Abstract
The microflora of Korean soy sauce (gangjang) play an important role in maintaining its quality and safety. Hence, it is important to study the microflora and the possible approaches to improve their composition. In this study, the effect of adding coriander during soy sauce fermentation on the microflora and biogenic amines was evaluated using metagenomics and 1H NMR analyses, respectively. The β-diversity showed a clear distinction between the microbiota of the coriander and control groups. Microbial composition analysis revealed noticeable shifts, as Firmicutes abundance was significantly higher in the coriander group (91.77%) than that in the control (38.78%). The dominant bacterial family in the coriander group was the Bacillaceae (57.94%), while Halomonadaceae was dominant in the control group (49.77%). At the species level, Chromohalobacter beijerinckii dominated the microbial community in the control group (49.54%), but not (4.43%) in the coriander group. Moreover, there was a negative correlation between the Bacillaceae and several other bacterial families, including Halomonadaceae, which indicated a possible antagonism and partly explained the reduction in Chromohalobacter abundance in the coriander group. The levels of the biogenic amines histamine, putrescine, and tyramine, which are considered potential health risk factors, were significantly lower in the coriander soy sauce than those in the control sauce. The results of this study suggest that the addition of coriander during Korean soy sauce fermentation is beneficial, as coriander significantly reduces the levels of biogenic amines and the bacteria that produce them.
Keywords: soy sauce, microbiome, coriander, Chromohalobacter
1. Introduction
Soy sauce is the most popular fermented soybean product, owing to its distinct intense umami taste; it is mainly used as a flavoring condiment worldwide [1]. In East Asia, each country has its unique soy sauce, which slightly differs based on the traditional method of preparation and the ingredients used [2]. In Korea, traditional fermented soy sauce (gangjang) is prepared from soybean blocks (meju), which are fermented by leveraging the fungal and bacterial populations naturally occurring in the raw materials. The dry, moldy blocks are then soaked in brine solution (~20% salt) for a second long-term fermentation that produces a solid component, doenjang, and a liquid component, gangjang [3]. An array of aromatic and flavoring compounds, as well as several bioactive compounds, have been detected in soy sauce [4]. Previous reports have indicated the potential health benefits of soy sauce, including a preventive effect on thrombus formation, as well as antioxidant, antitumor, antimicrobial activities [5,6,7,8].
The quality and properties of fermented soy sauce depend—for the most part—on the microbial composition during fermentation. As this fermentation is conducted under non-sterile conditions and relies on the spontaneous growth of bacteria and fungi, it is not surprising that there are huge variations in the composition of fermented soy sauce microbiota. Variations in the microbial composition of soy sauce may result in the following: (1) differences in the organoleptic properties and (2) the possible generation of undesirable metabolites, such as biogenic amines or toxins [9,10]. Biogenic amines are low-molecular weight organic compounds that are found in fermented foods; they are generated as a result of amino acid decarboxylation or the amination and transamination of aldehydes and ketones by specific microbes [11,12]. The consumption of large amounts of biogenic amines can result in physiological and toxicological effects that may lead to poisoning; hence, the content of biogenic amines in fermented foods should be minimized [12]. Therefore, it is of great importance to study the microbial composition and factors that affect and shape the microbial profile of traditional soy sauce.
There are a few reports on the microbial composition of fermented soy sauce. In general, the brine fermentation step restricts the growth of several undesirable microbes and creates more favorable conditions for the growth of halophilic lactic acid microbes, which dominate in soy sauce and are mainly responsible for the flavor [10]. 16S rRNA sequence analysis has been used to study the microbial composition of Korean soy sauce; it has revealed Halanaerobium, Tetragenococcus, Staphylococcus, and Bacillus spp. to be the dominant microbes [9]. Recently, culture-independent techniques utilizing advanced high-throughput sequencing have been used for microbial community profiling of several fermented foods [13,14]. However, only a limited number of studies have used such an advanced approach to study the microbial composition of traditional Korean soy sauce [15,16]. A recent study showed that halotolerant and halophilic microbes are mainly responsible for soy sauce fermentation, and that they are derived from the sea salt added before the brine fermentation step, whereas the non-halophilic microbes derived from meju are abundant during the early fermentation stages [15].
Herbs and spices are well-known food additives (with potential health benefits) that preserve food and exhibit antioxidant activity by virtue of their high phenolic contents [17,18]. Coriander (Coriandrum sativum L.) is a member of the Umbelliferae family; this annual, herbaceous plant has culinary and medicinal uses [19]. Coriander leaves and seeds are rich sources of antioxidants, and contain volatile compounds that have been reported to inhibit the growth of a range of microorganisms [20,21]. Therefore, we hypothesized that the incorporation of coriander during soy sauce production via fermentation would have a significant impact on the microbial composition, possibly leading to a reduction in the levels of harmful metabolites. The objective of this study was to evaluate the effect of adding coriander during the fermentation of Korean soy sauce on the microbial composition and biogenic amine contents using high-throughput metagenomic sequencing and proton nuclear magnetic resonance spectroscopy (1H NMR), respectively.
2. Materials and Methods
2.1. Preparation of Soy Sauce Samples
Soy sauce samples were prepared using the traditional two-stage Korean method [3]. Briefly, dry cooked soybean blocks (meju) were firstly fermented using naturally occurring bacteria and fungi. The prepared fermented moldy meju blocks (~2 kg, Gigang County, Korea) were then soaked in 6 L of 20% (w/v) solar salt solution (Shinan, Korea) in porcelain containers. Pure charcoal (3 pieces; 3 cm × 3 cm × 10 cm) and dried red pepper (5 pieces) purchased from the local market (Gigang County) were added to the mixture. In the treatment samples, 200 g of fresh coriander was added to the mixture after trimming the roots. The mixture was then fermented for 45 days under sunlight; the lid of the pot was open by day and closed by night. The solid component, fermented soy paste (doenjang), was separated from the liquid component, soy sauce (gangjang); this was followed by boiling for 10 min. The soy sauce samples were then stored at 4 °C until they were used for metagenomic sequencing and analysis.
2.2. DNA Extraction, Sequencing, and Metagenomic Analysis
The collected soy sauce samples were first centrifuged for 15 min at 10,000× g and the obtained pellet was washed with sterile distilled water to remove excess water and salts. A part of the obtained pellet (250 mg) was used for metagenomic DNA extraction using a PowerSoil® DNA Isolation Kit (MO BIO Laboratories, Carlsbad, CA, USA), based on the manufacturer’s protocol. The obtained DNA was checked for quality and concentration using agarose gel electrophoresis and a NanoDrop2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, NC, USA). Qualified samples were stored in Tris-EDTA buffer at −20 °C until further analysis.
The hypervariable regions, V3 and V4, of the 16S rRNA gene were used for the metagenomic analysis of the obtained DNA samples. The conditions of PCR amplification and sequencing, which were performed in an Illumina® MiSeq® platform at Macrogen (Seoul, South Korea), were based on the protocol provided along with the Herculase II fusion DNA polymerase Nextera XT Index Kit V2. The following primer pair was used:
(F), 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3′;
(R), 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC-3′.
The paired-end reads obtained from sequencing were merged using the fast length adjustment of short reads (FLASH; http://ccb.jhu.edu/software/FLASH/) [22]. The Illumina adaptors and the short and low-quality reads were trimmed, and the raw sequences were purified using the Scythe (v0.994) (https://github.com/vsbuffalo/scythe) and Sickle programs (https://github.com/najoshi/sickle). Following purification, clustering and annotation were performed using the CD-HIT-OTU-MiSeq and UCLUST algorithms, and qualified sequences were organized into respective operational taxonomic units (OTUs) at a cut off value of 97%, using the Greengenes database [23,24,25]. Analysis of the microbiota of soy sauce samples—including diversity statistics and taxonomic assignments of the obtained OTUs from the phylum to the species level—was performed using the Quantitative Insights into Microbial Ecology version 2 (QIIME2) pipeline [26]. The obtained sequences were deposited as a sequence read archive in the National Center for Biotechnology Information database (Bethesda, MD, USA) under the BioProject ID PRJNA640944.
2.3. Quantification of Biogenic Amines Using 1H NMR Spectroscopy
Biogenic amines (i.e., histamine, putrescine, and tyramine) in the prepared Korean soy sauce were analyzed using 1H NMR spectroscopy as per a previously described method [27]. Briefly, 1 mL—from each 10-fold diluted supernatant—of each Korean soy sauce sample was freeze-dried and dissolved in 600 μL of 99.9% deuterium oxide containing 2 mM trimethylsilylpropanoic acid (Sigma-Aldrich, St. Louis, MI, USA) for standardization. The solutions were then transferred into NMR tubes, and their 1H NMR spectra were recorded using a Bruker Avance 500 MHz spectrometer (Bruker Biospin, Rheinstetten, Germany). Histamine, putrescine, and tyramine were identified and quantified using the profiler option in the Chenomx NMR suite program (v8.6, Chenomx Inc., Edmonton, AB, Canada).
2.4. Statistical Analysis
Statistical analysis of the bacterial microbiome was performed using QIIME2 scripts, R (version 3.1.3), and the PAleontological STatistics software package (PAST) version 3.23 [28]. Principle coordinates analysis (PCoA) was performed based on Bray–Curtis and Euclidean distances, for assessing the β-diversity between groups. Student’s t-test was used for statistical analysis of the relative abundance of bacterial taxa at different taxonomic levels, and p-values < 0.05 were considered significant. Pearson’s correlation analysis and correlogram plotting were performed using PAST.
3. Results
3.1. Microbial Diversity in Soy Sauce Samples Prepared with or without Coriander
High-throughput sequencing resulted in 1,374,263 reads with an average of 229,044 reads per sample; the total bases, read counts, GC%, Q20%, and Q30% for each sample are shown in Table S1. Following pre-processing and clustering using CD–HIT–OTU to remove low-quality reads and chimeras, 470,857 total reads were obtained with an average of 78,476 ± 19,598 reads per sample, ranging from a minimum of 48,207 to a maximum of 100,810.
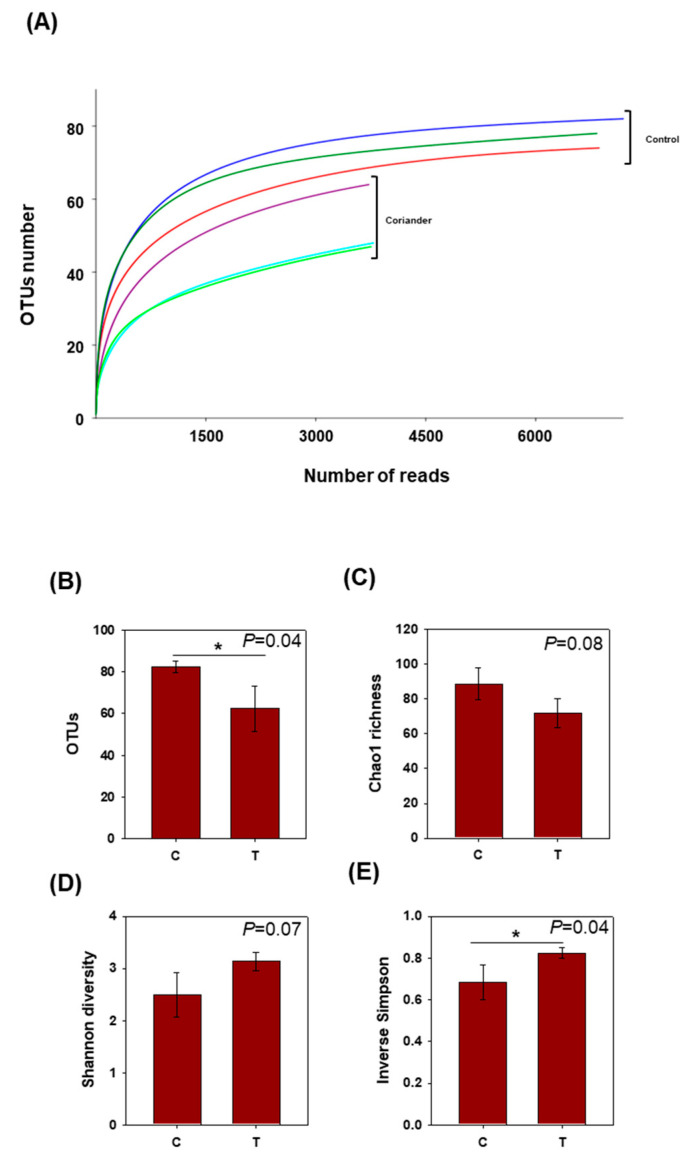
Rarefaction analysis on the sequences obtained from both soy sauce groups indicated satisfactory sequencing depth, as a near plateau level was achieved at approximately 2000 reads, particularly for the control group (Figure 1A). The number of OTUs was significantly (p < 0.05) higher in the control soy sauce group compared to that in the coriander group (Figure 1B). There was no significant difference between both groups with respect to Chao1 richness and Shannon alpha diversity index (Figure 1C,D). However, the inverse Simpson index was significantly higher for the coriander soy sauce group compared to that for the control (Figure 1E).
Figure 1.
(A) Rarefaction curves of the obtained 16S rRNA sequence reads against the assigned operational taxonomic units (OTUs). Alpha diversity in soy sauce samples prepared by adding coriander, compared to that in control. (B) Number of OTUs, (C) Chao1 richness index, (D) Shannon diversity index, and (E) inverse Simpson’s index. Data represent means and standard deviations of three replicates for each soy sauce group. *: a significant difference at p < 0.05. C = control, T = coriander treatment.
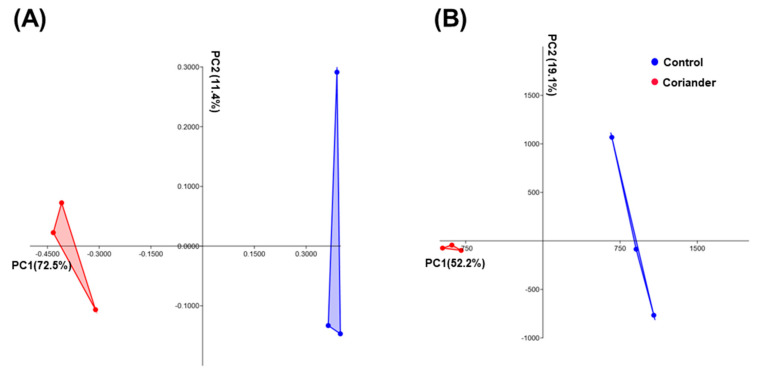
PCoA revealed an evident distinction between both soy sauce groups with respect to the β-diversity, as measured using the Bray–Curtis and Euclidean distance matrices (Figure 2A,B).
Figure 2.
Principle coordinates analysis of (A) Bray–Curtis and (B) Euclidean distances of soy sauce samples prepared by adding coriander during fermentation, compared to those of control. Each dot represents a replicate from the different groups of soy sauce.
3.2. Microbial Structure and Dominant Taxa in Coriander and Control Soy Sauce
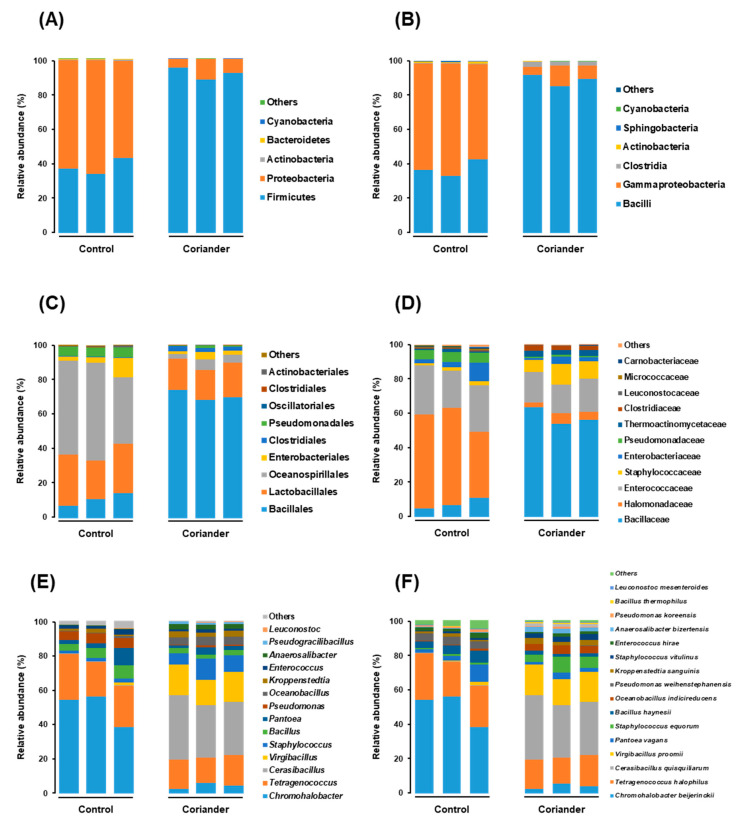
Noticeable differences were observed upon plotting the relative abundance of microbial taxa at different taxonomic levels on stacked bar graphs. At the phylum level, Firmicutes and Proteobacteria were the two major groups in all the samples. Firmicutes was abundant in all the coriander soy sauce samples, with a relative abundance average of 91.78% ± 0.03% compared to 38.03% ± 0.04% in the control group, whereas Proteobacteria was abundant in the control group, with an abundance average of 60.88% ± 0.04% compared to 8.12% ± 0.03% in the coriander group (Figure 3A). The Firmicutes and Proteobacteria in both samples were mainly composed of members of the Bacilli and Gammaproteobacteria classes, respectively (Figure 3B).
Figure 3.
Stacked bar graphs representing the microbiome structure in soy sauce samples prepared by adding coriander, compared to that of control: (A) phylum level, (B) class level, (C) order level, (D) family level, (E) genus level, and (F) species level.
At the order level, the main difference was the dominance of the Bacillales and Oceanospiralles in the coriander and control groups, respectively, with corresponding abundance averages of 70.71% ± 0.02% and 49.77% ± 0.08% (Figure 3C). At the family level, the coriander group was dominated by Bacillaceae, Enterococcaceae, Staphylococcaceae, and Halomonadaceae, with average relative abundances of 57.95% ± 0.04%, 17.94% ± 0.01%, 9.55% ± 0.01%, and 4.54% ± 0.02%, respectively. The control group was dominated by Halomonadaceae, Enterococcaceae, Bacillaceae, and Pseudomonadaceae, with average relative abundances of 49.77% ± 0.08%, 25.67% ± 0.03%, 7.63% ± 0.03%, and 5.51% ± 0.00%, respectively (Figure 3D). At the genus level, the coriander group was dominated by Cerasibacillus, Virgibacillus, Tetragenococcus, Staphylococcus, and Chromohalobacter spp., with average relative abundances of 32.82% ± 0.03%, 16.51% ± 0.01%, 16.48% ± 0.01%, 9.54% ± 0.02%, and 4.44% ± 0.01%, respectively. The control group was dominated by Chromohalobacter, Tetragenococcus, Bacillus, Pseudomonas, and Pantoea spp., with average relative abundances of 49.54% ± 0.08%, 23.53% ± 0.03%, 5.73% ± 0.01%, 5.51% ± 0.00%, and 5.00% ± 0.04%, respectively (Figure 3E).
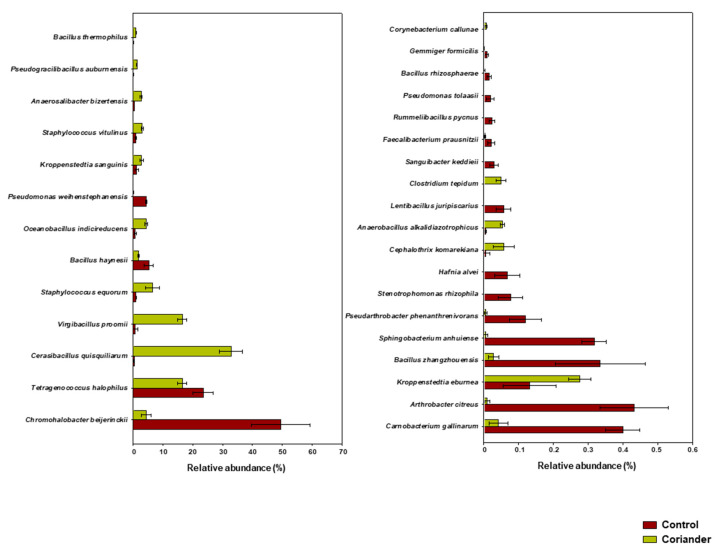
At the species level, the coriander group was dominated by Cerasibacillus quisquiliarum, Virgibacillus proomii, Tetragenococcus halophilus, Staphylococcus equorum, and Chromohalobacter beijerinckii, with average relative abundances of 32.82% ± 0.03%, 16.50% ± 0.01%, 16.48% ± 0.01%, 6.52% ± 0.02%, and 4.44% ± 0.01%, respectively. The control group was dominated by C. beijerinckii, T. halophilus, Bacillus haynesii, Pantoea vagans, and Pseudomonas weihenstephanensis, with average relative abundances of 49.54% ± 0.08%, 23.53% ± 0.03%, 5.24% ± 0.01%, 5.00% ± 0.04%, and 4.31% ± 0.00%, respectively (Figure 3F). The microbial taxa that existed at significant levels (p < 0.05) in the coriander and control soy sauce groups are shown in Figure 4.
Figure 4.
Bacterial species existing with significantly different (p < 0.05) relative abundance in soy sauce samples prepared by adding coriander, compared to those in control. Bars represent means and standard deviations.
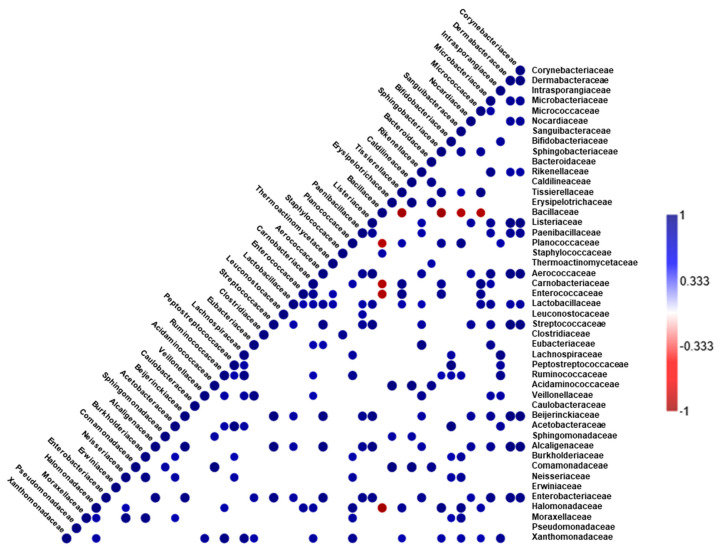
The Pearson’s correlation relationship among the microbial taxa at the family level indicated various positive correlations; only the Bacillaceae family showed significant negative correlation with the Halomonadaceae, Carnobacteriaceae, Enterococcaceae, Planococcaceae, Micrococcaceae, Sanguibacteriaceae, Sphingobacteriaceae, and Tissierellaceae families (Figure 5).
Figure 5.
Correlogram representing matrices of Pearson’s correlation coefficient (r) between different bacterial taxa in soy sauce samples (n = 6), at family level. Only significant (p < 0.05) positive (blue) and negative (red) correlations are shown in the graph.
3.3. Reduction in the Biogenic Amine Content in Korean Soy Sauce upon Addition of Coriander during Fermentation
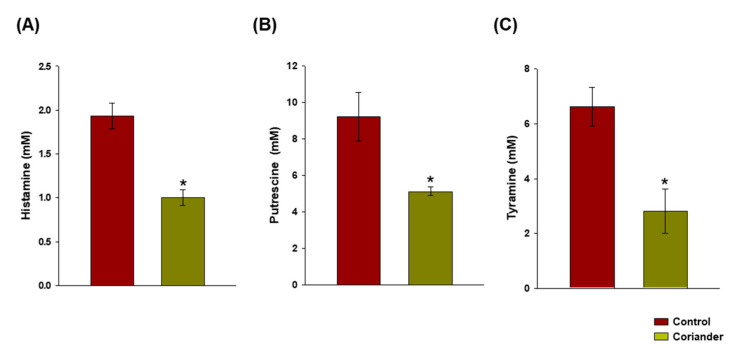
1H NMR quantification of biogenic amines revealed that the contents of histamine, putrescine, and tyramine were significantly (p < 0.05) lower in the Korean soy sauce samples prepared by adding coriander during fermentation, compared to those in the control (Figure 6). The percentages of inhibition were 48.03% ± 3.65%, 43.96% ± 3.27%, and 57.74% ± 4.89% for histamine, putrescine, and tyramine, respectively.
Figure 6.
Quantification of biogenic amines, (A) histamine, (B) putrescine, and (C) tyramine, in Korean soy sauce samples prepared by adding coriander during fermentation, compared to those in the control. *: a significant difference at p < 0.05.
4. Discussion
Fermented food microbiota are directly associated with the quality and safety aspects of the food, and may directly or indirectly affect human health [29]. Therefore, the ultimate objective for improving food quality is to ensure product safety by determining the microbial composition of fermented foods and analyzing the possibilities for deliberately manipulating the community structure [30]. In this study, the microbiota in Korean soy sauce were investigated, and the effect of coriander on the microbial composition and the content of biogenic amines was studied, with the intention of regulating the fermentation conditions to improve soy sauce quality.
An earlier study using a 16S rRNA PCR-based culture-independent approach indicated that Korean soy sauce microbiota are mainly composed of Haloanaerobium, Tetragenococcus, Staphylococcus, and Bacillus spp. [9]. A more recent study that utilized a next-generation high-throughput sequencing-based approach—similar to the approach used in the current study—indicated that the halotolerant and halophilic microbes derived from the sea salt added during fermentation—such as Tetragenococcus, Staphylococcus, and Chromohalobacter spp.—were responsible for Korean soy sauce fermentation [15]. Tetragenococcus was found in another study to be related to production of aroma-active and umami taste constituents such as aspartic acid, glutamic acid and alanine, indicating its important role in the fermentation of soy sauce [31]. Consistently, the control soy sauce group in the current study was dominated by halophilic Chromohalobacter and Tetragenococcus spp., whereas the coriander group was dominated by Tetragenococcus spp. and spore-forming Bacillaceae such as Cerasibacillus and Virgibacillus spp., indicating a clear shift between the soy sauce groups. Spore-forming Bacillaceae can survive extreme environmental conditions—such as high-salt and undernutrition environments—that may kill vegetative bacterial cells [32]. Non-halophilic microbes that survive during the second fermentation step are mainly derived from meju, and may not play a major role in soy sauce fermentation [15].
The significant shift in the microbial community in the coriander soy sauce group can be attributed to the antimicrobial activity of the bioactive compounds present in coriander. Coriander has been shown to contain volatile compounds that may be detrimental to the growth of certain bacterial groups [18]. The essential oils present in coriander have also been shown to exert antimicrobial activity against Gram-negative and Gram-positive foodborne pathogenic bacteria, such as Escherichia coli, Salmonella typhimurium, Listeria monocytogenes, and Staphylococcus aureus [21]. Moreover, coriander oil shows potent antimicrobial activity against pathogenic Campylobacter jejuni [33]. In the current study, there was a clear distinction between the microbial compositions of the soy sauce prepared using coriander and the control, as Firmicutes was dominant in coriander soy sauce, while Proteobacteria was dominant in the control.
The control soy sauce was dominated by C. beijerinckii with approximately 50% relative abundance, which dramatically decreased in the coriander soy sauce to less than 5%. Chromohalobacter beijerinckii is a psychrophilic, extremely halotolerant Gram-negative bacterium typically found in highly salted environments such as salty beans and herrings [34]. It is known to produce biogenic amines, such as putrescine, histamine, and tyramine, which are low-molecular weight nitrogenous microbial metabolites originating as a result of the decarboxylation of specific amino acids and nitrogen compounds during fermentation [12,35]. The excessive consumption of biogenic amines is associated with adverse toxicological effects [12]. Hence, it is important to monitor the potential microbial producers of biogenic amines in fermented foods and to manage their population.
In a previous study, simultaneous investigation of the metabolites and the bacterial community of Korean soy sauce showed that Chromohalobacter was the dominant genus toward the end of the fermentation, and that it closely correlated with the production of biogenic amines, including putrescine [27]. In the current study, the changes in the microbial composition could consistently explain the significant reduction in the levels of biogenic amines (histamine, putrescine, and tyramine) in the coriander soy sauce samples. The presence of histamine, putrescine, and tyramine, as well as other biogenic amines in food, is associated with health risks; owing to their potential toxicity, avoiding their accumulation in foods is advisable [36]. Histamine poisoning is a known risk factor associated with the consumption of histamine-rich foods and could lead to serious allergen-type reactions at high levels [37]. The presence of other types of biogenic amines such as putrescine enhance the toxicity of histamine [38]. In addition, acute and subacute putrescine and tyramine toxicity were confirmed in animal model studies and their consumption in large amounts was linked to dietary-induced migraines and hypertensive crisis [38,39]. Therefore, the significant reduction in C. beijerinckii abundance in coriander soy sauce suggests that coriander addition during the fermentation of soy sauce is beneficial as it controls the levels of biogenic amines.
A correlation analysis provided insights into the relationships among the detected microbes, although several other factors—during the fermentation process—should have been considered. The significant negative correlation between Bacillaceae and Halomonadaceae could be attributed to possible antagonistic activity that resulted in a reduction in the relative abundance in the coriander soy sauce group, which showed a higher relative abundance of Bacillaceae, but showed a low abundance of potential biogenic amine producers, i.e., Chromohalobacter spp.
The results of this study suggest that adding coriander during the fermentation of Korean soy sauce is beneficial for inhibiting the production of undesirable metabolites. In particular, the levels of the biogenic amines, histamine, putrescine, and tyramine, and the biogenic amine–producing bacterium C. beijerinckii were significantly lower in the coriander-supplemented Korean soy sauce compared to those in the control. Further studies that consider other quality and safety aspects related to the addition of coriander during soy sauce fermentation are required.
Acknowledgments
We would like to thank Hokeun Son for his advice in the analyses of metagenomics.
Supplementary Materials
The following are available online at https://www.mdpi.com/2304-8158/9/10/1346/s1, Table S1: Results of high-throughput sequencing, representing total bases, read counts, GC%, Q20%, and Q30%.
Author Contributions
Conceptualization, I.P. and Y.-S.S.; methodology, M.M. and I.P.; validation, M.M. and I.P.; formal analysis, M.M.; data curation, M.M., I.P. and Y.-S.S.; writing—original draft preparation, M.M. and I.P.; writing—review and editing, Y.-S.S.; project administration, Y.-S.S.; funding acquisition, I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by research fund 2020 through Youngsan University, Busan, Republic of Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Luh B.S. Industrial production of soy sauce. J. Ind. Microbiol. 1995;14:467–471. doi: 10.1007/BF01573959. [DOI] [Google Scholar]
- 2.Xu L., Li Y., Xu N., Hu Y., Wang C., He J., Cao Y., Chen S., Li D. Soy sauce classification by geographic region and fermentation based on artificial neural network and genetic algorithm. J. Agric. Food Chem. 2014;62:12294–12298. doi: 10.1021/jf504530w. [DOI] [PubMed] [Google Scholar]
- 3.Chung H.J., Sohn K.H. The changes of component in traditional Korean soy sauce during ripening period (I) Korean J. Soc. Food Sci. 1994;10:29–34. [Google Scholar]
- 4.Steinhaus P., Schieberle P. Characterization of the key aroma compounds in soy sauce using approaches of molecular sensory science. J. Agric. Food Chem. 2007;55:6262–6269. doi: 10.1021/jf0709092. [DOI] [PubMed] [Google Scholar]
- 5.Tsuchiya H., Sato M., Watanabe I. Antiplatelet activity of soy sauce as functional seasoning. J. Agric. Food Chem. 1999;47:4167–4174. doi: 10.1021/jf990147d. [DOI] [PubMed] [Google Scholar]
- 6.Long L.H., Kwee D., Halliwell B. The antioxidant activities of seasonings used in Asian cookings. Powerful antioxidant activity of dark soy sauce revealed using ABTS assay. Free Radic. Res. 2000;32:181–186. doi: 10.1080/10715760000300181. [DOI] [PubMed] [Google Scholar]
- 7.Masuda S., Hara-Kudo Y., Kumagai S. Reduction of Escherichia coli O157:H7 populations by soy sauce: A fermented seasoning. J. Food Prot. 1998;61:657–661. doi: 10.4315/0362-028X-61.6.657. [DOI] [PubMed] [Google Scholar]
- 8.Ito A., Watanabe H., Basaran N. Effects of soy products in reducing risk of spontaneous and neutron-induced liver tumors in mice. Int. J. Oncol. 1993;2:773–776. doi: 10.3892/ijo.2.5.773. [DOI] [PubMed] [Google Scholar]
- 9.Cho K.M., Seo W.T. Bacterial diversity in a Korean traditional soybean fermented foods (doenjang and ganjang) by 16S rRNA gene sequence analysis. Food Sci. Biotechnol. 2007;16:320–324. [Google Scholar]
- 10.Lee K.E., Lee S.M., Choi Y.H., Hurh B.S., Kim Y.S. Comparative volatile profiles in soy sauce according to inoculated microorganisms. Biosci. Biotechnol. Biochem. 2013;77:2192–2200. doi: 10.1271/bbb.130362. [DOI] [PubMed] [Google Scholar]
- 11.Yongmei L., Xiaohong C., Mei J., Xin L., Rahman N., Mingsheng D., Yan G. Biogenic amines in Chinese soy sauce. Food Control. 2009;20:593–597. doi: 10.1016/j.foodcont.2008.08.020. [DOI] [Google Scholar]
- 12.Silla Santos M.H. Biogenic amines: Their importance in foods. Int. J. Food Microbiol. 1996;29:213–231. doi: 10.1016/0168-1605(95)00032-1. [DOI] [PubMed] [Google Scholar]
- 13.Lee M., Song J.H., Jung M.Y., Lee S.H., Chang J.Y. Large-scale targeted metagenomics analysis of bacterial ecological changes in 88 kimchi samples during fermentation. Food Microbiol. 2017;66:173–183. doi: 10.1016/j.fm.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Mannaa M., Seo Y.S., Park I. Effect of seafood (gizzard shad) supplementation on the chemical composition and microbial dynamics of radish kimchi during fermentation. Sci. Rep. 2019;9:17693. doi: 10.1038/s41598-019-54318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han D.M., Chun B.H., Feng T., Kim H.M., Jeon C.O. Dynamics of microbial communities and metabolites in ganjang, a traditional Korean fermented soy sauce, during fermentation. Food Microbiol. 2020;92:103591. doi: 10.1016/j.fm.2020.103591. [DOI] [PubMed] [Google Scholar]
- 16.Liang R., Huang J., Wu X., Fan J., Xu Y., Wu C., Jin Y., Zhou R. Effect of raw material and starters on the metabolite constituents and microbial community diversity of fermented soy sauce. J. Sci. Food Agric. 2019;99:5687–5695. doi: 10.1002/jsfa.9830. [DOI] [PubMed] [Google Scholar]
- 17.Pereira M.P., Tavano O.L. Use of different spices as potential natural antioxidant additives on cooked beans (Phaseolus vulgaris). Increase of DPPH radical scavenging activity and total phenolic content. Plant Foods Hum. Nutr. 2014;69:337–343. doi: 10.1007/s11130-014-0439-4. [DOI] [PubMed] [Google Scholar]
- 18.El-Zaeddi H., Calín-Sánchez Á., Nowicka P., Martínez-Tomé J., Noguera-Artiaga L., Burló F., Wojdyło A., Carbonell-Barrachina Á.A. Preharvest treatments with malic, oxalic, and acetylsalicylic acids affect the phenolic composition and antioxidant capacity of coriander, dill and parsley. Food Chem. 2017;226:179–186. doi: 10.1016/j.foodchem.2017.01.067. [DOI] [PubMed] [Google Scholar]
- 19.Shahwar M.K., El-Ghorab A.H., Anjum F.M., Butt M.S., Hussain S., Nadeem M. Characterization of coriander (Coriandrum sativum L.) seeds and leaves: Volatile and non volatile extracts. Int. J. Food Prop. 2012;15:736–747. doi: 10.1080/10942912.2010.500068. [DOI] [Google Scholar]
- 20.Wangensteen H., Samuelsen A.B., Malterud K.E. Antioxidant activity in extracts from coriander. Food Chem. 2004;88:293–297. doi: 10.1016/j.foodchem.2004.01.047. [DOI] [Google Scholar]
- 21.Delaquis P.J., Stanich K., Girard B., Mazza G. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int. J. Food Microbiol. 2002;74:101–109. doi: 10.1016/S0168-1605(01)00734-6. [DOI] [PubMed] [Google Scholar]
- 22.Magoč T., Salzberg S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edgar R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 24.McDonald D., Price M.N., Goodrich J., Nawrocki E.P., DeSantis T.Z., Probst A., Andersen G.L., Knight R., Hugenholtz P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012;6:610–618. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li W., Chang Y. CD-HIT-OTU-MiSeq, an improved approach for clustering and analyzing paired end MiSeq 16S rRNA sequences. BioRxiv. 2017:153783. [Google Scholar]
- 26.Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Pena A.G., Goodrich J.K., Gordon J.I., et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung J.Y., Chun B.H., Jeon C.O. Chromohalobacter is a causing agent for the production of organic acids and putrescine during fermentation of ganjang, a Korean traditional soy sauce. J. Food Sci. 2015;80:2853–2859. doi: 10.1111/1750-3841.13114. [DOI] [PubMed] [Google Scholar]
- 28.Hammer O., Harper D.A., Ryan P.D. Palaeontological statistics software package for education and data analysis. Palaeontol. Electron. 2001;4:9. [Google Scholar]
- 29.Marco M.L., Heeney D., Binda S., Cifelli C.J., Cotter P.D., Foligné B., Gänzle M., Kort R., Pasin G., Pihlanto A., et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017;44:94–102. doi: 10.1016/j.copbio.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 30.De Filippis F., Parente E., Ercolini D. Metagenomics insights into food fermentations. Microb. Biotechnol. 2017;10:91–102. doi: 10.1111/1751-7915.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L., Zhang L., Xu Y. Effects of Tetragenococcus halophilus and Candida versatilis on the production of aroma-active and umami-taste compounds during soy sauce fermentation. J. Sci. Food Agric. 2020;100:2782–2790. doi: 10.1002/jsfa.10310. [DOI] [PubMed] [Google Scholar]
- 32.Nicholson W.L., Munakata N., Horneck G., Melosh H.J., Setlow P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 2000;64:548–572. doi: 10.1128/MMBR.64.3.548-572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rattanachaikunsopon P., Phumkhachorn P. Potential of coriander (Coriandrum sativum) oil as a natural antimicrobial compound in controlling Campylobacter jejuni in raw meat. Biosci. Biotechnol. Biochem. 2010;74:31–35. doi: 10.1271/bbb.90409. [DOI] [PubMed] [Google Scholar]
- 34.Peçonek J., Gruber C., Gallego V., Ventosa A., Busse H.J., Kämpfer P., Radax C., Stan-Lotter H. Reclassification of Pseudomonas beijerinckii Hof 1935 as Chromohalobacter beijerinckii comb. nov., and emended description of the species. Int. J. Sys. Evol. Microbiol. 2006;56:1953–1957. doi: 10.1099/ijs.0.64355-0. [DOI] [PubMed] [Google Scholar]
- 35.Beutling D.M., Peçonek J., Stan-Lotter H. Chromohalobacter beijerinckii: A psychrophilic, extremely halotolerant and enzymatically active microbe from salted food with the capacity for biogenic amine production. Eur. Food Res. Technol. 2009;229:725–730. doi: 10.1007/s00217-009-1106-0. [DOI] [Google Scholar]
- 36.Del Rio B., Redruello B., Linares D.M., Ladero V., Ruas-Madiedo P., Fernandez M., Martin M.C., Alvarez M.A. The biogenic amines putrescine and cadaverine show in vitro cytotoxicity at concentrations that can be found in foods. Sci. Rep. 2019;9:120. doi: 10.1038/s41598-018-36239-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugiura K., Sugiura M. Soy sauce allergy. J. Eur. Acad. Dermatol. Venereol. 2010;24:852–855. doi: 10.1111/j.1468-3083.2009.03512.x. [DOI] [PubMed] [Google Scholar]
- 38.Naila A., Flint S., Fletcher G., Bremer P., Meerdink G. Control of biogenic amines in food—existing and emerging approaches. J. Food Sci. 2010;75:R139–R150. doi: 10.1111/j.1750-3841.2010.01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Til H.P., Falke H.E., Prinsen M.K., Willems M.I. Acute and subacute toxicity of tyramine, spermidine, spermine, putrescine and cadaverine in rats. Food Chem. Toxicol. 1997;35:337–348. doi: 10.1016/S0278-6915(97)00121-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.