. Immunol. 2004;172:6751–6763. doi: 10.4049/jimmunol.172.11.6751. [DOI] [PubMed] [Google Scholar]
- Sohn J., Nam K., Hong H., Kim J.M., Lim D., Lee K.T., Do Y.J., Cho C.Y., Kim N., Chai H.H., Nam J.U. Whole genome and transcriptome maps of the entirely black native Korean chicken breed Yeonsan Ogye. Giga Science. 2018;7:1–14. doi: 10.1093/gigascience/giy086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh S., Sharma A., Lee S.H., Cho C.Y., Kim J.H., Choi S.B., Kim H., Seong H.H., Yeon S.H., Kim D.H., Ko Y.G. Genetic diversity and relationships of Korean chicken breeds based on 30 microsatellite markers. Asian-Australasian J. Anim. Sci. 2014;27:1399–1405. doi: 10.5713/ajas.2014.14016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen J.P., Delany M.E., Mullens B.A. MHC haplotype involvement in avian resistnce to an extoparasite. Immunogenetics. 2008;60:621–631. doi: 10.1007/s00251-008-0314-2. [DOI] [PubMed] [Google Scholar]
- Walugembe M., Bertolini F., Dematawewa C.M.B., Reis M.P., R Elbeltagy A., Schmidt C.J., Lamont S.J., Rothschild M.F. Detection of selection signatures among Brazilian, Sri Lankan, and Eegyptian chicken populations under different environmental conditions. Front. Genet. 2019;9:737. doi: 10.3389/fgene.2018.00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Li C., Li Q., Li B., Larkin D.M., Lee C., Storz J.F., Antunes A., Greenwold M., Meredith R., Ödeen A., Cui J., Zhou Q., Xu L., Pan H., Wang Z., Jin L., Zhang P., Hu H., Yang W., Hu J., Xiao J., Yang Z., Liu Y., Xie Q., Yu H., Lian J., Wen P., Zhang F., Li H., Zeng Y.Z., Xiong, Liu S., Zhou L., Huang Z., An N., Wang J., Zheng Q., Xiong Y., Wang G., Wang B., Wang J., Fan Y., Da Fonseca R., Alfaro-Núñez A., Schubert M., Orlando L., Mourier T., Howard J., Ganapathy G., Pfenning A., Whitney O., Rivas M., Hara E., Smith J., Farré M., Narayan J., Slavov G., Romanov M., Borges R., Machado J., Khan I., Springer M., Gatesy J., Hoffmann F., Opazo J., Håstad O., Sawyer R., Kim H., Kim K., Kim H., Cho S., Li N., Huang Y., Bruford M., Zhan X., Dixon A., Bertelsen M., Derryberry E., Warren W., Wilson R., Li S., Ray D., Green R., O'Brien S., Griffin D., Johnson W., Haussler D., Ryder O., Willerslev E., Graves G., Alström P., Fjeldså J., Mindell D., Edwards S., Braun E., Rahbek C., Burt D., Houde P., Zhang Y., Yang H., Wang J., Jarvis E., Gilbert M., Wang J., Ye C., Liang S., Yan Z., Zepeda M., Campos P., Velazquez A., Samaniego J., Avila-Arcos M., Martin M., Barnett R., Ribeiro A., Mello C., Lovell P., Almeida D., Maldonado E., Pereira J., Sunagar K., Philip S., Dominguez-Bello M., Bunce M., Lambert D., Brumfield R., Sheldon F., Holmes E., Gardner P., Steeves T., Stadler P., Burge S., Lyons E., Smith J., McCarthy F., Pitel F., Rhoads D., Froman D. Comparative genomics reveals insights into avian genome evolution and adaptation. Science. 2014;346:1311–1320. doi: 10.1126/science.1251385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D., O’Keefe G., Li L., Johnson L.W., Ewald S.J. A PCR-method foe typing B-LBII (Class II MH C) allele in broiler chickens. Anim. Genet. 1999;30:109–119. doi: 10.1046/j.1365-2052.1999.00460.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.