Abstract
This experiment was conducted to evaluate the effects of wheat bran (WB) and antibiotics on growth performance, intestinal immunity, barrier function, and microbial composition in broiler chickens. A total of 168 one-day-old male Arbor Acre chicks were allocated to 3 treatments consisting of 7 replicates with 8 birds per replicate. The 3 treatments were: an antibiotic-free control diet (control, CON), CON + 75 mg/kg chlortetracycline as an antibiotic growth promoter (AGP), and CON + 3% WB. Birds fed AGP and WB had greater (P < 0.05) ADG during days 1 to 21 and lower (P < 0.05) feed-to-gain ratio during each phase than those fed CON. The WB supplementation reduced (P < 0.05) serum concentrations of tumor necrosis factor-α and diamine oxidase activity compared with CON on both day 21 and 42. The AGP and WB supplementation decreased (P < 0.05) interleukin-1β concentration in jejunal mucosa on day 21 and increased (P < 0.05) secretory immunoglobulin A concentration in jejunal mucosa on day 21 and 42. The relative expression of occludin in jejunal mucosa was upregulated (P < 0.05) in WB than in CON on day 21. Moreover, both AGP and WB supplementation upregulated (P < 0.05) the relative expression of zonula occludens-1 in jejunal mucosa on day 21 and 42. The WB supplementation enhanced the α-diversity of cecal microbiota, as evidenced by the increased Shannon index (P < 0.05). At the phylum level, the phylum Firmicutes was enriched (P < 0.05) in WB. At the genus level, the WB supplementation enriched (P < 0.05) Lachnoclostridium and Butyricicoccus. The WB supplementation increased (P < 0.05) cecal total short chain fatty acids concentrations on day 21 and 42, and butyric acid concentrations on day 42 compared with CON. Collectively, supplementation of 3% WB could promote growth by improving intestinal immunity, barrier function, and microbial composition in broilers. Thus, WB may have a role in replacing antibiotics for improved growth performance and intestinal health in broilers.
Key words: barrier function, broiler, immunity, microbial composition, performance
Introduction
Antibiotic growth promoters (AGP) have been used in poultry production for decades to maintain intestinal health, promote growth performance, and prevent diseases (Dibner and Richards, 2005). However, with the long-term abuse of AGP, public concerns have increased regarding antibiotic drug residues and resistant bacteria, which pose a potential risk to human health (Yang et al., 2019). Consequently, many countries, especially in Europe, have banned the use of AGP in poultry diets (Castanon, 2007). In China, in-feed antibiotics as growth promoters will be banned beginning in 2020, which has increased pressure to develop safe and effective strategies that can maintain intestinal health and performance in poultry.
Dietary fiber (DF) consists of carbohydrate polymers that are resistant to digestion by endogenous enzymes in the small intestine with complete or partial fermentation in the large intestine (Rebello et al., 2016). In recent years, DF is drawing increasing attention due to its various beneficial effects especially in maintaining gut health (Gemen et al., 2011). Maintaining gut health is essential to improve growth performance and the overall health of animals, implying that DF may be a potential alternative to antibiotics in poultry diets (Jha et al., 2019). However, results regarding the effects of DF on poultry have been inconsistent, probably due to its different physicochemical properties (He et al., 2015; Donadelli et al., 2019). Soluble dietary fiber (SDF) generally increases digesta viscosity, delays gastric emptying, and thereby reduces nutrient digestibility and feed consumption, eventually resulting in growth inhibition (Mateos et al., 2012). In contrast, insoluble dietary fiber (IDF) at moderate levels (2–3%) has been shown to stimulate gizzard development and digestive enzyme secretions, resulting in better nutrient utilization and growth (Donadelli et al., 2019; Jiménez-Moreno et al., 2019). Therefore, it may be useful to supplement moderate levels of IDF rather than SDF in poultry diets.
Wheat bran (WB) is a source of insoluble fiber, rich in arabinoxylan and cellulose (Onipe et al., 2015). Previous studies with other species have shown that WB had anti-inflammatory effects, and improved intestinal barrier function and microbial composition (Stevenson et al., 2012; Chen et al., 2017; Suriano et al., 2018). There was also evidence showing beneficial effects of WB-derived arabinoxylan on overall poultry health (Courtin et al., 2008; Akhtar et al., 2012). However, little information is available concerning the comparative effects of WB at moderate levels and antibiotics on performance and, in particular, intestinal health in broilers. Therefore, the present study was conducted to evaluate the effects of 3% WB in comparison to antibiotics on growth performance, intestinal immunity, barrier function, and microbial composition in broiler chickens.
Materials and methods
All experimental protocols used in the study were reviewed and approved by the Institutional Animal Care and Use Committee of China Agricultural University (Beijing, China).
Birds and Husbandry
A total of 168 one-day-old male Arbor Acre broiler chickens (weighing 46.31 ± 0.81 g) were obtained from Arbor Acres Poultry Breeding Company (Beijing, China). All birds were raised in wire-floored cages in an environmentally controlled room with continuous light and had free access to feed and water throughout the experiment. The room temperature was maintained at 33°C for the initial 3 D, and gradually decreased to 24°C at a rate of 3°C each week, and then maintained until the end of the experiment. The experiment was conducted in 2 phases: starter phase (1–21 D) and finisher phase (22–42 D). All birds were inoculated with inactivated Newcastle disease vaccine on day 7 and 28 and inactivated infectious bursa disease vaccine on day 14 and 21.
Experimental Design and Diets
Upon arrival, birds were individually wing-tagged, weighed, and randomly allocated to 3 treatments with 7 replicate cages per treatment and 8 birds per cage (0.9 × 0.6 × 0.4 m). The 3 treatments were: an antibiotic-free corn-soybean meal control diet (control, CON), CON + 75 mg/kg chlortetracycline (Charoen Pokphand Group, Bangkok, Thailand) as an AGP, and CON + 3% WB. Analyzed composition of WB (as-fed basis) was 89.37% DM, 5.82% ash, 83.55% organic matter, 17.12% CP, 17.01 MJ/kg gross energy, 37.36% neutral detergent fiber, 11.55% acid detergent fiber, 44.57% total dietary fiber (TDF), 3.89% SDF, and 40.68% IDF. All diets were formulated to meet or exceed the nutrient requirements recommended by the NRC (1994) and fed in mash form (Table 1).
Table 1.
Composition and nutrient levels of the experimental diets (%, as-fed basis).
| Item | Starter phase (days 1–21) |
Finisher phase (days 22–42) |
||||
|---|---|---|---|---|---|---|
| Control | Antibiotic | Wheat bran | Control | Antibiotic | Wheat bran | |
| Corn | 58.17 | 58.17 | 53.97 | 64.26 | 64.26 | 24.05 |
| Soybean meal | 30.44 | 30.44 | 30.44 | 24.05 | 24.05 | 60.06 |
| Corn gluten meal | 2.00 | 2.00 | 2.00 | 2.50 | 2.50 | 2.50 |
| Fish meal | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Wheat bran | – | – | 3.00 | – | – | 3.00 |
| Soybean oil | 3.38 | 3.38 | 4.58 | 3.60 | 3.60 | 4.80 |
| Dicalcium phosphate | 1.50 | 1.50 | 1.50 | 1.04 | 1.04 | 1.04 |
| Limestone | 1.30 | 1.30 | 1.30 | 1.35 | 1.35 | 1.35 |
| Salt | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| L-Lysine HCl, 78% | 0.01 | 0.01 | 0.01 | 0.08 | 0.08 | 0.08 |
| DL-Methionine, 98% | 0.14 | 0.14 | 0.14 | 0.04 | 0.04 | 0.04 |
| L-Threonine, 98% | 0.01 | 0.01 | 0.01 | 0.03 | 0.03 | 0.03 |
| Chromium oxide | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Vitamin–mineral premix1 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Analyzed nutrient composition | ||||||
| DM | 91.18 | 90.98 | 90.94 | 91.20 | 91.25 | 91.16 |
| Ash | 5.11 | 5.05 | 5.22 | 4.99 | 4.88 | 5.06 |
| CP | 21.08 | 21.04 | 21.00 | 19.00 | 19.03 | 19.08 |
| Gross energy, kcal/kg | 4,186 | 4,178 | 4,212 | 4,202 | 4,215 | 4,253 |
| Calcium | 1.04 | 1.06 | 1.11 | 0.92 | 0.90 | 0.95 |
| Total phosphorus | 0.70 | 0.72 | 0.72 | 0.59 | 0.60 | 0.61 |
| Sodium | 0.19 | 0.20 | 0.18 | 0.15 | 0.16 | 0.16 |
| Total dietary fiber | 13.94 | 14.16 | 14.72 | 13.47 | 13.53 | 14.24 |
| Insoluble dietary fiber | 1.81 | 1.86 | 1.85 | 1.77 | 1.78 | 1.81 |
| Soluble dietary fiber | 12.13 | 12.30 | 12.87 | 11.70 | 11.75 | 12.43 |
| Neutral detergent fiber | 11.38 | 11.50 | 12.03 | 11.02 | 11.22 | 11.68 |
| Acid detergent fiber | 4.96 | 5.04 | 5.16 | 4.56 | 4.69 | 4.76 |
| Chlortetracycline | – | 0.0074 | – | – | 0.0071 | – |
| Lysine, total | 1.20 | 1.18 | 1.17 | 1.10 | 1.08 | 1.11 |
| Methionine, total | 0.55 | 0.53 | 0.56 | 0.42 | 0.44 | 0.40 |
| Threonine, total | 0.82 | 0.84 | 0.86 | 0.76 | 0.77 | 0.79 |
| Tryptophan, total | 0.31 | 0.33 | 0.30 | 0.26 | 0.25 | 0.23 |
| Calculated nutrient composition | ||||||
| ME, kcal/kg | 3,050 | 3,050 | 3,050 | 3,150 | 3,150 | 3,150 |
| Available phosphorus | 0.45 | 0.45 | 0.45 | 0.35 | 0.35 | 0.36 |
Control: antibiotic-free control diet; antibiotic: control diet + 75 mg/kg chlortetracycline; wheat bran: control diet + 3% wheat bran.
Premix supplied per kg diet: vitamin A, 11,000 IU; vitamin D, 3,025 IU; vitamin E, 22 mg; vitamin K3, 2.2 mg; thiamine, 1.65 mg; riboflavin, 6.6 mg; pyridoxine, 3.3 mg; cobalamin, 17.6 μg; nicotinic acid, 22 mg; pantothenic acid, 13.2 mg; folic acid, 0.33 mg; biotin, 88 μg; choline chloride, 500 mg; iron, 48 mg; zinc, 96.6 mg; manganese, 101.76 mg; copper, 10 mg; selenium, 0.05 mg; iodine, 0.96 mg; cobalt, 0.3 mg.
Sample Collection
Individual BW and feed consumption per cage were recorded on day 21 and 42 after 12-hour fast to calculate ADG, ADFI, and feed-to-gain ratio (F:G).
On day 21 and 42, 1 bird per cage with the BW close to the mean BW of the cage (7 birds per treatment) was selected. Blood samples were collected by wing vein puncture, centrifuged at 3,000 × g for 15 min at 4°C, and stored at −80°C until analysis. After blood sample collection, birds were euthanized by electrical stunning and bleeding of the carotid artery. Thereafter, the gastrointestinal tract was carefully excised. The jejunum section (from the pancreatic ducts to Meckel's diverticulum) was gently flushed with 0.9% ice-cold saline, then the mucosa was scraped with a sterile glass slide, snap frozen in liquid nitrogen, and stored at −80°C. Cecal digesta was also collected, snap frozen in liquid nitrogen, and stored at −80°C until further analysis.
Chemical Analysis
Samples of ingredients and diets were ground through a 1-mm screen, and then analyzed for DM (AOAC, 2007; method 930.15), CP (AOAC, 2007; method 976.05), and ash (AOAC, 2007; method 942.15). The neutral detergent fiber and acid detergent fiber were determined using a fiber analyzer (Ankom Technology, Macedon, NY) according to Van Soest et al. (1991) with heat-stable α-amylase and sodium sulfite and expressed inclusive of residual ash. The gross energy was determined using an automatic adiabatic oxygen bomb calorimeter (Parr 6300 Calorimeter; Parr Instrument Company, Moline, IL). The TDF and IDF were analyzed using AOAC (2007) methods 985.29 and 991.43, respectively. The SDF was calculated as the difference between TDF and IDF.
Analysis of Immune Parameters in Serum and Jejunal Mucosa
Diamine oxidase (DAO) activity and endotoxin concentrations in serum were determined in accordance with Zhang et al. (2016). Tumor necrosis factor-α (TNF-α), and interleukin (IL)-1β and IL-6 concentrations in serum and jejunal mucosa were measured by commercial ELISA kits (Beijing Sino-UK Institute of Biological Technology, Beijing, China) using the method of Grilli et al. (2015). Secretory immunoglobulin A (sIgA) concentration in jejunal mucosa was measured using a commercially available chicken ELISA kit (R&D Systems, Minneapolis, MN) according to Levkut et al. (2017). Total protein concentration in jejunal mucosa was measured using the method described by Smith (1985). Values in jejunal mucosa were expressed as units/g protein.
Analysis of Tight Junction Proteins Expression in Jejunal Mucosa
Expression of zonula occludens-1 (ZO-1), claudin-1, and occludin genes in jejunal mucosa was determined by real-time PCR. Total RNA was extracted from jejunal mucosal samples using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The quality and quantity of RNA were determined using a spectrophotometer (NanoDrop ND-1000; Thermo Fisher Scientific, Wilmington, DE). The integrity of RNA was determined by agarose gel electrophoresis. Then the RNA was treated with DNase I (TaKaRa Biotechnology, Dalian, China) and used for reverse transcription and PCR. First-strand cDNA was synthesized using a reverse transcription kit (Invitrogen). Software (Oligo 6.0; Molecular Biology Insights, Cascade, CO) was used to design primers, which are listed in Table 2. Real-time PCR was performed with a volume of 10 μL containing 1 μL cDNA template, 5 μL SYBR Green Mix, 0.2 μL ROX Reference Dye (50 times), and 0.2 μL each of forward and reverse primers. The thermal cycling conditions were as follows: pre-denaturation (10 s at 95°C); 40 cycles of amplification (5 s at 95°C and 20 s at 60°C); and melting curve construction (60°C–99°C with heating rate of 0.1°C/second and fluorescence measurements). Relative gene expression was expressed as a ratio of the target gene to the control genes using the 2−ΔΔCt method.
Table 2.
Primer sequences for real-time PCR assays.
| Gene | Gene bank ID | Primer sequence (5′-3′) | Length (bp) |
|---|---|---|---|
| Claudin-1 | NM_001013611.2 | F: TGATTGCTTCCAACCAGGCT | 89 |
| R: CACACGGCTCTCCTTGTCTA | |||
| Occludin | NM_205128.1 | F: ATCGCCTCCATCGTCTACATC | 90 |
| R: GCTGCACATGGCCAACAAG | |||
| ZO-1 | XM_413773.4 | F: TGGGCCTCACGGACTAAAAT | 118 |
| R: GTTTGCTCCAACAAGATAGTTTGG | |||
| GAPDH | NM_204305.1 | F: GGAAAGTCATCCCTGAGCTGAAT | 90 |
| R: GGCAGGTCAGGTCAACAACA |
Abbreviation: ZO-1, zonula occludens-1.
DNA Extraction, PCR and 16S rRNA Sequencing
Total genomic DNA was extracted from cecal digesta using the DNA Kit (Omega Bio-tek, Norcross, GA) according to the manufacturer's instructions. The V3–V4 hypervariable regions of the bacterial 16S rRNA gene were amplified using primers F338 (5′-ACTCCTACGGGAGGCAGCAG-3′) and R806 (5′-GGACTACHVGGGTWTCTAAT-3′). The PCR conditions were pre-denaturation at 95°C for 3 min, 27 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, elongation at 72°C for 30 s, and final extension at 72°C for 10 min. Amplicons were extracted from 2% agarose gels, and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA) and quantified using QuantiFluor-ST (Promega Corporation, Madison, WI). Purified amplicons were pooled in equimolar concentrations and paired-end sequenced (2 × 300) on an Illumina MiSeq platform (Illumina, San Diego, CA) according to the standard protocols. Raw FASTQ files were demultiplexed, and quality-filtered using QIIME (version 1.17; GitHub, San Francisco, CA). Operational taxonomic units were clustered with 97% similarity cutoff using UPARSE (Edgar, 2013) and chimeric sequences were identified and removed using UCHIME (Edgar et al., 2011). The taxonomy of each 16S rRNA gene sequence was analyzed by RDP Classifier (http://rdp.cme.msu.edu/) against the Silva (SSU128) 16S rRNA database using a confidence threshold of 80%.
Analysis of Short Chain Fatty Acids (SCFA) in Cecum
Samples of cecal digesta were thawed on ice and mixed thoroughly. Then 1.0 g samples were suspended in 8 mL deionized water, and ultrasonic irradiation for 20 min, and then centrifuged at 12,000 × g for 10 min. The supernatant was diluted 50 times and filtered through a 0.22-mm filter. Extracted sample solution was analyzed by an ICS-3000 high-performance ion chromatography system (Dionex, Sunnyvale, CA). The SCFA concentrations were expressed as mg/g of digesta.
Statistical Analysis
This experiment was performed as a complete randomized design (n = 7). Microbial diversity metrics were determined from normalized operational taxonomic unit reads using R software (version 3.2.2; R Foundation for Statistical Computing, Vienna, Austria). The results of α-diversity were analyzed by Kruskal-Wallis H test. The remaining data were analyzed with cage as an experimental unit using GLM procedures of SAS (version 9.2; SAS Institute Inc., Cary, NC) followed by Tukey's tests. Significant difference was declared at P < 0.05, and tendency was declared at 0.05 ≤ P < 0.10.
Results
Growth Performance
The effects of WB supplementation on growth performance of broilers are presented in Table 3. The ADFI during each phase and overall did not differ among the 3 treatments. However, birds fed WB and AGP had lower (P < 0.05) F:G than those fed CON during each phase. In addition, the ADG was greater (P < 0.05) in WB and AGP than in CON during days 1 to 21, but this significant effect was not observed thereafter. As a consequence of the increase in ADG, birds fed WB were heavier than those fed CON during days 1 to 21. There were no significant effects observed for any of the performance parameters between WB and AGP.
Table 3.
Effects of wheat bran supplementation on growth performance of broilers.
| Item | CON | AGP | WB | SEM | P-value |
|---|---|---|---|---|---|
| Day 1 BW, g | 46.52 | 46.43 | 45.92 | 0.34 | 0.42 |
| Day 21 BW, g | 607.89b | 632.62a,b | 637.36a | 7.53 | 0.03 |
| Day 42 BW, g | 1,936.84 | 2,006.48 | 2,022.20 | 30.58 | 0.14 |
| Days 1–21 | |||||
| ADG, g/day | 26.66b | 28.21a | 28.42a | 0.42 | 0.02 |
| ADFI, g/day | 36.07 | 35.63 | 35.12 | 0.79 | 0.70 |
| F:G | 1.35a | 1.26b | 1.23b | 0.02 | 0.01 |
| Days 22–42 | |||||
| ADG, g/day | 66.36 | 69.22 | 69.86 | 1.50 | 0.24 |
| ADFI, g/day | 107.05 | 104.54 | 106.21 | 2.46 | 0.77 |
| F:G | 1.61a | 1.51b | 1.52b | 0.02 | 0.01 |
| Days 1–42 | |||||
| ADG, g/day | 46.12 | 47.80 | 48.55 | 0.78 | 0.11 |
| ADFI, g/day | 70.69 | 69.25 | 69.80 | 1.41 | 0.77 |
| F:G | 1.53a | 1.45b | 1.44b | 0.02 | 0.01 |
a,bMeans in the same row with different superscripts differ significantly (P < 0.05).
Abbreviations: AGP, CON + 75 mg/kg chlortetracycline (antibiotic growth promoter); CON, antibiotic-free control diet: F:G, feed-to-gain ratio; WB, CON + 3% wheat bran.
Values are means, n = 7 per treatment.
Serum Immune Parameters
The effects of WB supplementation on serum immune parameters of broilers are shown in Table 4. On day 21, serum concentrations of TNF-α, DAO, and endotoxin were reduced (P < 0.05) by AGP and WB supplementation when compared with CON. Birds fed WB also tended to have lower (P = 0.06) serum concentrations of IL-1β than those fed CON. On day 42, the serum concentrations of IL-1β, IL-6, and endotoxin did not differ among the 3 treatments. However, WB supplementation decreased (P < 0.05) serum concentrations of TNF-α compared with CON. In addition, birds fed both WB and AGP had decreased (P < 0.05) serum concentrations of DAO than those fed CON. All the above indexes did not differ between AGP and WB on either day 21 or 42.
Table 4.
Effects of wheat bran supplementation on serum immune parameters of broilers.
| Item | CON | AGP | WB | SEM | P-value |
|---|---|---|---|---|---|
| Day 21 | |||||
| TNF-α, pg/mL | 57.46a | 40.53b | 45.17b | 3.01 | 0.01 |
| IL-1β, pg/mL | 26.23 | 24.04 | 22.29 | 1.07 | 0.06 |
| IL-6, pg/mL | 127.74 | 124.25 | 122.34 | 2.16 | 0.23 |
| DAO, U/L | 23.35a | 19.12b | 18.93b | 0.99 | 0.01 |
| Endotoxin, EU/mL | 1.38a | 0.98b | 0.96b | 0.10 | 0.01 |
| Day 42 | |||||
| TNF-α, pg/mL | 47.47a | 43.11a,b | 40.08b | 1.59 | 0.02 |
| IL-1β, pg/mL | 26.43 | 25.11 | 22.20 | 1.59 | 0.34 |
| IL-6, pg/mL | 136.32 | 129.34 | 127.36 | 2.93 | 0.11 |
| DAO, U/L | 21.66a | 18.48b | 18.39b | 0.81 | 0.02 |
| Endotoxin, EU/mL | 0.97 | 0.95 | 0.92 | 0.03 | 0.55 |
a,bMeans in the same row with different superscripts differ significantly (P < 0.05).
Abbreviations: AGP, CON + 75 mg/kg chlortetracycline (antibiotic growth promoter); CON, antibiotic-free control diet; DAO, diamine oxidase; IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; WB, CON + 3% wheat bran.
Values are means, n = 7 per treatment.
Inflammatory Cytokines and sIgA in Jejunal Mucosa
The effects of WB supplementation on inflammatory cytokines and sIgA concentrations in jejunal mucosa are presented in Table 5. On day 21, birds fed AGP and WB had lower (P < 0.05) IL-1β concentration and greater (P < 0.05) sIgA concentration in the jejunal mucosa than those fed CON. The TNF-α concentration in jejunal mucosa was reduced (P < 0.05) in WB than in CON, but was similar to AGP. There was no difference in the concentration of IL-6 among treatments. On day 42, the TNF-α concentration in jejunal mucosa was decreased (P < 0.05) in WB compared with CON, but not different from AGP. Both AGP and WB supplementation increased (P < 0.05) the concentration of sIgA in jejunal mucosa compared with CON. The concentrations of IL-1β and IL-6 in jejunal mucosa did not vary significantly among the 3 treatments.
Table 5.
Effects of wheat bran supplementation on the concentrations of inflammatory cytokines and sIgA in jejunal mucosa of broilers.
| Item | CON | AGP | WB | SEM | P-value |
|---|---|---|---|---|---|
| Day 21 | |||||
| TNF-α, pg/mg | 6.67a | 6.25a,b | 5.96b | 0.18 | 0.04 |
| IL-1β, pg/mg | 2.90a | 2.43b | 2.28b | 0.13 | 0.01 |
| IL-6, pg/mg | 126.08 | 121.08 | 118.57 | 2.47 | 0.13 |
| sIgA, μg/mg | 1.24b | 1.55a | 1.63a | 0.08 | <0.01 |
| Day 42 | |||||
| TNF-α, pg/mg | 7.16a | 6.86a,b | 6.49b | 0.15 | 0.02 |
| IL-1β, pg/mg | 2.41 | 2.47 | 2.30 | 0.12 | 0.61 |
| IL-6, pg/mg | 129.61 | 122.19 | 123.30 | 2.85 | 0.17 |
| sIgA, μg/mg | 1.23b | 1.50a | 1.61a | 0.07 | 0.01 |
a,bMeans in the same row with different superscripts differ significantly (P < 0.05).
Abbreviations: AGP, CON + 75 mg/kg chlortetracycline (antibiotic growth promoter); CON, antibiotic-free control diet; IL-1β, interleukin-1β; IL-6, interleukin-6; sIgA, secretory immunoglobulin A; TNF-α, tumor necrosis factor-α; WB, CON + 3% wheat bran.
Values are means, n = 7 per treatment.
Tight Junction Proteins Expression in Jejunal Mucosa
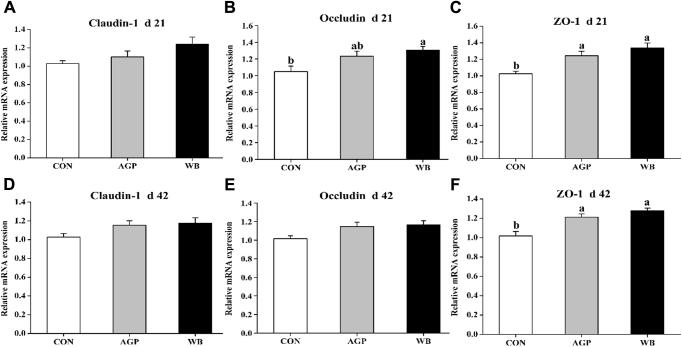
The effects of WB supplementation on the relative mRNA expression of tight junction proteins in jejunal mucosa are shown in Figure 1. On day 21, dietary treatments did not affect the relative expression of claudin-1 in jejunal mucosa (Figure 1A). However, the relative expression of occludin was upregulated (P < 0.05) in WB than in CON, but not different from AGP (Figure 1B). Moreover, both AGP and WB supplementation upregulated (P < 0.05) the relative expression of ZO-1 (Figure 1C). On day 42, the relative expression of claudin and occludin did not significantly differ among treatments (Figures 1D, 1E). However, the relative expression of ZO-1 was upregulated (P < 0.05) by AGP and WB supplementation (Figure 1F).
Figure 1.
Effects of wheat bran supplementation on the relative mRNA expression of tight junction proteins in jejunal mucosa. (A–C) Claudin-1, occludin, and ZO-1 expression on day 21. (D–F) Claudin-1, occludin, and ZO-1 expression on day 42. Values are presented as mean ± SEM, n = 7 per treatment. a,bMeans without common letters differ at P < 0.05. AGP, CON +75 mg/kg chlortetracycline (antibiotic growth promoter). Abbreviations: CON, antibiotic-free control diet; WB, CON +3% wheat bran; ZO-1, zonula occludens-1.
Cecal Microbiota
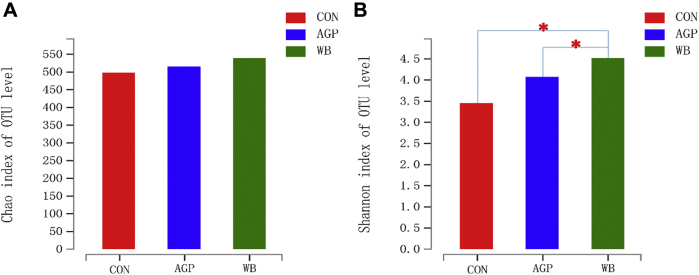
The effects of WB supplementation on α-diversity of cecal microbial communities are shown in Figure 2. Dietary WB supplementation markedly enhanced the α-diversity of cecal microbiota compared with CON and AGP, as evidenced by the increased Shannon index (P < 0.05; Figure 2A). However, the Chao index was not affected by dietary treatments (Figure 2B).
Figure 2.
Effects of wheat bran supplementation on microbial α-diversity in cecum on day 42. (A) Chao index, (B) Shannon index. The results were analyzed by Kruskal-Wallis H test and presented as mean values of different bacteria. ∗Means the difference was significant (P < 0.05). AGP, CON +75 mg/kg chlortetracycline (antibiotic growth promoter). Abbreviations: CON, antibiotic-free control diet; OTU, operational taxonomic units; WB, CON +3% wheat bran.
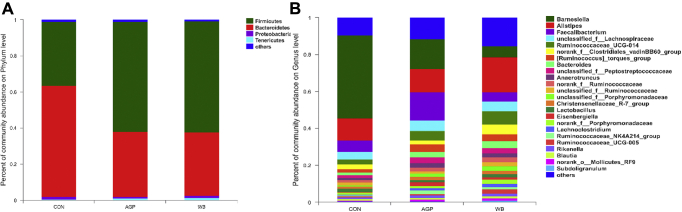
The effects of WB supplementation on cecal microbial community composition are shown in Figure 3. At the phylum level, Firmicutes and Bacteroidetes were the most dominant phyla in the cecal digesta of broilers, followed by Proteobacteria and Tenericutes (Figure 3A). Supplementation with AGP and WB increased the percentage of Firmicutes phylum, but decreased the percentage of Bacteroidetes phylum. At the genus level, Barnesiella, Alistipes, Faecalibacterium, unclassified_f_Lachnospiraceae, and Ruminococcaceae_UCG-014 were the abundant genera in the cecal digesta of broilers (Figure 3B).
Figure 3.
Effects of wheat bran supplementation on microbial community in cecum on day 42. (A) Microbial community barplot at the phylum level. (B) Microbial community barplot at the genus level. AGP, CON +75 mg/kg chlortetracycline (antibiotic growth promoter). Abbreviations: CON, antibiotic-free control diet; WB, CON +3% wheat bran.
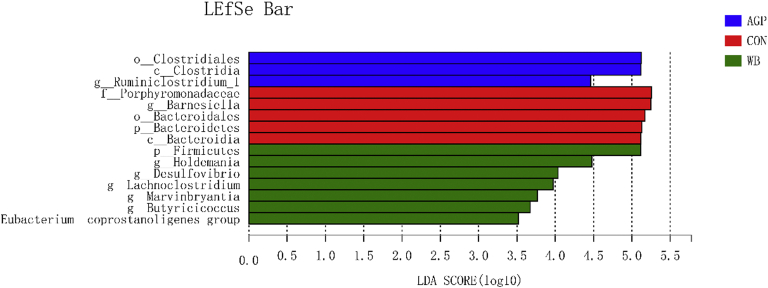
The bacteria that differed significantly among treatments were further analyzed by linear discriminant analysis coupled with effect size (Figure 4). At the phylum level, the phylum Firmicutes was significantly enriched (P < 0.05) in WB, while the phylum Bacteroidetes was significantly enriched (P < 0.05) in CON. At the genus level, WB supplementation significantly enriched (P < 0.05) Holdemania, Desulfovibrio, Lachnoclostridium, Marvinbryantia, Butyricicoccus, and Eubacterium_coprostanoligenes_group. The genera Barnesiella and Ruminiclostridium_1 were significantly enriched (P < 0.05) in CON and AGP, respectively.
Figure 4.
Effects of wheat bran supplementation on LDA score in cecum on day 42. AGP, CON +75 mg/kg chlortetracycline (antibiotic growth promoter). Abbreviations: CON, antibiotic-free control diet; LDA, linear discriminant analysis; LEfSe, linear discriminant analysis coupled with effect size; WB, CON +3% wheat bran.
SCFA in Cecum
The effects of WB supplementation on SCFA concentrations in the cecum of broilers are presented in Table 6. On day 21, the concentrations of most SCFA including acetic acid, propionic acid, isobutyric acid, valeric acid, and isovaleric acid did not differ among treatments. However, WB supplementation tended (P = 0.06) to increase the concentrations of butyric acid and significantly increased (P < 0.05) the concentration of total SCFA compared with CON. On day 42, the concentrations of butyric acid and total SCFA were greater (P < 0.05) in WB than in CON, but not different from AGP. There were no significant differences in the concentrations of acetic acid, propionic acid, isobutyric acid, valeric acid, and isovaleric acid among treatments.
Table 6.
Effects of wheat bran supplementation on SCFA concentrations in cecum of broilers (mg/g of digesta).
| Item | CON | AGP | WB | SEM | P-value |
|---|---|---|---|---|---|
| Day 21 | |||||
| Acetic acid | 7.91 | 7.79 | 8.40 | 0.30 | 0.34 |
| Propionic acid | 1.17 | 1.18 | 1.32 | 0.10 | 0.50 |
| Butyric acid | 2.36 | 2.53 | 3.05 | 0.20 | 0.06 |
| Isobutyric acid | 0.03 | 0.04 | 0.04 | 0.01 | 0.46 |
| Valeric acid | 0.19 | 0.29 | 0.24 | 0.05 | 0.33 |
| Isovaleric acid | 0.04 | 0.05 | 0.05 | 0.01 | 0.83 |
| Total SCFA | 11.70b | 11.86a,b | 13.10a | 0.37 | 0.03 |
| Day 42 | |||||
| Acetic acid | 10.35 | 10.83 | 12.03 | 0.65 | 0.20 |
| Propionic acid | 2.39 | 2.53 | 3.34 | 0.37 | 0.17 |
| Butyric acid | 5.18b | 5.46a,b | 6.33a | 0.27 | 0.02 |
| Isobutyric acid | 0.08 | 0.07 | 0.09 | 0.01 | 0.43 |
| Valeric acid | 0.41 | 0.44 | 0.51 | 0.04 | 0.16 |
| Isovaleric acid | 0.10 | 0.08 | 0.08 | 0.02 | 0.45 |
| Total SCFA | 18.50b | 19.41a,b | 22.39a | 0.83 | 0.01 |
a,bMeans in the same row with different superscripts differ significantly (P < 0.05).
Abbreviations: AGP, CON + 75 mg/kg chlortetracycline (antibiotic growth promoter); CON, antibiotic-free control diet; SCFA, short chain fatty acids; WB, CON + 3% wheat bran.
Values are means, n = 7 per treatment.
Discussion
In the present study, supplementation of 3% WB increased the ADG of broilers in the starter phase when compared with CON. Because WB supplementation reduced F:G without influencing ADFI, the improved ADG was likely due to increased nutrient utilization. In addition, broilers fed WB had similar growth performance compared to those fed AGP, indicating that WB may be an effective alternative to antibiotics in poultry diets. Similar beneficial effects of DF on broiler performance were also reported in previous studies (Adibmoradi et al., 2016; Jiménez-Moreno et al., 2016). However, there are also some conflicting findings suggesting that DF had no effects or negative effects on the growth performance in broilers (Sacranie et al., 2012; Walugembe et al., 2014). These contradictory results may depend upon many factors. First, the source of fiber is an important influencing factor. Jimenez-Moreno et al. (2010) reported that insoluble fiber, such as oat hulls, improved weight gain and feed conversion ratio while soluble fiber, such as sugar beet pulp, had no effect. Second, the level of fiber has an impact on the response to the fiber in broilers, as evidenced by Jiménez-Moreno et al. (2011), who demonstrated that inclusion of 5% pea hulls improved weight gain and feed conversion ratio in broilers, but a further increase to 7.5% hindered the performance. Third, the age of birds also contributed to the inconsistent responses. González-Alvarado et al. (2010) reported that SBP inclusion improved growth performance of broilers from 1 to 10 D of age but not thereafter.
Cytokines are a group of regulatory and immunomodulatory proteins involved in a number of physiological processes, among which pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, promote systemic inflammation and tissue damage (Cunningham and Green, 1994; Kim and Moudgil, 2017). The present study demonstrated that WB supplementation reduced concentrations of TNF-α and IL-1β in serum, as well as the concentration of TNF-α in jejunal mucosa, indicating that WB supplementation may have a beneficial effect on attenuation of the inflammatory response. Consistent with current results, Chen et al. (2017) observed that WB downregulated the gene expression of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) in weaned pigs. Various other studies also have shown that fiber intake decreased inflammation-associated biomarkers such as TNF-α, and protected the host against inflammation (Kaczmarczyk et al., 2012; Hung and Suzuki, 2016), while lower fiber intake is usually associated with systemic inflammation (Leinig et al., 2019). The beneficial effects of WB on inflammation may be attributed to its fermentation products SCFA (Koh et al., 2016).
Secretory immunoglobulin A, secreted by plasma cells, is the most prominent antibody present in mucosal surfaces and protects intestinal mucosa against the invasion of enteric toxins and pathogenic microorganisms (Min et al., 2016). In the present study, greater sIgA concentrations in birds fed WB suggested enhanced intestinal mucosal immunity, which may in turn protect the intestine against pathogen adherence and thereby contribute to lower pro-inflammatory cytokine production in the intestine. The reason for the increased sIgA concentration may be attributed to the altered microbial composition following WB supplementation, as the gut microbiota interacts directly or indirectly with the host immune system (Tilg and Moschen, 2015).
An intact intestinal barrier is essential for maintaining health by protecting the intestine against invasion of antigens, pathogens, toxins, and other pro-inflammatory substances (Turner, 2009). Tight junction proteins including claudins, occludin, and ZO, are multiprotein complexes located at the apical end of the lateral membrane of epithelial cells and play an important role in the regulation of the intestinal barrier (Gadde et al., 2017). In present study, supplementation of WB upregulated the expression level of occludin and ZO-1 in the ileum compared with CON, suggesting improved intestinal barrier function. Beneficial effects of WB on intestinal barrier function were also reported in weaned pigs in which the ZO-1 mRNA levels in the ileum and colon were upregulated by WB fiber supplementation (Chen et al., 2013). It has been well demonstrated that pro-inflammatory cytokines promote barrier dysfunction by inhibiting the transcription of junction proteins and inducing cytoskeleton-mediated redistribution of tight junction proteins (Choi et al., 2012). Therefore, the improved intestinal barrier function in WB may be due to the reduced pro-inflammatory cytokines as evidenced by lower concentrations of TNF-α and IL-1β in the serum and jejunal mucosa.
The gastrointestinal tract of poultry is densely colonized with diverse microorganisms, which are considered to have vitally important influences on host health and growth performance (Gong et al., 2019). In the present study, birds with WB supplementation had the highest α-diversity. It has been shown that increased microbial diversity could help to maintain gut ecosystems that are more stable and more likely to inhibit pathogen proliferation (Oviedo-Rondón, 2019). Consequently, the increased microbial diversity may be responsible for the improved intestinal immunity and barrier function in birds fed WB. Firmicutes and Bacteroidetes were predominant in the ceca with a relative abundance of more than 95% in this study, which was consistent with previous findings (Corrigan et al., 2015; Awad et al., 2016). In addition, the percentage of the Bacteroidetes phylum was decreased, while the percentage of Firmicutes phylum was increased by AGP and WB supplementation. It has been established that a higher Firmicutes to Bacteroidetes ratio could promote broiler growth (Hong et al., 2019), which may be a possible reason for growth promotion in AGP and WB. Further linear discriminant analysis coupled with effect size analysis revealed that the abundance of Lachnoclostridium and Butyricicoccus was increased in birds fed WB. Lachnoclostridium and Butyricicoccus were proposed as butyrate producers that could improve feed conversion, inhibit the proliferation of pathogens and alleviate intestinal inflammation (Eeckhaut et al., 2016; Wang et al., 2019). Taken together, the WB supplementation improved diversity and composition of cecal microbial communities.
Short chain fatty acids (mainly acetate, propionate, and butyrate) are the major end products of the microbial fermentation of undigested carbohydrates in the intestine and have broad impacts on various aspects of host physiology (Koh et al., 2016; Zhang et al., 2019). In this study, WB supplementation significantly increased the concentrations of total SCFA in the cecum when compared with CON, suggesting greater bacterial fermentation in the intestine. The results may be due to possible increases in bacterial proliferation which resulted from the increased content of DF in WB diet (Walugembe et al., 2015). The increased concentrations of SCFA could, in turn, increase intestinal acidity, which is associated with pathogen suppression and maintain intestinal health (Rehman et al., 2007). In addition, greater concentration of butyrate in the cecum of birds fed with WB was also found in this study, which was consistent with the enrichment of butyrate producing bacteria Lachnoclostridium and Butyricicoccus. Butyrate, the primary energy source of intestinal colonocytes, has been shown to inhibit inflammation and improve intestinal barrier function (Jiang et al., 2015; Song et al., 2017), which may be partly responsible for the reduced inflammatory cytokines and improved barrier function of intestine in birds fed WB diets.
In summary, supplementation of 3% WB could enhance growth by improving intestinal immune response, barrier function, and microbial composition in broilers. Thus, WB may play a role in replacing antibiotics for improved growth performance in broilers.
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (31772612) and the Beijing Municipal Natural Science Foundation (6202019).
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Adibmoradi M., Navidshad B., Jahromi M.F. The effect of moderate levels of finely ground insoluble fibre on small intestine morphology, nutrient digestibility and performance of broiler chickens. Ital. J. Anim. Sci. 2016;15:310–317. [Google Scholar]
- Akhtar M., Tariq A.F., Awais M.M., Iqbal Z., Muhammad F., Shahid M., Hiszczynska-Sawicka E. Studies on wheat bran Arabinoxylan for its immunostimulatory and protective effects against avian coccidiosis. Carbohyd. Polym. 2012;90:333–339. doi: 10.1016/j.carbpol.2012.05.048. [DOI] [PubMed] [Google Scholar]
- AOAC. 18th ed. AOAC International; Arlington, VA: 2007. Official Methods of Analysis. [Google Scholar]
- Awad W.A., Mann E., Dzieciol M., Hess C., Schmitz-Esser S., Wagner M., Hess M. Age-related differences in the luminal and mucosa-associated gut microbiome of broiler chickens and shifts associated with Campylobacter jejuni infection. Front. Cell. Infect. Mi. 2016;6:154. doi: 10.3389/fcimb.2016.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon J. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 2007;86:2466–2471. doi: 10.3382/ps.2007-00249. [DOI] [PubMed] [Google Scholar]
- Chen H., Chen D., Qin W., Liu Y., Che L., Huang Z., Luo Y., Zhang Q., Lin D., Liu Y. Wheat bran components modulate intestinal bacteria and gene expression of barrier function relevant proteins in a piglet model. Int. J. Food Sci. Nutr. 2017;68:65–72. doi: 10.1080/09637486.2016.1212817. [DOI] [PubMed] [Google Scholar]
- Chen H., Mao X., He J., Yu B., Huang Z., Yu J., Zheng P., Chen D. Dietary fibre affects intestinal mucosal barrier function and regulates intestinal bacteria in weaning piglets. Br. J. Nutr. 2013;110:1837–1848. doi: 10.1017/S0007114513001293. [DOI] [PubMed] [Google Scholar]
- Choi H.J., Kim J., Park S., Do K.H., Yang H., Moon Y. Pro-inflammatory NF-κB and early growth response gene 1 regulate epithelial barrier disruption by food additive carrageenan in human intestinal epithelial cells. Toxicol. Lett. 2012;211:289–295. doi: 10.1016/j.toxlet.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Corrigan A., de Leeuw M., Penaud-Frézet S., Dimova D., Murphy R.A. Phylogenetic and functional alterations in bacterial community compositions in broiler ceca as a result of mannan oligosaccharide supplementation. Appl. Environ. Microbiol. 2015;81:3460–3470. doi: 10.1128/AEM.04194-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtin C.M., Broekaert W.F., Swennen K., Lescroart O., Onagbesan O., Buyse J., Decuypere E., Van de Wiele T., Marzorati M., Verstraete W. Dietary inclusion of wheat bran arabinoxylooligosaccharides induces beneficial nutritional effects in chickens. Cereal Chem. 2008;85:607–613. [Google Scholar]
- Cunningham J.M., Green I.C. Cytokines, nitric oxide and insulin secreting cells. Growth. Regul. 1994;4:173–180. [PubMed] [Google Scholar]
- Dibner J.J., Richards J.D. Antibiotic growth promoters in agriculture: history and mode of action. Poult. Sci. 2005;84:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- Donadelli R.A., Stone D.A., Aldrich C.G., Beyer R.S. Effect of fiber source and particle size on chick performance and nutrient utilization. Poult. Sci. 2019;98:5820–5830. doi: 10.3382/ps/pez382. [DOI] [PubMed] [Google Scholar]
- Eeckhaut V., Wang J., Van Parys A., Haesebrouck F., Joossens M., Falony G., Raes J., Ducatelle R., Van Immerseel F. The probiotic Butyricicoccus pullicaecorum reduces feed conversion and protects from potentially harmful intestinal microorganisms and necrotic enteritis in broilers. Front. Microbiol. 2016;7:1416. doi: 10.3389/fmicb.2016.01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- Edgar R.C., Haas B.J., Clemente J.C., Quince C., Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadde U.D., Oh S., Lee Y., Davis E., Zimmerman N., Rehberger T., Lillehoj H.S. Dietary Bacillus subtilis-based direct-fed microbials alleviate LPS-induced intestinal immunological stress and improve intestinal barrier gene expression in commercial broiler chickens. Res. Vet. Sci. 2017;114:236–243. doi: 10.1016/j.rvsc.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Gemen R., de Vries J.F., Slavin J.L. Relationship between molecular structure of cereal dietary fiber and health effects: focus on glucose/insulin response and gut health. Nutr. Rev. 2011;69:22–33. doi: 10.1111/j.1753-4887.2010.00357.x. [DOI] [PubMed] [Google Scholar]
- Gong Y., Yang H., Wang X., Xia W., Lv W., Xiao Y., Zou X. Early intervention with cecal fermentation broth regulates the colonization and development of gut microbiota in broiler chickens. Front. Microbiol. 2019;10:1422. doi: 10.3389/fmicb.2019.01422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alvarado J.M., Jiménez-Moreno E., González-Sánchez D., Lázaro R., Mateos G.G. Effect of inclusion of oat hulls and sugar beet pulp in the diet on productive performance and digestive traits of broilers from 1 to 42 days of age. Anim. Feed. Sci. Tech. 2010;162:37–46. [Google Scholar]
- Grilli E., Tugnoli B., Passey J.L., Stahl C.H., Piva A., Moeser A.J. Impact of dietary organic acids and botanicals on intestinal integrity and inflammation in weaned pigs. Bmc Vet. Res. 2015;11:96. doi: 10.1186/s12917-015-0410-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L.W., Meng Q.X., Li D.Y., Zhang Y.W., Ren L.P. Influence of feeding alternative fiber sources on the gastrointestinal fermentation, digestive enzyme activities and mucosa morphology of growing Greylag geese. Poult. Sci. 2015;94:2464–2471. doi: 10.3382/ps/pev237. [DOI] [PubMed] [Google Scholar]
- Hong Y., Cheng Y., Li Y., Li X., Zhou Z., Shi D., Li Z., Xiao Y. Preliminary study on the effect of bacillus amyloliquefaciens TL on cecal bacterial community structure of broiler chickens. Biomed. Res. Int. 2019;2019:5431354. doi: 10.1155/2019/5431354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung T.V., Suzuki T. Dietary fermentable fiber reduces intestinal barrier defects and inflammation in colitic mice. J. Nutr. 2016;146:1970–1979. doi: 10.3945/jn.116.232538. [DOI] [PubMed] [Google Scholar]
- Jha R., Fouhse J.M., Tiwari U.P., Li L., Willing B.P. Dietary fiber and intestinal health of monogastric animals. Front. Vet. Sci. 2019;6:48. doi: 10.3389/fvets.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Zhang W., Gao F., Zhou G. Effect of sodium butyrate on intestinal inflammatory response to lipopolysaccharide in broiler chickens. Can J. Anim. Sci. 2015;95:389–395. [Google Scholar]
- Jiménez-Moreno E., Chamorro S., Frikha M., Safaa H.M., Lázaro R., Mateos G.G. Effects of increasing levels of pea hulls in the diet on productive performance, development of the gastrointestinal tract, and nutrient retention of broilers from one to eighteen days of age. Anim. Feed. Sci. Tech. 2011;168:100–112. [Google Scholar]
- Jiménez-Moreno E., de Coca-Sinova A., González-Alvarado J.M., Mateos G.G. Inclusion of insoluble fiber sources in mash or pellet diets for young broilers. 1. Effects on growth performance and water intake. Poult. Sci. 2016;95:41–52. doi: 10.3382/ps/pev309. [DOI] [PubMed] [Google Scholar]
- Jiménez-Moreno E., González-Alvarado J.M., de Coca-Sinova A., Lázaro R.P., Cámara L., Mateos G.G. Insoluble fiber sources in mash or pellets diets for young broilers. 2. Effects on gastrointestinal tract development and nutrient digestibility. Poult. Sci. 2019;98:2531–2547. doi: 10.3382/ps/pey599. [DOI] [PubMed] [Google Scholar]
- Jimenez-Moreno E., Gonzalez-Alvarado J.M., Gonzalez-Sanchez D., Lazaro R., Mateos G.G. Effects of type and particle size of dietary fiber on growth performance and digestive traits of broilers from 1 to 21 days of age. Poult. Sci. 2010;89:2197–2212. doi: 10.3382/ps.2010-00771. [DOI] [PubMed] [Google Scholar]
- Kaczmarczyk M.M., Miller M.J., Freund G.G. The health benefits of dietary fiber: beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism. 2012;61:1058–1066. doi: 10.1016/j.metabol.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.Y., Moudgil K.D. Immunomodulation of autoimmune arthritis by pro-inflammatory cytokines. Cytokine. 2017;98:87–96. doi: 10.1016/j.cyto.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh A., De Vadder F., Kovatcheva-Datchary P., Ckhed F.B. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Leinig C.E., Pecoits-Filho R., Kunii L., Claro L.M., Merlin J., de Almeida N.R., Carvalho C.R.D.S., de Moraes T.P. Low-fiber intake is associated with high production of intraperitoneal inflammation biomarkers. J. Ren. Nutr. 2019;29:322–327. doi: 10.1053/j.jrn.2018.10.013. [DOI] [PubMed] [Google Scholar]
- Levkut M., Husáková E., Bobíková K., Karaffová V., Levkutová M., Ivani Inová O., Gre Áková U., Obanová K., Reiterová K., Levkut M. Inorganic or organic zinc and MUC-2, IgA, IL-17, TGF-β4 gene expression and sIgA secretion in broiler chickens. Food Agr. Immunol. 2017;28:801–802. [Google Scholar]
- Mateos G.G., Jiménez-Moreno E., Serrano M.P., Lázaro R.P. Poultry response to high levels of dietary fiber sources varying in physical and chemical characteristics. J. Appl. Poult. Res. 2012;21:156–174. [Google Scholar]
- Min Y.N., Yang H.L., Xu Y.X., Gao Y.P. Effects of dietary supplementation of synbiotics on growth performance, intestinal morphology, sIgA content and antioxidant capacities of broilers. J. Anim. Physiol. N. 2016;100:1073–1080. doi: 10.1111/jpn.12479. [DOI] [PubMed] [Google Scholar]
- NRC. National Academy Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Onipe O.O., Jideani A.I., Beswa D. Composition and functionality of wheat bran and its application in some cereal food products. Int. J. Food Sci. Tech. 2015;50:2509–2518. [Google Scholar]
- Oviedo-Rondón E.O. Holistic view of intestinal health in poultry. Anim. Feed. Sci. Tech. 2019;250:1–8. [Google Scholar]
- Rebello C.J., O Neil C.E., Greenway F.L. Dietary fiber and satiety: the effects of oats on satiety. Nutr. Rev. 2016;74:131–147. doi: 10.1093/nutrit/nuv063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman H.U., Vahjen W., Awad W.A., Zentek J. Indigenous bacteria and bacterial metabolic products in the gastrointestinal tract of broiler chickens. Arch. Anim. Nutr. 2007;61:319–335. doi: 10.1080/17450390701556817. [DOI] [PubMed] [Google Scholar]
- Sacranie A., Svihus B., Denstadli V., Moen B., Iji P.A., Choct M. The effect of insoluble fiber and intermittent feeding on gizzard development, gut motility, and performance of broiler chickens. Poult. Sci. 2012;91:693–700. doi: 10.3382/ps.2011-01790. [DOI] [PubMed] [Google Scholar]
- Smith P.K. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Song B., Li H., Wu Y., Zhen W., Wang Z., Xia Z., Guo Y. Effect of microencapsulated sodium butyrate dietary supplementation on growth performance and intestinal barrier function of broiler chickens infected with necrotic enteritis. Anim. Feed. Sci. Tech. 2017;232:6–15. [Google Scholar]
- Stevenson L., Phillips F., O'Sullivan K., Walton J. Wheat bran: its composition and benefits to health, a European perspective. Int. J. Food Sci. Nutr. 2012;63:1001–1013. doi: 10.3109/09637486.2012.687366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suriano F., Neyrinck A.M., Verspreet J., Olivares M., Leclercq S., Van de Wiele T., Courtin C.M., Cani P.D., Bindels L.B., Delzenne N.M. Particle size determines the anti-inflammatory effect of wheat bran in a model of fructose over-consumption: Implication of the gut microbiota. J. Funct. Foods. 2018;41:155–162. [Google Scholar]
- Tilg H., Moschen A.R. Food, immunity, and the microbiome. Gastroenterology. 2015;148:1107–1119. doi: 10.1053/j.gastro.2014.12.036. [DOI] [PubMed] [Google Scholar]
- Turner J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009;9:799. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- Van Soest P.V., Robertson J.B., Lewis B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Walugembe M., Hsieh J.C.F., Koszewski N.J., Lamont S.J., Persia M.E., Rothschild M.F. Effects of dietary fiber on cecal short-chain fatty acid and cecal microbiota of broiler and laying-hen chicks. Poult. Sci. 2015;94:2351–2359. doi: 10.3382/ps/pev242. [DOI] [PubMed] [Google Scholar]
- Walugembe M., Rothschild M.F., Persia M.E. Effects of high fiber ingredients on the performance, metabolizable energy and fiber digestibility of broiler and layer chicks. Anim. Feed. Sci. Tech. 2014;188:46–52. [Google Scholar]
- Wang W., Jia H., Zhang H., Wang J., Lv H., Wu S., Qi G. Supplemental plant extracts from flos ionicerae in combination with Baikal skullcap attenuate intestinal disruption and modulate gut microbiota in laying hens challenged by Salmonella pullorum. Front. Microbiol. 2019;10:1681. doi: 10.3389/fmicb.2019.01681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Liu Y., Yan F., Yang C., Yang X. Effects of encapsulated organic acids and essential oils on intestinal barrier, microbial count, and bacterial metabolites in broiler chickens. Poult. Sci. 2019;98:2858–2865. doi: 10.3382/ps/pez031. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Chen H., Zhu W., Yu K. Cecal infusion of sodium propionate promotes intestinal development and jejunal barrier function in growing pigs. Animals. 2019;9:284. doi: 10.3390/ani9060284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zhang L., Zhan X., Zeng X., Zhou L., Cao G., Chen A., Yang C. Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88. J. Anim. Sci. Biotechno. 2016;7:3. doi: 10.1186/s40104-016-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]