Abstract
Two meat-type broiler strains, strain A and strain B, were reared in floor pens (25 birds/pen; 45 pens/strain) for pectoralis (P) major collagen and mixed muscle protein turnover (PT) study from 0–56 D using primary breeder nutrition and husbandry guidelines. Forty broilers (n = 10/strain for collagen PT; n = 10/strain for mixed muscle PT) were selected at each sampling age at day 21, 28, 35, 42, and 56 and infused with 1-13C proline (Pro) and 15N-phenylalanine (Phe) which are used as amino acid tracers for collagen and mixed muscle PT measurements, respectively. Muscle and plasma samples were collected, and enrichments of 1-13C Pro and 15N-Phe were determined using mass spectrometry. Fractional synthesis rate (FSR) and fractional degradation rate (FDR) were measured for collagen and mixed muscle using precursor-product principle. At day 42, after separating the sampled broilers as myopathy (woody breast [WB] score > 1) and nonmyopathy (WB = 0), plasma metabolites were screened for differential 3-methyhistidine (3-MH) expression for both strains. Data were analyzed using one-way ANOVA using t test. Results showed that collagen and mixed muscle FSR and FDR in pectoralis major decreased (P < 0.05) for both strains as the broilers aged. FSR for collagen and FDR for mixed muscle were higher for strain B than those for strain A (P < 0.05). Total collagen was higher (P < 0.05) for strain B. Differentially expressed 3-MH in plasma was higher (P < 0.05) for myopathy-affected broilers indicating greater muscle degradation occurring in myopathy-affected broiler types for both strains. 3-MH Expression in plasma was higher for strain B than for strain A. The research findings showing an increased collagen content per unit muscle weight in pectoralis major in strain B (than in strain A) could be due to higher mixed muscle FDR and increased collagen FSR occurring during the grow-out period. The increased degradation of muscle fibers and probable replacement of muscle-specific protein with connective tissue, mainly collagen, was an evident pathophysiological phenomenon occurring in myopathy-affected broilers.
Key words: broiler strain, collagen, mixed muscle, synthesis, degradation, myopathy
Introduction
Selection for quantitative traits such as breast yield and growth rate exerts physiological pressure leading to antemortem histological and biochemical alterations in muscle tissues in modern broiler strains. The industry has recently witnessed a myopathy condition affecting pectoralis major (breast muscle) of broilers called woody breast (WB), an issue subject to both economic losses and broiler welfare (Kuttappan et al., 2016; Thaxton et al., 2016). A recent study reported WB myopathy incidence of ∼9% for 10,483 fillets evaluated in high breast meat yielding strain from a flock which produced larger broilers (2.72–4.53 kg) (Wold et al., 2017). The WB myopathy condition is mainly characterized by macroscopic stiffness in breast tissue affecting the appearance and protein quality (Petracci et al., 2012; Kuttappan et al., 2016). Histologically, it exhibits moderate to severe polyphasic myodegeneration with variable degrees of interstitial connective tissue accretion or fibrosis (Sihvo et al., 2014; Maharjan et al., 2020a).
Understanding possible biological mechanisms and pathways associated with the onset and progression of WB myopathy can be crucial to minimizing the incidence of muscle myopathy. The exact etiology for WB myopathy is still unclear to scientific community. Current research reports have shown micrographic evidence of excessive accumulation of intramuscular collagen occurring in breast muscle; therefore, collagen could be considered the key protein involved in WB myopathy condition (Petracci et al., 2012; Kuttappan et al., 2017; Maharjan et al., 2020a). Collagen is an extracellular connective tissue matrix (ECM) in muscle fibers that forms collagen fibrils, which then crosslink to give stiffness to muscle tissues (Gelse et al., 2003). The quantitative intramuscular collagen content, collagen arrangement types, and extent of cross-linking between collagen fibrils can vary between broiler lines (Vellemen et al., 2015) which in turn could determine the degree of WB myopathy condition. In this study, collagen turnover in pectoralis major of 2 high-yielding meat-type broiler lines were studied for collagen protein turnover during their grow-out cycle.
The modern broiler strains consume less feed per unit of body weight (BW) gain; however, their needs for dietary amino acids have increased compared to previous years (Dozier et al., 2007). Formulating diets with higher amino acids is intended for optimizing breast yield as amino acids play a critical role in muscle development (Bolster et al., 2004). It is shown that the availability of amino acids can be a crucial factor to stimulate skeletal muscle protein synthesis in animals (Lundhold and Schersten, 1975; Wolfe, 2002). However, the physiological threshold or maximal anabolic state for PT of skeletal muscle does exist beyond which higher dietary intake of protein for higher rate of protein synthesis is halted (Muramatsu et al., 1963; Symons et al., 2009; Maharjan et al., 2020a). Furthermore, there are almost no existing research available in literature that distincts on how normal vs. myopathy-affected broilers are turning over skeletal muscle when fed primary breeder nutrition during their grow-out period. The objectives of present study were to investigate the mixed muscle PT changes occurring in pectoralis major in broiler lines with typical corn-soy diets that fulfilled the recommended amino acid requirement, while also differentiating PT changes occurring under WB pathological conditions. The findings of mixed muscle protein turnover changes in the present study may complement the collagen PT dynamics (Maharjan et al., 2020a) as collagen protein could be replacing the space generated by degenerating myofiber in pectoralis major. To the best of authors' knowledge, the present study is the first investigation to simultaneously evaluate in vivo collagen and mixed muscle PT changes in pectoralis major during grow-out. Understanding mixed muscle and collagen turnover changes occurring in pectoralis major in broiler lines during the grow-out period can be advantageous in formulating strain-specific dietary regimes aimed at modulating biosynthesis of protein types that will ultimately help curtail incidence of WB myopathy.
Methods
Broiler Type and Husbandry and Sampling
Two meat-type broiler strains, strain A (fast-growing) and strain B (high-yielding), were reared in 90 floor pens (25 broilers/pen; 45 pens/strain) from 0–56 D using recommended primary breeder nutrition and husbandry guidelines under approved Institutional Animal Care and Use Committee protocol #17080. Table 1 gives the dietary formulations used for the study for all feeding phases. Broilers were randomly selected from 14 pens (7 pens/strain) each at 21, 28, 35, 42, and 56 D to determine collagen PT and mixed muscle PT with total of 70 pens used for PT study during the trial period. Labeled amino acids, 1-13C proline and 15N-phenylalanine, were used as metabolic tracers for measuring collagen and mixed muscle PT, respectively. Forty broilers were used at each sampling age: 20 broilers for collagen PT study (10 broilers/strain) and 20 broilers for mixed PT study (10 broilers/strain). All broilers sampled that were used in the PT study in each sampling age were subjected to WB scoring (Tijare et al., 2017); the cumulative WB score is given in Table 2. An additional 5 broilers (n = 5) per strain were selected at each sampling age and scanned for body composition using dual energy x-ray absorptiometry equipped with Lunar Prodigy small animal software to determine protein composition for each sampling age (Caldas et al., 2018) (Figure 1). All broilers selected for study were within 1 SD of average pen mean weight for that age group. The remaining 20 pens (10 pens/strain) were used for performance study (Table 3).
Table 1.
Test diets used in the experiment.1
| Ingredients | Starter |
Grower |
Finisher I |
Finisher II |
|---|---|---|---|---|
| 1–10 D |
11–20 D |
21–42 D |
43–56 D |
|
| % | % | % | % | |
| Yellow corn 7.4% | 53.78 | 62 | 63.25 | 65.66 |
| Soybean meal, 44% | 35.5 | 27.07 | 27.49 | 23.76 |
| Proplus | 6.06 | 5.69 | 2.26 | 3.7 |
| Poultry fat | 2.78 | 2.66 | 4 | 4 |
| Dicalcium phosphate | 0.63 | 0.61 | 1.78 | 0.83 |
| Limestone | 0.25 | |||
| Salt | 0.38 | 0.46 | 0.35 | 0.43 |
| Methionine 98.5% | 0.31 | 0.3 | 0.26 | 0.24 |
| Lysine | 0.11 | 0.2 | 0.13 | 0.16 |
| Choline chloride-60 | 0.15 | 0.15 | 0.15 | 0.15 |
| Vitamin premix | 0.1 | 0.1 | 0.1 | 0.1 |
| Mineral premix | 0.1 | 0.1 | 0.1 | 0.1 |
| Threonine 98% | 0.01 | 0.05 | 0.04 | 0.04 |
| Selenium premix 0.06% | 0.02 | 0.02 | 0.02 | 0.02 |
| Monsanto santoquin ethoxyquin | 0.02 | 0.02 | 0.02 | 0.02 |
| Mold curb | 0.05 | 0.05 | 0.05 | 0.05 |
| Calculated values | ||||
| Metabolisable energy (kcal/kg) | 3,035 | 3,108 | 3,180 | 3,215 |
| Digestible arginine | 1.49 | 1.23 | 1.15 | 1.08 |
| Digestible lysine | 1.22 | 1.08 | 0.98 | 0.93 |
| Digestible methionine | 0.61 | 0.55 | 0.5 | 0.48 |
| Digestible methionine and cystine | 0.91 | 0.82 | 0.76 | 0.72 |
| Digestible leucine | 1.75 | 1.53 | 1.47 | 1.4 |
| Digestible isoleucine | 0.89 | 0.73 | 0.7 | 0.65 |
| Digestible threonine | 0.79 | 0.71 | 0.67 | 0.63 |
| Digestible valine | 0.98 | 0.83 | 0.78 | 0.74 |
| Analyzed composition | ||||
| Crude protein, % | 24.3 | 20.97 | 18.63 | 18.45 |
| Calcium, % | 0.79 | 0.66 | 0.68 | 0.55 |
| Total phosphorus, % | 0.71 | 0.61 | 0.55 | 0.53 |
Supplies per kilogram of diet: antioxidant, 200 mg; retinyl acetate, 21 mg; cholecalciferol, 110 μg; D-α-tocopherol acetate, 132 mg; menadione, 6 mg; riboflavin, 15.6 mg; D-calcium pantothenate, 23.8 mg; niacin, 92.6 mg; folic acid, 7.1 mg; cyanocobalamin, 0.032 mg; pyridoxine, 22 mg; biotin, 0.66 mg; thiamine, 3.7 mg; choline chlorine, 1,200 mg; Mn, 100 mg; Mg, 27 mg; Zn, 100 mg; Fe, 50 mg; Cu, 10 mg; I, 1 mg; Se, 200 μg.
Table 2.
Cumulative woody breast (WB) scores for broilers used (n = 20 each strain) in a protein turnover (PT) study at each sampling age.
| Collagen PT |
Mixed muscle PT |
|||
|---|---|---|---|---|
| Strain A | Strain B | Strain A | Strain B | |
| day 21 | 8.5 | 7.5 | 7 | 8.5 |
| day 28 | 10.5 | 14 | 7 | 9.5 |
| day 35 | 14.5 | 27 | 15.5 | 28 |
| day 42 | 9 | 23.5 | 13.5 | 27.5 |
| day 56 | 27.5 | 34.5 | 25.5 | 28.5 |
Each broiler was scored for WB score of 0–3, and then all the scores were added to obtain cumulative score.
Figure 1.
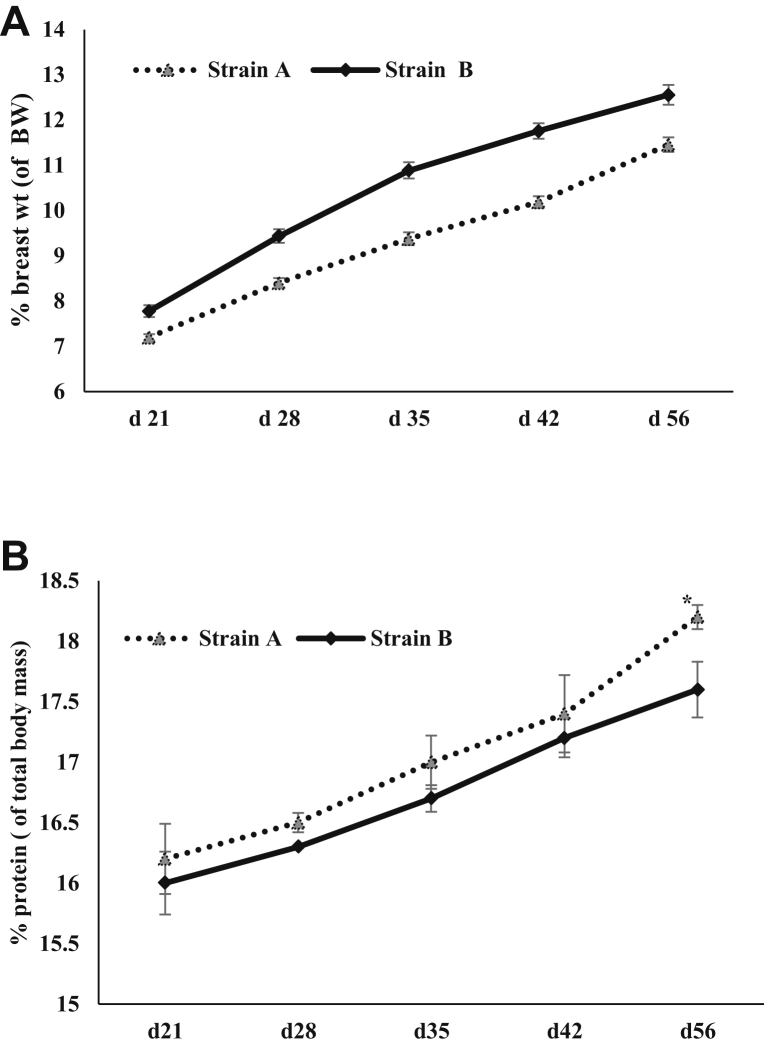
(A) Percent pectoralis major weight (hot-deboned) of total body weight (BW) for strain A and strain B (P < 0.05 for all ages) (n = 25/strain). (B) Percent protein in pectoralis major of total body mass for strain A and strain B as given by dual energy x-ray absorptiometry measurements (n = 5/strain) (P < 0.05 at day 56).
Table 3.
Body weight and FCR of 2 broiler strains studied for collagen and mixed muscle protein turnover at various feeding phases.
| Strain | FCR day 0-10 | BW day 10 | FCR day 10-20 | BW day 20 | FCR day 20-41 | BW day 41 | FCR day 42-56 | BW day 56 |
|---|---|---|---|---|---|---|---|---|
| Strain A | 1.41 | 227.54 | 1.46 | 892.77 | 1.77 | 2,977.73 | 2.08 | 4,392 |
| Strain B | 1.43 | 234.71 | 1.45 | 859.99 | 1.75 | 2,904.39 | 2.05 | 4,426 |
| SD | 0.04 | 24 | 0.07 | 77 | 0.046 | 99 | 0.18 | 91 |
No difference in BW or FCR was observed between these strains (P > 0.05).
Abbreviation: FCR, feed conversion ratio.
Measurement of Collagen Protein Turnover
Isotope Preparation and Infusion
L-proline-1-13C (Sigma Aldrich, 81,202-06-4) isotopic solution at 20 atomic percent excess (APE) was prepared fresh for each sampling age. Selected broilers (n = 10 each strain; total 20 broilers each age group) were infused with the solution through brachial vein at the rate of 10 mL/kg BW with 1-min interval (flooding dose). Noninfused broilers were treated as control to know the baseline enrichment.
Blood and Tissue Sample Collection
After infusion of an isotopic solution, arterialized venous blood was collected (∼1 mL) at 3 different occasions (20-min intervals), using brachial vein, and broilers were then sacrificed to collect breast tissue samples (∼150-gm wet tissue, cranio-ventral portion). Collected breast samples were immediately frozen in liquid nitrogen and stored at −80°C until analysis. Plasma was separated from the blood and stored similarly at −80°C until analysis.
Blood and breast tissue samples from the control broiler were similarly collected for all sampling ages to understand the baseline enrichment of 1-13C proline.
Determination of 13C-proline Enrichment in Tissue and Plasma
Collagen protein fractions (insoluble [I] and soluble [S]) were initially separated from collected breast muscle tissue. Briefly, tissue samples were lyophilized, ground to powder, and added to homogenization buffer with protease and phosphatase inhibitor (0.15 M NaCl, 0.1% Triton X-100, 0.02 M Tris-HCl buffer, 5 mM EDTA, pH = 7.4). The tissue solution was incubated for 3 h. The solution was then sequentially centrifuged (1,600 × g, 20 min, and 4°C) to separate out collagen fraction. The collagen protein fraction was subjected to overnight incubation with 0.1% pepsin and 0.5-mol acetic acid. The solution was then centrifuged to precipitate pellet (I-collagen fraction). The supernatant fraction that had S-collagen was precipitated after centrifugation, using salt (0.9-mol NaCl). The protein fractions (I- and S-collagen) were acid hydrolyzed (6-M HCl) for 24 h at 110°C to release protein-bound amino acids and then purified using the Dowex 50W-X8 H+ (Alfa Aesar, MA) ion-exchange resin. Purified amino acids (including proline) were derivatized with N-methyl-N-tert-butyldimethylsilyltrifluoroacetamide (Sigma, CAS Number 77377-52-7) to tert-butyldimethylsilyl (t-BDMS) compounds to get tBDMS-proline. Derivatized samples were subjected to gas chromatography-mass spectrometer (GC MS) (Agilent Tech, 7890A GC system, 5975C VL MSD, Santa Clara, CA) analysis, and the mass spectrum (ion fragmentation pattern) of tBDMS-proline was obtained.
Free amino acids including proline from plasma samples were isolated, and proline was derivatized to tBDMS-proline before the mass spectra from GC MS were generated. Selected ion monitoring was performed for mass to charge ratio (m/z) of 73, 147, 184, 258, and 286 (El-Harake et al., 1998). Enrichment (E), which is the isotopic 13C-to-12C ratio, was measured using the ratio of fragments 287 to 286. Enrichment was then adjusted to APE values.
Determination of Fractional Synthesis Rate and Fractional Degradation Rate
Insoluble (I) collagen and soluble (S) collagen protein fractional synthesis rates (FSRs) and fractional degradation rates (FDRs) were measured as described elsewhere (Babraj et al., 2005; Mittendorfer et al., 2005). FSR was determined using traditional precursor-product principal (Hansen et al., 2008), which is given by
where “ΔEp” is the difference in enrichment of 1-13C proline measured in terms of APE between infused and control (basal) tissue samples. Therefore,
where Ei = enrichment of tissue or plasma sample in infused birds; Ec = enrichment (baseline) of tissue or plasma in control birds.
A is the average enrichment of tracer (1-13C proline) determined as the area under the time-plasma enrichment curve, and t is the time (in hr) of tracer (1-13C proline) incorporation in product.
Growth rate (FGR) for collagen tissue, for S-collagen and I-collagen, was initially measured for measuring FDR by quantifying collagen using hydroxyproline assay (HP) assay (explained in methods discussed in the following sections). The FGR is expressed as
FGR = ([weight of protein fraction (W2) at time t1 – weight of protein fraction (W1) at time t2]/[weight of protein fraction (W2) at time t1]) ∗ 100.
Then, the FDR was measured using the following equation:
For calculating predictive FSR values, WB scores and the corresponding FSR values were cumulated for each replicate broiler, and then the FSR values were obtained for each sampling age after the WB scores were normalized to WB score value of 1. The linear positive relationship was applied for obtaining predictive FSR values for WB scores of 2 and 3 (Maharjan et al., 2020c).
Tissue Collagen Quantitation
Broilers used for dual energy x-ray absorptiometry scanning (n = 5/strain for each age group) were also used for taking breast tissue samples for collagen quantitation. For the collected breast tissue samples, collagen in pectoralis major was quantified using HP assay (Woessner, 1961). Briefly, separated S-collagen and I-collagen fractions were lyophilized, acid hydrolyzed for 24 h using 6-mol HCl (5 mg/mL) at 115°C, and then neutralized with base. The sample solution was mixed with buffered chloramine T reagent (5 mL of chloramine T solution, then diluted with 7.5 mL of n-propanol and 12.5 mL of acetate citrate buffer). Ehrlich's reagent (2 gm of 4-dimethylamino benzaldehyde [Sigma Aldrich product #156477] in 3 mL of 60% (v/v) perchloric acid mixed with 13 mL of n-propanol) was added to the solution mixture, and the solution was incubated at 60°C for 20 min. After incubation, absorbance of the solution was measured at 550 nm to determine the HP concentration. Standard curves of trans-4-hydroxy-L proline (Sigma Aldrich Product #H54409) were produced to find the unknown concentration of HP in sample solutions.
Measurement of Mixed Muscle Protein Turnover
Isotope Preparation and Infusion
Isotopic solution of 15N-phenylalanine (150 mM, 40% APE) was prepared and given to broilers (n = 10 each strain; total 20 broilers each strain) as an intravenous flooding-dose for PT determination (Garlick et al., 1989; Vignale et al., 2016). The dose was administered at 10 mL per kg of BW.
Tissue and Digesta Sample Collection and Processing
Ten min after infusion, broilers were euthanized by CO2 inhalation and immediately sampled for pectoralis major tissue samples. Illeal digesta (from Meckel's diverticulum to ileocecal junction) sample was also taken from each infused bird. The digesta and breast muscle samples were immediately frozen in liquid nitrogen for PT determination. Protein turnover was determined by measuring 15N-phenylalanine enrichment in breast muscle and 3-methylhistidine (3-MH) quantitation in muscle and excreta using same GC-MS for collagen PT study. Breast and digesta samples were also analyzed in GC MS from noninfused broiler (n = 1) to understand baseline enrichment for 15N-phenylalanine.
Before the tissue or digesta samples were run in GC-MS, sample preparations were carried out (Garlick et al., 1989). Briefly, the acid-soluble fraction containing free amino acid was removed by addition of 2% (w/v) perchloric acid. This was achieved by first homogenizing breast muscle tissue with the acid and then centrifuging at 3,000 × g. The supernatant containing free amino acid was removed. The protein precipitate was washed 3 times with 2% perchloric acid before it was hydrolyzed in 6N HCl. The supernatant and precipitate, respectively, were then eluted through an ion-exchange column packed with Dowex 50WX8-200. Phenylalanine and 3-MH were eluted with 2 mL of 4N NH4OH and 1 mL of nanopure H2O into a new vial and dried under vacuum. Then the tBDMS derivative was formed by the addition of 600 μL of C2CH3CN-MTBSTFA (1:1) and incubated at 110°C for 120 min. Ileal digesta was processed without the removal of the acid-soluble fraction. Three-methylhistidine was determined on the same mass spectrometer. Ion fragments were selectively monitored for mass-to-charge (m/z) ratio of 73, 91, 147, 234, and 302. The ratio of 302 and 303 m/z, representing the M, and M + 1 fragments of phenylalanine, respectively, were used to measure the 15N-phenylalanine enrichment. The 238-m/z fragment of 3-MH was monitored for selected ion monitoring carried out for m/z ratio of 73, 96, 147, 210, 238, and 302.
Determination of FSR and FDR
Precursor-product principle was used to calculate FSR as given elsewhere (Danicke et al., 2001; Ekmay et al., 2013), which is given by the following equation:
APEb = 15N atom percent excess (relative to natural abundance) of phenylalanine in the protein; APEf = 15N atom percent excess of free phenylalanine in tissues, assumed as the precursor pool; t = time [D].
Degradation rate was measured using the following expression:
In addition, broilers sampled from day 42 and 56 were pooled and separated as myopathy (WB score ≥ 1–3) and nonmyopathy broilers (WB score = 0) groups, and the weighted average for the FSR and FDR values was calculated to differentiate existing differences in FSR or FDR between the 2 myopathy treatment groups for both strains.
Differential Expression of 3-MH in Plasma Samples
Blood samples were taken at day 42 after separating the sampled broilers as myopathy (WB score > 1) and nonmyopathy (WB = 0) (n = 5 each group for each strain) groups. Plasma was separated from collected blood samples, and samples were analyzed for differential 3-MH expression. Targeted metabolomics of 3-MH was performed on a triple quadrupole MS coupled to an I-class UPLC system (Waters) for differential expression. Procedures for handling plasma samples in a laboratory, data processing, and bioinformatics were carried out as described in the article by Maharjan et al., 2020c.
Data Analysis
The data obtained were analyzed by one-way ANOVA using JMPro 14 (SAS Institute, Inc., Cary, NC). Mean values were obtained for variables measured (collagen and mixed muscle FSR and FDR, collagen content) for different sampling ages for both strains. Significant means for the measured variables were separated using Student t test or HSD test where appropriate. Means were considered significant for P ≤ 0.05. FSR or FDR values over sampling ages for collagen protein were subjected for regression analysis (linear or polynomial fit) of data.
Results
Collagen Protein FSR and FDR
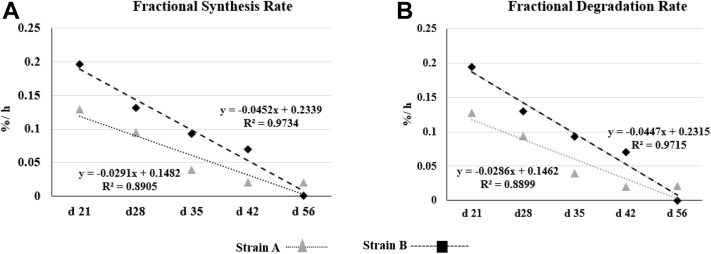
The FSR and FDR for I-collagen at different sampling ages for strain A and strain B were given in Figure 2. The FSR and FDR for I-collagen decreased for both strains as the broiler aged (Figures 2A, 2B). Strain B had higher FSR and FDR values throughout until day 42. The FSR and FDR values at day 21 for strain B was 0.196%/h and 0.194%/h, whereas it was 0.129%/h and 0.127%/h for strain A. The FSR and FDR values decreased to almost undetectable range on %/h basis by day 56 for both the strains. The decrease in FSR and FDR with age was greater for strain B than for strain A as indicated by higher negative slope values for strain B. A similar trend for FSR and FDR was observed for S-collagen, except lower FSR or FDR values were observed at each sampling age compared to I-collagen. The FSR values for line A and line B for S-collagen were 0.13%/h and 0.144%/h, respectively, and then dropped with age. Undetectable FSR or FDR values on %/h basis for S-2collagen after day 35 were observed for both the lines.
Figure 2.
(A) Fractional synthesis rates (FSR) for I-collagen in pectoralis major for strain A and strain B over sampling ages. (B) Fractional degradation rate (FDR) for I-collagen in pectoralis major for strain A and strain B over sampling ages. Higher FSR or FDR for strain A than for strain B over day 21–42. Dashed line indicates the linear fit for each strain for FSR or FDR.
The FSR values were higher than corresponding FDR values for each sampling age indicating that the collagen protein was accreting over the grow-out period. FSR and FDR both tend to be undetectable on %/h basis for both the strains at a later age.
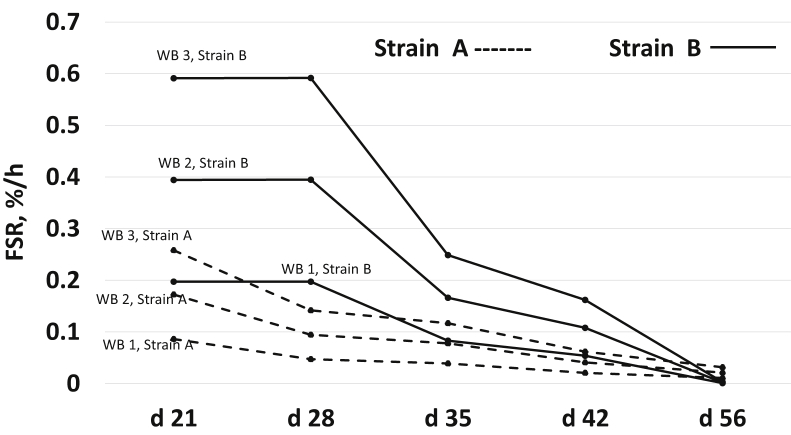
Figure 3 gives the predictive FSR values for collagen protein for both strains with their WB scores. Higher WB scores had higher FSR values for both strains. Higher predictive FSR values were reported for strain B than for strain A until day 42, followed by higher values for strain A than for strain B at day 56.
Figure 3.
Predictive fractional synthesis rate (FSR) values in pectoralis major for strain A and strain B over sampling ages for I-collagen based on woody breast (WB) scores. WB 1, WB 2, and WB 3 represent woody breast score of 1, 2, and 3, respectively, for strain A and strain B.
Collagen Quantitation
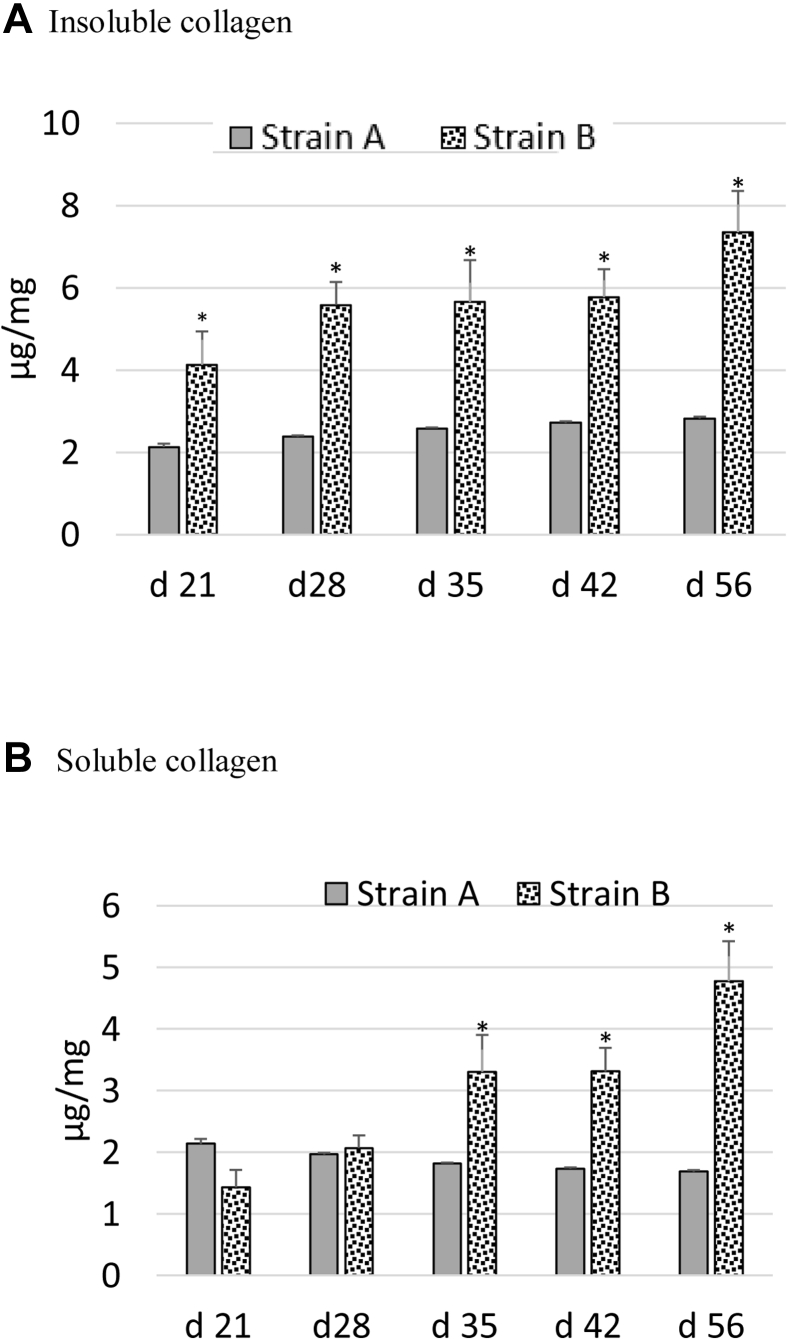
Figure 4 gives the amount of collagen in the form of I-collagen and S-collagen fractions (dry matter basis) at different ages of broiler grow-out cycle for both strains. I-collagen increased linearly (R2 = 0.84, slope = 0.66) from 4.13 μg/mg at day 21 to 7.35 μg/mg at day 56 for strain B, whereas it was 2.12 μg/mg at day 21 to 2.82 (R2 = 0.97, slope = 0.17) for strain A. S-collagen was higher for strain B from day 28 to 56. The I-collagen to S-collagen ratio was slightly higher than 1 for strain A for all ages, whereas it was ≥2 throughout sampling ages for strain B.
Figure 4.
(A) Insoluble (I)-collagen and (B) soluble (S)-collagen content (DM basis) in pectoralis major for strain A and strain B. Strain B had higher (P < 0.05) I-collagen and I-collagen:S-collagen ratio than strain A. Asterisks on the top of bars indicate significantly different mean values.
Mixed Muscle FSR and FDR
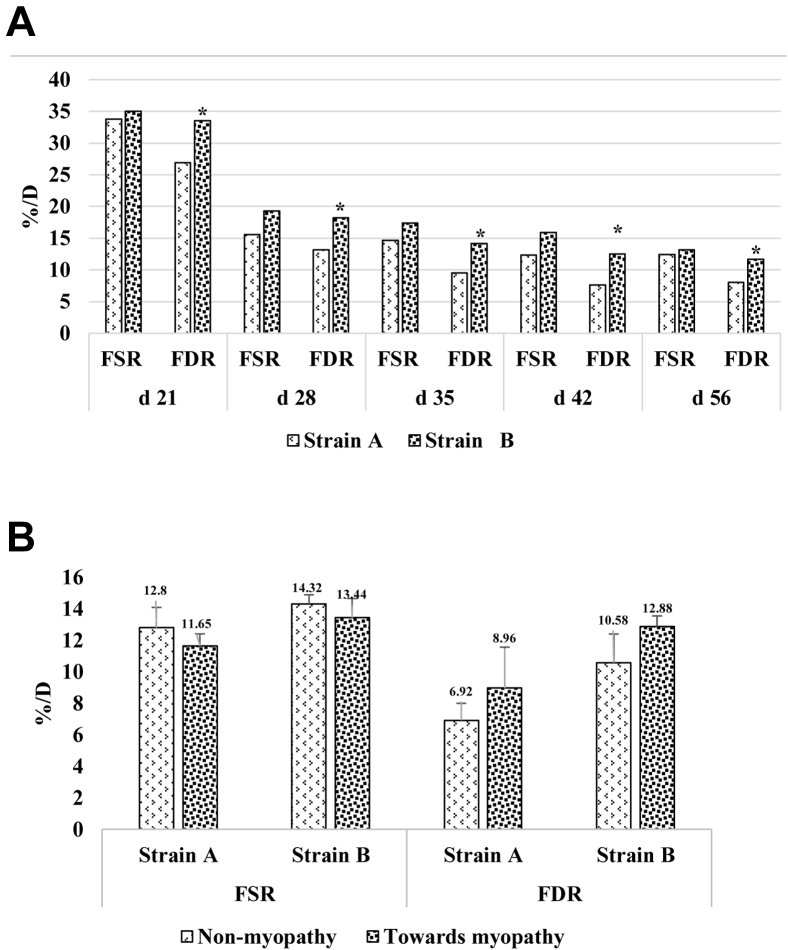
Figure 5 gives the mixed muscle synthesis rates for strain A and strain B for various ages of grow-out cycle. Mixed muscle FSR and FDR in pectoralis major dropped (P < 0.05) for both the strains as the broiler aged. FSR for strain A was 33.75%/D, whereas it was 35.03%/D for strain B at day 21 and gradually decreased over sampling age to 8.01%/D and 11.66%/D by day 56, respectively. Strain B had higher turnover of mixed muscle as exhibited by higher FSR and FDR occurring through the sampled ages than strain A. The FDR values for strain B were relatively higher in relation to corresponding FSR values at each sampling age compared to strain A.
Figure 5.
(A) Mixed muscle fractional synthesis rate (FSR) and mixed muscle fractional degradation rate (FDR) in pectoralis major for strain A and strain B. FSR or FDR decreased over age (P < 0.05) for both the strains. Higher FDR was observed for strain B than for strain A (P < 0.05). (B) FSR and FDR values for nonmyopathy (woody breast [WB] score = 0) and myopathy (WB score ≥ 1-3) for pooled sampled for day 42 and day 56 for strain A and strain B (P > 0.05). Asterisks on the top of bars indicate significantly different mean values.
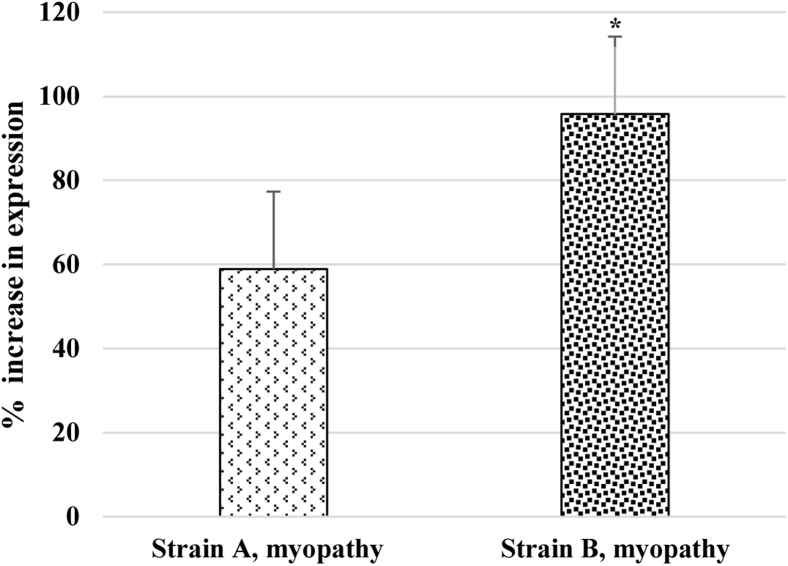
Figure 5B gives the average FSR and FDR values for nonmyopathy and myopathy broilers for strain A and strain B. For both strains, there was a reduced FSR value and higher FDR value observed for myopathy broilers than for the broilers in the nonmyopathy group. The higher protein degradation of mixed muscle occurring in the myopathy group was also exhibited by higher expression of 3-MH marker (P < 0.05) in plasma samples for the myopathy group (Figure 6).
Figure 6.
Percent increase in differential expression of 3-methyhistidine in plasma of myopathy-affected broilers (P < 0.05) in relation to nonmyopathy broilers for strain A and stain B. Between strain, there was difference (P < 0.05) in 3-methylhistidine expression. Asterisk on the top of bar indicates significantly different mean values.
Discussion
Collagen is a fibrous protein that provides strength and structure to muscle tissue. Collagen is synthesized intracellularly and is released outside of cell to become a component of ECM in tissue. The stable isotope method that was used in the present study to understand in vivo collagen PT changes occurring in pectoralis major muscle is novel for different broiler genotypes. Maharjan et al. (2020a) conducted an exploratory study for understanding in vivo collagen synthesis in pectoralis major in a broiler line. The earlier study by Maharjan et al. (2020a) used a higher dietary amino acid level (120% of recommended) even though significant differences in the collagen turnover results were not observed compared with the present study. The present study also considered broiler mixed muscle PT changes while measuring collagen PT occurring with the same flock and compared PT changes in 2 different broiler strains. The FSR or FDR values at each sampled ages for I-collagen as observed by Maharjan et al. (2020a) were similar to results in strain B for collagen in this study, except undetectable FSR and FDR values were recorded at day 56, which could be due to dietary amino acid level differences (higher in the reports of Maharjan et al., 2020a) between the studies. The day 21 FSR value for I-collagen synthesis (∼0.196%/h for strain B and 0.129%/h for strain A) found in this study was comparable to collagen synthesis rates in skeletal tissue in 1-month-old rats (Mays et al., 1991). Collagen synthesis in human skeletal muscle in an elderly man was found to be 0.023 ± 0.002%/h (Babraj et al., 2005). The FSR values obtained in humans were similar to collagen FSR values in pectoralis major muscle of a mature broiler (>5 wk) as reported in this present study.
Changes in collagen degradation can play a major role in regulating collagen mass in any tissue. The FSR and FDR values of I-collagen in pectoralis major were higher in younger broilers and decreased over the age of broilers for both strains. The differences in FSR and FDR values at each age were positive indicating accretion of I-collagen occurred in the maturing broiler. Higher turnover of collagen protein was occurring in strain B; thus, it was synthesizing more collagen than strain A. Although there was increased accretion of I-collagen for strain B, there was also a larger linear drop in FSR and FDR for strain B (as indicated by the larger negative slope [Figure 2]) that reached nondetectable levels (%/h basis) by day 56. Reduced synthesis and turnover of collagen at a later age of the grow-out period could be associated with bird's adaptive response against further synthesis than needed for physiological sustainability (Kjaer, 2004; Waterlow, 2006).
Increased I-collagen pool size with increasing age of the broiler potentially indicated higher availability of substrate (collagen fibrils) for crosslinking resulting in the toughness of muscles (Coro et al., 2002). Results depicted that amounts of total collagen were higher for strain B than those for strain A in pectoralis major throughout sampling ages indicating that strain B was more vulnerable to myopathy (Table 2; Figure 4). The increased collagen content in maturing broilers could be associated with myodegeneration and increased mixed muscle FDR (in relation to FSR) as observed in the present study (Figure 5) and subsequent replacement of muscle specific protein with collagenous tissue (Sihvo et al., 2014). The increment of collagen content in the maturing broiler was also observed in a past report showing a relative increase in nonfiber space per unit area of muscle tissue over the age of the broiler (Maharjan et al., 2020a).
Earlier studies that measured FSR in mixed muscle in pectoralis major in broilers over age found progressive and significant decrease in FSR values (22.4 to 12.5%/D) (Tesseraud et al., 1996; Urdaneta-Rincon and Leeson, 2004; Maharjan et al., 2020b). A decreasing mixed muscle FSR with increasing age of the broiler was also found in present study for both strains. Interestingly, the FSR (%/D) in modern broilers as observed in the present study was higher than that of broilers reared in the past decades (Jones et al., 1986; Tesseraud et al., 1996; Pym et al., 2004; Maharjan et al., 2020b). The higher synthesis rates in current lines of broilers were accompanied by higher degradation rates as well. This indicates that modern broiler strains are turning over mixed muscle protein in pectoralis major more rapidly than earlier genetic lines of broilers. The increased FSR and FDR for current broiler lines could be attributed to progress made in continuous genetic selection along with optimal nutritional programs. Both strains evaluated in the present study were considered meat strains with strain B being a high-yielding strain as exhibited by breast yield percent of live BW (hot-deboned) (Figure 1A). Strain B also had an overall higher FDR values over all sampling ages, which could be a probable reason that strain B possessed less protein % of total body mass (Figure 1B). Decreased protein synthesis and increased degradation is a physiological phenomenon that may contribute to muscle protein loss, whereas the proteolytic pathways (ubiquitin-proteasome, lysosomal, and calpain) may be more evident in enhanced degradation of muscle tissue (Khalil et al., 2018; McCarthy and Murach, 2019; Maharjan et al., 2020b). Furthermore, both strains had higher FDR values for myopathy-affected broilers than for nonmyopathy broilers, which was also validated by the relatively higher expression of 3-MH in plasma samples in myopathy-affected broilers for both strains (Maharjan et al., 2020c). Strain B had greater expression of 3-MH when compared between strains indicating higher mixed muscle degradation occurring than in strain A. 3-MH Is considered a unique biomarker of myofibrillar protein breakdown, and its appearance level in blood or urine is indicative of the degree of breakdown occurring in muscle tissue (Williamson et al., 1977; Tipton et al., 2018). Furthermore, Maharjan et al. (2020c) recently reported that myopathy-affected broilers had a differential expression of plasma metabolites that showed potential involvement of vascularity issues causing the poor arterial blood circulation in muscle tissue leading to localized hypoxia and increased muscle degradation and myodegeneration. Similarly, the relative gene expression of muscle-specific E3 ubiquitin ligases in broilers such as Muscle RING finger 1 and muscle atrophy F-box/atrogin-1 was found to be increased during myopathy condition (Vignale et al., 2016). The higher mixed muscle degradation occurring in myopathy-affected broilers could be one potential factor leading to fibrosis and replacement of muscle-specific protein with connective (mainly with collagen) tissue in strain B (P < 0.05) (Figure 1B). Consequently, strain B myopathy-affected broilers had a higher intramuscular collagen content per mm2 of tissue that was formed far beyond the physiological need.
Intervention strategies for reducing the myopathy incidence could be by either reducing muscle degradation or stimulating protein synthesis. The metabolic response of skeletal tissue growth and protein synthesis mediated by mechanistic target of rapamycin (mTOR) pathways can be affected by hormone or glycoprotein (follistatin) stimulation (Winbanks et al., 2012; Schiaffino et al., 2013). One strategy for increased stimulation of muscle PT synthesis would be by increasing balanced protein or amino acids in the diets that induce protein synthesis as the amino acid availability does act as upstream signal for mTOR kinase (Dillon et al., 2009). An effector of mTOR, eukaryotic initiation factor 4E, is freed after phosphorylation of phosphorylated heat-acid stabled protein, which accelerates translation initiation and stimulates protein synthesis. The reduction in excess deposition of cross-linked collagen in extracellular matrix could be a mechanistic target and another approach to intervene and control the WB myopathy issue. The presence of collagen within physiological limits may be essential for providing structural support and prevent the occurrence of other myopathies such as spaghetti muscle (Baldi et al., 2018; Huang and Ahn, 2018). This can be achieved by either reducing intracellular synthesis or increasing intracellular degradation of collagen peptides especially at an earlier age during broiler grow-out because more collagen synthesis is occurring at that time. Intracellular degradation of collagen peptides can be targeted by decreasing the expression or production of prolyl or lysyl hydroxylases as these enzymes are involved in posttranslational modifications of polypeptide chain and providing ultimate stability to procollagen. Another approach for reducing WB myopathy would be enhancing the extracellular degradation of collagen through ECM proteinases after collagen fibrils are exocytosed. The ECM proteinases are mainly matrix metalloproteinases (MMPs); upon their activation starts the proteolytic degradative pathways (Riso et al., 2016). Furthermore, MMPs are also thought to have regulatory roles on muscle growth and repair. Collagenases of MMPs family are MMP-1, MMP-8, MMP-13, and MMP-18; these collagenases are involved in cleaving native helical structure of collagen types I, II, and III. As discussed earlier, the stabilization of collagen fibrils is provided by covalent cross-links, which are formed by lateral interaction of 2 helices by conversion of lysine residues to aldehyde derivatives. The formation of aldol cross-links in the aldehyde derivatives is mediated by lysyl oxidase (Lodisth et al., 2000) which may be another target biomolecule to help control the pectoralis major WB myopathy issue.
Overall, both strains studied showed vulnerability to WB myopathy, with strain B showing relatively higher vulnerability to myopathy incidence. This could be due to higher collagen FSR occurring during early grow-out period leading to increased accumulation of collagen per unit of tissue weight. Further research on modulating the biosynthesis or degradation of collagen, mainly I-collagen in pectoralis major in maturing broilers, can be aimed to reduce substrate availability for collagen cross-linking. Findings suggest the excess accumulation of collagen in pectoralis major may be caused by higher mixed muscle degradation; therefore, dietary strategies that stimulate muscle synthesis or reduce degradation rates would be another approach to reduce the incidence of the prevailing myopathy in meat broiler strains.
Acknowledgements
The authors acknowledge Cobb Vantress, Inc., for their generous support by funding this project. Authors would also like to thank II-Young Kim and Rick H. Williams from Wolfe laboratory, Isotope Tracers in Metabolic Research, University of Arkansas for Medical Sciences, for answering queries related to methods on collagen protein turnover study.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Babraj J.A., Smith K., Cuthbertson D.J., Rickhuss P., Dorling J.S., Rennie M.J. Human bone collagen synthesis is a rapid, nutritionally modulated process. J. Bone. Min. Res. 2005;20:930–937. doi: 10.1359/JBMR.050201. [DOI] [PubMed] [Google Scholar]
- Baldi G., Soglia F., Mazzoni M., Sirri F., Canonico L., Babini E., Laghi L., Cavani C., Petracci M. Implications of white striping and spaghetti meat abnormalities on meat quality and histological features in broilers. Animal. 2018;12:164–173. doi: 10.1017/S1751731117001069. [DOI] [PubMed] [Google Scholar]
- Bolster D.R., Jefferson L.S., Kimball S.R. Regulation of protein synthesis associated with skeletal muscle hypertrophy by insulin-, amino acid-and exercise-induced signalling. Proc. Nutr. Soc. 2004;63:351–356. doi: 10.1079/PNS2004355. [DOI] [PubMed] [Google Scholar]
- Caldas J.V., Hilton K., Boonsinchai N., England J.A., Mauromoustakos A., Coon C.N. Dynamics of nutrient utilization, heat production, and body composition in broiler breeder hens during egg production. Poult. Sci. 2018;97:2845–2853. doi: 10.3382/ps/pey133. [DOI] [PubMed] [Google Scholar]
- Coro F.A., Youssef E.Y., Shimokomaki M. Age related changes in poultry breast meat collagen pyridinoline and texture. J. Food Biochem. 2002;26:533–541. [Google Scholar]
- Dänicke S., Böttcher W., Simon O., Jeroch H. The measurement of muscle protein synthesis in broilers with a flooding dose technique: Use of 15N-labelled phenylalanine, GC-MS and GC-C-IRMS. Isotopes Environ. Health Stud. 2001;37:213–225. doi: 10.1080/10256010108033297. [DOI] [PubMed] [Google Scholar]
- Dillon E.L., Sheffield-Moore M., Paddon-Jones D., Gilkison C., Sanford A.P., Casperson S.L., Jiang J., Chinkes D.L., Urban R.J. Amino acid supplementation increases lean body mass, basal muscle protein synthesis, and insulin-like growth factor-I expression in older women. J. Clin. Endocrinol. Metabol. 2009;94:1630–1637. doi: 10.1210/jc.2008-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier W., III, Corzo A., Kidd M., Branton S. Dietary apparent metabolizable energy and amino acid density effects on growth and carcass traits of heavy broilers. J. Appl. Poult. Res. 2007;16:192–205. [Google Scholar]
- El-Harake W.A., Furman M.A., Cook B., Nair K.S., Kukowski J., Brodsky I.G. Measurement of dermal collagen synthesis rate in vivo in humans. Am. J. Physiol-endocrinol. Metabol. 1998;274:586–591. doi: 10.1152/ajpendo.1998.274.4.E586. [DOI] [PubMed] [Google Scholar]
- Ekmay R.D., Salas C., England J., Cerrate S., Coon C.N. The effects of age, energy and protein intake on protein turnover and the expression of proteolysis-related genes in the broiler breeder hen. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 2013;164:38–43. doi: 10.1016/j.cbpb.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Garlick P.J., Wernerman J., McNurlan M.A., Essen P., Lobley G.E., Milne E., Calder G.A., Vinnars E. Measurement of the rate of protein synthesis in muscle of postabsorptive young men by injection of a 'flooding dose' of [1-13C] leucine. Clin. Sci. (Lond) 1989;77:329–336. doi: 10.1042/cs0770329. [DOI] [PubMed] [Google Scholar]
- Gelse K., Pöschl E., Aigner T. Collagens—structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Hansen M., Miller B.F., Holm L., Doessing S., Petersen S.G., Skovgaard D., Frystyk J., Flyvbjerg A., Koskinen S., Pingel J. Effect of administration of oral contraceptives in vivo on collagen synthesis in tendon and muscle connective tissue in young women. J. Appl. Physiol. 2009;106:1435–1443. doi: 10.1152/japplphysiol.90933.2008. [DOI] [PubMed] [Google Scholar]
- Huang X., Ahn D.U. The incidence of muscle abnormalities in broiler breast meat–A review. Korean Journal Food Science Animal Resources. 2018;38:835–850. doi: 10.5851/kosfa.2018.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S., Aberle E., Judge M. Skeletal muscle protein turnover in broiler and layer chicks. J. Anim. Sci. 1986;62:1576–1583. doi: 10.2527/jas1986.6261576x. [DOI] [PubMed] [Google Scholar]
- Khalil R.M., Abdo W.S., Saad A., Khedr E.G. Muscle proteolytic system modulation through the effect of taurine on mice bearing muscular atrophy. Mol. Cell. Biochem. 2018;444:161–168. doi: 10.1007/s11010-017-3240-5. [DOI] [PubMed] [Google Scholar]
- Kjær M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol. Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- Kuttappan V., Hargis B., Owens C. White striping and woody breast myopathies in the modern poultry industry: a review. Poult. Sci. 2016;95:2724–2733. doi: 10.3382/ps/pew216. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Bottje W., Ramnathan R., Hartson S.D., Coon C.N., Kong B., Owens C.M., Vazquez-Añon M., Hargis B.M. Proteomic analysis reveals changes in carbohydrate and protein metabolism associated with broiler breast myopathy. Poult. Sci. 2017;96:2992–2999. doi: 10.3382/ps/pex069. [DOI] [PubMed] [Google Scholar]
- Lodish H., Berk A., Zipursky S., Matsudaira P., Baltimore D., Darnell J. 2000. Collagen: the fibrous proteins of the matrix. In Molecular Cell Biology. 4th ed. W. H. Freeman, New York, NY. [Google Scholar]
- Lundholm K., Schersten T. Determination in vitro of the rate of protein synthesis and degradation in human-skeletal-muscle tissue. Eur. Journal Biochemistry. 1975;60:181–186. doi: 10.1111/j.1432-1033.1975.tb20990.x. [DOI] [PubMed] [Google Scholar]
- Maharjan P., Owens C.M., Coon C. In-vivo intramuscular collagen synthesis, muscle fiber growth and Histomorphology of pectoralis major of a fast-growing broiler strain Gallus gallus domesticus. Front. Vet. Sci. 2020;6:1–11. doi: 10.3389/fvets.2019.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharjan P., Mullenix G., Hilton K., Beitia A., Weil J., England J., Martinex D., Umberson C., Caldas J., Haro V.D.N., Coon C. Effects of dietary amino acid levels and ambient temperature on mixed muscle protein turnover in Pectoralis major during finisher feeding period in two broiler lines. J. Anim. Physiol. Anim. Nutr. 2020 doi: 10.1111/jpn.13363. In press. [DOI] [PubMed] [Google Scholar]
- Maharjan P., Hilton K., Weil J., Suesuttajit N., Beitia A., Owens C., Coon C. Characterizing woody breast myopathy in a meat broiler line by heat production, Microbiota, and plasma metabolites. Front.Vet. Sci. 2020;6:497. doi: 10.3389/fvets.2019.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays P.K., McAnulty R.J., Campa J.S., Laurent G.J. Age-related changes in collagen synthesis and degradation in rat tissues. Importance of degradation of newly synthesized collagen in regulating collagen production. Biochem. J. 1991;276:307–313. doi: 10.1042/bj2760307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J.J., Murach K.A. Nutrition and Enhanced Sports Performance. Academic Press; Cambridge, MA: 2019. Anabolic and Catabolic signaling pathways that regulate skeletal muscle mass; pp. 275–290. [Google Scholar]
- Mittendorfer B., Andersen J., Plomgaard P., Saltin B., Babraj J., Smith K., Rennie M. Protein synthesis rates in human muscles: neither anatomical location nor fibre-type composition are major determinants. J. Physiol. (Lond.) 2005;563:203–211. doi: 10.1113/jphysiol.2004.077180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu K., Sato T., Ashida K. Dietary protein level and the turnover rate of tissue proteins in rats. J. Nutr. 1963;81:427–433. doi: 10.1093/jn/81.4.427. [DOI] [PubMed] [Google Scholar]
- Petracci M., Cavani C. Muscle growth and poultry meat quality issues. Nutrients. 2012;4:1–12. doi: 10.3390/nu4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pym R., Leclercq B., Tomas F., Tesseraud S. Protein utilisation and turnover in lines of chickens selected for different aspects of body composition. Br. Poult. Sci. 2004;45:775–786. doi: 10.1080/00071660400012774. [DOI] [PubMed] [Google Scholar]
- Riso E.M., Kaasik P., Seene T. Composition and function of the extracellular matrix in the human body. Tech; Rijeka: 2016. Remodelling of skeletal muscle extracellular matrix: effect of unloading and reloading; pp. 45–68. [Google Scholar]
- Schiaffino S., Dyar K.A., Ciciliot S., Blaauw B., Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS Journal. 2013;280:4294–4314. doi: 10.1111/febs.12253. [DOI] [PubMed] [Google Scholar]
- Sihvo H., Immonen K., Puolanne E. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet. Pathol. 2014;51:619–623. doi: 10.1177/0300985813497488. [DOI] [PubMed] [Google Scholar]
- Symons T.B., Sheffield-Moore M., Wolfe R.R., Paddon-Jones D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J. Am. Diet. Assoc. 2009;109:1582–1586. doi: 10.1016/j.jada.2009.06.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesseraud S., Peresson R., Chagneau A. Age-related changes of protein turnover in specific tissues of the chick. Poult. Sci. 1996;75:627–631. doi: 10.3382/ps.0750627. [DOI] [PubMed] [Google Scholar]
- Thaxton V.Y., Christensen K.D., Mench J.A., Rumley E.R., Daugherty C., Feinberg B., Parker M., Siegel P., Scanes C.G. Symposium: animal welfare challenges for today and tomorrow. Poult. Sci. 2016;95:2198–2207. doi: 10.3382/ps/pew099. [DOI] [PubMed] [Google Scholar]
- Tijare V.V., Yang F., Kuttappan V., Alvarado C., Coon C., Owens C. Meat quality of broiler breast fillets with white striping and woody breast muscle myopathies. Poult. Sci. 2016;95:2167–2173. doi: 10.3382/ps/pew129. [DOI] [PubMed] [Google Scholar]
- Tipton K.D., Hamilton D.L., Gallagher I.J. Assessing the role of muscle protein breakdown in response to nutrition and exercise in humans. Sports Med. 2018;48:53–64. doi: 10.1007/s40279-017-0845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdaneta-Rincon M., Leeson S. Muscle (pectoralis major) protein turnover in young broiler chickens fed graded levels of lysine and crude protein. Poult. Sci. 2004;83:1897–1903. doi: 10.1093/ps/83.11.1897. [DOI] [PubMed] [Google Scholar]
- Velleman S.G., Clark D.L. Histopathologic and myogenic gene expression changes associated with wooden breast in broiler breast muscles. Avian Dis. 2015;59:410–418. doi: 10.1637/11097-042015-Reg.1. [DOI] [PubMed] [Google Scholar]
- Vignale K., Caldas J.V., England J.A., Boonsinchai N., Magnuson A., Pollock E.D., Dridi S., Owens C.M., Coon C.N. Effect of white striping myopathy on breast muscle (Pectoralis major) protein turnover and gene expression in broilers. Poult. Sci. 2016;96:886–893. doi: 10.3382/ps/pew315. [DOI] [PubMed] [Google Scholar]
- Waterlow J.C. CABI; Worcester, MA: 2006. Protein Turnover. [Google Scholar]
- Williamson D.H., Farrell R., Kerr A., Smith R. Muscle-protein catabolism after injury in man, as measured by urinary excretion of 3-methylhistidine. Clin. Sci. Mol. Med. 1977;52:527–533. doi: 10.1042/cs0520527. [DOI] [PubMed] [Google Scholar]
- Winbanks C.E., Weeks K.L., Thomson R.E., Sepulveda P.V., Beyer C., Qian H., Chen J.L., Allen J.M., Lancaster G.I., Febbraio M.A., Harrison C.A., McMullen J.R., Chamberlain J.S., Gregorevic P. Follistatin-mediated skeletal muscle hypertrophy is regulated by Smad3 and mTOR independently of myostatin. J. Cell Biol. 2012;197:997–1008. doi: 10.1083/jcb.201109091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner J., Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch. Biochem. Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Wold J.P., Veiseth-Kent E., Høst V., Løvland A. Rapid on-line detection and grading of wooden breast myopathy in chicken fillets by near-infrared spectroscopy. PloS One. 2017;12:0173384. doi: 10.1371/journal.pone.0173384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe R.R. Regulation of muscle protein by amino acids. J. Nutr. 2002;132:3219S–3224S. doi: 10.1093/jn/131.10.3219S. [DOI] [PubMed] [Google Scholar]