Abstract
This study was conducted to evaluate the effects of quercetin on the antioxidant ability, intestinal barrier functions, and cecal microbiota in broiler chickens fed with oxidized soya oil. Four hundred eighty male Arbor Acres broilers were randomly assigned to 5 treatments, each involving 8 cages (12 birds per cage). The treatment groups were as follows: the control group, birds fed with basal diets containing oxidized oil, and birds fed with basal diets containing oxidized oil and supplemented with 200 ppm of quercetin, 400 ppm of quercetin, and 800 ppm of quercetin. The results showed that dietary supplementation with quercetin at a dose of 400 ppm or 800 ppm alleviated the increased serum malondialdehyde (MDA) level induced by oxidized oil on day 11 (P = 0.005) and reversed the increased MDA level in the mucosa on day 11 (P = 0.021). Quercetin significantly upregulated the transcription of nuclear factor erythroid 2–related factor 2 (Nrf2) and its downstream genes such as catalase (P < 0.001), superoxide dismutase 1 (P < 0.001), glutathione peroxidase 2 (P = 0.018), heme oxygenase-1 (HO-1) (P = 0.0), and thioredoxin (P = 0.002) and reversed the mRNA expression of HO-1 (P = 0.007) in the ileal mucosa. Tight junction protein 1 was only downregulated by oxidized oil (P = 0.013). In addition, quercetin (800 ppm) alleviated the decreased mRNA expression of mucin 2 (MUC2), which contributed to the intestinal chemical barrier (P = 0.039). The supplemental dose of 400 ppm of quercetin was able to promote Lactobacillus in the cecum, which enhanced the gastrointestinal tract health. In summary, these results indicated that quercetin ameliorated the oxidized oil–induced oxidative stress by upregulating the transcription of Nrf2 and its downstream genes to restore redox balance and reinforced the intestinal barrier via higher expression and secretion of MUC2 and facilitating the growth of Lactobacillus in the cecum. Therefore, quercetin could be a potential feed additive that can be applied in poultry production for amelioration of oxidative stress caused by oxidized oil and preventing the potential invasion of exogenous pathogens.
Key words: broiler, quercetin, oxidized oil, antioxidant status, cecal microbiota
Introduction
Oxidative stress is a major concern in poultry production and derived from nutritional, environmental heat stress, and pathological factors, which negatively impact poultry health and production (Lin et al., 2017; Mishra and Jha, 2019). The great potential of intestinal epithelia for nutrient absorption contributes to fast growth of broilers (Mishra and Jha, 2019). As the intestinal epithelium is located at the interface of its luminal environments and organisms, it is prone to oxidative damage (Circu and Aw, 2012). Moreover, feed exposes the intestinal epithelia of broilers to a wide variety of exogenous potentially harmful substances, which leads to excessive generation of reactive oxygen species (ROS) and results in oxidative stress (Mishra and Jha, 2019).
Oil is commonly used in poultry feed to meet the energy requirement, especially during summer to alleviate acute heat stress (Tan et al., 2018). However, high contents of unsaturated fatty acids in soybean oils make them prone to oxidation in hot climates. Lipid peroxidation not only damages DNA, protein, and membrane lipids but also decreases feed utilization and growth performance (Liang et al., 2015). Broilers are easily exposed to cumulative oxidative by-products through oxidized oil intake, resulting in an imbalance of redox status especially in the liver, which is the target organ for detoxication (Wang et al., 2016). Oxidized fish oil diet also caused microbial disorder and decreased the species richness and intestinal microbial diversity in fish (Peng et al., 2019).
Owing to the commercially related stressor, additional supports for the antioxidant system in poultry are strongly needed (Surai et al., 2019). Quercetin, a flavonoid compound, is widely distributed in plants and reported to possess strong anti-inflammatory, antioxidant, and antibacterial effects (Duenas et al., 2010; Li et al., 2016; Wang et al., 2018). In vitro studies based on DPPH scavenging ability and ferric reducing antioxidant power (FRAP) assays showed that quercetin was the most potent antioxidant among its 6 metabolites and butylated hydroxytoluene (Lesjak et al., 2018). Quercetin could alleviate H2O2-induced cell damage, reduce the intracellular ROS level, and reverse the change of malondialdehyde (MDA) levels (Chen et al., 2018). Dietary quercetin reversed the reduced Chao1 and Shannon index and meanwhile counteracted the dextran sulfate sodium-induced increased myeloperoxidase levels and reduced glutathione, MDA, and serum nitrate concentration in mice (Hong and Piao, 2018).
But most studies focused on the influence of oxidized oil on broilers (Liang et al., 2015; Tan et al., 2019) or the modulation function of quercetin alone on broilers, especially the shelf life of poultry meat or oxidative status of hen layers (Goliomytis et al., 2014; Hager-Theodorides et al., 2014; Liu et al., 2014a,b). However, the effects of quercetin on broilers fed with oxidized oil remain to be further investigated. Here, we studied the effect of quercetin on growth performance, antioxidant status, and intestinal microbiota of broiler chickens under oxidative stress induced by oxidized oil.
Materials and methods
Bird, Diets, and Experimental Design
A total of four hundred eighty, 1-day-old, male, Arbor Acres broilers were randomly assigned to 5 treatments, each involving 8 cages (12 birds per cage, about 80 cm [wide] × 60 cm [high] × 200 cm [length]). The broilers were raised on a floor pen and fed with feed, and tap water was provided using a nipple-type drinker. Feed in pellet form and water were provided ad libitum. The basal diet was formulated to meet or exceed the nutrient requirement for broilers as recommended by NRC (1994). The ingredient and nutrient compositions of the basal diets for the starter (0 to 21 D) and grower (22 to 42 D) phases are shown in Table 1. The room temperature was maintained at 35°C during the first 3 D, followed by a reduction to 28°C–30°C during the next 2 wk and 25°C for the remainder of the trial. A standard lighting regime was followed: 23 h of light and 1 h of darkness for the first 5 D followed by 20 h of light and 4 h of darkness from day 6 until the end of the trail. The experiment procedures were approved and conducted under the guidelines of the China Agricultural University Animal Care and Use Committee (approval number: AW04129102-1-1).
Table 1.
Ingredient and nutrient compositions of the basal diets.
| Ingredients, % | Starter diet | Finisher diet |
|---|---|---|
| Corn | 51.169 | 56.559 |
| Soybean meal | 35.835 | 31.400 |
| Wheat flour | 5.000 | 3.219 |
| Soy oil1 | 4.000 | 5.500 |
| Limestone | 1.348 | 0.581 |
| Dicalcium phosphate | 1.248 | 1.533 |
| Salt | 0.300 | 0.300 |
| L-Lysine (78%) | 0.356 | 0.170 |
| DL-Methionine (98%) | 0.215 | 0.208 |
| Choline chloride (50%) | 0.150 | 0.150 |
| Mineral premix2 | 0.200 | 0.200 |
| Enzyme blend3 | 0.030 | 0.030 |
| Vitamin premix4 | 0.030 | 0.030 |
| Phytase (5,000 FTU/kg) | 0.020 | 0.020 |
| Additive of Exp5 | 0.100 | 0.100 |
| Nutrient composition, % | ||
| ME (kcal/kg) | 3,000 | 3,150 |
| CP, % | 21.50 | 19.50 |
| Lysine, % | 1.41 | 1.16 |
| Methionine, % | 0.53 | 0.50 |
| Methionine + Cysteine, % | 0.85 | 0.80 |
| Calcium, % | 1.00 | 0.79 |
| Available phosphorus, % | 0.35 | 0.39 |
The same percentage of soybean oil was used in different treatment groups. The control group (Ctr) was fed with fresh soybean oil, the Ox group was fed with oxidized oil, and the OxL, OxM, and OxH groups were fed with oxidized oil and different concentrations of quercetin. Ox: basal diet with oxidized oil; OxL: basal diet supplemented with oxidized oil and 200 ppm of quercetin; OxM: basal diet supplemented with oxidized oil and 400 ppm of quercetin; OxH: basal diet supplemented with oxidized oil and 800 ppm of quercetin.
The mineral premix provided per kg diet: CuSO4·5H2O, 8 mg; FeSO4, 80 mg; MnSO4·H2O, 100 mg; Na2SeO3, 0.15 mg; KI, 0.35 mg.
The enzyme provided per kg diet: xylanase, ≥2,250 viscosity unit; β-dextranase, ≥52 AGL U/g.
The vitamin premix provided per kg diet: vitamin A, 9,500 IU; vitamin D3, 62.5 μg; vitamin E, 300 IU; vitamin K3, 2.65 mg; vitamin B6, 6 mg; vitamin B12, 0.025 mg; biotin, 0.0325 mg; folic acid, 1.25 mg; pantothenic acid, 12 mg; nicotinic acid, 50 mg.
The control group was fed with the basal diet containing 0.100% zeolite powder as the additive of Exp. The other groups were fed with the basal diet supplemented with 200 ppm, 400 ppm, and 800 ppm of quercetin and 800 ppm, 600 ppm, and 200 ppm of zeolite powder as the carrier separately to make 0.100% additive of Exp.
The treatment groups were as follows: control group (Ctr), birds fed with basal diet containing oxidized oil (Ox), birds fed with basal diet containing oxidized oil and supplemented with 200 ppm of quercetin (OxL), 400 ppm of quercetin (OxM), and 800 ppm of quercetin (OxH). Quercetin (purity >95%) used in this study was obtained from Shaanxi Sciphar Natural Products Co. Ltd. (Shanxi, China).
Preparation of Oxidized Oil
Fresh soybean oil was purchased from the Guchuan Edible Oil Company (Beijing, China). Oxidized oil was prepared according to method of Liang et al., 2015 with some modifications. The oil was put in an open container and heated to 95°C with continuous air flow (6 L/min) for 96 h. Then, the processed oil was cooled down to room temperature before feed preparation, and the peroxide value was 184 meq/kg, with 2.2 meq/kg for the fresh oil.
Performance Parameters
Chickens and feed were weighed by cage (replicate) on the day of hatch, at day 21, and at day 42. Feed intake, body weight gain, and feed conversion ratio were calculated for each period and throughout the whole experiment. Mortalities and health status were observed and recorded daily throughout the experiment.
Sample Collection
On day 11, day 18, and day 42, 8 birds (one bird per cage) of each treatment were randomly selected and killed by intracardial administration of sodium pentobarbital (30 mg/kg of body weight) and jugular exsanguination. Blood from the wing sinus was collected in sterile vacuum blood collection tubes and kept in ice. Serum was obtained after centrifuging the blood at 4°C at 3,000g for 15 min and stored at −80°C until analysis. The liver, spleen, and bursa of Fabricius were obtained in the sterile atmosphere and weighed to calculate the organ index using the following formula: organ index = organ weight (g)/body weight (g) × 100. Then, a piece of the liver was taken and then snap frozen in liquid nitrogen and stored at −80°C for measurements of antioxidant status. Samples (~2 cm∗2) of the ileum were collected midway between Meckel's diverticulum and the ileocecal junction and were respectively washed with 0.9% physiological saline or 0.1% diethyl pyrocarbonate-treated water before obtaining the mucosa of the intestinal segment and then snap frozen in liquid nitrogen and stored at −80°C. Samples of the ileum (1 cm) were taken proximal to the ileocecal junction and fixed in 4% (m/vol) paraformaldehyde solution for histological examination. The contents of the cecum were collected aseptically, snap frozen, and stored at −80°C for 16S rRNA sequencing. Blood was collected from the wing vein, and the serum was isolated by centrifugation at 3,000 rpm for 10 min at 4°C and stored at −20°C until analysis.
Intestinal Histomorphology
Formalin-fixed ileum samples were prepared using paraffin embedding techniques. Consecutive sections (5 μm) were stained using hematoxylin and eosin for histomorphological observation and using periodic acid–Schiff and alcian blue–periodic acid–Schiff for observation of neutral and acidic mucous cells. For histomorphological observation, the villus height (from the tip of the villus to the crypt opening) and the crypt depth (from the base of the crypt to the crypt opening) were measured from 10 randomly selected villi and the associated crypt, with one section per chicken at 40× magnification. The villus height-to-crypt depth ratio was then calculated from these measurements. The measurements of goblet based on periodic acid–Schiff and alcian blue–periodic acid–Schiff staining were calculated by the ratio of positive cells per villus vs. the area of the associated villus.
RNA Isolation and Quantitative Reverse Transcription Polymerase Chain Reaction
The RNA was extracted from the ileal mucosa using the Eastep Super Total RNA Extraction Kit (Promaga Co., Shanghai, China). RNA quantity was measured using the Nanodrop (Thermo Fisher, Waltham, MA) at 260 nm and 280 nm. Then, total RNA was reversed transcribed into cDNA using the PrimeScrip RT reagent Kit with gDNA Eraser Perfect Real Time (Takara Biomedical Technology, Beijing, China), and the gene expression levels were determined using the SYBR Premix Ex Taq Tli RNaseH Plus (Takara Biomedical Technology) according to the product protocols. The primers of glutamate–cysteine ligase modifier subunit (GCLM) and β-actin are shown in Table 2, and 2−ΔΔCt was calculated to measure the GCLM expression level.
Table 2.
Primer used for real-time PCR of the ileal mucosa.
| Gene | Forward primer | Reverse primer | Accession number |
|---|---|---|---|
| β-actin | CCACCGCAAATGCTTCTAAAC | AAGACTGCTGCTGACACCTTC | NM_205518.1 |
| MUC2 | CCCTCACCCAGCCCGACTTC | GCCGTTGGTGGAGGTGTTACAG | JX284122.1 |
| ZO1 | CTTCAGGTGTTTCTCTTCCTCCTC | CTGTGGTTTCATGGCTGGATC | NM_001301025.3 |
| Claudin-2 | TCCTGTGCTGTCTCCTCCCTTG | ACTCGGTCCTTGGCTGGTGAG | NM_001277622.1 |
| Nrf2 | GGGACGGTGACACAGGAACAAC | GCTCTCCACAGCGGGAAATCAG | NM_205117.1 |
| GCLM | GCCATAGGCACCTCTGACCTTG | CGGCATCACGCAACATGAAGC | NM_001007953.1 |
| CAT | TCAGAGGGACGGGCCAATGTG | CTGAAACGGACATGCGGCTCTC | NM_001031215.2 |
| SOD1 | GGCAAGCAGCACGGTGGAC | CTTCTGCCACTCCTCCCTTTGC | NM_205064.1 |
| GPX2 | ACGGCACCAACGAGGAGATCC | CTTCCCGTTCACCTGGCACTTC | NM_001277854.2 |
| GLRDX | AGCGAACCGTCCCTCGTGTG | GCATCATGGGGAGCTGCTGTTC | NM_205160.1 |
| TXN | GGTGAAGAGCGTGGGCAATC | GGTCCACACCATGTGGCAGA | NM_205453.1 |
| HO-1 | GGCAGAGCTTCGCACAAGGAG | CCACCGCACCAGGGGAGAG | NM_205344.1 |
| NOX2 | TGCTGATTCTGCTGCCAGTATGTC | GTTCCTGTCCAGTTGCCGTCTTAC | NM_001100286.1 |
| IL-8 | ATGAACGGCAAGCTTGGAGCTG | TCCAAGCACACCTCTCTTCCATCC | NM_205018.1 |
Abbreviations: CAT, catalase; GCLM, glutamate–cysteine ligase modifier subunit; GLRDX, glutaredoxin; GPX2, glutathione peroxidase 2; HO-1, heme oxygenase-1; MUC2, mucin 2; Nrf2, nuclear factor erythroid 2–related factor 2; TXN, thioredoxin; SOD1, superoxide dismutase 1; ZO1, tight junction protein 1.
Antioxidant Status
The intestinal (ileal) mucosa and liver were homogenized in pre-cold 0.9% saline and centrifuged at 3,000 rpm for 10 min at 4°C. The supernatant and serum were preserved at −20°C for further analysis. The activity of total antioxidant capacity (TAOC), MDA, catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPX) was analyzed using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). In brief, TAOC activity was determined by the FRAP method. The MDA content was determined by the TBA reaction method. The activity of CAT was determined based on the reaction between ammonium molybdate and hydrogen peroxide under the catalytic action of CAT. Activity of SOD was detected by the WST method. The GPX activity was measured by the 5,5′-dithiobis-(2-nitrobenzoic acid) method. In the tissue homogenate, the values of TAOC, CAT, GPX, and SOD activities were expressed as units per milligram of protein, and MDA activity was expressed as nanomoles per milligram of protein. The cellular ROS level of the liver was measured using the probe 2',7'-dichlorodihydrofluorescein diacetate (Solarbio, Beijing, China) (Pang et al., 2016). In brief, ice-cold liver homogenates were centrifuged (10,000 × g, 15 min, 4°C). Then, the supernatants were incubated with 10 μM 2',7'-dichlorodihydrofluorescein diacetate for 1 h in the dark. Fluorescence was immediately read at an excitation wavelength of 485 nm and an emission wavelength of 525 nm using a spectrophotometer (Molecular Devices SpectraMax i3x plate reader, San Jose, CA). Protein concentrations in the supernatant were assayed using BCA kits.
Cecal Microbiota Populations
The cecum was removed from the chicken and put into the sterile tube before snap freezing in liquid nitrogen and stored at –80°C for further analysis. Multiplexed 16S rDNA libraries were prepared using standard 16S metagenomic sequencing library protocols from Illumina (San Diego, CA), which used the V3–V4 region of 16S rDNA for target amplification. The authors used Illumina Miseq (Illumina) to performed paired-end reads (300 bp) to generate ∼200,000 sequences per sample. Subsequent analysis was performed in Quantitative Insights Into Microbial Ecology based on barcode sequences, and paired-end reads were merged (minimum score <20, mismatch). Additional quality filter steps were applied to exclude short reads <230 bp and chimeras. To ensure high coverage in all samples, samples producing <35,000 reads were excluded. Sequences after processed were clustered at 97% sequence identity to generate operational taxonomic unit and each operational taxonomic unit was annotated using the Silva128 16S rRNA database (bacteria and archaea). The fastq files generated by Illumina sequencing output were uploaded in the NCBI BioProject database (BioProject ID, PRJNA595453; submission ID, SUB6687114).
Statistical Analysis
Statistical significance was analyzed using SPSS 17.0 (IBM, Armonk, NY). The t-test was used to compare the Ctr and Ox treatment groups. The Ctr group was used as a positive control, and we just analyzed 0 (Ox), 200 (OxL), 400 (OxM), and 800 (OxH) ppm levels of quercetin in oxidized oil diet to give linear or quadratic responses. When significant differences among the different levels of quercetin in oxidized oil treatments were found, the significance among the groups and oxidized oil treatment was identified using the Duncan test for multiple comparisons.
Results
Performance and Internal Organ Weights
As shown in Table 3, feed intake, body weight gain, and feed conversion ratio were not significantly influenced in the Ox group compared with the Ctr group from day 1 to day 42. On the other hand, quercetin with oxidized oil did not induce significant changes compared with oxidized oil treatment. As shown in Table 4, relative weight of the liver was significantly increased by oxidized oil treatment on day 18 (P = 0.005) and day 42 (P = 0.042). However, no significant difference in the relative weight of the liver was observed between the group treated with oxidized oil and that cotreated with quercetin and oxidized oil on day 11 (P = 0.102), day 18 (P = 0.197), and day 42 (P = 0.171). The relative weight of the spleen and bursa of Fabricius was not significantly influenced by oxidized oil and quercetin treatment on day 11, day 18, and day 42.
Table 3.
Effects of dietary quercetin on growth performance of broiler chickens fed with oxidized oil.
| Item | Treatment1 | P-value, t-test | Linear | Quadratic | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ctr | Ox | OxL | OxM | OxH | SEM | ||||||
| 1–21 D | FI,2 g/bird | 1,123 | 1,124 | 1,141 | 1,124 | 1,152 | 9.7 | 0.961 | 0.460 | 0.783 | 0.765 |
| BWG,3 g/bird | 802 | 795 | 783 | 779 | 785 | 4.3 | 0.659 | 0.571 | 0.320 | 0.716 | |
| FCR,4 g/g | 1.40 | 1.41 | 1.46 | 1.44 | 1.47 | 0.012 | 0.656 | 0.275 | 0.704 | 0.587 | |
| 21–42 D | FI, g/bird | 2.63 | 2.70 | 2.66 | 2.80 | 2.70 | 0.023 | 0.350 | 0.619 | 0.288 | 0.220 |
| BWG, g/bird | 1,800 | 1,820 | 1,770 | 1,840 | 1,790 | 13.0 | 0.592 | 0.719 | 0.821 | 0.402 | |
| FCR, g/g | 1.46 | 1.48 | 1.50 | 1.53 | 1.51 | 0.008 | 0.498 | 0.125 | 0.126 | 0.181 | |
| 1–42 D | FI, g/bird | 3,750 | 3,840 | 3,770 | 3,910 | 3,850 | 23.0 | 0.189 | 0.536 | 0.663 | 0.350 |
| BWG, g/bird | 2,600 | 2,620 | 2,550 | 2,610 | 2,570 | 15.0 | 0.745 | 0.619 | 0.904 | 0.490 | |
| FCR, g/g | 1.44 | 1.47 | 1.48 | 1.50 | 1.50 | 0.006 | 0.170 | 0.056 | 0.333 | 0.197 | |
| Mortality, % | 1.04 | 3.12 | 0.00 | 1.04 | 1.04 | ||||||
Means within 4 treatments (Ox, OxL, OxM, and OxH) lacking a common superscript differ (P < 0.05).
Abbreviation: Ctr, control group.
Ctr: basal diet; Ox: basal diet with oxidized oil; OxL: basal diet supplemented with oxidized oil and 200 ppm of quercetin; OxM: basal diet supplemented with oxidized oil and 400 ppm of quercetin; OxH: basal diet supplemented with oxidized oil and 800 ppm of quercetin.
FI: feed intake, g/bird.
BWG: body weight gain, g/bird.
FCR: feed conversion ratio.
Table 4.
Effects of dietary quercetin on the internal organ index of broiler chickens fed with oxidized oil.
| Item | Treatment1 |
P-value, t-test | Linear | Quadratic | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ctr | Ox | OxL | OxM | OxH | SEM | ||||||
| 11 D | Liver | 3.920 | 4.090 | 4.230 | 4.430 | 4.010 | 0.0560 | 0.272 | 0.600 | 0.022 | 0.102 |
| Spleen | 0.063 | 0.059 | 0.067 | 0.062 | 0.061 | 0.0020 | 0.631 | 0.914 | 0.356 | 0.493 | |
| Bursa of Fabricius | 0.200 | 0.186 | 0.196 | 0.180 | 0.204 | 0.0060 | 0.464 | 0.490 | 0.611 | 0.682 | |
| 18 D | Liver | 2.950 | 3.380∗ | 3.200 | 3.380 | 3.510 | 0.0510 | 0.005 | 0.141 | 0.297 | 0.197 |
| Spleen | 0.069 | 0.066 | 0.069 | 0.063 | 0.071 | 0.0020 | 0.625 | 0.625 | 0.601 | 0.704 | |
| Bursa of Fabricius | 0.255 | 0.201 | 0.239 | 0.224 | 0.187 | 0.0090 | 0.054 | 0.359 | 0.100 | 0.249 | |
| 42 D | Liver | 2.180 | 2.410∗ | 2.750 | 2.460 | 2.340 | 0.0590 | 0.042 | 0.346 | 0.193 | 0.171 |
| Spleen | 0.095 | 0.079 | 0.087 | 0.07 | 0.087 | 0.0040 | 0.145 | 0.582 | 0.994 | 0.800 | |
| Bursa of Fabricius | 0.038 | 0.041 | 0.046 | 0.046 | 0.041 | 0.0020 | 0.682 | 0.727 | 0.229 | 0.635 | |
∗Means were significantly different compared with the control. Means within 4 treatments (Ox, OxL, OxM, and OxH) lacking a common superscript differ (P < 0.05).
Abbreviation: Ctr, control group.
Ctr: basal diet; Ox: basal diet with oxidized oil; OxL: basal diet supplemented with oxidized oil and 200 ppm of quercetin; OxM: basal diet supplemented with oxidized oil and 400 ppm of quercetin; OxH: basal diet supplemented with oxidized oil and 800 ppm of quercetin.
Antioxidant Status
As shown in Table 5, Table 6, and Table 7, the activities of TAOC, MDA, GPX, CAT, SOD, and ROS are examined in the liver, serum, and intestinal mucosa. As shown in Table 5, oxidized oil significantly increased the ROS (P = 0.014) and MDA level (P < 0.001) and enhanced the activity of CAT (P = 0.16) and SOD (P = 0.020) in the liver compared with the Ctr group, but 200 ppm of quercetin alleviated the increase of ROS activity induced by oxidized oil on day 11 (P = 0.018). In addition, higher concentration of quercetin significantly decreased the activity of GPX (P = 0.001) and the level of MDA on day 11 (P = 0.028). Oxidized oil did not significantly influence the ROS, TAOC, MDA, GPX, CAT, and SOD activities in the liver on day 18 and day 42. However, 200 ppm of quercetin significantly decreased the level of MDA (P = 0.004) and the activity of GPX (P = 0.003) and SOD (P = 0.006) compared with the Ox group on day 18. And 200 ppm of quercetin significantly increased the TAOC (P = 0.009) activity compared with the Ox group on day 42.
Table 5.
ROS, TAOC, MDA, GPX, CAT, and SOD activity in the liver of broiler chickens fed with oxidized oil and different concentrations of quercetin.
| Item | Treatment1 |
P-value, t-test | Linear | Quadratic | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ctr | Ox | OxL | OxM | OxH | SEM | ||||||
| 11 D | ROS2 | 7.88 | 7.96∗,a | 7.87b | 7.91a,b | 7.92a,b | 0.018 | 0.014 | 0.426 | 0.016 | 0.018 |
| TAOC3 | 1.20 | 1.25 | 1.18 | 1.20 | 1.33 | 0.024 | 0.382 | 0.200 | 0.161 | 0.298 | |
| MDA4 | 0.55 | 1.15∗,a,b | 1.42a | 0.80b | 0.98b | 0.073 | <0.001 | 0.120 | 0.605 | 0.028 | |
| GPX5 | 15.27 | 13.89a | 13.51a | 14.96a | 10.68b | 0.395 | 0.155 | 0.003 | 0.012 | 0.001 | |
| CAT5 | 7.24 | 12.18∗ | 12.31 | 11.68 | 11.80 | 0.595 | 0.016 | 0.751 | 0.926 | 0.977 | |
| SOD5 | 145.96 | 163.56∗ | 150.44 | 151.5 | 162.79 | 2.760 | 0.020 | 0.788 | 0.076 | 0.313 | |
| 18 D | ROS2 | 8.22 | 8.19 | 8.33 | 8.26 | 8.21 | 0.023 | 0.663 | 0.759 | 0.082 | 0.173 |
| TAOC3 | 1.12 | 1.19a | 1.04b | 0.98b | 1.02b | 0.023 | 0.369 | 0.034 | 0.021 | 0.028 | |
| MDA4 | 1.35 | 1.67a | 1.04b | 0.99b | 1.32a,b | 0.076 | 0.255 | 0.226 | 0.001 | 0.004 | |
| GPX5 | 16.76 | 15.243a,b | 12.86c | 13.20b,c | 16.50a | 0.410 | 0.185 | 0.062 | 0.001 | 0.003 | |
| CAT5 | 7.51 | 9.79 | 10.31 | 9.95 | 10.02 | 0.387 | 0.101 | 0.942 | 0.828 | 0.974 | |
| SOD5 | 162.98 | 176.46a | 150.71c | 156.38b,c | 169.83a,b | 2.772 | 0.141 | 0.967 | 0.001 | 0.006 | |
| 42 D | ROS2 | 8.40 | 8.35 | 8.29 | 8.08 | 8.36 | 0.057 | 0.346 | 0.992 | 0.260 | 0.624 |
| TAOC3 | 1.30 | 1.34b | 1.72a | 1.49a,b | 1.24b | 0.047 | 0.730 | 0.115 | 0.012 | 0.009 | |
| MDA4 | 1.25 | 1.50 | 1.82 | 1.46 | 1.11 | 0.103 | 0.385 | 0.120 | 0.330 | 0.238 | |
| GPX5 | 24.46 | 23.76 | 28.22 | 26.47 | 22.35 | 0.762 | 0.786 | 0.226 | 0.026 | 0.062 | |
| SOD5 | 209.06 | 212.23 | 225.21 | 205.14 | 196.59 | 7.240 | 0.808 | 0.060 | 0.469 | 0.104 | |
∗Means were significantly different compared with the control. Means within 4 treatments (Ox, OxL, OxM, and OxH) lacking a common superscript differ (P < 0.05).
Abbreviations: CAT, catalase; Ctr, control group; GPX, glutathione peroxidase; MDA, malondialdehyde; ROS, reactive oxygen species; SOD, superoxide dismutase; TAOC, total antioxidant capacity.
Ctr: basal diet; Ox: basal diet with oxidized oil; OxL: basal diet supplemented with oxidized oil and 200 ppm of quercetin; OxM: basal diet supplemented with oxidized oil and 400 ppm of quercetin; OxH: basal diet supplemented with oxidized oil and 800 ppm of quercetin.
ROS: log (fluorescence value of ROS).
TAOC: U/mg of protein.
MDA: nM/mg of protein.
GPX, SOD, CAT: U/mg of protein.
Table 6.
TAOC, MDA, CAT, SOD, and GPX activity in the serum of broiler chickens fed with oxidized oil and different concentrations of quercetin.
| Item | Treatment1 |
P-value, t-test | Linear | Quadratic | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ctr | Ox | OxL | OxM | OxH | SEM | ||||||
| 11 D | TAOC 2 | 5.40 | 6.52 | 6.17 | 5.32 | 7.19 | 0.372 | 0.304 | 0.555 | 0.173 | 0.460 |
| MDA3 | 3.40 | 5.81a | 2.85b | 2.70b | 2.79b | 0.316 | 0.105 | 0.011 | 0.014 | 0.005 | |
| CAT4 | 1.93 | 2.81a | 2.13a,b | 0.98b | 1.84a,b | 0.200 | 0.152 | 0.067 | 0.011 | 0.021 | |
| SOD4 | 84.23 | 94.97 | 84.46 | 104.02 | 81.69 | 3.493 | 0.152 | 0.424 | 0.351 | 0.224 | |
| GPX4 | 1,674.11 | 1,629.20b | 1,899.11a,b | 1,624.79a,b | 2,158.01a | 50.466 | 0.669 | 0.001 | 0.233 | 0.001 | |
| 18 D | TAOC2 | 10.64 | 7.52∗ | 10.13 | 8.9 | 10.71 | 0.44 | 0.049 | 0.055 | 0.677 | 0.116 |
| MDA3 | 1.77 | 2.70∗ | 2.36 | 1.88 | 2.48 | 0.121 | 0.001 | 0.584 | 0.055 | 0.211 | |
| CAT4 | 2.02 | 2.61 | 1.96 | 1.88 | 1.87 | 0.151 | 0.387 | 0.212 | 0.270 | 0.390 | |
| SOD4 | 91.15 | 77.80 | 90.34 | 91.13 | 69.88 | 3.048 | 0.135 | 0.258 | 0.032 | 0.115 | |
| GPX4 | 2,139.99 | 2,197.03 | 2,168.06 | 2,145.2 | 2,127.31 | 47.347 | 0.747 | 0.627 | 0.886 | 0.966 | |
| 42 D | CAT4 | 1.18 | 1.69 | 1.57 | 1.61 | 1.92 | 0.093 | 0.085 | 0.110 | 0.205 | 0.239 |
| SOD4 | 100.92 | 101.58 | 105.67 | 105.05 | 107.87 | 2.998 | 0.956 | 0.564 | 0.883 | 0.934 | |
| GPX4 | 2,581.29 | 2,306.27b | 2,255.79b | 2,743.26a | 2,059.50b | 123.903 | 0.156 | 0.423 | 0.017 | 0.017 | |
∗Means were significantly different compared with the control. Means within 4 treatments (Ox, OxL, OxM, and OxH) lacking a common superscript differ (P < 0.05).
Abbreviations: CAT, catalase; Ctr, control group; MDA, malondialdehyde; GPX, glutathione peroxidase; SOD, superoxide dismutase; TAOC, total antioxidant capacity.
Ctr: basal diet; Ox: basal diet with oxidized oil; OxL: basal diet supplemented with oxidized oil and 200 ppm of quercetin; OxM: basal diet supplemented with oxidized oil and 400 ppm of quercetin; OxH: basal diet supplemented with oxidized oil and 800 ppm of quercetin.
TAOC: U/mg of protein.
MDA: nM/mg of protein.
CAT, SOD, GPX: U/mg of protein.
Table 7.
TAOC, MDA, CAT, SOD, and GPX activity in the ileal mucosa of broiler chickens fed with oxidized oil and different concentrations of quercetin on day 11.
| Item | Treatment1 |
P-value, t-test | Linear | Quadratic | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ctr | Ox | OxL | OxM | OxH | SEM | |||||
| TAOC2 | 1.15 | 0.93 | 0.97 | 1.03 | 0.95 | 0.034 | 0.066 | 0.837 | 0.306 | 0.740 |
| MDA3 | 0.51 | 0.89∗,a | 0.74a,b | 0.47c | 0.61b,c | 0.043 | 0.008 | 0.020 | 0.052 | 0.021 |
| CAT4 | 4.49 | 7.27 | 8.73 | 8.05 | 10.23 | 0.781 | 0.128 | 0.277 | 0.942 | 0.690 |
| SOD4 | 72.37 | 69.71 | 68.35 | 68.31 | 61.85 | 2.094 | 0.662 | 0.246 | 0.762 | 0.677 |
| GPX4 | 25.28 | 26.77b | 24.89b | 24.56b | 35.65a | 1.417 | 0.927 | 0.012 | 0.218 | 0.049 |
∗Means were significantly different compared with the control. Means within 4 treatments (Ox, OxL, OxM, and OxH) lacking a common superscript differ (P < 0.05).
Abbreviations: CAT, catalase; Ctr, control group; GPX, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase; TAOC, total antioxidant capacity.
Ctr: basal diet; Ox: basal diet with oxidized oil; OxL: basal diet supplemented with oxidized oil and 200 ppm of quercetin; OxM: basal diet supplemented with oxidized oil and 400 ppm of quercetin; OxH: basal diet supplemented with oxidized oil and 800 ppm of quercetin.
TAOC: U/mg of protein.
MDA: nM/mg of protein.
CAT, SOD, GPX: U/mg of protein.
As shown in Table 6, antioxidant status of serum was examined. The oxidized oil significantly increased the serum MDA concentration (P = 0.001) and decreased the TAOC (P = 0.049) activity on day 18, and quercetin (200 ppm to 800 ppm) decreased the level of MDA (P = 0.005); 800 ppm of quercetin increased the activity of GPX (P = 0.001) on day 11. Catalase, SOD, and GPX activities were not significantly influenced by quercetin with oxidized oil on day 18 and day 42 compared with the Ox group, except that treatment with 800 ppm of quercetin increased the activity of GPX compared with the oxidized oil treatment on day 11. On day 42, the only difference detected was in GPX activity between the OxM and OxL groups (P = 0.017), as shown in Table 6.
As shown in Table 7, quercetin at a concentration of 400 and 800 ppm alleviated the increased ileal mucosal MDA levels induced by oxidized oil in a dose-dependent manner (P = 0.021). And only treatment with 800 ppm of quercetin increased the activity of GPX compared with the oxidized oil treatment (P = 0.049).
Ileum Morphology
The characteristics of ileum morphology at day 11 are listed in Table 8. Compared with the Ctr group, the morphology characteristics of the ileum such as the villus height, crypt depth, and the density of the neutral/acidic goblet were not significantly influenced by oxidized oil treatment, but the ratio of villus height and crypt was significantly increased (P = 0.019). However, 200 ppm of quercetin increased the crypt depth (P = 0.045) and significantly decreased the ratio of villus height and crypt depth compared with Ox group (P < 0.001). On the other hand, the density of the neutral and acidic goblet was not significantly influenced by quercetin and oxidized oil.
Table 8.
Ileal morphology of broiler chickens fed with oxidized oil and quercetin on day 11.
| Item | Treatment1 |
P-value, t-test | Linear | Quadratic | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ctr | Ox | OxL | OxM | OxH | SEM | |||||
| Villus height, μm | 383.82 | 376.83 | 343.3 | 389.9 | 390.14 | 8.426 | 0.785 | 0.329 | 0.699 | 0.316 |
| Crypt depth, μm | 77.53 | 68.78b | 84.63a | 77.59a,b | 70.58b | 1.970 | 0.152 | 0.649 | 0.022 | 0.045 |
| V/C2 | 5.08 | 5.52∗,a | 4.24b | 5.14a | 5.58a | 0.113 | 0.019 | 0.119 | 0.003 | <0.001 |
| Number of neutral mucus goblet/(mm2) | 1,959 | 1,832 | 1,727 | 1,622 | 1,470 | 52.9 | 0.475 | 0.015 | 0.822 | 0.103 |
| Number of acidic mucus goblet/(mm2) | 1,917 | 171 | 1,738 | 1,439 | 1,401 | 60.9 | 0.368 | 0.043 | 0.726 | 0.137 |
∗Means were significantly different compared with the control. Means within 4 treatments (Ox, OxL, OxM, and OxH) lacking a common superscript differ (P < 0.05).
Abbreviation: Ctr, control group.
Ctr: basal diet; Ox: basal diet with oxidized oil; OxL: basal diet supplemented with oxidized oil and 200 ppm of quercetin; OxM: basal diet supplemented with oxidized oil and 400 ppm of quercetin; OxH: basal diet supplemented with oxidized oil and 800 ppm of quercetin.
V/C: (villus height)/(crypt depth), μm/μm.
Relative mRNA Expression of Antioxidant and Barrier Function–Related Genes in the Chicken Ileal Mucosa
As shown in Table 9, oxidized oil significantly downregulated the genes related to intestinal barriers such as tight junction protein 1 (P = 0.001) and mucin 2 (MUC2) (P = 0.013) compared with the Ctr group in the ileal mucosa. But 800 ppm of quercetin reversed the downregulation of MUC2 (P < 0.001) and upregulated the mRNA expression of Claudin-2 (P = 0.001). The concentration of 400 ppm of quercetin also reversed the downregulated transcription of tight junction protein 1 induced by oxidized oil (P = 0.039). As for the antioxidant genes, oxidized oil significantly decreased the mRNA expression of thioredoxin (TXN) (P = 0.037) and IL-8 (P = 0.024). However, 800 ppm of quercetin significantly increased expression of the antioxidant-related gene such as nuclear factor erythroid 2–related factor 2 (Nrf2), GCLM, CAT, superoxide dismutase 1, glutathione peroxidase 2, glutaredoxin, TXN, and heme oxygenase-1 (HO-1) compared with the Ox group (P-values are listed in Table 9). And quercetin decreased the mRNA expression of NOX2 compared with the Ctr group (P = 0.033) and reversed the decreased expression of IL-8 induced by oxidized oil (P = 0.001).
Table 9.
Relative mRNA expression of antioxidant- and inflammation-related genes in the broiler chicken ileal mucosa on day 11.
| Item | Treatment1 |
P-value, t-test | Linear | Quadratic | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ctr | Ox | OxL | OxM | OxH | SEM | |||||
| MUC2 | 1.00 | 0.15∗,b | 0.71b | 0.42b | 2.19a | 0.147 | 0.001 | <0.001 | 0.080 | <0.001 |
| ZO1 | 1.03 | 0.22∗,b | 0.34a,b | 0.41a | 0.27a,b | 0.063 | 0.013 | 0.413 | 0.006 | 0.039 |
| Claudin-2 | 1.00 | 0.71b | 1.53b | 0.67b | 3.34a | 0.23 | 0.441 | 0.001 | 0.059 | 0.001 |
| Nrf2 | 1.00 | 0.37b | 0.65b | 0.81b | 2.10a | 0.157 | 0.102 | <0.001 | 0.270 | 0.002 |
| GCLM | 1.65 | 0.67b | 0.66b | 0.57b | 1.35a | 0.142 | 0.192 | 0.002 | 0.011 | 0.002 |
| CAT | 1.00 | 0.50b | 0.87b | 0.96b | 2.30a | 0.146 | 0.121 | <0.001 | 0.182 | <0.001 |
| SOD1 | 1.00 | 0.40b | 0.97b | 0.52b | 1.92a | 0.142 | 0.178 | <0.001 | 0.154 | <0.001 |
| GPX2 | 1.00 | 1.01b | 1.40b | 1.28b | 2.21a | 0.131 | 0.970 | 0.003 | 0.494 | 0.018 |
| GLRDX | 1.15 | 0.90b | 1.55b | 1.17b | 2.39a | 0.150 | 0.412 | 0.002 | 0.525 | 0.008 |
| TXN | 1.14 | 0.77∗,b | 1.48a | 1.45a | 2.08a | 0.118 | 0.037 | <0.001 | 0.486 | 0.002 |
| HO-1 | 1.00 | 0.45b | 0.87a,b | 0.84a,b | 1.29a | 0.087 | 0.101 | 0.001 | 0.715 | 0.007 |
| NOX2 | 1.03 | 0.90a | 1.10a | 0.74a,b | 0.49b | 0.068 | 0.397 | 0.012 | 0.372 | 0.033 |
| IL-8 | 0.80 | 0.36∗,c | 0.51b | 0.47b,c | 0.65a | 0.033 | 0.024 | <0.001 | 0.996 | 0.001 |
∗Means were significantly different compared with the control. Means within 4 treatments (Ox, OxL, OxM, and OxH) lacking a common superscript differ (P < 0.05).
Abbreviations: CAT, catalase; GCLM, glutamate–cysteine ligase modifier subunit; GLRDX, glutaredoxin; GPX2, glutathione peroxidase 2; HO-1, heme oxygenase-1; MUC2, mucin 2; Nrf2, nuclear factor erythroid 2–related factor 2; TXN, thioredoxin; SOD1, superoxide dismutase 1; ZO1, tight junction protein 1.
Ctr: basal diet; Ox: basal diet with oxidized oil; OxL: basal diet supplemented with oxidized oil and 200 ppm of quercetin; OxM: basal diet supplemented with oxidized oil and 400 ppm of quercetin; OxH: basal diet supplemented with oxidized oil and 800 ppm of quercetin.
Cecal Microbiota
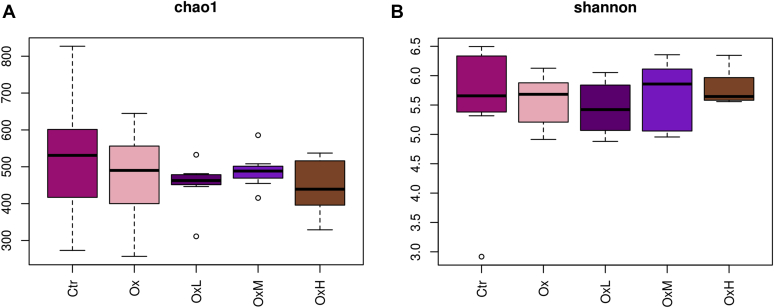
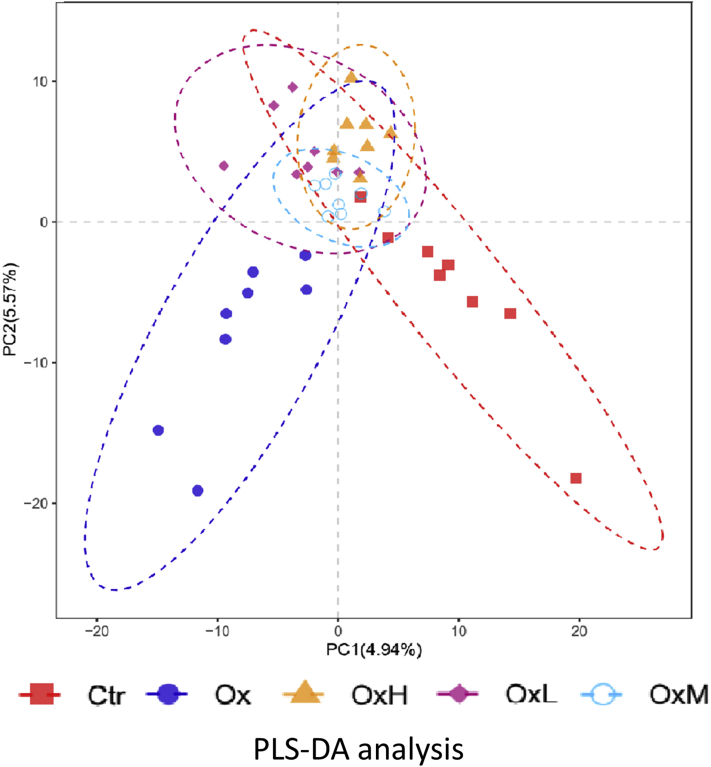
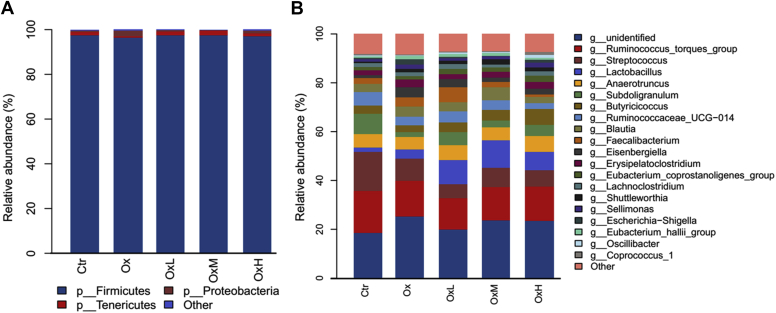
As shown in Figure 1, the alpha diversity of cecal microbiota was not significantly different between treatments. Beta diversity was analyzed by partial least squares discrimination analysis, as shown in Figure 2, which showed remarkable differentiation of the microbial community among the 5 groups. The Ctr group and oxidized oil group were separated, whereas the groups treated with quercetin and oxidized oil were in the transition status between these 2 groups. As shown in Figure 3A, at the phylum level (relative abundance >0.1%), Firmicutes (96.4–97.6%), Tenericutes (0.7–2.0%), Proteobacteria (0.3–2.4%), and Actinobacteria (0.2–0.5%) made up more than 99.9% of the total cecal microbiota. However, no significant difference was observed among treatments at the phylum level. In Figure 3B, the top 20 genera are listed. Among the 20 genera, the relative abundance of Lactobacillus was higher in the oxidized oil treatment group than in the Ctr group, and treatment with quercetin further increased the relative abundance of Lactobacillus in the cecum.
Figure 1.
Effects of quercetin on the α-diversity of cecal microbiota. The α-diversity was evaluated by (A) Chao1 index and (B) Shannon index. Abbreviations: Ctr, control group – basal diet; Ox, basal diet with oxidized oil; OxL, basal diet supplemented with oxidized oil and 200 ppm of quercetin; OxM, basal diet supplemented with oxidized oil and 400 ppm of quercetin; OxH, basal diet supplemented with oxidized oil and 800 ppm of quercetin.
Figure 2.
Effects of quercetin on the cecal microbiota: quercetin alleviated the change caused by oxidized oil in the cecum. Abbreviations: Ctr, control group – basal diet; Ox, basal diet with oxidized oil; OxL, basal diet supplemented with oxidized oil and 200 ppm of quercetin; OxM, basal diet supplemented with oxidized oil and 400 ppm of quercetin; OxH, basal diet supplemented with oxidized oil and 800 ppm of quercetin; PC, principal component; PLS-DA, partial least squares discrimination analysis.
Figure 3.
Effects of quercetin on the cecal microbiota. (A) No significant change was observed at phylum level. (B) Quercetin further increased the abundance of Lactobacillaceae induced by oxidized oil. Abbreviations: Ctr, control group – basal diet; Ox, basal diet with oxidized oil; OxL, basal diet supplemented with oxidized oil and 200 ppm of quercetin; OxM, basal diet supplemented with oxidized oil and 400 ppm of quercetin; OxH: basal diet supplemented with oxidized oil and 800 ppm of quercetin.
Discussion
As mentioned in Introduction, consumption of oxidized oil was reported to induce oxidative damage in broilers. However, dietary oxidized oil was not able to affect broiler growth during the early growth phase in our study. Similar results were found in the study of Tan et al. (2018). Moderately thermo-oxidized oil did not impair the nutrient digestibility of diet, and there was no significant effect of oxidized oil on digestibility coefficients of crude protein and ether extract (Açıkgöz et al., 2011). In addition, treatment with quercetin at a concentration of 200 ppm to 800 ppm with oxidized oil did not induce any significant changes in growth performance from day 1 to day 42. Similarly, dietary quercetin at a concentration of 200 ppm to 600 ppm had no significant effect on growth performance (Yang et al., 2020). In agreement with another study on pigs (Lu et al., 2014), oxidized oil increased the liver weight and liver index on day 18, which was as a result of the elevated weight of the liver. Oxidized oil could induce a higher rate of hepatic lipid metabolism, and the increase in liver weight may be the result of higher metabolic activity or reflection of an upregulation of mRNA expression for the fatty acid catabolism pathway (Liu et al., 2014a,b).
The liver is an important organ for nutrient metabolism and sensitive indicator of toxicity (Liu et al., 2014a,b). Based on previous studies, animal fed with oxidized oils experienced stress responses (Liu et al., 2014a,b). In our study, oxidized oil significantly increased the MDA and ROS level in the liver on day 11, and quercetin alleviated the increase in the ROS level induced by oxidized oil in the liver. Similar results were shown wherein quercetin prevented the in vitro production of ROS such as peroxides, superoxide, and reactive nitrogen species nitric oxide, induced by a proinflammatory cytokine mixture in liver cells (Crespo et al., 2008). However, the parameters related to oxidation in the liver on day 18 and 42 were less affected by oxidized oil. In this study, the peroxide value was much lower than that in other reports. And under mild oxidative stress, organisms usually block extensive biosynthesis to prevent or neutralize negative ROS effects, gradually leading to the adaptive response (Lushchak, 2014).
Oxidized oil in diets was absorbed by the small intestine, which was incorporated into chylomicrons and then circulated in blood, where they contributed to the total body pool of oxidized lipids (Staprans et al., 1994). Dietary quercetin alleviated the increase of the MDA level in serum on day 11. Most antioxidant enzymes were not significantly upregulated by quercetin except GPX on day 11. Similarly, quercetin was reported to elevate the serum FRAP and the content of small antioxidant molecules such as ascorbic acid and vitamin E in Wistar rats after they were fed with 0.5% quercetin for 2 wk (Zhao et al., 2011). Based on the changes of oxidative status and the protection effects of quercetin against oxidized oil during the whole trial, in this study, we then focused on the early stage after broilers were fed with oxidized oil.
As the first defense line against oxidative stress, the intestinal mucosa contains an extensive enzyme and nonenzyme antioxidant system. Meanwhile, the intestinal mucosa experiences oxidative stress when exposed to oxidized soybean oil after intake of highly oxidized diets (Liang et al., 2015). The redox status and the morphology of the ileum were examined in our studies. Because concentration of dietary quercetin was much higher in the lumen of the intestine than in plasma and tissue, the intestinal mucosa was undoubtedly the target of this flavonoid (Murota et al., 2004). In this experiment, dietary quercetin (400 ppm and 800 ppm) alleviated the increase of the MDA level in the ileal mucosa on day 11, and the tendency of upregulation of GPX was observed in the 800-ppm quercetin treatment compared with the oxidized oil treatment. In another study about transport stress of pigs, quercetin-supplemented pigs showed lower levels of ROS and MDA in the intestine than the those in the control group (Zou et al., 2016). As for the morphology changes, in an in vivo jejunal ischemia–reperfusion injury experiment in rats, the intestinal gland depth was significantly higher in the quercetin group, and deeper intestinal glands contributed to the faster recovery of the intestinal epithelium as it was the site of proliferation of epithelial cells (Curgali et al., 2018). In our study, 200 ppm of quercetin significantly increased the crypt depth and decreased the ratio of villus height and crypt depth, which indicated faster recovery of the epithelium.
Mucus is composed of 95% water, 1% salts, 1–2% lipids, and protein. The thickness of the intestinal mucus layer in the small intestine depends on the diet (Gonzales et al., 2016). However, in this study, the mucus goblets were not significantly influenced by different concentrations of quercetin.
To further elucidate the changes of redox status and mucin, mRNA expression was examined in the ileal mucosa. Mucus in the gastrointestinal tract was composed of both membrane-bound and secreted mucins, among which MUC2 was secreted in the intestine (Gonzales et al., 2016). Quercetin (800 ppm) significantly alleviated the downregulation of MUC2 mRNA expression induced by oxidized oil. In an in vitro experiment of LS174 T cells, a human intestinal goblet cell–like cell, quercetin could induce mRNA levels and secretion of MUC2 via the protein kinase C alpha/extracellular regulated protein kinases 1-2 pathway, and the same effects of quercetin also observed in the intestinal epithelial Caco-2 cells (Damiano et al., 2018). As a member of leucine zipper transcription factors, Nrf2 was essential for the induction of many stress-responsive or cytoprotective enzymes, such as CAT, SOD, GPX, HO-1, TXN, and glutamate–cysteine ligase (Surh et al., 2008). Quercetin could induce the upregulation and nuclear translocation of Nrf2 in several cells in vitro and in vivo (Tanigawa et al., 2007; Surh et al., 2008). In agreement with these observations, in our study, the group treated with quercetin (800 ppm) showed significantly increased expression of the antioxidant gene such as Nrf2 and downstream genes such as CAT, superoxide dismutase 1, glutathione peroxidase 2, glutaredoxin, and TXN compared with the Ctr group. Heme oxygenase-1 could catalyze the decomposition of hemin (a toxic oxidant) into bilirubin (a potent antioxidant), carbon monoxide (a novel gaseous signal messenger), and ferrous efflux. In an in vitro study on human hepatocytes, Nrf2 translocation into nuclei and subsequent upregulation of HO-1 induced by quercetin protected human hepatocytes from ethanol-induced oxidative stress (Yao et al., 2007). In the present study, quercetin reversed the decrease of HO-1 mRNA expression in the ileal mucosa induced by oxidized oil. NADPH oxidases (NOX) were the major sources of superoxide anions and mainly expressed in phagocytes, including chicken heterophils (Katsuyama, 2010; Nambooppha et al., 2018). In our study, the group treated with quercetin (800 ppm) showed significant downregulation in the mRNA expression of NOX2 compared with the oxidized oil treatment group. Cytosolic components p47phox, p67phox, NOXO1, and NOXA1 were required for NOX2 activation, while quercetin was able to downregulate the expression of the p47phox subunit, leading to the inhibition of NADPH oxidase (Yousefian et al., 2019). Similar results were also observed in the myocardial ischemia–reperfusion injury model in rabbits in vivo; quercetin inhibited myocardial ischemia–reperfusion injury–induced NOX2 mRNA and protein expression (Wan et al., 2009). IL-8 secreted by enterocytes attracted and activated neutrophils during the acute phase of inflammation (Martirosyan et al., 2016). IL-8 could rapidly be upregulated by proinflammatory cytokines, such as bacterial or viral products, and cell stresses (Hoffmann et al., 2002). In response to bacterial infection, heterophils were being secreted as a major source of proinflammatory cytokines such as IL-6 and IL-8 and other chemokines to communicate between innate and adaptive immune systems and affected tissues (Khampeerathuch et al., 2017). In our experiment, quercetin (800 ppm) alleviated the decrease in IL-8 expression levels caused by oxidized oil. In another study, isolated heterophils from hybrid chickens treated with quercetin also showed higher relative expression levels of IL-8, and the increase in proinflammatory gene expression such as that of IL-6 and IL-8 was suggested to be associated with the resistance to extraintestinal Salmonella enteritidis (Khampeerathuch et al., 2017).
Physiologically, ROS are generated by gastrointestinal tract epithelial cells as a result of oxygen metabolism or by enteric commensal bacteria and involved in the regulation of gut health (Mishra and Jha, 2019). And the antioxidant system maintains the microbiota in the luminal epithelium (Mishra and Jha, 2019). Enterocytes are able to absorb quercetin glucosides through transport and hydrolysis. However, owing to the highly hydrophobic property of quercetin and the highly hydrophilic property of the intestinal mucus layer, most of the dietary quercetin was not absorbed by the intestine, and the dietary quercetin could show a beneficial effect in the unabsorbed form in the gut lumen (Murota et al., 2004; Gonzales et al., 2016). In vitro coculture of quercetin and different Lactobacillus strains showed that quercetin increased the cell surface hydrophobicity and improved the autoaggregation and coaggregation capacity of the examined Lactobacillus strains (Dos Santos et al., 2019). And cell surface hydrophobicity and aggregation were strongly related to the ability of probiotics to adhere to the intestinal mucosa, which supported the beneficial modulatory effects exerted by quercetin on the examined Lactobacillus strains (Dos Santos et al., 2019). In this study, quercetin significantly enhanced the relative abundance of Lactobacillus in the cecum, which contributed to the gastrointestinal tract health.
Based on these results, quercetin ameliorated the oxidized oil–induced oxidative stress by restoring the redox balance, reinforcing the intestinal barrier, and facilitating the growth of Lactobacillus in the cecum. During this oxidative stress process caused by oxidized oil, quercetin at a concentration of 800 ppm enhanced the inherent antioxidant genes Nrf2 and its downstream genes such as SOD, CAT, GPX, TXN, and HO-1 to maintain the redox balance and reinforced the intestinal chemical barrier. The concentration of 400 ppm of quercetin also contributed to restore the redox balance and facilitated the growth of Lactobacillus in the cecum. Therefore, quercetin could be a potential feed additive that can be applied in poultry production for amelioration of oxidative stress and preventing the potential invasion of exogenous pathogens.
Acknowledgments
This work was supported by the National Key R&D Program of China (2017YFE0129900) and the Funding of Young Talent Supporting Program of the College of Animal Science and Technology of the China Agricultural University Education Foundation (2017DKA002).
Conflict of Interest Statement: The authors declare no competing financial interest.
References
- Açıkgöz Z., Bayraktar H., Altan Ö., Akhisaroglu S.T., Kırkpınar F., Altun Z. The effects of moderately oxidised dietary oil with or without vitamin E supplementation on performance, nutrient digestibility, some blood traits, lipid peroxidation and antioxidant defence of male broilers. J. Sci. Food Agr. 2011;91:1277–1282. doi: 10.1002/jsfa.4311. [DOI] [PubMed] [Google Scholar]
- Chen Z., Yuan Q., Xu G., Chen H., Lei H., Su J. Effects of quercetin on proliferation and H2O2-induced Apoptosis of intestinal Porcine enterocyte cells. Molecules. 2018;23:2012. doi: 10.3390/molecules23082012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Circu M.L., Aw T.Y. Intestinal redox biology and oxidative stress. Semin. Cell Dev. Biol. 2012;23:729–737. doi: 10.1016/j.semcdb.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo I., Garcia-Medlavilla M.V., Almar M., Gonzalez P., Tunon M.J., Sanchez-Campos S., Gonzalez-Gallego J. Differential effects of dietary flavonoids on reactive oxygen and nitrogen species generation and changes in antioxidant enzyme expression induced by proinflammatory cytokines in Chang Liver cells. Food Chem. Toxicol. 2008;46:1555–1569. doi: 10.1016/j.fct.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Curgali K., Toth S., Jonecova Z., Maretta M., Kalpakidis T., Petriskova I., Kusnier M., Soltes J., Svana M., Caprnda M., Delev D., Rodrigo L., Mechirova E., Kruzliak P. Quercetin protects jejunal mucosa from experimental intestinal ischemia reperfusion injury by activation of CD68 positive cells. Acta Histochem. 2018;120:28–32. doi: 10.1016/j.acthis.2017.11.001. [DOI] [PubMed] [Google Scholar]
- Damiano S., Sasso A., De Felice B., Di Gregorio I., La Rosa G., Lupoli G.A., Belfiore A., Mondola P., Santillo M. Quercetin increases MUC2 and MUC5AC gene expression and secretion in intestinal goblet cell-like LS174T via PLC/PKC alpha/ERK1-2 pathway. Front Physiol. 2018;9 doi: 10.3389/fphys.2018.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos A.S., Rodrigues De Albuquerque T.M., de Brito Alves J.L., de Souza E.L. Effects of quercetin and Resveratrol on in vitro properties related to the Functionality of potentially probiotic lactobacillus strains. Front Microbiol. 2019;10 doi: 10.3389/fmicb.2019.02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duenas M., Gonzalez-Manzano S., Gonzalez-Paramas A., Santos-Buelga C. Antioxidant evaluation of O-methylated metabolites of catechin, epicatechin and quercetin. J. Pharmaceut. Biomed. 2010;51:443–449. doi: 10.1016/j.jpba.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Goliomytis M., Tsoureki D., Simitzis P.E., Charismiadou M.A., Hager-Theodorides A.L., Deligeorgis S.G. The effects of quercetin dietary supplementation on broiler growth performance, meat quality, and oxidative stability. Poult. Sci. 2014;93:1957–1962. doi: 10.3382/ps.2013-03585. [DOI] [PubMed] [Google Scholar]
- Gonzales G.B., Van Camp J., Smagghe G., Raes K., Mackie A. Flavonoid–gastrointestinal mucus interaction and its potential role in regulating flavonoid bioavailability and mucosal biophysical properties. Food Res. Int. 2016;88:342–347. [Google Scholar]
- Hager-Theodorides A.L., Goliomytis M., Delis S., Deligeorgis S. Effects of dietary supplementation with quercetin on broiler immunological characteristics. Anim. Feed Sci. Tech. 2014;198:224–230. [Google Scholar]
- Hoffmann E., Dittrich-Breiholz O., Holtmann H., Kracht M. Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 2002;72:847–855. [PubMed] [Google Scholar]
- Hong Z., Piao M. 2018. Effect of quercetin Monoglycosides on oxidative stress and gut microbiota diversity in mice with dextran sodium Sulphate-induced colitis. Biomed. Res. Int. 2018:1–7. doi: 10.1155/2018/8343052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuyama M. NOX/NADPH oxidase, the superoxide-Generating enzyme: its transcriptional regulation and physiological Roles. J. Pharmacol. Sci. 2010;114:134–146. doi: 10.1254/jphs.10r01cr. [DOI] [PubMed] [Google Scholar]
- Khampeerathuch T., Mudsak A., Srikok S., Vannamahaxay S., Chotinun S., Chuammitri P. Differential gene expression in heterophils isolated from commercial hybrid and Thai indigenous broiler chickens under quercetin supplementation. J. Appl. Anim. Res. 2017;46:804–812. [Google Scholar]
- Lesjak M., Beara I., Simin N., Pintać D., Majkić T., Bekvalac K., Orčić D., Mimica-Dukić N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods. 2018;40:68–75. [Google Scholar]
- Li Y., Yao J., Han C., Yang J., Chaudhry M., Wang S., Liu H., Yin Y. Quercetin, inflammation and Immunity. Nutrients. 2016;8:167. doi: 10.3390/nu8030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F., Jiang S., Mo Y., Zhou G., Yang L. Consumption of oxidized soybean oil increased intestinal oxidative stress and affected intestinal immune Variables in Yellow-feathered broilers. Asian Austral J. Anim. 2015;28:1194–1201. doi: 10.5713/ajas.14.0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W.C., Lee M.T., Chang S.C., Chang Y.L., Shih C.H., Yu B., Lee T.T. Effects of mulberry leaves on production performance and the potential modulation of antioxidative status in laying hens. Poult. Sci. 2017;96:1191. doi: 10.3382/ps/pew350. [DOI] [PubMed] [Google Scholar]
- Liu H.N., Liu Y., Hu L.L., Suo Y.L., Zhang L., Jin F., Feng X.A., Teng N., Li Y. Effects of dietary supplementation of quercetin on performance, egg quality, cecal microflora populations, and antioxidant status in laying hens. Poult. Sci. 2014;93:347–353. doi: 10.3382/ps.2013-03225. [DOI] [PubMed] [Google Scholar]
- Liu P., Chen C., Kerr B.J., Weber T.E., Johnston L.J., Shurson G.C. Influence of thermally oxidized vegetable oils and animal fats on growth performance, liver gene expression, and liver and serum cholesterol and triglycerides in young pigs. J. Anim. Sci. 2014;92:2960–2970. doi: 10.2527/jas.2012-5709. [DOI] [PubMed] [Google Scholar]
- Lu T., Harper A.F., Zhao J., Estienne M.J., Dalloul R.A. Supplementing antioxidants to pigs fed diets high in oxidants: I. Effects on growth performance, liver function, and oxidative status. J. Anim. Sci. 2014;92:5455. doi: 10.2527/jas.2013-7109. [DOI] [PubMed] [Google Scholar]
- Lushchak V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem-Biol Interact. 2014;224:164–175. doi: 10.1016/j.cbi.2014.10.016. [DOI] [PubMed] [Google Scholar]
- Martirosyan A., Grintzalis K., Polet M., Laloux L., Schneider Y. Tuning the inflammatory response to silver nanoparticles via quercetin in Caco-2 (co-)cultures as model of the human intestinal mucosa. Toxicol. Lett. 2016;253:36–45. doi: 10.1016/j.toxlet.2016.04.018. [DOI] [PubMed] [Google Scholar]
- Mishra B., Jha R. Oxidative stress in the poultry gut: potential Challenges and Interventions. Fron. Vet. Sci. 2019;6 doi: 10.3389/fvets.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murota K., Mitsukuni Y., Ichikawa N., Tsushida T., Miyamoto S., Terao J. Quercetin-4' glucoside is more potent than quercetin-3-glucoside in protection of rat intestinal mucosa homogenates against iron ion-induced lipid peroxidation. J. Agr Food Chem. 2004;52:1907–1912. doi: 10.1021/jf035151a. [DOI] [PubMed] [Google Scholar]
- Nambooppha B., Photichai K., Wongsawan K., Chuammitri P. Quercetin manipulates the expression of genes involved in the reactive oxygen species (ROS) process in chicken heterophils. J. Vet. Med. Sci. 2018;80:1204–1211. doi: 10.1292/jvms.17-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC . 9th rev. Natl. Acad. Press; Washington. DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Pang C., Zheng Z., Shi L., Sheng Y., Wei H., Wang Z., Ji L. Caffeic acid prevents acetaminophen-induced liver injury by activating the Keap1-Nrf2 antioxidative defense system. Free Radic. Bio Med. 2016;91:236–246. doi: 10.1016/j.freeradbiomed.2015.12.024. [DOI] [PubMed] [Google Scholar]
- Peng M., Luo H., Kumar V., Kajbaf K., Hu Y., Yang G. Dysbiosis of intestinal microbiota induced by dietary oxidized fish oil and recovery of diet-induced dysbiosis via taurine supplementation in rice field eel (Monopterus albus) Aquaculture. 2019;512:734288. [Google Scholar]
- Staprans I., Rapp J.H., Pan X.M., Kim K.Y., Feingold K.R. Oxidized lipids in the diet are a source of oxidized lipid in chylomicrons of human serum. Arterioscler Thromb. 1994;14:1900–1905. doi: 10.1161/01.atv.14.12.1900. [DOI] [PubMed] [Google Scholar]
- Surai P.F., Kochish I.I., Fisinin V.I., Kidd M.T. Antioxidant defence systems and oxidative stress in poultry biology: an Update. Antioxidants (Basel) 2019;8 doi: 10.3390/antiox8070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh Y., Kundu J.K., Na H. Nrf2 as a Master redox Switch in Turning on the cellular signaling involved in the induction of cytoprotective genes by some Chemopreventive Phytochemicals. Planta Med. 2008;74:1526–1539. doi: 10.1055/s-0028-1088302. [DOI] [PubMed] [Google Scholar]
- Tan L., Rong D., Yang Y., Zhang B. The effect of oxidized fish oils on growth performance, oxidative status, and intestinal barrier function in broiler chickens. J. Appl. Poult. Res. 2019;28:31–41. [Google Scholar]
- Tan L., Rong D., Yang Y., Zhang B. Effect of oxidized soybean oils on oxidative status and intestinal barrier function in broiler chickens. Braz. J. Poult. Sci. 2018;20:333–342. [Google Scholar]
- Tanigawa S., Fujii M., Hou D. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic. Bio Med. 2007;42:1690–1703. doi: 10.1016/j.freeradbiomed.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Wan L.L., Xia J., Ye D., Liu J., Chen J., Wang G. Effects of quercetin on gene and protein expression of NOX and NOS after myocardial ischemia and reperfusion in rabbit. Cardiovasc. Ther. 2009;27:28–33. doi: 10.1111/j.1755-5922.2009.00071.x. [DOI] [PubMed] [Google Scholar]
- Wang J., Zhang H.J., Xu L., Long C., Samuel K.G., Yue H.Y., Sung L.L., Wu S.G., Qi G.H. Dietary supplementation of pyrroloquinoline quinone disodium protects against oxidative stress and liver damage in laying hens fed an oxidized sunflower oil-added diet. Animal. 2016;10:1129–1136. doi: 10.1017/S175173111600001X. [DOI] [PubMed] [Google Scholar]
- Wang S., Yao J., Zhou B., Yang J., Chaudry M.T., Wang M., Xiao F., Li Y., Yin W. Bacteriostatic effect of quercetin as an Antibiotic Alternative in vivo and its antibacterial Mechanism in vitro. J. Food Protect. 2018;81:68–78. doi: 10.4315/0362-028X.JFP-17-214. [DOI] [PubMed] [Google Scholar]
- Yang J.X., Maria T.C., Zhou B., Xiao F.L., Wang M., Mao Y.J., Li Y. Quercetin improves immune function in Arbor Acre broilers through activation of NF-kappaB signaling pathway. Poult. Sci. 2020;99:906–913. doi: 10.1016/j.psj.2019.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao P., Nussler A., Liu L., Hao L., Song F., Schirmeier A., Nussler N. Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J. Hepatol. 2007;47:253–261. doi: 10.1016/j.jhep.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Yousefian M., Shakour N., Hosseinzadeh H., Hayes A.W., Hadizadeh F., Karimi G. The natural phenolic compounds as modulators of NADPH oxidases in hypertension. Phytomedicine. 2019;55:200–213. doi: 10.1016/j.phymed.2018.08.002. [DOI] [PubMed] [Google Scholar]
- Zhao L., Wu J., Yang J., Wei J., Gao W., Guo C. Dietary quercetin supplementation increases serum antioxidant capacity and alters hepatic gene expression profile in rats. Exp. Biol. Med. 2011;236:701–706. doi: 10.1258/ebm.2011.010258. [DOI] [PubMed] [Google Scholar]
- Zou Y., Wei H.K., Xiang Q., Wang J., Zhou Y., Peng J. Protective effect of quercetin on pig intestinal integrity after transport stress is associated with regulation oxidative status and inflammation. J. Vet. Med. Sci. 2016;78:1487–1494. doi: 10.1292/jvms.16-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]