Abstract
The chitooligosaccharide (COS) and chlorella polysaccharide (CPS) have been used as feed supplements in the poultry industry for improving growth performance and immunity. However, the benefits of these prebiotics on the gut health of chickens when used in early nutrition are unknown. This study evaluated the effects of in ovo feeding of COS and CPS on the cecal microbiome, metabolic pathways, and fermentation metabolites of chickens. A total of 240 fertile eggs were divided into 6 groups (n = 4; 10 eggs/replicate): 1) no-injection control, 2) normal saline control, 3) COS 5 mg, 4) COS 20 mg, 5) CPS 5 mg, and 6) CPS 20 mg injection. On day 12.5 of egg incubation, test substrate was injected into the amniotic sac of eggs in respective treatments. The hatched chicks were raised for 21 D under standard husbandry practices. On day 3 and 21, cecal digesta were collected to determine microbiota by shotgun metagenomic sequencing and short-chain fatty acids by gas chromatography. The cecal microbial composition was not different (P > 0.05) among the treatment groups on day 3 but was different (P < 0.05) on day 21. At the species level, the polysaccharide-utilizing bacteria including Lactobacillus johnsonii, Bacteroides coprocola, and Bacteroides salanitronis were higher in the COS group, whereas the relative abundance of some opportunistic pathogenic bacteria were lower than those in the CPS and control groups. At the functional level, the pathways of gluconeogenesis, L-isoleucine degradation, L-histidine biosynthesis, and fatty acid biosynthesis were enriched in the COS group. In addition, propionic acid content was higher (P < 0.05) in the COS group. A network based on the correlation between the COS and other factors was constructed to illuminate the potential action mechanism of the COS in chicken early nutrition. In conclusion, in ovo inoculation of COS 5 mg showed positive effects on the cecal microbiota, metabolic pathways, and propionic acid, thus can be used as in ovo feeding to modulate the gut health of chickens.
Key words: in ovo feeding, prebiotics, intestinal microbiota, short-chain fatty acids, shotgun metagenomic sequencing
Introduction
Poultry production is the fast-growing animal industry and is expected to continue growing to meet the increasing demand for animal protein for the ever-increasing global population. The growth of the industry was based primarily on the nutritionally balanced feeding program combined with antibiotic growth promoters (AGP), among others. However, AGP are banned or regulated in different jurisdictions of the world in food animal production, and consumers are demanding chicken grown free of AGP. The situation has necessitated finding alternatives to AGP for healthy animal production. Different alternatives to AGP, including oligosaccharides and polysaccharides as prebiotics, have been evaluated with some success (Jha et al., 2019a; Yadav and Jha, 2019). These options are sought to maintain or improve the gut health of animals, as is essential for optimum growth, better feed efficiency, and overall health. Emerging evidence indicates that there is a close relationship between intestinal microbiota and nutrition utilization (Jha et al., 2019a), which in turn affect the overall performance and health of animals (Jha and Berrocoso, 2016). Intestinal microbiota can be manipulated by nutrition programming in poultry, during both the prehatch and posthatch period of life (Jha et al., 2019b). Generally, fibers (including oligosaccharides and polysaccharides) are fermented in the lower gut of animals producing metabolites such as short-chain fatty acids (SCFA), which in turn affects associated metabolic pathways and the intestinal microbial ecology of host animals (Pieper et al., 2008; Jha and Berrocoso, 2015). Among fibers, galactooligosaccharides (GOS) and fructooligosaccharides (FOS) are widely studied in poultry. In a recent study, in ovo injection of GOS increased the relative abundance of Lactobacillus and Bifidobacterium in the cecum of chickens and upregulated receptors of free fatty acid in the intestine (Slawinska et al., 2019). Similarly, FOS supplementation increased the alpha diversity of chicken intestinal microbiota (Park et al., 2017). Moreover, similar to the aforementioned GOS results, the beneficial microbes including Bifidobacterium and Lactobacillus not only produce extracellular enzymes to degrade FOS but also compete with other species of intestinal microorganisms and suppress the growth of pathogenic bacteria (Wan et al., 2020). However, different types of fibers vary in their fermentation characteristics and physiological effects on the gut ecology of the host animal (Jha et al., 2010; Jha and Leterme, 2012). In addition, dose-dependent responses of prebiotics are found on gut health variables of broiler chickens (Berrocoso et al., 2017). Thus, any new potential prebiotics needs to be evaluated before being used in animals to get the optimum gut health benefits.
Chitosan is primarily extracted from the shells of crustaceans including shrimp and crabs and is widely available (Guzman et al., 2003). Chitooligosaccharide (COS), derived by chemical and enzymatic hydrolysis of chitosan, has higher biological activity and more physiological functions than chitosan (Laokuldilok et al., 2017; Guan et al., 2019). Similarly, the chlorella polysaccharide (CPS) is extracted from the chlorella, which is a common microalga widely distributed in freshwater and well known for its high nutritional value and various bioactive compounds with functional and health benefits (Wang et al., 2018). The COS and CPS are comparatively novel and less frequently used as feed additives in animal nutrition. However, both have been tried as feed supplements in poultry for improving the growth performance, egg quality, intestinal histomorphology, and immunity (Shi et al., 2005; Deng et al., 2008; Khambualai et al., 2009; Zhou et al., 2009; Jiao et al., 2019). In a mice model, COS supplementation promoted the growth of the beneficial gut microbes, increased the intestinal immunity, and regulated the glucose–lipid metabolism through the gut–liver axis (Wang et al., 2020). By performing histopathology and biochemistry analyses, Wan et al. (2018) reported the hypolipidemic effect and gut microbiota regulation of the CPS on diet-induced obese rats. They confirmed that the CPS could improve plasma and liver lipid metabolism and accelerate the metabolism of the bile acids and cecal SCFA. In addition, the CPS diet changed the composition of intestinal microbiota including increasing the relative abundance of Coprococcus, Lactobacillus, and Turicibacter, whereas decreasing the Ruminococcus gauvreauii species. However, there is limited or no information on the effect of COS and CPS on the intestinal microbiome, metabolic pathways, and fermentation metabolites in broiler chickens. Moreover, most of the previous studies evaluated the response of COS and CPS when fed in diets posthatch, while in ovo feeding is an effective strategy to deliver supplements for efficient use and optimum gut health benefits to broiler chickens (Jha et al., 2019b; Slawinska et al., 2019).
During the prehatch period, the chicken's embryo develops by getting nutrients through oral consumption of the amniotic fluid, accumulation of glycogen reserves in muscle and liver tissues, and glycogenolysis (Moran, 2007). Accordingly, the dramatic physiological and metabolic changes occur, and any disturbances during this period may significantly affect the hatchability and subsequently growth performance and gut health of the chicken (Jha et al., 2019b; Yadav and Jha, 2019). To improve the nutritional status of hatchlings and intestinal functionality, a method for feeding the embryo (in ovo feeding) was developed in the past, and a method of injecting nutrient solutions into the embryonic amniotic fluid was created for poultry subsequently (Uni et al., 2005). By now, many potential nutrients and supplements had been evaluated using in ovo feeding, which indicates the wider applicability and potential of this technique to optimize growth performance and gut health of chickens (Siwek et al., 2018; Jha et al., 2019b).
This study aims to provide insights into the mechanisms that drive the beneficial effects of COS and COP when fed in ovo to broiler chickens. It focuses on the evaluation of in ovo feeding of COS and COP at different dose rates on the modulation of intestinal microbiota, selected metabolic pathways, and fermentation metabolites as a function of the microbiota–host interaction.
Materials and methods
All the animal procedures were performed following the protocol approved by the Institutional Animal Care and Use Committee of the University of Hawaii.
Experimental Design and Egg Incubation
A total of 240 fertile eggs from a breeder flock (Cobb 500) were obtained from a commercial hatchery (Asagi Hatchery Inc., Honolulu, HI) on the 10th day of incubation. On arrival, the eggs were individually weighed, numbered, and incubated at 37.5°C and relative humidity of 58% in an incubator (GQF incubator, Savannah, GA) in the Small Animal Facility of University of Hawaii at Manoa (Honolulu, HI). After the eggs were acclimatized in the incubator for >8 h, all the eggs were equally and randomly assigned to each of 6 prespecified treatment groups on each of 4 replicate tray levels (10 eggs per treatment in each tray level). Eggs were incubated under standard commercial conditions. On the 12th day of incubation, eggs were candled, and those unfertilized or with dead embryos were discarded. On day 12.5, fertile eggs were injected with allocated treatment solution and continued incubation under standard conditions. On day 19, the eggs were transferred to a preset hatcher set at 37°C and relative humidity 75% (GQF incubator, Savannah, GA). On hatch, chicks from each treatment were weighed individually and wing tagged. Depending on the hatch, 30 chicks from each treatment were randomly allocated within the treatment group to 6 replicate pens (4–6 chicks/pen) and raised under standard commercial conditions until day 21. The birds were fed with standard starter ration and had access to feed and water ad libitum. On day 3 and 21, 1 to 2 birds from each pen (n = 6/treatment) were euthanized by CO2 inhalation. The digesta from 1 cecum of each bird was collected in a Whirl-Pak bag with gentle milking and was transferred to −20°C until further analysis of SCFA. In addition, another set of cecal digesta was collected for DNA extraction, which was snap-frozen in liquid nitrogen and kept at −80°C until further analysis for microbiota.
In Ovo Injection
On day 12.5, each replicate group of eggs were taken out for in ovo injection in a biosafety cabinet, and the eggs were placed out of the incubator for less than 15 min. The broadside of the eggs was disinfected with a 10% povidone–iodine solution, and a punch hole (shell perforation) was made with a stabbing awl. After every punch, the tip of the awl was disinfected with 70% ethanol and wiped with sterile gauze. Each egg was injected with their specified treatment solution in their amniotic sac using a blunt-tip 21-gauge sterile needle, except the eggs in the noninjection control group. The hole in all eggs was sealed immediately after injection using sterile nontoxic glue. A total of 6 treatment groups 1) noninjection control; 2) 0.85% normal saline (NS); 3) 0.5 mL 0.85% NS containing 5 mg COS (Sigma-Aldrich Inc., St. Louis, MO); 4) 0.5 mL 0.85% NS containing 20 mg COS; 5) 0.5 mL 0.85% NS containing 5 mg CPS (FEBICO, Ningbo, China); and 6) 0.5 mL 0.85% NS containing 20 mg CPS were set in the study.
DNA Extraction and Shotgun Metagenomic Sequencing
The frozen cecal digesta samples were thawed, and metagenomic DNA was extracted from the cecal content using a QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) following the manufacturer's instruction. Purified genomic DNA was isolated by removing the RNA and proteins using QIAamp Mini spin columns. The concentration of the eluted DNA was determined by using NanoPhotometer P330 (IMPLEN, Los Angeles, CA), and DNA was preserved at −20°C until used. Libraries were prepared with a fragment length of approximately 300 bp. Paired-end reads were generated using 100 bp in the forward and reverse directions. The reads were trimmed using Sickle and were subsequently aligned to the human genome to remove the host DNA fragments. An average of 3 to 4 gigabases of high-quality paired-end reads were obtained from each sample, totaling about 300 gigabases of high-quality data that were free of human DNA and adaptor contaminants.
Metagenomic Species, Microbial Functional Genes, and Metabolic Pathway Annotation
The shotgun reads were assembled into contigs and scaffolds using MegaHiT (Li et al., 2015) with the default parameter (megahit -1 1.fq -2 2.fq -m 1000000 -t 12 -o--min-contig-len 500). For metagenomic species annotation, MetaPhlAn2 pipeline was applied. For metagenomic functional features and metabolic pathway annotation, HUMAnN2 (humann2 --threads 12 --input examples/demo.fasta--output $OUTPUT_DIR) was performed by using the UniRef90 database (Franzosa et al., 2018). More information was listed in “code availability.” Accordingly, we got the relative abundance of intestinal microbial taxonomic, gene families, and metabolic pathway profiles.
Short-Chain Fatty Acid Determination
The SCFA was analyzed by a gas chromatography–mass spectrometry (MS) assay as previously described (Zhang et al., 2017) with some modifications. Briefly, the cecal samples were thawed, weighed, and diluted 1:10 (w/v) in isooctane. The mixture was homogenized for 15 min, followed by centrifugation for 10 min at 5,000 × g. Five hundred microliters of supernatant was dried with a SpeedVac overnight. The dried metabolite extracts were dissolved in 50 μL of the methoxyamine hydrochloride solution in pyridine (20 mg/mL) and vortex-mixed for 2 min. Methoxymation was carried out at 70°C for 30 min. After the addition of 40 μL of N-(tert-butyldimethylsilyl)-N methyltrifluoroacetamide mixed with 1% tertbutyl-dimethylchlorosilane, derivatization was carried out at 70°C for 1 h. Gas chromatography (Agilent 7890B; Agilent, Santa Clara, CA) coupled with MS (Agilent 5977A; Agilent, Santa Clara, CA) was used for the analysis of the samples. The separation was achieved using an HP-5 MS column (30 m × 0.25 mm i.d. coated with 0.25 μm film thickness; Agilent, Santa Clara, CA). The gas chromatography temperature program was as follows: 50°C held for 1.0 min, increased to 200°C by 10°C/min, 200°C held for 5.0 min, increased to 220°C by 5°C/min, 220°C held for 10.0 min, and increased to 250°C for 10 min by 15°C/min. The inlet temperature was set at 250°C. The mass range was set as 35 to 400 m/z. The ion source chamber was set at 230°C with the transfer line temperature set to 250°C and the electron energy of 70 eV.
Statistical Analysis
All statistical analyses were performed using R software. Principal coordinate analysis (PCoA) was performed in R using the ade4 package (Jombart, 2008). The heatmap was constructed using the “pheatmap” package. The differential abundances of various profiles were tested with the Wilcoxon rank sum test and were considered significantly different at P < 0.05. For box plot construction, the package “ggpubr” (Whitehead et al., 2019) was used. The edges of the network were calculated by Spearman's rank correlation coefficient and visualized in Cytoscape (version 3.7.1).
Results
Alteration of the Structure of Intestinal Microbiota in the Chicken of Different Treatment Groups on Day 21 Posthatch
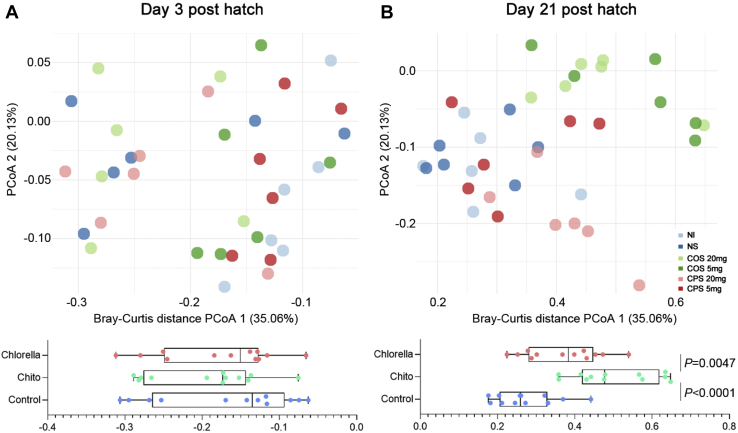
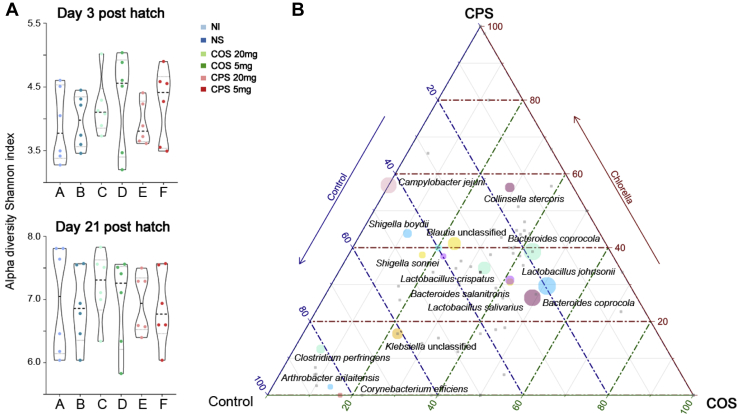
The principal coordinate analysis was performed based on the Bray–Curtis distances of the metagenomic sequencing profiles at the species level (Figure 1). In Figure 1A, the different color points represent the intestinal microbiota of each sample on day 3, showing mixed values, which suggests that there was no significant difference (P > 0.05) in intestinal microbial composition among different treatment groups in the early life of the chicken. However, on day 21, the intestinal microbiota in the control, COS, and CPS groups were distinct for organismal structure. To quantify the difference, we calculated the P values (Wilcoxon rank sum tests) of PC1 based on Bray–Curtis distances, which indicated that in ovo feeding with COS to the chicken has significant (P < 0.0001) impact on the intestinal microbiota. In addition, it was observed that the microbial Bray–Curtis distances between the control group and the CPS group were not significant (P > 0.05), which implied no effect of in ovo feeding with CPS. The results were confirmed with the microbial alpha diversity metrics (Shannon index) in which we observed a higher alpha diversity of the samples in the COS group (Figure 2A).
Figure 1.
Metagenomic species–based Bray–Curtis distances principal coordinate analysis (PCoA) of samples in control, NS, COS, and CPS groups on day 3 (A) and day 21. (B) The points in different colors represented the intestinal microbial structure of the samples in each group. The PC1 was extracted and compared using a box plot, the P-value represented the significance between the 2 groups (Wilcoxon rank sum tests). Abbreviations: COS, chitooligosaccharide; CPS, chlorella polysaccharide; NI, noninjection control; NS, normal saline.
Figure 2.
(A) Alpha diversity metric showing a higher alpha diversity of the samples in the COS group. (B) Ternary diagram showing significantly different metagenomic species among the groups. Abbreviations: COS, chitooligosaccharide; CPS, chlorella polysaccharide.
The Difference in Specific Intestinal Metagenomic Species Among the Treatment Groups on Day 21 Posthatch
Based on the taxonomic level annotated by MetaPhlAn2 pipeline, we calculated the significant difference species among the different treatment groups and visualized them as a ternary diagram (Figure 2B). It was observed that the polysaccharide-utilizing species including Lactobacillus crispatus, Lactobacillus johnsonii, Lactobacillus salivarius, Bacteroides coprophilus, Bacteroides coprocola, and Bacteroides salanitronis increased in the COS group, whereas the relative abundance of some chicken-source pathogens and opportunistic pathogens including Campylobacter jejuni, Clostridium perfringens, Collinsella stercoris, Corynebacterium efficiens, Fusobacterium mortiferum, Klebsiella unclassified, Shigella boydii, and Shigella sonnei were lower than that in the control and CPS groups.
The Difference in Specific Intestinal Metabolic Pathways and Metabolites Among the Treatment Groups on Day 21 Posthatch
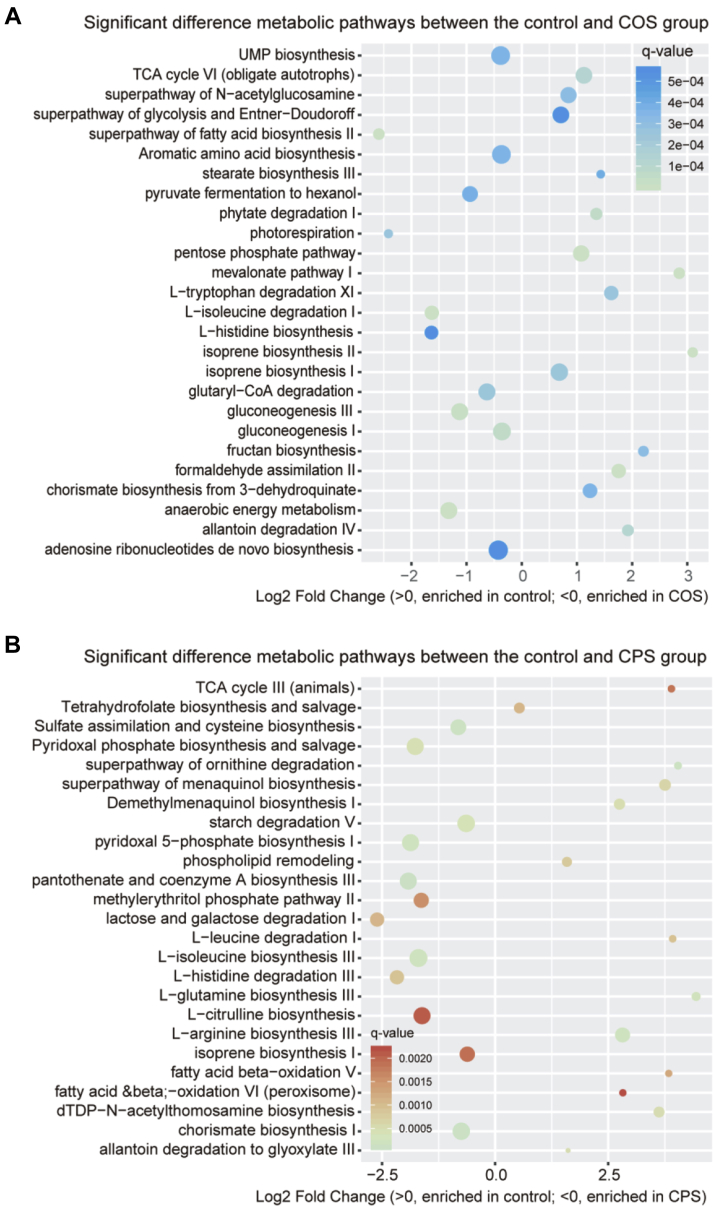
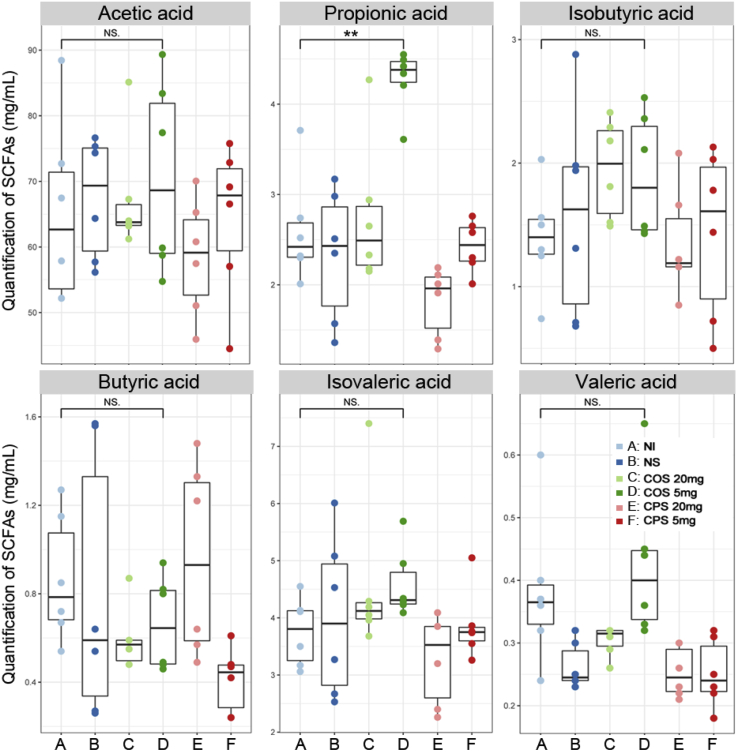
After confirming the difference at the species level, we further explored the changes in the microbial metabolic pathway, as well as SCFA, representing microbial fermentation metabolites among the groups on day 21 (Figure 3). By comparing the metabolic pathway between the COS group and the other 2 groups, we found more than 30 different enrichment pathways. Among these pathways, the pathways of gluconeogenesis, anaerobic energy metabolism, L-isoleucine degradation, L-histidine biosynthesis, and fatty acid biosynthesis were enriched in the COS group, whereas the pathways of isoprene biosynthesis, mevalonate pathway, fructan biosynthesis, allantoin degradation, and formaldehyde assimilation were enriched in the control and CPS groups. In addition, we determined the contents of SCFA including acetic acid, propionic acid, isobutyric acid, butyric acid, isovaleric acid, and valeric acid in the cecal contents of each group on day 21 (Figure 4). Among the SCFA, only propionic acid was significantly (P < 0.05) increased in the group injected with 0.5 mL 0.85% NS containing 5 mg COS.
Figure 3.
(A) The differentially enriched microbial metabolic pathways between the control and the COS groups. (B) The differentially enriched microbial metabolic pathways between the COS and the CPS groups. Abbreviations: COS, chitooligosaccharide; CPS, chlorella polysaccharide.
Figure 4.
The significantly different intestinal microbial SCFA including acetic acid, propionic acid, isobutyric acid, butyric acid, isovaleric acid, and valeric acid in the cecal digesta of each treatment group on day 21. Abbreviation: SCFA, short-chain fatty acid.
The Potential Effective Mechanism of Early Nutrition Promoting the Homeostasis of the Chicken Intestinal Microbiome
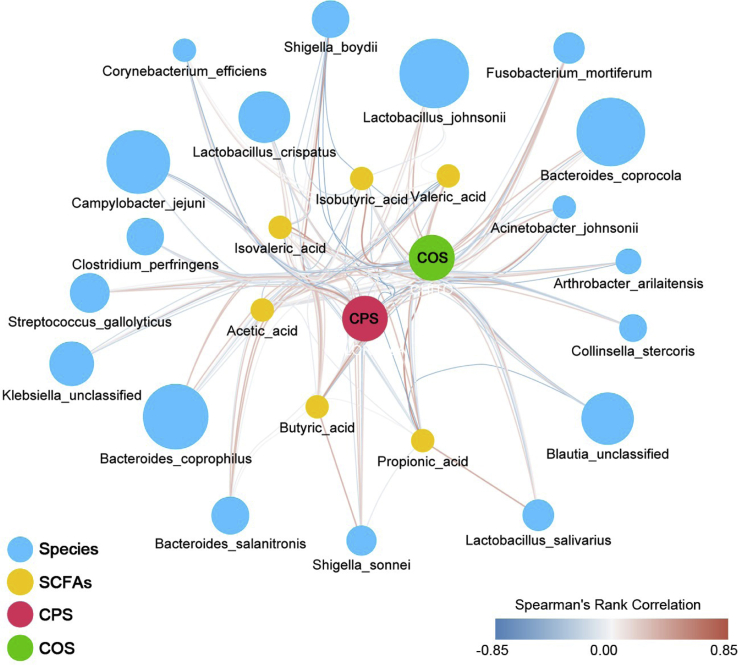
After confirming the benefits of COS in early nutrition for promoting the intestinal microbiome homeostasis, we were interested to explore the question, how does it work? To address this question, we had to illuminate the correlation and the interaction between the COS-injected group and the host symbiotic intestinal microbes by constructing a bundle network based on the determined Spearman's rank correlation coefficients (Figure 5). As shown in Figure 5, on one hand, a generally positive correlation was observed between the COS and the polysaccharide-utilizing species including L. crispatus, L. johnsonii, L. salivarius, B. coprophilus, B. coprocola, and B. salanitronis, which were able to produce more propionic acid. On the other hand, a common negative correlation was observed between the COS and the S. boydii and S. sonnei, represented pathogens, and opportunistic pathogens.
Figure 5.
A network showing the potential effective mechanism of early nutrition promoting the homeostasis of the chicken intestinal microbiome. The edge in red or blue represent the positive or negative correlation between the microbial species and the treatments (Spearman's rank correlation coefficient). All the species represented nodes are colored in blue and all the SCFA represented nodes are colored in orange. The factors of COS and CPS represented nodes are colored in red and green. The node sizes are proportional to the mean abundance in the respective factors. Abbreviations: COS, chitooligosaccharide; CPS, chlorella polysaccharide; SCFA, short-chain fatty acid.
Discussion
In general, the chicken embryos have no chance to get nutrition from outside during the embryonic stages. After being laid, the egg has a definite nutrient composition supporting avian embryo development at all stages (Samadi and Liebert, 2007). Accordingly, in ovo feeding technology is the only method that provides external nutrition to the chicken embryo for their development and directly increase the robustness of the neonate posthatch (Uni et al., 2005). Prebiotics are composed of natural, fermentable oligosaccharides that are not digested by the host (Gibson et al., 2004). The benefits of prebiotics had been manifested by the development of a healthy microbiome but also by the proper functioning of the immune system, metabolism, and physiology of the host (Jha and Berrocoso, 2015; Berrocoso et al., 2017; Slawinska et al., 2019). By applying the in ovo feeding techniques, the potential prebiotics of COS and CPS were injected into the chicken eggs on day 12.5 of incubation to evaluate the impacts of the 2 potential prebiotics on the host's intestinal microbiome and related metabolic pathways and fermentation metabolites. Generally, for the in ovo delivery of prebiotics, an earlier time point (day 12.5 of incubation) has been proven to be more effective (Villaluenga et al., 2004). The injected solution is deposited inside the air cell of the incubating eggs. The prebiotic, owing to its high solubility in the water, is transported into the bloodstream and the developing intestinal tract.
Posthatch, we focused on the development of the intestinal microbiome. To address the question of how chicken intestinal microbiota responds to COS and CPS intervention in early life, we compared the structure of chicken intestinal microbes in different treatment groups on day 3 and day 21 posthatch by shotgun metagenomic sequencing and related metabolic pathways and fermentation metabolites. Interestingly, no significant difference in intestinal microbiota was found on day 3 posthatch. However, on day 21 posthatch, the intestinal microbiotas from the chickens in the groups of control, COS, and CPS were distinct for organismal structure, suggesting the response of prebiotics with the increase in the age. The COS represented prebiotic, owing to its high solubility in the water, is transported into the bloodstream and into the developing intestinal tract. Accordingly, the polysaccharide-utilizing species including L. johnsonii and B. salanitronis were increased in the COS group with age, whereas the relative abundance of some chicken-source pathogens and opportunistic pathogens were lower than that in the control and CPS groups. L. johnsonii is essential in maintaining chicken health by stimulating natural immunity and contributing to the balance of the microbiota (Wang et al., 2017). B. salanitronis helps in the breakdown of food and produce nutrients and energy that the chicken needs, and it was reported to have anti-inflammatory properties and contribute to improving gut health through butyrate production (Lan et al., 2006). Accordingly, increased levels of these species in the gut lead to the production of SCFA, which can also induce the differentiation of Treg by inhibiting the deacetylation of histidine, promoting the repair of tissue damage (Chen et al., 2017), inhibiting the inflammatory response, and having a beneficial effect on the overall health of the host (Huuskonen et al., 2004).
Lactobacillus was able to produce the abundant SCFA by their metabolic pathway of fatty acid biosynthesis. In particular, L. salivarius significantly increased the amount of propionate after the in vitro incubation of cecal cultures of chickens (Meimandipour et al., 2009, 2010). The SCFA are considered as the key bacterial metabolites for immune response and the anti-inflammatory factors in the gut (Kim et al., 2016). Short-chain fatty acids can affect bacterial fitness by acid stress and act as a signal to regulate virulence genes in common enteric pathogens (Sun and O'Riordan, 2013). In the present study, we observed Lactobacillus sp., including L. salivarius and fatty acid biosynthesis, were enriched in the COS group; significant SCFA alterations and lower pathogens and opportunistic pathogens also were explored. It is reported that 1.76 mg of inulin in ovo injection exhibited the lower SCFA level than the control group (Miśta et al., 2017), whereas increased immune-related gene expression was found with the in ovo injection of 1.76 mg of inulin (Plowiec et al., 2015). Collectively, it indicated 5 mg low-dose but not 20 mg high-dose COS delivered in ovo might modulate gene expression related to intestinal immune responses in broiler chickens, and similar conclusions also have been confirmed by 5 mg of raffinose (Berrocoso et al., 2017) and 3.5 mg of GOS (Slawinska et al., 2019).
In conclusion, the present research revealed the impacts of in ovo feeding of chicken embryos with COS and CPS on gut health of broilers. It indicated the feasibility of early nutrition with 5 mg COS to modulate the gut microbiome, related metabolic pathways, and SCFA production, which in turn, affect gut health and overall health and production performance of broiler chickens. In addition, it extends our understanding of prebiotics in early nutrition and the improvements in gut health of poultry.
Acknowledgment
This research was supported by the Key Research and Development Project of Hainan Province (No. ZDYF2019150) and USDA/NIFA-Multistate Fund, managed by the College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa.
Data Availability: The sequence data reported in this article have been deposited in the NCBI database (metagenomic sequencing data: PRJNA628092).
Conflict of Interest Statement: The authors do not have any conflict of interest.
References
- Berrocoso J.D., Kida R., Singh A.K., Kim Y.S., Jha R. Effect of in ovo injection of raffinose on growth performance and gut health parameters of broiler chicken. Poult. Sci. 2017;96:1573–1580. doi: 10.3382/ps/pew430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Kim C.Y., Kaur A., Lamothe L., Shaikh M., Keshavarzian A., Hamaker B.R. Dietary fibre-based SCFA mixtures promote both protection and repair of intestinal epithelial barrier function in a Caco-2 cell model. Food Funct. 2017;8:1166–1173. doi: 10.1039/c6fo01532h. [DOI] [PubMed] [Google Scholar]
- Deng X.Z., Li X.J., Liu P., Yuan S.L., Zang J.J., Li S.Y., Piao X.S. Effect of chito-oligosaccharide supplementation on immunity in broiler chickens. Asian-Australas J. Anim. Sci. 2008;21:1651–1658. [Google Scholar]
- Franzosa E.A., McIver L.J., Rahnavard G., Thompson L., Schirmer M., Weingart G., Schwarzberg K., Knight R., Caporaso J.G., Segata N., Huttenhower C. Species-level functional profiling of metagenomes and metatranscriptomes. Nat. Methods. 2018;15:962–968. doi: 10.1038/s41592-018-0176-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G.R., Probert H.M., Loo J.V., Rastall R.A., Roberfroid M.B. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr. Res. Rev. 2004;17:259–275. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- Guan G., Azad M.A.K., Lin Y., Kim S.W., Tian Y., Liu G., Wang H. Biological effects and applications of chitosan and chito-oligosaccharides. Front. Physiol. 2019;10:516. doi: 10.3389/fphys.2019.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman S., Gato A., Lamela M., Freire-Garabal M., Calleja J.M. Anti-inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother. Res. 2003;17:665–670. doi: 10.1002/ptr.1227. [DOI] [PubMed] [Google Scholar]
- Huuskonen J., Suuronen T., Nuutinen T., Kyrylenko S., Salminen A. Regulation of microglial inflammatory response by sodium butyrate and short-chain fatty acids. Br. J. Pharmacol. 2004;141:874–880. doi: 10.1038/sj.bjp.0705682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha R., Berrocoso J.D. Review: dietary fiber utilization and its effects on physiological functions and gut health of swine. Animal. 2015;9:1441–1452. doi: 10.1017/S1751731115000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha R., Berrocoso J.F.D. Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: a review. Anim. Feed Sci. Technol. 2016;212:18–26. [Google Scholar]
- Jha R., Leterme P. Feed ingredients differing in fermentable fibre and indigestible protein content affect fermentation metabolites and faecal nitrogen excretion in growing pigs. Animal. 2012;6:603–611. doi: 10.1017/S1751731111001844. [DOI] [PubMed] [Google Scholar]
- Jha R., Rossnagel B., Pieper R., Van Kessel A., Leterme P. Barley and oat cultivars with diverse carbohydrate composition alter ileal and total tract nutrient digestibility and fermentation metabolites in weaned piglets. Animal. 2010;4:724–731. doi: 10.1017/S1751731109991510. [DOI] [PubMed] [Google Scholar]
- Jha R., Fouhse J.M., Tiwari U.P., Li L., Willing B.P. Dietary fiber and intestinal health of monogastric animals. Front. Vet. Sci. 2019;6:48. doi: 10.3389/fvets.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha R., Singh A.K., Yadav S., Berrocoso J.F.D., Mishra B. Early nutrition programming (in ovo and post-hatch feeding) as a strategy to modulate gut health of poultry. Front. Vet. Sci. 2019;6:82. doi: 10.3389/fvets.2019.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y., Jha R., Zhang W.L., Kim I.H. Effects of chitooligosaccharide supplementation on egg production, egg quality and blood profiles in laying hens. Indian J. Anim. Res. 2019;53:1199–1204. [Google Scholar]
- Jombart T. Adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics. 2008;24:1403–1405. doi: 10.1093/bioinformatics/btn129. [DOI] [PubMed] [Google Scholar]
- Khambualai O., Yamauchi K., Tangtaweewipat T., Cheva-Isarakul B. Growth performance and intestinal histology in broiler chickens fed with dietary chitosan. Br. Poult. Sci. 2009;50:592–597. doi: 10.1080/00071660903247182. [DOI] [PubMed] [Google Scholar]
- Kim M., Qie Y., Park J., Kim C.H. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe. 2016;20:202–214. doi: 10.1016/j.chom.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan P.T., Sakamoto M., Sakata S., Benno Y. Bacteroides barnesiae sp. nov., Bacteroides salanitronis sp. nov. and Bacteroides gallinarum sp. nov., isolated from chicken caecum. Int. J. Syst. Evol. Microbiol. 2006;56:2853–2859. doi: 10.1099/ijs.0.64517-0. [DOI] [PubMed] [Google Scholar]
- Laokuldilok T., Potivas T., Kanha N., Surawang S., Seesuriyachan P., Wangtueai S., Phimolsiripol Y., Regenstein J.M. Physicochemical, antioxidant, and antimicrobial properties of chitooligosaccharides produced using three different enzyme treatments. Food Biosci. 2017;18:28–33. [Google Scholar]
- Li D., Liu C.M., Luo R., Sadakane K., Lam T.W. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics. 2015;31:1674–1676. doi: 10.1093/bioinformatics/btv033. [DOI] [PubMed] [Google Scholar]
- Meimandipour A., Shuhaimi M., Hair-Bejo M., Azhar K., Kabeir B.M., Rasti B., Yazid A.M. In vitro fermentation of broiler cecal content: the role of lactobacilli and pH value on the composition of microbiota and end products fermentation. Lett. Appl. Microbiol. 2009;49:415–420. doi: 10.1111/j.1472-765X.2009.02674.x. [DOI] [PubMed] [Google Scholar]
- Meimandipour A., Shuhaimi M., Soleimani A.F., Azhar K., Hair-Bejo M., Kabeir B.M., Javanmard A., Muhammad Anas O., Yazid A.M. Selected microbial groups and short-chain fatty acids profile in a simulated chicken cecum supplemented with two strains of Lactobacillus. Poult. Sci. 2010;89:470–476. doi: 10.3382/ps.2009-00495. [DOI] [PubMed] [Google Scholar]
- Miśta D., Króliczewska B., Pecka-Kiełb E., Kapuśniak V., Zawadzki W., Graczyk S., Kowalczyk A., Łukaszewicz E., Bednarczyk M. Effect of in ovo injected prebiotics and synbiotics on the caecal fermentation and intestinal morphology of broiler chickens. Anim. Prod. Sci. 2017;57:1884–1892. [Google Scholar]
- Moran E.T., Jr. Nutrition of the developing embryo and hatchling. Poult. Sci. 2007;86:1043–1049. doi: 10.1093/ps/86.5.1043. [DOI] [PubMed] [Google Scholar]
- Park S.H., Perrotta A., Hanning I., Diaz-Sanchez S., Pendleton S., Alm E., Ricke S.C. Pasture flock chicken cecal microbiome responses to prebiotics and plum fiber feed amendments. Poult. Sci. 2017;96:1820–1830. doi: 10.3382/ps/pew441. [DOI] [PubMed] [Google Scholar]
- Pieper R., Jha R., Rossnagel B., Van Kessel A., Souffrant W.B., Leterme P. Effect of barley and oat cultivars with different carbohydrate compositions on the intestinal bacterial communities in weaned piglets. FEMS Microbiol. Ecol. 2008;66:556–566. doi: 10.1111/j.1574-6941.2008.00605.x. [DOI] [PubMed] [Google Scholar]
- Plowiec A., Slawinska A., Siwek M.Z., Bednarczyk M.F. Effect of in ovo administration of inulin and Lactococcus lactis on immune-related gene expression in broiler chickens. Am. J. Vet. Res. 2015;76:975–982. doi: 10.2460/ajvr.76.11.975. [DOI] [PubMed] [Google Scholar]
- Samadi, Liebert F. Threonine requirement of slow-growing male chickens depends on age and dietary efficiency of threonine utilization. Poult. Sci. 2007;86:1140–1148. doi: 10.1093/ps/86.6.1140. [DOI] [PubMed] [Google Scholar]
- Shi B.L., Li D.F., Piao X.S., Yan S.M. Effects of chitosan on growth performance and energy and protein utilisation in broiler chickens. Br. Poult. Sci. 2005;46:516–519. doi: 10.1080/00071660500190785. [DOI] [PubMed] [Google Scholar]
- Siwek M., Slawinska A., Stadnicka K., Bogucka J., Dunislawska A., Bednarczyk M. Prebiotics and synbiotics - in ovo delivery for improved lifespan condition in chicken. BMC Vet. Res. 2018;14:402. doi: 10.1186/s12917-018-1738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawinska A., Dunislawska A., Plowiec A., Radomska M., Lachmanska J., Siwek M., Tavaniello S., Maiorano G. Modulation of microbial communities and mucosal gene expression in chicken intestines after galactooligosaccharides delivery in ovo. PLoS One. 2019;14:e0212318. doi: 10.1371/journal.pone.0212318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., O'Riordan M.X. Regulation of bacterial pathogenesis by intestinal short-chain fatty acids. Adv. Appl. Microbiol. 2013;85:93–118. doi: 10.1016/B978-0-12-407672-3.00003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uni Z., Ferket P.R., Tako E., Kedar O. In ovo feeding improves energy status of late-term chicken embryos. Poult. Sci. 2005;84:764–770. doi: 10.1093/ps/84.5.764. [DOI] [PubMed] [Google Scholar]
- Villaluenga C.M., Wardenska M., Pilarski R., Bednarczyk M., Gulewicz K. Utilization of the chicken embryo model for assessment of biological activity of different oligosaccharides. Folia. Biol. (Krakow) 2004;52:135–142. doi: 10.3409/1734916044527502. [DOI] [PubMed] [Google Scholar]
- Wan X., Li T., Liu D., Chen Y., Liu Y., Liu B., Zhang H., Zhao C. Effect of marine microalga chlorella pyrenoidosa ethanol extract on lipid metabolism and gut microbiota composition in high-fat diet-fed rats. Mar. Drugs. 2018;16:E498. doi: 10.3390/md16120498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X.Z., Ai C., Chen Y.H., Gao X.X., Zhong R.T., Liu B., Chen X.H., Zhao C. Physicochemical characterization of a polysaccharide from green microalga chlorella pyrenoidosa and its hypolipidemic activity via gut microbiota regulation in rats. J. Agric. Food Chem. 2020;68:1186–1197. doi: 10.1021/acs.jafc.9b06282. [DOI] [PubMed] [Google Scholar]
- Wang H., Ni X., Liu L., Zeng D., Lai J., Qing X., Li G., Pan K., Jing B. Controlling of growth performance, lipid deposits and fatty acid composition of chicken meat through a probiotic, Lactobacillus johnsonii during subclinical Clostridium perfringens infection. Lipids Health Dis. 2017;16:38. doi: 10.1186/s12944-017-0408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Jiang Y., Luo X., Wang C., Wang N., He H., Zhang T., Chen L. Chitooligosaccharides modulate glucose-lipid metabolism by suppressing SMYD3 pathways and regulating gut microflora. Mar. Drugs. 2020;18:69. doi: 10.3390/md18010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wang X., Jiang H., Cai C., Li G., Hao J., Yu G. Marine polysaccharides attenuate metabolic syndrome by fermentation products and altering gut microbiota: an overview. Carbohydr. Polym. 2018;195:601–612. doi: 10.1016/j.carbpol.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Whitehead M.J., McCanney G.A., Willison H.J., Barnett S.C. MyelinJ: an ImageJ macro for high throughput analysis of myelinating cultures. Bioinformatics. 2019;35:4528–4530. doi: 10.1093/bioinformatics/btz403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S., Jha R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019;10:2. doi: 10.1186/s40104-018-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Yang G., Wen Y., Liu S., Li C., Yang R., Li W. Intestinal microbiota are involved in the immunomodulatory activities of longan polysaccharide. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.201700466. [DOI] [PubMed] [Google Scholar]
- Zhou T.X., Chen Y.J., Yoo J.S., Huang Y., Lee J.H., Jang H.D., Shin S.O., Kim H.J., Cho J.H., Kim I.H. Effects of chitooligosaccharide supplementation on performance, blood characteristics, relative organ weight, and meat quality in broiler chickens. Poult. Sci. 2009;88:593–600. doi: 10.3382/ps.2008-00285. [DOI] [PubMed] [Google Scholar]