Abstract
Vaccination is an effective method to prevent Newcastle disease (ND) in chickens. Marcol 52 and #10 white oil are mineral-based adjuvants and can be found in commercial inactivated ND virus vaccines. The present study demonstrated that a vegetable origin oil E515-D had lower polycyclic aromatic hydrocarbons and higher flash point than the commercial products Marcol 52 and #10 white oil. E515-D could be mixed with an aqueous phase containing ND virus antigen to form a stable water-in-oil vaccine emulsion and exhibited more potent adjuvant effects on the immune response than Marcol 52 and #10 white oil. Moreover, the absorption of E515-D–adjuvanted vaccine was faster than absorption of Marcol 52- and #10 white oil-adjuvanted vaccines when ND virus vaccines were injected in broilers. Therefore, E515-D was safe and could be a suitable adjuvant used in vaccines for food animals. In addition,E515-D is not easy to be flammable during shipping and storage owing to its higher flash point.
Key words: vegetable oil, ginseng stem–leaf saponin, adjuvant, Newcastle disease vaccine
Introduction
Newcastle disease (ND) is caused by the ND virus (NDV) that is an avian paramyxovirus type I serotype of genus Avulavirus belonging to the Paramyxoviridae family (Hines and Miller, 2012; Orabi et al., 2017). When infected with the NDV, the chickens show clinical signs such as reduction in egg production, respiratory distress, listlessness, central nervous signs, and even death, causing huge amount of economic losses to the poultry industry (Kapczynski et al., 2013). Vaccination is one of the conventional approaches to the control of the disease (Kiril et al., 2017; Ma et al., 2019). To improve the immunization, inactivated NDV vaccine is usually supplemented with adjuvants, and the vaccine is usually made from emulsification of an aqueous phase containing antigen with an adjuvant oil phase. Generally, the adjuvant used for NDV vaccine originates from the mineral oil (Ben Arous et al., 2013; Lone et al., 2017). In China, immunization of NDV vaccine is compulsory for fowl (Yu et al., 2015). In accordance with the statistics, about 10 billion broilers are sold in the Chinese market in 2017 and 10.8 billion in 2018 (Fan et al., 2019; Ruidong Zhai et al., 2020). If each bird receives immunization of NDV vaccine with 0.3 mL for each shot, about 3 million L of NDV vaccine and 2 million L of adjuvant oil (aqueous phase: oil phase = 1:2) are consumed only for NDV vaccination annually. Although the adjuvant oil is made by refining and distilling, it is difficult to remove all the impurities from the oils, which has caused public concern (Kimber and Carrillo, 2016). And, the safety of mineral oil in vaccines is frequently questioned (Stone, 1993; Petermann et al., 2017). For example, polycyclic aromatic hydrocarbons (PAH) left in the oil was reported to be carcinogenic (Bulder et al., 2008; Pirow et al., 2019; Li et al., 2020). The metabolic fate of mineral oil in chickens has been found very slow, and the oil residue has been detected in the muscles, stomach, and other internal organs (Liu et al., 2010; EFSA Panel on Contaminants in the Food Chain (CONTAM), 2012; Liu et al., 2012). These adverse reactions have been even found at 12 wk after injection of mineral oil–adjuvanted NDV vaccine in chickens (Yamanaka et al., 1993).
The stem and leaves of Panax ginseng C.A. Meyer (GSLS) have been reported to display an adjuvant effect on poultry vaccines. Zhai et al. (2011a) showed that oral administration of GSLS could enhance ND vaccine and inactivated avian influenza vaccine (Zhai et al., 2011b), Ma et al. (2019) observed that GSLS and Se synergistically enhanced the immune effect of live bivalent vaccine, and Yu et al. (2015) demonstrated that oral administration of GSLS had capacity in increasing antibody responses of chickens to a bivalent NDV and avian influenza virus vaccine under the oxidative stress condition. In addition, Li et al. (2012) reported that GSLS combined with mineral oil adjuvant could promote the immune responses of NDV vaccine. Besides, compared with the mineral-originated oil, vegetable oil is renewable, edible, and safe. Although previous studies have demonstrated the adjuvant effect of some vegetable oil formulations in mice, swine, and sheep (Zhang et al., 2014, Zhang et al., 2018; Cui et al., 2020), the vegetable oil as an adjuvant in poultry vaccine is barely reported. Our preliminary studies found that a vegetable origin oil E515-D, which comprised sunflower seed oil and ginseng saponins from GSLS, could form a stable water-in-oil (W/O) emulsion (Yuan et al., 2020). In the present study, E515-D, Marcol 52, and #10 white oil were first compared for their flash points and the concentrations of PAH; then, the NDV vaccines adjuvanted with E515-D, Marcol 52, and #10 white oil were characterized for their emulsions and induced immune responses in broilers. In addition, as broilers grow fast with approximately 50 D of growth period (Lee and Leeson, 2001; Broomhead et al., 2002) and fast absorption of the vaccine is important for the consumers, the residues were evaluated at the immunization sites. As Marcol 52 and #10 white oil were commonly used adjuvants for poultry vaccines, they were used for comparisons in this study.
Materials and methods
Animals
One-day-old yellow broilers were purchased from Ningbo Zhenning Animal Husbandry Co., Ltd. (Ningbo, China). Chickens were housed in separate cages and given free access to feed and water. Temperature was controlled at 35°C during the first 3 D and then gradually reduced to 26°C. The animals were acclimatized for 2 wk until the maternal antibody was less than 4 log2. The protocol on handling animals was approved by the Animals Ethics Committee (ZJU20160377) of Zhejiang University.
Antigen and Adjuvants
Inactivated NDV antigen (strain LaSota) was from Zhejiang Ebvac Biotechnology Co., Ltd. (Hangzhou, China); Marcol 52 was made in ExxonMobil, France; #10 white oil was from Sinopec Hangzhou Refinery (Hangzhou, China); E515-D was made from sunflower seed oil (Yihai Kerry Group Co., Ltd., China) and standardized ginseng stem–leaf saponins (GSLS) that contain ginsenosides Re (16.4%), Rd (9.0%), Rg1 (6.0%), Rb2 (3.8%), Rc (3.7%), Rb1 (2.4%), and Rf (0.1%) based on HPLC analysis (Hongjiu Ginseng Industry Co., Ltd., Jilin, China).
Diagnostic Kits
The NDV antibody test kit was the product of IDEXX Laboratories Inc. (Westbrook, MA). Newcastle disease virus antigen and positive control serum for hemagglutination inhibition (HI) test were purchased from Qingdao Yebio Biological Engineering Co., Ltd. (Qingdao, China).
Determination of PAH in Adjuvants
HPLC was used to assay PAH in the adjuvants in accordance with GB 5009.265-2016 (China State Bureau of Standards, 2016). Briefly, dichloromethane and N-hexane were used to extract PAH from the oil adjuvant and activate the solid-phase extraction column of florey silica soil to get the testing sample. Then, an ultra-performance liquid chromatograph–equipped Agilent 1,260 Infinity Fluorescence Detector was used to analyze the sample. The column was Agilent C18 (4.6 mm i.d., 250 mm long, particle diameter 5 μm). The mobile phase was a gradient of degassed water and acetonitrile at a flow rate of 1.5 mL/min. Naphthalene, acenaphthylene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo [a] anthracene, benzo [k] fluoranthene, and benzo [a] pyrene were determined using the fluorescence detector. The excitation/emission wavelengths were 270/324 nm for naphthalene, acenaphthylene, and fluorene; 248/375 nm for phenanthrene and anthracene; 280/462 nm for fluoranthene; 270/385 nm for pyrene and benzo [a] anthracene; and 292/410 nm for benzo [k] fluoranthene and benzo [a] pyrene.
Determination of Flash Point of Adjuvants
A flash point detector SYD-26-I (Shanghai, China) was used to test flash point of E515-D, Marcol 52, and #10 white oil, and the method was in accordance with GB/T 261-2008 (China State Bureau of Standards, 2008). Briefly, adjuvant oil was kept in the sample tank that was heated automatically to a candidate flash point temperature. When the temperature of the tank reached to 90°C, the flame was moved to the sample for every 2°C until there was a flame emerging in the sample. The flash point was recorded when the flame was observed in the sample.
Vaccine Preparation and Identification of Emulsion Type
Newcastle disease virus vaccines were made by emulsifying the aqueous phase with antigen with the oil phase E515-D, Marcol 52, or #10 white oil as previously described by Li et al. (2012). Two methods were used to identify the type of the vaccine emulsion. 1) Dilution method: 1 mL of NDV vaccine emulsion was dropped into a tube containing water or oil. The emulsion of W/O will be dispersed in oil while the emulsion of oil in water (O/W) will be dispersed in water. 2) Staining method: 0.5 mL of NDV vaccine emulsion was dropped on a slide. Then, a drop of eosin Y (water-soluble dye) or Sudan III (liposoluble dye) was added to the surface of emulsion. Eosin Y will be dispersed in O/W emulsion, whereas Sudan III dispersed in W/O emulsion.
Test for Stability of the Vaccine Emulsions
Newcastle disease virus vaccines were emulsified by E515-D, Marcol 52, and #10 white oil. Then, stability of the vaccine emulsions was evaluated by centrifugation method as per the Chinese Veterinary Pharmacopoeia. Briefly, 10 mL of vaccine emulsions samples were centrifuged at 3,000 rpm for 15 min, and emulsions were regarded stable when volume of the aqueous phase at the bottom of the centrifugal tube was less than 0.5 mL after centrifugation.
Test for the Immune Responses of Broilers to NDV Vaccines
One hundred twenty broilers at 14 D of age were randomly divided into 10 groups (n = 12/groups) and received subcutaneous (s.c.) or intramuscular (i.m.) injection with 0.3 mL of NDV antigen in saline or adjuvanted with E515-D, Marcol 52, or #10 white oil. Birds injected with saline solution served as a control. Blood samples were collected at 1, 2, 3, 4, 5, and 6 wk after immunization for testing NDV-specific HI titers. At 6 wk after immunization, blood was collected to test NDV-specific antibody titer and neutralizing antibody titer.
Observation of Residue of NDV Vaccine in the Injection Sites
Fifty-four broilers at 14 D of age were randomly divided into 5 groups. Four groups (n = 12/groups) were i.m. injected with 0.3 mL of a commercial NDV vaccine (Marcol 52 as adjuvant) or the vaccine adjuvanted with E515-D, Marcol 52, or #10 white oil at the left chest muscles. Birds injected with saline (n = 6) served as a control group. Two birds from each immunized group and 1 bird from control group were euthanized once a wk for checking the vaccine residue at the injection sites.
Hemagglutination Inhibition Test
Serum NDV-specific HI titer was measured mainly as described by Zhai et al. (2011a). In brief, serum was diluted in double (from 1:2 to 1:1024) in a 96-well microtiter plates with V-shaped bottom, NDV antigen (25 μl; 4 hemagglutination units) was added to each well. After incubation at 37°C for 30 min, 25 μl of 1% rooster erythrocyte suspension was added and incubated at 37°C for 30 min. Hemagglutination inhibition titer was defined as the reciprocal of the final dilution of serum, which would completely inhibit hemagglutination. Positive and negative serum controls were set on each plate, and all samples were tested in triplicate. Data were presented as log2 of the highest dilution of HI.
ELISA for NDV-specific Antibody Titer
An ELISA kit was used to detect serum NDV-specific antibody titer as previously described by Wang et al. (2013). Briefly, 100 μL of diluted serum sample (1:500) was added to an NDV antigen–coated 96-well plate, and the well was set in duplicate. After incubation at room temperature for 30 min, the plate was washed, and 100 μL of goat anti-chicken antibody-horseradish peroxidase conjugate was added. After 30 min incubation at room temperature, 100 μl of 3,3',5,5'-tetramethylbenzidine solution was added and incubated at room temperature for 15 min. Afterward, 100 μl of stop solution was added to terminate the reaction. The plate was read at 650 nm using an automatic ELISA reader for optical density value. The ratio of sample to positive (S/P) was calculated by (mean of sample optical density-mean of negative control optical density)/(mean of positive control optical density−mean of negative control optical density), and the endpoint titer was estimated by the equation log10 titer = 1.09 (log 10 S/P) +3.36 (Flock check program, IDEXX).
Determination of Neutralizing Antibody Titer
Neutralizing antibody was carried out as previously described by Ma et al. (2019). In brief, serum was diluted in serial four-fold dilution beginning at 1:4 with medium in tubes. Then, 100 TCID50 (50% tissue culture infective dose) of NDV (Strain LaSota) was mixed with an equal volume of the diluted serum samples. After incubation at 37°C for 1 h, the mixtures were added to chicken embryo fibroblasts in 96-well plates. The plates were incubated at 40°C in 5% CO2 for 144 h, and then, the cytopathic effect was recorded and calculated as per the Reed–Muench method. Serum virus neutralizing titers were expressed as mean ± SD.
Statistical Analysis
One-way ANOVA was used to analyze the data by GraphPad Prism 7.0 software (San Diego, CA). P value < 0.05 was considered as statistically significant difference. Data were expressed as mean ± SD.
Results
Emulsion Type and Stability of NDV Vaccines
Newcastle disease virus vaccines adjuvanted with E515-D, Marcol 52, and #10 white oil were the stable white emulsion, and no separation was found after centrifugation at 3,000 rpm for 15 min (Figure 1A). All 3 vaccines were W/O emulsion as they stained with liposoluble dye Sudan III (Figure 1B) but not with water-soluble dye eosin Y (Figure 1C).
Figure 1.
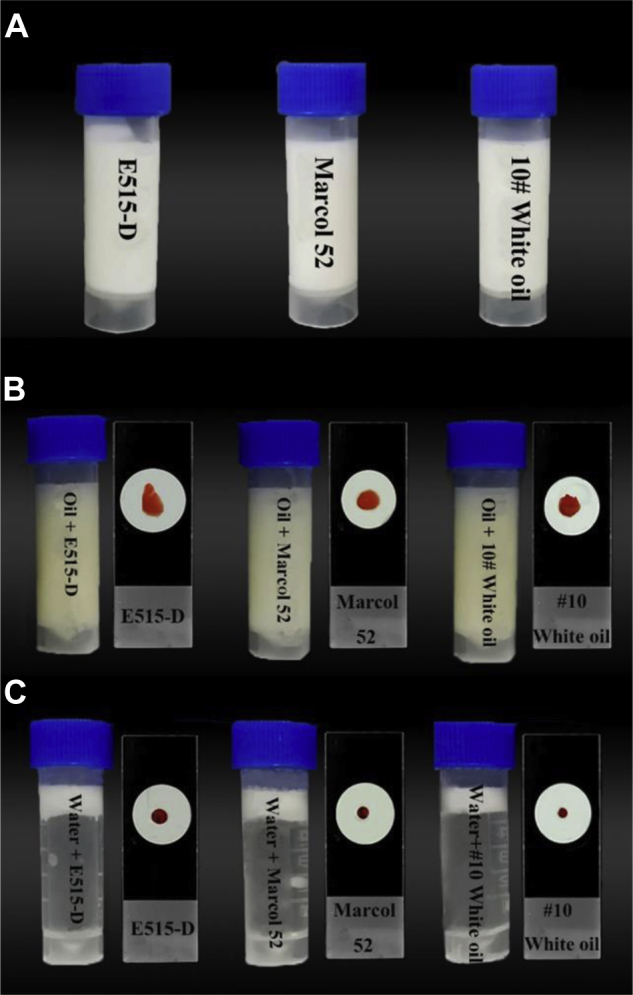
Identification of vaccine emulsion forms. NDV vaccines were emulsified in E515-D, Marcol 52 and #10 white oil (A). The vaccine emulsion was dispersed in vegetable oil or stained with liposoluble dye Sudan III (B) but not dispersed in water or stained with water-soluble dye eosin Y (C), indicating all the vaccine emulsions were water in oil (W/O). Abbreviation: NDV, Newcastle disease virus.
Flash Point of Oil Adjuvants
To investigate the inflammability the adjuvant oils, flash point was measured. Table 1 shows that the flash points from high to low were E515-D (228°C) > Marcol 52 (158°C) and #10 white oil (155°C).
Table 1.
Flash point (°C) of adjuvant oils.
| E515-D | Marcol 52 | #10 white oil | |
|---|---|---|---|
| Flash point | 228 | 158 | 155 |
The flash point was determined by a flash point detector SYD-26-I in accordance with GB/T 261-2008.
Polycyclic Aromatic Hydrocarbons in Oil Adjuvants
Polycyclic aromatic hydrocarbons are a class of organic compounds containing 2 or more benzenes and include naphthalene, acenaphthylene, fluorene, phenanthrene, phenanthrene, fluoranthene, pyrene, benzo [a] anthracene, benzo [k] fluoranthene, and benzo [a] pyrene. Table 2 shows that a total of PAH in E515-D was 50- and 12-fold lower than that in Marcol 52 and #10 white oil, respectively.
Table 2.
Polycyclic aromatic hydrocarbons (PAH) detected in E515-D, Marcol 52, and #10 white oil (μg/kg).
| PAH | E515-D | Marcol 52 | #10 white oil |
|---|---|---|---|
| Naphthalene | 5,090 | 567 | |
| Acenaphthylene | |||
| Fluorene | 12.5 | 64.5 | 32.1 |
| Phenanthrene | 61.4 | 533 | |
| Anthracene | 28 | ||
| Fluoranthene | 10.7 | 46.3 | |
| Pyrene | 14.9 | 20.9 | |
| Benz [a] anthracene | |||
| Benzo [k] fluoranthene | 2.34 | 7.6 | 4.4 |
| Benzo [a] pyrene | |||
| Total | 101.84 | 5,162.1 | 1,231.7 |
The level of PAH was determined by HPLC in accordance with GB 5009.265-2016.
E515-D–Enhanced NDV-specific Antibody Responses
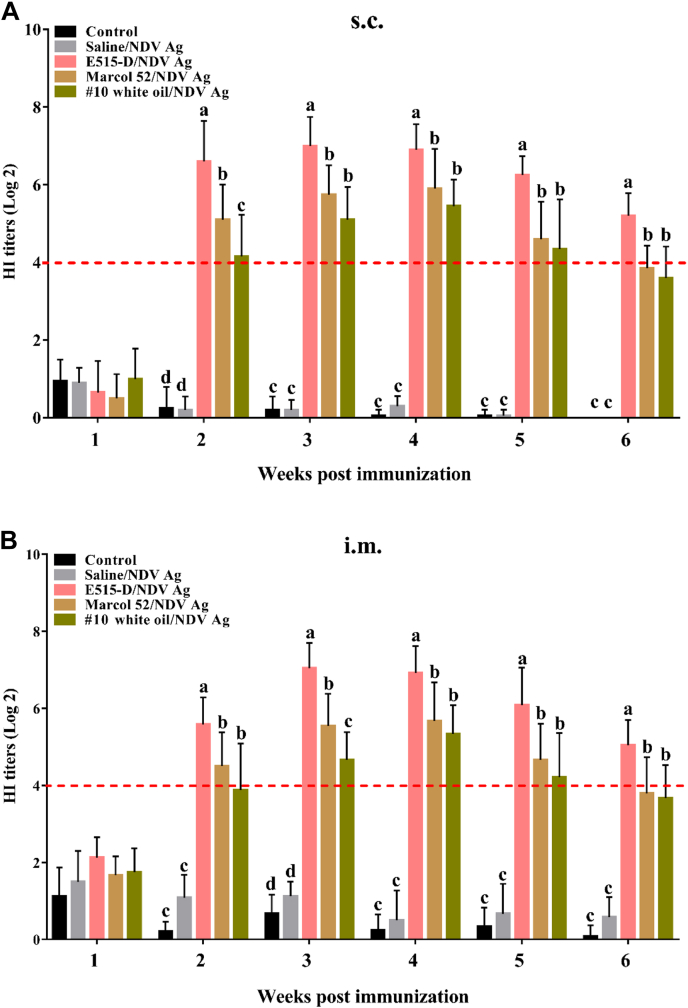
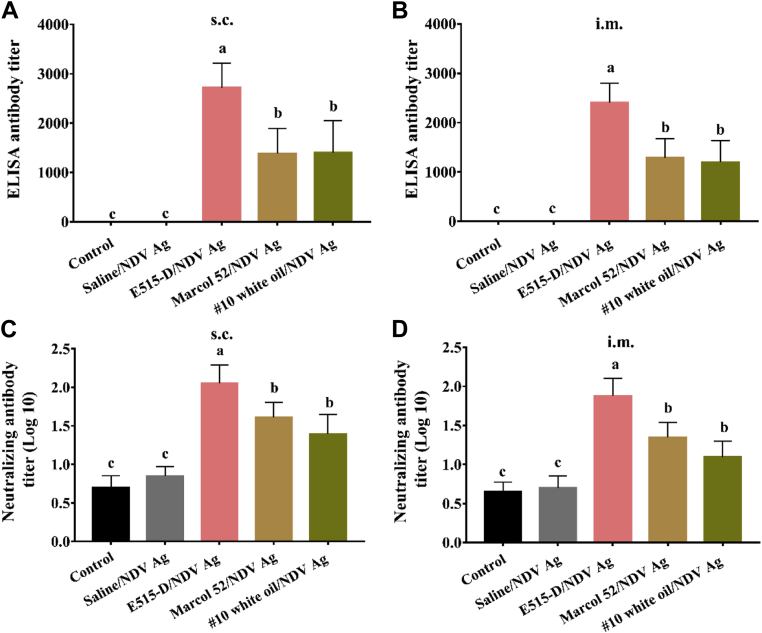
To compare the adjuvant effects of E515-D, Marcol 52, and #10 white oil on the immune responses to NDV vaccines, broilers were s.c. or i.m. immunized with NDV vaccines. Blood samples were collected after immunization to analyze antibody responses. Figure 2 showed that E515-D, Marcol 52, and #10 white oil had an adjuvant effect with the highest serum HI titer detected in the E515-D group and then was in Marcol 52 and #10 white oil groups. At 6 wk after immunization, HI titers in the Marcol 52 (1:14 for s.c.; 1:13 for i.m.) and #10 white oil (1:12 for s.c.; 1:12 for i.m.) groups decreased lower but in the E515-D group (1:37 for s.c.; 1:33 for i.m.) remained higher than 4 log2. Antibody titers measured by ELISA were significantly higher in the E515-D group (2,717 for s.c.; 2,407 for i.m.) than in HI titers in the Marcol 52 (1,384 for s.c.; 1,288 for i.m.) and #10 white oil groups (1,400 for s.c.; 1,198 for i.m.) (Figures 3A and 3B). Importantly, the neutralizing antibody titer (Figures 3C and 3D) in the E515-group (1:113 for s.c.; 1:76 for i.m.) was significantly higher than in the Marcol 52 (1:41 for s.c.; 1:22 for i.m.) and #10 white oil (1:25 for s.c.; 1:13 for i.m.) groups.
Figure 2.
HI titers induced by NDV vaccines. Broilers (n = 12/group) were s.c. (A) or i.m. (B) injected with NDV antigen (Ag) in saline or emulsified in E515-D, Marcol 52, or #10 white oil. Birds injected with saline alone served as a control. Blood samples were collected at 1, 2, 3, 4, 5, and 6 wk after immunization and used for analysis of NDV-specific HI titer. Data are represented as mean ± SD. Bars with different letters are statistically different (P < 0.05). Abbreviations: HI, hemagglutination inhibition; i.m., intramuscularly; NDV, Newcastle disease virus; s.c., subcutaneously.
Figure 3.
NDV-specific antibody and neutralizing antibody induced by NDV vaccines. Broilers (n = 12/group) were s.c. or i.m. immunized with NDV Ag in saline or emulsified in E515-D, Marcol 52, or #10 white oil. Birds injected with saline solution alone served as a control. Blood samples were collected at 6 wk post immunization for analysis of NDV-specific antibody by ELISA (A, B) and neutralizing antibody titers (C, D). Data are represented as mean ± SD. Bars with different letters are statistically different (P < 0.05). Abbreviations: i.m., intramuscularly; NDV, Newcastle disease virus; s.c., subcutaneously.
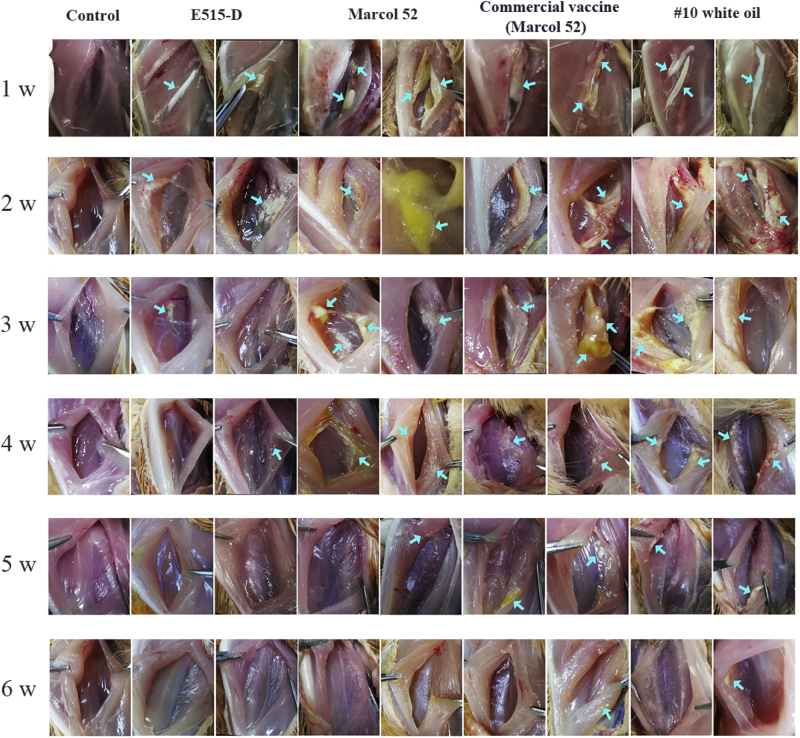
Residues in the Injection Sites after Immunization With NDV Vaccines
To investigate the safety of the oil adjuvant NDV vaccines, the emulsion residue in the injection site was observed. Birds received i.m. injection with different NDV vaccines at the chest. In Figure 4, we observed that the NDV vaccine formulated by E515-D began to be absorbed by bird from 3 wk after the injection. However, bird inoculated with the commercial NDV vaccine and the vaccines adjuvanted with Marcol 52 and #10 white oil showed poor absorption ability until 5 wk after the injection. Moreover, the residue of mineral oil adjuvant Marcol 52 and #10 white oil could induce observed severe inflammatory reaction than the E515-D in the injection site. These observations indicate that the NDV vaccine adjuvanted with E515-D is a safe adjuvant for chickens.
Figure 4.
The residues of different NDV vaccines in the injection sites. Broilers (n = 12/group) were i.m. injected in the left chest with commercial NDV vaccine (emulsified in Marcol 52) or NDV vaccines emulsified with E515-D, Marcol 52, and #10 white oil and broilers (n = 6) were i.m. injected 0.3 mL saline as control. Two birds from each immunized group and 1 bird from control group were randomly euthanized to check the injection sites at 1, 2, 3, 4, 5, and 6 wk after immunization. The places with azure arrows were observed to have the residues of vaccine emulsion. Abbreviations: i.m., intramuscularly; NDV, Newcastle disease virus; s.c., subcutaneously.
Discussion
The present study showed that a vegetable origin oil E515-D had lower PAH (Table 2) and higher flash point (Table 1) than the commercial products Marcol 52 and #10 white oil. E515-D could be mixed with an aqueous phase containing NDV antigen to form a stable W/O vaccine emulsion and exhibited more potent adjuvant effect on the immune response than Marcol 52 and #10 white oil (Figure 1, Figure 2, Figure 3). Moreover, the absorption of E515-D–adjuvanted vaccine was faster than Marcol 52– and #10 white oil–adjuvanted vaccine when NDV vaccines were injected in broilers (Figure 4).
Today, most adjuvants for inactivated poultry vaccines are mineral oil based. Marcol 52 and #10 white oil are the mineral oils found in many commercial poultry vaccines in China. These products contain hydrocarbons including normal, isoalkanes, and cycloalkanes as well as PAH (Kimber and Carrillo, 2016). In 1995, the scientific community on foods evaluated the safety of mineral oil in rats and found that accumulation of hydrocarbons was observed in the liver and lymph nodes associated with a granulomatous response (FAO/WHO, 1995). In 2002, the Joint FAO/WHO Expert Committee on Food Additives found all mineral oil products accumulated in tissues in dose- and time-dependent manners, causing hepatic damage in Fischer rats (FAO/WHO, 2002). Polycyclic aromatic hydrocarbons are formed by the organic compounds that possess 2 or more fused aromatic rings of carbon and hydrogen atoms, and some of them have carcinogenic and mutagenic potentials (Bansal and Kim, 2015). Although the mineral oils used as a vaccine adjuvant are highly refined, the present study found that Marcol 52 and #10 white oil remained to have the concentrations of PAH (5,162.1 and 1,231.7 μg/kg, respectively), which were 50- and 12-fold higher than those of E515-D (101.84 μg/kg), suggesting that E515-D is much safe for food. Flash point is another concern that should be addressed when oil adjuvants are selected for the use in poultry vaccine. The flash point is usually used to measure the tendency of the test specimen to form a flammable mixture with air in response to heat and an ignition source. Higher flash point means it less volatile and safer regarding the storage and transport (Mullin and Champ, 2003; Gulum et al., 2015). The present study showed that E515-D had higher flash point (228°C) than those of Marcol 52 and #10 white oil (158°C and 155°C, respectively), indicating that E515 is not easy to flammable in shipping and storage.
Three types of oil emulsion including O/W, W/O, and water in oil in water can be formed depending on the ratio of oil and aqueous phases and the surfactants used. It was reported that the W/O emulsion showed more potent adjuvant on vaccines against the avian influenza virus, infectious bronchitis virus, or NDV than O/W or water-in-oil-in-water emulsion in chickens (Jansen et al., 2006; Lone et al., 2017). The reason is because W/O emulsion sustainably releases the antigen to stimulate the lymph tissues from the vaccine depot at the injection sites (Spickler and Roth, 2003; Bonam et al., 2017). Figure 1 shows E515-D, Marcol 52, and #10 white oil could develop a stable W/O emulsion when emulsified with an aqueous phase.
Newcastle disease virus–specific antibody immune responses exert a vital role in protecting chickens from NDV infection. Usually, HI assay was conducted to detect levels of specific antibody against hemagglutinin neuraminidase protein (Bello et al., 2018). In Figure 2, vaccine adjuvanted with E515-D induced significantly higher serum NDV-specific HI titers than vaccines formulated by Marcol 52 and #10 white oil did in chickens. Generally, chickens are believed to be susceptible to infections of NDV when the serum HI titer level is less than 4 log2 (Li et al., 2004). Compared with the E515-D group, serum NDV-specific HI titers in both the Marcol 52 and #10 white oil groups were less than 4 log2, whereas the E515-D group had higher HI titers than 4 log2 at 6 wk after immunization. This result means that NDV vaccine emulsified in E515-D could provide higher protection against NDV than that of Marcol 52 and #10 white oil. Several ELISA kits based on whole or part of the virus antigen have been developed for rapid diagnosis of ND (Berinstein et al., 2005; Bello et al., 2018). Many of these kits are commercially available in the form of sandwich, competitive, or indirect ELISA (De Oliveira et al., 2013; Desingu et al., 2014). They are highly sensitive and produce results that pretty well correlate with HI test results. The ELISA kit was used to test antibody titers against all proteins in NDV particle in this study. Figures 3A and 3B shows that the highest antibody titers among all groups were detected by the ELISA method at 6 wk after vaccination. Serum NDV-neutralizing antibody is a marker for efficient protection against NDV replication and complete protection against NDV disease and death (Nayak et al., 2012). As shown in Figures 3C and 3D, E515-D significantly improved the serum NDV-neutralizing antibody response when compared with Marcol 52 and #10 white oil, which paralleled data of HI and antibody. These observations suggested that E515-D could induce potent humoral immune responses against the NDV vaccine.
Absorption of the vaccine at the injection sites is another point that should be considered when immunization is administered in food animals. Domestic animals grow much faster than their ancestors owing to modern genetic and breeding technology. For example, only 49 D are needed for broilers growing up to be consumed (Broomhead et al., 2002). However, Yamanaka et al. found that granulomatous reaction and cyst formulation still occurred in the injection sites of birds at 12 wk after the injection of mineral oil–adjuvanted NDV vaccine (Yamanaka et al., 1993). Similarly, Stone (1997) reported that immunization with mineral oil–adjuvanted vaccines resulted in undesirable tissue reaction that could persist for mo. Similar results were observed in our study when broilers were immunized with vaccines adjuvanted with mineral oils. We found that at least 5 or 6 wk were required for the absorption of vaccine emulsion at the injection sites (Figure 4), whereas only 4 wk were needed when vaccine was adjuvanted with E515-D. Therefore, the vaccine with E515-D was absorbed faster than the vaccine with mineral oil and is suitable to be used in vaccines for food animals.
In conclusion, the present study demonstrated that a vegetable origin oil E515-D had lower PAH and higher flash point than the commercial products Marcol 52 and #10 white oil. E515-D could be mixed with an aqueous phase containing NDV antigen to form a stable W/O vaccine emulsion and exhibited more potent adjuvant effect on the immune response than Marcol 52 and #10 white oil. Moreover, the absorption of E515-D–adjuvanted vaccine was faster than Marcol 52– and #10 white oil–adjuvanted vaccines when NDV vaccines were injected in broilers. Therefore, E515-D was safe and could be a suitable adjuvant used in vaccines for food animals. In addition, E515-D is not easy to be flammable during shipping and storage owing to its higher flash point.
Acknowledgments
This study was supported by the National Key R&D Program of China (project no.: 2017YFD0502200) and the National Natural Science Foundation of China (grant No. 31672598) The authors are thankful to the students in the Laboratory of Traditional Chinese Veterinary Medicine (TCVM Lab) for their assistance in this study.
Conflict of interest: The authors declare no conflict of interest.
References
- Bansal V., Kim K.-H. Review of PAH contamination in food products and their health hazards. Environ. Int. 2015;84:26–38. doi: 10.1016/j.envint.2015.06.016. [DOI] [PubMed] [Google Scholar]
- Bello M.B., Yusoff K., Ideris A., Hair-Bejo M., Peeters B.P.H., Omar A.R. Diagnostic and vaccination approaches for Newcastle disease virus in poultry: the current and emerging perspectives. Biomed Res. Int. 2018;2018:1–18. doi: 10.1155/2018/7278459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Arous J., Deville S., Pal J.K., Baksi S., Bertrand F., Dupuis L. Reduction of Newcastle disease vaccine dose using a novel adjuvant for cellular immune response in poultry. Procedia. Vaccinol. 2013;7:28–33. [Google Scholar]
- Berinstein A., Vazquez-Rovere C., Asurmendi S., Gomez E., Zanetti F., Zabal O., Tozzini A., Grand D.C., Taboga O., Calamante G., Barrios H., Hopp E., Carrillo E. Mucosal and systemic immunization elicited by Newcastle disease virus (NDV) transgenic plants as antigens. Vaccine. 2005;23:5583–5589. doi: 10.1016/j.vaccine.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Bonam S.R., Partidos C.D., Halmuthur S.K.M., Muller S. An overview of novel adjuvants designed for improving vaccine efficacy. Trends Pharmacol. Sci. 2017;38:771–793. doi: 10.1016/j.tips.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Broomhead J.N., Ledoux D.R., Bermudez A.J., Rottinghaus G.E. Chronic effects of Fumonisin B-1 in broilers and turkeys fed dietary treatments to market age. Poult. Sci. 2002;81:56–61. doi: 10.1093/ps/81.1.56. [DOI] [PubMed] [Google Scholar]
- Bulder A.S., Bouwmeester H. Risk assessment of nickel, mineral oils, polycyclic aromatic hydrocarbons and volatile organic compounds in animal feed materials, Report 2007.020. Wageningen University and Research Centre; the Netherlands: 2008. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM) Scientific opinion on mineral oil hydrocarbons in food. EFSA J. 2012;10 2704. [Google Scholar]
- China State Bureau of Standards . China Standards Press; Beijing, China: 2008. National Standard of the People's Republic of China (GB/T 261- 2008): Determination of Flash Point-Pensky-Martens Closed Cup Method. [Google Scholar]
- China State Bureau of Standards . China Standards Press; Beijing, China: 2016. National Standard of the People's Republic of China (GB 5009.265-2016): National Food Safety Standard-Determination of Polycyclic Aromatic Hydrocarbons in Foods. [Google Scholar]
- Cui X., Wang Y., Guan R., Lu M., Yuan L., Xu W., Hu S. Enhanced immune responses with serum proteomic analysis of Hu sheep to foot-and-mouth disease vaccine emulsified in a vegetable oil adjuvant. Vaccines. 2020;8:180. doi: 10.3390/vaccines8020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Oliveira E.S., Silva K.R., Fernando F.S., Mello Goncalves M.C., Fernandes C.C., Borzi M.M., dos Santos R.M., Feres Tamanini M.d.L., da Silva Montassier M.d.F., Montassier H.J. A liquid-phase-blocking concanavalin A enzyme-linked immunosorbent assay for the detection of antibodies against Newcastle disease virus in serum of free-ranging pigeons. J. Vet. Diagn. Invest. 2013;25:720–726. doi: 10.1177/1040638713503656. [DOI] [PubMed] [Google Scholar]
- Desingu P.A., Singh S.D., Dhama K., Kumar O.R.V., Singh R., Singh R.K. Development of slide ELISA (SELISA) for detection of four poultry viral pathogens by direct heat fixation of viruses on glass slides. J. Virol. Methods. 2014;209:76–81. doi: 10.1016/j.jviromet.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Fan W., Tang N., Dong Z., Chen J., Zhang W., Zhao C., He Y., Li M., Wu C., Wei T., Huang T., Mo M., Wei P. Genetic analysis of avian coronavirus infectious bronchitis virus in yellow chickens in Southern China over the past decade: revealing the changes of genetic diversity, dominant genotypes, and selection pressure. Viruses-Basel. 2019;11:898. doi: 10.3390/v11100898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO (Food and Agriculture Organization/World Health Organization) Summary of Evaluations Performed by the Joint FAO/WHO Expert Committee on Food Additives. Mineral Oil. 1995. http://www.inchem.org/documents/jecfa/jeceval/jec_1655.htm Accessed Aug. 2020.
- FAO/WHO (Food and Agriculture Organization/World Health Organization). Evaluation of certain food additives: fifty-ninth report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organization; Geneva, Switzerland: 2002. [PubMed] [Google Scholar]
- Gulum M., Bilgin A. Density, flash point and heating value variations of corn oil biodiesel-diesel fuel blends. Fuel Process. Technol. 2015;134:456–464. [Google Scholar]
- Hines N.L., Miller C.L. Avian paramyxovirus serotype-1: a review of disease distribution, clinical symptoms, and laboratory diagnostics. Vet. Med. Int. 2012;2012:17. doi: 10.1155/2012/708216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen T., Hofmans M.P.M., Theelen M.J.G., Manders F., Schijns V.E.J.C. Structure- and oil type-based efficacy of emulsion adjuvants. Vaccine. 2006;24:5400–5405. doi: 10.1016/j.vaccine.2006.03.074. [DOI] [PubMed] [Google Scholar]
- Kapczynski D.R., Afonso C.L., Miller P.J. Immune responses of poultry to Newcastle disease virus. Dev. Comp. Immunol. 2013;41:447–453. doi: 10.1016/j.dci.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Kimber I., Carrillo J.-C. Oral exposure to mineral oils: is there an association with immune perturbation and autoimmunity? Toxicology. 2016;344:19–25. doi: 10.1016/j.tox.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Kiril M., Dimitrov C.L.A., Yu Q., Miller P.J. Newcastle disease vaccines—a solved problem or a continuous challenge? Vet. Microbiol. 2017;206:126–136. doi: 10.1016/j.vetmic.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.H., Leeson S. Performance of broilers fed limited quantities of feed or nutrients during seven to fourteen days of age. Poult. Sci. 2001;80:446–454. doi: 10.1093/ps/80.4.446. [DOI] [PubMed] [Google Scholar]
- Li H., Jiang T., Li Q., Gu H., Wu T., Qu H., Wang Z. Studies on the relationship of challenge against Newcastle disease and serology (HI) test. Chin. J. Vet. Drug. 2004;8:5–8. [Google Scholar]
- Li S., Yang Y., Lin X., Li Z., Ma G., Su Z., Zhang S. Biocompatible cationic solid lipid nanoparticles as adjuvants effectively improve humoral and T cell immune response of foot and mouth disease vaccines. Vaccin. 2020;38:2478–2486. doi: 10.1016/j.vaccine.2020.02.004. [DOI] [PubMed] [Google Scholar]
- Li Y., Yan X., Hu S. Ginseng stem-leaf saponins and oil adjuvant synergistically promote the immune responses to Newcastle disease in chickens. J. Anim. Vet. Adv. 2012;11:2423–2428. [Google Scholar]
- Liu L., Tang S., Chen N., Liu N. GC-MS investigation of dynamic changes of mineral oil components in broilers. Food Sci. 2010;31:87–91. [Google Scholar]
- Liu L., Tang S., Li Y., Jiang Y. Determination of mineral oil residues in chicken's kidney by GC-MS. J. Food Saf. Qual. 2012;3:185–189. [Google Scholar]
- Lone N.A., Spackman E., Kapczynski D. Immunologic evaluation of 10 different adjuvants for use in vaccines for chickens against highly pathogenic avian influenza virus. Vaccine. 2017;35:3401–3408. doi: 10.1016/j.vaccine.2017.05.010. [DOI] [PubMed] [Google Scholar]
- Ma X., Bi S., Wang Y., Chi X., Hu S. Combined adjuvant effect of ginseng stem-leaf saponins and selenium on immune responses to a live bivalent vaccine of Newcastle disease virus and infectious bronchitis virus in chickens. Poult. Sci. 2019;98:3548–3556. doi: 10.3382/ps/pez207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin J.V., Champ M.A. Introduction/overview to in situ burning of oil spills. Spill Sci. Technology Bull. 2003;8:323–330. [Google Scholar]
- Nayak B., Dias F.M., Kumar S., Paldurai A., Collins P.L., Samal S.K. Avian paramyxovirus serotypes 2-9 (APMV-2-9) vary in the ability to induce protective immunity in chickens against challenge with virulent Newcastle disease virus (APMV-1) Vaccine. 2012;30:2220–2227. doi: 10.1016/j.vaccine.2011.12.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orabi A., Hussein A., Saleh A.A., Abu El-Magd M., Munir M. Evolutionary insights into the fusion protein of Newcastle disease virus isolated from vaccinated chickens in 2016 in Egypt. Arch. Virol. 2017;162:3069–3079. doi: 10.1007/s00705-017-3483-1. [DOI] [PubMed] [Google Scholar]
- Petermann J., Bonnefond R., Mermoud I., Rantoen D., Meynard L., Munro C., Lua L.H.L., Hue T. Evaluation of three adjuvants with respect to both adverse effects and the efficacy of antibody production to the Bm86 protein. Exp. Appl. Acarol. 2017;72:303–315. doi: 10.1007/s10493-017-0156-4. [DOI] [PubMed] [Google Scholar]
- Pirow R., Blume A., Hellwig N., Herzler M., Huhse B., Hutzler C., Pfaff K., Thierse H.-J., Tralau T., Vieth B., Luch A. Mineral oil in food, cosmetic products, and in products regulated by other legislations. Crit. Rev. Toxicol. 2019;49:742–789. doi: 10.1080/10408444.2019.1694862. [DOI] [PubMed] [Google Scholar]
- Ruidong Zhai B.F., Shi X., Sun C., Liu Z., Wang S., Shen Z., Walsh T.R., Chang C., Wang Y., Wu C. Contaminated in-house environment contributes to the persistence and transmission of NDM-producing bacteria in a Chinese poultry farm. Environ. Int. 2020;139:105715. doi: 10.1016/j.envint.2020.105715. [DOI] [PubMed] [Google Scholar]
- Spickler A.R., Roth J.A. Adjuvants in veterinary vaccines: modes of action and adverse effects. J. Vet. Intern. Med. 2003;17:273–281. doi: 10.1111/j.1939-1676.2003.tb02448.x. [DOI] [PubMed] [Google Scholar]
- Stone H.D. Efficacy of experimental animal and vegetable oil-emulsion vaccines for Newcastle-disease and avian influenza. Avian Dis. 1993;37:399–405. [PubMed] [Google Scholar]
- Stone H.D. Newcastle disease oil emulsion vaccines prepared with animal, vegetable, and synthetic oils. Avian Dis. 1997;41:591–597. [PubMed] [Google Scholar]
- Wang M., Meng X., Yang R., Qin T., Li Y., Zhang L., Fei C., Zhen W., Zhang K., Wang X., Hu Y., Xue F. Cordyceps militaris polysaccharides can improve the immune efficacy of Newcastle disease vaccine in chicken. Int. J. Biol. Macromol. 2013;59:178–183. doi: 10.1016/j.ijbiomac.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Yamanaka M., Okabe T., Nakai M., Goto N. Local pathological reactions and immune response of chickens to ISA-70 and other adjuvants containing Newcastle disease virus antigen. Avian Dis. 1993;37:459–466. [PubMed] [Google Scholar]
- Yu J., Shi F.S., Hu S. Improved immune responses to a bivalent vaccine of Newcastle disease and avian influenza in chickens by ginseng stem-leaf saponins. Vet. Immunol. Immunopathol. 2015;167:147–155. doi: 10.1016/j.vetimm.2015.07.017. [DOI] [PubMed] [Google Scholar]
- Yuan L., Wang Y., Ma X., Cui X., Lu M., Guan R., Chi X., Xu W., Hu S. Sunflower seed oil combined with ginseng stem-leaf saponins as an adjuvant to enhance the immune response elicited by Newcastle disease vaccine in chickens. Vaccine. 2020;38:5343–5354. doi: 10.1016/j.vaccine.2020.05.063. [DOI] [PubMed] [Google Scholar]
- Zhai L., Li Y., Wang W., Wang Y., Hu S. Effect of oral administration of ginseng stem-and-leaf saponins (GSLS) on the immune responses to Newcastle disease vaccine in chickens. Vaccine. 2011;29:5007–5014. doi: 10.1016/j.vaccine.2011.04.097. [DOI] [PubMed] [Google Scholar]
- Zhai L., Li Y., Wang W., Wang Y., Hu S. Enhancement of humoral immune responses to inactivated Newcastle disease and avian influenza vaccines by oral administration of ginseng stem-and-leaf saponins in chickens. Poult. Sci. 2011;90:1955–1959. doi: 10.3382/ps.2011-01433. [DOI] [PubMed] [Google Scholar]
- Zhang C., Wang Y., Wang M., Su X., Lu Y., Su F., Hu S. Rapeseed oil and ginseng saponins work synergistically to enhance Th1 and Th2 immune responses induced by the foot-and-mouth disease vaccine. Clin. Vaccin. Immunol. 2014;21:1113–1119. doi: 10.1128/CVI.00127-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Xu W., Chen J., Guan R., Bi S., Jin H., Cui X., Shi F., Hu S. Soybean oil containing ginseng saponins as adjuvants promotes production of cytokines and enhances immune responses to foot-and-mouth disease vaccine. Microbiol. Immunol. 2018;62:187–194. doi: 10.1111/1348-0421.12567. [DOI] [PubMed] [Google Scholar]