Abstract
Caged layer osteoporosis (CLO) is a common bone metabolism diseases and poses a great threat to the production of laying hens. So far, there is no effective nutrition intervention to prevent CLO. The objective of this study was to evaluate the effects of dietary total flavonoids from Rhizoma Drynariae (TFRD), a Chinese herbal, on bone health, egg quality, and serum antioxidant capacity of caged laying hens. A total of two hundred sixteen, 54-wk-old Lohmann Pink-shell laying hens at were allocated to 3 groups with 6 replicates of 12 hens per replicate. The control group was fed a basal diet (BD) and 2 treatment groups additionally supplied with 0.5 or 2.0 g/kg TFRD, respectively. Results showed that supplying 2.0 g/kg TFRD enhanced the activities of serum total antioxidant capacity (P < 0.01) and glutathione peroxidase (P < 0.05) and had higher femur and tibia bone mineral density (both P < 0.05) compared with the control group. Dietary 2.0 g/kg TFRD also reduced the activities of serum alkaline phosphatase (P < 0.01), tartrate resistant acid phosphatase (P < 0.01), and the contents of osteocalcin (P < 0.01). Furthermore, tibia histomorphology observation showed that the microstructure of bone tissue was improved after TFRD treatment. Egg quality was not affected by TFRD while the egg weight significantly increased (P < 0.01). These findings suggested that TFRD has beneficial effects on bone health in older caged laying hens.
Key words: osteoporosis, bone metabolism, laying hen, total flavonoids of Rhizoma Drynariae
Introduction
Bone health has always been the focal point of laying hen breeding industry (Tarlton et al., 2013; Nie et al., 2018). Hens have a unique bone physiology because of the demands placed on them through egg production (Whitehead, 2004). Laying hens have 3 distinctive kinds of bones related to egg formation: cortical, cancellous, and medullary bones (Kim et al., 2012). Under the pressure of constant egg production, the cortical bone of the laying hen is progressively lost during the laying period, resulting in skeletal fragility and great susceptibility to fractures which was descripted as caged layer osteoporosis (CLO) (Jiang et al., 2019). With the breeding density of laying hens increasing, the limitation of sport environment has also increased the loss of bone mass (Aguado et al., 2015; Rodriguez-Navarro et al., 2018). What worse, laying hens with skeletal problems usually have a poor egg laying performance (Rufener et al., 2018). Caged layer osteoporosis has caused huge economic losses and involved in animal welfare issues (Riber et al., 2018; Hardin et al., 2019). So far, there is no effective nutrition intervention to prevent CLO.
Rhizoma Drynariae (Gu-Sui-Bu in Chinese) is one of the most frequently used traditional Chinese medicines which can replenish the kidney, strengthen the bones, promote the healing fracture, and relieve pain (Liu et al., 2012a,b). Studies have demonstrated that Rhizoma Drynariae contains flavonoids, triterpenes, and phenylpropanoids (Yuan et al., 2019). Among them, the study on the active components of Rhizoma Drynariae mainly focused on total flavonoids. Total flavonoids from Rhizoma Drynariae (TFRD) is a Chinese herbal product extracted from the dried root of Rhizoma Drynariae (Liu et al., 2012a,b), which has been developed into a postmarketing Chinese medicine called Qianggu capsule. Total flavonoids from Rhizoma Drynariae is widely used for the treatment of bone fractures or related diseases in Asian countries. It has been proven that TFRD has antiosteoporosis activity by inhibiting the bone resorption and stimulating bone formation (Wang et al., 2011) and finally achieves the goal of prevention and treatment of osteoporosis in humans and rat model (Song et al., 2016). Recently, studies found that TFRD has therapeutic effect on tibial dyschondroplasia in broilers (Yao et al., 2018). However, it is unclear whether TFRD has beneficial effects on older caged laying hens.
Therefore, this study was conducted to determine the effects of dietary TFRD supplementation on laying performance, egg quality, antioxidant capacity, and bone health in older caged laying hens.
Materials and methods
Birds, Diets, and Management
All procedures involving animals was approved by the Institutional Animal Care and Use Committee of Huazhong Agricultural University, China. A total of 216, 54-wk-old Lohmann Pink-shell laying hens with an average laying rate of 91% were obtained from a commercial farm in the Hubei province of China. The hens were randomly allocated to 3 groups (CON, TFRD1, and TFRD2) with 6 replicates of 12 hens per replicate. The CON group was fed a corn–soybean basal diet (BD) (Table 1) according to National Research Council (Dale, 1994), and the 2 experimental groups were fed BD supplemented with 0.5 and 2.0 g/kg TFRD. The TFRD used in this study was an extract from Rhizoma Drynariae and was purchased from Xi'an Kailai Biological Engineering Co., Ltd (Xi'an, China), and the total flavonoid content is 90.25% by HPLC analysis. The hens were randomly assigned to cages (80 cm-width × 80 cm-length × 40 cm-height) of 6 hens per cage based on completely randomized design. The hens were kept in an environmentally controlled room with ad libitum feeding and watering and with the temperature controlled at 22°C and 16 h/D of illumination throughout the entire experimental period. The experiment lasted 13 wk, included a 1-wk acclimation period and a 12-wk experimental period.
Table 1.
Basal diet formulation and nutrient levels.
| Dietary ingredient | Content (%)1 |
|---|---|
| Corn | 62.7 |
| Soybean meal | 26.3 |
| Limestone | 8.5 |
| DL-Methionine | 0.1 |
| Calcium hydrogen phosphate | 1.0 |
| Choline chloride (50%) | 0.1 |
| NaCl | 0.3 |
| Vitamin and trace mineral premix2 | 1.0 |
| Total | 100 |
| Nutrient levels (calculated) | |
| Metabolizable energy, MJ/kg | 11.09 |
| Crude protein, % | 16.61 |
| Calcium, % | 3.5 |
| Available phosphorus, % | 0.35 |
| Methionine, % | 0.35 |
| Lysine, % | 0.85 |
Values are expressed on as-fed basis.
Premix provided per kilogram of diet: vitamin A (retinyl palmitate), 7,715 IU; vitamin D3 (cholecalciferol), 2,755 IU; vitamin E (dl-a-tocopheryl acetate), 8.8 IU; vitamin K (menadione sodium bisulfate complex), 2.2 mg; vitamin B12 (cobalamin), 0.01 mg; menadione (menadione sodium bisulfate complex), 0.18 mg; riboflavin, 4.41 mg; pantothenic acid (d-calcium pantothenate), 5.51 mg; niacin, 19.8 mg; folic acid, 0.28 mg; pyridoxine (pyridoxine hydrochloride), 0.55 mg; manganese (manganese sulfate), 50 mg; iron (ferrous sulfate), 25 mg; copper (copper sulfate), 2.5 mg; zinc (zinc sulfate), 50 mg; iodine (calcium iodate), 1.0 mg; and selenium (sodium selenite), 0.15 mg.
Sample Collections
The number of eggs and egg weight was recorded everyday (at 13:00) throughout experiment on a replication basis, and hen-day laying rate was calculated. Average egg weight was calculated as the mean weight of all eggs from each replicate. Feed consumption was measured weekly on a replication basis. Average daily feed intake (ADFI) was calculated using the following equation: ADFI = feed consumption (g)/(hen number × time (D)), and feed conversion ratio was calculated as (feed intake/(egg weight × egg production)). At 5, 9, and 13 wk, 2 eggs were randomly selected from each replicate for egg quality determination. At the end of the experiment (13 wk), 1 hen from each replicate was randomly selected, and blood samples were individually collected from the wing vein and then were centrifuged at 3,000 × g for 10 min at 4°C to obtain serum. In addition, hens were slaughtered to collect femurs and tibias.
Egg Quality Determination
Egg shape index was calculated by height/width. The eggs were weighed before being cracked. The eggshell strength was evaluated using an egg shell force gauge model II (Robotmation Co., Ltd., Tokyo, Japan). Eggshell thickness was measured on the large end, equatorial region, and small end, respectively, using an eggshell thickness gauge (Robotmation Co., Ltd). Egg weight, albumen height, yolk color, and Haugh unit were measured by using a digital egg tester (DET-6000, Nabel Co., Ltd, Kyoto, Japan).
Antioxidant Capacity Determination
The activities of total antioxidant capacity contents, total superoxide dismutase, glutathione peroxidase, and the contents of malondialdehyde in serum were analyzed using analysis kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions.
Bone Mineral Density Measurement
The bone mineral density (BMD) was measured with dual energy X-ray absorptiometry (InAlyzer; MEDIKORS Inc., Gyeonggi-Do, Korea). The detection sensitivity of the instrument was 0.001 g/cm2. A standard calibration block was used to calibrate the device before measurements were made, according to the operator's manual.
Bone Histomorphometry Analysis
The left tibias were removed from hens and fixed overnight at 4°C in 4% paraformaldehyde (Luo et al., 2019), then decalcified in 10% EDTA at room temperature for 2 wk and embedded into paraffin. Serial sections (5 μm) were cut and stained with Goldner Trichrome stain. In addition, the static parameters of bone histomorphometry were analyzed.
Bone Metabolism Biomarkers Analysis
The activities of alkaline phosphatase (ALP), tartrate-resistant acid phosphatase (TRACP), and the contents of calcium in serum were measured by specific assay kits from the Nanjing Jiancheng Bioengineering Institute of China. The concentrations of osteocalcin (OCN) in serum were measured with the use of ELISA kits (CH50029; Bio-Swamp, China) according to the manufacturer's instructions.
Total RNA Extraction and Real-Time Quantitative PCR
According to the manufacturer's protocol, the total RNA was extracted from the tissues of the femur by adding TRIzol Reagent (Invitrogen, Waltham, MA). After extraction with chloroform, isopropanol was added to make the RNA precipitate. After washing with 75% ethanol, the RNA was eluted in ribonuclease-free water. The cDNA was synthesized using ABScript II RT Master Mix (ABclonal Technology, Wuhan, China). Runt-related transcription factor 2 (RUNX2) is a multifunctional transcription factor that controls skeletal development by regulating the differentiation of mesenchymal stem cells into osteoblasts and the expression of other related genes during osteoblast differentiation (Vimalraj et al., 2015). Osteoprotegerin (OPG), a soluble receptor that binds to receptor activator of nuclear factor kappa-B ligand (RANKL), is the natural inhibitor of RANKL and protects bone from excessive resorption (Tanaka et al., 2011). The forward and reverse primer sequences of RUNX2, OPG, and RANKL were designed based on available sequences on the NCBI GenBank and are listed in Table 2. β-actin was chosen as an internal standard to control for normalization purposes. Quantitation of the mRNA level by QPCR was performed on a real-time PCR system using iTaq Universal SYBR Green Supermix (Bio-Rad, Richmond, CA). The threshold cycle indicated the fractional cycle number at which the amount of amplified target reached a fixed threshold, so we can obtain the relative gene expression level by the 2−ΔΔCT method for fold induction. All PCR operations were performed in triplicate.
Table 2.
Primers used for the quantitative polymerase chain reaction.
| Genes | GenBank ID | Primers sequence (5′ to 3′) | Products (bp) |
|---|---|---|---|
| RUNX2 | NM_204128.1 | F: GATTACAGACCCCAGGCAGG | 75 |
| R: TGGCTCAAGTAGGACGGGTA | |||
| OPG | NM_001033641.1 | F: GTTCCTACTCGTTCCACACC | 115 |
| R: GCTCTTGTGAACTGTGCCTTTG | |||
| RANKL | NM_001083361.1 | F: AGGAGAAATAAGCCCGAGAA | 108 |
| R: TTTGTTATGATGCCAGGATGTA | |||
| β-actin | NM_205518.1 | F: CACGATCATGTTTGAGACCTT | 100 |
| R: CATCACAATACCAGTGGTACG |
Abbreviations: OPG, osteoprotegerin; RUNX2, runt-related transcription factor 2; RANKL, receptor activator of nuclear factor kappa-B ligand.
Statistical Analysis
Data were analyzed using the SPSS statistical software (SPSS version 22.0, for windows, SPSS Inc., Chicago, IL). One-way ANOVA was used for the analysis of group differences. For measures that presented heterogeneous variances, a Welch-ANOVA was employed. Post hoc analyses were performed by means of Bonferroni test or Games–Howell post hoc test (following the Welch-ANOVA) to characterize the significant effects. Differences were considered statistically significant at P < 0.05. The results are presented as the means and SEMs.
Results
Performance of Laying Hens
As shown in Table 3, laying rate, ADFI, and feed conversion ratio did not differ among groups (P > 0.05). Dietary TFRD supplementation significantly increased the average egg weight (P < 0.01) compared with the CON group.
Table 3.
Effects of dietary TFRD on laying performance in caged laying hens.
| Items1 | CON | TFRD1 | TFRD2 | SEM | P-value |
|---|---|---|---|---|---|
| Laying rate, % | 86.90 | 86.86 | 87.78 | 1.23 | 0.949 |
| AEW, g | 60.97b | 62.92a | 63.07a | 0.29 | 0.001 |
| ADFI, g | 121.86 | 121.83 | 121.79 | 0.04 | 0.844 |
| FCR | 2.31 | 2.23 | 2.22 | 0.02 | 0.099 |
Means within a row lacking a common superscript differ (P < 0.05), n = 6 per group.
TFRD, total flavonoids of Rhizoma Drynariae; AEW, average egg weight; ADFI, average daily feed intake; FCR, feed conversion ratio; CON, basal diet; TFRD1, basal diet supplemented with 0.5 g/kg TFRD; TFRD2, basal diet supplemented with 2.0 g/kg TFRD.
Egg Quality
No significant difference was found in egg quality parameters (P > 0.05) (Table 4).
Table 4.
Effects of dietary TFRD on egg quality in caged laying hens.
| Items1 | CON | TFRD1 | TFRD2 | SEM | P-value |
|---|---|---|---|---|---|
| Egg shape index | |||||
| 5 wk | 1.30 | 1.31 | 1.31 | 0.01 | 0.908 |
| 9 wk | 1.30 | 1.32 | 1.30 | 0.01 | 0.630 |
| 13 wk | 1.31 | 1.32 | 1.31 | 0.01 | 0.958 |
| Eggshell strength, N | |||||
| 5 wk | 44.75 | 48.02 | 46.02 | 0.70 | 0.156 |
| 9 wk | 39.65 | 44.95 | 42.63 | 1.05 | 0.118 |
| 13 wk | 34.74 | 39.38 | 36.92 | 0.87 | 0.088 |
| Eggshell thickness, mm | |||||
| 5 wk | 0.36 | 0.38 | 0.38 | 0.01 | 0.165 |
| 9 wk | 0.34 | 0.35 | 0.35 | 0.004 | 0.157 |
| 13 wk | 0.30 | 0.32 | 0.32 | 0.006 | 0.234 |
| Yolk color | |||||
| 5 wk | 13.30 | 13.10 | 13.64 | 0.14 | 0.311 |
| 9 wk | 14.01 | 13.86 | 13.84 | 0.12 | 0.813 |
| 13 wk | 13.98 | 13.66 | 14.01 | 0.11 | 0.402 |
| Albumen height, mm | |||||
| 5 wk | 7.41 | 7.85 | 7.33 | 0.15 | 0.309 |
| 9 wk | 7.40 | 6.93 | 6.99 | 0.13 | 0.292 |
| 13 wk | 7.54 | 8.29 | 7.98 | 0.18 | 0.260 |
| Haugh unit | |||||
| 5 wk | 85.17 | 87.46 | 83.49 | 1.04 | 0.308 |
| 9 wk | 85.35 | 83.11 | 82.96 | 0.87 | 0.477 |
| 13 wk | 83.45 | 91.74 | 87.96 | 1.50 | 0.077 |
Means within a row lacking a common superscript differ (P < 0.05), n = 12 per group.
TFRD, total flavonoids of Rhizoma Drynariae; CON, basal diet; TFRD1, basal diet supplemented with 0.5 g/kg TFRD; TFRD2, basal diet supplemented with 2.0 g/kg TFRD.
Serum Antioxidant Capacity
Table 5 shows the effects of dietary TFRD on serum redox indicators in caged laying hens. The contents of malondialdehyde and the activities of total superoxide dismutase did not differ among groups (P > 0.05). Dietary TFRD supplementation significantly increased the levels of total antioxidant capacity (P < 0.01). Supplying 2.0 g/kg TFRD in diet had a higher activities of glutathione peroxidase compared with the control group (P < 0.05).
Table 5.
Effects of dietary TFRD on serum antioxidant indicators in caged laying hens.
| Items1 | CON | TFRD1 | TFRD2 | SEM | P-value |
|---|---|---|---|---|---|
| MDA, nmol/mL | 3.90 | 3.80 | 3.69 | 0.12 | 0.787 |
| T-AOC, U/mL | 6.40b | 8.58a | 8.92a | 0.34 | 0.001 |
| T-SOD, U/mL | 207 | 224 | 232 | 4.82 | 0.090 |
| GSH-Px, U/mL | 994b | 1060a,b | 1247a | 44.8 | 0.045 |
Means within a row lacking a common superscript differ (P < 0.05), n = 6 per group.
TFRD, total flavonoids of Rhizoma Drynariae; MDA, malondialdehyde; T-AOC, total antioxidant capacity; T-SOD, total superoxide dismutase; GSH-Px, glutathione peroxidase; CON, basal diet; TFRD1, basal diet supplemented with 0.5 g/kg TFRD; TFRD2, basal diet supplemented with 2.0 g/kg TFRD.
Femur and Tibia BMD
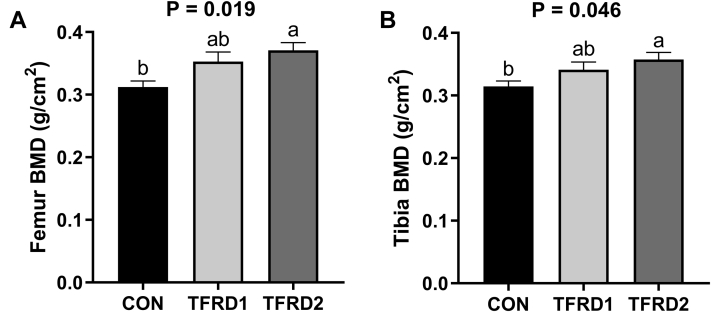
Figure 1 shows the effects of dietary TFRD on BMD of femur and tibia in caged laying hens. Dietary 0.5 g/kg TFRD supplementation did not induce an increase in BMD, whereas supplying 2.0 g/kg TFRD significantly increased the femur BMD by 19.4% and tibia BMD by 13.9% (both P < 0.05).
Figure 1.
Effects of dietary TFRD on BMD in caged laying hens. (A) Femur BMD and (B) tibia BMD. Values are means ± SEM (n = 6 per group). The a, b, and c means every bars without same letter differ significantly (P < 0.05). Abbreviations: BMD, bone mineral density; TFRD, total flavonoids of Rhizoma Drynariae; CON, basal diet; TFRD1, basal diet supplemented with 0.5 g/kg TFRD; TFRD2, basal diet supplemented with 2.0 g/kg TFRD.
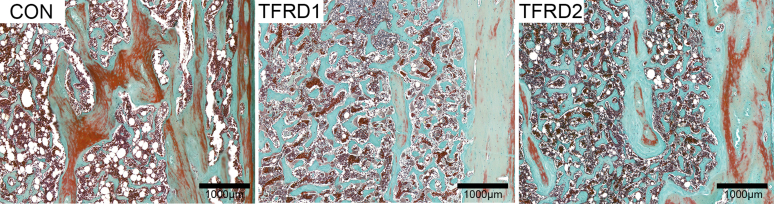
Tibia Histomorphometry
Figure 2 shows that, in the control group, many large absorption cavities appeared on the cortical bone, and the cortical bone area and width were reduced. The trabecular bone became loose or broken with decreased area. When TFRD was supplied, the absorption cavity was reduced, and the trabecular bone structure was more complete. In addition, the static parameters of bone histomorphometry were analyzed (Table 6). Compared with the control group, a significantly higher (P < 0.05) percent trabecular area and trabecular thickness was observed in all TFRD treated groups. Dietary supplemented with 2.0 g/kg TFRD enhanced cortical area ration and cortical width (P < 0.05) than that of control hens. Furthermore, a lower (P < 0.05) trabecular separation was observed with TFRD treatment. Trabecular number among groups did not differ in the study (P > 0.05).
Figure 2.
Effects of dietary TFRD on the microstructure of tibia tissue in caged laying hens. Goldner's trichrome staining (magnification, 10×). Abbreviations: CON, basal diet; TFRD1, basal diet supplemented with 0.5 g/kg TFRD; TFRD2, basal diet supplemented with 2.0 g/kg TFRD; TFRD, total flavonoids from Rhizoma Drynariae.
Table 6.
Effects of dietary TFRD on bone histomorphometry parameters in caged laying hens.
| Items1 | CON | TFRD1 | TFRD2 | SEM | P-value |
|---|---|---|---|---|---|
| Ct.Ar, % | 11.42b | 15.42a,b | 17.82a | 0.90 | 0.010 |
| CW, mm | 0.29b | 0.38a,b | 0.46a | 0.02 | 0.005 |
| Tb.Ar, % | 24.82b | 41.07a | 34.15a | 2.47 | 0.005 |
| Tb.Th, um | 16.27b | 25.58a | 22.93a | 2.11 | 0.014 |
| Tb.Sp, um | 49.33a | 40.61b | 43.21b | 1.15 | 0.001 |
| Tb.N, #/mm | 15.28 | 14.61 | 15.29 | 0.50 | 0.927 |
Means within a row lacking a common superscript differ (P < 0.05), n = 6 per group.
TFRD, total flavonoids of Rhizoma Drynariae; Ct.Ar, cortical area ration; CW, cortical width; Tb.Ar, percent trabecular area; Tb.Th, trabecular thickness; Tb.Sp, trabecular separation; Tb.N, trabecular number; CON, basal diet; TFRD1, basal diet supplemented with 0.5 g/kg TFRD; TFRD2, basal diet supplemented with 2.0 g/kg TFRD.
Serum Biomarkers of Bone Metabolism
Dietary TFRD supplementation decreased the activities of TRACP (P < 0.01) in serum (Table 7). Compared with the control group, supplying 2.0 g/kg TFRD significantly reduced the levels of ALP and OCN (both P < 0.05). No significant difference was observed in serum calcium (P > 0.05).
Table 7.
Effects of dietary TFRD on serum bone metabolism biomarkers in caged laying hens.
| Items1 | CON | TFRD1 | TFRD2 | SEM | P-value |
|---|---|---|---|---|---|
| ALP, U/L | 750a | 677a,b | 589b | 23.4 | 0.009 |
| OCN, ng/mL | 27.80a | 25.04a,b | 20.83b | 0.95 | 0.003 |
| TRACP, U/L | 78.42a | 61.56b | 56.45b | 2.80 | <0.001 |
| Ca, mmol/L | 2.54 | 2.57 | 2.64 | 0.04 | 0.585 |
Means within a row lacking a common superscript differ (P < 0.05), n = 6 per group.
TFRD, total flavonoids of Rhizoma Drynariae; ALP, alkaline phosphatase; OCN, osteocalcin; TRACP, tartrate-resistant acid phosphatase; CON, basal diet; TFRD1, basal diet supplemented with 0.5 g/kg TFRD; TFRD2, basal diet supplemented with 2.0 g/kg TFRD.
Real-Time Quantitative PCR
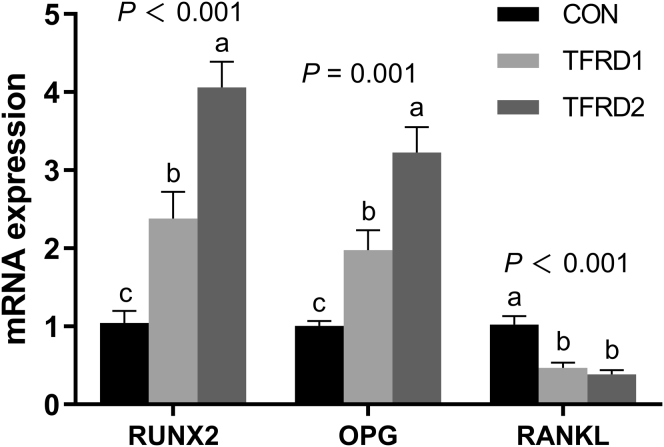
As shown in Figure 3, dietary 0.5 g/kg TFRD supplementation increased the mRNA expression of RUNX2 (P < 0.05) and OPG (P < 0.05) and decreased RANKL (P < 0.01). Compared with the control group, supplying 2.0 g/kg TFRD had a higher mRNA expression of RUNX2 (P < 0.01) and OPG (P < 0.01) and a lower RANKL (P < 0.01).
Figure 3.
Effects of dietary TFRD on RUNX2, OPG, and RANKL mRNA expressions in caged laying hens. Values are means ± SEM (n = 6 per group). The a, b, and c means every bars without same letter differ significantly (P < 0.05). Abbreviations: TFRD, total flavonoids from Rhizoma Drynariae; RUNX2, runt-related transcription factor 2; OPG, TNF receptor super family member 11b; RANKL, tumor necrosis factor super family member 11; CON, basal diet; TFRD1, basal diet supplemented with 0.5 g/kg TFRD; TFRD2, basal diet supplemented with 2.0 g/kg TFRD.
Discussion
At present, there is no research directly indicating the effect of TFRD on the performance and egg quality of laying hens, but some of the previous studies involved the effects of the main active ingredients of TFRD (e.g., naringin and naringenin) on laying hens. In a study, naringenin (0.5 g/kg) for 30-wk-old Leghorn laying hens found that egg production and eggshell quality did not differ significantly among groups (Lien et al., 2008). In other similar study, dietary supplementation of naringin (0.5, 0.75, or 1.5 g/kg) also did not affect performance and eggshell quality (Iskender et al., 2017; Goliomytis et al., 2019). In the present study, consistent with previous results, dietary supplementation of TFRD did not affect laying rate and egg quality. However, TFRD significantly increased average egg weight, which may be related to the estrogen-like effects of TFRD (Wong et al., 2013). It has been proved that estrogen is important in controlling egg weight, and mean plasma estradiol concentrations were very highly correlated with the changes in egg weights (Whitehead et al., 1993).
In addition, the results of serum antioxidant indicators showed that TFRD enhanced the antioxidant capacity of caged laying hens. Recently, there has been a tendency toward using antioxidants, especially from natural sources, for the protection of animal health (Xu et al., 2017). Total flavonoids from Rhizoma Drynariae have received enormous attention because they are plant-derived antioxidants and have the ability to suppress the formation of reactive oxygen species (ROS) (Matkowski et al., 2013). Many studies have indicated that ROS might have a role in osteoporosis development, which involves bone resorption activation and bone formation suppression (Huang et al., 2014). It is worth noting that in addition to TFRD, flavonoids from other sources also have shown a regulatory effect on ROS generation not only through gene expression and cell signaling pathways to activate enzymes that eliminate oxygen radicals but can also sequester potential oxidants (Weaver et al., 2012).
Traditionally, TFRD have long been considered an ideal drug for the treatment of bone-related disease (Song et al., 2016). Osteoporosis in caged laying hens is a common bone metabolic disease which leads to bone fragility, and the incidence rates of fracture up to 30% during the laying period (Whitehead and Fleming, 2000; Johnsson et al., 2015). Our results showed that supplying TFRD in diets increased the femur and tibia BMD in caged laying hens, which can reduce the risk of osteoporosis. Bone mineral density is the gold standard for the osteoporosis diagnosis and is useful in evaluating the risk of fracture (Richards et al., 2008). Previous study demonstrated that treatment with TFRD increased BMD and prevented bone loss in ovariectomized rats, thereby exerting antiosteoporotic effects (Yu et al., 2019). In vivo research also indicated that administration of TFRD significantly increased the BMD and mechanical strength and prevented bone loss induced by hindlimb unloading in rats (Song et al., 2017). Furthermore, clinical practice have proved that TFRD has therapeutic effect on osteoporotic fractures by improving BMD (Zhang et al., 2017).
Through bone histomorphometry observation, it was found that TFRD improved the microstructure of bone tissue, which was manifested in raised cortical area, cortical width, trabecular area, and trabecular thickness. Cortical bone is a structural bone that plays a critical role in bone strength (Seeman and Delmas, 2006). Preservation of the trabecular bone architecture significantly promotes bone strength and may be more important in decreasing fracture risk than improving BMD (Turner, 2002). Therefore, bone microarchitecture determinants are necessary to evaluate the true impact of a treatment on bone quality.
Caged laying hens are in a state of imbalance of bone metabolism during laying period, which ultimately reduces the bone strength of the laying hens and causes fractures (Kim et al., 2012). Some markers of bone metabolism may be conveniently classified either as indicators of bone formation and bone resorption (Christenson, 1997). Markers of bone formation assess either osteoblastic synthetic activity or postrelease metabolism of procollagen (Thiel et al., 2018). Resorption markers reflect osteoclast activity and collagen degradation (Soysa and Alles, 2016). Serum levels of total ALP provide a good identification of the extent of new bone formation and osteoblast activity (Naylor and Eastell, 2012). Osteocalcin, a hydroxyapatite-binding protein, which is considered as a specific marker of osteoblast function and bone metabolism (Chapurlat and Confavreux, 2016). Tartrate-resistant acid phosphatase is a potent enzyme that plays an important role in the bone resorption process (Linder et al., 2017). In our study, dietary TFRD supplementation decreased the levels of serum ALP, OCN, and TRACP, which indicated TFRD could affect osteoblast and osteoclast activities, thereby improving bone metabolism in older caged laying hens.
Runt-related transcription factor 2 is a multifunctional transcription factor that controls skeletal development by regulating the differentiation of osteoblasts and the expression of many extracellular matrix protein genes during osteoblast differentiation (Komori, 2010). Receptor activator of nuclear factor kappa-B ligand promotes the differentiation and activation of osteoclasts and stimulates and maintains their resorption activity (Martin and Sims, 2015). Previous studies revealed that the administration of soluble RANKL results in an increase in the formation and activation of osteoclasts that lead to osteoporosis in mice (Piemontese et al., 2016). On the contrary, OPG is a potent inhibitor of osteoclast formation and acts as a decoy receptor for RANKL (Behera et al., 2018). In vivo experiments showed that OPG knockout mice developed severe osteoporosis (Zhao et al., 2019). Dietary TFRD enhanced the mRNA expressions of RUNX2 and OPG/RANKL in older caged laying hens. It was suggested that TFRD could protect the bone health via regulating osteoblast and osteoclasts activity.
Conclusion
In summary, dietary TFRD supplementation did not affect egg quality, whereas significantly increased the egg weight. Supplying 2.0 g/kg TFRD to diet significantly enhanced serum antioxidant capacity and the BMD of femur and tibia of older caged laying hens. Furthermore, bone tissue microstructure and bone metabolism were improved with TFRD treatment. These findings suggested that TFRD has beneficial effects on bone health in older caged laying hens.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (No. 2017YFD0502200, No. 2016YFD0501210) and Fundamental Research Funds for the Central Universities (No. 2662014BQ023).
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Aguado E., Pascaretti-Grizon F., Goyenvalle E., Audran M., Chappard D. Bone mass and bone quality are altered by hypoactivity in the chicken. PLoS One. 2015;10:e116763. doi: 10.1371/journal.pone.0116763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera J., George A.K., Voor M.J., Tyagi S.C., Tyagi N. Hydrogen sulfide epigenetically mitigates bone loss through OPG/RANKL regulation during hyperhomocysteinemia in mice. Bone. 2018;114:90–108. doi: 10.1016/j.bone.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapurlat R.D., Confavreux C.B. Novel biological markers of bone: from bone metabolism to bone physiology. Rheumatology. 2016;55:1714–1725. doi: 10.1093/rheumatology/kev410. [DOI] [PubMed] [Google Scholar]
- Christenson R.H. Biochemical markers of bone metabolism: an overview. Clin. Biochem. 1997;30:573–593. doi: 10.1016/s0009-9120(97)00113-6. [DOI] [PubMed] [Google Scholar]
- Dale N. National research council nutrient requirements of poultry–ninth revised edition. J. Appl. Poult. Res. 1994;3:101. [Google Scholar]
- Goliomytis M., Simitzis P., Papalexi A., Veneti N., Hager-Theodorides A.L., Charismiadou M.A., Deligeorgis S.G. Influence of citrus flavonoids on laying hen performance, inflammatory immune response, egg quality and yolk oxidative stability. Br. Poult. Sci. 2019;60:1–7. doi: 10.1080/00071668.2019.1587150. [DOI] [PubMed] [Google Scholar]
- Hardin E., Castro F., Kim W.K. Keel bone injury in laying hens: the prevalence of injuries in relation to different housing systems, implications, and potential solutions. World Poult. Sci. J. 2019;75:285–292. [Google Scholar]
- Huang Q., Gao B., Wang L., Hu Y., Lu W., Yang L., Luo Z., Liu J. Protective effects of myricitrin against osteoporosis via reducing reactive oxygen species and bone-resorbing cytokines. Toxicol. Appl. Pharm. 2014;280:550–560. doi: 10.1016/j.taap.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Iskender H., Yenice G., Dokumacioglu E., Kaynar O., Hayirli A., Kaya A. Comparison of the effects of dietary supplementation of flavonoids on laying hen performance, egg quality and egg nutrient profile. Br. Poult. Sci. 2017;58:550–556. doi: 10.1080/00071668.2017.1349297. [DOI] [PubMed] [Google Scholar]
- Jiang S., Wu X.L., Jin M.L., Wang X.Z., Tang Q., Sun Y.X., Cheng H.W. Pathophysiological characteristics and gene transcriptional profiling of bone microstructure in a low calcium diet fed laying hens. Poult. Sci. 2019;98:4359–4368. doi: 10.3382/ps/pez271. [DOI] [PubMed] [Google Scholar]
- Johnsson M., Jonsson K.B., Andersson L., Jensen P., Wright D. Genetic regulation of bone metabolism in the chicken: similarities and differences to mammalian systems. Plos Genet. 2015;11:e1005250. doi: 10.1371/journal.pgen.1005250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W.K., Bloomfield S.A., Sugiyama T., Ricke S.C. Concepts and methods for understanding bone metabolism in laying hens. World Poult. Sci. J. 2012;68:71–82. [Google Scholar]
- Komori T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 2010;339:189–195. doi: 10.1007/s00441-009-0832-8. [DOI] [PubMed] [Google Scholar]
- Lien T., Yeh H., Su W. Effect of adding extracted hesperetin, naringenin and pectin on egg cholesterol, serum traits and antioxidant activity in laying hens. Arch. Anim. Nutr. 2008;62:33–43. doi: 10.1080/17450390701780318. [DOI] [PubMed] [Google Scholar]
- Linder C.H., Ek-Rylander B., Krumpel M., Norgård M., Narisawa S., Millán J.L., Andersson G., Magnusson P. Bone alkaline phosphatase and tartrate-resistant acid phosphatase: potential co-regulators of bone mineralization. Calcified Tissue Int. 2017;101:92–101. doi: 10.1007/s00223-017-0259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Qu W., Liang J. Progress on chemical constituents and biological activities of Drynaria fortunei. Strait Pharm. J. 2012;24:4–7. [Google Scholar]
- Liu X., Zhang S., Lu X., Zheng S., Li F., Xiong Z. Metabonomic study on the anti-osteoporosis effect of Rhizoma Drynariae and its action mechanism using ultra-performance liquid chromatography-tandem mass spectrometry. J. Ethnopharmacol. 2012;139:311–317. doi: 10.1016/j.jep.2011.11.017. [DOI] [PubMed] [Google Scholar]
- Luo J., Zhang Y., Sun H., Wei J., Khalil M.M., Wang Y., Dai J., Zhang N., Qi D., Sun L. The response of glandular gastric transcriptome to T-2 toxin in chicks. Food Chem. Toxicol. 2019;132:110658. doi: 10.1016/j.fct.2019.110658. [DOI] [PubMed] [Google Scholar]
- Martin T.J., Sims N.A. RANKL/OPG; Critical role in bone physiology. Rev. Endocr. Metab. Dis. 2015;16:131–139. doi: 10.1007/s11154-014-9308-6. [DOI] [PubMed] [Google Scholar]
- Matkowski A., Jamiolkowska-Kozlowska W., Nawrot I. Chinese medicinal herbs as source of antioxidant compounds–where tradition meets the future. Curr. Med. Chem. 2013;20:984–1004. [PubMed] [Google Scholar]
- Naylor K., Eastell R. Bone turnover markers: use in osteoporosis. Nat. Rev. Rheumatol. 2012;8:379–389. doi: 10.1038/nrrheum.2012.86. [DOI] [PubMed] [Google Scholar]
- Nie W., Wang B., Gao J., Guo Y., Wang Z. Effects of dietary phosphorous supplementation on laying performance, egg quality, bone health and immune responses of laying hens challenged with Escherichia coli lipopolysaccharide. J. Anim. Sci. Biotechno. 2018;9:53. doi: 10.1186/s40104-018-0271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piemontese M., Xiong J., Fujiwara Y., Thostenson J.D., O'Brien C.A. Cortical bone loss caused by glucocorticoid excess requires RANKL production by osteocytes and is associated with reduced OPG expression in mice. Am. J. Physiol-endoc. M. 2016;311:587–593. doi: 10.1152/ajpendo.00219.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riber A.B., Casey-Trott T.M., Herskin M.S. The influence of keel bone damage on welfare of laying hens. Front. Vet. Sci. 2018;5:6. doi: 10.3389/fvets.2018.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J.B., Rivadeneira F., Inouye M., Pastinen T.M., Soranzo N., Wilson S.G., Andrew T., Falchi M., Gwilliam R., Ahmadi K.R. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. The Lancet. 2008;371:1505–1512. doi: 10.1016/S0140-6736(08)60599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Navarro A.B., McCormack H.M., Fleming R.H., Alvarez-Lloret P., Romero-Pastor J., Dominguez-Gasca N., Prozorov T., Dunn I.C. Influence of physical activity on tibial bone material properties in laying hens. J. Struct. Biol. 2018;201:36–45. doi: 10.1016/j.jsb.2017.10.011. [DOI] [PubMed] [Google Scholar]
- Rufener C., Baur S., Stratmann A., Toscano M.J. Keel bone fractures affect egg laying performance but not egg quality in laying hens housed in a commercial aviary system. Poult. Sci. 2018;98:1589–1600. doi: 10.3382/ps/pey544. [DOI] [PubMed] [Google Scholar]
- Seeman E., Delmas P.D. Bone quality—the material and structural basis of bone strength and fragility. New Engl. J. Med. 2006;354:2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- Song S., Zhai Y., Li C., Yu Q., Lu Y., Zhang Y., Hua W., Wang Z., Shang P. Effects of total flavonoids from Drynariae Rhizoma prevent bone loss in vivo and in vitro. Bone Reports. 2016;5:262–273. doi: 10.1016/j.bonr.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S., Gao Z., Lei X., Niu Y., Zhang Y., Li C., Lu Y., Wang Z., Shang P. Total flavonoids of Drynariae Rhizoma prevent bone loss induced by Hindlimb unloading in rats. Molecules. 2017;22:1033. doi: 10.3390/molecules22071033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soysa N.S., Alles N. Osteoclast function and bone-resorbing activity: an overview. Biochem. Bioph. Res. Co. 2016;476:115–120. doi: 10.1016/j.bbrc.2016.05.019. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Mine T., Ogasa H., Taguchi T., Liang C.T. Expression of RANKL/OPG during bone remodeling in vivo. Biochem. Bioph. Res. Co. 2011;411:690–694. doi: 10.1016/j.bbrc.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Tarlton J.F., Wilkins L.J., Toscano M.J., Avery N.C., Knott L. Reduced bone breakage and increased bone strength in free range laying hens fed omega-3 polyunsaturated fatty acid supplemented diets. Bone. 2013;52:578–586. doi: 10.1016/j.bone.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Thiel A., Reumann M.K., Boskey A., Wischmann J., von Eisenhart-Rothe R., Mayer-Kuckuk P. Osteoblast migration in vertebrate bone. Biol. Rev. 2018;93:350–363. doi: 10.1111/brv.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner C.H. Biomechanics of bone: determinants of skeletal fragility and bone quality. Osteoporos. Int. 2002;13:97–104. doi: 10.1007/s001980200000. [DOI] [PubMed] [Google Scholar]
- Vimalraj S., Arumugam B., Miranda P.J., Selvamurugan N. Runx2: structure, function, and phosphorylation in osteoblast differentiation. Int. J. Biol. Macromol. 2015;78:202–208. doi: 10.1016/j.ijbiomac.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Wang X., Zhen L., Zhang G., Wong M., Qin L., Yao X. Osteogenic effects of flavonoid aglycones from an osteoprotective fraction of Drynaria fortunei—an in vitro efficacy study. Phytomedicine. 2011;18:868–872. doi: 10.1016/j.phymed.2011.01.022. [DOI] [PubMed] [Google Scholar]
- Weaver C.M., Alekel D.L., Ward W.E., Ronis M.J. Flavonoid intake and bone health. J. Nutrition Gerontology Geriatrics. 2012;31:239–253. doi: 10.1080/21551197.2012.698220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead C.C. Overview of bone biology in the egg-laying hen. Poult. Sci. 2004;83:193–199. doi: 10.1093/ps/83.2.193. [DOI] [PubMed] [Google Scholar]
- Whitehead C.C., Bowman A.S., Griffin H.D. Regulation of plasma oestrogen by dietary fats in the laying hen: relationships with egg weight. Br. Poult. Sci. 1993;34:999–1010. doi: 10.1080/00071669308417659. [DOI] [PubMed] [Google Scholar]
- Whitehead C.C., Fleming R.H. Osteoporosis in cage layers. Poult. Sci. 2000;79:1033–1041. doi: 10.1093/ps/79.7.1033. [DOI] [PubMed] [Google Scholar]
- Wong K., Pang W., Wang X., Mok S., Lai W., Chow H., Leung P., Yao X., Wong M. Drynaria fortunei-derived total flavonoid fraction and isolated compounds exert oestrogen-like protective effects in bone. Br. J. Nutr. 2013;110:475–485. doi: 10.1017/S0007114512005405. [DOI] [PubMed] [Google Scholar]
- Xu D., Li Y., Meng X., Zhou T., Zhou Y., Zheng J., Zhang J., Li H. Natural antioxidants in foods and medicinal plants: extraction, assessment and resources. Int. J. Mol. Sci. 2017;18:96. doi: 10.3390/ijms18010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W., Zhang H., Jiang X., Mehmood K., Iqbal M., Li A., Zhang J., Wang Y., Waqas M., Shen Y., Li J. Effect of total flavonoids of Rhizoma drynariae on tibial dyschondroplasia by regulating BMP-2 and Runx2 expression in Chickens. Front. Pharmacol. 2018;9:1251. doi: 10.3389/fphar.2018.01251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Lv Q., Tong Z., Song W., Zhao Z., Yuan B., Liang H. Effect of total flavonoids from Drynaria rhizome on bone loss in ovariectomized rats. Trop. J. Pharm. Res. 2019;18:1285–1289. [Google Scholar]
- Yuan B., Fang Y., Song W., Zhao Z., Liang H. Optimization of extraction of total flavonoids from Drynaria rhizome, and its effect on osteoclast differentiation. Trop. J. Pharm. Res. 2019;18:829–835. [Google Scholar]
- Zhang Y., Jiang J., Shen H., Chai Y., Wei X., Xie Y. Total flavonoids from Rhizoma Drynariae (Gusuibu) for treating osteoporotic fractures: implication in clinical practice. Drug Design, Development Therapy. 2017;11:1881–1890. doi: 10.2147/DDDT.S139804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D., Shu B., Wang C., Zhao Y., Cheng W., Sha N., Li C., Wang Q., Lu S., Wang Y. Oleanolic acid exerts inhibitory effects on the late stage of osteoclastogenesis and prevents bone loss in osteoprotegerin knockout mice. J. Cell. Biochem. 2019;120:1–13. doi: 10.1002/jcb.28994. [DOI] [PubMed] [Google Scholar]