Abstract
In this study, fermentation with Lactobacillus plantarum and Saccharomyces cerevisiae was applied to improve the flavor of cured duck leg meat. Odor and taste evaluations, lipid oxidation, volatile flavor substances, and protein degradation were determined to investigate the effects of microbial fermentation on flavor improvement. The results showed that the utilization of L. plantarum represented the most significant effect on lipid peroxidation inhibition (the lowest value of thiobarbituric acid reactive substances and the highest content of polyunsaturated fatty acids) and also enhanced the generation of volatile flavor substances than nonfermented duck meat. Microbial fermentation accelerated protein degradation in duck meat. S. cerevisiae could produce glutamate to promote the umami taste flavor of cured duck leg meat, and L. plantarum significantly improved the sweet taste by releasing alanine. Meanwhile, mixed fermentation with the two microbial species resulted in the combination of both of their advantages. These findings not only indicate the potential application of microbial fermentation in characteristic duck meat but also indicate that fermentation improves sensory properties of duck products significantly.
Key words: cured duck leg, fermentation, Lactobacillus plantarum, Saccharomyces cerevisiae, sensory quality
Introduction
Fermented meat products, such as Chinese bacon and ham, are popular among consumers for their distinctive aroma and taste (Papavergou et al., 2012). Microbial fermentation can also improve color, microbiological safety, tenderness, and other desirable attributes of meat products (Ockerman and Basu, 2010). Pork meat is usually processed by fermentation (Huang et al., 2016), but little attention has been paid to poultry meat, for example, duck meat. Duck meat is a nutritious food source owing to its high content of protein, iron, vitamins, selenium, and niacin and low content of fat and cholesterol (Adzitey, 2012). The most used methods to process duck meat are dry pickling, salting, roasting, and boiling (Xu et al., 2008). However, the application of microbial fermentation in duck meat processing has not been well evaluated yet.
Lactic acid bacteria are widely used in food fermentation (Leroy and Vuyst, 2004). Lactobacillus plantarum, a versatile species of lactic acid bacteria, has been frequently applied in fermentation of dairy, meat, and vegetables (Vries et al., 2006). It has been reported that L. plantarum can not only promote the safety of fermented sausages but also increase the release of free amino acids and peptides in meat (Fadda et al., 2010). L. plantarum is also used as a flavor enhancer during production of low-salt bread (Valerio et al., 2017). Yeast has the capacity of producing a unique yeast flavor and stabilizing meat color (Campagnol et al., 2011). Saccharomyces cerevisiae, a species of yeast, has been shown to improve the taste and aroma of fermented sausages by influencing the compositions of free amino acids and volatile compounds (Chaveslópez et al., 2011).
As duck meat is an important animal meat source, the application of microbial fermentation in duck meat processing should be potentially valuable for development of characteristic meat products. We hypothesize that microbial fermentation endows duck meat with distinctive flavors. Therefore, the aim of this study was to investigate the effects of fermentation with L. plantarum and S. cerevisiae on the flavors of cured duck legs (a famous meat product), including odor and taste. This work contributes to expanding our knowledge of duck meat production.
Materials and methods
Bacterial Strains and Culture Conditions
The L. plantarum and S. cerevisiae strains used in this study were laboratory preserved. L. plantarum was first inoculated and cultured at 37°C in de Man, Rogosa and Sharpe medium for 6–8 h and then cultured in new de Man, Rogosa and Sharpe medium at 37°C for another 10–12 h. S. cerevisiae was inoculated in yeast extract–peptone–dextrose medium and cultured at 30°C for 24 h.
Preparation of Cured Duck Legs
A total of 100 raw duck legs (about 250 g each, from Cherry-Valley ducks) were purchased from a local market. The duck legs were cured at 4°C for 24 h with salt (15 g/kg), sodium nitrite (0.15 g/kg), ascorbic acid (0.8 g/kg), sucrose (15 g/kg), glucose (10 g/kg), monosodium glutamate (2 g/kg), white pepper (0.8 g/kg), cooking wine (40 mL/kg), Chinese prickly ash (1.5 g/kg), onion powder (2 g/kg), and soy sauce (65 mL/kg). All the 100 duck legs were randomly divided into 4 groups: CK, control group without inoculation; MF, samples inoculated with both L. plantarum and S. cerevisiae (1:2, n/n); LP, samples inoculated with L. plantarum; and SC, samples inoculated with S. cerevisiae. The 4 inoculation concentrations were all modified to 1 × 106 cfu/g, and the inoculation amount was 2% of each leg weight. Subsequently, the fermented duck legs were placed in a fermentation chamber (model HSW; Zhejiang Ningbo Jiangnan Instrument Factory, Ningbo, China) and kept at 30°C and 50% relative humidity for 24 h. After fermentation was completed, the duck legs were steamed for 30 min to obtain the final products.
Sensory Evaluations
Sensory evaluations were performed according to the method described by Yang et al. (2018) with slight modifications. Twenty panelists (10 men and 10 women, aged from 20 to 40 yr) were selected and trained to participate in odor and taste evaluations. The odor attributes included meat flavor, pickled sweet, sourness, aroma of roast meat, grease odor, scorched aroma, and overall aroma. Each panelist was asked to score the aroma qualities between one and 9. One corresponds to the lowest intensity, and 9 corresponds to the highest intensity. For taste evaluation, the panelists were asked to describe the sourness, sweetness, saltiness, bitterness, umami, aftertaste, and overall taste of the duck leg meat subjected to different fermentation treatments. Before the analysis, 5 solutions were prepared for the panelists to familiarize the taste attributes: citric acid (sour), caffeine (bitter), sucrose (sweet), sodium glutamate (umami), and sodium chloride (salty). Each panelist was asked to score the taste qualities between one and 6. For each parameter, one corresponds to the lowest intensity, and 6 corresponds to the highest intensity. Before each taste evaluation, all the assessors were asked to clean their mouths with filtered water. Each sample was scored within 2 min, and an interval of 10 min was set between each of the two scorings. Each sensory evaluation was performed in triplicate.
Discrimination of Odor and Taste
The odor and taste of the duck leg meat subjected to different fermentation treatments were analyzed based on the slightly modified method of Song et al. (2013). Each meat sample (5 g) was prepared in a sampling bottle, and the parameters were analyzed after incubation at 30°C for 10 min. Headspace sampling was applied. The detection time was 450 s, the cleaning time was 150 s, and the injection volume was 300 mL/min. Linear discriminant analysis (LDA) was performed on the 440–441 s data of the electronic nose (model PEN3; Airsense, Schwerin, Mecklenburg, Germany) with the matching WinMuster software. Each odor determination was repeated 5 times.
Ten grams of duck leg meat was accurately weighed, minced, and homogenized in 100 mL of ddH2O at 10,000 rpm for 10 s. The homogenization was repeated 3 times in an ice-water bath, and then, the mixture was centrifuged at 9,000 rpm at 4°C for 10 min. The supernatant was filtered through a two-layer qualitative filter paper. The filtrate (15 mL) was placed in a special beaker of the electronic tongue (Smartongue; Shanghai Ruiyu International Trade Co., Ltd., Shanghai, China) and analyzed using the automatic sample analysis device. The acquisition time was 120 s, and the average of the last 30-s values was considered the result of sample detection. After the measurement, the sensor cleaning time was 300 s. Each assay was duplicated three times.
Determination of Thiobarbituric Acid Reactive Substances
The thiobarbituric acid reactive substances (TBARS) values were determined following the modified method of Witte et al. (2010). Ten grams of duck leg meat was minced and homogenized in 50 mL of 7.5% trichloroacetic acid (TCA, containing 0.1% EDTA) at 10,000 rpm in an ice-water bath for 10 s. The homogenization was repeated 6 times. The homogenate was filtered, and the filtrate was mixed with the same volume of 0.02 mol 2-thiobarbituric acid solution. The mixture was incubated in a boiling water bath for 40 min, cooled, and then centrifuged at 5,000 rpm for 5 min. An equal volume of chloroform was added to the supernatant. After layering, the absorbance of the supernatant was measured at 532 nm and 600 nm, respectively. The TBARS value was calculated using the following formula: TBARS (mg/kg) = (A532-A600)/155 × 72.06 × 1,000. The values were expressed as milligram of malondialdehyde per kilogram of meat. Each determination was repeated three times.
Free Fatty Acid Determination
The contents of free fatty acids in duck leg meat were determined based on the method described by Yang et al. (2018). Each meat sample (3.0 g) was minced and homogenized in chloroform–methanol (2:1, v/v) at 3,000 rpm for 10 s. The homogenization was repeated three times. Then, the homogenate was adjusted to 45 mL. After allowing the homogenate to stand for 2 h, it was filtered through a two-layer qualitative filter paper, and the filtrate was washed with 20% volume of the solution (containing 0.5 g/L of CaCl2 and 7.3 g/L of NaCl) and centrifuged at 4,500 g for 20 min. The lower phase was harvested and dried using a rotary evaporator at 44°C, and the obtained total lipid was stored at −20°C until analysis.
The total lipid (20 mg) was dissolved in 1.0 mL of chloroform. Then, 0.5 mL of the solution was added to an aminopropylsilica minicolumn (100 mg; Varian, Palo Alto, CA), which was previously activated with 1.0 mL of chloroform. The minicolumn was washed using 2.0 mL of chloroform–2-propanol (2:1, v/v) to detach the neutral lipid. After elution with 3.0 mL of acetic acid (2%, w/w) in diethyl ether, the free fatty acids were obtained.
The free fatty acid elute was evaporated under nitrogen and mixed with 2.0 mL of 14% mass fraction of boron fluoride–methanol. Methyl heptadecanoate was used as the internal standard. The mixture was methylated at 60°C for 30 min, and 3 drops of 2,2-dimethoxy propane were added to the mixture to eliminate water. After cooling, 1.0 mL of ddH2O and 1.0 mL of n-hexane were added to the mixture, and the mixture was shaken for 5 s and allowed to stand for 1 h. Finally, the upper phase was transferred to a sample vial, evaporated under nitrogen, and dissolved in 0.5 mL of hexane. Fatty acid methyl esters (1.5 μL) were assayed using a gas chromatograph (5,977A-7,890 B; Agilent Technologies, Santa Clara, CA) equipped with a split injector, a flame ionization detector, and a capillary column (CP-Sil 88 for Fame, 50 m × 0.25 mm × 0.20 μm). The temperature of the injector and detector was maintained at 280°C, and the temperature of the oven was increased from 160°C to 220°C at the rate of 6°C/min and maintained at 220°C for 30 min. The pressure of the carrier gas (N2) maintained at 80 kPa, and the split ratio was 1:40.
Analysis of Volatile Flavor Compounds
The volatile flavor compounds of the fermented duck legs were analyzed by gas chromatography–mass spectrometry (GC–MS) according to the method used by Lorenzo and Lorenzo (2014). Each meat sample (3.0 g) was weighed, frozen in liquid nitrogen for 30 min, minced, and moved into a 20-mL extraction flask. The 50/30-μm carboxen/divinylbenzene/polydimethylsiloxane extraction head was inserted into a sealed extraction flask, and extraction was carried out at 60°C for 30 min. The extraction head was then treated at 250°C for 5 min in a gas phase injection port for thermal desorption.
The volatile compounds were analyzed by GC–MS (7,890B-7,000 C, Agilent, Santa Clara, CA). The GC system was equipped with an HP-5MS elastic quartz capillary column (30 m × 0.25 mm, 0.25 μm; Supelco, Bellefonte, PA). Helium was used as the carrier gas, with a velocity of 1.0 mL/min. The sample was injected in splitless mode, and the temperature of the injection port was set at 260°C. The start temperature was maintained at 40°C for 5 min, increased to 180°C isothermally at the rate of 5°C/min, then increased to 250°C at the rate of 15°C/min, and maintained for 8 min. The mass spectra were obtained using a mass selective detector, which was operated in electronic impact mode at 70 eV. The temperature of the ion source was 230°C. The scanned range was 35–500 m/z. Compounds were identified by comparison with the mass spectrum with the mass spectral database in the libraries of NIST and WILEY 7.0.
Determination of Nonprotein Nitrogen
Ten grams of duck leg meat was weighed, homogenized in 25 mL of 5% TCA at 10,000 rpm in an ice-water bath for 10 s, transferred to a 250-mL beaker, and allowed to stand for 30 min. Then, the homogenate was filtered through a nitrogen-free filter paper, washed using 50 mL of 5% TCA, and adjusted to 250 mL. Six grams of K2SO4, 0.2 g of CuSO4, and 20 mL of H2SO4 were added to the sample (10–20 mL), and the mixture was heated until the content was carbonized completely. After foaming stopped completely, the liquid was boiled slightly until its color turned to bluish green; then, the liquid was cooled, and 20 mL of H2O was added to it; the resultant was transferred to a 100-mL volumetric flask. The determination of nitrogen content was carried out following the method used by Gao et al. (2015). Each assay was performed in triplicate.
Free Amino Acid Determination
According to the slightly modified method of Zhang et al. (2014), 10 mL of 10% sulfosalicylic acid was added to each meat sample (1.0 g); the meat sample was homogenized at 10,000 rpm for 1 min and allowed to stand at 4°C for 17 h. The mixture was filtered through a two-layer qualitative filter paper. The pH of the filtrate was adjusted to 6.0 using NaOH solution. And 10 mL of the filtrate was filtered again through a 0.22-μm filter. Detection was performed using an automatic amino acid analyzer (model L-8800; Hitachi, Tokyo, Japan). Analytical 2622# (4.6 mm × 60 mm) and guard 2650# (4.6 mm × 40 mm) columns (Hitachi) were used for free amino acid determination. Immediately after the injection of the sample into the columns, an autosampler was used for inline derivatization by ninhydrin postcolumn derivatization. The ninhydrin-derivatized amino acids were monitored at 570 nm and 440 nm.
Statistical Analysis
For sensory analysis, TBARS, nonprotein nitrogen (NPN), free fatty acid and free amino acid, and volatile flavors were evaluated via the one-way analysis of variance procedure (Duncan's multiple range test) using SAS 8.0 software (SAS Institute, Cary, NC). The results were expressed as mean ± standard deviation, and the significance of difference was set at P <0.05.
Results and discussion
Odor Evaluation of Cured Duck Legs Subjected to Different Fermentation Treatments
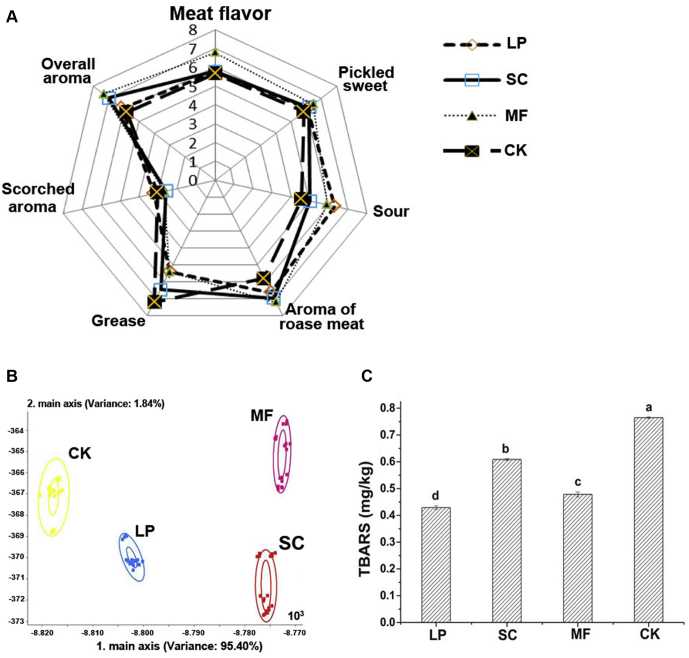
The odor sensory evaluation was carried out to assess the aroma variations of cured duck legs caused by different microbial fermentations, and the results are shown in Figure 1A. It indicated that the sourness of the LP group was heavy and that the grease odor of the CK group and the SC group was intensive. The main flavor characteristic of the MF group was strong meat flavor, and the other odor components were not significant (P > 0.05). The overall aroma score of the MF group was higher than that of the other groups; the difference between the SC group and the MF group was not significant (P > 0.05), and the difference between the LP group and the CK group was not significant (P > 0.05).
Figure 1.
Comparison of the odor profile (A), electronic nose LDA (B), and TBARS values (C) of cured duck legs subjected to various fermentations. Different letters mean significant differences at P < 0.05. Abbreviations: LDA, linear discriminant analysis; TBARS, thiobarbituric acid reactive substances; LP, samples inoculated with L. plantarum; SC, samples inoculated with S. cerevisiae; MF, samples inoculated with L. plantarum and S. cerevisiae; CK, control group without inoculation.
The electronic nose was used to discriminate the aromas of different groups, and the LDA results are presented in Figure 1B. Each ellipse in the electronic nose represented the data collection point under the same conditions. The distances between different data points represent the repeatability of the samples (Brudzewski et al., 2010). By LDA, the samples of the fermentation groups could be well distinguished, with the total variance of 97.24%. LD1 and LD2 accounted for a total variance of 76.5% and 15.2%, respectively, which covered almost all the variable information of the samples. There were no overlapped clusters in the figure. The odors of the LP group and CK group were similar. In the same way, the odors of the MF group and the SC group were similar. These results were consistent with the odor sensory scores.
Lipid Oxidation Affected by Microbial Fermentation
As a result of oxidative decomposition of unsaturated fatty acids in animal lipids, TBARS is usually used as an indicator of lipid oxidation (Demeyer et al., 2010). The TBARS values of the 4 treatment groups were determined to evaluate the oxidation status of duck leg meat affected by microbial fermentation (Figure 1C). The results showed that all the three fermentation treatments significantly decreased the TBARS values of cured duck legs (P < 0.05). Among the three fermentation groups, the order of TBARS values was LP < MF < SC (P < 0.05). This indicated that L. plantarum possessed more significant suppressive effect on lipid peroxidation than S. cerevisiae. It might be due to the production of lactic acid in the LP group, which resulted in a decrease in pH and the inhibition of fat oxidation (Loh et al., 2014).
Lipids are the precursors of flavors in fermented meat products, and the oxidative products of lipids play important roles in flavor formation of fermented meat products. However, peroxidation was reported to cause meat flavor deterioration (Stetzer et al., 2008). Therefore, it is necessary to control lipid oxidation of meat products. The data in this study showed that the fermentations with L. plantarum and S. cerevisiae could significantly inhibit lipid peroxidation in cured duck legs.
Fermentation Affects the Contents of Free Fatty Acids in Duck Meat
During meat processing, free fatty acids are released through lipid hydrolysis (Toldrá et al., 1997). Free fatty acids are important precursors of flavor compounds in meat products, and the main volatile flavor compounds (aldehydes, ketones, alcohols, esters, and so on) are generated by free fatty acids (Zhou et al., 2015). Studies had found that unsaturated fatty acids are the main materials causing lipid oxidation, especially polyunsaturated fatty acids (PUFA), which are more susceptible to oxidation in the free state than in the bound state (Vigor et al., 2014). In this study, the compositions of free fatty acids in fermented duck legs were determined and are listed in Table 1. The results showed that the content of PUFA in the LP group was the highest (P < 0.05), and the content in the MF group was significantly higher than that in the SC group (P < 0.05). It was consistent with the TBARS results that L. plantarum had a greater capacity of suppressing lipid peroxidation than S. cerevisiae. It has been reported that L. plantarum had certain ability to degrade lipids (Axling et al., 2012). However, it was also found that S. cerevisiae had a higher lipase activity in dry-cured sausage, which was conducive to flavor formation (Nie et al., 2014). The differences should be associated with the meat sources or products. Moreover, the content of total free fatty acids in the MF group was found to be significantly higher than that in all the other three groups (P < 0.05). Except the SC group, the rest of the fermentation groups had higher contents of total free fatty acids than the CK group (P < 0.05). It implied that there might be an interaction between L. plantarum and S. cerevisiae for promoting the accumulation of free fatty acids in cured duck legs.
Table 1.
The contents of free fatty acids in fermented cured duck legs.
| Free fatty acids | Content (mg/g fat) |
|||
|---|---|---|---|---|
| LP2 | SC2 | MF2 | CK2 | |
| C12:0 | 0.013 ± 0.004b | 0.0103 ± 0.0001b,c | 0.0871 ± 0.0044a | 0.0069 ± 0.0001c |
| C14:0 | 0.129 ± 0.017a | 0.106 ± 0.014a,b | 0.0990 ± 0.0021a,b | 0.0820 ± 0.0013b |
| C14:1 | 0.0163 ± 0.0044a | 0.0144 ± 0.0004a | 0.0112 ± 0.0021a,b | 0.0091 ± 0.0017b |
| C15:0 | 0.0189 ± 0.0015 | 0.0167 ± 0.0201 | 0.0152 ± 0.0078 | 0.0127 ± 0.0009 |
| C16:0 | 14.59 ± 0.33b | 13.58 ± 1.08b | 23.12 ± 0.92a | 12.99 ± 0.42b |
| C16:1 | 0.778 ± 0.042a | 0.611 ± 0.020b | 0.445 ± 0.043c | 0.446 ± 0.009c |
| C17:0 | 0.0304 ± 0.0002a | 0.0271 ± 0.0007a,b | 0.0259 ± 0.0000a,b | 0.0219 ± 0.0075b |
| C18:0 | 9.25 ± 0.21b | 7.41 ± 0.50c | 12.22 ± 0.54a | 6.97 ± 0.32c |
| C18:1n-9c | 8.26 ± 0.37b | 6.87 ± 0.45b,c | 12.21 ± 0.39a | 5.71 ± 0.28c |
| C18:2n-6c | 17.21 ± 0.64a | 13.34 ± 2.80b | 10.11 ± 0.84b | 12.85 ± 0.63b |
| C18:3n-6r | 0.0141 ± 0.0021b | 0.0101 ± 0.0007c | 0.0905 ± 0.0041a | 0.0081 ± 0.0032c |
| C18:3n-3 | 0.152 ± 0.014a | 0.114 ± 0.023b | 0.113 ± 0.015b | 0.101 ± 0.027b |
| C20:0 | 0.0155 ± 0.0032 | 0.0148 ± 0.0008 | 0.0146 ± 0.0000 | 0.0131 ± 0.0002 |
| C20:1 | 0.0636 ± 0.0050a | 0.0559 ± 0.0023a | 0.0475 ± 0.0014b | 0.0471 ± 0.0009b |
| C20:2 | 0.033 ± 0.009 | 0.027 ± 0.030 | 0.025 ± 0.004 | 0.023 ± 0.014 |
| C20:3n-6 | 0.0265 ± 0.0032a | 0.0172 ± 0.0000b | 0.0163 ± 0.0002b | 0.0153 ± 0.0004b |
| C23:0 | 0.0471 ± 0.0041a | 0.0372 ± 0.0007b | 0.0419 ± 0.0002a,b | 0.0387 ± 0.0005b |
| SFA1 | 24.094 ± 0.539b | 21.202 ± 2.686c | 35.624 ± 0.623a | 20.135 ± 1.451c |
| MFA1 | 0.858 ± 0.372 | 0.681 ± 0.455 | 0.504 ± 0.393 | 0.502 ± 0.278 |
| PUFA1 | 25.696 ± 0.694a | 20.378 ± 2.796c | 22.565 ± 1.110b | 18.707 ± 1.754c |
| Total | 48.442 ± 1.382b | 42.262 ± 1.984c | 61.692 ± 2.950a | 39.345 ± 2.715c |
a–cDifferent superscript letters within the same row stand for significant differences at P < 0.05.
SFA: saturated fatty acid; MFA: monounsaturated fatty acid; PUFA: polyunsaturated fatty acid.
LP: samples inoculated with L. plantarum; SC: samples inoculated with S. cerevisiae; MF: samples inoculated with L. plantarum and S. cerevisiae; CK: control group without inoculation.
Analysis of Volatile Flavor Compounds
All volatile flavor compounds of the duck leg samples were analyzed by GC–MS and are listed in Table 2. The detected volatile compound varieties in the LP, SC, MF, and CK groups were 39, 52, 60, and 34, respectively. This indicated that cured duck legs fermented with L. plantarum and S. cerevisiae generated the most diversified volatile compounds. Both L. plantarum and S. cerevisiae contributed to the volatile flavor of cured duck legs. Compared with the LP or CK group, more volatile flavor substances were detected in the SC group, which also had more components of esters and alcohols. It can be explained that S. cerevisiae could form a variety of esters under the action of esterase or alcohol acyltransferase during fermentation (Saerens et al., 2008), and alcohols were also reported as by-products of S. cerevisiae fermentation (Spinosa et al., 2016). Moreover, S. cerevisiae could endow substrates with the characteristic yeast flavor and promote the decomposition of fat and protein to improve flavor (Kong et al., 2016). The alcohols, esters, aliphatic hydrocarbons, and aromatic compounds were more in the fermentation groups than in the CK group. Of the volatile flavors, 1-octene-3-ol content was the highest in the MF group and SC group, and D-limonene content was the highest in the LP group and CK group. This indicated that terpenes and alcohols are the main volatile flavors of the fermented duck legs.
Table 2.
Volatile flavor compounds of duck legs fermented by different inoculation methods.
| Volatile flavor compounds | RT1 (min) | Relative content (%) |
|||
|---|---|---|---|---|---|
| LP2 | SC2 | MF2 | CK2 | ||
| Alcohols | |||||
| 1-Octene-3-ol | 16.303 | 10.44 ± 0.13c | 16.53 ± 0.76a | 13.20 ± 0.29b | 6.88 ± 0.10d |
| Isoamyl alcohol | 18.022 | 3.30 ± 0.98a,b | 2.82 ± 0.78b | 0.93 ± 0.86c | 4.01 ± 1.55a |
| 3-Buten-2-ol | 18.322 | - | 1.26 ± 1.15a | - | - |
| N-pentanol | 19.541 | - | 0.69 ± 0.06a | 0.21 ± 0.05c | 0.38 ± 0.05b |
| (2S,3S)-(+)-2,3-butanediol | 21.299 | - | - | 1.09 ± 0.29a | - |
| N-hexanol | 23.820 | 2.51 ± 0.53c | 3.91 ± 0.23b,c | 4.24 ± 0.46b | 5.83 ± 1.09a |
| 2-Heptanol | 24.783 | - | 0.48 ± 0.01a | - | - |
| Heptanol | 27.725 | - | - | 0.84 ± 0.21a | - |
| Eucalyptus | 30.664 | 3.24 ± 0.57a | 3.53 ± 0.60a | 1.82 ± 0.71b | 3.32 ± 0.44a |
| 2-Methyl-5-(1-methylethyl)-(1α,2β,5α)-bicyclo[3.1.0]hexan-2-ol | 31.876 | 0.42 ± 0.01c | 1.25 ± 0.11a | 0.80 ± 0.29a,b | - |
| Isopropanol | 31.881 | 3.70 ± 2.54a | - | - | - |
| Linalool | 32.423 | - | 1.90 ± 0.20a | - | - |
| Trans-1-methyl-4-(1-methylvinyl)cyclohex-2-en-1-ol | 33.211 | - | 0.66 ± 0.07a | - | - |
| Terpene alcohol | 36.074 | 0.83 ± 0.14a | 0.37 ± 0.17b | 0.47 ± 0.06b | 0.87 ± 0.17a |
| L-α-terpineol | 36.527 | 0.44 ± 0.02b | 0.28 ± 0.02c | 0.80 ± 0.18a | - |
| Aldehydes | |||||
| Isovaleraldehyde | 14.867 | - | - | 0.63 ± 0.08b | 3.61 ± 0.45a |
| 2-Methylbutanal | 15.283 | - | - | 1.26 ± 0.09b | 3.88 ± 0.15a |
| Valeraldehyde | 16.911 | - | - | 1.29 ± 0.15a | 0.40 ± 0.09b |
| N-hexanal | 21.412 | 1.08 ± 0.29b | 3.00 ± 0.07a,b | 1.74 ± 0.55b | 3.26 ± 0.16a |
| 3-Methylthiopropanal | 27.402 | 0.31 ± 0.02c | - | 0.62 ± 0.12b | 3.24 ± 0.24a |
| Furfural | 32.801 | - | 0.31 ± 0.45b | - | 1.31 ± 0.24a |
| Trans-2,4-nonadienal | 39.482 | - | - | 0.30 ± 0.06b | 0.55 ± 0.07a |
| Acids | |||||
| Caproic acid | 27.393 | 0.46 ± 0.08a | - | 0.16 ± 0.00b | - |
| Ketones | |||||
| 2-Butanone | 12.351 | - | - | 0.29 ± 0.07b | 1.69 ± 0.22a |
| 2-Pentanone | 16.414 | - | 0.39 ± 0.02b | - | 0.90 ± 0.08a |
| 2-Pentadecanone | 16.554 | 0.36 ± 0.03b | 1.16 ± 0.30a | 0.39 ± 0.02b | - |
| 2,3-pentanedione | 16.861 | - | - | 0.42 ± 0.60b | 3.97 ± 0.23a |
| 3-Hydroxy-2-butanone | 18.169 | 8.00 ± 0.61a | 3.07 ± 0.66b | 1.09 ± 0.58c | - |
| 1-Hydroxy-2-butanone | 18.215 | - | - | 0.85 ± 0.57b | 2.94 ± 0.96a |
| Acetone | 18.940 | - | - | 1.34 ± 0.04b | 1.65 ± 0.17a |
| 2-Heptanone | 25.036 | - | 0.52 ± 0.27b | - | 3.21 ± 0.29a |
| 6-Methyl-5-hepten-2-one | 28.94 | - | - | 0.23 ± 0.02 | - |
| 2-Undecanone | 38.553 | - | 0.59 ± 0.05b | - | 1.33 ± 0.15a |
| 2-Isopropyl-5-methyl-3-cyclohexen-1-one | 39.404 | - | 0.35 ± 0.02a | 0.24 ± 0.06b | - |
| Esters | |||||
| 4-Penten-1-yl acetate | 8.135 | - | 0.69 ± 0.07 | - | - |
| Methyl acetate | 9.122 | - | 0.31 ± 0.31 | - | - |
| Vinyl acetate | 11.98 | 0.35 ± 0.01 | - | - | - |
| Ethyl acetate | 12.686 | 0.50 ± 0.06d | 2.21 ± 0.32b | 3.18 ± 0.23a | 1.51 ± 0.17c |
| Ethyl butyrate | 20.951 | - | - | 1.58 ± 0.24 | - |
| Heptyl formate | 21.023 | 0.43 ± 0.54 | - | - | - |
| Hexyl acetate | 21.557 | 0.48 ± 0.01b | - | 0.55 ± 0.25b | 3.36 ± 0.26a |
| Methyl valerate | 22.05 | - | 0.44 ± 0.05 | 0.54 ± 0.12 | - |
| Formic acid acetate | 23.747 | - | 0.83 ± 0.01 | - | - |
| Methyl caproate | 26.022 | - | 0.33 ± 0.16 | - | - |
| Propyl propionate | 26.936 | 0.39 ± 0.18 | - | - | - |
| Ethyl hexanoate | 28.65 | 0.40 ± 0.02b | 0.46 ± 0.04b | 0.60 ± 0.05a | - |
| Linalyl acetate | 32.428 | 3.83 ± 0.52b | 0.28 ± 0.05c | 5.26 ± 0.19a | 5.33 ± 0.85a |
| Ethyl sorbate | 32.975 | - | 0.38 ± 0.05 | - | - |
| Methyl octanoate | 33.034 | - | 0.47 ± 0.20 | - | - |
| Aliphatic hydrocarbons | |||||
| N-pentane | 6.622 | 7.38 ± 0.60 | - | - | - |
| Hexane | 10.203 | 4.58 ± 0.74 | 4.39 ± 0.78 | 5.70 ± 0.60 | - |
| Heptane | 14.786 | - | 4.69 ± 0.57a | 1.86 ± 0.26b | - |
| 4-Methylene-1-(1-methylethyl)-bicyclo[3.1.0]hexane | 17.961 | 0.41 ± 0.05a | - | - | - |
| N-octane | 19.301 | 6.29 ± 0.12b | 8.59 ± 0.22a | 5.36 ± 0.26c | - |
| 2-Methyl-5-(1-methylethyl)-bicyclo[3.1.0]hex-2-ene | 25.651 | 1.68 ± 0.05a | 1.10 ± 0.15b | 1.17 ± 0.30a,b | - |
| 1-Methyl-4-(1-methylethyl)-1,3-cyclohexadiene | 29.578 | 0.94 ± 0.17a | 0.42 ± 0.33b | 0.90 ± 0.08a | - |
| 1,1-Dimethylcyclopentane | 31.357 | - | - | 0.28 ± 0.02 | - |
| 2-Methylpropane | 42.540 | - | - | - | 3.26 ± 0.16 |
| 1,3,3-Trimethyl-tricyclo[2.2.1.0(2,6)]heptane | 44.723 | - | - | - | 2.04 ± 0.49 |
| 2-Methylene 4,8,8 trimethyl-4-vinyl-bicyclo[5.2.0]decane | 44.95 | 1.24 ± 0.23b | 1.74 ± 0.46a | 1.29 ± 0.41a,b | - |
| Aromatic compounds | |||||
| Toluene | 20.046 | 2.41 ± 0.45a | 1.29 ± 0.75a,b | 1.07 ± 0.27b | - |
| Ethylbenzene | 24.176 | 0.83 ± 0.06 | 0.83 ± 0.17 | 0.79 ± 0.09 | - |
| 1,3-Xylene | 24.389 | - | 0.79 ± 0.15 | - | - |
| Paraxylene | 24.398 | 2.20 ± 0.26 | - | 2.01 ± 0.03 | - |
| Meta-xylene | 25.700 | - | - | 0.78 ± 0.15a | - |
| Styrene | 25.815 | - | - | - | 0.41 ± 0.20 |
| 4-Ethylbenzoic acid cyclobutyl ester | 26.936 | - | - | - | 0.65 ± 0.27 |
| Fennel brain | 28.388 | - | 0.36 ± 0.08b | 3.00 ± 0.75a | - |
| Benzaldehyde | 29.646 | - | - | 6.15 ± 0.50 | - |
| 1-Isopropyl-2-methylbenzene | 30.302 | - | - | 1.19 ± 0.84 | - |
| P-umbrella (p-isopropyl toluene) | 30.315 | 4.24 ± 0.50 | - | - | - |
| O-isopropylbenzene | 30.361 | - | 4.19 ± 0.84 | - | - |
| 3-Benzyloxy-2-fluoro-4-methoxy-benzaldehyde | 32.726 | - | - | 0.30 ± 0.07 | - |
| Phenylacetaldehyde | 32.795 | 0.24 ± 0.35 | - | - | - |
| Terpenes | |||||
| 1-Methyl-4-(1-methylvinyl)-1,3-cyclohexadiene | 25.642 | - | - | 2.49 ± 0.89 | - |
| (1S)-(1)-β-pinene | 28.026 | 2.03 ± 0.23c | 2.91 ± 0.37b | 2.08 ± 0.03c | 3.92 ± 0.36a |
| Celery | 29.162 | - | 0.62 ± 0.08b | 1.36 ± 0.37a | 0.47 ± 0.03c |
| 2-Methyl-5-(1-methylethyl)-1,3-cyclohexadiene | 29.271 | 0.47 ± 0.03b | 0.36 ± 0.01c | 0.62 ± 0.08a | - |
| 3-Decene | 29.356 | 1.90 ± 0.19b | 3.67 ± 0.75a | 3.39 ± 0.47a | 2.72 ± 0.54a |
| Terpene | 29.840 | - | 1.66 ± 0.43a | - | 0.62 ± 0.06b |
| D-limonene | 30.035 | 15.22 ± 0.15a | 11.54 ± 0.36b | 6.98 ± 0.97c | 14.39 ± 1.24a |
| D-decadiene | 30.099 | 0.73 ± 0.87a | - | 0.32 ± 0.41b | 0.37 ± 0.55b |
| Terpinene | 30.311 | 1.13 ± 0.11c | 1.96 ± 0.34b | 1.24 ± 0.55b,c | 5.72 ± 0.50a |
| 3-ylidene | 37.241 | - | 1.17 ± 0.30 | - | - |
| (1S)-(+)- 3-decene | 37.246 | - | - | 1.00 ± 0.33 | - |
| Nitrogen-containing sulfur compounds | |||||
| 3-Methylthiophene | 20.652 | - | - | 0.44 ± 0.12 | - |
| 2,5-Dimethylthio | 26.416 | - | - | 0.20 ± 0.03 | - |
| 2,3-Dimethylthio | 26.429 | - | 0.25 ± 0.01 | - | - |
| 4-Ethyl-2-methyl-1H-pyrrole | 33.22 | - | - | 0.34 ± 0.14 | - |
| Furan | |||||
| 2-Pentylfuran | 28.515 | - | - | 0.22 ± 0.09 | - |
| Others | |||||
| (1-Methylbutyl)-ethylene oxide | 14.844 | - | 0.51 ± 0.05 | - | - |
| 1,2-Epoxy-5-methylhexane | 14.848 | 3.51 ± 0.02 | - | - | |
| 2,3-Epoxy-4,4-dimethylpentane | 17.97 | 1.10 ± 0.13 | 1.49 ± 0.29 | - | - |
| 2-(1-Methylbutyl)-ethylene oxide | 23.747 | - | - | 0.51 ± 0.05 | - |
a–dDifferent superscript letters within the same row mean significant differences among different treatments (P < 0.05).
RT: retention times of the detected volatile compounds.
LP: samples inoculated with L. plantarum; SC: samples inoculated with S. cerevisiae; MF: samples inoculated with L. plantarum and S. cerevisiae; CK: control group without inoculation.
Alcohols, especially aliphatic alcohols, which have a significant effect on meat volatile flavor, are derived from reduction of aldehydes, produced by the oxidation of lipid and amino acids (Insausti et al., 2010). This might explain the higher scores of aroma of roast meat for the SC and MF groups. The content of 1-octene-3-ol in the 4 groups was relatively high. 1-Octene-3-ol is generally described as an important component of meat volatiles and is a common oxidation product. It is considered to be the autoxidation product of linoleic acid or other PUFA and has the flavor characteristics of meat fats (Estévez et al., 2003). The autoxidation product, in fact, is found in soy oil, dairy products, coffee, cocoa, tea, and berries (Noyori et al., 2003).
The CK group had more types of aldehydes than other groups, and the aldehydes have been reported as a particularly important component for imparting fat odor and are secondary products in the lipid oxidation process (Ruths et al., 1998). Aldehydes have a grease odor and low thresholds (Fan et al., 2015). Among aldehydes, hexanal is the most significant volatile compound in cooked meat, and its content is directly related to the TBARS value and negatively correlated to flavor acceptability (Shahidi and Pegg, 1994; Martin et al., 2008). This might explain the heavy grease odor of the CK group in odor sensory evaluation. Furans have a heavy meat aroma and baked sweet aroma with low threshold (Shahidi and Pegg, 1994). Rather than the other groups, the MF group contained furan substances, which could explain its heavy meat aroma. The volatile flavor substances in the inoculated groups were more than those in the CK group, indicating that the fermentation process could significantly contribute to the aroma flavor of meat products.
Taste Differences Induced by Microbial Fermentation
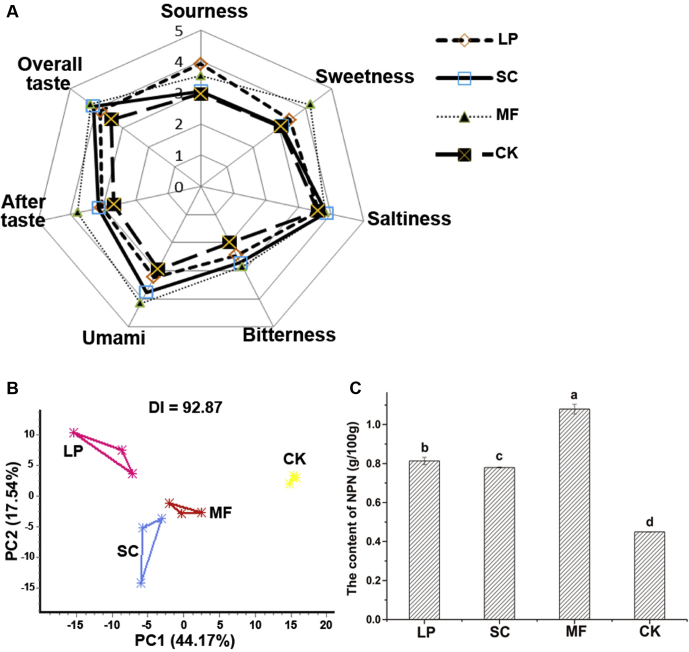
As shown in Figure 2A, the sour taste score of the LP group was the highest, and the sweetness of the MF group was the most significant. The overall scores of the inoculated groups were significantly higher than those of the CK group (P < 0.05). However, there was no significant difference between the inoculated groups (P > 0.05), indicating that their tastes were similar.
Figure 2.
Comparison of the taste profile (A), electronic tongue PCA (B), and NPN contents (C) of cured duck legs subjected to various fermentations. Different letters stand for significant differences at P < 0.05. Abbreviations: PCA, principal component analysis; NPN, nonprotein nitrogen; LP, samples inoculated with L. plantarum; SC, samples inoculated with S. cerevisiae; MF, samples inoculated with L. plantarum and S. cerevisiae; CK, control group without inoculation.
The electronic tongue was used to discriminate the tastes of duck legs subjected to different treatments. As shown in Figure 2B, the samples had a principal component discrimination index value of 92.87 (the larger the discrimination index value, the greater the difference between them), and the tastes of all 4 groups were well distinguishable. It also showed that the three fermentation groups were closer to each other rather than the CK group. This indicated that the fermentation added specific taste flavor to cured duck legs, which was consistent with the results of taste sensory evaluation.
Effects of Fermentation on Protein Degradation in Duck Meat
As a product of protein degradation, NPN is usually used as an important indicator for characterizing protein degradation. The hydrolysis of protein can produce small molecular components, for example, peptides, free amino acid, and amines, which would lead to the generation of NPN compounds through further enzymatic and chemical reactions (Hierro et al., 1999). Protein degradation has an important impact on the flavor of meat products (Wang et al., 2014).
As shown in Figure 2C, the content of NPN in the MF group was the highest (P < 0.05), indicating that the content of peptides and amino acids in the mixed-fermented duck leg meat was the highest. There was no significant difference in NPN contents between the SC and LP groups (P > 0.05). The CK group had the lowest content (P < 0.05). This indicated that protein degradation of duck meat could be promoted by microbial fermentations.
Microbial Fermentations Promote the Release of Free Amino Acids
Free amino acids are the end products of protein degradation (Je et al., 2005), which contribute to the favorite taste of meat products (Lorenzo and Franco, 2012). The contents of free amino acids in cured duck legs were determined and were compared with thresholds, which are listed in Tables 3 and 4, respectively. The results showed that the compositions of the free amino acids in the 4 treatment groups did not change, and the lowest content was detected in the CK group, corresponding to its lowest NPN content. This indicated that microbial fermentations could promote protein degradation in duck meat. The contents of free amino acids in both the SC and MF groups were significantly higher than those in the LP group (P < 0.05). However, no significant difference was observed between the SC and MF groups (P > 0.05). This implied the more remarkable effects of S. cerevisiae on protein degradation, rather than L. plantarum. Two taste-active (>threshold) amino acids were detected in cured duck legs, glutamate (umami) and alanine (sweet). The data showed that the glutamate contents in the MF and SC groups were significantly higher than those in the LP and CK groups (P < 0.05), and the alanine contents in the LP and MF groups were significantly higher than those in the CK group (P < 0.05), but not than those in the SC group (P > 0.05). This indicated that S. cerevisiae had the capacity of releasing glutamate to promote the umami taste of cured duck legs, whereas L. plantarum tended to improve the sweet taste better than S. cerevisiae by generating alanine, although the difference was not significant. These data corresponded to the taste sensory evaluation results. Overall, microbial fermentations promoted the taste flavor of cured duck legs. However, considering all the results obtained in this study, the best ratio of the two microbial species used in cured duck legs still needs to be further investigated.
Table 3.
The contents of free amino acids in fermented duck legs.
| Free amino acids | Contents (mg/100 g) |
|||
|---|---|---|---|---|
| LP1 | SC1 | MF1 | CK1 | |
| Glutamate | 260.67 ± 15.69b | 360.33 ± 10.12a | 380.43 ± 9.13a | 220.12 ± 9.96b |
| Alanine | 67.83 ± 9.83a | 56.00 ± 4.69a,b | 68.07 ± 6.54a | 50.27 ± 1.50b |
| Valine | 22.47 ± 3.28 | 24.30 ± 3.44 | 28.83 ± 2.72 | 20.67 ± 3.69 |
| Lysine | 37.07 ± 2.40a,b | 35.90 ± 2.75a,b | 40.07 ± 1.89a | 30.38 ± 1.83b |
| Histidine | 13.20 ± 0.87b | 11.23 ± 1.00b | 11.51 ± 0.51b | 34.67 ± 0.28a |
| Aspartate | 13.07 ± 1.50b | 14.57 ± 0.96b | 17.46 ± 0.18a | 8.82 ± 0.33b |
| Threonine | 38.73 ± 3.84a | 40.30 ± 2.86a | 37.51 ± 0.61a | 8.40 ± 0.09b |
| Serine | 24.33 ± 3.49a | 26.87 ± 1.39a | 24.47 ± 1.04a | 14.36 ± 0.14b |
| Glycine | 18.57 ± 2.80a,b | 15.53 ± 0.70b | 20.86 ± 2.68a | 17.30 ± 0.53a |
| Methionine | 7.60 ± 0.38a | 7.90 ± 1.31a | 8.19 ± 1.59a | 5.90 ± 0.87b |
| Isoleucine | 13.30 ± 2.63b,c | 15.60 ± 1.78a,b | 17.74 ± 1.36a | 11.07 ± 1.40c |
| Leucine | 27.07 ± 3.28b,c | 33.90 ± 4.31a,b | 35.33 ± 1.79a | 22.33 ± 2.47c |
| Tyrosine | 13.23 ± 1.50 | 12.19 ± 0.79 | 12.76 ± 1.35 | 15.73 ± 0.84 |
| Phenylalanine | 29.13 ± 1.42a | 31.00 ± 2.27a | 33.56 ± 2.77a | 22.52 ± 1.80b |
| Arginine | 16.09 ± 0.31b | 17.07 ± 2.28b | 20.30 ± 1.63b | 32.31 ± 0.42a |
| Proline | 11.10 ± 1.87b | 9.43 ± 1.33b | 12.70 ± 0.60a | 4.42 ± 0.33c |
| Tryptophan | 0.94 ± 0.02b | 0.98 ± 0.10b | 2.82 ± 0.33a | 0.80 ± 0.09b |
| Total | 614.4 ± 50.01b | 713.03 ± 29.59a | 772.31 ± 30.13a | 515.07 ± 10.87c |
a–cDifferent letters within same row mean significant differences at P < 0.05.
LP: samples inoculated with L. plantarum; SC: samples inoculated with S. cerevisiae; MF: samples inoculated with L. plantarum and S. cerevisiae; CK: control group without inoculation.
Table 4.
Taste activity values (TAV) of free amino acids in cured duck legs.
| Amino acids | Taste attributes | Taste threshold1 (mg/g) | TAV2 |
|||
|---|---|---|---|---|---|---|
| LP3 | SC3 | MF3 | CK3 | |||
| Glutamate | Umami | 0.3 | 8.69 | 12.01 | 12.68 | 7.34 |
| Alanine | Sweet | 0.6 | 1.13 | 0.93 | 1.13 | 0.84 |
| Valine | Sweet | 0.4 | 0.56 | 0.61 | 0.72 | 0.52 |
| Lysine | Sweet | 0.5 | 0.74 | 0.72 | 0.80 | 0.61 |
| Histidine | Bitter | 0.2 | 0.66 | 0.56 | 0.58 | 1.73 |
| Aspartate | Umami | 1 | 0.13 | 0.15 | 0.17 | 0.09 |
| Threonine | Sweet | 2.6 | 0.15 | 0.16 | 0.14 | 0.03 |
| Serine | Sweet | 1.5 | 0.16 | 0.18 | 0.16 | 0.10 |
| Glycine | Sweet | 1.3 | 0.14 | 0.12 | 0.16 | 0.13 |
| Methionine | Bitter | 0.3 | 0.25 | 0.26 | 0.27 | 0.20 |
| Isoleucine | Bitter | 0.9 | 0.15 | 0.17 | 0.20 | 0.12 |
| Leucine | Bitter | 1.9 | 0.14 | 0.18 | 0.19 | 0.12 |
| Tyrosine | Bitter | 0.906 | 0.15 | 0.13 | 0.14 | 0.17 |
| Phenylalanine | Bitter | 0.9 | 0.32 | 0.34 | 0.37 | 0.25 |
| Arginine | Bitter | 0.5 | 0.32 | 0.34 | 0.41 | 0.65 |
| Proline | Sweet | 3 | 0.04 | 0.03 | 0.04 | 0.01 |
| Tryptophan | Bitter | NF4 | - | - | - | - |
Recognition threshold in water (Chen and Zhang, 2007).
TAV were calculated by dividing the concentrations of free amino acids by their taste thresholds in water. The compounds whose TAV was higher than 1 were considered as active in meat taste.
LP: samples inoculated with L. plantarum; SC: samples inoculated with S. cerevisiae; MF: samples inoculated with L. plantarum and S. cerevisiae; CK: control group without inoculation.
NF: not found in the literature.
Conclusions
The application of microbial fermentations can improve the flavor of cured duck legs, and the specific contributions of the two used microbial species were characterized in this study. L. plantarum has the capacity of alleviating lipid oxidation in cured duck leg meat, whereas S. cerevisiae tends to improve the meat taste better. Furthermore, mixed fermentations with the species promote both odor and taste flavors. These results provide new directions for processing characteristic meat products and also indicate the flavor diversifications of aroma and taste with various microbial fermentations.
Acknowledgments
This work was supported by the Science and Technology Programs of Zhejiang (2019C02085) and Ningbo (2019C10017), the Modern Agricultural Technical Foundation of China (CARS-42-25), and the Kuancheng Wong Magna Fund at Ningbo University.
Conflict of Interest Statement: The authors declare that they have no conflicts of interest.
References
- Adzitey F. Production potentials and the physicochemical composition of selected duck strains: a mini review. Online J. Anim. Feed Res. 2012;2:89–94. [Google Scholar]
- Axling U., Olsson C., Xu J., Fernandez C., Larsson S., Ström K., Ahrné S., Holm C., Molin G., Berger K. Green tea powder and Lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice. Nutr. Metab. 2012;9:105. doi: 10.1186/1743-7075-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudzewski K., Osowski S., Ulaczyk J. Differential electronic nose of two chemo sensor arrays for odor discrimination. Sensor. Actuat. B-chem. 2010;145:246–249. [Google Scholar]
- Campagnol P.C.B., dos Santos B.A., Wagner R., Terra N.N., Mar P. The effect of yeast extract addition on quality of fermented sausages at low NaCl content. Meat Sci. 2011;87:290–298. doi: 10.1016/j.meatsci.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Chaveslópez C., Paparella A., Tofalo R., Suzzi G. Proteolytic activity of Saccharomyces cerevisiae strains associated with Italian dry-fermented sausages in a model system. Int. J. Food Microbiol. 2011;150:50–58. doi: 10.1016/j.ijfoodmicro.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Chen D.W., Zhang M. Non-volatile taste active compounds in the meat of Chinese mitten crab (Eriocheir sinensis) Food Chem. 2007;104:1200–1205. [Google Scholar]
- Demeyer D., Hoozee J., Mesdom H. Specificity of lipolysis during dry sausage ripening. J. Food Sci. 2010;39:293–296. [Google Scholar]
- Estévez M., Morcuende D., Ventanas S., Cava R. Analysis of volatiles in meat from Iberian pigs and lean pigs after refrigeration and cooking by using SPME-GC-MS. J. Agr. Food Chem. 2003;51:3429–3435. doi: 10.1021/jf026218h. [DOI] [PubMed] [Google Scholar]
- Fadda S., Vildoza M.J., Vignolo G. The acidogenic metabolism of Lactobacillus plantarum CRL 681 improves sarcoplasmic protein hydrolysis during meat fermentation. J. Muscle Foods. 2010;21:12. [Google Scholar]
- Fan W., Yi Y., Zhang Y., Pan D. Effect of an antioxidant from bamboo leaves combined with tea polyphenol on biogenic amine accumulation and lipid oxidation in pork sausages. Food Sci. Biotechnol. 2015;24:421–426. [Google Scholar]
- Gao P., Li Z., Zan L., Yue T., Bo S. A non-protein nitrogen index for discriminating raw milk protein adulteration via the Kjeldahl method. Anal. Methods. 2015;7:9166–9170. [Google Scholar]
- Hierro E., Hoz L.D.L., Ordóñez J.A. Contribution of microbial and meat Endogenous Enzymes to the lipolysis of dry fermented sausages. J.Agr. Food Chem. 1999;45:2989–2995. doi: 10.1021/jf980834p. [DOI] [PubMed] [Google Scholar]
- Huang A., Sirisansaneeyakul S., Chisti Y. Yunnan fermented meat: Xuanwei ham, Huotui. Tradit. Foods. 2016;10:235–250. [Google Scholar]
- Insausti K., Beriain M.J., Gorraiz C., Purroy A. Volatile compounds of raw beef from 5 local Spanish cattle breeds stored under Modified atmosphere. J. Food Sci. 2010;67:1580–1589. [Google Scholar]
- Je J.Y., Park P.J., Jung W.K., Kim S.K. Amino acid changes in fermented oyster (Crassostrea gigas) sauce with different fermentation periods. Food Chem. 2005;91:15–18. [Google Scholar]
- Kong Z., Li M., An J., Chen J., Bao Y., Frédéric F., Dai X. The fungicide triadimefon affects beer flavor and composition by influencingSaccharomyces cerevisiaemetabolism. Sci. Rep. 2016;6:33552. doi: 10.1038/srep33552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy F., Vuyst L.D. Lactic acid bacteria as functional starter cultures for the food fermentation industry. China Dairy Ind. 2004;15:67–78. [Google Scholar]
- Loh T.C., Di W.C., Foo H.L., Sazili A.Q., Bejo M.H. Effects of feeding different postbiotic metabolite combinations produced by Lactobacillus plantarum strains on egg quality and production performance, faecal parameters and plasma cholesterol in laying hens. BMC Vet. Res. 2014;10:149. doi: 10.1186/1746-6148-10-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo J.M., Franco D. Fat effect on physico-chemical, microbial and textural changes through the manufactured of dry-cured foal sausage Lipolysis, proteolysis and sensory properties. Meat Sci. 2012;92:704–714. doi: 10.1016/j.meatsci.2012.06.026. [DOI] [PubMed] [Google Scholar]
- Lorenzo J.M., Lorenzo J.M. Changes on physico-chemical, textural, lipolysis and volatile compounds during the manufacture of dry-cured foal “cecina”. Meat Sci. 2014;96:256–263. doi: 10.1016/j.meatsci.2013.06.026. [DOI] [PubMed] [Google Scholar]
- Martin D., Antequera T., Muriel E., Perez-Palacios T., Ruiz J. Effect of dietary conjugated linoleic acid in combination with monounsaturated fatty acids on the meat composition and quality traits of dry-cured loin. Meat Sci. 2008;80:1309–1319. doi: 10.1016/j.meatsci.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Nie X., Zhang Q., Lin S. Biogenic amine accumulation in silver carp sausage inoculated with Lactobacillus plantarum plus Saccharomyces cerevisiae. Food Chem. 2014;153:432–436. doi: 10.1016/j.foodchem.2013.12.093. [DOI] [PubMed] [Google Scholar]
- Noyori R., Aoki M., Sato K. Green oxidation with Aqueous Hydrogen Peroxide. Chem. Commun. 2003;34:1977–1986. doi: 10.1039/b303160h. [DOI] [PubMed] [Google Scholar]
- Ockerman H.W., Basu L. Blackwell Publishing; 2010. Production and Consumption of Fermented Meat Products. (Chapter 2) [Google Scholar]
- Papavergou E.J., Savvaidis I.N., Ambrosiadis I.A. Levels of biogenic amines in retail market fermented meat products. Food Chem. 2012;135:2750–2755. doi: 10.1016/j.foodchem.2012.07.049. [DOI] [PubMed] [Google Scholar]
- Ruths S.M., Cheraghi T., Roozen J.P. Blackie Academic & Professional; New York: 1998. Lipid-derived Off-Flavours in Meat By-Products: Effect of Antioxidants and Maillard Reactants. (Chapter in scientific book) [Google Scholar]
- Saerens S.M.G., Delvaux F., Verstrepen K.J., Dijck P.V., Thevelein J.M., Delvaux F.R. Parameters affecting Ethyl ester production by Saccharomyces cerevisiae during fermentation. Appl. Environ. Microbiol. 2008;74:454–461. doi: 10.1128/AEM.01616-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi F., Pegg R.B. Hexanal as an indicator of the flavor deterioration of meat and meat products. ACS Symp. Ser. 1994;558:256–279. [Google Scholar]
- Song S., Yuan L., Zhang X., Hayat K., Chen H., Liu F., Xiao Z., Niu Y. Rapid measuring and modelling flavour quality changes of oxidised chicken fat by electronic nose profiles through the partial least squares regression analysis. Food Chem. 2013;141:4278–4288. doi: 10.1016/j.foodchem.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Spinosa W.A., Vitório D.S.J., Galvan D., Fiorio J.L., Gomez R.J.H.C. Fermentation Kinetics of Rice Syrup, with high content of Dextrose Equivalent, by Saccharomyces cerevisiae and Characterization of volatile compounds from wine. J. Food Process. Pres. 2016;40:1199–1205. [Google Scholar]
- Stetzer A.J., Cadwallader K., Singh T.K., Mckeith F.K., Brewer M.S. Effect of enhancement and ageing on flavor and volatile compounds in various beef muscles. Meat Sci. 2008;79:13–19. doi: 10.1016/j.meatsci.2007.07.025. [DOI] [PubMed] [Google Scholar]
- Toldrá F., Flores M., Sanz Y. Dry-cured ham flavour: enzymatic generation and process influence. Food Chem. 1997;59:523–530. [Google Scholar]
- Valerio F., Conte A., Di B.M., Lattanzio V.M., Lonigro S.L., Padalino L., Pontonio E., Lavermicocca P. Formulation of yeast-leavened bread with reduced salt content by using a Lactobacillus plantarum fermentation product. Food Chem. 2017;221:582–589. doi: 10.1016/j.foodchem.2016.11.135. [DOI] [PubMed] [Google Scholar]
- Vigor C., Bertrand-Michel J., Pinot E., Oger C., Vercauteren J., Faouder P.L., Galano J.M., Lee C.Y., Durand T. Non-enzymatic lipid oxidation products in biological systems: Assessment of the metabolites from polyunsaturated fatty acids. J. Chromatogr. B. 2014;964:65–78. doi: 10.1016/j.jchromb.2014.04.042. [DOI] [PubMed] [Google Scholar]
- Vries M.C.D., Vaughan E.E., Kleerebezem M., Vos W.M.D. Lactobacillus plantarum-survival, functional and potential probiotic properties in the human intestinal tract. Int. Dairy J. 2006;16:1018–1028. [Google Scholar]
- Wang D.Y., Zhang M.H., Bian H., Dong H., Xu W.M., Xu X.L., Zhu Y.Z., Liu F., Geng Z.M., Zhou G.H. Proteolysis and cathepsin activities in the processing of dry-cured duck. Poult. Sci. 2014;93:687–694. doi: 10.3382/ps.2013-03335. [DOI] [PubMed] [Google Scholar]
- Witte V.C., Krause G.F., Bailey M.E. A new extraction method for determining 2-thiobarbituric acid values of pork and beef during storage. J. Food Sci. 2010;35:582–585. [Google Scholar]
- Xu W., Xu X., Zhou G., Wang D., Li C. Changes of intramuscular phospholipids and free fatty acids during the processing of Nanjing dry-cured duck. Food Chem. 2008;110:279–284. doi: 10.1016/j.foodchem.2007.11.044. [DOI] [PubMed] [Google Scholar]
- Yang Y., Pan D., Sun Y., Wang Y., Xu F., Cao J. 1H NMR-based metabolomics profiling and taste of stewed pork-hock in soy sauce. Food Res. Int. 2018;121 doi: 10.1016/j.foodres.2018.12.035. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wang Z.Y., Yang X., Zhao H.T., Zhang Y.C., Dong A.J., Jing J., Wang J. Determination of free amino acids and 18 elements in freeze-dried strawberry and blueberry fruit using an Amino Acid Analyzer and ICP-MS with micro-wave digestion. Food Chem. 2014;147:189–194. doi: 10.1016/j.foodchem.2013.09.118. [DOI] [PubMed] [Google Scholar]
- Zhou J., Han Y., Zhuang H., Tao F., Xu B. Influence of the type of extraction Conditions and Fiber Coating on the meat of sauced duck Neck volatile compounds extracted by Solid-phase Microextraction (SPME) Food Anal. Method. 2015;8:1661–1672. [Google Scholar]