Abstract
The risk factors for severe COVID-19 are diverse, yet closely resemble the clinical manifestations of catecholamine excess states (eg, hypertension, cardiovascular disease, immune dysregulation, and hyperglycaemia), suggesting a potentially common basis for disease. Unfortunately, severe illness (eg, respiratory failure, compromised cardiac function, and shock) incurred by COVID-19 hinders the direct study of catecholamines in these patients, especially among those on multiple medications or those on adrenaline or noradrenaline infusions, or both. Phaeochromocytoma and paraganglioma (PPGL) are tumours that secrete catecholamines, namely adrenaline and noradrenaline, often in excess. PPGL are well studied disease processes in which the effects of catecholamines are easily discernible and therefore their potential biochemical and physiological influences in patients with COVID-19 can be explored. Because catecholamines are expected to have a role in patients with critical illness, patients on vasopressor infusions, and patients who sustain some acute and chronic physical stresses, the challenges involved in the management of catecholamine excess states are directly relevant to the treatment of patients with COVID-19. In this Personal View, we discuss the complex interplay between catecholamines and COVID-19, and the management of catecholamine excess states, while referencing relevant insights derived from the study of PPGL.
Introduction
The COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been a devastating global health burden. Susceptibility to SARS-CoV-2 is significantly increased in patients who have pre-existing cardiovascular and respiratory disorders, diabetes, cancer, and immune system dysregulation.1, 2, 3, 4, 5
Catecholamines have an essential role in the physiological regulation of the cardiovascular, respiratory, metabolic, and immune systems—those most heavily affected by COVID-19. Phaeochromocytoma and paraganglioma (PPGL) are chromaffin cell tumours that produce and secrete catecholamines, namely adrenaline and noradrenaline, often in supraphysiological amounts.6 Consequently, catecholamine excess states such as PPGL can cause substantial dysregulation of physiological systems, and lead to pronounced changes in pulmonary (vasoplegia), coronary (myocardial infarction), cerebrovascular (stroke), and remaining systemic vascular tone (hypertension), as well as myocardial disease (cardiomyopathies), tachyarrhythmias (benign and fatal), hypercoagulability (thromboembolism), immune dysregulation (cytokine storm), and diabetogenic states; these outcomes are the same as the risk factors that lead to adverse outcomes from COVID-19.2, 6, 7, 8, 9, 10, 11, 12 These findings suggest that catecholamines might be key mediators in COVID-19. As PPGL is an extensively studied catecholamine excess state, we believe that it might serve as a practical model for exploring the pathological and beneficial actions of catecholamines in patients with COVID-19.
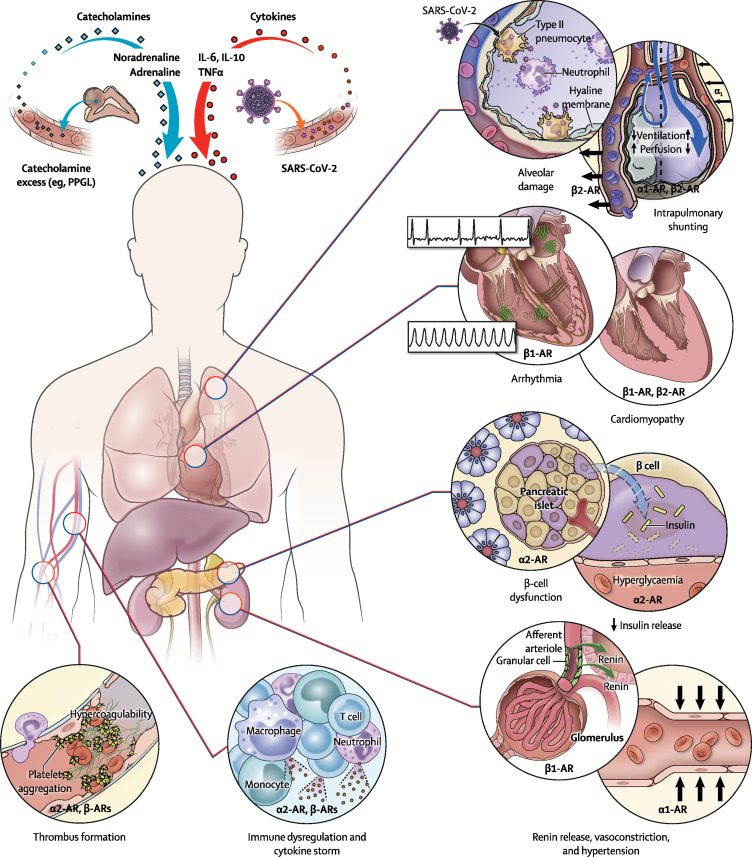
Four main systems or axes are potentially affected by catecholamines in patients with COVID-19, namely: (1) cytokines and the immune and haematological systems; (2) renin, angiotensin, aldosterone, and the cardiovascular system; (3) ventilation, perfusion, and the pulmonary system; and (4) glucose metabolism and the endocrine system. Further, these systems or axes are interconnected; eg, catecholamines can lead to the release of cytokines and vice versa.13 Given these points of interaction, patients with coexistent PPGL and COVID-19 could potentially provide valuable insights into the role of catecholamines in the pathophysiology of COVID-19. These patients could also serve as surrogates for patients with COVID-19 in whom catecholamine excess is relevant but cannot be directly studied (eg, in patients with noradrenaline or adrenaline infusion, recent surgery, or severe trauma leading to catecholamine release). Furthermore, such patients with coexisting PPGL and COVID-19 would require appropriate and early intervention to avoid catastrophic outcomes such as stroke, myocardial infarction, circulatory collapse, and death from superimposed risk (figure ).14
Figure.
The various potential interactions between COVID-19 and PPGL
The figure was designed by Alan Hoofring from the Medical Arts Design Section of the US National Institute of Health. AR=adrenoceptor. IL=interleukin. PPGL=phaeochromocytoma and paraganglioma. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. TNF=tumour necrosis factor.
Cytokines and the immune and haematological systems
A subset of patients with COVID-19 develop cytokine storm syndrome or multisystem inflammatory syndrome in children, a hyperinflammatory state resulting from uncontrolled immune system activation.13, 15, 16, 17, 18 Cytokine storm syndrome or multisystem inflammatory syndrome in children results from an elevation of macrophage-derived cytokines, specifically interleukin-6 (IL-6), IL-10, tumour necrosis factor α (TNFα), granulocyte colony stimulating factor, and probably IL-2.19 Increased concentrations of these cytokines and their downstream acute phase reactants (eg, ferritin) have been associated with a higher likelihood of severe disease and mortality in patients with COVID-19.19, 20 Catecholamines augment the production of IL-6, IL-10, and other cytokines through a self-amplifying feed-forward loop within myeloid cells, an effect mediated through α1-adrenoceptors.21 An elevation in cytokine concentrations leads to a hypercoagulable state and a greater risk of thrombosis.22 Thrombosis has been associated with adverse outcomes in patients with COVID-19.22 Additionally, catecholamines binding to α2-adrenoceptors can potentiate second messenger cascades that result in platelet aggregation and thrombosis.23, 24 On the contrary, adrenaline, probably via β-adrenoceptor stimulation, exerts antithrombotic effects through activation of fibrinolysis (eg, tissue type plasminogen activator and plasmin-α2-antiplasmin) in human models of lipopolysaccharide endotoxaemia.25 Therefore, excess catecholamines (mainly noradrenaline), as seen in patients with PPGL and critical illness, can augment cytokine production and increase the risk for hypercoagulability in patients with COVID-19.26, 27, 28
Catecholamines also modulate several components of the immune system, including α2-adrenoceptor-mediated inhibition of antigen-presenting cells (eg, Langerhans cells), suppression of T-lymphocyte proliferation, and stimulation of cytokine production.29, 30 These effects might facilitate increased viral replication in patients with COVID-19. In general, catecholamines seem to inhibit the T-helper type 1 cell-mediated cytokine response (including IL-1, IL-2, IL-12, interferon–γ, and TNFα), and augment the T-helper type 2 cell-mediated cytokine response (including IL-6 and IL-10; key players in cytokine storm syndrome or multisystem inflammatory syndrome in children).13, 31, 32 Infusion of adrenaline in murine models of sepsis leads to a β-adrenoceptor-mediated lymphocyte depletion.31 Lymphopenia, observed in patients with COVID-19, is a prognostic indicator of disease severity.33 In addition, local neurohormonal effects of catecholamines can influence immune function. The actions of B-lymphocytes and T-lymphocytes, which almost exclusively express β2-adrenoceptors, are influenced by stressors that activate the sympathetic nervous system, which predominantly contains noradrenaline.34 Although noradrenaline is a weak β2-adrenoceptor agonist, it is probable that its effects on immune cells are exerted in the spleen where local concentrations of noradrenaline from nerve terminals that innervate the organ are high enough to act on β2-adrenoceptors and modulate lymphocyte function.35 Severe COVID-19 is also associated with a reduction in the number of natural killer cells.36 Natural killer cell numbers have been shown to substantially increase immediately following intravenous adrenaline or noradrenaline infusion, whereas prolonged catecholamine infusion (>4 weeks) can decrease natural killer cells. Thus, chronic catecholamine elevations as studied in PPGL, and seen in hospitalised patients or patients with critical illness who often experience persistent physiological stress, might lead to a decline in circulating natural killer cells, B-lymphocytes, and T-lymphocytes.31 Therefore, it might be possible for patients with COVID-19 and chronic catecholamine elevations to be at higher risk for an attenuated immune response during COVID-19 infection than patients with COVID-19 without chronic catecholamine elevations.
Renin, angiotensin, aldosterone, and the cardiovascular system
About 85–90% of patients with PPGL have pre-existing hypertension (either sustained or paroxysmal). Such hypertension can be highly resistant and can require multiple anti-hypertensives.6 Several studies have consistently shown that hypertension is associated with poor clinical outcomes in patients with COVID-19.1, 12, 20, 37 Hypertension was the most common comorbidity (56·6%) among a cohort of 5700 patients with COVID-19 admitted to various hospitals in and around New York City.12 The number of deaths among patients with COVID-19 was 2·3 times higher in those with hypertension than among those without hypertension.12 In the OpenSAFELY study, hypertension was associated with higher odds of COVID-19-related death (age-adjusted and sex-adjusted hazard ratio [HR] 1·09, 95% CI 1·05–1·14).2 Data from 1099 patients with COVID-19 from 552 hospitals in China revealed that hypertension was associated with a higher likelihood for admission to an intensive care unit, mechanical ventilation, or death when compared with patients without hypertension (35·8% vs 13·7%).1 A key pathophysiological mechanism might involve the interaction between SARS-CoV-2 spike protein and the host cell membrane-bound angiotensin-converting enzyme 2 (ACE2), which facilitates viral entry into the host cell.38 The ACE2 enzyme converts angiotensin II, a vasoconstrictive, profibrotic peptide, to angiotensin 1–7, a vasodilatory, anti-inflammatory peptide.39 On viral entry of SARS-CoV-2 into target cells, ACE2 is proteolytically degraded, thus, eliminating its enzymatic activity.40 This degradation might lead to an excess of circulating angiotensin II, which exerts vasoconstrictive effects by binding to angiotensin II type 1 receptor.41, 42 The superimposed angiotensin II-mediated vasoconstriction and the catecholamine excess from PPGL can lead to profound cardiovascular effects through several mechanisms. First, stimulation of α1-adrenoceptors by catecholamines in the systemic vasculature can act synergistically with excess angiotensin II (the result of SARS-CoV-2 infection), causing severe vasoconstriction and a marked rise in systemic vascular resistance.14, 43 Second, the action of catecholamines on β1-adrenoceptors within the kidney can increase secretion of renin, which in turn can increase the production of angiotensin II, leading to further worsening of hypertension. Moreover, in several patients with COVID-19, hypertension can be pre-existent as a part of metabolic syndrome, manifesting along with impaired glucose homoeostasis, and obesity.44, 45 This observation is also relevant in patients with COVID-19 with excess catecholamines, because blood pressure—even among patients with PPGL—has been shown to positively correlate with body-mass index.9 On the other hand, some patients with catecholamine excess and severe COVID-19 infection who would have otherwise developed distributive (septic) or cardiogenic shock might be paradoxically protected from developing a shock state due to the vasoconstrictive and cardio-stimulatory effects of excess circulatory catecholamines; these physiological effects are relevant to patients with COVID-19 who might have compensated shock with the provision of endogenous or exogenous adrenaline or noreadrenaline.14
Familiarity with the biologically active catecholamines noradrenaline and adrenaline provides valuable insight when considering the influence of a particular catecholamine on the cardiovascular system.14 Noradrenaline preferentially stimulates α1-adrenoceptors on peripheral vessels leading to marked vasoconstriction.14 By contrast, adrenaline often stimulates β-adrenoceptors leading to severe tachyarrhythmia through cardiac β1-adrenoceptors and, in some patients with PPGL, to hypotension through overstimulation of β2-adrenoceptors on peripheral vessels.14 However, cardiac β1-adrenoceptor stimulation and inotropic effects can also occur from synaptic noradrenaline produced locally in the postganglionic sympathetic neurons that innervate the heart.46 The net cardiovascular effects of catecholamines on blood pressure and heart rate depend largely on catecholamine actions via adrenoceptors on vasculature and various other organs, their interplay with the immune system, the host response to SARS-CoV-2, and the severity of the infection.
Catecholamines, as mentioned, also mediate compensatory and beneficial haemodynamic effects47 in the state of cardiogenic or distributive shock. Conversely, we believe that catecholamines might predispose to or precipitate life-threatening decompensations, especially in the setting of COVID-19.14 Up to 71% of patients with PPGL die of cardiovascular causes, with 19·3% experiencing acute, sometimes fatal, cardiovascular complications.9, 10, 48 These complications include heart failure, myocardial infarction, arrhythmias, and ischaemic and haemorrhagic strokes.7, 10 Catecholamines have chronic and acute influences on the cardiovascular system.49 Chronically elevated catecholamine concentrations and associated hypertension lead to deleterious remodelling of coronary, cerebrovascular, and remaining systemic arteries, predisposing to stroke, myocardial infarction, and microvascular ischaemia in the setting of superimposed haemodynamic stressors.7, 50 Furthermore, chronic hypertension and high afterload lead to myocardial thickening and eventual heart failure with preserved ejection fraction.51 Even more perplexing, yet not well understood, is the development of heart failure with reduced ejection fraction in the setting of catecholamine excess.52 This disease state can be mediated by toxic concentrations of catecholamines leading to myocardial cell death, fibrosis, and ventricular dilatation with systolic dysfunction.50 In our opinion, acute catecholamine surges, seen in patients with underlying PPGL and in critically ill patients with sudden haemodynamic stressors or vasopressor infusion, might increase the risk of lethal complications in the setting of COVID-19. These complications might include fatal arrhythmias, hypertensive emergencies with stroke, aortic dissection, demand and vasospastic myocardial infarction, myocarditis, and stress-induced (takotsubo) cardiomyopathy.7
Ventilation, perfusion, and the pulmonary system
Chronic obstructive lung disease and asthma are associated with substantial morbidity and mortality in patients with COVID-19.2, 28 Furthermore, autopsy specimens in these patients show diffuse alveolar damage and, in some cases, extensive granulocytic infiltration of the bronchi and alveoli, resulting in ventilation defects.26, 28, 53, 54, 55 Adrenaline causes β2-adrenoceptor-mediated broncho-dilation whereas noradrenaline causes α1-adrenoceptor-mediated reductions in bronchial gland secretions.43, 56 These effects might improve ventilation in patients with COVID-19. Excess catecholamines can cause α1-adrenoceptor-mediated vasoconstriction, which can reduce blood flow to well ventilated alveoli.43 Catecholamine excess can also cause β2-adrenoceptor-mediated vaso-dilation, which can reverse the compensatory hypoxic vasoconstriction in the vasculature that surrounds poorly ventilated alveoli.57 These effects might cause a mismatch between ventilation and perfusion, leading to intrapulmonary shunting that could potentially worsen the already present hypoxaemia in patients with COVID-19.5, 58, 59, 60
Glucose metabolism and the endocrine system
Both type 1 and type 2 diabetes are risk factors for severe COVID-19, and uncontrolled hyperglycaemia in both forms of diabetes is significantly independently associated with COVID-19-related mortality.61 Patients with COVID-19 and diabetes are at a higher risk for developing acute respiratory distress syndrome, septic shock, acute kidney injury, and hypercoagulability with thrombi or emboli than patients with COVID-19 but no diabetes.2, 12, 62 These conditions necessitate care in an intensive care unit, often with mechanical ventilation. Diabetes was a common comorbidity (33·8%) in a study done on 5700 hospitalised patients with COVID-19 in New York, USA.12 An analysis of data obtained from over 17·2 million individuals in the UK (OpenSAFELY study) showed that uncontrolled diabetes was an independent risk factor for death from COVID-19 (age-adjusted and sex-adjusted HR 2·61, 95% CI 2·46–2·77), even after adjusting for co-variables.2 A multicentre, retrospective study on 7337 patients with COVID-19 in Hubei province, China, showed that patients who had type 2 diabetes had significantly higher mortality than patients without diabetes.62 51–75% of patients with PPGL have fasting hyperglycaemia as a result of β-cell α2-adrenoceptor-mediated inhibition of insulin secretion.63 Although PPGL-related hyperglycaemia is usually mild, it can require insulin and, in severe cases, precipitate diabetic ketoacidosis. Moreover, hyperglycaemia is associated with dysregulated immunity, including impaired neutrophil chemotaxis, defective intracellular destruction of microbes, blunted antiviral interferon responses, delayed activation of T-helper type 1 cell-mediated immunity, and a late hyperinflammatory response, all of which can worsen the severity of COVID-19.64 In our opinion, excess catecholamines might oppose hypoglycaemia in patients with COVID-19, especially those with severe sepsis or septic shock.
Effects of catecholamine excess in PPGL and their implications for patients with COVID-19
As previously discussed, catecholamines have opposing effects—from hypertensive crises to hypotensive shock—on various systems, sometimes augmenting cardiac output while also potentially causing acute decompensated heart failure, metabolic disturbances, and immune dysregulations. Thus, to avert these devastating catecholamine-induced outcomes, we will discuss the pertinent therapeutic principles that are foundational to the management of catecholamine excess, particularly in PPGL (table ). PPGLs can store and therefore secrete great amounts of catecholamines into circulation.65 The pursuant severe clinical consequences are the best clinical examples for appreciating the effects of circulating adrenaline and noradrenaline. Within a moment, a patient can develop haemodynamic instability resulting in cardiac arrest.66 Thus, beyond the potential for acute decompensation, the cardiovascular, pulmonary, haematological, endocrine, and immunological changes incurred by catecholamines establish risk factors for morbidity and mortality in patients with COVID-19.6, 10, 30, 49, 50, 56, 67 These risk factors include hypertension, diabetes, and cardiovascular disease; and in patients with PPGL, there is a potential for the additional burden of malignancy, which in itself was shown to be an important risk factor for COVID-19-related death even after multivariate analysis.2 By contrast, catecholamines might sustain beneficial cardiopulmonary effects in patients with COVID-19 pneumonia and associated distributive (eg, septic) or cardiogenic shock. Interfering with the influence of catecholamines on the pulmonary and cardiovascular system (table) in such a balanced compensatory state might lead to cardiovascular and respiratory failure. Thus, rather than striving for optimal management when considering patients with excess catecholamines, a multidisciplinary team needs to consider the complex beneficial and harmful effects of catecholamines in patients with COVID-19 (table).
Table.
Potential interactions between catecholamines, adrenoceptors, and physiological systems, and implications in patients with COVID-19
| Deleterious effects of catecholamines | Beneficial effects of catecholamines | ||
|---|---|---|---|
| Cardiovascular system | |||
| Arrhythmogenic effects | |||
| β1-AR | adrenaline > noradrenaline: arrhythmias, deteriorate into cardiac arrest; most common arrhythmias: sinus tachycardia, atrial fibrillation or flutter, ventricular tachycardia | .. | |
| Haemodynamic effects | |||
| α1-AR | noradrenaline > adrenaline: vasoconstriction, hypertension | noradrenaline > adrenaline: catecholamine-induced vasoconstriction might compensate for COVID-19-distributive or cardiogenic shock | |
| β1-AR | noradrenaline and adrenaline: renin release and COVID-19-mediated ACE2 degradation; both can lead to increased angiotensin II, potentially resulting in hypertensive crises | noradrenaline and adrenaline: cardiac stimulation might compensate for COVID-19-distributive or cardiogenic shock | |
| β2-AR | adrenaline or noradrenaline with α1-AR blockade: can precipitate hypotensive shock or distributive (septic) shock in patients with COVID-19 | .. | |
| Cardiomyopathic effects | |||
| β1-AR and β2-AR | adrenaline > noradrenaline: acute heart failure or decompensation, takotsubo cardiomyopathy, myocarditis, chronic catecholamine cardiomyopathies | .. | |
| Pulmonary system | |||
| α1-AR | noradrenaline > adrenaline: vasoconstriction to well ventilated alveoli, intrapulmonary shunt, hypoxaemia | noradrenaline > adrenaline: decreased bronchial gland secretion, improved ventilation | |
| β2-AR | adrenaline: vasodilation or opposition to hypoxic vasoconstriction to poorly ventilated alveoli, intrapulmonary shunt, hypoxaemia | adrenaline: bronchodilation, improved ventilation | |
| Haematological system | |||
| β-ARs | noradrenaline and adrenaline: hypercoagulability, thrombosis | adrenaline > noradrenaline: activation of fibrinolysis, anti-thrombotic | |
| α2-AR | adrenaline and noradrenaline: platelet aggregation, thrombosis | .. | |
| Immunological system | |||
| α2-ARs | probably noradrenaline and adrenaline: inhibition of antigen presentation, lymphocyte proliferation, cytokine production, greater susceptibility to SARS-CoV-2 infection | .. | |
| β-ARs | likely adrenaline: lymphopenia and chronic catecholamines cause NK cell reduction | likely adrenaline: acute catecholamine elevation, increased NK cells, improved host viral defence | |
| Endocrine system | |||
| α2-AR | adrenaline > noradrenaline: β-cell dysfunction, hyperglycaemia | adrenaline > noradrenaline: catecholamine-induced hyperglycaemia, could counteract hypoglycaemia in patients with severe COVID-19 infection | |
ACE2=angiotensin-converting enzyme 2. AR=adrenoceptor. NK cell=natural killer cell. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Regarding patients with PPGL, it is very well accepted in clinical practice that catecholamine excess can be treated by α-adrenoceptor or β-adrenoceptor blockers, catecholamine synthesis inhibitors, calcium channel blockers, and the more recent hyperpolarisation-activated cyclic nucleotide-gated channel blocker ivabradine.68, 69 Adrenoceptor blockade, including α1-adrenoceptor blockers (eg, doxazosin or prazosin), non-specific α-adrenoceptor blockers (eg, phenoxybenzamine), and β-adrenoceptor blockers (eg, atenolol or metoprolol), are the most commonly used medications for management of catecholamine excess in patients with PPGLs.68 Using metyrosine (Demser), which inhibits catecholamine synthesis by blocking tyrosine hydroxylase, a rate-limiting enzyme of catecholamine synthesis, prevents high catecholamine concentrations, which would otherwise bind to cognate adrenoceptors.68 Metyrosine might offer additional benefits, as treatment will not only dampen catecholamine synthesis and action on various organs via adrenoceptors, but could also attenuate the hyperinflammatory response, cytokine storm syndrome, or multisystem inflammatory syndrome in children. In a well designed experiment by Staedtke and colleagues,21 inhibition of tyrosine hydroxylase with metyrosine decreased excess cytokine release in response to bacterial infections, T-cell targeting antibodies, and chimeric antigen receptor T cells in mice. In the same study, α1-adrenoceptor blockade, but not β-adrenoceptor blockade, limited the lipopolysaccharide-induced hyperinflammatory response. In a retrospective analysis of data from a population with pneumonia complicated by acute respiratory distress syndrome, patients who were given α1-adrenoceptor blockers in the year preceding hospitalisation had a significantly lower risk for mechanical ventilation and mortality than those not on α1-adrenoceptor blockers.32 Therefore, among patients with COVID-19, α1-specific adrenoceptor-blockade with doxazosin or prazosin, or non-specific α-adrenoceptor-blockade with phenoxybenzamine, might substantially reduce the catecholamine-mediated hyperinflammatory response, cytokine storm syndrome, or multisystem inflammatory syndrome in children.13, 21 The PREVENT-COVID trial (ClinicalTrials.gov, NCT04365257) is a phase 2 randomised, open-label, clinical trial that is currently recruiting patients to evaluate the efficacy and safety of the α1-adrenoceptor blocker prazosin in preventing cytokine storm syndrome among hospitalised patients with COVID-19. Furthermore, the use of metyrosine can be very beneficial in patients with PPGL and COVID-19 because of its well known effect on catecholamine synthesis. In addition, drugs that mitigate cytokine storm syndrome or multisystem inflammatory syndrome in children through inhibition of cytokine production, such as the IL-6 inhibitors tocilizumab and sarilumab, the IL-1 receptor antagonist anakinra, or anti-TNF therapy, could be of particular use in this unique cohort.70, 71 However, the cost and adverse effects of these agents might limit their use. Additionally, the use of ivabradine, a sinus node If current inhibitor, might successfully treat tachycardia refractory to β-adrenoceptor and calcium channel blockades.69 In our opinion, the use of adrenoceptor blockers in drug-naive patients (ie, patients with no previous therapeutic exposure to adrenoceptor-blockers) might lead to hypotension in the setting of SARS-CoV-2 infection. Additionally, these medications might cause orthostatic hypotension during the first few days of therapy, especially among those patients with intermittent hypertension.68 Physicians should be aware that catecholamines are necessary for maintaining vascular tone, and blocking the synthesis of catecholamines or their actions on adrenoceptors in patients with COVID-19 could increase the risk for hypotension. In patients with PPGL, appropriate (often vigorous) administration of fluids and ingestion of salt will offset such adverse outcomes. Other stress hormones such as glucocorticoids might act synergistically with catecholamines, leading to deleterious clinical outcomes.72 The interactions between catecholamines and glucocorticoids are intricate. Catecholamines influence the hypothalamic–pituitary–adrenal axis and, in turn, affect glucocorticoid-mediated immune signals.73 On the other hand, intra-adrenal glucocorticoids contribute to adrenaline production in the adrenal medulla.74 Patients with COVID-19 have been shown to mount a robust hypercortisolaemic response during the infection, and higher serum cortisol values are associated with reduced median survival and increased mortality.75 However, glucocorticoids might mitigate some effects related to catecholamine excess, such as excess cytokine production, and inflammation.73 Such beneficial effects might have contributed to the favourable outcomes in patients with COVID-19 and hypoxaemia who were given dexamethasone in the RECOVERY trial.76 It is also crucial to be cognisant of glucocorticoid therapy in patients with catecholamine excess, and this is particularly relevant in patients with coexistent PPGL and COVID-19.11, 77 When a patient with COVID-19 is suspected to have an underlying PPGL, physicians need to be wary of elevations in plasma and urine metanephrines that can be observed in severe acute illness due to sympathoadrenal activation, vasopressor infusions, or other medications, even without an underlying PPGL.78 In these situations, plasma metanephrines can elevate to concentrations similar to those observed in patients with catecholamine-producing PPGLs, precluding an unequivocal diagnosis.78, 79 Nevertheless, if the clinical course of a patient allows, evaluation for PPGL can be deferred until the resolution of acute COVID-19 illness. A subset of patients who develop severe COVID-19 and require intensive care unit admission could be at risk for developing post-intensive care unit syndrome, characterised by new or persistent physical, psychological, and cognitive impairments after hospital discharge.80 Catecholamines have been implicated in the development of these syndromes,81, 82 and their manifestations might be exaggerated among patients with COVID-19 with catecholamine excess states such as PPGL.
In our opinion, patients with PPGL and mild COVID-19 infection or suspected COVID-19 infection not requiring hospitalisation, who are already on adrenoceptor blockers or other anti-hypertensives, can continue with these medications, and these anti-hypertensive regimens should not be interrupted as long as the blood pressure and heart rate remain stable. Patients should contact their physician if they experience erratic changes in blood pressure or heart rate or worsening of respiratory symptoms. Because patients with coexistent PPGL and COVID-19 are at risk for hyperglycaemia, optimal glycaemic control might be necessary for better outcomes.62 For patients with PPGL and mild COVID-19 infection or patients with pre-existing hyperglycaemia, blood glucose should be closely monitored at home, with the provision of anti-hyperglycaemic agents and insulin on a case-by-case basis. Active surveillance and in-person evaluation (eg, in the clinic) should be considered for patients who meet one or more of the following criteria: have a newly diagnosed secretory PPGL; have catecholaminergic symptoms; are undergoing peptide receptor radionuclide therapy, chemotherapy, or radiation; and have evidence of clinical, biochemical, or radiological progression of disease.83 On the other hand, patients who are potential candidates for telemedicine consultations are those that have a non-functioning PPGL, have stable (including metastatic) disease, have had a resection of an uncomplicated PPGL 1–3 months ago, or those in whom more than 4 weeks has elapsed since peptide receptor radionuclide therapy or ablation, and who remain asymptomatic.83
Surgical intervention should be offered to PPGL patients in a timely manner when warranted.84 However, if a patient remains stable on adrenoceptor blockade or there is no previously detected rapid tumour growth (regardless of secretory status), it is not unreasonable to actively monitor and defer surgery in a patient with COVID-19 and PPGL until the resolution of the infection or the patient tests negative for SARS-CoV-2 on a nasopharyngeal swab.83 Several risk factors, including the patient's age, comorbidities, biochemical phenotype of PPGL (catecholamine secretor vs catecholamine non-secretor), tumour size, local invasion into surrounding tissues, extent of disease, and severity of symptoms and signs are to be considered and weighed against the benefits of timely surgical intervention in these patients. Prompt initiation of preoperative adrenoceptor blockade, preferably 7–14 days before surgery, is crucial for prevention of hypertensive crisis during surgery.68, 84, 85, 86 A high-sodium diet and adequate fluid intake is necessary to minimise preoperative catecholamine-induced blood volume contraction to prevent postoperative hypotension.85
As outlined in this Personal View, the effect of elevated catecholamine concentrations is modelled by patients with PPGL and relevant to patients with critical illness. Catecholamines, especially in excess concentrations, cause dysregulation of physiological cascades. This dysregulation subsequently leads to various conditions, many of which are risk factors closely linked to adverse outcomes in patients with COVID-19. Despite the detrimental effects of catecholamines, counteracting their influence with various agents should be done cautiously. This cautious approach should be taken because of the various potential beneficial effects of catecholamines on pulmonary ventilation, vascular tone, and cardiac function, which can be vital compensatory influences in the setting of COVID-19. Further studies aimed at exploring the role of catecholamines in the pathophysiology of COVID-19 might reveal novel therapeutic strategies and druggable targets in the management of COVID-19.
Search strategy and selection criteria
The literature review was done on PubMed using the search terms “phaeochromocytoma”, “paraganglioma”, “COVID-19”, “coronavirus”, “immune system”, “hypercoagulation”, “hypertension”, “diabetes”, and “physiology” in combination with the term “catecholamines”. Data were reviewed from full-length articles, including case reports, case series, observational retrospective studies, systematic reviews, and meta-analyses published on any date before Aug 31, 2020. There were no language restrictions applied to our search. The final reference list of literature was created on the basis of originality and relevance to this Personal View.
Acknowledgments
Acknowledgments
This work was supported by the Intramural Research Program of the US National Institutes of Health, the US Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the US National Institute of Diabetes and Digestive and Kidney Diseases.
Contributors
SG and MAN did the literature search, prepared the first draft of the manuscript, and revised the manuscript. DT and JK-G reviewed and revised the manuscript. KP conceptualised the manuscript, contributed towards the preparation of the first draft of the manuscript, did the literature search, and revised the manuscript.
Declaration of interests
We declare no competing interests.
References
- 1.Guan WJ, Ni ZY, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williamson EJ, Walker AJ, Bhaskaran K. OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie J, Tong Z, Guan X, Du B, Qiu H. Clinical characteristics of patients who died of coronavirus disease 2019 in China. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia LF. Immune response, inflammation, and the clinical spectrum of COVID-19. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gattinoni L, Chiumello D, Caironi P. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–675. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 7.Petrak O, Rosa J, Holaj R. Blood pressure profile, catecholamine phenotype, and target organ damage in pheochromocytoma/paraganglioma. J Clin Endocrinol Metab. 2019;104:5170–5180. doi: 10.1210/jc.2018-02644. [DOI] [PubMed] [Google Scholar]
- 8.Majtan B, Zelinka T, Rosa J. Long-term effect of adrenalectomy on cardiovascular remodeling in patients with pheochromocytoma. J Clin Endocrinol Metab. 2017;102:1208–1217. doi: 10.1210/jc.2016-2422. [DOI] [PubMed] [Google Scholar]
- 9.Geroula A, Deutschbein T, Langton K. Pheochromocytoma and paraganglioma: clinical feature-based disease probability in relation to catecholamine biochemistry and reason for disease suspicion. Eur J Endocrinol. 2019;181:409–420. doi: 10.1530/EJE-19-0159. [DOI] [PubMed] [Google Scholar]
- 10.Zelinka T, Petrak O, Strauch B. Elevated inflammation markers in pheochromocytoma compared to other forms of hypertension. Neuroimmunomodulation. 2007;14:57–64. doi: 10.1159/000107289. [DOI] [PubMed] [Google Scholar]
- 11.Tomoyasu M, Mori Y, Fukase A, Kushima H, Hirano T. Pheochromocytoma presenting with severe hyperglycemia and metabolic acidosis following intra-articular glucocorticoid administration: a case report. J Med Case Reports. 2019;13:3. doi: 10.1186/s13256-018-1945-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richardson S, Hirsch JS, Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konig MF, Powell M, Staedtke V. Preventing cytokine storm syndrome in COVID-19 using alpha-1 adrenergic receptor antagonists. J Clin Invest. 2020;130:3345–3347. doi: 10.1172/JCI139642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nazari MA, Rosenblum JS, Haigney MC, Pacak K. Current view: pathophysiology and acute management of tachyarrhythmias in pheochromocytoma. J Am Coll Cardiol. 2020;74:451–464. doi: 10.1016/j.jacc.2020.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta P, McAuley DF, Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belhadjer Z, Meot M, Bajolle F. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.048360. https://doi.org/CIRCULATIONAHA.120.048360 published May 17. [DOI] [PubMed] [Google Scholar]
- 17.Feldstein LR, Rose EB, Horwitz SM. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020 doi: 10.1056/NEJMc2026136. [DOI] [PubMed] [Google Scholar]
- 18.Dufort EM, Koumans EH, Chow EJ. Multisystem inflammatory syndrome in children in New York state. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen G, Wu D, Guo W. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staedtke V, Bai RY, Kim K. Disruption of a self-amplifying catecholamine loop reduces cytokine release syndrome. Nature. 2018;564:273–277. doi: 10.1038/s41586-018-0774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bikdeli B, Madhavan MV, Jimenez D. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birk AV, Leno E, Robertson HD, Bolotina VM, Szeto HH. Interaction between ATP and catecholamines in stimulation of platelet aggregation. Am J Physiol Heart Circ Physiol. 2003;284:H619–H625. doi: 10.1152/ajpheart.00110.2002. [DOI] [PubMed] [Google Scholar]
- 24.Olbrich C, Aepfelbacher M, Siess W. Epinephrine potentiates calcium mobilization and activation of protein kinases in platelets stimulated by ADP through a mechanism unrelated to phospholipase C. Cell Signal. 1989;1:483–492. doi: 10.1016/0898-6568(89)90033-8. [DOI] [PubMed] [Google Scholar]
- 25.van der Poll T, Levi M, Dentener M. Epinephrine exerts anticoagulant effects during human endotoxemia. J Exp Med. 1997;185:1143–1148. doi: 10.1084/jem.185.6.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ackermann M, Verleden SE, Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klok FA, Kruip M, van der Meer NJM. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wichmann D, Sperhake JP, Lutgehetmann M. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seiffert K, Hosoi J, Torii H. Catecholamines inhibit the antigen-presenting capability of epidermal Langerhans cells. J Immunol. 2002;168:6128–6135. doi: 10.4049/jimmunol.168.12.6128. [DOI] [PubMed] [Google Scholar]
- 30.Bao JY, Huang Y, Wang F, Peng YP, Qiu YH. Expression of alpha-AR subtypes in T lymphocytes and role of the alpha-ARs in mediating modulation of T cell function. Neuroimmunomodulation. 2007;14:344–353. doi: 10.1159/000129670. [DOI] [PubMed] [Google Scholar]
- 31.Oberbeck R. Catecholamines: physiological immunomodulators during health and illness. Curr Med Chem. 2006;13:1979–1989. doi: 10.2174/092986706777584997. [DOI] [PubMed] [Google Scholar]
- 32.Vogelstein JT, Powell M, Koenecke A. Alpha-1 adrenergic receptor antagonists for preventing acute respiratory distress syndrome and death from cytokine storm syndrome. ArXiv. 2020 published online April 21. https://doi.org/arXiv:2004.10117v5 (preprint) [Google Scholar]
- 33.Tan L, Wang Q, Zhang D. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanders V, Kasprowicz D, Kohm A, Swanson M. Neurotransmitter receptors on lymphocytes and other lymphoid cells. Psychoneuroimmunology. 2001;1:161–196. [Google Scholar]
- 35.Sanders VM. The beta2-adrenergic receptor on T and B lymphocytes: do we understand it yet? Brain Behav Immun. 2012;26:195–200. doi: 10.1016/j.bbi.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiffrin EL, Flack JM, Ito S, Muntner P, Webb RC. Hypertension and COVID-19. Am J Hypertens. 2020;33:373–374. doi: 10.1093/ajh/hpaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmann M, Kleine-Weber H, Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gheblawi M, Wang K, Viveiros A. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo J, Huang Z, Lin L, Lv J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bavishi C, Maddox TM, Messerli FH. Coronavirus disease 2019 (COVID-19) infection and renin angiotensin system blockers. JAMA Cardiol. 2020;5:745–747. doi: 10.1001/jamacardio.2020.1282. [DOI] [PubMed] [Google Scholar]
- 43.Pacak K, Keiser HR, Eisenhofer G. Pheochromocytoma. In: DeGroot LJ, Jamenson JL, editors. Endocrinology. 5th edn. Elsevier Saunders; Philadelphia: 2006. pp. 2501–2534. [Google Scholar]
- 44.Bansal R, Gubbi S, Muniyappa R. Metabolic syndrome and COVID 19: endocrine-immune-vascular interactions shapes clinical course. Endocrinology. 2020;161 doi: 10.1210/endocr/bqaa112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simonnet A, Chetboun M, Poissy J. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Esler M, Jennings G, Lambert G, Meredith I, Horne M, Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiol Rev. 1990;70:963–985. doi: 10.1152/physrev.1990.70.4.963. [DOI] [PubMed] [Google Scholar]
- 47.Goldstein B, Woolf PD. The autonomic and neurohumoral response to critical illness. Clin Intensive Care. 1996;7:297–307. [Google Scholar]
- 48.Sutton MG, Sheps SG, Lie JT. Prevalence of clinically unsuspected pheochromocytoma. Review of a 50-year autopsy series. Mayo Clin Proc. 1981;56:354–360. [PubMed] [Google Scholar]
- 49.Prejbisz A, Lenders JW, Eisenhofer G, Januszewicz A. Cardiovascular manifestations of phaeochromocytoma. J Hypertens. 2011;29:2049–2060. doi: 10.1097/HJH.0b013e32834a4ce9. [DOI] [PubMed] [Google Scholar]
- 50.Santos JRU, Brofferio A, Viana B, Pacak K. Catecholamine-induced cardiomyopathy in pheochromocytoma: how to manage a rare complication in a rare disease? Horm Metab Res. 2019;51:458–469. doi: 10.1055/a-0669-9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yildiz M, Oktay AA, Stewart MH, Milani RV, Ventura HO, Lavie CJ. Left ventricular hypertrophy and hypertension. Prog Cardiovasc Dis. 2020;63:10–21. doi: 10.1016/j.pcad.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 52.Kassim TA, Clarke DD, Mai VQ, Clyde PW, Mohamed Shakir KM. Catecholamine-induced cardiomyopathy. Endocr Pract. 2008;14:1137–1149. doi: 10.4158/EP.14.9.1137. [DOI] [PubMed] [Google Scholar]
- 53.Xu Z, Shi L, Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tian S, Xiong Y, Liu H. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schaller T, Hirschbuhl K, Burkhardt K. Postmortem examination of patients with COVID-19. JAMA. 2020;323:2518–2520. doi: 10.1001/jama.2020.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reinhardt D. Adrenoceptors and the lung: their role in health and disease. Eur J Pediatr. 1989;148:286–293. doi: 10.1007/BF00444116. [DOI] [PubMed] [Google Scholar]
- 57.Sylvester JT, Shimoda LA, Aaronson PI, Ward JP. Hypoxic pulmonary vasoconstriction. Physiol Rev. 2012;92:367–520. doi: 10.1152/physrev.00041.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lang M, Som A, Mendoza DP. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30367-4. published online April 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323:2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 60.Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care. 2020;24:154. doi: 10.1186/s13054-020-02880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holman N, Knighton P, Kar P. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8:823–833. doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu L, She ZG, Cheng X. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1068. doi: 10.1016/j.cmet.2020.04.021. 77.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Isles CG, Johnson JK. Phaeochromocytoma and diabetes mellitus: further evidence that alpha 2 receptors inhibit insulin release in man. Clin Endocrinol (Oxf) 1983;18:37–41. doi: 10.1111/j.1365-2265.1983.tb03184.x. [DOI] [PubMed] [Google Scholar]
- 64.Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:e736–e741. doi: 10.1152/ajpendo.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev. 2004;56:331–349. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- 66.Loscalzo J, Roy N, Shah RV. Case 8–2018: a 55-year-old woman with shock and labile blood pressure. N Engl J Med. 2018;378:1043–1053. doi: 10.1056/NEJMcpc1712225. [DOI] [PubMed] [Google Scholar]
- 67.Venugopal B, Sharon R, Abramovitz R, Khasin A, Miskin R. Plasminogen activator inhibitor-1 in cardiovascular cells: rapid induction after injecting mice with kainate or adrenergic agents. Cardiovasc Res. 2001;49:476–483. doi: 10.1016/s0008-6363(00)00271-6. [DOI] [PubMed] [Google Scholar]
- 68.Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92:4069–4079. doi: 10.1210/jc.2007-1720. [DOI] [PubMed] [Google Scholar]
- 69.Malaza G, Brofferio A, Lin F, Pacak K. Ivabradine in catecholamine-induced tachycardia in a patient with paraganglioma. N Engl J Med. 2019;380:1284–1286. doi: 10.1056/NEJMc1817267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020;92:814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feldmann M, Maini RN, Woody JN. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;395:1407–1409. doi: 10.1016/S0140-6736(20)30858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Axelrod J, Reisine TD. Stress hormones: their interaction and regulation. Science. 1984;224:452–459. doi: 10.1126/science.6143403. [DOI] [PubMed] [Google Scholar]
- 73.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 74.Zuckerman-Levin N, Tiosano D, Eisenhofer G, Bornstein S, Hochberg Z. The importance of adrenocortical glucocorticoids for adrenomedullary and physiological response to stress: a study in isolated glucocorticoid deficiency. J Clin Endocrinol Metab. 2001;86:5920–5924. doi: 10.1210/jcem.86.12.8106. [DOI] [PubMed] [Google Scholar]
- 75.Tan T, Khoo B, Mills EG. Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol. 2020;8:659–660. doi: 10.1016/S2213-8587(20)30216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Horby P, Lim WS, Emberson JR. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. published online July 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barrett C, van Uum SH, Lenders JW. Risk of catecholaminergic crisis following glucocorticoid administration in patients with an adrenal mass: a literature review. Clin Endocrinol (Oxf) 2015;83:622–628. doi: 10.1111/cen.12813. [DOI] [PubMed] [Google Scholar]
- 78.Amar L, Eisenhofer G. Diagnosing phaeochromocytoma/paraganglioma in a patient presenting with critical illness: biochemistry versus imaging. Clin Endocrinol (Oxf) 2015;83:298–302. doi: 10.1111/cen.12745. [DOI] [PubMed] [Google Scholar]
- 79.Eisenhofer G, Goldstein DS, Walther MM. Biochemical diagnosis of pheochromocytoma: how to distinguish true- from false-positive test results. J Clin Endocrinol Metab. 2003;88:2656–2666. doi: 10.1210/jc.2002-030005. [DOI] [PubMed] [Google Scholar]
- 80.Hosey MM, Needham DM. Survivorship after COVID-19 ICU stay. Nat Rev Dis Primers. 2020;6:60. doi: 10.1038/s41572-020-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21:1190–1222. doi: 10.1016/j.jagp.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 82.Schelling G. Post-traumatic stress disorder in somatic disease: lessons from critically ill patients. Prog Brain Res. 2008;167:229–237. doi: 10.1016/S0079-6123(07)67016-2. [DOI] [PubMed] [Google Scholar]
- 83.Casey RT, Valk GD, Schalin-Jäntti C, Grossman AB, Thakker RV. Endocrinology in the time of COVID-19: clinical management of neuroendocrine neoplasms (NENs) Eur J Endocrinol. 2020;183:G79–G88. doi: 10.1530/EJE-20-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Neumann HPH, Young WF, Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381:552–565. doi: 10.1056/NEJMra1806651. [DOI] [PubMed] [Google Scholar]
- 85.Lenders JW, Duh QY, Eisenhofer G. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:1915–1942. doi: 10.1210/jc.2014-1498. [DOI] [PubMed] [Google Scholar]
- 86.Buitenwerf E, Osinga TE, Timmers H. Efficacy of α-blockers on hemodynamic control during pheochromocytoma resection: a randomized controlled trial. J Clin Endocrinol Metab. 2020;105:2381–2391. doi: 10.1210/clinem/dgz188. [DOI] [PMC free article] [PubMed] [Google Scholar]