Abstract
This study investigated the effects of dietary chitosan oligosaccharides (COS) supplementation on growth performance; corticosterone, growth hormone, and insulin-like growth factor-1 concentration; relative organ weight; liver function; meat quality; muscle glycolytic metabolism; and oxidative status in yellow-feather broilers under heat stress. A total of 108 35-day-old Chinese yellow-feather broilers (BW, 470.31 ± 13.15 g) was randomly allocated to 3 dietary treatments as follow: control group, basal diet and raised under normal temperature (24°C); HS group, basal diet and raised under cycle heat stress (34°C from 10:00 to 18:00 and 24°C for the rest time); and HSC group, basal diet with 200 mg/kg COS supplementation and raised under cycle heat stress. Each treatment had 6 replication pens and 6 broilers per pen. Results indicated that heat stress decreased ADG, ADFI, gain:feed ratio, the relative weight of thymus, bursa of Fabricius, pancreas, proventriculus, gizzard, and liver, growth hormone concentration, pH24h, muscle glycogen content, muscle superoxide dismutase and glutathione peroxidase activity, as well as increased corticosterone, alanine aminotransferase and aspartate aminotransferase level, cooking loss, muscle lactate and malondialdehyde content. Compared with the HS group, broilers in the HSC group had higher ADG, the relative weight of thymus, bursa of Fabricius, and liver, growth hormone concentration, pH24h, muscle glycogen content, muscle superoxide dismutase and glutathione peroxidase activity, and lower serum corticosterone, alanine aminotransferase and aspartate aminotransferase level, cooking loss, and muscle lactate and malondialdehyde content. In conclusion, the results suggested that COS could be used as an effective feed additive to maintain growth performance, liver function, meat quality, muscle glycolytic metabolism, and oxidative status of yellow-feather broilers under heat stress. The improved meat quality is possibly through reducing muscle glycolysis metabolism and improving muscle oxidative status by dietary COS supplementation in broilers under heat stress.
Key words: chitosan oligosaccharide, heat stress, meat quality, muscle oxidative status, yellow-feather broiler
Introduction
Heat stress is one of the major environment stressors in poultry production because it adversely affects health status and production performance of broilers, causing direct economic losses (St-Pierre et al., 2003). Broilers exposed to heat stress would induce multiple physiology disturbances, such as endocrine disorders (Rajaei-Sharifabadi et al., 2017), immune suppression (Wang et al., 2018), and oxidative stress (Ganesan et al., 2017; Zhang et al., 2018) and adversely affect the meat quality by changing muscle glycolysis metabolism and oxidative status (Zaboli et al., 2019). Either acute or chronic heat stress had detrimental effects on meat quality by increasing postmortem glycolytic metabolisms (Zhang et al., 2012; Zaboli et al., 2019) and induced a lower ultimate pH accompanied with variation in water-holding capacity, meat color, and tenderness of meat, resulting in a lower purchasing desire (Zhang et al., 2012; Wang et al., 2017). In recent years, numerous nutritional strategies have been used to alleviate the detrimental effects of heat stress on animals, and dietary supplementation with functional oligosaccharides attracted increasing attention (Cheng et al., 2019; Tavaniello et al., 2020). Chitosan oligosaccharides (COS), a functional oligosaccharide, are a natural alkaline polymer of glucosamine and obtained by chemical and enzymatic hydrolysis of chitosan (Zou et al., 2016) and considered as an antibiotic alternative in animal production (Zhou et al., 2009; Li et al., 2019). Previous studies reported that dietary COS supplementation could improve growth performance (Li et al., 2007; Zhou et al., 2009), antioxidant capacity (Li et al., 2017), intestinal morphology and barrier function, and immunity (Li et al., 2019) of broilers under normal condition. Moreover, it also reported that dietary chitosan or COS could improve growth performance, antioxidant status, and immune response of animals exposed to various stressors (Qiao et al., 2011; Xu et al., 2018). However, there was limited research studying the effects of dietary COS supplementation on alleviating the negative effects of heat stress, especially, on meat quality, muscle glycolytic metabolism, and antioxidant status. Only Lan et al. (2019) reported that dietary COS supplementation could alleviate oxidative damage and inflammatory response in heat-stressed rats. In addition, it has been reported that dietary mannan oligosaccharides supplementation could improve growth performance, intestinal oxidative status, and barrier integrity, as well as meat quality and oxidative status of breast muscle in broilers under heat stress (Cheng et al., 2018, 2019). Based on the similar biological function of oligosaccharides, we hypothesized that dietary COS supplementation could alleviate the negative effects of broilers under heat stress. Therefore, the aim of this study was to evaluate the effects of dietary COS supplementation on growth performance; serum corticosterone (Cort), growth hormone (GH), and insulin-like growth factor-1 (IGF-1) concentration; relative organ weight; liver function; meat quality; and muscle glycogen and lactate content, as well as muscle oxidative status in yellow-feather broilers under heat stress.
Materials and methods
Experiment Design and Dietary Treatments
The experimental procedures were approved by the Animal Care and Use Committee of Guangdong Ocean University (SYXK-2018-0147). A total of 108 35-day-old Chinese indigenous yellow-feather broilers (Frizzled chicken, BW 470.31 ± 13.15 g) were purchased from local breeding company (Zhanjiang, Guangdong province, China) and randomly allocated to 3 treatments. Each treatment had 6 replication pens and 6 broilers per pen. Dietary treatments were control group, basal diet and raised under normal temperature (24°C); HS group, basal diet and raised under cycle heat stress (34°C from 10:00 to 18:00 and 24°C for the rest time); and HSC group, basal diet with 200 mg/kg COS and raised under cycle heat stress. Broilers of each replication were assigned in battery pens (124 cm length × 64 cm width × 40 cm height). The basal diets (Table 1) were formulated to meet or exceed the nutrient requirement of the Feeding Standard of Chicken, China (NY/T 33-2004). Chitosan oligosaccharides was purchased from Jiangsu Xinrui Biotechnology Co., Ltd. (HPLC purity 95%, deacetylation degree more than 95%, and average molecular weight lower than 32 kDa). The feed was provided in mash form and the supplementation of COS to the basal diet at the expense of corn.
Table 1.
Basal diet composition (as-fed basis).
| Ingredients | Content, % |
|---|---|
| Corn | 69.95 |
| Soybean | 22.10 |
| Soybean oil | 2.70 |
| Calcium hydrogen phosphate | 1.70 |
| Shell power | 1.93 |
| Salt | 0.35 |
| Met | 0.10 |
| Lys | 0.05 |
| Zeolite powder | 0.80 |
| Vitamin premix1 | 0.16 |
| Mineral premix2 | 0.16 |
| Total | 100.00 |
| Nutrient level | |
| ME, MJ/kg | 12.65 |
| CP, % | 16.29 |
| Calcium, % | 1.18 |
| Total phosphorus, % | 0.62 |
| Available phosphorous, % | 0.41 |
| Met, % | 0.36 |
| Lys, % | 0.87 |
| Met + Cys, % | 0.64 |
Provided per kilogram of complete diet: 12,8000 IU vitamin A; 1,600 IU vitamin D3; 60 IU vitamin E; 1.6 mg vitamin K3; 0.12 mg biotin; 50 mg choline; 1.2 mg folic acid; 32 mg nicotinic acid; 16 mg pantothenic acid; 4.8 mg riboflavin; 2.4 mg thiamine (VB1); 3.2 mg vitamin B6; and 0.03 mg vitamin B12.
Provided per kilogram of diet: Mg, 79 mg as manganese oxide; Zn, 60 mg as zinc oxide; Cu 100 mg as copper sulfate; Fe, 120 mg as iron sulfate; I, 0.96 mg as potassium iodine; Co, 0.16 mg as cobalt sulfate and Se, 0.24 mg as sodium selenite.
Growth Performance
The BW was recorded on pen basis at the beginning and the end of the experiment to calculate ADG. Feed consumption was recorded on pen basis every week to calculate ADFI and gain:feed ratio (G:F).
Serum Parameters
At the end of the experiment, after 12-h fast, 6 broilers per treatment (1 broiler from each replication pen) were randomly selected, and the broilers were individually weighted; then, blood samples were collected from the brachial vein into nonheparinized tubes and centrifuged at 3,000 g for 10 min at 4°C to obtain serum. The serum samples stored at −20°C until analysis. The serum Cort, GH, IGF-1, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) concentration were measured with commercial enzyme immunoassay kits (Cusabio Biotech. Co., Ltd., Wuhan, Hubei, China) as per the manufacturers' instruction.
Relative Organ Weight
After blood sample collection, the broilers were killed by cervical dislocation and exsanguinated. The proventriculus, gizzard, pancreas, liver, heart, thymus, spleen, bursa of Fabricius, and abdominal fat (fat surrounding the cloaca and gizzard) were excised and measured. The relative organ weight (g/kg) was expressed as the organ weight (g)/broiler weight (kg).
Meat Quality
Both sides of breast muscle were sampled for further analysis. The left part was transported to the laboratory where meat quality analysis was immediately carried out, whereas the right part was immediately frozen and stored at −20°C for further analysis. The pH value measured on breast muscle at a depth of 2.5 cm below the surface using a pH meter at 24 h (pH24h) postmortem (Fisher Scientific, Pittsburgh, PA). Meat color of the lightness, redness, and yellowness were measured using a Model CR-410 Chroma meter (Konica Minolta Sensing Inc., Osaka, Japan) at 45 min postmortem. For cooking loss determination, approximately 4 g of the sample was weighed, placed in a plastic bag, and cooked to an internal temperature of 75°C using a water bath; then, the cooked samples were cooled for 30 min, blotted dry, and weighed. Cooking loss values were calculated based on the following equation: cooking loss (%) = (raw weight-cooked weight)/raw weight × 100. The drip loss was measured using approximately 4 g of meat sample, according to the method described by Honikel (1998), briefly; the samples were packaged in a transparent polythene bag and stored at 4°C for 1, 3, 5, 7 D, after which the excess moisture was wiped out, and the breast samples were weighed (on day 1, 3, 5, 7). Dripping loss values were calculated based on the following equation: dripping loss (%) = (raw weight-stored weight)/raw weight × 100.
Muscle Glycogen and Lactate Content and Muscle Oxidative Status
About 1 g of muscle sample was homogenized at a ratio of 1: 9 (weight/volume) with ice-cold phosphate-buffered saline. Homogenate was centrifuged at 3,000 g for 10 min at 4°C to obtain supernatant, and the analysis was immediately conducted. The breast muscle protein concentration was determined using the Bradford method (Bradford, 1976) with BSA as the standard. The activities of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) and the content of malondialdehyde (MDA), glycogen, and lactate in breast muscle were measured with corresponding assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer's instructions. All results were normalized to the total protein concentration in each sample for intersample comparison. The breast sample concentration was detected as per the Bradford method (Bradford, 1976).
Statistical Analysis
The pen was used as the experiment unit, and all data were analyzed with SAS 2003 (v. 9.1, SAS Institute Inc., Cary, NC). All data were analyzed using 1-way ANOVA followed by Duncan's multiple range test to analyze differences among treatments. Differences were considered significant at P < 0.05.
Results
Growth Performance
Compared with the control group, heat stress significantly reduced (P < 0.05) ADG, ADFI, and G:F (Table 2). Compared with the HS group, dietary COS supplementation increased (P < 0.05) ADG.
Table 2.
Effects of chitosan oligosaccharides on growth performance of yellow-feather broilers under heat stress.
| Item | Control | HS | HSC | SEM |
|---|---|---|---|---|
| ADG, g | 21.17a | 16.27b | 18.50c | 0.65 |
| ADFI, g | 65.39a | 61.25b | 63.24a,b | 0.73 |
| G:F | 0.32a | 0.27b | 0.29a,b | 0.01 |
a,b,cWithin the same row with different superscripts differ (P < 0.05).
Abbreviations: COS, chitosan oligosaccharides; Control group, basal diet and raised under normal temperature (24°C); G:F, gain:feed ratio; HS group, basal diet and raised under cycle heat stress (34°C from 10:00 to 18:00 and 24°C for the rest time); HSC group, basal diet with 200 mg/kg COS supplementation and raised under cycle heat stress.
Serum Cort, GH, and IGF-1 Level
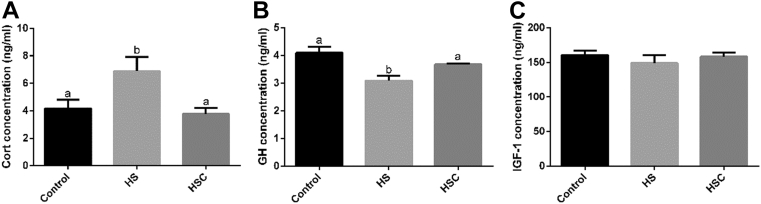
Heat stress significantly increased (P < 0.05) serum Cort and decreased GH concentration compared with the control group (Figure 1). Dietary COS supplementation significantly decreased (P < 0.05) Cort and increased (P < 0.05) GH concentration compared with the HS group.
Figure 1.
Effects of chitosan oligosaccharides (COS) on serum corticosterone (Cort), growth hormone (GH), and insulin-like growth factor-1 (IGF-1) concentration in yellow-feather broilers under heat stress. Values are mean ± SE. The values have different superscript letters are different (P < 0.05). Abbreviations: Control group, basal diet and raised under normal temperature (24°C); HS group, basal diet and raised under cycle heat stress (34°C from 10:00 to 18:00 and 24°C for the rest time); HSC group, basal diet with 200 mg/kg COS supplementation and raised under cycle heat stress.
Relative Organ Weight
Compared with the control group, heat stress had no significant effects on the relative weight of spleen, heart, or abdominal fat but decreased (P < 0.05) the relative weight of thymus, bursa of Fabricius, pancreas, proventriculus, gizzard, and liver (Table 3). Compared with the HS group, dietary COS supplementation had no significant effects on the relative weight of the spleen, pancreas, proventriculus, gizzard, heart, and abdominal fat but increased (P < 0.05) the relative weight of the thymus, bursa of Fabricius, and liver.
Table 3.
Effects of chitosan oligosaccharides on relative weight (g/kg BW) of yellow-feather broilers under heat stress.
| Item | Control | HS | HSC | SEM |
|---|---|---|---|---|
| Thymus | 2.95a | 1.84b | 2.52a | 0.26 |
| Spleen | 1.88 | 1.62 | 1.72 | 0.13 |
| Bursa of Fabricius | 1.13a | 0.72b | 1.12a | 0.11 |
| Pancreas | 1.92a | 1.37b | 1.41b | 0.12 |
| Proventriculus | 3.38a | 2.44b | 2.70b | 0.18 |
| Gizzard | 20.91a | 14.58b | 15.58b | 1.35 |
| Liver | 20.62a | 15.93b | 18.14c | 0.76 |
| Heart | 4.42 | 3.97 | 4.39 | 0.14 |
| Abdominal fat | 12.30 | 13.41 | 11.64 | 1.92 |
a,bWithin the same row with different superscripts differ (P < 0.05).
Abbreviations: COS, chitosan oligosaccharides; Control group, basal diet and raised under normal temperature (24°C); HS group, basal diet and raised under cycle heat stress (34°C from 10:00 to 18:00 and 24°C for the rest time); HSC group, basal diet with 200 mg/kg COS supplementation and raised under cycle heat stress.
Liver Function
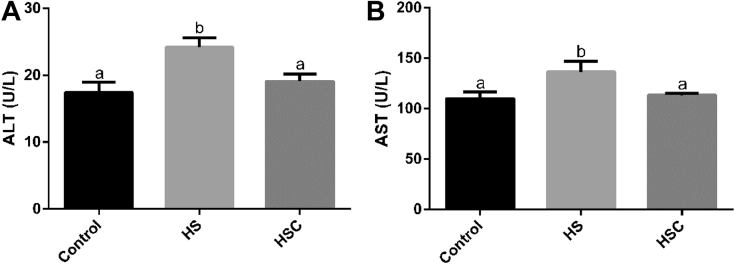
Compared with the control group, heat stress increased (P < 0.05) serum ALT and AST concentration (Figure 2). Compared with the HS group, dietary COS supplementation decreased (P < 0.05) serum ALT and AST concentration.
Figure 2.
Effects of chitosan oligosaccharides (COS) on serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in yellow-feather broilers under heat stress. Values are mean ± standard error. The values have different superscript letters are different (P < 0.05). Abbreviations: Control group, basal diet and raised under normal temperature (24°C); HS group, basal diet and raised under cycle heat stress (34°C from 10:00 to 18:00 and 24°C for the rest time); HSC group, basal diet with 200 mg/kg COS supplementation and raised under cycle heat stress.
Meat Quality and Muscle Glycogen and Lactate Content
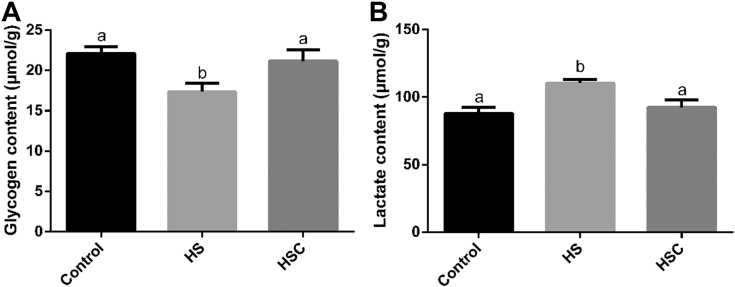
Compared with the control group, heat stress had no significant effect on meat color (lightness, redness, and yellowness) or drip loss (day 1, 3, 5 and 7), decreased (P < 0.05) pH24h (Table 4) and glycogen content (Figure 3), as well as increased (P < 0.05) cooking loss (Table 4) and lactate content (Figure 3). Compared with the HS group, dietary COS supplementation had no significant effects on meat color (lightness, redness, and yellowness) or drip loss (day 1, 3, 5 and 7), increased (P < 0.05) pH24h and glycogen content, as well as decreased (P < 0.05) cooking loss and lactate content.
Table 4.
Effects of chitosan oligosaccharides on meat quality of yellow-feather broilers under heat stress.
| Item | Control | HS | HSC | SEM |
|---|---|---|---|---|
| Meat color | ||||
| L∗ (lightness) | 49.61 | 50.37 | 50.61 | 1.07 |
| a∗ (redness) | 10.33 | 10.39 | 10.39 | 0.16 |
| b∗ (yellowness) | 9.05 | 8.88 | 8.82 | 0.17 |
| pH24h | 5.73a | 5.30b | 5.62a | 0.06 |
| Drip loss, % | ||||
| Day 1 | 3.00 | 3.17 | 3.17 | <0.00 |
| Day 3 | 5.67 | 5.17 | 5.17 | <0.00 |
| Day 5 | 10.33 | 10.00 | 10.00 | <0.00 |
| Day 7 | 13.17 | 13.33 | 13.17 | <0.00 |
| Cooking loss, % | 15.66b | 20.83a | 16.67b | 0.01 |
a,bWithin the same row with different superscripts differ (P < 0.05).
Abbreviations: COS, chitosan oligosaccharides; Control group, basal diet and raised under normal temperature (24°C); HS group, basal diet and raised under cycle heat stress (34°C from 10:00 to 18:00 and 24°C for the rest time); HSC group, basal diet with 200 mg/kg COS supplementation and raised under cycle heat stress.
Figure 3.
Effects of chitosan oligosaccharides (COS) on muscle glycogen and lactate content in yellow-feather broilers under heat stress. Values are mean ± standard error. The values have different superscript letters are different (P < 0.05). Abbreviations: Control group, basal diet and raised under normal temperature (24°C); HS group, basal diet and raised under cycle heat stress (34°C from 10:00 to 18:00 and 24°C for the rest time); HSC group, basal diet with 200 mg/kg COS supplementation and raised under cycle heat stress.
Muscle Antioxidant Status
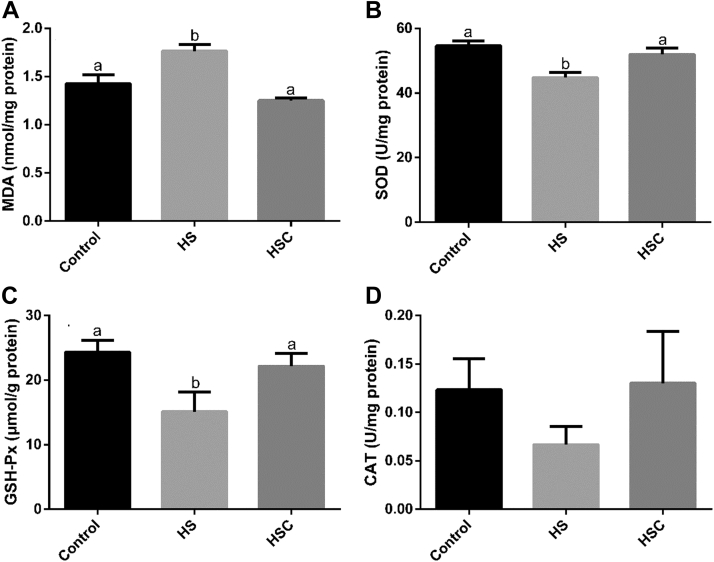
Compared with the control group, heat stress had no significant effect on muscle CAT activity, increased (P < 0.05) muscle MDA content, and decreased (P < 0.05) muscle SOD and GSH-Px activity (Figure 4). Compared with the HS group, dietary COS supplementation decreased (P < 0.05) muscle MDA content and increased (P < 0.05) muscle SOD and GSH-Px activity.
Figure 4.
Effects of chitosan oligosaccharides (COS) on anti-oxidant status in breast muscle of yellow-feather broilers under heat stress. Values are mean ± SE. The values have different superscript letters are different (P < 0.05). Abbreviations: CAT, catalase; Control group, basal diet and raised under normal temperature (24°C); GSH-Px, glutathione peroxidase; HS group, basal diet and raised under cycle heat stress (34°C from 10:00 to 18:00 and 24°C for the rest time); HSC group, basal diet with 200 mg/kg COS supplementation and raised under cycle heat stress; MDA, malondialdehyde; SOD, superoxide dismutase.
Discussion
The main purpose of poultry production is to maintain broilers' health status, enhance the productivity and better meat quality. It was indubitable that heat stress adversely affected the growth performance of broilers by inducing lower feed efficiency, intestinal damage, metabolism disturbance, immune suppression and oxidative stress (Cheng et al., 2018, 2019; He et al., 2019). In this study, dietary COS supplementation increased ADG of broilers under heat stress. Consistent with our results, Li et al. (2007) and Zhou et al. (2009) reported dietary COS supplementation improved BW gain of broilers. Li et al. (2019) reported dietary COS supplementation increased G:F, accompanied with increasing intestinal barrier function, antioxidant capacity and immunity. Xu et al. (2018) demonstrated that dietary chitosan supplementation increased ADG, G:F, and antioxidant enzymes activities and regulated cytokines of oxidative-stressed piglets. The positive effects of COS on the growth performance in this study may attribute to the improvement of feed intake, feed efficiency, intestinal function, antioxidant capacity, and immune response.
Serum Cort level is a convenient index for monitor stress status of animals. Numerous studies showed that heat stress increased serum Cort level (Quinterio-Filho et al., 2010; Cheng et al., 2018). Similarly, in this study, heat stress increased serum Cort level. As expected, dietary COS supplementation decreased serum Cort level, which was consistent with the results of Li et al. (2013), who indicated that dietary chitosan supplementation decreased the level of cortisol in weaning pigs. The decreased serum Cort level in the HSC group suggested that COS could alleviate heat stress, which might explain the improved growth performance of broilers in the HSC group.
GH and IGF-1 level are related to physiological process of animal growth (Sirotkin, 2010). Liu et al. (2013) reported that heat stress significantly decreased serum GH and IGF-1 level in black-boned chicken. Consistently, we also observed that heat stress decreased serum GH level in yellow-feather broilers. In this study, dietary COS supplementation increased GH concentration, which was in agreement with the study of Li et al. (2007), who reported that dietary COS supplementation increased serum GH in broilers. Dietary COS supplementation increased serum GH of broilers under heat stress, suggesting that COS had a vital role in maintaining normal GH level and may be an adaptive response to alleviate heat stress–induced damage, which may explain the better growth performance of broilers with dietary COS supplementation in this study.
The relative organ weight could reflect the growth and development of organ in some degree. Previous studies reported that heat stress decreased the relative weight of the thymus, spleen, and bursa of Fabricius in broilers (Calefi et al., 2016; He et al., 2019). In this study, we also found that heat stress significantly decreased the relative weight of the thymus and bursa of Fabricius, as well as the relative weight of the pancreas, proventriculus, gizzard, and liver. These results indicated that heat stress caused damage to the growth and development of organs, whereas dietary COS supplementation increased the relative weight of the thymus, bursa of Fabricius, and liver. In agreement with our results, Deng et al. (2008) reported that dietary COS supplementation increased the relative weight of the thymus, spleen, and bursa of Fabricius in broilers under normal conditions. Similarly, Zhou et al. (2009) reported dietary COS supplementation increased the relative weight of thr liver. These results suggested that COS have potential capacity to inhibit organ dysplasia and atrophy in broilers under heat stress.
The serum ALT and AST level are widely used to evaluate liver function, owing to both ALT and AST are the earliest and most sensitive indicators of liver injury. During liver damage, the release of ALT and AST into the circulation lead to higher ALT and AST level in serum. Therefore, the ALT and AST level in the serum are used as a reliable and specific marker of liver damage. Heat stress is known to induce tissue injury and severe liver damage in ducks (Zeng et al., 2014), the increased serum ALT and AST level reflected the destruction of hepatocytes and liver function (Tessari et al., 2010). Former studies indicated that heat stress enhanced the serum ALT and AST level in broilers (Attia et al., 2017). In this study, we also observed heat stress increased the serum ALT and AST level in yellow-feather broilers, which suggested that heat stress induced liver damage. However, dietary COS supplementation decreased the serum ALT and AST level of broilers under heat stress. These results indicated that COS may provide a potential nutritional strategy to maintaining liver function in yellow-feather broilers under heat stress. Consistently, Qiao et al. (2011) reported that COS protected the adverse effects of lipopolysaccharide on the liver of rats by decreasing the serum ALT and AST level. Kong et al. (2018) also reported that COS inhibited the D-galactose–induced upregulation of the serum ALT and AST level.
Former studies reported that heat stress had detrimental effects on meat quality and even resulted in pale, soft, and exudative-like meat, decreasing consumers' acceptability and economic loss (Zaboli et al., 2019). In this study, heat stress induced poor meat quality by lowering pH24h and increasing cooking loss. Dietary supplementation with probiotics (Cramer et al., 2018), prebiotics (Tavaniello et al., 2020), and ginger (Wen et al., 2020) were proved as effective additives to alleviate the negative effects of heat stress on meat quality of broilers. Chitosan oligosaccharides also reported as a promising feed additive to alleviate heat stress. As expected, dietary COS supplementation improved the meat quality of broilers by higher pH24h and lower cooking loss under heat stress conditions. The beneficial effects of COS on meat quality were highly related to muscular energy metabolism and muscle antioxidant status, which were important factors affecting meat quality (Zhang et al., 2019). Glycogen was the main energy reserve in muscle. It is known that aerobic glycolysis generates a maximum of 38 molecules of ATP per molecule of glucose, whereas anaerobic glycolysis generates only 2 net molecules of ATP per molecule of glucose alone with lactate fermentation. The enhanced anaerobic glycolysis of muscle glycogen resulted in lactate accumulation and further decreased muscle pH (Zhang et al., 2014). Therefore, the muscle glycogen and lactate content were used to evaluate muscle energy metabolism. Former studies indicated that heat stress increased postmortem glycolytic metabolism, which resulted in decreasing muscle glycogen content, lactate accumulation, and pH reduction (Wang et al., 2017; Zhao et al., 2019). In this study, we also observed that heat stress decreased muscle glycogen content and increased lactate content, which might explain the lower pH24h and higher cooking loss of broilers in the HS group, whereas dietary COS supplementation increased muscle glycogen content and decreased lactate content, accompanied with higher pH24h and lower cooking loss, suggesting that COS could improve meat quality through decreasing the muscle glycogen glycolytic metabolism and lactate accumulation.
It has been reported that heat stress could induce overproduction of reactive oxygen species in skeletal muscle (Mujahid et al., 2009), which may damage the integrity of muscle cell membrane (Huang et al., 2015), accelerate lipid and protein oxidation (Estévez, 2015), and result in the imbalance of antioxidant enzymes (Azard et al., 2010), eventually leading to the change of meat quality. Therefore, the antioxidant capacity of muscle is also crucial to meat quality (Pan et al., 2018). Previous studies indicated that heat stress had detrimental effects on muscle antioxidant status and increased MDA content (Cramer et al., 2018; Wen et al., 2020). Similarly, in this study, we observed that heat stress increased muscle MDA content and decreased SOD and GSH-Px activity, which indicated a decreased muscle antioxidant capacity, alone with increased lipid peroxidation. Li et al. (2019) reported that dietary COS supplementation increased SOD activity in the duodenum and decreased MDA content in the jejunum of broilers. As expected, dietary COS supplementation increased muscle antioxidant status (higher muscle SOD and GSH-Px activity) and decreased lipid peroxidation (lower MDA content) in broilers under heat stress, suggesting that COS could improve meat quality of broilers under heat stress via direct antioxidant capacity.
Conclusion
In conclusion, dietary COS supplementation can be used as an effective feed additive to maintain growth performance, liver function, meat quality, muscle glycolysis metabolism, and oxidative status of yellow-feather broilers under heat stress. The reduction of muscle glycolysis metabolism and better oxidative status are probably the reason for improving meat quality by dietary COS supplementation in broilers under heat stress.
Acknowledgments
Financial support provided by program for scientific research start-up funds of Guangdong Ocean University (101402/R18005) and Key Platform Project of Innovation strong school Engineering by Department of Education of Guangdong Province (2018302), is gratefully acknowledged.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Attia Y.A., Bakhashwain A.A., Bertu N.K. Thyme oil (Thyme vulgaris L.) as a natural growth promoter for broiler chickens reared under hot climate. Ital. J. Anim. Sci. 2017;16:275–282. [Google Scholar]
- Azad M.A.K., Kikusato M., Maekawa T., Shirakawa H., Toyomizu M. Metabolic characteristics and oxidative damage to skeletal muscle in broiler chickens exposed to chronic heat stress. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2010;155:401–406. doi: 10.1016/j.cbpa.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Calefi A.S., de Siqueira A., Namazu L.B., Costola-de-Souza C., Honda B.B., Ferreira A.J., Quinteiro-Filho W.M., Da S.F.J., Palermo-Neto J. Effects of heat stress on the formation of splenic germinal centres and immunoglobulins in broilers infected by Clostridium perfringens type A. Vet. Immunol. Immunopathol. 2016;171:38–46. doi: 10.1016/j.vetimm.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Chen Y., Chen R., Su Y., Zhang R., He Q., Wang K., Wen C., Zhou Y. Dietary mannan oligosaccharide ameliorates cyclic heat stress-induced damages on intestinal oxidative status and barrier integrity of broilers. Poult. Sci. 2019;98:4746–4776. doi: 10.3382/ps/pez192. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Du M., Xu Q., Chen Y., Wen C., Zhou Y. Dietary mannan oligosaccharide improves growth performance, muscle oxidative status, and meat quality in broilers under cyclic heat stress. J. Therm. Biol. 2018;75:106–111. doi: 10.1016/j.jtherbio.2018.06.002. [DOI] [PubMed] [Google Scholar]
- Cramer T.A., Kim H.W., Chao Y., Wang W., Wang H.W., Kim Y.H.B. Effects of probiotic (Bacillus subtilis) supplementation on meat quality characteristics of breast muscle from broilers exposed to chronic heat stress. Poult. Sci. 2018;97:3358–3368. doi: 10.3382/ps/pey176. [DOI] [PubMed] [Google Scholar]
- Deng X.Z., Li X.J., Liu P., Yuan S.L., Piao X.S. Effect of chito-oligosaccharide supplementation on immunity in broiler chickens. Asian Austral. J. Anim. Sci. 2008;21:81–88. [Google Scholar]
- Estévez M. Oxidative damage to poultry: from farm to fork. Poult. Sci. 2015;94:1368–1378. doi: 10.3382/ps/pev094. [DOI] [PubMed] [Google Scholar]
- Ganesan S., Summers C.M., Pearce S.C., Gabler N.K., Valentine R.J., Baumgard L.H., Rhoads R.P., Selsby J.T. Short-term heat stress causes altered intracellular signaling in oxidative skeletal muscle. J. Anim. Sci. 2017;95:2438–2451. doi: 10.2527/jas.2016.1233. [DOI] [PubMed] [Google Scholar]
- He S., Yu Q., He Y., Hu R., Xia S., He J. Dietary resveratrol supplementation inhibits heat stress-induced high-activated innate immunity and inflammatory response in spleen of yellow-feather broilers. Poult. Sci. 2019;98:6378–6387. doi: 10.3382/ps/pez471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honikel K.O. Reference methods for the assessment of physical characteristics of meat. Meat. Sci. 1998;49:447–457. doi: 10.1016/s0309-1740(98)00034-5. [DOI] [PubMed] [Google Scholar]
- Huang C., Jiao H., Song Z., Zhao J., Wang X., Lin H. Heat stress impairs mitochondria functions and induces oxidative injury in broiler chickens. J. Anim. Sci. 2015;93:2144–2153. doi: 10.2527/jas.2014-8739. [DOI] [PubMed] [Google Scholar]
- Kong S.Z., Li J.C., Li S.D., Liao M.N., Li C.P., Zheng P.J., Guo M.H., Tan W.X., Zheng Z.H., Hu Z. Anti-aging effect of chitosan oligosaccharide on d-galactose-induced subacute aging in mice. Mar. Drugs. 2018;16:181–194. doi: 10.3390/md16060181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan R.X., Li S.Q., Chang Q.Q., Zhao Z.H. Chitosan oligosaccharides protect sprague dawley rats from cyclic heat stress by attenuation of oxidative and inflammation stress. Animals. 2019;9:1074–1084. doi: 10.3390/ani9121074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Cheng Y., Chen Y., Qu H., Zhao Y., Wen C., Zhou Y. Dietary chitooligosaccharide inclusion as an alternative to antibiotics improves intestinal morphology, barrier function, antioxidant capacity, and immunity of broilers at early age. Animals (Basel) 2019;9:493. doi: 10.3390/ani9080493. 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.C., Ding X.M., Peng X., Chi X.F., Cui H.M., Zuo Z.C., Fang J. Effect of chitosan oligosaccharides on antioxidant function, lymphocyte cycle and apoptosis in ileum mucosa of broiler. Kafkas. Univ. Vet. Fak. Derg. 2017;23:571–577. [Google Scholar]
- Li X., Piao X., Kim S., Liu P., Wang L., Shen Y., Jung S., Lee H. Effects of chito-oligosaccharide supplementation on performance, nutrient digestibility, and serum composition in broiler chickens. Poult. Sci. 2007;86:1107–1114. doi: 10.1093/ps/86.6.1107. [DOI] [PubMed] [Google Scholar]
- Li J., Shi B., Yan S., Lu J., Guo Y. Effects of dietary supplementation of chitosan on stress hormones and antioxidative enzymes in weaned piglets. J. Anim. Vet. Adv. 2013;12:650–654. [Google Scholar]
- Liu L., He J., Xie H., Yang Y., Li J., Zou Y. Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poult. Sci. 2013;93:54–62. doi: 10.3382/ps.2013-03423. [DOI] [PubMed] [Google Scholar]
- Mujahid A., Akiba Y., Toyomizu M. Olive oil-supplemented diet alleviates acute heat stress-induced mitochondrial ROS production in chicken skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:R690–R698. doi: 10.1152/ajpregu.90974.2008. [DOI] [PubMed] [Google Scholar]
- Pan L., Ma X., Zhao P., Shang Q., Long S., Wu Y., Piao X. Forsythia suspensa extract attenuates breast muscle oxidative injury induced by transport stress in broilers. Poult. Sci. 2018;97:1554–1563. doi: 10.3382/ps/pey012. [DOI] [PubMed] [Google Scholar]
- Qiao Y., Bai X.F., Du Y.G. Chitosan oligosaccharides protect mice from LPS challenge by attenuation of inflammation and oxidative stress. Int. Immunopharmacol. 2011;11:121–127. doi: 10.1016/j.intimp.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho W.M., Ribeiro A., Ferraz-de-Paula V., Pinheiro M.L., Sakai M., Sa L.R.M., Ferreira A.J.P., Palermo-Neto J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010;89:1905–1914. doi: 10.3382/ps.2010-00812. [DOI] [PubMed] [Google Scholar]
- Rajaei-Sharifabadi H., Ellestad L., Porter T., Donoghue A., Bottje W.G., Dridi S. Noni (Morinda citrifolia) modulates the hypothalamic expression of stress- and metabolic-related genes in broilers exposed to acute heat stress. Front. Genet. 2017;8:192. doi: 10.3389/fgene.2017.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotkin A.V. Effect of two types of stress (heat shock/high temperature and malnutrition/serum deprivation) on porcine ovarian cell functions and their response to hormones. J. Exp. Biol. 2010;213:2125–2130. doi: 10.1242/jeb.040626. [DOI] [PubMed] [Google Scholar]
- St-Pierre N.R., Cobanov B., Schnitkey G. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 2003;86(E. Suppl):E52–E77. [Google Scholar]
- Tavaniello S., Slawinska A., Prioriello D., Petrecca V., Bertocchi M., Zampiga M., Salvatori G., Maiorano G. Effect of galactooligosaccharides delivered in ovo on meat quality traits of broiler chickens exposed to heat stress. Poult. Sci. 2020;99:612–619. doi: 10.3382/ps/pez556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessari E.N., Kobashigawa E., Cardoso A.L.S., Ledoux D.R., Rottinghaus G.E., Oliveira C.A. Effects of aflatoxin B1 and fumonisin B1 on blood biochemical parameters in broilers. Toxins. 2010;2:453–460. doi: 10.3390/toxins2040453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.H., Liang R.R., Lin H., Zhu L.X., Zhang Y.M., Mao Y.W., Dong P.C., Niu L.B., Zhang M.H., Luo X. Effect of acute heat stress and slaughter processing on poultry meat quality and postmortem carbohydrate metabolism. Poult. Sci. 2017;96:738–746. doi: 10.3382/ps/pew329. [DOI] [PubMed] [Google Scholar]
- Wang W.C., Yan F.F., Hu J.Y., Amen O.A., Cheng H.W. Supplementation of Bacillus subtilis-based probiotic reduces heat stress-related behaviors and inflammatory response in broiler chickens. J. Anim. Sci. 2018;96:1654–1666. doi: 10.1093/jas/sky092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C., Liu Y., Ye Y.W., Tao Z.G., Cheng Z.J., Wang T. Effects of gingerols-rich extract of ginger on growth performance, serum metabolites, meat quality and antioxidant activity of heat-stressed broilers. J. Therm. Biol. 2020;89:102544. doi: 10.1016/j.jtherbio.2020.102544. [DOI] [PubMed] [Google Scholar]
- Xu Y., Xing Y., Wang Z., Yan S., Shi B. Pre-protective effects of dietary chitosan supplementation against oxidative stress induced by diquat in weaned piglets. Cell. Stress Chapero. 2018;23:703–710. doi: 10.1007/s12192-018-0882-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaboli G., Huang X., Feng X., Ahn D.U. How can heat stress affect chicken meat quality?-a review. Poult. Sci. 2019;98:1551–1556. doi: 10.3382/ps/pey399. [DOI] [PubMed] [Google Scholar]
- Zeng T., Li J.J., Wang D.Q., Li G.Q., Wang G.L., Lu L.Z. Effects of heat stress on antioxidant defense system, inflammatory injury, and heat shock proteins of Muscovy and Pekin ducks: evidence for differential thermal sensitivities. Cell. Stress Chaperones. 2014;19:895–901. doi: 10.1007/s12192-014-0514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.Y., Jia G.Q., Zuo J.J., Zhang Y., Lei J., Ren L., Feng D.Y. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult. Sci. 2012;91:2931–2937. doi: 10.3382/ps.2012-02255. [DOI] [PubMed] [Google Scholar]
- Zhang J.F., Bai K.W., Su W.P., Wang A.A., Zhang L.L., Huang K.H., Wang T. Curcumin attenuates heat-stressinduced oxidant damage by simultaneous activation of GSH-related antioxidant enzymes and Nrf2-mediated phase II detoxifying enzyme systems in broiler chickens. Poult. Sci. 2018;97:1209–1219. doi: 10.3382/ps/pex408. [DOI] [PubMed] [Google Scholar]
- Zhang C., Geng Z., Chen K., Zhao X., Wang C. L-theanine attenuates transport stress-induced impairment of meat quality of broilers through improving muscle antioxidant status. Poult. Sci. 2019;98:4648–4655. doi: 10.3382/ps/pez164. [DOI] [PubMed] [Google Scholar]
- Zhang L., Li J.L., Gao T., Lin M., Wang X.F., Zhu X.D., Gao F., Zhou G.H. Effects of dietary supplementation with creatine monohydrate during the finishing period on growth performance, carcass traits, meat quality and muscle glycolytic potential of broilers subjected to transport stress. Animal. 2014;8:1955–1962. doi: 10.1017/S1751731114001906. [DOI] [PubMed] [Google Scholar]
- Zhao J.S., Deng W., Liu H.W. Effects of chlorogenic acid-enriched extract from Eucommia ulmoides leaf on performance, meat quality, oxidative stability, and fatty acid profile of meat in heat-stressed broilers. Poult. Sci. 2019;98:3040–3049. doi: 10.3382/ps/pez081. [DOI] [PubMed] [Google Scholar]
- Zhou T., Chen Y., Yoo J., Huang Y., Lee J., Jang H., Shin S., Kim H., Cho J., Kim I. Effects of chitooligosaccharide supplementation on performance, blood characteristics, relative organ weight, and meat quality in broiler chickens. Poult. Sci. 2009;88:593–600. doi: 10.3382/ps.2008-00285. [DOI] [PubMed] [Google Scholar]
- Zou P., Yang X., Wang J., Li Y.F., Yu H.L., Zhang Y.X., Liu G.Y. Advances in characterisation and biological activities of chitosan and chitosan oligosaccharides. Food Chem. 2016;190:1174–1181. doi: 10.1016/j.foodchem.2015.06.076. [DOI] [PubMed] [Google Scholar]