Abstract
Rearing system is a critical nongenetic factor influencing meat quality of ducks. In this study, a total of 360 birds were randomly allocated into floor rearing system (FRS) and net rearing system (NRS) to compare their effects on intramuscular fat (IMF) deposition, fatty acid composition, and related gene expression in muscles of Nonghua ducks. Sawdust bedding and stainless mesh bed were equipped in FRS and NRS, respectively. At the eighth week (8w) and 13th week (13w), the breast and thigh muscles of ducks were collected to determine the profiles of lipids composition and the expressions of lipid metabolism–related genes. The IMF content was higher in 13w-FRS than 8w-FRS and 8w-NRS in breast muscle, whereas it was higher in 13w-NRS than other groups in thigh muscle (P < 0.05). C16:1, C20:5(n-3) of muscles were higher in 8w-NRS than 8w-FRS, whereas C18:1(n-9)c, C18:2(n-6)c, Ʃ monounsaturated fatty acid (MUFA), and ƩMUFA/Ʃsaturated fatty acid (SFA) ratio of muscles were higher in 13w-NRS than 8w-FRS and 8w-NRS (P < 0.05). C22:6(n-3), C20:4(n-6) of breast muscle and C20:3(n-6) of thigh muscle were higher in 13w-NRS than 13w-FRS (P < 0.05). Fatty acids variation was studied by principal component analysis, exhibiting extensive positive loadings on principal components. SREBP1, ACADL, and FABP3 were downregulated in breast muscle, whereas PPARα and ELOVL5 were upregulated in thigh muscle of NRS ducks at 13w. Principal components were extensively correlated with lipids composition parameters, and principal components of breast muscle 1 and principal components of thigh muscle 1 were correlated with SREBP1 and PPARα, respectively (P < 0.05). In conclusion, with increasing age, FRS enhanced IMF deposition in breast muscle, and the same promotion in thigh muscle was because of NRS. The variation of fatty acids in muscles was uniform, and the change of single fatty acid was unable to distinguish NRS and FRS. However, as NRS downregulated SREBP1, ACADL and FABP3 in breast muscle and upregulated PPARα and ELOVL5 in thigh muscle, NRS could improve nutrient value and meat quality by increasing ƩMUFA, ƩMUFA/ƩSFA ratio, and important PUFA levels. Therefore, NRS was more recommended than FRS for Nonghua ducks during week 8 to 13 posthatching.
Key words: rearing system, intramuscular fat content, fatty acid composition, lipid metabolism–related gene, Nonghua duck
Introduction
With the rapid development of duck husbandry, the traditional free-range rearing model has quickly transformed into dryland intensive or semi-intensive rearing model (Zhao et al., 2019). Net rearing system (NRS) and floor rearing system (FRS) are currently the most common dryland rearing models in China (Zhang et al., 2018), which greatly protect ducks from intestinal infectious disease by minimizing the need for waterbodies (Zhao et al., 2019). The NRS is to place a suitable size of plastic or stainless steel mesh bed on the already built shelf at a height of 50 to 75 cm above the ground. The feces of waterfowls are directly dropped through the mesh holes, which reduces the opportunity of ducks contacting with their excreta and makes it easy to clean up. But compared with FRS, NRS ducks are prone to get leg lesions, and duck activity is kind of constrained. In FRS, the thick bedding made of sawdust, straw, or rice husk is tiled on the concrete floor to stick feces, absorb moisture, and protect duck chest from directly contacting with solid ground. However, because the bedding materials need to be replenished or replaced regularly, it increases the cost of breeding in disguise.
Studies on NRS and FRS showed various effects on ducks, including production performance, meat quality, rearing environment, serum biochemical parameters, and health status, etc. (Kolluri et al., 2014; Almeida et al., 2017, 2018; Zhang et al., 2018; Zhao et al., 2019). Intramuscular fat (IMF) and fatty acids are key factors correlated with the flavor, tenderness, and succulence of duck meat (Liu et al., 2011a; Qiu et al., 2017). Meanwhile, the ratios of different kinds of fatty acids are also important in maintaining human health (Swanson et al., 2012). By comparing deep-litter rearing and free-range rearing, Michalczuk et al., 2016, Michalczuk et al., 2017 concluded the rearing conditions did not significantly affect the fat content of breast muscle in Muscovy and Pekin ducks. But Erdem et al. (2015) reported Pekin ducks reared in the optimal environmental conditions for the first week exhibited higher fat contents in breast and thigh muscles. Further studies mentioned the selection of rearing system also affected fatty acid composition of birds muscle (Castellini et al., 2002; Husak et al., 2008). Owing to studies on functional genomics, fatty acids are acknowledged as the bioregulators and important substrates in many complicated physiological processes regulated by series of genes. Peroxisome proliferator-activated receptors (PPAR) and sterol regulatory-element binding proteins (SREBP) are 2 key regulatory factors that mainly regulated lipids transport and metabolism via PPAR signaling pathway and SREBP signaling pathway (Mandard et al., 2004; Deckelbaum et al., 2006; Tzeng et al., 2015; Xie et al., 2017). Apart from PPAR and SREBP, other genes associated with β-oxidation (ACADL, CPT1A) (Bruce et al., 2009; Turner et al., 2014), fatty acids desaturation (SCD1) (Wang et al., 2013; Pioche et al., 2020), de novo synthesis (ACACA) (Liu et al., 2019), fatty acids transport (FATP1) (Mandard et al., 2004; Li et al., 2018), intracellular trafficking (FABP3) (Storch and Thumser, 2000; Abasht et al., 2019), and elongation (ELOVL5) (Gregory and James, 2014; Zhang et al., 2016) are also worthy investigating to demonstrate how IMF and fatty acids are affected by NRS and FRS. According to the study on NRS and FRS, Zhang et al. (2018) reported IMF content in breast muscle of Chaohu ducks was higher in NRS than FRS, but this study lacks a systematical exploration of fatty acids and genes related to lipid metabolism. Previous study on the effect of FRS and NRS on ducks IMF and fatty acids was not clearly elucidated yet, requiring further study to demonstrate the mechanisms of regulating fatty acids in duck muscle.
Therefore, the objective of current study was to explore the effects of FRS and NRS on IMF content, fatty acid composition, and candidate gene expression in breast and thigh muscles of Nonghua ducks to confirm the effects of FRS and NRS on lipids and conduct a preliminary study on its mechanism of influence.
Materials and methods
Birds and Sample Collection
All animal-related works were performed in compliance with the requirements of the Institutional Animal Care and Use Committee of Sichuan Agricultural University (No. DKY-B20141401). A total of 360 healthy 14-day-old male Nonghua ducks were selected by initial body weight and tagged with unique wing numbers and foot numbers, then they were randomly allocated into 2 groups (with 10 replicates of 18 ducks each), the first group of ducks was reared in FRS with 5 cm thick sawdust bedding covering the concrete floor, the beddings were replenished and cleaned weekly during the experiment period. Another group of ducks was reared in NRS, where the stainless mesh bed with 1.0 cm diameter mesh holes was set at a height of 50 cm above the ground. The feces of ducks were also cleaned weekly. All ducks were reared in the same well-ventilated breeding house with the same environmental conditions at the experimental waterfowl breeding farm of Sichuan Agricultural University (Sichuan, China). The temperature was set at 25°C at the beginning, then it was gradually decreased to 15°C to 20°C. The light was provided naturally, and the stocking density was maintained at a consistent 5 ducks per m2 by changing the fence perimeter after each sampling. All ducks were fed with the same basal diets (Table 1) formulated according to NY/T 2122-2012. The feed and water were provided ad libitum during the experimental period. After fasting for 12 h, 15 ducks from each group were randomly selected and euthanized at the eighth (8w) and 13th week (13w) after birth. After exsanguination, breast muscle (pectoralis major) and thigh muscle (peroneus longus) from the left side were rapidly collected and stored in freezer at −80°C for further analysis.
Table 1.
Ingredients and nutrients composition of basal diets (%, as fed).
| Items | 3–13 W |
|---|---|
| Ingredients | |
| Corn | 48.30 |
| Soybean meal | 23.10 |
| Wheat middling | 10.00 |
| Rice bran | 9.00 |
| Wheat bran | 6.00 |
| Calcium phosphate | 1.40 |
| Limestone powder | 0.90 |
| Vitamin and mineral premix 1 | 1.00 |
| NaCl | 0.30 |
| Total | 100 |
| Nutrients | |
| Metabolizable Energy, Mcal/kg | 2.80 |
| Crude Protein | 17.00 |
| Crude fat | 4.00 |
| Crude fiber | 3.88 |
| Crude ash | 6.05 |
| Calcium | 0.80 |
| Total Phosphorus | 0.70 |
| Available phosphorus | 0.38 |
| Lysine | 0.80 |
| Methionine | 0.38 |
| Methionine + Cystine | 0.67 |
| Threonine | 0.63 |
| Tryptophan | 0.23 |
Vitamin and mineral premix provided the following per kg of diet: Vitamin A (retinyl acetate), 10,000 IU; vitamin D3 (cholecalciferol), 2,250 IU; vitamin E (DL-α-tocopheryl acetate), 20 IU; vitamin K3 (menadione sodium bisulfate), 2.5 mg; vitamin B1 (thiamine mononitrate), 2.5 mg; vitamin B2, 8 mg; vitamin B6 (pyridoxine hydrochloride), 3.4 mg; vitamin B12 (cobalamin), 0.024 mg; choline chloride, 1,000 mg; calcium-d-pantothenate, 25.5 mg; nicotinic acid, 50 mg; folic acid, 1 mg; biotin, 0.25 mg; Cu (CuSO4·5H2O), 10.4 mg; Fe (FeSO4·7H2O), 60 mg; Mn (MnSO4·H2O), 70 mg; Zn (ZnO), 35 mg; Se (NaSeO3), 0.30 mg; I (KI), 0.35 mg.
Determination of IMF Content and Triglyceride
The IMF content was determined by using Soxhlet extraction. 5.0 g of lyophilized muscle samples were pulverized and then mixed with suitable amount of sea sand during the evaporation. After the evaporation, the mixture was transferred into a filtration paper cylinder with a degreasing cotton covering the top, then placed it into a drying oven at 103°C for 2 h, after that the dried filtration paper cylinder was placed into the Soxhlet extractor for 12 h extraction with petroleum ether. The remained petroleum ether was evaporated, and the extract was dried to constant weight to weigh precisely when it was cooled to room temperature. The triglyceride (TG) profile was measured by using a commercial assay kit (Qingdao Sci-tech Innovation Co., Ltd., China) according to the manufacturer's instructions.
Determination of Fatty Acid Profiles
The determination of fatty acids was referred to GB 5009.168-2016 (2016). A total of 200 mg lyophilized sample was used for dilute acid hydrolysis. Briefly, a mixture of 100 mg pyrogallic acid, 2 mL of 95% ethanol, and 10 mL of 8.3 mol/L HCl was added into a flask containing the muscle sample. Then the flask was placed into a water bath for incubation at 70°C to 80°C for 40 min. The total lipids of hydrolyzed samples were mixed with 10 mL of 95% ethanol and then extracted by adding a mixture of 50 mL of diethyl ether and petroleum ether (1:1 vol/vol) into the separating funnel. After shaking for 5 min and standing for 10 min, the ether layer extract was collected. Repeating the above steps for 3 times, the extract was placed into a drying oven at 103°C for 2 h to constant weight. Saponification and esterification were performed by adding 2 mL of 2% sodium hydroxide-methanol solution into a centrifugal tube with extracted lipids, and after a water bath incubation (85°C for 30 min), 3 mL of 14% boron trifluoride-methanol solution was added into the tube for water bath incubation (85°C for 30 min) again. 1 mL of n-Hexane was then added for 2 min extraction with shaking, and after standing for 1 h, 100 μL of supernatant was then collected and made up to 1 mL with n-Hexane. Filtering through a 0.45 μL membrane, the solution was ready for gas chromatography analysis.
The separation of fatty acid methyl esters (FAME) was performed on an Agilent 7890A gas chromatograph (Agilent technologies, Santa Clara, CA) equipped with a flame ionization detector and a CD-2560 capillary column (100 m × 0.25 mm × 0.25 μm) (CNW). The column oven parameters were set as follows: the initial temperature was held at 130°C for 5 min, then it was programmed to increase at 4°C/min to 240°C and held for 30 min. The carrier gas used was nitrogen at a flow rate of 0.5 mL/min. The injector and detector temperature was kept at 250°C, and the injection volume was 1 μL at a split ratio of 10:1. Identification of FAMEs was performed by comparing the retention times with authentic standards (FAME mix 35 components). The results were expressed as mg/kg of FAMEs identified. The amount and ratios of saturated fatty acid (SFA), monounsaturated fatty acid (MUFA), polyunsaturated fatty acids (PUFA), n-3 fatty acids (n-3), and n-6 fatty acids (n-6) were also calculated.
Total RNA Extraction and cDNA Synthesis
Three samples from each group were used for gene expression analysis. Total RNA was isolated from breast and thigh muscles by using Trizol reagent (Takara, Dalian, China). The integrity and concentration of extracted total RNA were measured by electrophoresis and NanoDrop 2000 within the A260:A280 ratio range of 1.8∼2.1. Reverse transcription from 1 μg RNA was conducted by using a cDNA synthesis kit (Takara) and then stored at −20°C before RT-PCR analysis.
Quantitative Real-time PCR Analysis
Primers shown in Table 2 were designed by Primer Premier 5.0 according to GenBank sequences. All primers were obtained from Beijing Tsingke Biotechnology Co., Ltd. (Beijing, China). Quantitative real-time PCR were performed with SYBR Premix ExTaq II (Takara) using the CFX96 Real-Time PCR detection system (Bio-Rad, Hercules, CA) according to manufacturer's instructions. The reaction volume of a 12.5 μL mixture contained 6.25 μL of the SYBR Premix ExTaq II, 0.5 μL of each forward and reverse primer, 4.25 μL of ddH2O, and 1 μL of cDNA template. PCR conditions were set as follows: 3 min predenaturation at 95°C, 40 cycles of 10 s at 95°C, 30 s annealing at primer-specific temperature, and 30 s extension at 72°C, a melting curve was followed to verify primers specificity. Each sample was measured in triplicate, and the average values were obtained. Relative mRNA expressions were calculated by using the 2−ΔΔCT method against the reference gene (β-ACTIN).
Table 2.
Primers used for quantitative real-time PCR.
| Gene | Primer sequences (5′-3′) | GenBank accession | Amplicon (bp) | Annealing temperature (°C) |
|---|---|---|---|---|
| β-actin | Forward: GCTATGTCGCCCTGGATTTC | EF667345 | 98 | 60 |
| Reverse: CACAGGACTCCATACCCAAGAA | ||||
| FATP1 | Forward: CGGCGAGTTCTACGGAGC | XM_005018222 | 286 | 58.3 |
| Reverse: GTAAGCAATCTTCTTGTGGGTG | ||||
| FABP3 | Forward: GGGCTGACCAAACCCACCACC | XM_027443958 | 223 | 68.1 |
| Reverse: GCTCCCGCACTAGCGATGTCTC | ||||
| SREBP1 | Forward: CGAGTACATCCGCTTCCTGC | AY613441 | 92 | 61.2 |
| Reverse: TGAGGGACTTGCTCTTCTGC | ||||
| SCD1 | Forward: GCTTCTTCATTCCAGCCATCC | KF185111 | 328 | 63.6 |
| Reverse: CCATCTCCAGTCCGCATTTTCC | ||||
| ACACA | Forward: TCTTCCCAACCCGCTAAACC | EF990143 | 337 | 59.9 |
| Reverse: TATTCCCTCCGAAACGAGTAAC | ||||
| CPT1A | Forward: CAGATGTTATGACAGGTGGTTTG | XM_027457811 | 138 | 58.4 |
| Reverse: TCAGTTGCCATTACATTCTCCC | ||||
| ELOVL5 | Forward: TTTGGCTTGGACCCAGAGAC | NM_001310419 | 192 | 59.6 |
| Reverse: ACAGGGAAAGCAGCGTGAGT | ||||
| PPARα | Forward: TGCTTGTGAAGGTTGTAAGGGTT | NM_001310383 | 120 | 61.3 |
| Reverse: CGACAGTATTGGCACTTATTACGATTT | ||||
| ACADL | Forward: GGATTCTTAACGGGAGTAAGGTATT | XM_027461394 | 431 | 60.7 |
| Reverse: TTTCATTTCTGCCAACTTGTGCT |
Statistical Analysis
Experimental data were analyzed by using the GLM procedure of SPSS 22.0 program. The statistical models included the main effects of rearing system (FRS or NRS), age (8w or 13w), and their interactions. Significant results were subjected to post hoc analysis by using Duncan's multiple-range tests. Principal component analysis (PCA) was carried out to identify the main factors that contributed to fatty acid composition, and the Pearson's correlation analysis was carried out to assess the relationships of principal components (PC) with lipids composition and genes expression. Mean values with SEM were presented, and values of P<0.05 were considered statistically significant.
Results
Lipid Contents of Muscles Varied Between FRS and NRS
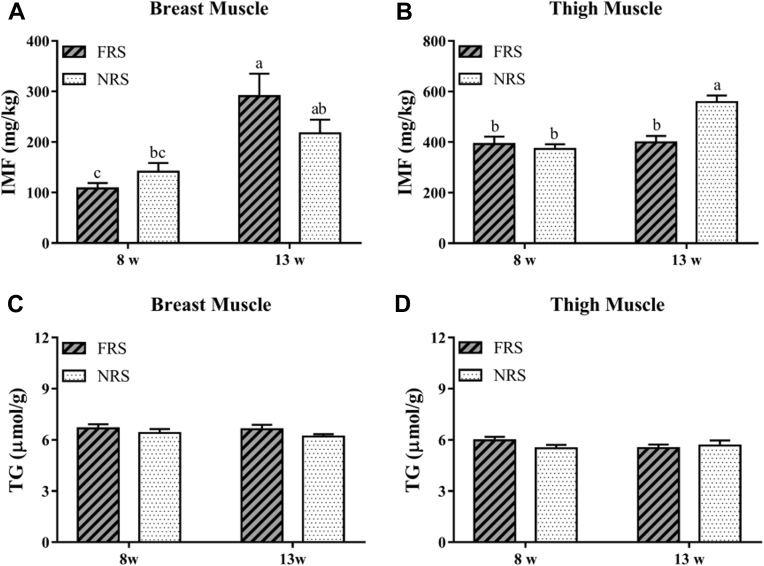
The results of IMF content and TG profile were depicted in Figure 1. In breast muscle, NRS ducks had a tendency of higher IMF content at 8w but lower in 13w than FRS ducks (P > 0.05). With the age increasing, all groups exhibited an increase in IMF content, which 13w-FRS ducks were significantly higher than 8w-FRS or 8w-NRS ducks (P < 0.05). However, in thigh muscle, this increase with age could not be found in FRS ducks but remained detectable in NRS ducks, whose IMF content was significantly higher than any other groups (P < 0.05). Though TG is an important component of IMF, the current study showed no significance in TG profile (P > 0.05).
Figure 1.
The content of IMF (A-B) and TG (C-D) in breast and thigh muscles of ducks under FRS and NRS. a-c indicated a significance (P < 0.05) among 8w-FRS, 8w-NRS, 13w-FRS, and 13w-NRS. Abbreviations: IMF, intramuscular fat; FRS, floor rearing system; NRS, net rearing system; TG, triglyceride; 8w, eight week; 13w, 13th week.
Fatty Acid Composition of NRS Ducks Differed From FRS Ducks
Fatty acid composition of breast muscle and thigh muscle were summarized in Table 3 and Table 4, respectively. In breast muscle, the significance between NRS and FRS ducks exhibited a same trend of higher fatty acids profiles in NRS ducks. More precisely, C22:0, C16:1, C18:1(n-9)t, C20:5(n-3), and the ratio of ƩMUFA/ƩSFA of NRS ducks were significantly higher than FRS ducks at 8w (P<0.05), whereas C20:4(n-6), C22:6(n-3), Ʃn-3, Ʃn-6, ƩPUFA, and the ratio of ƩMUFA/ƩSFA were significantly higher than FRS ducks at 13w (P<0.05). In thigh muscle, apart from the declined C17:1 (P<0.05), SFA (i.e., C14:0, C16:0, C21:0, C22:0), MUFA (i.e., C14:1, C16:1, C18:1(n-9)c), and PUFA (i.e., C20:5(n-3)) were found having the similar trend of significantly higher in NRS ducks than FRS ducks at 8w (P<0.05), whereas a general increase in ƩSFA, ƩMUFA, and the ratio of ƩMUFA/ƩSFA was also observed in NRS ducks (P<0.05). Compared with FRS ducks at 13w, NRS ducks were significantly higher in C18:1(n-9)c, ƩMUFA, C20:3(n-6), and the ratio of ƩMUFA/ƩSFA (P<0.05).
Table 3.
The effects of FRS and NRS on fatty acid composition of duck breast muscle.
| Items1 | Breast muscle |
|||
|---|---|---|---|---|
| 8w |
13w |
|||
| FRS | NRS | FRS | NRS | |
| C12:0 | – | – | – | – |
| C13:0 | 0.165 ± 0.026 | 0.115 ± 0.026 | 0.181 ± 0.024 | 0.224 ± 0.028 |
| C14:0 | 0.670 ± 0.069c | 0.839 ± 0.075b,c | 1.066 ± 0.144a,b | 1.238 ± 0.121a |
| C15:0 | 0.100 ± 0.007 | 0.110 ± 0.010 | 0.146 ± 0.017 | 0.146 ± 0.013 |
| C16:0 | 32.990 ± 2.064c | 39.615 ± 2.994b,c | 46.758 ± 5.080a,b | 52.392 ± 3.997a |
| C17:0 | 0.259 ± 0.015b | 0.265 ± 0.015b | 0.346 ± 0.038a | 0.394 ± 0.029a |
| C18:0 | 18.882 ± 0.681 | 20.443 ± 0.970 | 21.001 ± 1.619 | 22.885 ± 1.345 |
| C20:0 | 0.270 ± 0.015 | 0.289 ± 0.016 | 0.256 ± 0.024 | 0.268 ± 0.020 |
| C21:0 | – | – | – | 0.050 ± 0.007 |
| C22:0 | 0.284 ± 0.048 | 0.542 ± 0.0722 | 0.37 ± 0.049 | 0.279 ± 0.039 |
| C24:0 | - | - | 0.282 ± 0.031 | 0.239 ± 0.039 |
| ƩSFA | 53.633 ± 2.733c | 62.241 ± 4.036b,c | 70.375 ± 6.951a,b | 78.017 ± 5.486a |
| C14:1 | 0.078 ± 0.012 | 0.078 ± 0.009 | 0.098 ± 0.014 | 0.117 ± 0.016 |
| C16:1 | 2.409 ± 0.266c | 3.903 ± 0.460b,2 | 4.980 ± 0.555a,b | 5.591 ± 0.517a |
| C17:1 | 0.735 ± 0.058 | 0.809 ± 0.033 | 0.978 ± 0.054 | 1.089 ± 0.084 |
| C18:1(n-9)t | 0.500 ± 0.042 | 0.667 ± 0.0552 | 0.620 ± 0.063 | 0.722 ± 0.060 |
| C18:1(n-9)c | 43.217 ± 3.896c | 58.517 ± 6.407b,c | 64.657 ± 7.981a,b | 81.327 ± 7.215a |
| C20:1 | 0.501 ± 0.046 | 0.587 ± 0.047 | 0.543 ± 0.073 | 0.636 ± 0.070 |
| C22:1(n-9) | 0.123 ± 0.008 | 0.133 ± 0.009 | 0.116 ± 0.016 | 0.120 ± 0.010 |
| ƩMUFA | 47.517 ± 4.249c | 64.685 ± 6.980b,c | 71.991 ± 8.704a,b | 89.601 ± 7.881a |
| C18:2(n-6)t | – | – | 0.083 ± 0.007 | 0.093 ± 0.011 |
| C18:2(n-6)c | 33.501 ± 2.180b | 37.131 ± 2.842b | 50.402 ± 5.993a | 56.830 ± 4.054a |
| C18:3(n-6) | 0.201 ± 0.029b | 0.215 ± 0.026b | 0.246 ± 0.033a,b | 0.301 ± 0.027a |
| C20:2(n-6) | 0.924 ± 0.048a | 0.891 ± 0.030a | 0.677 ± 0.054b | 0.699 ± 0.065b |
| C20:3(n-6) | 1.478 ± 0.100a | 1.509 ± 0.089a | 1.084 ± 0.072b | 1.083 ± 0.083b |
| C20:4(n-6) | 39.859 ± 1.999b | 39.107 ± 3.779b | 41.216 ± 3.863b | 52.283 ± 2.618a,2 |
| Ʃn-6 | 75.981 ± 3.342b | 78.853 ± 5.871b | 93.666 ± 8.645b | 111.235 ± 6.041a,2 |
| C18:3(n-3) | 1.101 ± 0.107c | 1.345 ± 0.128b,c | 1.802 ± 0.267a,b | 2.055 ± 0.152a |
| C20:3(n-3) | 0.126 ± 0.015 | 0.107 ± 0.013 | 0.108 ± 0.014 | 0.125 ± 0.012 |
| C20:5(n-3) | 0.290 ± 0.025b | 0.388 ± 0.032a,2 | - | 0.053 ± 0.012c |
| C22:6(n-3) | 2.273 ± 0.163b | 2.509 ± 0.247b | 2.502 ± 0.136b | 3.300 ± 0.258a,2 |
| Ʃn-3 | 3.781 ± 0.219b | 4.348 ± 0.319b | 4.431 ± 0.342b | 5.493 ± 0.304a,2 |
| ƩPUFA | 79.763 ± 3.512b | 83.201 ± 6.157b | 98.097 ± 8.962b | 116.727 ± 6.206a,2 |
| ƩMUFA/ƩSFA | 0.866 ± 0.035c | 1.010 ± 0.042b,2 | 1.002 ± 0.026b | 1.134 ± 0.035a,2 |
| ƩPUFA/ƩSFA | 1.513 ± 0.061 | 1.353 ± 0.086 | 1.443 ± 0.084 | 1.535 ± 0.057 |
| Ʃn-6/Ʃn-3 | 20.433 ± 0.688 | 18.289 ± 0.580 | 21.028 ± 0.888 | 20.623 ± 0.972 |
a-cIndicated a significance (P <0.05) of Duncan's multiple-range tests among 8w-FRS, 8w-NRS, 13w-FRS, and 13w-NRS in breast muscle.
Abbreviations: FRS, floor rearing system; MUFA, monounsaturated fatty acid; NRS, net rearing system; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acid; 8w, eight week; 13w, 13th week.
The items displayed in the table were selected from 35 kinds of fatty acids with detectable rates higher than 30%, otherwise were not shown in the table or displayed in “–”.
Indicated a significance between FRS ducks and NRS ducks at the certain age of 8w or 13w.
Table 4.
The effects of FRS and NRS on fatty acid composition of duck thigh muscle.
| Items1 | Thigh muscle |
|||
|---|---|---|---|---|
| 8w |
13w |
|||
| FRS | NRS | FRS | NRS | |
| C12:0 | 0.167 ± 0.019c | 0.222 ± 0.021b,c | 0.328 ± 0.039a,b | 0.294 ± 0.017a |
| C13:0 | – | – | – | – |
| C14:0 | 1.742 ± 0.162c | 2.565 ± 0.236b | 2.888 ± 0.433a,b | 3.432 ± 0.186a |
| C15:0 | 0.18 ± 0.017b | 0.245 ± 0.022b | 0.325 ± 0.040a | 0.357 ± 0.019a |
| C16:0 | 68.522 ± 6.193c | 100.037 ± 9.851b | 112.151 ± 12.861a,b | 131.487 ± 7.607a |
| C17:0 | 0.463 ± 0.036c | 0.597 ± 0.056b,c | 0.731 ± 0.082a,b | 0.895 ± 0.050a |
| C18:0 | 27.475 ± 2.008 | 21.903 ± 2.730 | 18.743 ± 2.423 | 17.541 ± 2.556 |
| C20:0 | 0.463 ± 0.033 | 0.581 ± 0.053 | 0.706 ± 0.079 | 0.749 ± 0.051 |
| C21:0 | 0.069 ± 0.006 | 0.102 ± 0.0112 | 0.109 ± 0.015 | 0.104 ± 0.008 |
| C22:0 | 0.893 ± 0.080 | 1.219 ± 0.1122 | 0.729 ± 0.11 | 0.82 ± 0.113 |
| C24:0 | 0.074 ± 0.019 | 0.111 ± 0.025 | 0.138 ± 0.028 | 0.161 ± 0.046 |
| ƩSFA | 100.033 ± 6.875 | 127.569 ± 9.5462 | 136.834 ± 13.467 | 155.828 ± 7.199 |
| C14:1 | 0.181 ± 0.021 | 0.305 ± 0.0252 | 0.347 ± 0.045 | 0.334 ± 0.021 |
| C16:1 | 8.001 ± 0.866 | 14.125 ± 1.5082 | 15.111 ± 1.771 | 16.493 ± 0.922 |
| C17:1 | 0.729 ± 0.0412 | 0.554 ± 0.033 | 0.561 ± 0.052 | 0.515 ± 0.033 |
| C18:1(n-9)t | 0.976 ± 0.080 | 0.807 ± 0.151 | 1.018 ± 0.123 | 1.064 ± 0.223 |
| C18:1(n-9)c | 138.323 ± 14.658c | 213.313 ± 23.501b,2 | 217.065 ± 24.660b | 301.926 ± 20.378a,2 |
| C20:1 | 1.485 ± 0.141 | 2.026 ± 0.205 | 2.442 ± 0.277 | 2.891 ± 0.185 |
| C22:1(n-9) | 0.224 ± 0.018 | 0.279 ± 0.029 | 0.375 ± 0.040 | 0.375 ± 0.027 |
| ƩMUFA | 149.919 ± 15.699c | 231.247 ± 25.143b,2 | 236.783 ± 26.605b | 323.031 ± 21.332a,2 |
| C18:2(n-6)t | 0.082 ± 0.003 | 0.088 ± 0.008 | 0.085 ± 0.008 | 0.091 ± 0.004 |
| C18:2(n-6)c | 86.124 ± 7.299c | 113.434 ± 10.106b,c | 141.69 ± 16.164a,b | 166.306 ± 9.849a |
| C18:3(n-6) | 0.531 ± 0.057 | 0.699 ± 0.083 | 0.728 ± 0.097 | 0.917 ± 0.053 |
| C20:2(n-6) | 1.039 ± 0.052b | 1.02 ± 0.068b | 1.27 ± 0.116a,b | 1.377 ± 0.088a |
| C20:3(n-6) | 1.405 ± 0.083 | 1.587 ± 0.116 | 1.427 ± 0.086 | 1.815 ± 0.1192 |
| C20:4(n-6) | 43.979 ± 1.207 | 41.283 ± 1.507 | 42.693 ± 0.933 | 43.136 ± 1.591 |
| Ʃn-6 | 133.111 ± 6.956c | 158.095 ± 11.236b,c | 187.871 ± 16.348a,b | 213.635 ± 11.073a |
| C18:3(n-3) | 3.401 ± 0.325 | 4.834 ± 0.456 | 4.864 ± 0.868 | 5.644 ± 0.478 |
| C20:3(n-3) | 0.074 ± 0.009 | 0.092 ± 0.013 | 0.081 ± 0.008 | 0.084 ± 0.006 |
| C20:5(n-3) | 0.183 ± 0.015 | 0.265 ± 0.0282 | 0.237 ± 0.014 | 0.219 ± 0.019 |
| C22:6(n-3) | 4.014 ± 0.184 | 3.955 ± 0.251 | 3.728 ± 0.241 | 3.99 ± 0.324 |
| Ʃn-3 | 7.666 ± 0.366 | 9.115 ± 0.562 | 8.878 ± 0.866 | 9.881 ± 0.663 |
| ƩPUFA | 140.777 ± 7.284c | 167.209 ± 11.744b,c | 196.749 ± 17.138a,b | 223.517 ± 11.633a |
| ƩMUFA/ƩSFA | 1.452 ± 0.066c | 1.756 ± 0.080b,2 | 1.71 ± 0.055b | 2.057 ± 0.071a,2 |
| ƩPUFA/ƩSFA | 1.437 ± 0.039 | 1.329 ± 0.040 | 1.47 ± 0.046 | 1.435 ± 0.034 |
| Ʃn-6/Ʃn-3 | 17.366 ± 0.435b | 17.323 ± 0.57b | 22.433 ± 1.623a | 22.671 ± 1.57a |
a-cIndicated a significance (P <0.05) of Duncan's multiple-range tests among 8w-FRS, 8w-NRS, 13w-FRS, and 13w-NRS in thigh muscle.
Abbreviations: FRS, floor rearing system; MUFA, monounsaturated fatty acid; NRS, net rearing system; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acid; 8w, eight week; 13w, 13th week.
The items displayed in the table were selected from 35 kinds of fatty acids with detectable rates higher than 30%, otherwise were not shown in the table or displayed in “–”.
Indicated a significance between FRS ducks and NRS ducks at the certain age of 8w or 13w.
Taking ages into consideration, the Duncan's multiple-range tests indicated that C14:0, C16:0, C17:0, C18:1(n-9)c, C18:2(n-6)c, ƩMUFA, Ʃn-6, ƩPUFA, and the ratio of ƩMUFA/ƩSFA of breast and thigh muscles were significantly higher in 13w-NRS ducks than 8w-FRS or 8w-NRS ducks (P<0.05). Other parameters in breast muscle, including C16:1, C18:3(n-6), C20:4(n-6), C18:3(n-3), C22:6(n-3), ƩSFA, and Ʃn-3 were also significantly higher in 13w-NRS ducks than 8w-FRS or 8w-NRS ducks (P<0.05), whereas opposite to the trend of C20:2(n-6), C20:3(n-6), and C20:5(n-3) (P<0.05). As for thigh muscle, C12:0, C15:0, C20:2(n-6), and the ratio of Ʃn-6/Ʃn-3 were significantly higher at 13w-NRS ducks than 8w-FRS or 8w-NRS ducks (P < 0.05).
PCA Analysis of Fatty Acids in Breast and Thigh Muscles
To decrease the number of variables without much loss of the data set, PCA was conducted to analyze the variance of fatty acids compositions of breast and thigh muscles. Based on the correlation matrix, the extreme significance (Sig.<0.001) of Bartlett test of sphericity and the figures of Kaiser-Meyer-Olkin measure of sampling adequacy >0.8, 3 orthogonal PC (principal components of breast muscle [BPC]1, BPC2, and BPC3) accounting for 83.11% of variance in breast muscle, and 4 PC (principal components of thigh muscle [TPC]1, TPC2, TPC3, and TPC4) accounting for 81.93% of variance in thigh muscle were generated by PCA with Kaiser's rule of eigenvalues >1. As shown in Table 5 and Table 6, BPC1 had the highest eigenvalue of 7.634 and described 34.70% of the variance, the positive loadings of C14:0 (0.768), C16:0 (0.744), C22:0 (0.802), C14:1 (0.846), C16:1 (0.858), and C18:1n9c (0.777) indicated their high contributions to BPC1. BPC2 also had a high eigenvalue of 7.481, accounting for 34.01% of the variance with high positive loadings of C15:0 (0.742), C17:0 (0.809), C18:0 (0.736), C20:0 (0.794), C22:1(n-9) (0.703), and C20:2(n-6) (0.887). BPC1 and BPC2 were mainly contributed by SFA and MUFA, whereas apart from C17:1 (0.600), BPC3 was most described by PUFA including C20:3(n-6) (0.669), C20:4(n-6) (0.894), C22:6(n-3) (0.706), and C20:3(n-3) (-0.582). In thigh muscle, TPC1 had the highest eigenvalue of 13.542 and described 61.55% of the total variance. Most of fatty acids in thigh muscle were highly contributed to TPC1 with positive loadings. TPC2 accounting for 8.14% of the variance was mainly contributed by C20:4(n-6) (0.751) and C22:6(n-3) (0.722). TPC3 had a positive loading of C18:1(n-9)t (0.770) and a negative loading of C17:1 (−0.770), indicating these 2 variables were negatively correlated in TPC3. TPC4 with the lowest contribution of 5.48% was most described by C20:3(n-3) (0.825).
Table 5.
Eigenvalues of the correlation matrix.
| Items1 | Eigenvalue | Variance contribution(%) | Cumulative contribution(%) |
|---|---|---|---|
| Breast Muscle | |||
| BPC1 | 7.634 | 34.70 | 34.70 |
| BPC2 | 7.481 | 34.01 | 68.71 |
| BPC3 | 3.169 | 14.41 | 83.11 |
| Thigh Muscle | |||
| TPC1 | 13.542 | 61.55 | 61.55 |
| TPC2 | 1.790 | 8.14 | 69.69 |
| TPC3 | 1.488 | 6.76 | 76.45 |
| TPC4 | 1.205 | 5.48 | 81.93 |
BPC, principal components of breast muscle; TPC, principal components of thigh muscle.
Table 6.
Statistical loadings of variables from PCA of breast and thigh muscles.
| Items1 | Breast muscle2 |
Thigh muscle2 |
|||||
|---|---|---|---|---|---|---|---|
| BPC1 | BPC2 | BPC3 | TPC1 | TPC2 | TPC3 | TPC4 | |
| C14:0 | 0.768 | 0.573 | 0.143 | 0.928 | 0.105 | 0.185 | 0.138 |
| C15:0 | 0.499 | 0.742 | −0.332 | 0.970 | 0.098 | 0.087 | 0.131 |
| C16:0 | 0.744 | 0.630 | 0.148 | 0.974 | 0.085 | 0.111 | 0.024 |
| C17:0 | 0.489 | 0.809 | 0.144 | 0.944 | 0.176 | 0.066 | 0.160 |
| C18:0 | 0.513 | 0.736 | 0.191 | −0.210 | −0.519 | −0.166 | 0.152 |
| C20:0 | 0.507 | 0.794 | 0.104 | 0.964 | 0.058 | 0.025 | 0.025 |
| C22:0 | 0.802 | 0.135 | −0.112 | 0.804 | −0.070 | 0.087 | −0.020 |
| C14:1 | 0.846 | 0.276 | 0.107 | 0.843 | −0.116 | 0.101 | 0.109 |
| C16:1 | 0.858 | 0.435 | 0.090 | 0.935 | 0.011 | 0.098 | −0.020 |
| C17:1 | 0.247 | 0.304 | 0.600 | −0.092 | 0.102 | −0.770 | 0.034 |
| C18:1(n-9)t | 0.703 | 0.663 | −0.116 | 0.138 | 0.023 | 0.770 | 0.087 |
| C18:1(n-9)c | 0.777 | 0.571 | 0.119 | 0.965 | 0.097 | 0.109 | −0.052 |
| C20:1 | 0.672 | 0.678 | 0.128 | 0.973 | 0.049 | 0.037 | 0.015 |
| C22:1(n-9) | 0.504 | 0.703 | 0.018 | 0.919 | 0.032 | 0.039 | 0.031 |
| C18:2(n-6)c | 0.638 | 0.626 | 0.399 | 0.971 | 0.126 | 0.104 | 0.086 |
| C18:3(n-6) | 0.655 | 0.587 | 0.324 | 0.906 | 0.206 | 0.064 | 0.192 |
| C20:2(n-6) | 0.234 | 0.887 | −0.008 | 0.786 | 0.266 | −0.015 | 0.337 |
| C20:3(n-6) | 0.156 | 0.525 | 0.669 | 0.821 | 0.398 | 0.109 | 0.181 |
| C20:4(n-6) | 0.036 | 0.091 | 0.894 | 0.032 | 0.751 | −0.301 | 0.441 |
| C18:3(n-3) | 0.702 | 0.524 | 0.404 | 0.921 | 0.125 | 0.203 | 0.069 |
| C20:3(n-3) | 0.176 | 0.095 | −0.582 | 0.195 | −0.165 | 0.077 | 0.825 |
| C22:6(n-3) | 0.290 | −0.439 | 0.706 | 0.013 | 0.722 | −0.094 | −0.155 |
Abbreviation: PCA, principal component analysis.
The loadings displayed in boldface were variables contributed greatly to the principal components.
BPC, principal components of breast muscle; TPC, principal components of thigh muscle.
The coefficients used for calculating the scores of each PC were shown in Table 7. The scores were calculated by using Eqs: Fj = aj1X1 +aj2X2 +aj3X3 + … +ajpXp, j = 1, 2, 3, …, k; where Fj: the scores of PC; aj1 ∼ ajp: the factor score coefficients of PC on variables; X1 ∼ Xp: the fatty acids profiles of each sample; j represented the number of PC; p represented the number of variables. In this study, the scores of PC were calculated and used for further correlation analysis with lipids composition parameters and genes expression.
Table 7.
Factor score coefficients from PCA of breast and thigh muscles.
| Items1 | Breast muscle |
Thigh muscle |
|||||
|---|---|---|---|---|---|---|---|
| BPC1 | BPC2 | BPC3 | TPC1 | TPC2 | TPC3 | TPC4 | |
| C14:0 | 0.124 | −0.027 | −0.006 | 0.056 | 0.008 | 0.066 | 0.045 |
| C15:0 | −0.022 | 0.144 | −0.153 | 0.072 | −0.016 | −0.018 | 0.029 |
| C16:0 | 0.092 | 0.007 | −0.002 | 0.082 | −0.015 | −0.007 | −0.070 |
| C17:0 | −0.086 | 0.177 | 0.015 | 0.062 | 0.033 | −0.018 | 0.055 |
| C18:0 | −0.052 | 0.137 | 0.030 | 0.028 | −0.357 | −0.182 | 0.185 |
| C20:0 | −0.069 | 0.164 | 0.000 | 0.093 | −0.045 | −0.079 | −0.071 |
| C22:0 | 0.316 | −0.231 | −0.099 | 0.084 | −0.103 | −0.033 | −0.088 |
| C14:1 | 0.268 | −0.184 | −0.025 | 0.078 | −0.142 | −0.027 | 0.034 |
| C16:1 | 0.217 | −0.119 | −0.032 | 0.090 | −0.060 | −0.027 | −0.105 |
| C17:1 | −0.045 | 0.045 | 0.194 | 0.065 | −0.047 | −0.588 | −0.004 |
| C18:1(n-9)t | 0.130 | −0.031 | −0.015 | 0.087 | −0.002 | −0.010 | −0.142 |
| C18:1(n-9)c | 0.082 | 0.036 | −0.093 | −0.083 | 0.119 | 0.608 | 0.108 |
| C20:1 | 0.044 | 0.054 | −0.004 | 0.094 | −0.050 | −0.072 | −0.081 |
| C22:1(n-9) | −0.031 | 0.126 | −0.030 | 0.088 | −0.056 | −0.066 | −0.059 |
| C18:2(n-6)c | 0.028 | 0.044 | 0.094 | 0.072 | 0.008 | −0.002 | −0.014 |
| C18:3(n-6) | 0.055 | 0.020 | 0.066 | 0.053 | 0.054 | −0.009 | 0.087 |
| C20:2(n-6) | −0.215 | 0.303 | −0.019 | 0.029 | 0.084 | −0.042 | 0.229 |
| C20:3(n-6) | −0.170 | 0.174 | 0.225 | 0.022 | 0.196 | 0.066 | 0.076 |
| C20:4(n-6) | −0.084 | 0.028 | 0.313 | −0.072 | 0.420 | −0.104 | 0.359 |
| C18:3(n-3) | 0.093 | −0.024 | 0.091 | 0.058 | 0.028 | 0.081 | −0.020 |
| C20:3(n-3) | 0.088 | −0.023 | −0.218 | −0.053 | −0.160 | 0.056 | 0.779 |
| C22:6(n-3) | 0.235 | −0.296 | 0.227 | −0.037 | 0.469 | 0.029 | −0.194 |
Abbreviation: PCA, principal component analysis.
BPC, principal components of breast muscle; TPC, principal components of thigh muscle.
Effects of NRS and FRS on Lipid Metabolism-Related Genes Expression in Breast and Thigh Muscles of Ducks
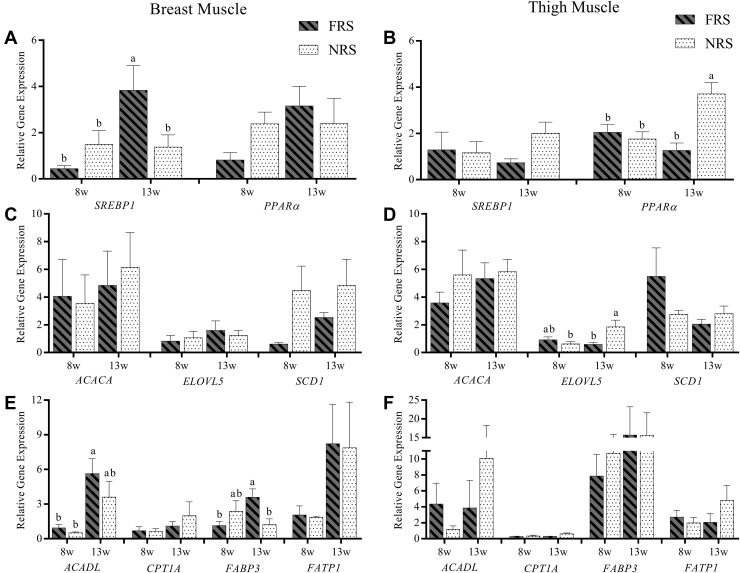
Profiles of ACACA, ACADL, CPT1A, FABP3, FATP1, ELOVL5, SCD1, SREBP1, and PPARα expressions were depicted in Figure 2. In breast muscle, a tendency of higher expressed FABP3, SCD1, and PPARα was found in NRS ducks at 8w, whereas a trend of higher expressed ACACA, ACADL, FABP3, SREBP1, and PPARα was found in FRS ducks at 13w. ACADL was found significantly higher in 13w-FRS than 8w-FRS and 8w-NRS (P < 0.05). FABP3 was found significantly higher in 13w-FRS than 8w-FRS and 13w-NRS (P < 0.05). SREBP1 was found significantly higher in 13w-FRS than 8w-FRS, 8w-NRS, and 13w-NRS (P < 0.05). In thigh muscle, expressions of ELOVL5 and SCD1 were lower in NRS ducks at 8w, whereas a tendency of higher expressed ACADL, CPT1A, FATP1, ELOVL5, SREBP1, and PPARα was found in NRS ducks at 13w. ELOVL5 was found significantly higher in 13w-NRS than 8w-NRS and 13w-FRS (P < 0.05), and PPARα was significantly higher in 13w-NRS than 8w-FRS, 8w-NRS, and 13w-NRS (P < 0.05).
Figure 2.
The lipid metabolism-related genes expression of breast and thigh muscles of ducks under FRS and NRS. (A), (C), and (E) depicted related genes expressions in breast muscle; (B), (D), and (F) depicted related genes expressions in thigh muscle. Genes of transcription factors were shown in (A) and (B), genes related to fatty acids synthesis were shown in (C) and (D), and genes related to fatty acids uptake and utilization were shown in (E) and (F). Abbreviations: FRS, floor rearing system; NRS, net rearing system; 8w, eight week; 13w, 13th week. a-c indicated a significance (P<0.05) among 8w-FRS, 8w-NRS, 13w-FRS and 13w-NRS. Genes: acetyl-CoA carboxylase, ACACA; acyl-CoA dehydrogenase long chain, ACADL; carnitine palmitoyltransferase 1A, CPT1A; fatty acid binding protein 3, FABP3, fatty acid transport protein-1, FATP1; elongation of very long chain fatty acids/fatty acid elongase-5, ELOVL5; stearoyl-CoA desaturase, SCD1; sterol regulatory element binding protein 1, SREBP1 and peroxisome proliferator-activated receptor-α, PPARα.
Correlations of PC With Lipids Composition Parameters and Genes Expression
A Pearson's correlation analysis was performed to determine the relationships of PC with IMF, TG, different categories of fatty acids, and lipid metabolism–related genes in breast and thigh muscles. According to Table 8, ƩPUFA, Ʃn-3, and Ʃn-6 were positively correlated with BPC (P < 0.05). Intramuscular fat, ƩSFA, ƩMUFA, and ƩMUFA/ƩSFA were positively correlated with BPC1 and BPC2 (P < 0.05). ƩPUFA/ƩSFA was negatively correlated with BPC1 while positively correlated with BPC3 (P < 0.05), whereas Ʃn-6/Ʃn-3 was positively correlated with BPC1 and BPC2 (P < 0.05). In thigh muscle, IMF, ƩSFA, ƩMUFA, ƩPUFA, Ʃn-3, Ʃn-6, and ƩMUFA/ƩSFA were positively correlated with TPC2, TPC3, and TPC4 (P < 0.05), whereas TPC1 was negatively correlated with ƩMUFA/ƩSFA as well as TPC2 was negatively correlated with TG (P < 0.05). As for PC and genes, BPC1 was positively correlated with SERBP1 and ELOVL5 (P < 0.05), whereas BPC3 was positively correlated with CPT1A, FATP1, and PPARα (P < 0.05). TPC1 was negatively associated with PPARα, whereas TPC2 and TPC4 were positively associated with ELOVL5 (P < 0.05).
Table 8.
The Pearson's correlation analysis of principal components, IMF contents, TG profiles, and genes expression.
| Items1 | Breast muscle |
Thigh muscle |
|||||
|---|---|---|---|---|---|---|---|
| BPC1 | BPC2 | BPC3 | TPC1 | TPC2 | TPC3 | TPC4 | |
| Genes | |||||||
| ACACA | −0.005 | 0.179 | 0.438 | 0.198 | −0.064 | 0.104 | 0.008 |
| ACADL | 0.498 | 0.542 | 0.569 | −0.361 | −0.007 | 0.130 | 0.059 |
| CPT1A | 0.119 | 0.427 | 0.767∗∗ | −0.175 | 0.255 | 0.381 | 0.364 |
| FABP3 | 0.408 | 0.331 | 0.170 | 0.024 | −0.143 | 0.019 | −0.064 |
| FATP1 | 0.194 | 0.383 | 0.650∗ | −0.393 | −0.002 | 0.145 | 0.119 |
| PPARα | −0.065 | 0.131 | 0.661∗ | −0.577∗ | 0.339 | 0.479 | 0.541 |
| SCD1 | 0.049 | 0.203 | 0.572 | −0.038 | −0.261 | −0.443 | −0.214 |
| SREBP1 | 0.592∗ | 0.527 | 0.355 | −0.332 | 0.267 | 0.194 | 0.344 |
| ELOVL5 | 0.631∗ | 0.559 | 0.303 | −0.464 | 0.607∗ | 0.532 | 0.728∗∗ |
| Lipids composition | |||||||
| IMF | 0.521∗∗ | 0.407∗∗ | −0.085 | −0.252 | 0.391∗∗ | 0.398∗∗ | 0.336∗∗ |
| TG | −0.122 | −0.089 | 0.056 | 0.020 | −0.262∗ | −0.229 | −0.191 |
| ƩSFA | 0.948∗∗ | 0.974∗∗ | 0.166 | 0.025 | 0.831∗∗ | 0.931∗∗ | 0.879∗∗ |
| ƩMUFA | 0.976∗∗ | 0.937∗∗ | 0.097 | −0.237 | 0.950∗∗ | 0.999∗∗ | 0.915∗∗ |
| ƩPUFA | 0.651∗∗ | 0.877∗∗ | 0.703∗∗ | −0.006 | 0.930∗∗ | 0.946∗∗ | 0.878∗∗ |
| Ʃn-3 | 0.579∗∗ | 0.661∗∗ | 0.596∗∗ | −0.102 | 0.852∗∗ | 0.841∗∗ | 0.787∗∗ |
| Ʃn-6 | 0.649∗∗ | 0.880∗∗ | 0.702∗∗ | −0.001 | 0.927∗∗ | 0.944∗∗ | 0.877∗∗ |
| ƩMUFA/ƩSFA | 0.790∗∗ | 0.635∗∗ | −0.027 | −0.517∗∗ | 0.859∗∗ | 0.839∗∗ | 0.685∗∗ |
| ƩPUFA/ƩSFA | −0.494∗∗ | −0.212 | 0.797∗∗ | −0.075 | 0.112 | −0.146 | −0.178 |
| Ʃn-6/Ʃn-3 | 0.149 | 0.407∗∗ | 0.262∗ | 0.141 | 0.074 | 0.135 | 0.128 |
∗ and ∗∗ indicated the significance of correlations.
Abbreviation: IMF, intramuscular fat; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acid; TG, triglyceride.
BPC, principal components of breast muscle; TPC, principal components of thigh muscle.
Discussion
The current study revealed the slaughtering age was a variable that should be taken into consideration when evaluating the effects of rearing systems on lipids deposition. The study on Chaohu ducks reported an increase of IMF content in the breast muscle of NRS ducks (Zhang et al., 2018), which was different from the current study. The reason could be attributed to the different breeds and slaughtering ages. In Nonghua ducks, the IMF content of breast muscle increased with age, in accordance with findings on Sheldrake ducks (He et al., 2018), Beijing You-chickens, and Arbor Acres broilers (Zhou et al., 2010). But when compared with FRS, NRS ducks had relatively higher IMF content in breast muscle at 8w, but it was relatively lower at 13w. While in thigh muscle, little difference was found at 8w, but a significant increase in NRS ducks was found at 13w. Therefore, the effects of rearing systems on lipid deposition in muscles may be different in different breeding stages. The TG and fatty acid–rich phospholipids are the main components of IMF. The current study showed no significant changes in TG profiles, indicating a considerable change in fatty acids composition.
Similarly with Cherry Valley ducks (Qiao et al., 2017), breast and thigh muscles of Nonghua ducks were different in fatty acids components. For instance, C13:0 was only detectable in breast muscle, whereas C12:0 was only detectable in thigh muscle. Besides, 5 fatty acids in breast muscle compared with 11 fatty acids in thigh muscle were directly affected by rearing system at 8w, indicating thigh muscle was more susceptible to NRS than breast muscle at 8w. With new findings of fatty acids serving as important nutrients in post-exercise recovery (Lundsgaard et al., 2020), this could be attributed to the more exercise taken by thigh muscle, because FRS ducks walk more frequently than NRS ducks during rearing. Notably, the significance between NRS and FRS at the same age exhibited a consistent tendency of higher profiles in NRS, indicating a capability of promoting fatty acids profiles in muscles, which was probably because FRS ducks suffered more from the temperature changes than in NRS. For instance, Tardo-Dino et al. (2019) found the temperature increase lead to less efficient oxidative phosphorylation of fatty acids in the soleus of rats. As for the interactions of rearing systems and age, 13w-NRS ducks exhibited higher profiles of C14:0, C16:0, C17:0, C18:1(n-9)c, C18:2(n-6)c, ƩMUFA, Ʃn-6, ƩPUFA, and the ratio of ƩMUFA/ƩSFA than 8w-FRS ducks and 8w-NRS ducks in breast and thigh muscles, whereas other fatty acids (i.e., C18:3(n-3), C20:4(n-6)) were also higher in specific tissues. By conducting a PCA analysis, BPC1 and BPC2 were defined as describing SFA and MUFA, whereas TPC1 was extensively contributed by assorted fatty acids. The loadings of variables indicated the fatty acids contributed greatly to BPC1 and BPC2 were mainly positively correlated with each other, based on their positions with respect to one another in a two-dimensional space generated by BPC1 and BPC2. Similarly in TPC1 and TPC2, fatty acids that contributed greatly were also positively correlated with each other, as they mainly focused on the positive axis of TPC1 and were also close to each other on the axis of TPC2. Despite the negative loading of C20:3(n-3) in BPC3 and negatively correlated C17:1 and C18:1(n-9)t in TPC3 because of their low profiles, when combined with factor scores calculated by coefficients, results of PCA revealed the effects of NRS and FRS on fatty acids compositions of muscles were uniform, and the difference in a single fatty acid was not suitable to be used as the signature variable to distinguish these 2 rearing systems.
However, significances in fatty acids profiles were worth noting from other perspectives. Fatty acids are important flavor precursors (Khan et al., 2015; Arshad et al., 2018), even a small proportion of fatty acids are sufficient to alter flavor when oxidized (Khan et al., 2015). Close (1997) reported the rise in ƩMUFA + SFA was beneficial to meat flavor. Zhu (2013) also reported MUFA was positively correlated with meat flavor, whereas PUFA had a negative impact on meat flavor. Net rearing system ducks in current study exhibited the escalated ƩMUFA, ƩMUFA/ƩSFA, and the increasing trend of ƩSFA extensively, implying a positive impact of NRS on meat flavor improvement. Current study also showed fatty acids including C20:5(n-3), C20:4(n-6), C22:6(n-3), C18:2(n-6)c, C18:3(n-3), and C20:3(n-6) were higher in NRS ducks, among which C18:2(n-6)c, C18:3(n-3), and C20:4(n-6) were essential fatty acids (Dal Bosco et al., 2016), C18:2(n-6)c and C18:3(n-3) were also important substrates for the synthesis of n-6 and n-3 long chain-polyunsaturated fatty acids (LC-PUFA), including C22:5(n-3), C20:5(n-3), and C22:6(n-3) (Ganesh and Hettiarachchy, 2016). This finding indicated NRS ducks might have better absorption of essential fatty acids and less fatty acids utilization than FRS ducks, which may result from the fewer exercise ducks would take in NRS, because physical exercise results in fatty acids oxidation in muscles (Musi et al., 2003). This was supported by Helge et al. (1999), who reported the unsaturation index, LC-PUFA levels, and Δ5-desaturase activity were lower in the muscles of rats with chronic exercise. Besides, the fatty acids mentioned above played an important role in maintaining body health, brain development, and antiaging. C20:5(n-3) and C22:6(n-3) were already proved beneficial to fetal development, cardiovascular diseases prevention, the immunity of pregnant women and fetuses, etc. (Swanson et al., 2012; Nain et al., 2015). Thus, the muscles of NRS ducks might provide higher nutritional value than FRS ducks.
Moreover, fatty acids participated in many biochemical processes regulated by series of genes. For instance, C16:1 and C18:1 can be derived from the desaturation of 16:0 and C18:0 catalyzing by stearoyl-CoA desaturase (SCD) encoded by SCD1 (Zhu, 2013). C18:2(n-6)c and C18:3(n-3) were able to synthesize LC-PUFA by Δ-5 and Δ-6 desaturases (Glaser et al., 2010) as well as the elongation driven by ELOVL2 and ELOVL5 (Gregory and James, 2014; Zhang et al., 2016). Besides, fatty acids transport and trafficking, β-oxidation, and de novo synthesis were also important processes in regulating fatty acid biosynthesis and metabolism (Mandard et al., 2004; Bionaz M and J. 2008; Wang et al., 2013), in which PPAR signaling pathway and SREBP signaling pathway were the most important regulatory pathways (Mandard et al., 2004; Deckelbaum et al., 2006; Bionaz M and J. 2008; Tzeng et al., 2015; Xie et al., 2017).
To give a specific demonstration, we selected ACACA, ACADL, CPT1A, FABP3, FATP1, ELOVL5, SCD1, SREBP1, and PPARα to study the effects of FRS and NRS on genes related to lipid metabolism. Acetyl-CoA carboxylase (ACACA) is the key enzyme for de novo biosynthesis (Duan et al., 2016; Liu et al., 2019). Unsurprisingly, there was no significance on ACACA in breast and thigh muscles because lipogenesis took place primarily in the liver, accounting for 95% of de novo fatty acid synthesis (Liu et al., 2011b; Li et al., 2017). It is also generally believed that almost all fatty acids were either synthesized from liver or derived from diet (Liu et al., 2011b; Zhang et al., 2013; Li et al., 2017). However, 2 important regulatory factors and genes involved in fatty acids uptake and β-oxidation were significantly affected. FABP3 and FATP1 were closely related to fatty acids transport and uptake (Vusse et al., 2002; Zhang et al., 2013). FABP3 was significantly higher in FRS ducks at 13w in breast muscle and was highly expressed in thigh muscle, whereas FATP1 showed a tendency of higher in NRS ducks at 13w in thigh muscle, indicating the effects of NRS and FRS on fatty acids composition of skeletal muscle were probably to influence the process of fatty acids trafficking. SREBP were pivotal transcription factors regulating the biosynthesis of cholesterol and fatty acids (Wang et al., 2017). SREBP1 was significantly lower in breast muscle of NRS ducks at 13w. From genes we detected, ACACA, CPT1A, and SCD1 are known being regulated by SREBP1 (Röhrig and Schulze, 2016; Wang et al., 2017), and they exhibited a consistent trend of elevation in NRS at 13w. Wang et al. (2017) summarized the activation of SREBP would lead to an increase of fatty acid transportation. This may explain why ACADL and FABP3 in breast muscle were relatively higher in 13w-FRS.
PPARα was a ligand-induced transcription factor that stimulates the target genes involved in peroxisomal and mitochondrial β-oxidation (ACO, ACADL, CPT1), lipogenesis (ME), fatty acid binding, and transport (ACBP, FABP3, FATP1) (Finck et al., 2002, 2005; Mandard et al., 2004; Burri et al., 2010). Research on broilers mentions the activation of PPARα induced the upregulation of fatty acid transport and β-oxidation (Tian et al., 2019). By overexpressing PPARα in mice, Finck et al. (2002) also found an increased fatty acid uptake and oxidation in muscle. Similarly in current study, PPARα was significantly higher in NRS ducks at 13w in thigh muscle, whereas a trend of escalated ACADL, CPT1A, and FATP1 were also observed, indicating the fatty acid uptake and oxidation were somehow strengthened by PPARα. FATP was also presumed functional in catalyzing fatty acids to fatty acyl-CoA for β-oxidation (Vusse et al., 2002). As ACADL encoded the enzyme for initial step of LC-PUFA β-oxidation and CPT1A encoded rate-limiting enzyme of β-oxidation, this could explain their uniformly higher expressions with PPARα. This is supported by the study on chickens, and Qiu et al. (2017) reported FATP1 overexpression increased CPT1A mRNA, whereas FATP1 knockdown decreased CPT1A mRNA via PPAR signaling pathway. As the rate-limiting enzyme of MUFA synthesis, SCD was capable of desaturating 16:0 and C18:0 to 16:1 and C18:1. Thus, the higher expression of SCD1 in breast muscle of NRS ducks explained the higher C16:1 and C18:1 than FRS ducks. Similarly, the higher expressed ELOVL5 in 13w-NRS ducks resulted in the significances of PUFA, as ELOVL5 and ELOVL2 encoded the key enzymes for LC-PUFA synthesis from C18:2(n-6)c and C18:3(n-3) (Glaser et al., 2010).
According to Pearson's correlation analysis, ƩSFA, ƩMUFA, and ƩMUFA/ƩSFA were positively correlated with BPC1and BPC2, in accordance with their definition of explaining SFA and MUFA. Because of the uniform fatty acids variation, the parameters of lipids composition were mainly positively correlated with multiple PC at the same time. Interestingly for PC and genes, the PC describing the most of variance (BPC1 and TPC1) were correlated with transcription factors (SREBP1 in breast muscle and PPARα in thigh muscle) respectively, demonstrating the key roles of SREBP1 and PPARα in regulating fatty acids metabolism of breast muscle and thigh muscle. With great contributions of PUFA in BPC3, the positive correlation with CPT1A, FATP1, and PPARα indicated the cellular metabolism processes with PUFA in breast muscle were mainly regulated by CPT1A, FATP1, and PPARα because of the capability of FATP1 to affect IMF deposition by regulating CPT1A via PPAR signaling pathway (Qiu et al., 2017). Moreover, TPC2 and TPC4 were positively correlated with ELOVL5. This may be associated with the elongation driven by ELOVL5 to synthesize LC-PUFA because TPC2 and TPC4 were greatly contributed by C20:4(n-6), C22:6(n-3), and C20:3(n-3). However, the categorical mechanisms need further research to study.
Conclusion
With age increasing, the IMF deposition in breast muscle was enhanced by FRS, and the same promotion in thigh muscle was because of NRS. Because of the uniform variation of fatty acids in both breast and thigh muscles, the change in a single fatty acid was not suitable to be used as the signature variable to distinguish NRS and FRS. But as NRS downregulated SREBP1, ACADL, and FABP3 in breast muscle and upregulated PPARα and ELOVL5 in thigh muscle, NRS was more conducive to improve nutrient value and meat quality by increasing the levels of some important PUFA, ƩMUFA, and the ratio of ƩMUFA/ƩSFA in muscles. Though the categorical mechanism need further study to elucidate, NRS was more recommended than FRS for dryland rearing popularization.
Acknowledgments
This work was financially supported by the China Agricultural Research System (CARS-42-4), the Livestock & Poultry Breeding Research Project of Sichuan Province (No. 2016NYZ0044) and the Project of National Science and Technology Plan for the Rural Development in China (2015BAD03B06).
Conflict of Interest Statement: The authors declare no conflict of interest regarding the publication of this article.
References
- Abasht B., Zhou N., Lee W.R., Zhuo Z., Peripolli E. The metabolic characteristics of susceptibility to wooden breast disease in chickens with high feed efficiency. Poult. Sci. 2019;98:3246–3256. doi: 10.3382/ps/pez183. [DOI] [PubMed] [Google Scholar]
- Almeida E. de A., Arantes De Souza L.F., Sant'anna A.C., Bahiense R.N., Macari M., Furlan R.L. Poultry rearing on perforated plastic floors and the effect on air quality, growth performance, and carcass injuries—experiment 1: Thermal comfort. Poult. Sci. 2017;96:3155–3162. doi: 10.3382/ps/pex131. [DOI] [PubMed] [Google Scholar]
- Almeida E.A., Sant’anna A.C., Crowe T.G., Macari M., Furlan R.L. Poultry rearing on perforated plastic floors and the effect on air quality, growth performance, and carcass injuries–Experiment 2: Heat stress situation. Poult. Sci. 2018;97:1954–1960. doi: 10.3382/ps/pey048. [DOI] [PubMed] [Google Scholar]
- Arshad M.S., Sohaib M., Ahmad R.S., Nadeem M.T., Imran A., Arshad M.U., Kwon J.-H., Amjad Z. Ruminant meat flavor influenced by different factors with special reference to fatty acids. Lipids Health Dis. 2018;17:223. doi: 10.1186/s12944-018-0860-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bionaz M., J J L. Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genomics. 2008;9:366. doi: 10.1186/1471-2164-9-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce C.R., Hoy A.J., Turner N., Watt M.J., Allen T.L., Carpenter K., Cooney G.J., Febbraio M.A., Kraegen E.W. Overexpression of Carnitine Palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet-induced insulin resistance. Diabetes. 2009;59:550–558. doi: 10.2337/db08-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burri L., Thoresen G.H., Berge R.K. The role of PPARα activation in liver and muscle. PPAR Res. 2010. 2010 doi: 10.1155/2010/542359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellini C., Mugnai C., Dal Bosco A. Effect of organic production system on broiler carcass and meat quality. Meat Sci. 2002;60:219–225. doi: 10.1016/s0309-1740(01)00124-3. [DOI] [PubMed] [Google Scholar]
- Close W.H. Biotechnology in the Feed Industry Proceedings of Alltech’s 13th Annual Symposium. Nottingham University Press; Nottingham: 1997. Nutritional manipulation of meat quality in pigs and poultry; pp. 181–192. [Google Scholar]
- Dal Bosco A., Mugnai C., Mattioli S., Rosati A., Ruggeri S., Ranucci D., Castellini C. Transfer of bioactive compounds from pasture to meat in organic free-range chickens. Poult. Sci. 2016;95:2464–2471. doi: 10.3382/ps/pev383. [DOI] [PubMed] [Google Scholar]
- Deckelbaum R.J., Worgall T.S., Seo T. n-3 fatty acids and gene expression. Am. J. Clin. Nutr. 2006;83:1520S–1525S. doi: 10.1093/ajcn/83.6.1520S. [DOI] [PubMed] [Google Scholar]
- Duan Y.H., Duan Y.M., Li F.N., Li Y.H., Guo Q.P., Ji Y.J., Tan B., Li T.J., Yin Y.L. Effects of supplementation with branched-chain amino acids to low-protein diets on expression of genes related to lipid metabolism in skeletal muscle of growing pigs. Amino Acids. 2016;48:2131–2144. doi: 10.1007/s00726-016-2223-2. [DOI] [PubMed] [Google Scholar]
- Erdem E., Onabaşilar E.E., Kocakaya A., YalÇin S. Effects of brooder machine rearing method in the first week on fattening performance, carcass characteristics, meat quality and some blood parameters of Pekin ducks. Ankara Üniv Vet. Fak Derg. 2015;62:237–242. [Google Scholar]
- Finck B.N., Bernal Mizrachi C., Han D.H., Coleman T., Sambandam N., Lariviere L.L., Holloszy J.O., Semenkovich C.F., Kelly D.P. A potential link between muscle peroxisome proliferator- activated receptor-α signaling and obesity-related diabetes. Cell Metab. 2005;1:133–144. doi: 10.1016/j.cmet.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Finck B.N., Lehman J.J., Leone T.C., Welch M.J., Bennett M.J., Kovacs A., Han X.L., Gross R.W., Kozak R., Lopaschuk G.D. The cardiac phenotype induced by PPARα overexpression mimics that caused by diabetes mellitus. J. Clin. Invest. 2002;109:121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh V., Hettiarachchy N.S. A review: supplementation of Foods with essential fatty acids—can it Turn a Breeze without further Ado? Crit. Rev. Food Sci. 2016;56:1417–1427. doi: 10.1080/10408398.2013.765383. [DOI] [PubMed] [Google Scholar]
- Glaser C., Heinrich J., Koletzko B. Role of FADS1 and FADS2 polymorphisms in polyunsaturated fatty acid metabolism. Metabolism. 2010;59:993–999. doi: 10.1016/j.metabol.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Gregory M.K., James M.J. Functional Characterization of the duck and Turkey fatty acyl elongase enzymes ELOVL5 and ELOVL2. J. Nutr. 2014;144:1234–1239. doi: 10.3945/jn.114.194159. [DOI] [PubMed] [Google Scholar]
- He J., Zheng H.Y., Pan D.D., Liu T., Sun Y.Y., Cao J.X., Wu Z., Zeng X.Q. Effects of aging on fat deposition and meat quality in Sheldrake duck. Poult. Sci. 2018;97:2005–2010. doi: 10.3382/ps/pey077. [DOI] [PubMed] [Google Scholar]
- Helge J.W., Ayre K.J., Hulbert A.J., Kiens B., Storlien L.H. Regular exercise Modulates muscle membrane phospholipid profile in rats. J. Nutr. 1999;129:1636–1642. doi: 10.1093/jn/129.9.1636. [DOI] [PubMed] [Google Scholar]
- Husak R.L., Sebranek J.G., Bregendahl K. A survey of commercially available broilers marketed as organic, free-range, and conventional broilers for cooked meat yields, meat composition, and relative value. Poult. Sci. 2008;87:2367–2376. doi: 10.3382/ps.2007-00294. [DOI] [PubMed] [Google Scholar]
- Khan M.I., Jo C., Tariq M.R. Meat flavor precursors and factors influencing flavor precursors—a systematic review. Meat Sci. 2015;110:278–284. doi: 10.1016/j.meatsci.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Kolluri G., Ramamurthy N., Churchil R.R., Dhinakar Raj G., Kannaki T.R. Influence of age, sex and rearing systems on Toll-like receptor 7 (TLR7) expression pattern in gut, lung and lymphoid tissues of indigenous ducks. Br. Poult. Sci. 2014;55:59–67. doi: 10.1080/00071668.2013.867926. [DOI] [PubMed] [Google Scholar]
- Li S., Vestergren A.S., Wall H., Trattner S., Pickova J., Ivarsson E. Feeding steam-pelleted rapeseed affects expression of genes involved in hepatic lipid metabolism and fatty acid composition of chicken meat. Poult. Sci. 2017;96:2965–2974. doi: 10.3382/ps/pex047. [DOI] [PubMed] [Google Scholar]
- Li J., Zhao Z., Xiang D., Zhang B., Ning T., Duan T. Expression of APOB, ADFP, and FATP1 and their correlation with fat deposition in Yunnan’s top six famous chicken breeds. Br. Poult. Sci. 2018;59:494–505. doi: 10.1080/00071668.2018.1490494. [DOI] [PubMed] [Google Scholar]
- Liu B.Y., Wang Z.Y., Yang H.M., Wang J.M., Xu D., Zhang R., Wang Q. Influence of rearing system on growth performance, carcass traits, and meat quality of Yangzhou geese. Poult. Sci. 2011;90:653–659. doi: 10.3382/ps.2009-00591. [DOI] [PubMed] [Google Scholar]
- Liu Y.L., Wan D., Zhou X.H., Ruan Z., Zhang T.Y., Wu X., Yin Y.L. Effects of dynamic feeding low-and high-methionine diets on the variation of glucose and lipid metabolism-related genes in the liver of laying hens. Poult. Sci. 2019;98:2231–2240. doi: 10.3382/ps/pey589. [DOI] [PubMed] [Google Scholar]
- Liu W.M., Zhang J., Lu L.Z., Shi F.X., Niu D., Wang D.L., Yu B., Tao Z.R., Shen J.D., Wang D.Q. Effects of perilla extract on productive performance, serum values and hepatic expression of lipid-related genes in Shaoxing ducks. Br. Poult. Sci. 2011;52:381–387. doi: 10.1080/00071668.2011.577053. [DOI] [PubMed] [Google Scholar]
- Lundsgaard A.M., Fritzen A.M., Kiens B. The importance of fatty acids as nutrients during post-exercise recovery. Nutrients. 2020;12:280. doi: 10.3390/nu12020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandard S., Müller M., Kersten S. Peroxisome proliferator-activated receptor α target genes. Cell Mol. Life Sci. 2004;61:393–416. doi: 10.1007/s00018-003-3216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalczuk M., Damaziak K., Pietrzak D., Marzec A., Chmiel M., Adamczak L., Florowski T. Influence of housing system on selected quality characteristics of duck meat. Chapter 1. Pekin duck. Ann. Warsaw Univ. Life Sci. – SGGW. Anim. Sci. 2016;55:89–97. [Google Scholar]
- Michalczuk M., Damaziak K., Pietrzak D., Marzec A., Chmiel M., Adamczak L., Florowski T. Influence of housing system on selected quality characteristics of duck meat. Chapter 2. Muscovy duck. Ann. Warsaw Univ. Life Sci. – Sggw, Anim. Sci. 2017;56:277–285. [Google Scholar]
- Musi N., Yu H., Goodyear L.J. AMP-activated protein kinase regulation and action in skeletal muscle during exercise. Biochem. Soc. Trans. 2003;31:191–195. doi: 10.1042/bst0310191. [DOI] [PubMed] [Google Scholar]
- Nain S., Oryschak M.A., Betti M., Beltranena E. Camelina sativa cake for broilers: effects of increasing dietary inclusion from 0 to 24% on tissue fatty acid proportions at 14, 28, and 42 d of age. Poult. Sci. 2015;94:1247–1258. doi: 10.3382/ps/pev080. [DOI] [PubMed] [Google Scholar]
- Pioche T., Skiba F., Bernadet M.D., Seilez I., Massimino W., Houssier M., Tavernier A., Ricaud K., Davail S., Skiba-Cassy S. Kinetic study of the expression of genes related to hepatic steatosis, glucose and lipid metabolism and cellular stress during overfeeding in mule ducks. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020;318:R453–R467. doi: 10.1152/ajpregu.00198.2019. [DOI] [PubMed] [Google Scholar]
- Qiao Y., Huang J.C., Chen Y.L., Chen H.C., Zhao L., Huang M., Zhou G.H. Meat quality, fatty acid composition and sensory evaluation of Cherry Valley, Spent Layer and Crossbred ducks. Anim. Sci. J. 2017;88:156–165. doi: 10.1111/asj.12588. [DOI] [PubMed] [Google Scholar]
- Qiu F.F., Xie L., Ma J.E., Luo W., Zhang L., Chao Z., Chen S.H., Nie Q.H., Lin Z.M., Zhang X.Q. Lower expression of SLC27A1 enhances intramuscular fat deposition in chicken via down-regulated fatty acid oxidation mediated by CPT1A. Front. Physiol. 2017;8:449. doi: 10.3389/fphys.2017.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhrig F., Schulze A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer. 2016;16:732. doi: 10.1038/nrc.2016.89. [DOI] [PubMed] [Google Scholar]
- Storch J., Thumser E.A.A. The fatty acid transport function of fatty acid-binding proteins. Biochim. Biophys. Acta. 2000;1486:0–44. doi: 10.1016/s1388-1981(00)00046-9. [DOI] [PubMed] [Google Scholar]
- Swanson D., Block R., Mousa S.A. Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv. Nutr. 2012;3:1. doi: 10.3945/an.111.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardo-Dino P.-E., Touron J., Bauge S., Bourdon S., Malgoyre A. The effect of a physiological increase in temperature on mitochondrial fatty acid oxidation in rat myofibres. J. Appl. Physiol. 2019;127:312–319. doi: 10.1152/japplphysiol.00652.2018. [DOI] [PubMed] [Google Scholar]
- The National Health and Family Planning Commission of the People's Republic of China (NHFPC/PRC) Standards Press of China; Beijing, China: 2016. GB/T 5009.168-2016. National Food Safety Standard: Determination of Fatty Acid in Foods. [Google Scholar]
- Tian D.L., Guo R.J., Li Y.M., Chen P.P., Zi B.B., Wang J.J., Liu R.F., Min Y.N., Wang Z.P., Niu Z.Y. Effects of lysine deficiency or excess on growth and the expression of lipid metabolism genes in slow-growing broilers. Poult. Sci. 2019;98:2927–2932. doi: 10.3382/ps/pez041. [DOI] [PubMed] [Google Scholar]
- Turner N., Cooney G.J., Kraegen E.W., Bruce C.R. Fatty acid metabolism, energy expenditure and insulin resistance in muscle. J. Endocrinol. 2014;220:T61–T79. doi: 10.1530/JOE-13-0397. [DOI] [PubMed] [Google Scholar]
- Tzeng T.F., Liou S.S., Chang C., Liu I.M. 6-Gingerol protects against nutritional Steatohepatitis by regulating key genes related to Inflammation and lipid metabolism. Nutrients. 2015;7:999–1020. doi: 10.3390/nu7020999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vusse G.J.V.D., Bilsen M.V., Jan F.C.G., Danny M.H., Joost J.F.P.L. Critical steps in cellular fatty acid uptake and utilization. Mol. Cell. Biochem. 2002;239:9–15. doi: 10.1007/978-1-4419-9270-3_2. [DOI] [PubMed] [Google Scholar]
- Wang G.Q., Kim W.K., Cline M.A., Gilbert E.R. Factors affecting adipose tissue development in chickens: a review. Poult. Sci. 2017;96:3687–3699. doi: 10.3382/ps/pex184. [DOI] [PubMed] [Google Scholar]
- Wang W., Xue W.D., Jin B.Q., Zhang X.X., Ma F., Xu X.F. Candidate gene expression affects intramuscular fat content and fatty acid composition in pigs. J. Appl. Genet. 2013;54:113–118. doi: 10.1007/s13353-012-0131-z. [DOI] [PubMed] [Google Scholar]
- Xie Z., Zhang J., Ma S., Huang X., Huang Y. Effect of Chinese herbal medicine treatment on plasma lipid profile and hepatic lipid metabolism in Hetian broiler. Poult. Sci. 2017;96:1918–1924. doi: 10.3382/ps/pew456. [DOI] [PubMed] [Google Scholar]
- Zhang C., Ah Kan Razafindrabe R.H., Chen K.K., Zhao X.H., Yang L., Wang L., Chen X.Y., Jin S., Geng Z.Y. Effects of different rearing systems on growth performance, carcass traits, meat quality and serum biochemical parameters of Chaohu ducks. Anim. Sci. J. 2018;89:672–678. doi: 10.1111/asj.12976. [DOI] [PubMed] [Google Scholar]
- Zhang J.Y., Kothapalli K.S.D., Brenna J.T. Desaturase and elongase-limiting endogenous long-chain polyunsaturated fatty acid biosynthesis. Curr. Opin. Clin. Nutr. 2016;19:103. doi: 10.1097/MCO.0000000000000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Shi H., Liu W., Wang Y., Wang Q., Li H. Differential expression of L-FABP and L-BABP between fat and lean chickens. Genet. Mol. Res. 2013;12:4192–4206. doi: 10.4238/2013.October.7.5. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Li X.H., Sun S.W., Chen L., Jin J.J., Liu S.Z., Song X.Z., Wu C.Q., Lu L.Z. Protective role of dryland rearing on netting floors against mortality through gut microbiota-associated immune performance in Shaoxing ducks. Poult. Sci. 2019;98:4530–4538. doi: 10.3382/ps/pez268. [DOI] [PubMed] [Google Scholar]
- Zhou X.J., Zhu N.H., Zhang R.J. Effects of variety, age and feeding method on inosine acid and intramuscular fat content in chicken. Chin. J. Anim. Nutr. 2010;22:128–133. (In Chinese) [Google Scholar]
- Zhu C.Y. Univ. Yangzhou; Yangzhou: 2013. Cloning of SCD1 Gene from Xuhuai Goat and Preparation of Transgenic Mice and Transgenic Sheep. M.A. Diss. [Google Scholar]