Abstract
Campylobacter jejuni is a major foodborne pathogen that causes gastroenteritis in humans. Chickens act as the reservoir host for C. jejuni, wherein the pathogen asymptomatically colonizes the ceca leading to contamination of carcasses during slaughter. The major colonization factors in C. jejuni include motility, intestinal epithelial attachment, acid/bile tolerance, and quorum sensing. Reducing the expression of the aforementioned factors could potentially reduce C. jejuni colonization in chickens. This study investigated the efficacy of subinhibitory concentration (SIC; compound concentration not inhibiting bacterial growth) of carvacrol in reducing the expression of C. jejuni colonization factors in vitro. Moreover, the effect of carvacrol on the expression of C. jejuni proteome was investigated using liquid chromatography-tandem mass spectrometry. The motility assay was conducted at 42°C, and the motility zone was measured after 24 h of incubation. For the adhesion assay, monolayers of primary chicken enterocytes (∼105 cells/well) were inoculated with C. jejuni (6 log cfu/well) either in the presence or absence of carvacrol, and the adhered C. jejuni were enumerated after 90 min of incubation at 42°C. The effect of carvacrol on C. jejuni quorum sensing and susceptibility to acid/bile stress was investigated using a bioluminescence assay and an acid–bile survival assay, respectively. The SIC (0.002%) of carvacrol reduced the motility of C. jejuni strains S-8 and NCTC 81-176 by ∼50 and 35%, respectively (P < 0.05). Carvacrol inhibited C. jejuni S-8 and NCTC 81-176 adhesion to chicken enterocytes by ∼0.8 and 1.5 log cfu/mL, respectively (P < 0.05). Moreover, carvacrol reduced autoinducer-2 activity and increased the susceptibility of C. jejuni to acid and bile in both the strains (P < 0.05). Liquid chromatography-tandem mass spectrometry revealed that the SIC of carvacrol reduced the expression of selected C. jejuni colonization proteins critical for motility (methyl-accepting chemotaxis protein), adhesion (GroL), growth and metabolism (AspA, AcnB, Icd, Fba, Ppa, AnsA, Ldh, Eno, PurB-1), and anaerobic respiration (NapB, HydB, SdhA, NrfA) (P < 0.05). Results suggest the mechanisms by which carvacrol could reduce C. jejuni colonization in chickens.
Key words: carvacrol, C. jejuni, adhesion, quorum sensing, proteomics
Introduction
Campylobacteriosis is one of the most common causes of bacterial gastroenteritis in the United States, affecting more than 1.3 million people annually (Scallan et al., 2011; Marder et al., 2017). Campylobacter jejuni alone causes ∼90% of the reported Campylobacter infections in humans (Cody et al., 2013). In addition, this pathogen increases the risk of Guillain–Barré syndrome and reactive arthritis in patients, thereby leading to significant health concerns and economic burden (Spiller, 2007; Gradel et al., 2009; Hoffmann et al., 2011).
Chickens are the natural reservoir host for C. jejuni, wherein the bacteria asymptomatically colonize the intestinal tract in high numbers (∼8 log cfu/g of cecal contents) by 3 to 4 wk of age (Humphery et al., 1993; Dhillon et al., 2006; Hue et al., 2010). This high level of cecal colonization leads to a high prevalence of the pathogen at the flock level and frequent contamination of carcass during slaughter and processing (Allen et al., 2007). Approximately 80% of human campylobacteriosis cases are linked to contaminated poultry (EFSA, 2010). Intensive research in the past 2 decades has increased our understanding of the various mechanisms used by C. jejuni to colonize chickens. The tolerance of C. jejuni to acidic pH and alkaline bile is critical for the survival of the bacterium in sufficiently high numbers (>2 Log) during gut transit and successful colonization in the ceca (Beery et al., 1988). Motility of bacteria, together with chemotaxis, promotes migration of C. jejuni toward a protective niche such as the crypts of the ceca (Hermans et al., 2011a). Evidence exists that motile C. jejuni colonizes the ceca in higher numbers as compared with nonmotile mutants indicating the necessity of motility for persistent colonization in birds (Morooka et al., 1985; Mertins et al., 2013). Once C. jejuni reaches the cecal crypts, it attaches to the epithelial cells followed by colonization (Beery et al., 1988). Quorum sensing in C. jejuni via the production of signal molecules (autoinducer-2 [AI-2]) allows communication among themselves in response to change in the bacterial population and the environment (Bassler et al., 1994; Castillo et al., 2014). Thus, attenuating the aforementioned colonization factors could potentially lead to a reduction in C. jejuni colonization in birds.
Several intervention strategies to control colonization of C. jejuni have been tested (Hermans et al., 2011b) with varying degrees of success. These approaches include supplementation of bacteriocins (Stern et al., 2005; Svetoch and Stern, 2010), bacteriophages (Carrillo et al., 2005; Wagenaar et al., 2005), probiotics (Arsi et al., 2015; Shrestha et al., 2017), and vaccination (Buckley et al., 2010; Chintoan-Uta et al., 2016). With an increasing demand for antibiotic-free and organic chickens, novel antimicrobial approaches are desired, which are safe, effective, and environmentally friendly. The use of plant-derived antimicrobials represents such an approach.
Since ancient times, humans have used plant-derived compounds as food preservatives and health promotors. Several components of plants have exhibited antibacterial activity against a wide range of pathogens (Burt, 2004; Holley and Patel, 2005). Carvacrol is the active component of oregano oil (Origanum glandulosum). It is classified as generally recognized as safe by the US Food and Drug Administration for use in food products (Food and Drug Administration, 2012). We previously reported that carvacrol affects the virulence attributes of C. jejuni critical for causing infection in humans (Upadhyay et al., 2017). However, the ability of carvacrol to change the colonization potential of C. jejuni in chickens is relatively unknown. Few previous in vivo studies have tested the efficacy of infeed supplementation of carvacrol to reduce C. jejuni colonization with inconsistent results (Arsi et al., 2014; Kelly et al., 2017). This situation warrants further investigations in the potential anti-Campylobacter mechanism(s) of action of carvacrol in chickens. The research reported here was undertaken to investigate the efficacy of subinhibitory concentration (SIC, highest concentration not inhibiting bacterial growth) of carvacrol in reducing the expression of C. jejuni colonization factors critical for persistence in the chicken gut. Moreover, the effect of carvacrol on the proteome of C. jejuni was investigated by liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis.
Materials and methods
Bacterial Strains and Culture Conditions
Two strains of C. jejuni (wild-type KADAMBIS8 and NCTC 81-176) were used for all the experiments. The wild-type KADAMBIS8 strain of C. jejuni was isolated from commercial poultry in our laboratory, and its genome sequence was published in GenBank under accession no. SFCH00000000 (called S-8 strain) (Wagle et al., 2020). Each C. jejuni strain was cultured separately in 10 mL of sterile Campylobacter Enrichment broth (CEB; catalog no. 7526A, Neogen Corp., Lansing, MI) and incubated at 42°C for 48 h under microaerophilic conditions (5% O2, 10% CO2, and 85% N2). After the growth, each strain was centrifuged at 3,000 rpm for 10 min and appropriately diluted and plated to investigate the growth pattern.
Determination of the SIC of Carvacrol
Carvacrol was purchased from Sigma-Aldrich Co., St. Louis, MO (catalog no. W224502). The SIC of carvacrol was determined using a previously published protocol (Amalaradjou et al., 2011) with slight modifications. Sterile 96-well polystyrene plates (Costar; Corning Incorporated, Corning, NY) containing serial dilutions of carvacrol in CEB (100 μL/well) were inoculated with ∼6 log cfu of C. jejuni wild-type S-8 or NCTC 81-176 in equal volume (100 μL) of CEB, followed by incubation at 42°C for 24 h. Bacterial growth was determined by culturing on Campylobacter Line Agar (CLA) plates (Line, 2001). The highest concentration of carvacrol that did not inhibit the growth of C. jejuni after 24 h of incubation was selected as the SIC for the study. Because 100% ethanol (catalog no. E7023; Sigma-Aldrich) was used as a diluent of carvacrol, it was included as a control in all the experiments.
Effect of Carvacrol on Viability and Proliferation of Chicken Enterocytes
Chicken enterocytes were harvested and cultured in Dulbecco's Modified Eagle Medium (DMEM; catalog no. L0101-0500; VWR life science, NY) containing additional growth factor supplements (10% heat-inactivated fetal bovine serum [catalog no. 10082147; Thermo Fisher Scientific, Carlsbad, CA], IX insulin-transferrin-selenite [catalog no. I3146; Sigma-Aldrich], and 1X epithelial cell growth supplement [catalog no. PHG0313; Sigma-Aldrich]) as described previously (Rath et al., 2018). The effect of carvacrol on viability and proliferation of chicken enterocytes was determined using an MTT [3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide] Cell Proliferation Assay Kit (ATCC 30-1010K; Manassas, VA) as per the standard published method (Zheng and Kunlong, 1992). Briefly, the monolayers of primary chicken enterocytes were grown in 96-well tissue culture plates (Costar) at ∼105 cells per well for 24 h. The enterocytes were treated with ethanol in DMEM (0.018%), carvacrol in DMEM (0.002%), or DMEM (control) for 2 h. The MTT reagent was added to each well, including blanks, and incubated at room temperature in the dark for 2 h. After the appearance of purple precipitation, detergent was added, and the absorbance was recorded at 570 nm by using a spectrophotometric microplate reader (Bio-Rad Laboratories, Hercules, CA).
Effect of Carvacrol on Motility of C. jejuni
The effect of the SIC of carvacrol on the motility of C. jejuni wild-type S-8 and NCTC 81-176 was determined as described previously (Niu and Gilbert, 2004) with modifications. Separate petri dishes containing 25 mL of motility test medium (catalog no. 211436; Becton, Dickinson and Company, Sparks, MD) alone (control) or with ethanol (0.018%) or SIC of carvacrol were prepared. A mid-log culture (10 h) of C. jejuni was centrifuged at 3,000 rpm for 15 min and washed 2 times with Butterfield's phosphate diluent (BPD; 0.625 mmol/L potassium dihydrogen phosphate, pH 6.67). Five microliters of washed culture (∼7 log cfu/mL) was stab inoculated at the center of the motility medium. After incubation under a microaerophilic environment at 42°C for 24 h, the zone of motility (bacterial migration distance from the site of stab to the periphery of agar plate) was measured.
Effect of Carvacrol on Adhesion of C. jejuni to Chicken Enterocytes
The effect of SIC of carvacrol on adhesion of C. jejuni wild-type S-8 and NCTC 81-176 to primary chicken enterocytes was determined as previously reported (Koo et al., 2012). Chicken enterocytes were harvested and cultured as described previously (Rath et al., 2018). Monolayers of primary chicken enterocytes were grown in 24-well tissue culture plates (Costar) at ∼105 cells per well and inoculated with C. jejuni ∼6 log cfu/well (multiplicity of infection–10:1) either alone (control) or in combination with ethanol or SIC of carvacrol. The inoculated monolayers were incubated at 42°C for 1.5 h under a microaerophilic environment. After incubation, the inoculated monolayers were rinsed 3 times in BPD and lysed with 1 mL of 0.1% Triton X-100 (catalog no. 171315-01; Invitrogen, Carlsbad, CA) treatment for 15 min. The number of C. jejuni that adhered to the enterocytes was determined by serial dilution and plating of BPD containing enterocyte lysate on CLA plates followed by incubation under microaerophilic conditions at 42°C for 48 h.
Effect of Carvacrol on Quorum Sensing (AI-2) Activity of C. jejuni
The effect of SIC of carvacrol on AI-2 levels of C. jejuni wild-type S-8 and NCTC 81-176 was investigated by Vibrio harveyi bioluminescence assay as per the standard published protocol with slight modifications (Bassler et al., 1994; Castillo et al., 2014). Briefly, C. jejuni was cultured to mid-log phase (10 h) in the presence or absence of 0.002% carvacrol followed by centrifugation at 4,000 rpm. The cell-free supernatant (CFS) was collected and filtered using a 0.2-μm syringe filter. Similarly, V. harveyi strain BB152 was grown overnight in Luria Bertani broth (catalog no. M1245; HiMedia Laboratories Pvt. Ltd., Mumbai, India) at 30°C, and the supernatant was prepared. The reporter strain of V. harveyi (BB170) was also cultured in Luria Bertani broth at 30°C for 24 h and diluted 1:5,000 with autoinducer assay medium (Inoculum ∼ 3 log cfu/mL). Ninety microliters of diluted reported strain were dispensed into 96-well microtiter plates followed by addition of 10 μL of CFS of carvacrol-treated or untreated C. jejuni or CFS of V. harveyi strain BB152 (positive control) or autoinducer assay medium (negative control). The plate was incubated at 30°C continuously, and the luminescence of mixture was measured every 20 min for 8 h using a Cytation 5 multi-mode reader (BioTek Instruments, Inc., Winooski, VT). The self-induction of luminescence in V. harveyi strain BB170 due to their growth in negative controls was deducted from positive controls and treatments before data analysis.
Effect of Carvacrol on Susceptibility of C. jejuni to Acid and Bile
C. jejuni encounters acid and bile in the proventriculus and duodenum, respectively, during passage through the gastrointestinal tract of poultry before colonization in the cecum. Therefore, we evaluated the effect of carvacrol on the susceptibility of C. jejuni to acid and bile using a previously described method (Beumer et al., 1992). Briefly, hydrochloric acid (catalog no. H1758; Sigma-Aldrich) at 0.000316 N and Oxgall bile (catalog no. 01585; Chem-Impex Int'l Inc., Wood Dale, IL) at 0.3% were used to prepare solutions similar to gastric juice (pH 3.5) and bile (pH 6.0), respectively. A mid-log culture of C. jejuni S-8 or NCTC 81-176 in the presence or absence of SIC of carvacrol was prepared as described previously. The mid-log culture was inoculated in the acid followed by incubation at 42°C for 1 h to represent food retention time in the proventriculus. The acid-exposed C. jejuni cells were then transferred to bile solution and incubated at 42°C for 5 min (average food retention time in the duodenum). The numbers of surviving C. jejuni were enumerated by dilution and plating on CLA at each step.
Proteomic Analysis of C. jejuni Exposed to Carvacrol
The effect of carvacrol on the proteome of C. jejuni wild-type S-8 and NCTC 81-176 was determined using LC-MS/MS as described previously (Miyamoto et al., 2015; Wagle et al., 2019). Briefly, C. jejuni (∼6 log cfu/mL) was incubated in the presence or absence of 0.002% carvacrol at 42°C for 10 h. The planktonic cells were washed with nuclease-free water (catalog no. AM9937; Thermo Fisher), and proteins were extracted using the B-Per bacterial protein extraction reagent (catalog no. 90084; Thermo Fisher). The samples were then subjected to SDS-PAGE (catalog no. NP0327BOX, NuPage 4 to 12% Bis-Tris protein gel; Thermo Fisher) at 220 V for 35 min. Each lane of gel was excised to 1 mm2 pieces and destained with 50% acetonitrile (catalog no. AX0145; Sigma-Aldrich) in ammonium bicarbonate (catalog no. A6141; Sigma-Aldrich) for 45 min. The gel pieces were dehydrated by adding acetonitrile and vacuum dried for 10 min. Dehydrated gels were treated with dithiothreitol (15 mg/mL in 25 mmol/L ammonium bicarbonate; catalog no. D9779; Sigma-Aldrich) to reduce disulfide bonds in proteins followed by reduction with iodoacetamide (37 mg/mL in ammonium bicarbonate; catalog no. I1149; Sigma-Aldrich) for 1 h. The residuals of iodoacetamide were removed by washing with ammonium bicarbonate buffer, and the gel pieces were dehydrated again using acetonitrile. The proteins in the gel pieces were digested with trypsin (20 ng per μL in 25 mmol/L ammonium bicarbonate; catalog no. 786-690; Biosciences, St. Louis, MO) overnight at 37°C. The resultant peptides were analyzed by LC-MS/MS technique using an Agilent 1200 series microflow HPLC coupled to a Bruker AmaZon-SL quadrupole ion trap mass spectrometer (Bruker Daltonics Inc., Billerica, MA) with a captive spray ionization source. The proteins were identified by matching MS/MS spectra to protein sequences of C. jejuni NCTC 81-176 available at the uniprot.org using in house MASCOT software (Matrix Science Inc., Boston, MA) (Perkins et al., 1999). The proteins were identified based on <5% false discovery rate and 1 unique peptide from a protein.
Statistical Analyses
A completely randomized designed was used for the study. The bacterial counts were logarithmically transformed before analysis to achieve homogeneity of variance (Byrd et al., 2003). All experiments had triplicate samples and replicated 2 times (n = 6) on each strain of C. jejuni. Data from independent trials were pooled and analyzed using ANOVA with Fisher least significant difference test for multiple comparisons on GraphPad Prism, version 8.0 (GraphPad Software, San Diego, CA). For the proteomic analysis, Scaffold Proteome Software, version 4.8 (Proteome Software Inc., Portland, OR), was used to analyze MASCOT files. Spectral counting quantitative method in Scaffold determined differentially expressed proteins between carvacrol treated and untreated C. jejuni cells using Student t test. Differences were considered significant with P values < 0.05.
Results
Determination of SIC of Carvacrol
Based on growth curve analysis, the highest concentration of carvacrol that did not significantly inhibit the growth of C. jejuni (both strains) after incubation at 42°C for 24 h was 0.002% (Data not shown).
Effect of Carvacrol on Viability and Proliferation of Chicken Enterocytes
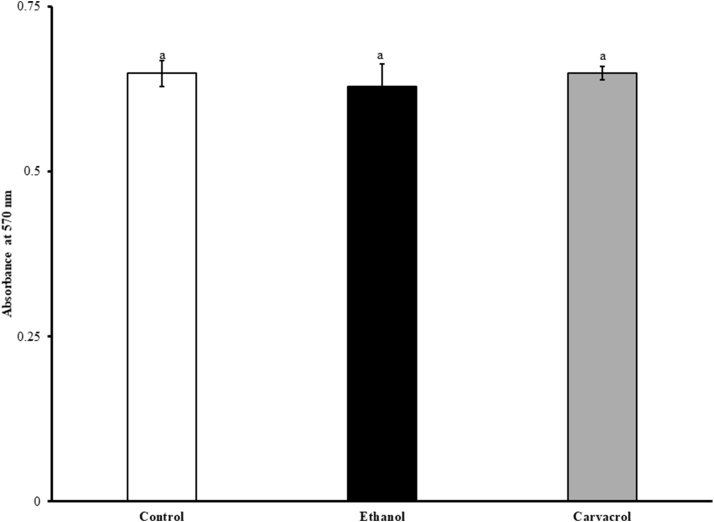
The effect of carvacrol on viability and proliferation of chicken enterocytes is shown in Figure 1. Carvacrol at the SIC level did not affect the viability of enterocytes as revealed by a nonsignificant change in the absorbance values between control and treatment. Similarly, ethanol did not affect the viability and proliferation of primary chicken enterocytes (P > 0.05).
Figure 1.
Effect of SIC (0.002%) of carvacrol on viability and proliferation of chicken enterocytes determined using MTT cell proliferation assay. Results are averages of 2 independent experiments, each containing triplicate samples (mean and SEM). Different letters across treatments indicate the statistical difference at P < 0.05. Abbreviations: MTT, 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide; SIC, subinhibitory concentration.
Effect of Carvacrol on C. jejuni Motility
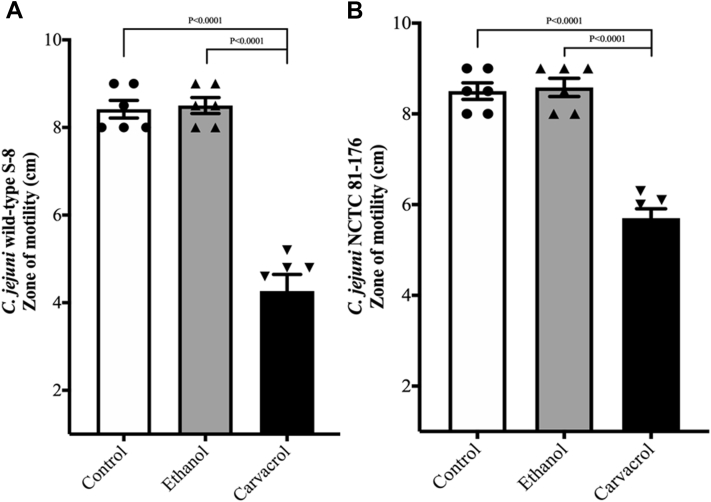
Figure 2 shows the effect of SIC of carvacrol on C. jejuni motility at 42°C. The SIC of carvacrol reduced the motility of C. jejuni wild-type S-8 (Figure 2A) and C. jejuni NCTC 81-176 (Figure 2B) without affecting the pH of the medium (P < 0.05). The control had a zone of 8.5 cm in both the strains, whereas carvacrol 0.002% decreased the motility by ∼50 and 35% in wild-type S-8 and NCTC 81-176, resulting in a zone of 4.26 and 5.7 cm in carvacrol treatment, respectively. Ethanol did not affect the motility of either strain of C. jejuni (P > 0.05).
Figure 2.
Effect of SIC (0.002%) of carvacrol on motility of Campylobacter jejuni wild-type S-8 (A) and NCTC 81-176 (B). Results are averages of 2 independent experiments, each containing triplicate samples (mean and SEM). Individual data points of control, ethanol, and carvacrol were indicated by symbols (•▲▼, respectively) at the top of each bar. Abbreviation: SIC, subinhibitory concentration.
Effect of Carvacrol on Adhesion of C. jejuni to Primary Chicken Enterocytes
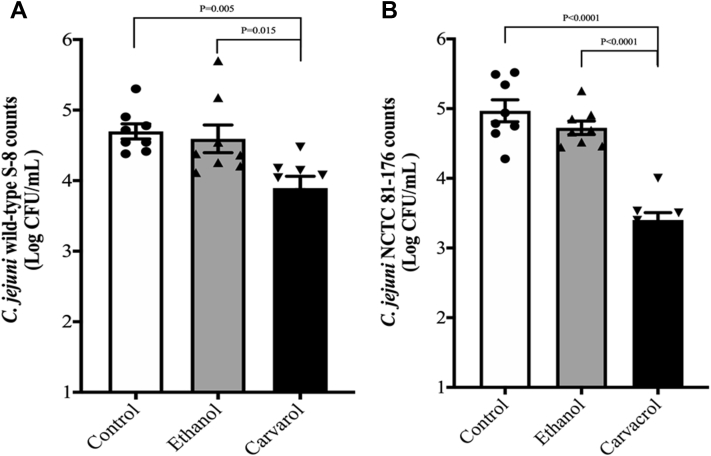
The effect of carvacrol on C. jejuni attachment to primary chicken enterocytes is presented in Figure 3. In the absence of carvacrol, ∼4.7 log cfu/mL of C. jejuni wild-type S-8 (Figure 3A) and ∼5 log cfu/mL of C. jejuni NCTC 81-176 (Figure 3B) adhered to primary chicken enterocytes. Carvacrol at 0.002% decreased attachment of C. jejuni wild-type S-8 and NCTC 81-176 by ∼0.8 and 1.5 log cfu/mL, respectively, as compared with their controls (P < 0.05). The presence of ethanol did not affect the attachment of both strains of C. jejuni to primary chicken enterocytes (P > 0.05).
Figure 3.
Effect of SIC (0.002%) of carvacrol on attachment of Campylobacter jejuni wild-type S-8 (A) and NCTC 81-176 (B) to primary chicken enterocytes. Results are averages of 2 independent experiments, each containing triplicate samples (mean and SEM). Individual data points of control, ethanol, and carvacrol were indicated by symbols (•▲▼, respectively) at the top of each bar. Abbreviation: SIC, subinhibitory concentration.
Effect of Carvacrol on Quorum Sensing (Autoinducer) Activity of C. jejuni
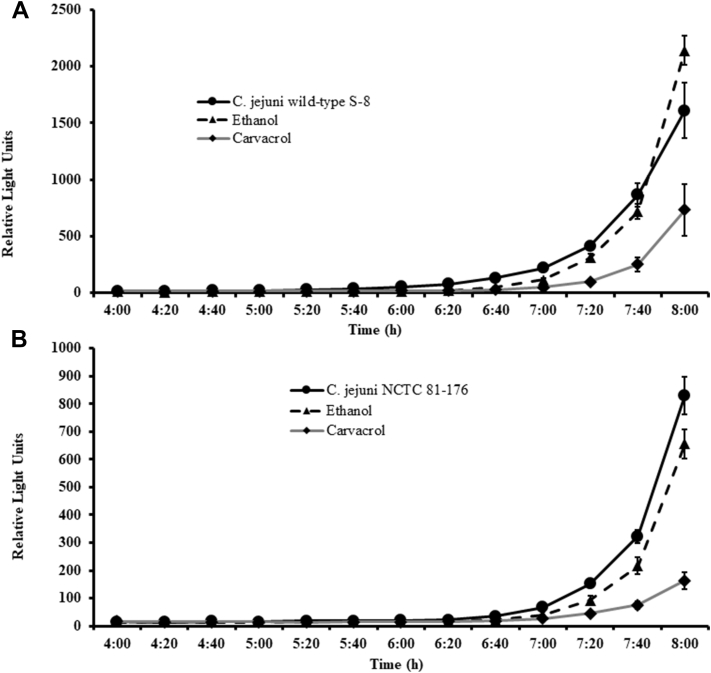
The effect of carvacrol on AI-2 levels of C. jejuni S-8 and NCTC 81-176 is shown in Figure 4. The AI-2 levels in the supernatant of untreated C. jejuni S-8 (Figure 4A) and NCTC 81-176 (Figure 4B) determined by luminescence measurement was ∼1,610 and 850 relative light units (RLU) (corresponds to 17 and 9% of positive control, V. harveyi BB152) at the end of 8 h. The presence of 0.002% carvacrol reduced AI-2 levels in C. jejuni S-8 and NCTC 81-176 to ∼730 RLU (54.65% reduction) and 160 RLU (81.17% reduction), respectively (P < 0.05). The presence of ethanol did not significantly affect AI-2 production in C. jejuni S-8; however, ethanol reduced AI-2 production by 23.52% in C. jejuni NCTC 81-176 at 8 h incubation (P < 0.05).
Figure 4.
Effect of SIC (0.002%) of carvacrol on AI-2 levels of Campylobacter jejuni wild-type S-8 (A) and NCTC 81-176 (B) determined by bioluminescence assay. Results are averages of 2 independent experiments, each containing triplicate samples (mean and SEM). Abbreviation: SIC, subinhibitory concentration.
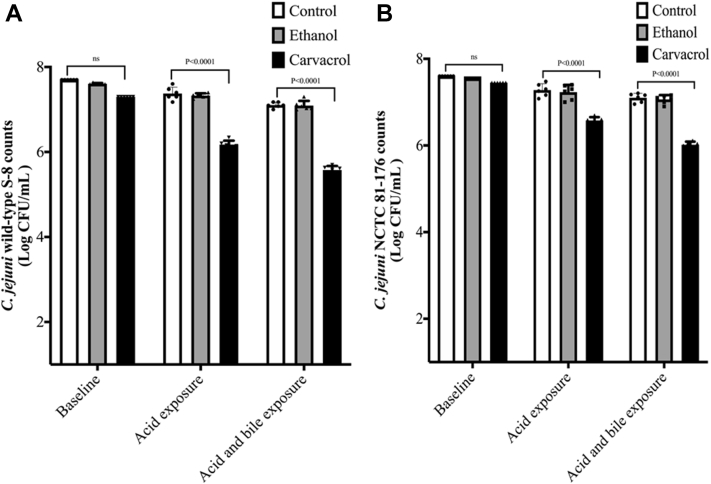
Effect of Carvacrol on Susceptibility of C. jejuni to Acid and Bile
Figure 5 shows the susceptibility of carvacrol-treated C. jejuni to acid and bile. There was no significant difference in the baseline (before exposure to acid and bile) between control and treatment in both strains. After exposure to acid, ∼7.4 log cfu/mL of C. jejuni wild-type S-8 survived in the controls (Figure 5A). Further exposure of C. jejuni S-8 to Oxgall bile did not reduce the counts (∼7.1 log cfu/mL) in the controls (P > 0.05). In contrast to controls, the presence of SIC of carvacrol resulted in ∼1.2 log cfu/mL reductions (from 7.3 to 6.1 log cfu/mL) after acid exposure (P < 0.05). Moreover, carvacrol resulted in additional 0.6 log cfu/mL reductions (6.1–5.5 log cfu/mL) after exposure to bile solution. Similar results were observed in C. jejuni NCTC 81-176 strain (Figure 5B). The reductions were ∼0.82 log cfu/mL after exposure with acid and an additional 0.56 log cfu/mL after exposure to bile (P < 0.05). The presence of ethanol did not affect the susceptibility of either strain of C. jejuni to acid and bile (P > 0.05).
Figure 5.
Effect of SIC (0.002%) of carvacrol on susceptibility of Campylobacter jejuni wild-type S-8 (A) and NCTC 81-176 (B) to acid and bile. Results are averages of 2 independent experiments, each containing triplicate samples (mean and SEM). Individual data points of control, ethanol, and carvacrol were indicated by symbols (•▲▼ respectively) at the top of each bar. Abbreviation: SIC, subinhibitory concentration.
Proteomic Profile of C. jejuni Exposed to Carvacrol
The effect of carvacrol on protein expression of C. jejuni wild-type S-8 and NCTC 81-176 is shown in Tables 1 and 2, respectively. The exposure of C. jejuni wild-type S-8 to SIC of carvacrol resulted in the downregulation of several proteins that play a major role in the cecal colonization in chickens (Table 1). Carvacrol downregulated proteins essential for flagellar chemotaxis (methyl-accepting chemotaxis protein [MCP]), interactions with host cells (AspA), anaerobic respiration (NapB), metabolism (AcnB, Icd, Fba, Ppa), and stress tolerance (Tpx) by at least 1.5 times. However, 2 other stress tolerance proteins (AhpC and KatA) were upregulated by approximately 2 times with carvacrol treatment. The ethanol treatment did not affect the expressions of aforementioned proteins of C. jejuni wild-type S-8 with the exception of inorganic pyrophosphatase (Ppa) (P > 0.05).
Table 1.
Differentially expressed proteins of Campylobacter jejuni strain wild-type S-8 among carvacrol (0.002%), ethanol (0.018%), and control samples.
| Protein description | Gene name | Functions | Fold change in expression (log2 transformed) |
||
|---|---|---|---|---|---|
| Control | Ethanol | Carvacrol | |||
| Downregulated expression | |||||
| Methyl-accepting chemotaxis protein | CJJ81176_1498 | Chemotaxis | 0a | 0.14a | −3.32b |
| Aspartate ammonia-lyase | aspA | Host-cell interactions | 0a | 0a | −3.32b |
| Periplasmic nitrate reductase | napB | Anaerobic respiration | 0a | 0a | −0.74b |
| Aconitate hydratase B | acnB | Metabolism | 0a | −0.15a | −0.74b |
| Isocitrate dehydrogenase | icd | Metabolism | 0a | 0.14a | −0.52b |
| Fructose-bisphosphate aldolase | fba | Metabolism | 0a | 0a | −3.32b |
| Inorganic pyrophosphatase | ppa | Metabolism | 0a | −0.51b | −1.74c |
| Probable thiol peroxidase | tpx | Stress response | 0a | −0.15a | −0.74b |
| Upregulated expression | |||||
| Catalase | katA | Stress response | 0a | 0a | 1b |
| Antioxidant, AhpC/Tsa family | CJJ81176_0356 | Stress response | 0a | 0a | 0.93b |
| CjaC protein | cjaC | ABC transport system | 0a | 0.38a | 0.6b |
Different letters within row indicate significant change in expression (P < 0.05).
Table 2.
Differentially expressed proteins of Campylobacter jejuni NCTC 81-176 among carvacrol (0.002%), ethanol (0.018%) and control samples.
| Protein description | Gene name | Functions | Fold change in expression (log2 transformed) |
||
|---|---|---|---|---|---|
| Control | Ethanol | Carvacrol | |||
| Downregulated expression | |||||
| Methyl-accepting chemotaxis protein | CJJ81176_0289 | Chemotaxis | 0a | 0.14a | −1b |
| Methyl-accepting chemotaxis protein | CJJ81176_0180 | Chemotaxis | 0a | 0.24a | −1b |
| 60 kDa chaperonin | groL | Adherence | 0a | −0.15a | −2.32b |
| Quinone-reactive Ni/Fe-hydrogenase | hydB | Anaerobic respiration | 0a | −0.12a | −1b |
| Cytochrome c552 | nrfA | Anaerobic respiration | 0a | −1.74b | −2.32c |
| Succinate dehydrogenase, flavoprotein subunit | sdhA | Anaerobic respiration | 0a | 0a | −3.32b |
| L-asparaginase | ansA | Growth and Metabolism | 0a | 0a | −1b |
| Pyruvate-flavodoxin oxidoreductase | CJJ81176_1469 | Growth and Metabolism | 0a | −0.15a | −1.74b |
| Aspartate–tRNA(Asp/Asn) ligase | aspS | Growth and Metabolism | 0a | 0.14a | −1.74b |
| Adenylosuccinate lyase | purB-1 | Growth and Metabolism | 0a | 0a | −1.32b |
| Cysteine desulfurase | CJJ81176_0265 | Growth and Metabolism | 0a | 0.15a | −1.32b |
| L-lactate dehydrogenase | ldh | Growth and Metabolism | 0a | 0.08a | −0.52b |
| Enolase | eno | Growth and Metabolism | 0a | −0.15a | −2.32b |
| Elongation factor Tu | tuf | Protein biosynthesis | 0a | −0.15a | −1.74b |
| Cysteine synthase A | cysK | Protein biosynthesis | 0a | −0.15a | −0.52b |
| GDP-mannose 4,6-dehydratase | wcbK | Capsule formation | 0a | −0.15a | −1.74b |
| Upregulated expression | |||||
| Putative peptidyl-prolyl cis-trans isomerase | cbf2 | Protein biosynthesis | 0b | −0.74c | 1.85a |
| Uroporphyrinogen decarboxylase | hemE | Heme biosynthesis | 0c | 1.26b | 3.58a |
| Phosphoheptose isomerase | gmhA-1 | Protein biosynthesis | 0b | 0b | 1.38a |
| Threonine synthase | thrC | Protein biosynthesis | 0b | 0b | 2.58a |
| Preprotein translocase, YajC subunit | yajC | Protein biosynthesis | 0b | 0.14b | 1.72a |
| Orotate phosphoribosyltransferase | pyrE | Metabolism | 0b | 0b | 1.38a |
Different letters within row indicate significant change in expression (P < 0.05).
The exposure of C. jejuni NCTC 81-176 to the SIC of carvacrol downregulated proteins essential for capsule formation (WcbK), chemotaxis (MCP), attachment to enterocytes (GroL), growth and metabolism (AnsA, AspA, Eno, Idh, PurB-1), protein biosynthesis (CysK, Tuf), and anaerobic respiration (NrfA, SdhA, HydB) (Table 2). The upregulated proteins (CBF2, HemE, GmhA-1, ThrC, YajC, PyrE) were mainly related to protein biogenesis. Among the aforementioned proteins, the majority of the proteins were not altered with ethanol treatment; however, it reduced expression of NrfA and CBF2 by ∼3.3 and 1.67 times, respectively, and upregulated expression of HemE by ∼2.4 times.
Discussion
Motility and adhesion are the 2 major factors responsible for the colonization of C. jejuni in birds (Van Deun et al., 2008). Tolerance to acid and bile further facilitates the colonization by increasing the number of C. jejuni that are able to reach the ceca. Moreover, AI-2 production in C. jejuni contributes to chicken colonization by coordinating the expression of virulence and host colonization factors (Quiñones et al., 2009). Therefore, attenuating the aforementioned factors could potentially reduce the colonization of C. jejuni in the poultry gut. With increasing antibiotic resistance in C. jejuni (Luangtongkum et al., 2009; Cha et al., 2016), there is a need for novel strategies to control C. jejuni in chickens. We investigated the efficacy of carvacrol in reducing C. jejuni colonization potential in vitro as a first step before conducting future in vivo experiments.
As an alternative to antibiotics, the anti-Campylobacter effect of carvacrol has been tested previously (Van Alphen et al., 2012; Upadhyay et al., 2017). However, these studies mainly evaluated the antiadhesion properties of carvacrol using human epithelial cells. In the present study, we used primary chicken enterocytes as a model to determine the efficacy of carvacrol in reducing the attachment ability of C. jejuni to chicken gut epithelium. The effect of carvacrol was investigated at SIC because the SIC of antimicrobial is known to modulate virulence properties of bacteria including C. jejuni (Van Alphen et al., 2012; Castillo et al., 2014; Upadhyay et al., 2017; Wagle et al., 2017a, b; Shrestha et al., 2019 a, b; Wagle et al., 2019) without killing them or inhibiting their growth.
Flagella-mediated motility of C. jejuni is imparted by polar flagella, which is crucial for reaching attachment sites for colonization in poultry gut (Morooka et al., 1985; Hermans et al., 2011a). Our results revealed that carvacrol significantly decreased C. jejuni motility in both the strains (Figure 2). Similar results were reported previously when C. jejuni motility was tested at human body temperature (37°C) in the presence and absence of carvacrol (Van Alphen et al., 2012), berries (Salaheen et al., 2014), and β-resorcylic acid (Wagle et al., 2017a). In contrast to these studies, we have conducted motility assay at 42°C to represent the temperature encountered by Campylobacter in the poultry gut. This selection of temperature is crucial because the respiratory proteins (NapA, NrfA, SdhA, HydB, MfrA) are expressed differently between the 2 temperatures (37°C and 42°C), thereby affecting the intensity and extent of C. jejuni motility (Kassem et al., 2012). A concurrent study by Šimunović et al. (2020) also investigated the effect of carvacrol on motility of C. jejuni NCTC 11168 at 42°C. The minimum inhibitory concentration (MIC) (0.0032%) estimations of Šimunović et al. (2020) were similar to our MIC values indicating similar interactions between carvacrol and C. jejuni. However, unlike our study, Šimunović et al. (2020) did not observe any inhibitory effect by carvacrol (0.25 MIC) on C. jejuni motility. This is probably because the test concentration used in Šimunović et al. (2020) study is 2.75 times lower than our test concentration. In our previous study (Upadhyay et al., 2017), we observed a dose-dependent reduction in C. jejuni motility on exposure to carvacrol. At 0.001% concentration, carvacrol was less effective than 0.002% in reducing C. jejuni motility. Therefore, it is definitely possible that a test dose of 0.0007% is not able to modulate the motility of C. jejuni as observed by Šimunović et al. (2020).
Adherence of C. jejuni to mucus lining of cecal crypts is the major prerequisite for successful colonization in poultry (Beery et al., 1988). Our results from cell culture revealed that SIC of carvacrol reduced adherence of C. jejuni to primary chicken enterocytes (Figure 3) without affecting the viability and proliferation of chicken enterocytes (Figure 1). This result agrees with our previous reports (Upadhyay et al., 2017) and others (Van Alphen et al., 2012), which also showed the antiadhesion property of carvacrol on human epithelial cells. Similar results have also been reported with berries (Salaheen et al., 2014), cranberry extract (Ramirez-Hernandez et al., 2015), Alpinia katsumadai extracts (Šikić Pogačar et al., 2015), grape extract (Klančnik et al., 2017), thyme extracts (Šikić Pogačar et al., 2016), herbal extracts (Bensch et al., 2011), β-resorcylic acid (Wagle et al., 2017a), and resveratrol (Klančnik et al., 2017). Adding to the knowledge of these studies, our data suggest that carvacrol exerts antiadhesion properties to primary chicken enterocytes at chicken body temperature (42°C). This is significant because C. jejuni adheres but does not invade chicken intestinal epithelial cells during colonization in birds (Byrne et al., 2007; Hermans et al., 2011a).
Quorum sensing plays an important role in motility and biofilm formation in C. jejuni (Hermans et al., 2011a). In addition, it was previously reported that AI-2 productions in C. jejuni NCTC 81-176 contributes to colonization in chickens and interaction with epithelial cells (Quiñones et al., 2009). In the present study, carvacrol significantly reduced AI-2 levels in both the strains indicating that it interferes with quorum sensing in C. jejuni (Figure 4). Similar results were reported in C. jejuni NCTC 11168 (strain isolated from humans) in the presence of citrus extracts (Castillo et al., 2014) and Euodia ruticarpa (Bezek et al., 2016). However, Šimunović et al. (2020) did not observe a reduction in quorum sensing of C. jejuni NCTC 11168 with carvacrol treatment. Because the antimicrobial efficacy is dose dependent, this variation between our study and that of the study by Šimunović et al. (2020) could be owing to differences in SIC doses of carvacrol (0.002 vs. 0.0007%, respectively) and C. jejuni strains (NCTC 81-176 vs. NCTC 11168) used for the study.
The survivability of C. jejuni on exposure to stress factors, especially acid and bile in the chicken gut, is essential for reaching bacterium at a level (>2 log) sufficient for colonization in the cecum (Beery et al., 1988; Hermans et al., 2011a). Our results indicate that exposure to carvacrol increased C. jejuni susceptibility to aforementioned stress factors (Figure 5). The SIC of carvacrol was also reported to increase the susceptibility of Salmonella Typhimurium to antibiotics (Johny et al., 2010). The increased susceptibility of C. jejuni in the presence of carvacrol could be because of the weakening of cell surface structure leading to increase cell membrane permeability (La Storia et al., 2011). Another reason for the increased susceptibility of C. jejuni could be a reduction in the expression of gene/proteins contributing to acid/bile stress tolerance. For example, Salaheen etal. (2018) observed that exposure of C. jejuni to phenolic extracts from blackberry and blueberry reduced the expression of tpx and ahpC genes.
To study the potential anti-Campylobacter mechanism of action(s) of carvacrol, LC-MS/MM–based proteome profiling was conducted. Liquid chromatography-tandem mass spectrometry analysis identified 232 and 313 proteins in C. jejuni S-8 and NCTC 81-176, respectively. We found that the exposure of C. jejuni to carvacrol led to modulation of select proteins essential for intestinal colonization in chickens in both the strains. For example, MCP is essential for sensing chemoattractants leading to activation of MCP-dependent signaling pathway and movement of C. jejuni to reach suitable sites for colonization in birds and humans (Li et al., 2014). Moreover, Vegge et al. (2009) reported that the deletion of select mcp genes (tlp1, tlp2, docB, docC) resulted in a 10-fold decrease in the ability of C. jejuni to invade human epithelial cells and chicken embryo cells. In the present study, the expression of MCP was reduced in both the strains of C. jejuni treated with carvacrol resulting in reduced motility as revealed by motility assay (Figure 2). Similarly, the reduction in adhered C. jejuni counts with carvacrol treatment could be owing to modulation of several proteins (AspA, AcnB, Icd, Fba, Ppa, AnsA, Ldh, Eno, SdhA, PurB-1) essential for growth and metabolism leading to deficiency of metabolic energy for various functions. The growth of C. jejuni by using amino acids (aspartate, glutamate, and proline) as an energy source is crucial for the persistence and colonization in the chicken gut. Guccione et al. (2008) had revealed that aspartate ammonia lyase (AspA) plays a key role in the amino acid–dependent growth of C. jejuni. Besides, AspA facilitates entry and survival of C. jejuni within epithelial cells (Novik et al., 2010). Similarly, chaperonin GroL strengthens adherence of C. jejuni to enterocytes (Ayllón et al., 2017). The anaerobic respiration in the gut is mediated by several respiratory proteins such as hydrogenase (HydB), dehydrogenase (SdhA), nitrate reductase (NapA, NapB), and nitrite reductase (NrfA), which play significant role in C. jejuni colonization in chickens (Weingarten et al., 2008; Kassem et al., 2012). Carvacrol reduced the expression of these proteins indicating that it hinders anaerobic respiration thereby inhibiting interactions with host cells. Although carvacrol affected proteins of similar functions in both the strains, the specific response of C. jejuni to the exposure of carvacrol varied between the strains. For example, carvacrol downregulated NapB in C. jejuni wild-type S-8, whereas the expression of NrfA, SdhA, and HydB proteins was reduced in C. jejuni NCTC 81-176 strain. In addition, exposure of C. jejuni wild-type S-8 to carvacrol altered expression of selected stress tolerance proteins (Table 1; upregulated AhpC and KatA, and downregulated Tpx); however, these proteins were not altered in C. jejuni NCTC 81-176 strain (Table 2). These findings indicate that carvacrol affects multiple proteins in C. jejuni, and the mechanism(s) of action could vary with strains.
Although found to be effective in reducing the expression of C. jejuni chicken colonization factors in vitro, the anti-Campylobacter efficacy of carvacrol needs further in vivo validation. Previous in vivo studies from our laboratory (Arsi et al., 2014) and elsewhere (Kelly et al., 2017) have produced inconsistent reductions in C. jejuni cecal counts in response to infeed supplementation of carvacrol. This result could be because of a variety of confounding factors such as binding of phytochemical to the feed, losses due to volatility, insufficient compound concentration in ceca, and so on. Future studies should investigate the potential of novel carrier systems (nanoparticles, emulsions) for enhanced/protected delivery of carvacrol to target sites such as the ceca.
In conclusion, we have demonstrated that carvacrol attenuates C. jejuni by decreasing motility, attachment, quorum sensing, and tolerance to stress in vitro. In addition, LC-MS/MS revealed modulation of select proteins that could potentially contribute to impaired colonization factor function in C. jejuni. However, in vivo studies are warranted to further validate these results.
Acknowledgments
This research was funded in part by the USDA-NIFA-OREI-2017-51300-26815. The authors would like to thank Dr. Joshua Lyte, USDA-ARS, Fayetteville, AR for the use of Cytation 5 for bioluminescence assay, and State Wide Mass Spectrometry Facility of University of Arkansas for the use of LC-MS/MS.
Disclaimer: Mention of a trade name, proprietary product, or specific equipment does not constitute a guarantee or warranty by the USDA and does not imply its approval to the exclusion of other products that may be suitable.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Allen V.M., Bull S.A., Corry J.E.L., Domingue G., Jørgensen F., Frost J.A., Whyte R., Gonzalez A., Elviss N., Humphrey T.J. Campylobacter spp. contamination of chicken carcasses during processing in relation to flock colonisation. Int. J. Food Microbiol. 2007;113:54–61. doi: 10.1016/j.ijfoodmicro.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Amalaradjou M.A.R., Narayanan A., Venkitanarayanan K. Trans-cinnamaldehyde decreases attachment and invasion of uropathogenic Escherichia coli in urinary tract epithelial cells by modulating virulence gene expression. J. Urol. 2011;185:1526–1531. doi: 10.1016/j.juro.2010.11.078. [DOI] [PubMed] [Google Scholar]
- Arsi K., Donoghue A.M., Venkitanarayanan K., Johny A.K., Fanatico A.C., Blore P.J., Donoghue D.J. The efficacy of the natural plant extracts, thymol and carvacrol against Campylobacter colonization in broiler chickens. J. Food Saf. 2014;34:321–325. [Google Scholar]
- Arsi K., Donoghue A.M., Woo-Ming A., Blore P.J., Donoghue D.J. The efficacy of selected probiotic and prebiotic combinations in reducing Campylobacter colonization in broiler chickens. J. Appl. Poult. Res. 2015;24:327–334. [Google Scholar]
- Ayllón N., Jiménez-Marín Á., Argüello H., Zaldívar-López S., Villar M., Aguilar C., Moreno A., La Fuente J.D., Garrido J.J. Comparative proteomics reveals differences in host-pathogen interaction between infectious and commensal relationship with Campylobacter jejuni. Front. Cell Infect. Microbiol. 2017;7:145. doi: 10.3389/fcimb.2017.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassler B.L., Wright M., Silverman M.R. Multiple signaling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol. Microbiol. 1994;13:273–286. doi: 10.1111/j.1365-2958.1994.tb00422.x. [DOI] [PubMed] [Google Scholar]
- Beery J.T., Hugdahl M.B., Doyle M.P. Colonization of gastrointestinal tracts of chicks by Campylobacter jejuni. Appl. Environ. Microbiol. 1988;54:2365–2370. doi: 10.1128/aem.54.10.2365-2370.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch K., Tiralongo J., Schmidt K., Matthias A., Bone K.M., Lehmann R., Tiralongo E. Investigations into the antiadhesive activity of herbal extracts against Campylobacter jejuni. Phytother. Res. 2011;25:1125–1132. doi: 10.1002/ptr.3384. [DOI] [PubMed] [Google Scholar]
- Beumer R.R., Vries J.D., Rombouts F.M. Campylobacter jejuni non-culturable coccoid cells. Int. J. Food Microbiol. 1992;15:153–163. doi: 10.1016/0168-1605(92)90144-r. [DOI] [PubMed] [Google Scholar]
- Bezek K., Kurinčič M., Knauder E., Klančnik A., Raspor P., Bucar F., Smole Možina S. Attenuation of adhesion, biofilm formation and quorum sensing of Campylobacter jejuni by Euodia ruticarpa. Phytother. Res. 2016;30:1527–1532. doi: 10.1002/ptr.5658. [DOI] [PubMed] [Google Scholar]
- Buckley A.M., Wang J., Hudson D.L., Grant A.J., Jones M.A., Maskell D.J., Stevens M.P. Evaluation of live-attenuated Salmonella vaccines expressing Campylobacter antigens for control of C. jejuni in poultry. Vaccine. 2010;28:1094–1105. doi: 10.1016/j.vaccine.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods-a review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Byrd J.A., Hargis B.M., Caldwell D.J., Bailey R.H., Herron K.L., McReynolds J.L., Brewer R.L., Anderson R.C., Bischoff K.M., Callaway T.R., Kubena L.F. Effect of lactic acid administration in the drinking water during preslaughter feed withdrawal on Salmonella and Campylobacter contamination of broilers. Poult. Sci. 2001;80:278–283. doi: 10.1093/ps/80.3.278. [DOI] [PubMed] [Google Scholar]
- Byrne C.M., Clyne M., Bourke B. Campylobacter jejuni adhere to and invade chicken intestinal epithelial cells in vitro. Microbiology. 2007;153:561–569. doi: 10.1099/mic.0.2006/000711-0. [DOI] [PubMed] [Google Scholar]
- Carrillo C.L., Atterbury R.J., El-Shibiny A., Connerton P.L., Dillon E., Scott A., Connerton I.F. Bacteriophage therapy to reduce Campylobacter jejuni colonization of broiler chickens. Appl. Environ. Microbiol. 2005;71:6554–6563. doi: 10.1128/AEM.71.11.6554-6563.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo S., Heredia N., Arechiga-Carvajal E., García S. Citrus extracts as inhibitors of quorum sensing, biofilm formation and motility of Campylobacter jejuni. Food Biotechnol. 2014;28:106–122. [Google Scholar]
- Cha W., Mosci R., Wengert S.L., Singh P., Newton D.W., Salimnia H., Lephart P., Khalife W., Mansfield L.S., Rudrik J.T., Manning S.D. Antimicrobial susceptibility profiles of human Campylobacter jejuni isolates and association with phylogenetic lineages. Front. Microbiol. 2016;7:589. doi: 10.3389/fmicb.2016.00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintoan-Uta C., Cassady-Cain R.L., Stevens M.P. Evaluation of flagellum-related proteins FliD and FspA as subunit vaccines against Campylobacter jejuni colonisation in chickens. Vaccine. 2016;34:1739–1743. doi: 10.1016/j.vaccine.2016.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody A.J., McCarthy N.D., van Rensburg M.J., Isinkaye T., Bentley S.D., Parkhill J., Dingle K.E., Bowler I.C., Jolley K.A., Maiden M.C. Real-time genomic epidemiological evaluation of human Campylobacter isolates by use of whole-genome multilocus sequence typing. J. Clin. Microbiol. 2013;51:2526–2534. doi: 10.1128/JCM.00066-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon A.S., Shivaprasad H.L., Schaberg D., Wier F., Weber S., Bandli D. Campylobacter jejuni infection in broiler chickens. Avian Dis. 2006;50:55–58. doi: 10.1637/7411-071405R.1. [DOI] [PubMed] [Google Scholar]
- EFSA Scientific opinion on quantification of the risk posed by broiler meat to human campylobacteriosis in the EU. EFSA J. 2010;8:1–89. [Google Scholar]
- Food and Drug Administration Code of Federal Regulations title 21 part 172. 2012. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?.CFRPart1/4172 Accessed Sept. 2019.
- Gradel K.O., Nielsen H.L., Schønheyder H.C., Ejlertsen T., Kristensen B., Nielsen H. Increased short- and long-term risk of inflammatory bowel disease after Salmonella or Campylobacter gastroenteritis. Gastroenterology. 2009;137:495–501. doi: 10.1053/j.gastro.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Guccione E., Leon-Kempis M.D.R., Pearson B.M., Hitchin E., Mulholland F., Van Diemen P.M., Stevens M.P., Kelly D.J. Amino acid-dependent growth of Campylobacter jejuni: key roles for aspartase (AspA) under microaerobic and oxygen-limited conditions and identification of AspB (Cj0762), essential for growth on glutamate. Mol. Microbiol. 2008;69:77–93. doi: 10.1111/j.1365-2958.2008.06263.x. [DOI] [PubMed] [Google Scholar]
- Hermans D., Van Deun K., Martel A., Van Immerseel F., Messens W., Heyndrickx M., Haesebrouck F., Pasmans F. Colonization factors of Campylobacter jejuni in the chicken gut. Vet. Res. 2011;42:82. doi: 10.1186/1297-9716-42-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans D., Van Deun K., Messens W., Martel A., Van Immerseel F., Haesebrouck F., Rasschaert G., Heyndrickx M., Pasmans F. Campylobacter control in poultry by current intervention measures ineffective: urgent need for intensified fundamental research. Vet. Microbiol. 2011;152:219–228. doi: 10.1016/j.vetmic.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Hoffmann S., Batz M.B., Morris J.G., Jr. Annual cost of illness and quality-adjusted life year losses in the United States due to 14 foodborne pathogens. J. Food Prot. 2012;75:1292–1302. doi: 10.4315/0362-028X.JFP-11-417. [DOI] [PubMed] [Google Scholar]
- Holley R.A., Patel D. Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol. 2005;22:273–292. [Google Scholar]
- Hue O., Le Bouquin S., Laisney M.J., Allain V., Lalande F.O., Petetin I., Rouxel S., Quesne S.G.N., Gloaguen P.Y., Picherot M.L., Santolini J., Salvat G., Bougeard S.P., Chemaly M. Prevalence of and risk factors for Campylobacter spp. contamination of broiler chicken carcasses at the slaughter- house. Food Microbiol. 2010;27:992–999. doi: 10.1016/j.fm.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Humphery T.J., Henley A., Lanning D.G. The colonization of broiler chickens with Campylobacter jejuni: some epidemiological investigations. Epidemiol. Infect. 1993;110:601–607. doi: 10.1017/s0950268800051025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johny A.K., Hoagland T., Venkitanarayanan K. Effect of subinhibitory concentrations of plant-derived molecules in increasing the sensitivity of multidrug-resistant Salmonella enterica serovar Typhimurium DT104 to antibiotics. Foodborne Pathog. Dis. 2010;7:1165–1170. doi: 10.1089/fpd.2009.0527. [DOI] [PubMed] [Google Scholar]
- Kassem I.I., Khatri M., Esseili M.A., Sanad Y.M., Saif Y.M., Olson J.W., Rajashekara G. Respiratory proteins contribute differentially to Campylobacter jejuni’s survival and in vitro interaction with hosts’ intestinal cells. BMC Microbiol. 2012;12:258. doi: 10.1186/1471-2180-12-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C., Gundogdu O., Pircalabioru G., Cean A., Scates P., Linton M., Pinkerton L., Magowan E., Stef L., Simiz E., Pet I. The in vitro and in vivo effect of carvacrol in preventing Campylobacter infection, colonization and in improving productivity of chicken broilers. Foodborne Pathog. Dis. 2017;14:341–349. doi: 10.1089/fpd.2016.2265. [DOI] [PubMed] [Google Scholar]
- Klančnik A., Šikić Pogačar M., Trošt K., Tušek Žnidarič M., Mozetič Vodopivec B., Smole Možina S. Anti-Campylobacter activity of resveratrol and an extract from waste Pinot noir grape skins and seeds, and resistance of C. jejuni planktonic and biofilm cells, mediated via the CmeABC efflux pump. J. Appl. Microbiol. 2017;122:65–77. doi: 10.1111/jam.13315. [DOI] [PubMed] [Google Scholar]
- Koo O.K., Amalaradjou M.A.R., Bhunia A.K. Recombinant probiotic expressing Listeria adhesion protein attenuates Listeria monocytogenes virulence in vitro. PLoS One. 2012;7:e29277. doi: 10.1371/journal.pone.0029277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Storia A., Ercolini D., Marinello F., Di Pasqua R., Villani F., Mauriello G. Atomic force microscopy analysis shows surface structure changes in carvacrol-treated bacterial cells. Res. Microbiol. 2011;162:164–172. doi: 10.1016/j.resmic.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Li Z., Lou H., Ojcius D.M., Sun A., Sun D., Zhao J., Lin X., Yan J. Methyl-accepting chemotaxis proteins 3 and 4 are responsible for Campylobacter jejuni chemotaxis and jejuna colonization in mice in response to sodium deoxycholate. J. Med. Microbiol. 2014;63:343–354. doi: 10.1099/jmm.0.068023-0. [DOI] [PubMed] [Google Scholar]
- Line J.E. Development of a selective differential agar for isolation and enumeration of Campylobacter spp. J. Food Prot. 2001;64:1711–1715. doi: 10.4315/0362-028x-64.11.1711. [DOI] [PubMed] [Google Scholar]
- Luangtongkum T., Jeon B., Han J., Plummer P., Logue C.M., Zhang Q. Antibiotic resistance in Campylobacter: emergence, transmission and persistence. Future Microbiol. 2009;4:189–200. doi: 10.2217/17460913.4.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E.P., Cieslak P.R., Cronquist A.B., Dunn J., Lathrop S., Rabatsky-Ehr T., Ryan P., Smith K., Tobin-D'Angelo M., Vugia D.J., Zansky S. Incidence and trends of infections with pathogens transmitted commonly through food and the effect of increasing use of culture-independent diagnostic tests on surveillance-foodborne diseases active surveillance network, 10 US Sites, 2013-2016. MMWR Morb. Mortal. Wkly. Rep. 2017;66:397–403. doi: 10.15585/mmwr.mm6615a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertins S., Allan B.J., Townsend H.G., Köster W., Potter A.A. Role of motAB in adherence and internalization in polarized Caco-2 cells and in cecal colonization of Campylobacter jejuni. Avian Dis. 2013;57:116–122. doi: 10.1637/10235-050412-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Miyamoto K.N., Monteiro K.M., da Silva Caumo K., Lorenzatto K.R., Ferreira H.B., Brandelli A. Comparative proteomic analysis of Listeria monocytogenes ATCC 7644 exposed to a sublethal concentration of nisin. J. Proteomics. 2015;119:230–237. doi: 10.1016/j.jprot.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Morooka T., Umeda A., Amako K. Motility as an intestinal colonization factor for Campylobacter jejuni. Microbiology. 1985;131:1973–1980. doi: 10.1099/00221287-131-8-1973. [DOI] [PubMed] [Google Scholar]
- Niu C., Gilbert E.S. Colorimetric method for identifying plant essential oil components that affect biofilm formation and structure. Appl. Environ. Microbiol. 2004;70:6951–6956. doi: 10.1128/AEM.70.12.6951-6956.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novik V., Hofreuter D., Galán J.E. Identification of Campylobacter jejuni genes involved in its interaction with epithelial cells. Infect. Immun. 2010;78:3540–3553. doi: 10.1128/IAI.00109-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins D.N., Pappin D.J., Creasy D.M., Cottrell J.S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Quiñones B., Miller W.G., Bates A.H., Mandrell R.E. Autoinducer-2 production in Campylobacter jejuni contributes to chicken colonization. Appl. Environ. Microbiol. 2009;75:281–285. doi: 10.1128/AEM.01803-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Hernandez A., Rupnow J., Hutkins R.W. Adherence reduction of Campylobacter jejuni and Campylobacter coli strains to HEp-2 cells by mannan oligosaccharides and a high-molecular-weight component of cranberry extract. J. Food Prot. 2015;78:1496–1505. doi: 10.4315/0362-028X.JFP-15-087. [DOI] [PubMed] [Google Scholar]
- Rath N.C., Liyanage R., Gupta A., Packialakshmi B., Lay J.O., Jr. A method to culture chicken enterocytes and their characterization. Poult. Sci. 2018;97:4040–4047. doi: 10.3382/ps/pey248. [DOI] [PubMed] [Google Scholar]
- Salaheen S., Nguyen C., Hewes D., Biswas D. Cheap extraction of antibacterial compounds of berry pomace and their mode of action against the pathogen Campylobacter jejuni. Food Control. 2014;46:174–181. [Google Scholar]
- Salaheen S., Tabashsum Z., Gaspard S., Dattilio A., Tran T.H., Biswas D. Reduced Campylobacter jejuni colonization in poultry gut with bioactive phenolics. Food Control. 2018;84:1–7. [Google Scholar]
- Scallan E., Hoekstra R.M., Angulo F.J., Tauxe R.V., Widdowson M., Roy S.L., Jones J.L., Griffin P.M. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 2011;17:7. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S., Wagle B.R., Upadhyay A., Arsi K., Donoghue D.J., Donoghue A.M. Carvacrol antimicrobial wash treatments reduce Campylobacter jejuni and aerobic bacteria on broiler chicken skin. Poult. Sci. 2019;98:4073–4083. doi: 10.3382/ps/pez198. [DOI] [PubMed] [Google Scholar]
- Shrestha S., Wagle B.R., Upadhyay A., Arsi K., Upadhyaya I., Donoghue D.J., Donoghue A.M. Edible coatings fortified with carvacrol reduce Campylobacter jejuni on chicken wingettes and modulate expression of select virulence genes. Front. Microbiol. 2019;10:583. doi: 10.3389/fmicb.2019.00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S., Arsi K., Wagle B.R., Donoghue A.M., Donoghue D.J. The ability of select probiotics to reduce enteric Campylobacter colonization in broiler chickens. Int. J. Poult. Sci. 2017;16:37–42. [Google Scholar]
- Šikić Pogačar M., Klančnik A., Bucar F., Langerholc T., Smole Možina S. Alpinia katsumadai extracts inhibit adhesion and invasion of Campylobacter jejuni in animal and human foetal small intestine cell lines. Phytother. Res. 2015;29:1585–1589. doi: 10.1002/ptr.5396. [DOI] [PubMed] [Google Scholar]
- Šikić Pogačar M., Klančnik A., Bucar F., Langerholc T., Smole Možina S. Anti-adhesion activity of thyme (Thymus vulgaris L.) extract, thyme post-distillation waste, and olive (Olea europea L.) leaf extract against Campylobacter jejuni on polystyrene and intestine epithelial cells. J. Sci. Food Agric. 2016;96:2723–2730. doi: 10.1002/jsfa.7391. [DOI] [PubMed] [Google Scholar]
- Šimunović K., Ramić D., Xu C., Smole Možina S. Modulation of Campylobacter jejuni motility, adhesion to polystyrene surfaces, and invasion of INT407 cells by quorum-sensing inhibition. Microorganisms. 2020;8:104. doi: 10.3390/microorganisms8010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller R.C. Role of infection in irritable bowel syndrome. J. Gastroenterol. 2007;42:41–47. doi: 10.1007/s00535-006-1925-8. [DOI] [PubMed] [Google Scholar]
- Stern N.J., Svetoch E.A., Eruslanov B.V., Kovalev Y.N., Volodina L.I., Perelygin V.V., Mitsevich E.V., Mitsevich I.P., Levchuk V.P. Paenibacillus polymyxa purified bacteriocin to control Campylobacter jejuni in chickens. J. Food Prot. 2005;68:1450–1453. doi: 10.4315/0362-028x-68.7.1450. [DOI] [PubMed] [Google Scholar]
- Svetoch E.A., Stern N.J. Bacteriocins to control Campylobacter spp. in poultry-a review. Poult. Sci. 2010;89:1763–1768. doi: 10.3382/ps.2010-00659. [DOI] [PubMed] [Google Scholar]
- Upadhyay A., Arsi K., Wagle B.R., Upadhyaya I., Shrestha S., Donoghue A.M., Donoghue D.J. Trans-cinnamaldehyde, carvacrol, and eugenol reduce Campylobacter jejuni colonization factors and expression of virulence genes in vitro. Front. Microbiol. 2017;2017:8. doi: 10.3389/fmicb.2017.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Alphen L.B., Burt S.A., Veenendaal A.K.J., Bleumink-Pluym N.M.C., Van Putten J.P.M. The natural antimicrobial carvacrol inhibits Campylobacter jejuni motility and infection of epithelial cells. PLoS One. 2012;7:e45343. doi: 10.1371/journal.pone.0045343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deun K., Pasmans F., Ducatelle R., Flahou B., Vissenberg K., Martel A., Van den Broeck W., Van Immerseel F., Haesebrouck F. Colonization strategy of Campylobacter jejuni results in persistent infection of the chicken gut. Vet. Microbiol. 2008;130:285–297. doi: 10.1016/j.vetmic.2007.11.027. [DOI] [PubMed] [Google Scholar]
- Vegge C.S., Brøndsted L., Li Y., Bang D.D., Ingmer H. Energy taxis drives Campylobacter jejuni toward the most favorable conditions for growth. Appl. Environ. Microbiol. 2009;75:5308–5314. doi: 10.1128/AEM.00287-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenaar J.A., Van Bergen M.A., Mueller M.A., Wassenaar T.M., Carlton R.M. Phage therapy reduces Campylobacter jejuni colonization in broilers. Vet. Microbiol. 2005;109:275–283. doi: 10.1016/j.vetmic.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Wagle B.R., Upadhyay A., Arsi K., Shrestha S., Venkitanarayanan K., Donoghue A.M., Donoghue D.J. Application of β-resorcylic acid as potential antimicrobial feed additive to reduce Campylobacter colonization in broiler chickens. Front. Microbiol. 2017;8:599. doi: 10.3389/fmicb.2017.00599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagle B.R., Arsi K., Upadhyay A., Shrestha S., Venkitanarayanan K., Donoghue A.M., Donoghue D.J. β-resorcylic acid, a phytophenolic compound, reduces Campylobacter jejuni in postharvest poultry. J. Food Prot. 2017;80:1243–1251. doi: 10.4315/0362-028X.JFP-16-475. [DOI] [PubMed] [Google Scholar]
- Wagle B.R., Upadhyay A., Upadhyaya I., Shrestha S., Arsi K., Liyanage R., Venkitanarayanan K., Donoghue D.J., Donoghue A.M. Trans-cinnamaldehyde, eugenol and carvacrol reduce Campylobacter jejuni biofilms and modulate expression of select genes and proteins. Front. Microbiol. 2019;10:1837. doi: 10.3389/fmicb.2019.01837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagle B.R., Marasini D., Upadhyaya I., Shrestha S., Arsi K., M Donoghue A., Carbonero F., Donoghue D.J., Maas K., Upadhyay A. Draft genome sequences of Campylobacter jejuni strains isolated from poultry. Microbiol. Resour. Announc. 2020;9:7. doi: 10.1128/MRA.01272-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten R.A., Grimes J.L., Olson J.W. Role of Campylobacter jejuni respiratory oxidases and reductases in host colonization. Appl. Environ. Microbiol. 2008;74:1367–1375. doi: 10.1128/AEM.02261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Kunlong B. Use of MTT assay for the determination of cell viability and proliferation. Immunol. J. 1992;4:016. [Google Scholar]