Abstract
Aim:
Over 7 million U.S. adults and about 20% of the military population have post-traumatic stress disorder (PTSD), a debilitating condition that is independently linked to a significantly greater risk of developing cardiovascular disease (CVD). Women have twice the probability of developing PTSD after experiencing a traumatic event compared to men. Existing literatures have reported higher inflammation and autonomic dysfunction including impaired baroreflex sensitivity, increased sympathetic reactivity and decreased parasympathetic activity in PTSD. However, most of these findings stem from studies conducted predominantly in males.
Methods:
We attempt in this narrative review to summarize the mixed literature available on sex differences in autonomic dysfunction and inflammation in PTSD, at rest and in response to stress in PTSD.
Results:
This review reveals that there is a paucity of research exploring autonomic function in females with PTSD. Recent studies have included female participants without probing for sex differences. A small number of studies have been conducted exclusively in women. Available data suggest that sympathetic nervous system output tends to be heightened, while parasympathetic activity and arterial baroreflex sensitivity appear more blunted in females with PTSD. Although few studies have investigated sex differences in inflammation in PTSD, data within females suggest chronic increases in inflammation with PTSD. This autonomic dysregulation and inflammation have also been described in males with PTSD.
Conclusion:
In sum, given the inherent biological differences in CVD clinical presentation and characteristics between men and women, human and animal studies aiming at elucidating sex differences in the pathophysiology of PTSD are needed.
Keywords: PTSD, Sex Differences, MSNA, Heart Rate Variability, Baroreflex, inflammation
INTRODUCTION
Adverse health outcomes linked to chronic stress exposure, including post-traumatic stress disorder (PTSD), are a major public health concern in the US and worldwide [1]. PTSD is a debilitating psychiatric illness characterized by persistent emotional and mental stress following trauma exposure (i.e., exposure to an event including death or threatened death, actual or threatened serious injury, or actual or threatened sexual violence) [2]. The health consequences of PTSD are substantial, affecting multiple organ systems. Multiple large epidemiological studies have demonstrated that PTSD is independently associated with a significantly greater risk of developing hypertension, other cardiovascular diseases (CVD), such as coronary artery disease, and mortality [3,4]. The mechanisms underlying increased hypertension and cardiovascular risk in PTSD remain unclear, but prior studies including from our laboratory and others report higher inflammation [5-7], alterations in autonomic function, including impaired baroreflex sensitivity, decreased resting parasympathetic nervous system (PNS) activity, and heightened reactivity of the sympathetic nervous system (SNS) during mental stress [7,8], all independent risk factors for CVD [9]. However, most of these studies were primarily conducted in men, with a few conducted exclusively in women and even fewer probing for sex differences within the PTSD patient population.
While premenopausal women are relatively protected from CVD compared to males, largely due to the protective effects of estrogen, a diagnosis of PTSD in women increases their CVD risk by up to 3-fold [10]. The lack of studies probing for sex differences in the biological mechanisms underlying risk for CVD in PTSD is problematic, as PTSD disproportionally affects women over men [11,12]. The lifetime prevalence of PTSD is about 10–12% in women and 5–6% in men [13]. This difference in PTSD prevalence mirrors the sex difference for comorbid disorders such as major depression and anxiety disorders. It has been hypothesized that the high prevalence of PTSD in women may be due to a greater vulnerability to trauma [13,14], abnormal immune responses [15] or dysregulation of neuroendocrine pathways involved in fear processing [16]. In women, trauma has been associated with the development of more severe psychiatric disorders compared to men [14]. Furthermore, men and women experience different types of trauma, both in private life and at work, with women being exposed to more high-impact trauma (e.g. sexual trauma) than men [13]. However, the observed sex difference in vulnerability to trauma is not entirely due to women experiencing more interpersonal violence, such as sexual assaults, than men [17]. The sex difference in PTSD risk may also be attributed to biological differences in how women respond to traumatic events [18]. For example, women present with higher re-experiencing symptoms such as physiological cue reactivity to familiar trauma [18]. Moreover, sex differences in fear processing (acquisition/learning and extinction), with women showing higher fear acquisition and intrusive memories, may also contribute to the sex difference in development of PTSD [19,20]. In sum, the predisposition to comorbid psychiatric disorders, high interpersonal violence (sexual assaults), heightened fear acquisition, impaired fear extinction and frequent intrusive memories contribute to increased incidence and prevalence of PTSD in women. These factors have resulted in an alarmingly growing rate of young women (both military veterans and civilian) with significantly greater CVD risk by virtue of having PTSD.
The current review is intended to be a narrative review of studies that probed for sex differences in autonomic function and inflammation at rest and in response to stress in PTSD. We conducted an ad hoc search on PubMed in the fields of psychology and autonomic control and included studies that best highlighted the need to probe for sex differences in the pathophysiology of PTSD. We did not include or exclude studies based on their quality or potential biases in their design. We synthesized available data to support the viewpoint that inflammation and autonomic nervous system reactivity to acute stress may differ between males and females, and that human and animal studies aimed at elucidating sex differences in the pathophysiology of PTSD are needed.
OVERVIEW OF PTSD
PTSD is a chronic illness that develops following a traumatic event and is associated with significant morbidity and mortality [2]. PTSD is highly prevalent amongst both the military and general population; up 20% of post 9/11 military veterans [21] and 7.8% of the general population will meet the diagnostic criteria for PTSD in their lifetime. The incidence of PTSD is expected to continue to rise given prolonged and ongoing military conflicts, increased natural disasters, societal violence and sexual assaults [21]. Symptoms of PTSD include hyperarousal, flashbacks, intrusive thoughts, or nightmares, and avoidance of activities that trigger memories of the traumatic event. The American Psychiatric Association recently revised the diagnostic criteria for PTSD (Diagnostic and Statistical Manual of Mental Disorders (5th Edition), 2013 [22]). In order to be diagnosed with PTSD, one must have been exposed to one or more traumatic events that may include direct exposure, witnessing in person, indirect exposure, or repeated indirect exposure to death, actual or threatened serious injury, or actual or threatened sexual violence. The symptoms of PTSD are clustered into four categories: (1) intrusion symptoms, (2) avoidance symptoms (3) negative alterations in cognition and mood, and (4) alterations in arousal and reactivity [22]. The Clinician Administered PTSD Scale 5th edition (CAPS 5) is used to diagnose (score ≥ 25) and assess PTSD symptom severity [23]. PTSD is frequently associated with the occurrence of comorbid psychiatric disorders such as major depression and anxiety disorders in men and women [2,24], and other adverse health sequelae including cardiovascular diseases (CVD) [3].
The symptoms observed in PTSD are associated with profound and well-described brain modifications [25]. PTSD is characterized by morphological changes in brain areas implicated in the stress response including the prefrontal cortex, anterior cingulate, the insula, the amygdala and the hippocampus [25,15]. Findings from animal studies corroborate human studies showing morphological and functional changes in hippocampal and anterior cingulate volumes, amygdala function, medial prefrontal/anterior cingulate function in PTSD [25]. In particular, the hippocampus, the part of the brain that forms memories, shows a significant decrease in volume and plasticity in PTSD [26]. The amygdala, significant for creating affective feelings and enhancing memory consolidation by arousal, is perhaps the most strongly implicated brain structure in the pathophysiology of PTSD [27]. Significant associations between smaller amygdala volumes and PTSD have been reported [27]. Moreover, women tend to have a more sustained amygdala response to repeated negative stimuli compared to men [28], resulting in intense emotion such as fear. This might be one reason why women who have experienced certain traumatic events are more likely than men to develop PTSD, even when the type of trauma is similar for both [29].
PTSD: GENETICS AND EPIGENETICS
Twin studies of PTSD risk show that the disorder is 30-40% heritable [30]. Single nucleotide polymorphisms (SNP)-based heritability estimates of 5-20% have been demonstrated in a multi-ethnic cohort including over 30,000 PTSD cases and 170,000 controls [31]. It is important to point out that these genetic variations appear to vary with sex. Among the genome-wide significant loci identified, analyses stratified by sex found three additional loci that confer risk in men [31]. Furthermore, a sex-specific association of pituitary adenylate cyclase-activating polypeptide (PACAP) blood levels confers risk for deficits in fear physiology, PTSD diagnosis and symptoms in females in a manner that is dependent on a SNP in the PACAP receptor gene (rs2267735) occuring in a putative estrogen response element [32]. Associations between PTSD and SNPs in genes important for the regulation the HPA axis, such as FKBP5 locus [33], and the immune system, such as C-Reactive Protein (CRP) gene [34,35] and the Human Leukocyte Antigen (HLA) genes [36] have also been described, supporting the notion that genetics play a role in contributing to individual risk for PTSD. The HLA, the major histocompatibility complex in humans, is an important part of the immune system.
Epigenetics are also implicated in risk for PTSD [37], as the development of PTSD is dependent upon trauma exposure [2]. The primary type of epigenetic modifications that have been associated with PTSD is DNA methylation [38-40], including methylation at gene loci involved in neuronal plasticty and hypothalamic–pituitary–adrenal (HPA) axis signaling [41]. Specifically, meta-analysis of PTSD epigenome-wide association studies in trauma-exposed civilians found two CpG sites within the protein coding region of the neuregulin 1 (NRG1; cg23637605) and the hepatocyte growth factor (HGS; cg19577098) genes that were significantly associated with PTSD [39]. These genes/proteins have been implicated in neural development and synaptic plasticity (NRG1) or associated with endocytosis and exocytosis which are important for immunity (HGS). Additionally, in a study of military members, blood-derived DNA methylation data collected before and after combat identified several epigenome-wide significant CpG islands (sites where cytosine lies next to guanine in the DNA sequence) [40], including the CpGs located in mitotic arrest deficient 1 like 1 (MAD1L1; cg12169700) and hexosaminidasecontaining protein (HEXDC; cg20756026), and four significant methylated regions situated in the HLA genomic region [40]. MAD1L1 ensures correct chromosome separation during mitosis and its malfunction could contribute to chromosome instability; while HEXDC is associated with rheumatoid arthritis, a chronic inflammatory condition. Of note, sex differences in DNA methylation profiles have recently been reported. Kim et al found elevated monocyte proportions in males but not females with lifetime history of PTSD [42], suggesting changes in the gene expression of these important immune cells. Given the focus of the current review, please refer to recent articles by Ryan et al. [41], Sheerin et al. [43] and others [44,45] for further insight into the genetics and epigenetics of PTSD.
PTSD AND SEX DIFFERENCES
PTSD is independently linked to a significantly greater risk of developing hypertension and CVD in both men and women [1]. While premenopausal women are relatively protected from CVD compared to males, largely due to the potential protective effects of estrogen, a diagnosis of PTSD in women increases their CVD risk by up to three-fold [10]. Of great public health concern, women are twice as likely as men to develop PTSD after a traumatic event [11,12]. These factors have resulted in an alarmingly growing rate of young women with significantly greater CVD risk by virtue of having PTSD. Factors driving high PTSD risk and associated CVD risk in women compared to men could be related to sex differences in hypothalamic pituitary adrenal (HPA) axis responsiveness, release of neuroendocrine steroid hormones, inflammatory response and autonomic reactivity to a stress stimulus, whether it is the initial traumatic event or subsequent stressors. Figure 1 summarizes putative factors contributing to CVD risk in women with PTSD as discussed below.
Figure 1: Mechanisms associated with PTSD risk and severity in women.
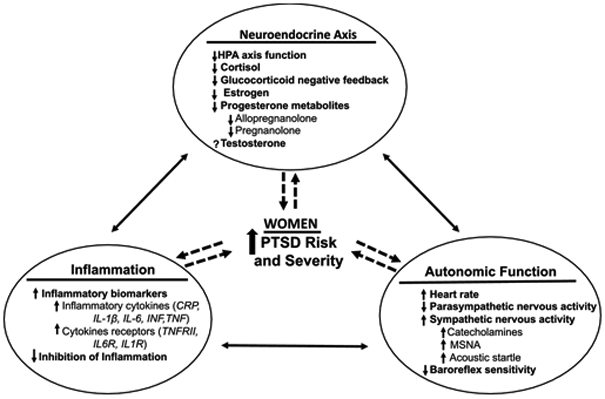
HPA, hypothalamo-pituitary axis; PTSD, post-traumatic stress disorder; CRP, C-reactive protein; IL-1, interleukin-1; IL-6, interleukin-6; INF, interferon; TNF, tumor necrotic factor; TNFR, tumor necrotic factor receptor; IL-6R, interleukin-6 receptor; IL-1R, interleukin-1 receptor; MSNA, muscle sympathetic nerve activity; Within each mechanism, arrow up = increase and arrow down = decrease; Between mechanisms, solid and dashed lines with arrows ↔, ← and → show the direction of the associations. Factors driving high PTSD risk and severity in women could be related to dysfunction in neuroendocrine axis, inflammation and autonomic function.
Sex Differences in Neuroendocrine Axis
HPA Axis
The neuroendocrine axis provides the structural and functional basis for interactions between the brain, hormones, and glands that allows the organism to respond to external stimuli like stress with complex, sometimes long-lasting physiological changes. PTSD is associated with dysfunction in the HPA axis and physiologic responses to stress [46]. Trauma survivors with PTSD show evidence of a highly sensitized HPA axis characterized by decreased basal cortisol levels and increased negative feedback regulation of the HPA axis [47]. Glucocorticoid response to an experimental stressor might have predictive value. Evidence suggests a state of low cortisol level in adult offspring of trauma survivors with PTSD, which in such offspring may increase the probability of PTSD development when exposed to a traumatic event [48]. Furthermore, an experiment in a large sample of healthy police officers revealed that those who responded to the stressor with significant increases in cortisol were more resilient or protected from developing chronic distress compared to those with no significant cortisol change in response to the challenge. Sex differences in HPA axis activation have also been reported. HPA axis has been found (in humans and rodents) to be more sensitive and to respond more strongly to acute stress in females than males [49,50], with HPA axis activity fluctuating with the ovarian cycle [50]. Interactions between circulating estradiol and the HPA axis might be one key mediator of the observed sex differences in PTSD susceptibility [51]. Estradiol especially seems to exert modulating effects on HPA functioning, including HPA responsiveness and its sensitivity to the negative feedback control of glucocorticoids [52]. For example, high estradiol has been shown to result in elevated cortisol response to stress [53]. Neuroimaging studies have shown greater activation of neural networks involved in fear acquisition when women are scanned during the early follicular (low estradiol) phase of their menstrual cycle relative to those scanned mid-cycle (high estradiol levels) [54]. Overall, these data seem to indicate that morphological and functional changes of the brain areas involved in the stress response, associated with dysfunction of the HPA axis, leads to blunted glucocorticoid negative feedback that contribute to the high risk of PTSD in women following a traumatic event. It can be hypothesized that on one hand, the greater stress reactivity observed in men leads to higher risk for diseases related to high levels of cortisol such as CVD and diabetes; while on the other hand, the lower cortisol response to stress observed in women stems from a hyporeactivity of the HPA, which is associated with an increased risk for stress disorders like PTSD.
Neuroendocrine steroid hormones/Gonadal steroid hormones.
The hypothalamic pituitary gonadal axis regulates the HPA axis activity via secretion of gonadal steroid hormones. Gonadal steroid hormones can also impact the HPA axis, which also have an important role in the neurobiology of PTSD. Reproductive hormones, such as estrogen, progesterone, and testosterone, have pivotal actions outside of the reproductive tract. Testosterone and estradiol are known, in both males and females, to influence brain function and behaviors [55]. Animal studies have shown that female rats that were conditioned and tested during proestrus (when estrogen levels are at their peak) showed less fear retention compared to both males and estrus females (when estrogen levels are lower) [56], implicating an important role of estrogen in hippocampal-dependent fear learning and memory. Estradiol supplementation in ovariectomized or aged animals have anxiety- and depressionreducing effects [57], and similar results were found in a human study [58]. In addition, testosterone replacement in older men have demonstrated improvement in spatial cognition, verbal memory, and working memory [59]. Several lines of evidence also suggest that testosterone supplementation might be helpful in the treatment of depressive disorders in men [60]. Finally, although estradiol-mediated effects seem to be the most potent modulators of stress regulation, some studies have also focused on the role of progesterone in fear, given that progesterone also fluctuates across phases of the menstrual cycle. In humans, women with PTSD showed impaired retention of extinction learning in the midluteal (high progesterone) phase of the menstrual cycle compared to women without PTSD [61], which was contrary to expected. However, a subsequent study in the same group of women examined metabolites of progesterone (allopregnanolone and pregnanolone) as a potential mechanism, given their anxiolytic properties. They found that women with PTSD had a deficit in the synthesis of those metabolites, suggesting that lower amounts of progesterone metabolites and not high progesterone per se, explain the findings related to poor extinction retention in PTSD [62]. For a comprehensive summary of studies on estradiol and progesterone in fear and PTSD, please refer to recent reviews [16,63]. In summary, although there has been limited focus on the role of progesterone and testosterone in fear memory processes compared to estradiol research, available evidence points to lower levels of estradiol and progesterone metabolites in the pathogenesis of PTSD.
Sex Differences in Autonomic Function
One of the hallmarks of PTSD is increased psychophysiological arousal driven by the autonomic nervous system. As seen in Table 1, over-activation of the sympathetic nervous system (SNS) including changes in heart rate (HR) and blood pressure (BP) [4,8], catecholamines [64], muscle sympathetic nerve activity (MSNA) [7,65], and skin conductance [66], have been described in PTSD, while parasympathetic nervous system (PNS) activity [67] is blunted. We have previously also reported high MSNA reactivity to acute stress and impaired arterial baroreflex sensitivity [7] in individuals with PTSD.
Table1:
Schematic representation of autonomic regulation in men and women with PTSD
| At Rest | BP | HR | PNS | SNS | BRS |
|---|---|---|---|---|---|
| Men | ⬌[6,7,92] | ⬌ [7,92] | ⬌? [6] | ⬌ [6,7] | |
| ⬆? [8] | ⬆[6,8,69-71] | ⬇[67,69,83,100] | ⬆[64,66,76] | ⬇[6,7] | |
| Women | ⬌? [65] | ⬌? [88] | |||
| ⬇? [72] | ⬆? [65] | ⬇? [84] | ⬆? [65] | ⬇? [93] | |
| ⬇? [72] | |||||
| Durinq Stress | BP | HR | PNS | SNS | BRS |
| Men | ⬌? [6] | ⬌ [6,69] | ⬌? [69] | ⬌? [6] | |
| ⬆? [7] | ⬆ [7,68,75] | ⬆? [87] | ⬆ [7,75] | ||
| ⬇? [6] | |||||
| Women | ⬌ [65,74] | ⬌? [87] | |||
| ⬆ [65,74] | ⬆? [73] | ⬇ [84,88] | ⬆ [65,74,99] | ⬇[92,93] |
BP, blood pressure; HR, heart rate; PNS, parasympathetic nervous system; SNS, sympathetic nervous system; BRS, baroreflex sensitivity. Left-right arrows denotes no differences when comparing PTSD to controls. Up arrows denote increases in PTSD when comparing to controls. Down arrows denote decreases in PTSD when comparing to controls. Question mark denotes unsure trend given the limited data (one study). The citations in bracket refer to the studies that have yielded those results.
Blood Pressure and Heart Rate
Previous studies assessing hemodynamic reactivity during mental stress in PTSD have shown mixed results. One of the most frequently reported indices of increased arousal in PTSD is elevated HR at rest and in response to negative stimuli [8,68]. While some have reported that PTSD patients had greater increases in HR and BP during combat related and non-combat related mental stress [4,7], others found that PTSD was characterized by a lack of hemodynamic responses to mental stress induced by recall of traumatic events [69]. Our laboratory showed that young veterans with PTSD have significantly greater increases in HR during virtual reality combat exposure compared to controls [7]. In the same study, we also noted heightened diastolic BP responses during mental arithmetic, suggesting that these exaggerated hemodynamic responses also extend to non-combat related mental stress in PTSD. Furthermore, in a study exploring the impact of symptom severity on cardiovascular measures, we found that resting HR tended to be higher in severe PTSD compared to controls [6]. In addition to its role in the symptomatology of PTSD, HR might also hold a predictive value. Elevated resting HR in the peritraumatic period in both males and females, is also considered as a predictor of the subsequent development of chronic PTSD [70,71]. Bryant et al. [71] also reported strong sensitivity and specificity in predicting PTSD using resting HR at discharge from the hospital, 2 to 26 days post-trauma exposure, but these predictions were not verified by other studies [72]. Therefore, although these studies showed early promise in using HR to predict PTSD, the reliance on hospital records and the intrinsic variability in HR post-trauma have limited its clinical usefulness.
The hemodynamic findings described above have been obtained in predominantly male populations. Few studies have examined sex differences in hemodynamic reactivity in PTSD. In a study of assault survivors, Kleim et al. reported that female participants who demonstrated increased HR in response to script-driven imagery had worse PTSD symptoms compared to male participants with increased HR response, and female participants who did not have an increased HR response [73]. Moreover, females with increased HR response were also three times more likely to have a PTSD diagnosis 6 months later [73]. Recently, in a study of 14 women with PTSD and 14 healthy controls, Yoo et al. found an early onset pressor response (despite comparable HR responses) at the first 30 seconds of static handgrip in women with PTSD compared to controls [74]. They also reported a significant association between early onset augmented exercise pressor response and greater awake systolic blood pressure variability in women with PTSD but not in healthy controls [74], and concluded that the early onset exercise pressor reflex in women with PTSD might be one mechanism underlying the link between PTSD and greater CVD risk. Additionally, the same group of women with PTSD exhibited a greater BP response to the cold pressor test compared with control subjects [65]. Together, these data support high resting HR and hemodynamic reactivity in women with PTSD.
Sympathetic Nervous System activity
Catecholamines.
Catecholamines are the end result of SNS activation and have been found to be elevated in plasma [75], urine [64] and cerebrospinal fluid [76] of patients with PTSD. Peripheral and central concentrations of norepinephrine are augmented in individuals with PTSD at baseline [76] and following exposure to stressful stimuli [75]. Likewise, urinary norepinephrine levels have also been shown to be significantly elevated in PTSD patients compared to controls without PTSD [64]. When sex differences were probed in a recent meta-analysis, no significant differences between PTSD and controls were found in relation to sex/gender of the study participants [77]. Increased norepinephrine production in PTSD in response to stressful or threatening stimuli can in turn induce cytokine release [78], resulting in increased inflammation. Increased catecholamines can also disrupt normal memory processing and could sustain distress associated with reexperiencing and reprocessing of the traumatic event in the initial post-trauma stages [15]. The estimation of SNS activation via catecholamine quantification in PTSD has been extensively covered in the following review [77].
Muscle Sympathetic Nerve Activity (MSNA).
While plasma norepinephrine levels provide an indirect measure of global SNS activation, microneurography remains the gold-standard for measuring SNS activity and reactivity in humans. Assessing MSNA in humans using microneurography in PTSD is a relatively new field of research with a PubMed search yielding seven results; the majority of studies in PTSD have previously relied on indirect measures of SNS activity. In our previous studies, where SNS activity was assessed using direct, intraneural measures of MSNA via microneurography [7], although resting MSNA was comparable between PTSD patients and controls, MSNA reactivity to acute mental stress was exaggerated in PTSD. Specifically, young veterans with PTSD had significantly greater increases in MSNA during virtual reality combat exposure compared to controls, suggesting a heightened sympathetic response to combat-related mental stress [7]. Likewise, MSNA responses to mental arithmetic were also higher in PTSD [7]. Although the sympathetic overactivity observed during virtual combat exposure extended to non-combat related mental stress in PTSD veterans, sympathetic reactivity was not augmented during non-baroreflex-mediated stimuli like cold pressor test. Thus, the augmented SNS responses in PTSD are specific to mental stress both related and unrelated to PTSD symptoms.
Although responses to the cold pressor test, a powerful sympathoexcitatory stimulus, was not augmented in PTSD patients in our previous study with predominantly male veterans [7], a recent study by Yoo et al. found that total sympathetic action potential discharge in response to the cold pressor test was markedly elevated in women with PTSD [65]. Women with PTSD exhibited increased firing of low-threshold axons as well as increased recruitment of latent subpopulations of larger-sized axons that are otherwise silent at baseline [65]. This finding might suggest that women with PTSD exhibit greater aberrant neural circulatory control in response to a sympathoexcitatory stimuli. Furthermore, in contrast to studies conducted in men, greater resting MSNA was reported in women with PTSD compared with healthy controls [65]. The same authors reported an earlier onset of MSNA activation during the first 30 seconds of static handgrip in women with PTSD compared with healthy controls [74]. The reported discrepancy between MSNA reactivity during cold pressor test and handgrip in women with PTSD can be attributed to the different reflex mechanisms stimulated by the cold pressor test versus static handgrip exercise. Although the cold pressor test stimulates the nociceptors and can be used to evaluate the central integration of vasomotor sympathetic processes and their efferent pathways, the exercise pressor reflex elicited through static handgrip exercise is dependent on a feedback loop arising from mechano- and metabo-sensitive afferent nerve endings in the skeletal muscle [74]. These two studies by Yoo et al. [65,74] expand our knowledge regarding physiological mechanisms that possibly contribute to an increased risk of developing CVD in women with PTSD.
Skin conductance and acoustic startle response.
Although microneurography is the gold-standard for measuring SNS activity in humans, it is an invasive and difficult technique not available to all. Therefore, an indirect measure of SNS activation that has received significant attention in the field of psychology is the skin conductance response. The skin conductance response is associated with both emotion and attention, and reflects sweat glad activity caused by arousal. In humans, the amplitude of skin conductance response is related to the level of arousal elicited by visual stimuli with either positive or negative emotional valence. Skin conductance response appears to be stronger at rest and in response to aversive stimuli among those with PTSD compared to control subjects [79]. In fearconditioning studies, both males and females with PTSD exhibit greater skin conductance to conditioned stimuli paired with aversive unconditioned stimuli during both fear acquisition and extinction phases than do trauma- and non-trauma-exposed controls without PTSD [79]. Additionally, skin conductance response to a trauma reminder in the Emergency Department in the acute aftermath of trauma exposure, positively correlates with the probability of developing chronic PTSD in a sample composed of both men and women [66]. In terms of sex differences, a study found that females with PTSD demonstrated stronger fear acquisition indexed by skin conductance response than males [80]. Furthermore, in a sample of trauma-exposed females, when skin conductance was used as the measure of conditioned fear, women with PTSD demonstrated worse retention of extinction learning, while controls without PTSD demonstrated better extinction retention [61]. Both groups were tested during the midluteal phase, suggesting that the levels of estradiol and progesterone in PTSD during the mid-luteal phase might not be as high as in the control group. With the paucity of studies investigating or reporting sex differences in SNS function in PTSD, much remains to be done to fully understand the development of PTSD and subsequent CVD in women.
Parasympathetic Nervous Activity
Another robust phenotype characteristic of PTSD that may contribute to increased cardiovascular risk is decreased parasympathetic drive as evidenced by decreased heart rate variability (HRV). HRV is a common measure of vagal control and represents the variability in time between heartbeats. Among individuals with PTSD, elevated resting HR may only be present among those with low HRV, suggesting that high HRV is a protective factor against the negative effects of elevated HR [81]. A prospective study of war-zone deployed male marines showed that post-deployment PTSD was higher in men with higher pre-deployment LF:HF ratios [82]. Studies in men and women with PTSD compared with trauma-exposed controls without PTSD, have demonstrated that HRV tends to be lower at rest and during challenge or provocation among those with PTSD, providing further evidence of decreased PNS activity in PTSD [83,100]. Hauschildt et al. reported similar reductions at baseline and in response to affective stimuli in a predominantly female sample of PTSD [84]. This impairment in cardiac PNS activity in PTSD seems to worsen in individuals with concomitant elevated resting blood pressure [85]. Additionally, we found that in response to acute mental stress via mental arithmetic, increasing PTSD symptom severity results in a greater reduction in HRV during mental stress in PTSD participants [6]. These findings suggest that PTSD severity is associated with greater parasympathetic nervous system withdrawal during acute stress.
Few studies have examined sex differences in PNS activity in patients with PTSD. A recent meta-analysis of conditions causing low resting respiratory sinus arrhythmia as observed in many mental health conditions, including anxiety disorders, found that females generally demonstrate greater HRV withdrawal during stress than males [86]. In contrast, Kamkwalala et al. did not observe a difference in HRV between highly traumatized females and controls [87], but instead observed an increase HRV in men with PTSD compared with men without PTSD. Interestingly, Keary et al. measured HRV in a group of 20 women with PTSD compared to 20 age-matched women controls, and found that women with PTSD had significantly greater reductions in high frequency HRV during a trauma-related and a non-trauma related stress task [88]. Taken together, these results suggest that PTSD might be associated with decreased parasympathetic control of the heart during stress in women.
Baroreflex Sensitivity
The arterial baroreflex modulates central sympathetic output in response to changes in blood pressure. Decreased arterial baroreflex sensitivity is an independent risk factor for hypertension and is associated with poorer clinical outcomes [89,90]. Chronic inflammation, a hallmark of PTSD, could contribute mechanistically to baroreflex sensitivity dysfunction via its direct effect on the vasculature, compromising the baroreceptor nerve endings or affecting its afferent and efferent tracts in the central nervous system prior to the development of hypertension [91]. A handful of studies have investigated the role of the baroreflex in the pathophysiology of PTSD. Our previous findings [7] and others [92,93] suggest that baroreflex sensitivity is impaired in PTSD and that the increased HR observed in PTSD participants, especially women [65] and individuals with severe PTSD [6], is possibly driven by differences in baroreceptor control. The failure of the baroreflex to elicit the appropriate changes in SNS activity and cardiac PNS activity may contribute to heightened neurocardiovascular reactivity during mental stress in PTSD. We demonstrated that PTSD patients have blunted baseline sympathetic and cardiovagal baroreflex sensitivity compared to controls [7]. Furthermore, we found that the impairment in cardiovagal baroreflex sensitivity might be more pronounced in those with more severe symptomatology, as cardiovagal baroreflex sensitivity remained intact in moderate PTSD [6]. Blunted cardiovagal BRS has been described in other patient populations characterized by increased CVD risk like hypertension [89] and atherosclerosis [90].
Of the very few studies that have examined baroreflex sensitivity in women with PTSD, Hughes et .al found reduced baroreflex sensitivity in 48 women with PTSD compared to 32 men with PTSD and 44 women without PTSD [92]. This blunted baroreflex sensitivity in women remained even after multivariate analyses controlling for major depression [93]. In response to an anger recall task, an acute psychological stressor, baroreflex sensitivity was further reduced in women with PTSD [93], The limited available literature on PTSD and baroreflex sensitivity thus suggest that the high CVD risk associated with PTSD may be linked to greater impairment in regulation of the autonomic nervous system by the arterial baroreflexes, and this impairment might be greater in women with PTSD than in men with PTSD.
Sex Differences in Inflammation
The inflammatory system has been increasingly studied over the last decade because of its role in the pathophysiology of chronic physical and mental illnesses like PTSD. The ultimate result of HPA axis activation in healthy individuals is increased level of circulating cortisol in the blood, and cortisol in turn inhibits inflammation. However, as highlighted above, PTSD is characterized by decreased HPA axis function and reduced ability of cortisol to inhibit inflammatory processes, which results in increased release of pro-inflammatory cytokines and overactivity of the sympathetic nervous system [15]. Several preclinical studies have shown that high levels of stress induce an exacerbation in the expression of pro-inflammatory cytokines such as interleukin (IL)-1β in both the plasma and brain, suggesting a mediating role of IL-1β in the neurochemical and behavioral consequences of stressors. Available data in PTSD have demonstrated elevated levels of several inflammatory biomarkers, such as C-reactive protein, IL-1β, IL-6, interferon gamma, and tumor necrosis factor alpha (TNF-α) [6,7,94]. Additionally, our laboratory recently showed elevated levels of TNFα, IL-1β and IL-6 and their related cytokine receptors (TNFRII, IL1RA and IL6R,) in a sample comprised of men and women with severe PTSD. Moreover, the notion that inflammation also confers risk for PTSD is supported by findings showing that higher concentrations of pre-deployment plasma CRP are associated prospectively with greater risk for post-deployment PTSD [95]. More recent data have shown however that lower TNFα and IFNγ concentrations in the immediate aftermath of trauma are associated with chronic PTSD development [96].
It is unclear if gonadal steroid hormones directly modulate the immunological response in PTSD, or achieve that effect via the HPA axis. While several studies have included female participants in their samples [79], only one recent study probed for sex differences in levels of pro-inflammatory cytokines in a sample of PTSD adults in comparison to age- and sex-matched controls [97]. The study demonstrated altered overnight levels of IL-6 and TNF-α in men and women with PTSD in comparison to healthy controls, but did not find any sex difference [97]. The paucity of studies highlights the need for additional sex-based research on the role of inflammation in the pathophysiology of PTSD. For more information on inflammation and the role of the immune system in PTSD, please refer to the following comprehensive reviews [15,98]. Ultimately, the overall increase in inflammatory cytokines in patients with PTSD may be mechanistically linked to autonomic dysfunction and higher CVD risk in both men and women with PTSD.
CONCLUSIONS/LIMITATIONS/FUTURE DIRECTIONS
The data presented here suggest that cycling estrogen levels during the menstrual cycle may modulate (see Summary Figure 2) the vulnerability to develop PTSD in women after trauma [99], as well as the risk for future CVD, such that traumatic events occurring during the low estrogen phase have a higher potential to cause PTSD-associated CVD. High estrogen may have neuroprotective actions on neuroendocrine stress pathways like the HPA axis and subsequent neuroinflammation activated by stress exposure [15]. More research is needed, especially research investigating sex differences in inflammation and autonomic activation within a representative population of PTSD patients. We do not suggest that our review is exhaustive or fully comprehensive. Moreover, the interpretation of the presented data was complicated by several methodological issues and limitations. For instance, most studies included almost exclusively males [7,6], while some tested mixed groups with either a majority of men [100] or women [72,84] and without reporting the effect of sex. A handful of studies focused exclusively on women [65,93,88] although sex differences in inflammation and neural mechanisms have not yet been clarified within the PTSD population. With more studies, systematic reviews and meta-analyses on these different outcomes could be conducted in the future. Prospective studies investigating changes in HPA, autonomic and inflammatory markers, before and after the diagnosis of PTSD would shed light on their respective roles in the pathophysiology of PTSD. Given reported [101] biological differences in CVD clinical presentation and characteristics between men and women, and the lack of studies focused on PTSD-related adverse health effects in women, there is a critical need to investigate early mechanistic pathways that increase CVD risk in preclinical populations of vulnerable women with PTSD. Elucidating such biological risk factors could potentially lead to novel treatment strategies.
Figure 2: Summary of stress-response mechanisms and modulatory role of estrogen.
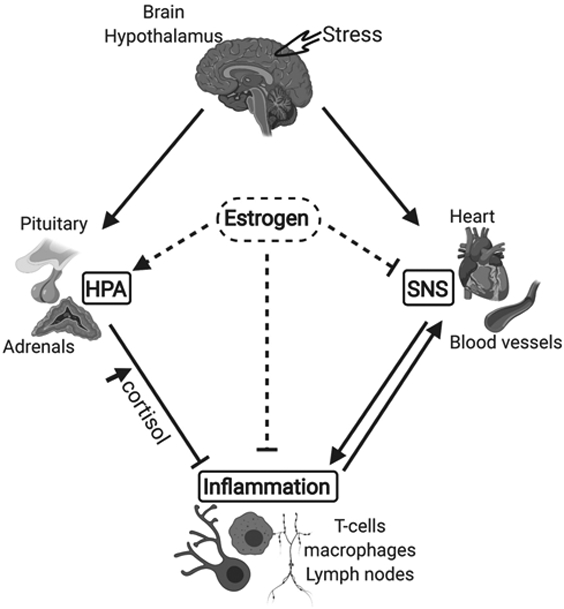
HPA, hypothalamic pituitary adrenal (HPA) axis; SNS, sympathetic nervous system. As a first response to a distress signal (Stress), the hypothalamus activates the SNS by sending signals through the sympathetic nerves to effector organs (heart, blood vessels). As the surge in sympathetic activity subsides, the hypothalamus activates the second component of the stress response system, the HPA axis. Cortisol, the end product of HPA axis stimulation exerts a negative control on inflammation, therefore inhibiting the inflammatory response and sympathetic stimulation. High circulating levels of estrogen stimulate the HPA axis while inhibiting inflammation and the SNS. Lines with arrow (↓) show excitatory effect. Lines with bar (⊥) show inhibitory effect.
Acknowledgments:
This work was supported by the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) under Award Numbers UL1TR002378 and KL2TR002381; NIH training grant T32 DK-00756; NIH Mental Health (NIMH) R01 MH115174; Emory Specialized Center of Research Excellence (SCORE) on Sex Differences, U54 AG062334; United States Department of Veterans Affairs Clinical Sciences Research and Development Program Merit Review Award I01CX001065; American Heart Association National Affiliate, Collaborative Sciences Award 15CSA24340001; NIH, National Heart Lung Blood Institute (NHLBI) R01 HL135183; NIH National Center for Complementary and Integrative Health (NCCIH) R61 AT010457; NIH training grant T32 DK-00756; Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and the Clinical Studies Center of the Atlanta VA Health Care System, Decatur, Georgia; and Foundation for Atlanta Veterans Education and Research (FAVER).
Funding: National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) under Award Numbers UL1TR002378 and KL2TR002381; NIH training grant T32 DK-00756; NIH Mental Health (NIMH) R01 MH115174; Emory Specialized Center of Research Excellence (SCORE) on Sex Differences, U54 AG062334; United States Department of Veterans Affairs Clinical Sciences Research and Development Program Merit Review Award I01CX001065; American Heart Association National Affiliate, Collaborative Sciences Award 15CSA24340001; NIH, National Heart Lung Blood Institute (NHLBI) R01 HL135183; NIH National Center for Complementary and Integrative Health (NCCIH) R61 AT010457; NIH training grant T32 DK-00756.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of interest/Competing interests: None
Ethics approval: 'Not applicable'
Consent to participate: 'Not applicable'
Consent for publication: Yes
Availability of data and material: All articles used in the review are published
Code availability: 'Not applicable'
REFERENCES
- 1.Edmondson D, von Känel R (2017) Post-traumatic stress disorder and cardiovascular disease. The lancet Psychiatry 4 (4):320–329. doi: 10.1016/s2215-0366(16)30377-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB (1995) Posttraumatic stress disorder in the National Comorbidity Survey. Archives of general psychiatry 52 (12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012 [DOI] [PubMed] [Google Scholar]
- 3.Edmondson D, Cohen BE (2013) Posttraumatic stress disorder and cardiovascular disease. Progress in cardiovascular diseases 55 (6):548–556. doi: 10.1016/j.pcad.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedi US, Arora R (2007) Cardiovascular manifestations of posttraumatic stress disorder. J Natl Med Assoc 99 (6):642–649 [PMC free article] [PubMed] [Google Scholar]
- 5.Pace TW, Heim CM (2011) A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain, behavior, and immunity 25 (1):6–13. doi: 10.1016/j.bbi.2010.10.003 [DOI] [PubMed] [Google Scholar]
- 6.Fonkoue IT, Marvar PJ, Norrholm S, Li Y, Kankam ML, Jones TN, Vemulapalli M, Rothbaum B, Douglas Bremner J, Le NA, Park J (2020) Symptom severity impacts sympathetic dysregulation and inflammation in post-traumatic stress disorder (PTSD). Brain, behavior, and immunity 83:260–269. doi: 10.1016/j.bbi.2019.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park J, Marvar PJ, Liao P, Kankam ML, Norrholm SD, Downey RM, McCullough SA, Le NA, Rothbaum BO (2017) Baroreflex dysfunction and augmented sympathetic nerve responses during mental stress in veterans with post-traumatic stress disorder. The Journal of physiology 595 (14):4893–4908. doi: 10.1113/jp274269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckley TC, Kaloupek DG (2001) A meta-analytic examination of basal cardiovascular activity in posttraumatic stress disorder. Psychosomatic medicine 63 (4):585–594 [DOI] [PubMed] [Google Scholar]
- 9.Grassi G (2009) Assessment of sympathetic cardiovascular drive in human hypertension: achievements and perspectives. Hypertension (Dallas, Tex : 1979) 54 (4):690–697. doi:HYPERTENSIONAHA.108.119883 [pii] 10.1161/HYPERTENSIONAHA.108.119883 [DOI] [PubMed] [Google Scholar]
- 10.Kubzansky LD, Koenen KC, Jones C, Eaton WW (2009) A prospective study of posttraumatic stress disorder symptoms and coronary heart disease in women. Health Psychol 28 (1):125–130. doi:2009-00026-006 [pii] 10.1037/0278-6133.28.1.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLean CP, Asnaani A, Litz BT, Hofmann SG (2011) Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. Journal of psychiatric research 45 (8):1027–1035. doi: 10.1016/j.jpsychires.2011.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL (1993) Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. Journal of consulting and clinical psychology 61 (6):984–991. doi: 10.1037//0022-006x.61.6.984 [DOI] [PubMed] [Google Scholar]
- 13.Olff M (2017) Sex and gender differences in post-traumatic stress disorder: an update. European journal of psychotraumatology 8 (sup4). doi: 10.1080/20008198.2017.1351204 [DOI] [Google Scholar]
- 14.Rivera JC, Hylden CM, Johnson AE (2015) Disability After Deployment Injury: Are Women and Men Service Members Different? Clinical orthopaedics and related research 473 (8):2448–2454. doi: 10.1007/s11999-015-4180-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T (2017) Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD, and Beyond. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 42 (1):254–270. doi: 10.1038/npp.2016.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravi M, Stevens JS, Michopoulos V (2019) Neuroendocrine pathways underlying risk and resilience to PTSD in women. Frontiers in neuroendocrinology 55:100790. doi: 10.1016/j.yfrne.2019.100790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tolin DF, Foa EB (2006) Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychological bulletin 132 (6):959–992. doi: 10.1037/0033-2909.132.6.959 [DOI] [PubMed] [Google Scholar]
- 18.Birkeland MS, Blix I, Solberg Ø, Heir T (2017) Gender Differences in Posttraumatic Stress Symptoms after a Terrorist Attack: A Network Approach. Frontiers in psychology 8:2091. doi: 10.3389/fpsyg.2017.02091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felmingham K, Williams LM, Kemp AH, Liddell B, Falconer E, Peduto A, Bryant R (2010) Neural responses to masked fear faces: sex differences and trauma exposure in posttraumatic stress disorder. Journal of abnormal psychology 119 (1):241–247. doi: 10.1037/a0017551 [DOI] [PubMed] [Google Scholar]
- 20.Olff M, Langeland W, Draijer N, Gersons BP (2007) Gender differences in posttraumatic stress disorder. Psychological bulletin 133 (2):183–204. doi: 10.1037/0033-2909.133.2.183 [DOI] [PubMed] [Google Scholar]
- 21.Committee on the Assessment of Ongoing Efforts in the Treatment of Posttraumatic Stress D, Board on the Health of Select P, Institute of M (2014). In: Treatment for Posttraumatic Stress Disorder in Military and Veteran Populations: Final Assessment. National Academies Press (US) Copyright 2014 by the National Academy of Sciences. All rights reserved, Washington (DC). doi: 10.17226/18724 [DOI] [Google Scholar]
- 22.First MB (2013) Diagnostic and statistical manual of mental disorders, 5th edition, and clinical utility. The Journal of nervous and mental disease 201 (9):727–729. doi: 10.1097/NMD.0b013e3182a2168a [DOI] [PubMed] [Google Scholar]
- 23.Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, Keane TM, Marx BP (2018) The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychological assessment 30 (3):383–395. doi: 10.1037/pas0000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rytwinski NK, Scur MD, Feeny NC, Youngstrom EA (2013) The co-occurrence of major depressive disorder among individuals with posttraumatic stress disorder: a meta-analysis. Journal of traumatic stress 26 (3):299–309. doi: 10.1002/jts.21814 [DOI] [PubMed] [Google Scholar]
- 25.Bremner JD (2006) Traumatic stress: effects on the brain. Dialogues in clinical neuroscience 8 (4):445–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS, Densmore M, Haswell CC, Ipser J, Koch SBJ, Korgaonkar M, Lebois LAM, Peverill M, Baker JT, Boedhoe PSW, Frijling JL, Gruber SA, Harpaz-Rotem I, Jahanshad N, Koopowitz S, Levy I, Nawijn L, O'Connor L, Olff M, Salat DH, Sheridan MA, Spielberg JM, van Zuiden M, Winternitz SR, Wolff JD, Wolf EJ, Wang X, Wrocklage K, Abdallah CG, Bryant RA, Geuze E, Jovanovic T, Kaufman ML, King AP, Krystal JH, Lagopoulos J, Bennett M, Lanius R, Liberzon I, McGlinchey RE, McLaughlin KA, Milberg WP, Miller MW, Ressler KJ, Veltman DJ, Stein DJ, Thomaes K, Thompson PM, Morey RA (2018) Smaller Hippocampal Volume in Posttraumatic Stress Disorder: A Multisite ENIGMA-PGC Study: Subcortical Volumetry Results From Posttraumatic Stress Disorder Consortia. Biological psychiatry 83 (3):244–253. doi: 10.1016/j.biopsych.2017.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morey RA, Gold AL, LaBar KS, Beall SK, Brown VM, Haswell CC, Nasser JD, Wagner HR, McCarthy G (2012) Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Archives of general psychiatry 69 (11):1169–1178. doi: 10.1001/archgenpsychiatry.2012.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andreano JM, Dickerson BC, Barrett LF (2014) Sex differences in the persistence of the amygdala response to negative material. Social cognitive and affective neuroscience 9 (9):1388–1394. doi: 10.1093/scan/nst127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breslau N (2009) The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma, violence & abuse 10 (3):198–210. doi: 10.1177/1524838009334448 [DOI] [PubMed] [Google Scholar]
- 30.Stein MB, Jang KL, Taylor S, Vernon PA, Livesley WJ (2002) Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. The American journal of psychiatry 159 (10):1675–1681. doi: 10.1176/appi.ajp.159.10.1675 [DOI] [PubMed] [Google Scholar]
- 31.Nievergelt CM, Maihofer AX, Klengel T, Atkinson EG, Chen CY, Choi KW, Coleman JRI, Dalvie S, Duncan LE, Gelernter J, Levey DF, Logue MW, Polimanti R, Provost AC, Ratanatharathorn A, Stein MB, Torres K, Aiello AE, Almli LM, Amstadter AB, Andersen SB, Andreassen OA, Arbisi PA, Ashley-Koch AE, Austin SB, Avdibegovic E, Babić D, Bæskvad-Hansen M, Baker DG, Beckham JC, Bierut LJ, Bisson JI, Boks MP, Bolger EA, Börglum AD, Bradley B, Brashear M, Breen G, Bryant RA, Bustamante AC, Bybjerg-Grauholm J, Calabrese JR, Caldas-de-Almeida JM, Dale AM, Daly MJ, Daskalakis NP, Deckert J, Delahanty DL, Dennis MF, Disner SG, Domschke K, Dzubur-Kulenovic A, Erbes CR, Evans A, Farrer LA, Feeny NC, Flory JD, Forbes D, Franz CE, Galea S, Garrett ME, Gelaye B, Geuze E, Gillespie C, Uka AG, Gordon SD, Guffanti G, Hammamieh R, Harnal S, Hauser MA, Heath AC, Hemmings SMJ, Hougaard DM, Jakovljevic M, Jett M, Johnson EO, Jones I, Jovanovic T, Qin XJ, Junglen AG, Karstoft KI, Kaufman ML, Kessler RC, Khan A, Kimbrel NA, King AP, Koen N, Kranzler HR, Kremen WS, Lawford BR, Lebois LAM, Lewis CE, Linnstaedt SD, Lori A, Lugonja B, Luykx JJ, Lyons MJ, Maples-Keller J, Marmar C, Martin AR, Martin NG, Maurer D, Mavissakalian MR, McFarlane A, McGlinchey RE, McLaughlin KA, McLean SA, McLeay S, Mehta D, Milberg WP, Miller MW, Morey RA, Morris CP, Mors O, Mortensen PB, Neale BM, Nelson EC, Nordentoft M, Norman SB, O'Donnell M, Orcutt HK, Panizzon MS, Peters ES, Peterson AL, Peverill M, Pietrzak RH, Polusny MA, Rice JP, Ripke S, Risbrough VB, Roberts AL, Rothbaum AO, Rothbaum BO, Roy-Byrne P, Ruggiero K, Rung A, Rutten BPF, Saccone NL, Sanchez SE, Schijven D, Seedat S, Seligowski AV, Seng JS, Sheerin CM, Silove D, Smith AK, Smoller JW, Sponheim SR, Stein DJ, Stevens JS, Sumner JA, Teicher MH, Thompson WK, Trapido E, Uddin M, Ursano RJ, van den Heuvel LL, Van Hooff M, Vermetten E, Vinkers CH, Voisey J, Wang Y, Wang Z, Werge T, Williams MA, Williamson DE, Winternitz S, Wolf C, Wolf EJ, Wolff JD, Yehuda R, Young RM, Young KA, Zhao H, Zoellner LA, Liberzon I, Ressler KJ, Haas M, Koenen KC (2019) International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nature communications 10 (1):4558. doi: 10.1038/s41467-019-12576-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V (2011) Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 470 (7335):492–497. doi: 10.1038/nature09856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ (2008) Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. Jama 299 (11):1291–1305. doi: 10.1001/jama.299.11.1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michopoulos V, Rothbaum AO, Jovanovic T, Almli LM, Bradley B, Rothbaum BO, Gillespie CF, Ressler KJ (2015) Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. The American journal of psychiatry 172 (4):353–362. doi: 10.1176/appi.ajp.2014.14020263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller MW, Maniates H, Wolf EJ, Logue MW, Schichman SA, Stone A, Milberg W, McGlinchey R (2018) CRP polymorphisms and DNA methylation of the AIM2 gene influence associations between trauma exposure, PTSD, and C-reactive protein. Brain, behavior, and immunity 67:194–202. doi: 10.1016/j.bbi.2017.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katrinli S, Lori A, Kilaru V, Carter S, Powers A, Gillespie CF, Wingo AP, Michopoulos V, Jovanovic T, Ressler KJ, Smith AK (2019) Association of HLA locus alleles with posttraumatic stress disorder. Brain, behavior, and immunity 81:655–658. doi: 10.1016/j.bbi.2019.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howie H, Rijal CM, Ressler KJ (2019) A review of epigenetic contributions to post-traumatic stress disorder . Dialogues in clinical neuroscience 21 (4):417–428. doi: 10.31887/DCNS.2019.21.4/kressler [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katrinli S, Stevens J, Wani AH, Lori A, Kilaru V, van Rooij SJH, Hinrichs R, Powers A, Gillespie CF, Michopoulos V, Gautam A, Jett M, Hammamieh R, Yang R, Wildman D, Qu A, Koenen K, Aiello AE, Jovanovic T, Uddin M, Ressler KJ, Smith AK (2020) Evaluating the impact of trauma and PTSD on epigenetic prediction of lifespan and neural integrity. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 45 (10):1609–1616. doi: 10.1038/s41386-020-0700-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uddin M, Ratanatharathorn A, Armstrong D, Kuan PF, Aiello AE, Bromet EJ, Galea S, Koenen KC, Luft B, Ressler KJ, Wildman DE, Nievergelt CM, Smith A (2018) Epigenetic meta-analysis across three civilian cohorts identifies NRG1 and HGS as blood-based biomarkers for post-traumatic stress disorder. Epigenomics 10 (12):1585–1601. doi: 10.2217/epi-2018-0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snijders C, Maihofer AX, Ratanatharathorn A, Baker DG, Boks MP, Geuze E, Jain S, Kessler RC, Pishva E, Risbrough VB, Stein MB, Ursano RJ, Vermetten E, Vinkers CH, Smith AK, Uddin M, Rutten BPF, Nievergelt CM (2020) Longitudinal epigenome-wide association studies of three male military cohorts reveal multiple CpG sites associated with post-traumatic stress disorder. Clinical epigenetics 12 (1):11. doi: 10.1186/s13148-019-0798-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryan J, Chaudieu I, Ancelin ML, Saffery R (2016) Biological underpinnings of trauma and post-traumatic stress disorder: focusing on genetics and epigenetics. Epigenomics 8 (11):1553–1569. doi: 10.2217/epi-2016-0083 [DOI] [PubMed] [Google Scholar]
- 42.Kim GS, Smith AK, Xue F, Michopoulos V, Lori A, Armstrong DL, Aiello AE, Koenen KC, Galea S, Wildman DE, Uddin M (2019) Methylomic profiles reveal sex-specific differences in leukocyte composition associated with post-traumatic stress disorder. Brain, behavior, and immunity 81:280–291. doi: 10.1016/j.bbi.2019.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheerin CM, Lind MJ, Bountress K, Nugent NR, Amstadter AB (2017) The Genetics and Epigenetics of PTSD: Overview, Recent Advances, and Future Directions. Current opinion in psychology 14:5–11. doi: 10.1016/j.copsyc.2016.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koenen KC, Nugent NR, Amstadter AB (2008) Gene-environment interaction in posttraumatic stress disorder: review, strategy and new directions for future research. European archives of psychiatry and clinical neuroscience 258 (2):82–96. doi: 10.1007/s00406-007-0787-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehta D, Binder EB (2012) Gene × environment vulnerability factors for PTSD: the HPA-axis. Neuropharmacology 62 (2):654–662. doi: 10.1016/j.neuropharm.2011.03.009 [DOI] [PubMed] [Google Scholar]
- 46.Yehuda R, Kahana B, Binder-Brynes K, Southwick SM, Mason JW, Giller EL (1995) Low urinary cortisol excretion in Holocaust survivors with posttraumatic stress disorder. The American journal of psychiatry 152 (7):982–986. doi: 10.1176/ajp.152.7.982 [DOI] [PubMed] [Google Scholar]
- 47.Yehuda R (1997) Sensitization of the hypothalamic-pituitary-adrenal axis in posttraumatic stress disorder. Annals of the New York Academy of Sciences 821:57–75. doi: 10.1111/j.1749-6632.1997.tb48269.x [DOI] [PubMed] [Google Scholar]
- 48.Yehuda R, Teicher MH, Seckl JR, Grossman RA, Morris A, Bierer LM (2007) Parental posttraumatic stress disorder as a vulnerability factor for low cortisol trait in offspring of holocaust survivors. Archives of general psychiatry 64 (9):1040–1048. doi: 10.1001/archpsyc.64.9.1040 [DOI] [PubMed] [Google Scholar]
- 49.Freidenberg BM, Gusmano R, Hickling EJ, Blanchard EB, Bremner JD, Frye C (2010) Women with PTSD have lower basal salivary cortisol levels later in the day than do men with PTSD: a preliminary study. Physiology & behavior 99 (2):234–236. doi: 10.1016/j.physbeh.2009.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oyola MG, Handa RJ (2017) Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity. Stress (Amsterdam, Netherlands) 20 (5):476–494. doi: 10.1080/10253890.2017.1369523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chrousos GP, Gold PW (1992) The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. Jama 267 (9):1244–1252 [PubMed] [Google Scholar]
- 52.Kudielka BM, Kirschbaum C (2005) Sex differences in HPA axis responses to stress: a review. Biological psychology 69 (1):113–132. doi: 10.1016/j.biopsycho.2004.11.009 [DOI] [PubMed] [Google Scholar]
- 53.Kirschbaum C, Schommer N, Federenko I, Gaab J, Neumann O, Oellers M, Rohleder N, Untiedt A, Hanker J, Pirke KM, Hellhammer DH (1996) Short-term estradiol treatment enhances pituitary-adrenal axis and sympathetic responses to psychosocial stress in healthy young men. The Journal of clinical endocrinology and metabolism 81 (10):3639–3643. doi: 10.1210/jcem.81.10.8855815 [DOI] [PubMed] [Google Scholar]
- 54.Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, Makris N (2005) Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. The Journal of neuroscience : the official journal of theSociety for Neuroscience 25 (40):9309–9316. doi: 10.1523/jneurosci.2239-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lan H-C, Lin J-H, Chen C-H, Chu P-W, Cheng C-P (2018) Estradiol level of male rat is correlated with depression and anxiety after traumatic stress. Journal of Medical Sciences 38:176 [Google Scholar]
- 56.Glover EM, Jovanovic T, Norrholm SD (2015) Estrogen and extinction of fear memories: implications for posttraumatic stress disorder treatment. Biological psychiatry 78 (3):178–185. doi: 10.1016/j.biopsych.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walf AA, Frye CA (2010) Estradiol reduces anxiety- and depression-like behavior of aged female mice. Physiology & behavior 99 (2):169–174. doi: 10.1016/j.physbeh.2009.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barth C, Villringer A, Sacher J (2015) Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Frontiers in neuroscience 9:37. doi: 10.3389/fnins.2015.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kenny AM, Fabregas G, Song C, Biskup B, Bellantonio S (2004) Effects of testosterone on behavior, depression, and cognitive function in older men with mild cognitive loss. The journals of gerontology Series A, Biological sciences and medical sciences 59 (1):75–78. doi: 10.1093/gerona/59.1.m75 [DOI] [PubMed] [Google Scholar]
- 60.Carnahan RM, Perry PJ (2004) Depression in aging men: the role of testosterone. Drugs & aging 21 (6):361–376. doi: 10.2165/00002512-200421060-00002 [DOI] [PubMed] [Google Scholar]
- 61.Pineles SL, Nillni YI, King MW, Patton SC, Bauer MR, Mostoufi SM, Gerber MR, Hauger R, Resick PA, Rasmusson AM, Orr SP (2016) Extinction retention and the menstrual cycle: Different associations for women with posttraumatic stress disorder. Journal of abnormal psychology 125 (3):349–355. doi: 10.1037/abn0000138 [DOI] [PubMed] [Google Scholar]
- 62.Pineles SL, Nillni YI, Pinna G, Irvine J, Webb A, Arditte Hall KA, Hauger R, Miller MW, Resick PA, Orr SP, Rasmusson AM (2018) PTSD in women is associated with a block in conversion of progesterone to the GABAergic neurosteroids allopregnanolone and pregnanolone measured in plasma. Psychoneuroendocrinology 93:133–141. doi: 10.1016/j.psyneuen.2018.04.024 [DOI] [PubMed] [Google Scholar]
- 63.Seligowski AV, Hurly J, Mellen E, Ressler KJ, Ramikie TS (2020) Translational studies of estradiol and progesterone in fear and PTSD. European journal of psychotraumatology 11 (1):1723857. doi: 10.1080/20008198.2020.1723857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wingenfeld K, Whooley MA, Neylan TC, Otte C, Cohen BE (2015) Effect of current and lifetime posttraumatic stress disorder on 24-h urinary catecholamines and cortisol: results from the Mind Your Heart Study. Psychoneuroendocrinology 52:83–91. doi: 10.1016/j.psyneuen.2014.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoo JK, Badrov MB, Huang M, Bain RA, Dorn RP, Anderson EH, Wiblin JL, Suris A, Shoemaker JK, Fu Q (2020) Abnormal sympathetic neural recruitment patterns and hemodynamic responses to cold pressor test in women with posttraumatic stress disorder. American journal of physiology Heart and circulatory physiology 318 (5):H1198–h1207. doi: 10.1152/ajpheart.00684.2019 [DOI] [PubMed] [Google Scholar]
- 66.Hinrichs R, van Rooij SJ, Michopoulos V, Schultebraucks K, Winters S, Maples-Keller J, Rothbaum AO, Stevens JS, Galatzer-Levy I, Rothbaum BO, Ressler KJ, Jovanovic T (2019) Increased Skin Conductance Response in the Immediate Aftermath of Trauma Predicts PTSD Risk. Chronic stress (Thousand Oaks, Calif) 3. doi: 10.1177/2470547019844441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shah AJ, Lampert R, Goldberg J, Veledar E, Bremner JD, Vaccarino V (2013) Posttraumatic stress disorder and impaired autonomic modulation in male twins. Biol Psychiatry 73 (11):1103–1110. doi: 10.1016/j.biopsych.2013.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ehlers A, Suendermann O, Boellinghaus I, Vossbeck-Elsebusch A, Gamer M, Briddon E, Martin MW, Glucksman E (2010) Heart rate responses to standardized trauma-related pictures in acute posttraumatic stress disorder. International journal of psychophysiology : official journal of the International Organization of Psychophysiology 78 (1):27–34. doi: 10.1016/j.ijpsycho.2010.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cohen H, Kotler M, Matar MA, Kaplan Z, Loewenthal U, Miodownik H, Cassuto Y (1998) Analysis of heart rate variability in posttraumatic stress disorder patients in response to a trauma-related reminder. Biol Psychiatry 44 (10):1054–1059. doi:S0006-3223(97)00475-7 [pii] [DOI] [PubMed] [Google Scholar]
- 70.Shalev AY, Sahar T, Freedman S, Peri T, Glick N, Brandes D, Orr SP, Pitman RK (1998) A prospective study of heart rate response following trauma and the subsequent development of posttraumatic stress disorder. Archives of general psychiatry 55 (6):553–559 [DOI] [PubMed] [Google Scholar]
- 71.Bryant RA, Harvey AG, Guthrie RM, Moulds ML (2000) A prospective study of psychophysiological arousal, acute stress disorder, and posttraumatic stress disorder. Journal of abnormal psychology 109 (2):341–344 [PubMed] [Google Scholar]
- 72.Blanchard EB, Hickling EJ, Galovski T, Veazey C (2002) Emergency room vital signs and PTSD in a treatment seeking sample of motor vehicle accident survivors. Journal of traumatic stress 15 (3):199–204. doi: 10.1023/a:1015299126858 [DOI] [PubMed] [Google Scholar]
- 73.Kleim B, Wilhelm FH, Glucksman E, Ehlers A (2010) Sex differences in heart rate responses to script-driven imagery soon after trauma and risk of posttraumatic stress disorder. Psychosomatic medicine 72 (9):917–924. doi: 10.1097/PSY.0b013e3181f8894b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoo JK, Badrov MB, Parker RS, Anderson EH, Wiblin JL, North CS, Suris A, Fu Q (2020) Early onset neurocirculatory response to static handgrip is associated with greater blood pressure variability in women with posttraumatic stress disorder. Am J Physiol Heart Circ Physiol 318 (1):H49–h58. doi: 10.1152/ajpheart.00490.2019 [DOI] [PubMed] [Google Scholar]
- 75.Blanchard EB, Kolb LC, Prins A, Gates S, McCoy GC (1991) Changes in plasma norepinephrine to combat-related stimuli among Vietnam veterans with posttraumatic stress disorder. The Journal of nervous and mental disease 179 (6):371–373. doi: 10.1097/00005053-199106000-00012 [DOI] [PubMed] [Google Scholar]
- 76.Geracioti TD Jr., Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, Schmidt D, Rounds-Kugler B, Yehuda R, Keck PE Jr., Kasckow JW (2001) CSF norepinephrine concentrations in posttraumatic stress disorder. The American journal of psychiatry 158 (8):1227–1230. doi: 10.1176/appi.ajp.158.8.1227 [DOI] [PubMed] [Google Scholar]
- 77.Pan X, Kaminga AC, Wen SW, Liu A (2018) Catecholamines in Post-traumatic Stress Disorder: A Systematic Review and Meta-Analysis. Frontiers in molecular neuroscience 11:450. doi: 10.3389/fnmol.2018.00450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP (2003) A mechanism converting psychosocial stress into mononuclear cell activation. Proceedings of the National Academy of Sciences of the United States of America 100 (4):1920–1925. doi: 10.1073/pnas.0438019100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seligowski AV, Harnett NG, Merker JB, Ressler KJ (2020) Nervous and Endocrine System Dysfunction in Posttraumatic Stress Disorder: An Overview and Consideration of Sex as a Biological Variable. Biological psychiatry Cognitive neuroscience and neuroimaging 5 (4):381–391. doi: 10.1016/j.bpsc.2019.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Inslicht SS, Metzler TJ, Garcia NM, Pineles SL, Milad MR, Orr SP, Marmar CR, Neylan TC (2013) Sex differences in fear conditioning in posttraumatic stress disorder. Journal of psychiatric research 47 :64–71. doi: 10.1016/j.jpsychires.2012.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hopper JW, Spinazzola J, Simpson WB, van der Kolk BA (2006) Preliminary evidence of parasympathetic influence on basal heart rate in posttraumatic stress disorder. Journal of psychosomatic research 60 (1):83–90. doi: 10.1016/j.jpsychores.2005.06.002 [DOI] [PubMed] [Google Scholar]
- 82.Minassian A, Maihofer AX, Baker DG, Nievergelt CM, Geyer MA, Risbrough VB (2015) Association of Predeployment Heart Rate Variability With Risk of Postdeployment Posttraumatic Stress Disorder in Active-Duty Marines. JAMA psychiatry 72 (10):979–986. doi: 10.1001/jamapsychiatry.2015.0922 [DOI] [PubMed] [Google Scholar]
- 83.Minassian A, Geyer MA, Baker DG, Nievergelt CM, O'Connor DT, Risbrough VB (2014) Heart rate variability characteristics in a large group of active-duty marines and relationship to posttraumatic stress. Psychosomatic medicine 76 (4):292–301. doi: 10.1097/psy.0000000000000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hauschildt M, Peters MJ, Moritz S, Jelinek L (2011) Heart rate variability in response to affective scenes in posttraumatic stress disorder. Biological psychology 88 (2-3):215–222. doi: 10.1016/j.biopsycho.2011.08.004 [DOI] [PubMed] [Google Scholar]
- 85.Fonkoue IT, Norrholm SD, Marvar PJ, Li Y, Kankam ML, Rothbaum BO, Park J (2018) Elevated resting blood pressure augments autonomic imbalance in posttraumatic stress disorder. American journal of physiology Regulatory, integrative and comparative physiology 315 (6):R1272–r1280. doi: 10.1152/ajpregu.00173.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beauchaine TP, Bell Z, Knapton E, McDonough-Caplan H, Shader T, Zisner A (2019) Respiratory sinus arrhythmia reactivity across empirically based structural dimensions of psychopathology: A meta-analysis. Psychophysiology 56 (5):e13329. doi: 10.1111/psyp.13329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kamkwalala A, Norrholm SD, Poole JM, Brown A, Donley S, Duncan E, Bradley B, Ressler KJ, Jovanovic T (2012) Dark-enhanced startle responses and heart rate variability in a traumatized civilian sample: putative sex-specific correlates of posttraumatic stress disorder. Psychosomatic medicine 74(1):153–159. doi: 10.1097/PSY.0b013e318240803a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Keary TA, Hughes JW, Palmieri PA (2009) Women with posttraumatic stress disorder have larger decreases in heart rate variability during stress tasks. International journal of psychophysiology : official journal of the International Organization of Psychophysiology 73 (3):257–264. doi: 10.1016/j.ijpsycho.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 89.Bristow JD, Honour AJ, Pickering GW, Sleight P, Smyth HS (1969) Diminished baroreflex sensitivity in high blood pressure. Circulation 39 (1):48–54. doi: 10.1161/01.cir.39.1.48 [DOI] [PubMed] [Google Scholar]
- 90.Nasr N, Pavy-Le Traon A, Larrue V (2005) Baroreflex sensitivity is impaired in bilateral carotid atherosclerosis. Stroke 36 (9):1891–1895. doi: 10.1161/01.STR.0000177890.30065.cb [DOI] [PubMed] [Google Scholar]
- 91.Chapleau MW, Li Z, Meyrelles SS, Ma X, Abboud FM (2001) Mechanisms determining sensitivity of baroreceptor afferents in health and disease. Ann N Y Acad Sci 940:1–19 [DOI] [PubMed] [Google Scholar]
- 92.Hughes JW, Feldman ME, Beckham JC (2006) Posttraumatic stress disorder is associated with attenuated baroreceptor sensitivity among female, but not male, smokers. Biol Psychol 71 (3):296–302. doi: 10.1016/j.biopsycho.2005.06.002 [DOI] [PubMed] [Google Scholar]
- 93.Hughes JW, Dennis MF, Beckham JC (2007) Baroreceptor sensitivity at rest and during stress in women with posttraumatic stress disorder or major depressive disorder. J Trauma Stress 20 (5):667–676. doi: 10.1002/jts.20285 [DOI] [PubMed] [Google Scholar]
- 94.Passos IC, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J, Salum G, Magalhaes PV, Kapczinski F, Kauer-Sant'Anna M (2015) Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. The lancet Psychiatry 2 (11):1002–1012. doi: 10.1016/s2215-0366(15)00309-0 [DOI] [PubMed] [Google Scholar]
- 95.Eraly SA, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A, O'Connor DT, Baker DG (2014) Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA psychiatry 71 (4):423–431. doi: 10.1001/jamapsychiatry.2013.4374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Michopoulos V, Beurel E, Gould F, Dhabhar FS, Schultebraucks K, Galatzer-Levy I, Rothbaum BO, Ressler KJ, Nemeroff CB (2020) Association of Prospective Risk for Chronic PTSD Symptoms With Low TNFa and IFNγ Concentrations in the Immediate Aftermath of Trauma Exposure. The American journal of psychiatry 177 (1):58–65. doi: 10.1176/appi.ajp.2019.19010039 [DOI] [PubMed] [Google Scholar]
- 97.Kóffer A, Straus LD, Prather AA, Inslicht SS, Richards A, Shigenaga JK, Madden E, Metzler TJ, Neylan TC, O'Donovan A (2019) Altered overnight levels of pro-inflammatory cytokines in men and women with posttraumatic stress disorder. Psychoneuroendocrinology 102:114–120. doi: 10.1016/j.psyneuen.2018.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Michopoulos V, Norrholm SD, Jovanovic T (2015) Diagnostic Biomarkers for Posttraumatic Stress Disorder: Promising Horizons from Translational Neuroscience Research. Biological psychiatry 78 (5):344–353. doi: 10.1016/j.biopsych.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ, Norrholm SD (2012) Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biological psychiatry 72 (1):19–24. doi: 10.1016/j.biopsych.2012.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chang HA, Chang CC, Tzeng NS, Kuo TB, Lu RB, Huang SY (2013) Decreased cardiac vagal control in drug-naive patients with posttraumatic stress disorder. Psychiatry Investig 10 (2):121–130. doi: 10.4306/pi.2013.10.2.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maas AH, Appelman YE (2010) Gender differences in coronary heart disease. Netherlands heart journal: monthly journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation 18 (12):598–602 [DOI] [PMC free article] [PubMed] [Google Scholar]


