Abstract
The behavior, growth and development, and production performance of poultry are affected by the light environment. The influence of light results from a combination of light sources, light intensity, light color, and the photoperiod regimen. With light-emitting diode (LED) lamps applied in poultry housing systems, specific light colors are desired for each time period for layer chickens. The objective of this study was to investigate the effects of a 2-phase mixed color lighting program (phase 1: blue-green, 1 D–13 wk; phase 2: yellow-orange, 14–20 wk) using LED lights on the blood parameters, skeletal development parameters, and sexual development parameters of caged layer chickens during their brooding and rearing periods. Fifty-two chickens were raised from 1 D to 20 wk of age in each of the 4 treatment groups with 3 replicates, with white (400–700 nm) light at phase 1 and phase 2 (WL treatment as the control); blue–green (435–565 nm) light at phase 1 followed by yellow–orange (565–630 nm) light at phase 2 (BG-YOL treatment); yellow–orange LED (565–630 nm) light at phase 1 and phase 2 (YOL treatment); and blue–green (435–565 nm) light at phase 1 and phase 2 (BGL treatment). The results showed that the serum Ig concentrations of the layer chickens in the BG-YOL treatment and BGL treatment were higher than those in the WL treatment at 13 wk of age (P < 0.05). At the age of 20 wk, the serum glucose concentration levels of the pullets after the WL and BGL treatments were lower than those after the YOL treatment (P < 0.05). Compared with the WL treatment, the YOL treatment significantly increased the bone mineral density of the layer chickens (P < 0.05), and BG-YOL treatment promoted the development of the sexual organs (oviducts and ovaries) of the laying hens at the age of 20 wk (P < 0.05). For the 50% egg production age, the YOL treatment was earlier than the other 3 treatments. This study demonstrated that appropriately staged spectral control using LED lights could have positive effects on the immune performance, bone development, and production performance of caged layer chickens during their brooding and rearing periods.
Key words: pullet, light-emitting diode, spectrum, growth and development, growing period
Introduction
Lighting is one of the essential environmental factors in confined poultry houses. Light not only provides illumination for the poultry but also influences their physiological responses, behavior, growth and development, and production performance (Lewis and Morris, 1998; Olanrewaju et al., 2006; Parvin et al., 2014; Borille et al., 2015). Currently, owing to the high energy efficiency, long working life, availability in different peak wavelengths, low electricity consumption, and low rearing cost (Hassan et al., 2013, 2014; Huber-Eicher et al., 2013; Sultana et al., 2013a,b; Liu et al., 2017), light-emitting diodes (LED) are gradually becoming a substitute for conventional incandescent and fluorescent lights for lighting in poultry houses (Yang et al., 2016; Li et al., 2018).
The quantity (intensity) and quality (color) of light are 2 important factors affecting poultry productivity (Manser, 1996; Prayitno et al., 1997; Parvin et al., 2014; Elkomy et al., 2019). Some researchers have indicated that high light intensity could increase poultry activity, feather pecking and cannibalism, and sexual development (Prayitno et al., 1997; Renema and Robinson, 2001; Shi et al., 2019) and increasing light intensity can reduce leg disease and total egg production of poultry (Newberry et al., 1988; Lewis and Morris, 1999). Light color is defined by the wavelength and can produce variable effects on poultry performance. Lights of different wavelengths have various stimulatory effects on the retina and can result in behavioral changes that affect the growth and development of chickens (Xie et al., 2008b). Studies have shown that blue and green light can improve the growth of layer chickens, help to calm them down, promote their immune performance (Xie et al., 2008a,b; Hassan et al., 2013; Zhang et al., 2014), and improve egg quality (Er et al., 2007). Red light has been shown to increase the levels of reproductive hormones, promote the development of sexual organs, influence the age of sexual maturity (SM) of pullets (Gongruttananun, 2011; Min et al., 2012; Hassan et al., 2013), improve production performance (Pyrzak et al., 1987; Min et al., 2012), and influence feather pecking and cannibalism (Rozenboim et al., 2004).
Blue–green light stimulated growth of chickens, whereas orange–red light stimulated reproduction development (Rozenboim et al., 1999). Moreover, many color LED lamps are currently available to meet the light environmental requirements of modern poultry houses (Rozenboim et al., 1998). The growing period for pullets can be divided into several different periods in accordance with their growth and development. In previous studies, the researchers reported that the effect of monochromatic light on the growth and development of the poultry was age related (Rozenboim et al., 1999). The white LED and monochrome LED lights that are widely applied in poultry production (Hassan et al., 2013, 2014; Liu et al., 2017) likely do not meet the requirements for the welfare and health of layer chickens, especially for the brooding and rearing periods (0–20 wk of age), when phased spectral control could be required to meet the growth of the immune and digestion system or skeletal and sexual development over the increasing chickens age.
We hypothesized that different color LED lights given during different growing phases would promote the growth and sexual development of layer chickens. However, there is limited information available regarding the effects of staged spectral control on the performance of layer chickens, especially during the brooding and rearing periods. A greater understanding of these effects could encourage industry to further use light to improve the production efficiency.
The objectives of this study were to evaluate the effects of a 2-phase mixed color lighting program using LED lights on the blood parameters, skeletal development parameters, and sexual development parameters in caged laying hens during their brooding and rearing periods.
Materials and methods
Hens and Treatments
All the layer chickens in this experiment were managed by trained staff with standing guidelines for the Jinghong laying hens (Beijing Yukou Poultry Co., Ltd., Beijing, China). Fifty-two layer chickens in the 4 treatment groups were raised from 1 D to 20 wk of age, which were divided into phase 1 (1 D–13 wk of age) and phase 2 (14 wk–20 wk of age) with 3 replicates. The 4 treatments were white (400–700 nm) light at phase 1 and phase 2 (WL treatment as the control); blue–green (435–565 nm) light at phase 1 followed by yellow–orange (565–630 nm) light at phase 2 (BG-YOL treatment); yellow–orange LED (565–630 nm) light at phase 1 and phase 2 (YOL treatment); and blue–green (435–565 nm) light at phase 1 and phase 2 (BGL treatment).
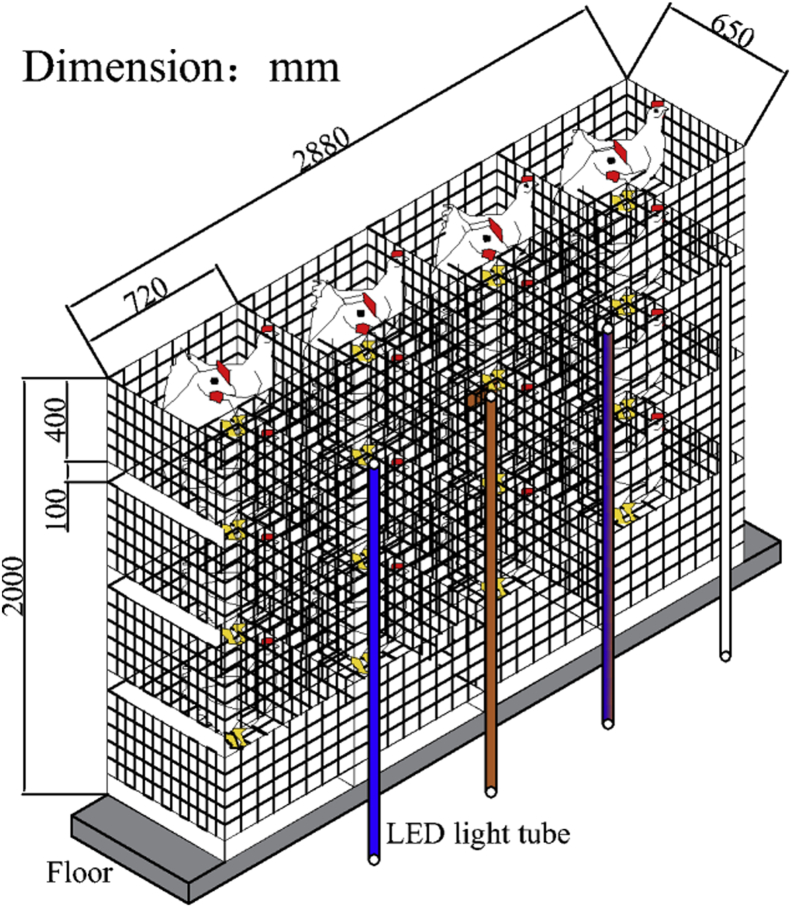
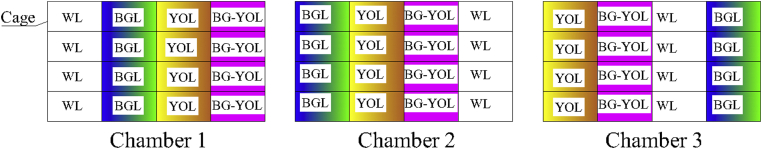
Each treatment had 4 cages (length × width × height, 72 cm × 65 cm × 40 cm) distributed at the 4 tiers of the stacked cage system, and the 4 different treatments were randomly assigned in 1 of the 3 chambers (each chamber was a replicate) (Figure 1 and Figure 2). A shading cloth was installed between the adjacent individual cages to avoid unintended irradiation to the hens from the lamps in the other cages. The surface of the light was wiped with 75% alcohol regularly to avoid excessive dust affecting the light intensity. The same physical design and environmental management in each chamber ensured similar and appropriate conditions in the 3 chambers.
Figure 1.
Oblique view of the 4 treatments in 1 chamber. Abbreviation: LED, light-emitting diode.
Figure 2.
The experimental treatment arrangements in the study. Abbreviations: BG-YOL group, blue–green (435–565 nm) light at phase 1 (1 D–13 wk) followed by yellow–orange (565–630 nm) light at phase 2 (14–20 wk); BGL group, blue–green (435–565 nm) light at phase 1 (1 D–13 wk) and phase 2 (14–20 wk); WL group, white (400–700 nm) light at phase 1 (1 D–13 wk) and phase 2 (14–20 wk); YOL group, yellow–orange LED (565–630 nm) light at phase 1 (1 D–13 wk) and phase 2 (14–20 wk).
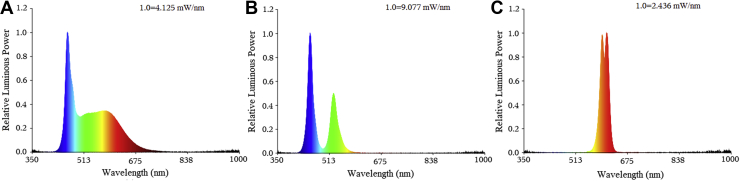
The air temperature and the relative humidity of the 3 chambers were maintained between 16°C and 36°C and between 40 and 60%, respectively, during the whole experiment, following the same environmental requirements for the birds at different ages. Specific colored light was provided by the LED light tubes (220 V 15 W; Huazhaohong Optoelectronic Technology Co., Ltd., Wuxi, China) in each chamber. The light intensity, as measured by an illuminometer (SRI 2000; Shangze photoelectric Co., Ltd., Hsinchu, Taiwan, China), was averaged at 5 to 60 lux at the level of the birds' head in the middle of each tier of cages, which was adjusted with the age of the layer chickens. The spectral characteristics involved in this study are shown in Figure 3. Hens were fed restrictively twice per day at 08:00 am and 02:00 pm, and unlimited water was provided during the whole experiment period. The photoperiod and light intensity in this experiment are shown in Table 1.
Figure 3.
Spectral characteristics: (A) white light-emitting diode light (WL); (B) blue-green light-emitting diode light (BGL); (C) yellow-orange light-emitting diode light (YOL).
Table 1.
Photoperiod and light intensity in this experiment.
| D/Wk | Photoperiodic (h) | Light intensity (lux) | D/Wk | Photoperiodic (h) | Light intensity (lux) |
|---|---|---|---|---|---|
| 1∼3 | 24 | 40∼60 | 6 wk | 12 | 5∼10 |
| 4∼7 D | 22 | 40∼60 | 7 wk | 10 | 5∼10 |
| 2 wk | 20 | 20∼40 | 8∼13 wk | 9 | 5∼10 |
| 3 wk | 18 | 10∼20 | 14∼18 wk | 9 | 5∼10 |
| 4 wk | 16 | 5∼10 | 19 wk | 10 | 10∼20 |
| 5 wk | 14 | 5∼10 | 20 wk | 11 | 10∼20 |
Blood Sample Collection and Analysis
We collected blood samples in anticoagulant blood containers from the right jugular vein of the same 1 bird, which was randomly chosen in each cage (12 birds for each treatment) at the ages of 7 wk (50 D), 13 wk (92 D), and 20 wk (141 D) during the experiment. The collected samples were stored under conditions of −20°C before being delivered to Beijing Huaying Biotechnology Research Institute (Beijing, China) for tests the same day. The Ig G (IgG) levels were determined at the age of 7, 13, and 20 wk. The glucose (GLU), total protein, triglyceride (TG), phosphorus (P), and calcium (Ca) contents were measured at the age of 20 wk.
Bone Sample Collection and Analysis
A bird was randomly selected from each cage (12 birds for each treatment) and euthanized at the age of 7, 13, and 20 wk during the experiment. The left tibia was immediately removed, and the tibia traits (bone mineral density [BMD], bone mineral content [BMC], and bone area [BA]) were detected by a dual-energy X-ray BMD instrument (Lunar-iDXA; GE Healthcare, Madison, WI).
Sexual Organ Sample Collection and Analysis
Six layer chickens were randomly selected from each treatment group and were killed humanly by neck dislocation. Then, the length and weight of the oviduct and the weight of the ovary were recorded at the age of 20 wk. The age of the first chicken and of 50% of chickens starting to lay eggs in each treatment was recorded.
Statistical Analysis
The data are presented as the means ± SE. All statistical analyses were performed using linear mixed models parameterized with SPSS (IBM SPSS Statistics 22.0, New York, NY). The linear mixed model included the chamber, the cage, the sample order, the sampling week, the light treatment, and the interaction between the week and the light treatment (week × light treatment). The effects in the statistical model were tested simultaneously, and the effects were removed from the original model when they were not significant. When the effect was statistically different (P < 0.05), further analysis was needed. One-way repeated measures ANOVA was applied for post hoc group comparisons.
Results and discussions
Blood Parameters
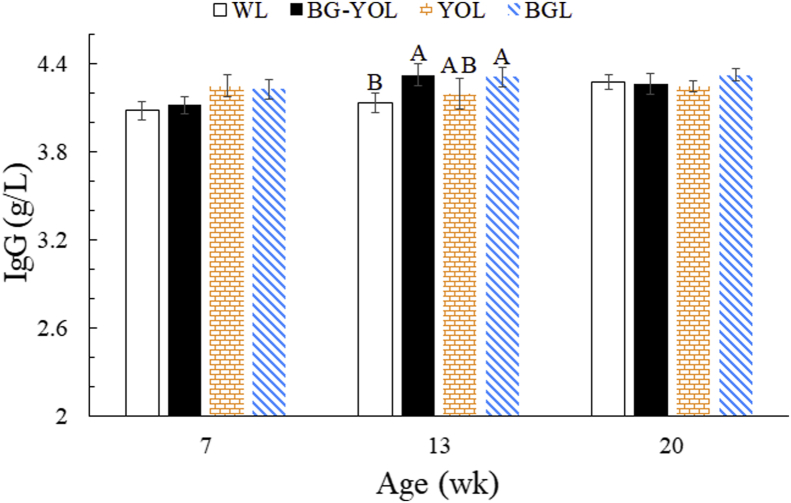
It is well known that antibodies, such as Ig, are an important component of the body's humoral immune system to protect the body from bacteria and viruses (Narat, 2003). The Ig concentrations reflect the performance of the immune system. Figure 4 shows the changes in the IgG level of the layer chickens under different LED light treatments. No significant differences in IgG concentration levels in the pullets were found in the different treatment groups at 7 wk and 20 wk of age (P > 0.05). Our results are not consistent with a previous result where the immune function of hens in the red light group was significantly greater than in the green and blue light groups (Scott and Siopes, 1994). One main reason might be the difference in the light sources, photostimulation time, and chicken breed (we used local mountainous laying hens). Only when these factors get a certain threshold, the layer chickens may make a full response to the stimulation of the light spectrum. However, at 13 wk of age, the IgG contents from the BGL group and the BG-YOL group were higher than that for the WL group. This confirms the results of a previous study where the immune function of poultry was affected by light color (Rozenboim et al., 1999; Cao et al., 2008), and blue light has been shown to enhance the immune response (Xie et al., 2008a; Hassan et al., 2013; Zhang et al., 2014). These results might have been due to the calming effect of the blue treatment on chicken activity (Prayitno et al., 1997). Moreover, blue light would alleviate the negative effects induced by the stress response, subsequently leading to a well-balanced immune response status. Hence, blue light may play a vital role in alleviating the stress response and improve the immune level in poultry (Xie et al., 2008a). Light color can affect the immune performance of chickens in the early part of the rearing period and may subsequently influence the mortality of layers during the laying period.
Figure 4.
The effect of different mixed color LED treatments on serum IgG content in laying hens. Data are presented as means ± SE. A, BDifferent capital letters within a column indicate significant differences at same week of age (P < 0.05). Abbreviations: BG-YOL group, blue–green (435–565 nm) light at phase 1 (1 D–13 wk) followed by yellow–orange (565–630 nm) light at phase 2 (14–20 wk); BGL group, blue–green (435–565 nm) light at phase 1 (1 D–13 wk) and phase 2 (14–20 wk); LED, light-emitting diode; WL group, white (400–700 nm) light at phase 1 (1 D–13 wk) and phase 2 (14–20 wk); YOL group, yellow–orange LED (565–630 nm) light at phase 1 (1 D–13 wk) and phase 2 (14–20 wk).
In addition, the photoreceptors in the hypothalamus of the poultry are more sensitive to blue and green light than red light (Osorio and Vorobyev, 2008). Pullets receive external light mainly through the eyes (Hart, 2001) during their growing periods. The retina receptors in the eye respond to spectral stimuli easily to form vision and arrive at the hypothalamus to adjust the circadian rhythm and promote the growth of birds. Thus, we concluded that short-wavelength (blue and green) light through the eye, arriving at the hypothalamus, helped the chickens remain calm and quiet and reduced the response on the environment, promoting the immune performance of the pullets. Some studies thought that the light color might affect the immune function indirectly via hormonal intermediates, such as affecting the melatonin or prolactin levels (Scott and Siopes, 1994). However, the mechanism by which blue and green light affects the immune performance of laying hens remains to be studied.
The biochemical blood parameters of the laying hens during the full trial period (0–20 wk of age) are summarized in Table 2. The total protein content at 20 wk of age significantly differed between the BGL group and the other 2 treatments (the BG-YOL group and YOL group). This did not correspond to the results of the study by Hassan et al. (2013). These might be because of the differences in the photoperiod or time of exposure to the different light combinations or the age of the layer chickens. In addition, the increasing of TP content level enhances the body immunity.
Table 2.
The effects of different mixed color light-emitting diode (LED) lights on the biochemical blood parameters of laying hens at 20 wk of age.
| Parameter | Treatment group |
|||
|---|---|---|---|---|
| WL group | BG-YOL group | YOL group | BGL group | |
| TP (g/L) | 39.54 ± 0.86a,b | 37.59 ± 0.84b | 37.91 ± 1.11b | 40.67 ± 0.84a |
| GLU (mmol/L) | 15.82 ± 0.18a | 15.53 ± 0.37a,b | 15.71 ± 0.15a | 14.92 ± 0.20b |
| TG (mmol/L) | 13.49 ± 2.10b | 11.76 ± 2.25b | 13.09 ± 2.92b | 20.54 ± 2.01a |
| Ca (mmol/L) | 8.27 ± 0.73b | 7.91 ± 0.74b | 10.18 ± 0.87a | 9.64 ± 0.43a,b |
| P (mmol/L) | 4.33 ± 0.16a,b | 4.32 ± 0.12a,b | 4.06 ± 0.16b | 4.56 ± 0.12a |
a,bDifferent lowercase letters within a row indicate significant differences (P < 0.05).
Data are presented as means ± SE.
Abbreviations: BG-YOL group, blue–green (435–565 nm) light at phase 1 (1 D–13 wk) followed by yellow–orange (565–630 nm) light at phase 2 (14–20 wk); BGL group, blue–green (435–565 nm) light at phase 1 (1 D–13 wk) and phase 2 (14–20 wk); Ca, calcium; GLU, glucose; P, phosphorus; TG, triglyceride; TP, total protein; WL group, white (400–700 nm) light at phase 1 (1 D–13 wk) and phase 2 (14–20 wk); YOL group, yellow–orange LED (565–630 nm) light at phase 1 (1 D–13 wk) and phase 2 (14–20 wk).
At the age of 20 wk, the blood GLU concentrations were improved after the YOL group treatment, which corresponded to the results of the study by Hassan et al. (2013). One reason was that the hens raised under red light were more active and generated more energy (Sultana et al., 2013a,b). There were significant differences in the TG contents at 20 wk of age between the BGL group and the other 3 groups. This may have been caused by the calming effect of the short wavelength on the poultry activity (Prayitno et al., 1997), which would cause fat accumulation in the bodies of laying hens. The decrease of activities and energy maintenance levels of hens would permit a greater proportion of energy to become lipid (Renema et al., 2001).
The blood GLU and TG concentration levels were previously used for stress indicators and as an indicator of the welfare status of hens (Yilmaz Dikmen et al., 2016). Therefore, we can see that blue and green light could improve the health and welfare of laying hens during the brooding and rearing periods. At 20 wk of age, the YOL treatment increased the serum Ca concentrations and decreased the serum P concentrations, which was consistent with the previous research that both Ca and P showed antagonism effects (Li et al., 2014b). On the other hand, the increase of Ca absorption can promote the skeleton mineralization (Zhang et al., 2006). When the serum Ca concentration decreases, Ca is mobilized from the bones to maintain the physiological blood level (Amoroso et al., 2013). Therefore, YOL may be useful for the skeleton growth and development of layer chickens by increasing the serum Ca concentrations during the brooding and rearing periods. However, whether this would have any long-term health benefits in reducing osteoporosis in laying hens at the end of the laying period is unclear, and a large amount of trial data is needed to confirm this experimental result.
Skeletal Development Parameters
The effect of different mixed color LED lights on the skeletal parameters of laying hens during the brooding and rearing periods is shown in Table 3. The BMD, BMC, and BA were often used as important indicators to evaluate the bone status (Park et al., 2003). Table 3 shows that the BMD level of the laying hens during the brooding and rearing periods increased as the birds aged. There were no significant changes in the BMD between 7 and 13 wk of age. However, at 20 wk of age, the BMD of the layer chickens in the YOL group was more than the BMD of the layer chickens in the WL group. No significant difference in the BMC level was detected in the different treatment groups at 7 and 13 wk of age (P > 0.05).
Table 3.
The effects of different mixed color LED treatments on the skeletal parameters of laying hens during the brooding and rearing periods.
| Age (wk) | Treatment group | Bone mineral density (g/cm2) | Bone mineral content (g) | Area (cm2) |
|---|---|---|---|---|
| 7 | WL | 0.144 ± 0.002 | 0.76 ± 0.02 | 5.27 ± 0.14b |
| BG-YOL | 0.143 ± 0.002 | 0.79 ± 0.03 | 5.49 ± 0.14a | |
| YOL | 0.142 ± 0.001 | 0.75 ± 0.02 | 5.26 ± 0.13b | |
| BGL | 0.141 ± 0.002 | 0.78 ± 0.03 | 5.57 ± 0.18a | |
| 13 | WL | 0.169 ± 0.002 | 1.33 ± 0.03 | 7.87 ± 0.13a |
| BG-YOL | 0.171 ± 0.003 | 1.32 ± 0.04 | 7.70 ± 0.16b | |
| YOL | 0.172 ± 0.001 | 1.29 ± 0.02 | 7.51 ± 0.09c | |
| BGL | 0.169 ± 0.003 | 1.29 ± 0.03 | 7.62 ± 0.14b | |
| 20 | WL | 0.206 ± 0.005b | 1.64 ± 0.05a | 7.92 ± 0.09a |
| BG-YOL | 0.210 ± 0.004a,b | 1.57 ± 0.06b | 7.47 ± 0.23c | |
| YOL | 0.215 ± 0.003a | 1.64 ± 0.06a | 7.60 ± 0.26b | |
| BGL | 0.209 ± 0.002a,b | 1.66 ± 0.04a | 7.97 ± 0.17a |
a–cDifferent lowercase letters within a column indicate significant differences at same week of age (P < 0.05).
Data are presented as means ± SE.
Abbreviations: BG-YOL group, blue–green (435–565 nm) light at phase 1 (1 D–13 wk) followed by yellow–orange (565–630 nm) light at phase 2 (14–20 wk); BGL group, blue–green (435–565 nm) light at phase 1 (1 D–13 wk) and phase 2 (14–20 wk); LED, light-emitting diode; WL group, white (400–700 nm) light at phase 1 (1 D–13 wk) and phase 2 (14–20 wk); YOL group, yellow–orange LED (565–630 nm) light at phase 1 (1 D–13 wk) and phase 2 (14–20 wk).
At 20 wk of age, the BMC level of the layer chickens in the BG-YOL group was more than that of the layer chickens in other 3 groups. A significant difference in the BA level was detected in the different groups at 7, 13, and 20 wk of age. However, there are no obvious conclusions regarding the BA level of the hens over the whole experiment. Previously published results demonstrated that the bone development of chickens during the brooding and rearing periods determined the egg production performance and mortality in the late egg production stage (Hester et al., 2011). In addition, the BMD, a very important measure of bone quality, is usually proportional to the bone quality (Riczu et al., 2004) and positively related to the bone breaking strength (Mccoy et al., 1996).
Being raised in cages is the primary reason for osteoporosis development in chickens (Rennie et al., 1997), rather than nutrition, though poor nutrition (in terms of Ca and P content or availability) may exacerbate the osteoporosis condition (Bishop et al., 2000). Allowing birds more exercise will improve their bone strength (Whitehead and Fleming, 2000). Moreover, laying hens raised under the red light were more active and performed more walking behaviors (Sultana et al., 2013a,b). Thus, we propose that YOL can increase the activity of layer chickens to increase the BMD content.
Researchers proved that hens spent more inactive time sitting or sleeping under blue light, whereas birds illuminated with red light were more active and engaged in more ground scratching behaviors (Hassan et al., 2014; Sultana et al., 2013a,b). Hence, YOL could have a positive effect on improving the bone quality of pullets. However, longer-term studies and a larger sample size are needed to validate that possibility.
Sexual Development Parameters
There were no significant changes in the development of the sexual organs in different groups at the age of 16 wk (Table 4). At the age of 20 wk, the length of the oviduct, the weight of the oviduct, and the weight of the ovary of laying hens in the BG-YOL group were more than those in the WL group. This disagrees with the previously published results that gonadal development was not affected by light color (Pyrzak and Siopes, 1986); the difference in light sources, photoperiod, light intensity, age, and nutrition may be the reasons for the different observations of the 2 studies.
Table 4.
The effects of different mixed color LED treatments on the sexual organs of laying hens at 16 and 20 wk of age.
| Age (wk) | Treatment group | The length of oviduct (cm) | The weight of oviduct (g) | The weight of ovary (g) |
|---|---|---|---|---|
| 16 | WL | 10.07 ± 0.46 | 1.8 ± 0.17 | 0.9 ± 0.12 |
| BG-YOL | 9.95 ± 0.58 | 1.9 ± 0.13 | 0.8 ± 0.07 | |
| YOL | 9.17 ± 0.45 | 1.8 ± 0.11 | 0.9 ± 0.09 | |
| BGL | 9.50 ± 0.31 | 1.7 ± 0.09 | 0.8 ± 0.07 | |
| 20 | WL | 42.42 ± 7.18b | 36.6 ± 10.08b | 19.0 ± 6.21b |
| BG-YOL | 59.17 ± 3.83a | 63.3 ± 9.37a | 25.0 ± 6.46a | |
| YOL | 45.67 ± 14.09b | 45.1 ± 19.42b | 20.2 ± 8.85a,b | |
| BGL | 62.17 ± 4.56a | 60.6 ± 10.66a | 22.6 ± 5.46a,b |
a,bDifferent lowercase letters within a column indicate significant differences at same week of age (P < 0.05).
Data are presented as means ± SE.
Abbreviations: BG-YOL group, blue–green (435–565 nm) light at phase 1 (1 D–13 wk) followed by yellow–orange (565–630 nm) light at phase 2 (14–20 wk); BGL group, blue–green (435–565 nm) light at phase 1 (1 D–13 wk) and phase 2 (14–20 wk); LED, light-emitting diode; WL group, white (400–700 nm) light at phase 1 (1 D–13 wk) and phase 2 (14–20 wk); YOL group, yellow–orange LED (565–630 nm) light at phase 1 (1 D–13 wk) and phase 2 (14–20 wk).
In addition, YOL can penetrate the skull of poultry to the hypothalamus, stimulating hormone secretion (Lewis and Morris, 2000). Therefore, when the light stimulates the hypothalamus, the body secretes gonadotropin-releasing hormone, which promotes the secretion of follicle-stimulating hormone and luteinizing hormone in the anterior pituitary and finally promotes the development of the oviduct and ovary. All in all, these sex hormones can enhance the growth and number of ovarian follicles, which may be associated with the rate of total egg production. However, the length and weight of the oviduct of hens in the BGL groups were higher than those of the hens in the YOL group. The weight of the ovary of hens in the BGL groups were similar to those of hens in the YOL group. In contrast, some researchers reported that red light increased the weights of reproductive organs (higher weight of the ovary and oviduct) of laying hens (Reddy et al., 2012; Li et al., 2014a). This might be owing to the influence of the light color on the oviducts of poultry as a result of the gonadal hormone concentrations and other hormone levels or other physiological factors. We assumed that BGL might affect the dietary behavior of hens to promote the growth and development of the oviduct. The sexual organs (oviduct and ovary) are only one part of the reproductive traits, and the development of the oviduct might not exactly reflect the overall sexual development.
Unlike mammals, the nonvisual effects of hens are more important than the visual effects, and the perception of light during reproduction does not depend on the photoreceptors of the eye (Jácome et al., 2014). Studies demonstrated that the photoreceptor of the hypothalamus is a bioconverter that converts photon energy into nerve impulses, which affects and controls the endocrine system of ovarian activity, which in turn affects the reproductive, behavioral, and secondary sexual characteristics of laying hens (Morris, 1973). Long-wavelength light can pass through the skull more easily than short-wavelength light and acts on the extraretinal photoreceptors of the hypothalamus (Lewis and Morris, 2000; Mobarkey et al., 2010; Huber-Eicher et al., 2013). Therefore, YOL is more likely to act on the hypothalamus than do BGL or WL, and this promotes the SM of laying hens. In accordance with these results, we can conclude that YOL can affect the reproductive development of the pullets, and we predict that the productivity of the layers may be affected by YOL.
Table 5 shows the effect of different mixed color LED treatments on the reproduction performance. The 50% egg production age of layer chickens in the BG-YOL group and YOL group was earlier than the other 2 groups. The YOL group improved the production uniformity, the BG-YOL group and the WL group were second, and the BGL group was the last. These results confirmed that long-wavelength (red, orange, and yellow) light can expedite the age of sexual maturation and promote sexual development of poultry, whereas short-wavelength (green) light can delay sexual maturation (Liu et al., 2017; Elkomy et al., 2019), as the long wavelength containing more energy would stimulate extraretinal photoreceptors, which reflects on the pituitary gland and deep brain and influences gonadal hormone (follicle-stimulating hormone, luteinizing hormone, and estradiol) secretion and the subsequent rapid development of the oviduct and ovary to promote egg laying (Hartwig and van Veen, 1979). However, the regulation of gonadotropin secretion in poultry is very complex (Li et al., 2014a). The mechanisms of how monochromatic light affects reproduction in layer chickens are still worth discussing.
Table 5.
The effects of different mixed color LED treatments on the reproduction performance.
| Treatment group | The age of first egg (D) | The age of 50% egg (D) | The age from first egg to 50% egg (D) |
|---|---|---|---|
| WL | 127 | 137 | 10 |
| BG-YOL | 124 | 133 | 9 |
| YOL | 126 | 133 | 7 |
| BGL | 121 | 135 | 14 |
Abbreviations: BG-YOL group, blue–green (435–565 nm) light at phase 1 (1 D–13 wk) followed by yellow–orange (565–630 nm) light at phase 2 (14–20 wk); BGL group, blue–green (435–565 nm) light at phase 1 (1 D–13 wk) and phase 2 (14–20 wk); LED, light-emitting diode; WL group, white (400–700 nm) light at phase 1 (1 D–13 wk) and phase 2 (14–20 wk); YOL group, yellow–orange LED (565–630 nm) light at phase 1 (1 D–13 wk) and phase 2 (14–20 wk).
Furthermore, researchers demonstrated that red, orange, or yellow light, as long-wavelength radiation, can pass through the hypothalamic extraretinal photoreceptors of poultry and stimulate the hypothalamus–pituitary growth axis to release the related hormones (Lewis and Morris, 2000), thereby having an accelerating effect on activity stimulation, sexual development, and the maturity of poultry (Baxter et al., 2014; Li et al., 2014a). In addition, this may be owing to the photostimulation of retinal photoreceptors, which are sensitive to short-wavelength (purple, blue, and green) light and appear to inhibit reproductive activity in birds (Mobarkey et al., 2010, 2013), as well as inhibit iodopsin (present in relatively large amounts in the chickens (Crescitelli et al., 1964)), which also affects the sexual development. The results in this study also supported the aforementioned theoretical analysis.
However, the age of 50% of pullets starting to lay eggs under blue and green light was earlier than those under white light, which disagreed with the previous observation that short-wavelength light could delay the SM of poultry (Min et al., 2012). There might exist a threshold for visual sensitivity in poultry's response to long-wavelength radiation, where long-wavelength light effects may occur only when the intensity of the short-wavelength light reaches a certain level (Benoit, 2006; Liu et al., 2017). For example hypothalamic photoreceptors of poultry might be stimulated by lower wavelength but high-intensity light is required to produce a clear response (Pang et al., 1974). More research regarding the comprehensive effect between light intensity and color on caged laying hens is needed to confirm the appropriate spectrum parameters.
The color and illumination intensity of light are believed to affect reproduction and production of poultry (Manser, 1996). Light sources of different wavelengths may be considered to have different intensities, and therefore, it is difficult to separate the effects on poultry of these 2 light characteristics (Prayitno et al., 1997). In this study, long-wavelength light can promote SM of chickens during the brooding and rearing periods, but the subsequent reproductive performance and productivity of layers during the egg laying period subjected to different light color and light intensity is still worth exploring.
Conclusions
Our results demonstrated that phased spectral control using LED lights could be beneficial to the growth and development of pullets. The BGL at phase 1 (0–13 wk of age) increased the serum Ig concentrations and serum GLU concentration levels of layer chickens. The YOL at phase 2 (14–20 wk of age) increased the BMD concentrations, promoted sexual organ (oviduct and ovary) development, advanced the age of sexual maturation, and improved the production uniformity of layer chickens. The results of this research should stimulate further studies on the effects of the exposure of chickens to different mixed color LED lights. To promote the welfare and health of laying hens, further research is needed on how the hormones in the serum and body are synthesized.
Acknowledgments
The animal study proposal was approved by The Laboratory Animal Ethical Committee of China Agricultural University. This study was funded by the National Key R&D Program of China (2017YFB0404000). Thanks to the colleagues of Shangzhuang experimental station and the department of Agricultural Structure and Bioenvironmental Engineering, College of Water Resources and Civil Engineering, China Agricultural University, Beijing, China.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Amoroso L., Baraldi A., Barreiro F., Pacheco M., Alva J., Soares N., Pacheco L., Melaré M. Bone densitometry and calcium serum levels in chickens treated with filtered or unfiltered water. Rev. Bras. Ciênc. Avíc. 2013;15:379–384. [Google Scholar]
- Baxter M., Joseph N., Osborne V.R., Bédécarrats G.Y. Red light is necessary to activate the reproductive axis in chickens independently of the retina of the eye. Poult. Sci. 2014;93:1289–1297. doi: 10.3382/ps.2013-03799. [DOI] [PubMed] [Google Scholar]
- Benoit J. The role of the eye and of the hypothalamus in the photostimulation of gonads in the duck. Ann. N. Y. Acad. Sci. 2006;117:204–215. doi: 10.1111/j.1749-6632.1964.tb48175.x. [DOI] [PubMed] [Google Scholar]
- Bishop S.C., Fleming R.H., McCormack H.A., Flock D.K., Whitehead C.C. Inheritance of bone characteristics affecting osteoporosis in laying hens. Br. Poult. Sci. 2000;41:33–40. doi: 10.1080/00071660086376. [DOI] [PubMed] [Google Scholar]
- Borille R., Garcia R.G., Nääs I.A., Caldara F.R., Santana M.R. Monochromatic light-emitting diode (LED) source in layers hens during the second production cycle. Rev. Bras. Eng. Agr. Amb. 2015;19:877–881. [Google Scholar]
- Cao J., Liu W., Wang Z., Xie D., Jia L., Chen Y. Green and blue monochromatic lights promote growth and development of broilers via stimulating testosterone secretion and myofiber growth. J. Appl. Poult. Res. 2008;17:211–218. [Google Scholar]
- Crescitelli F., Wilson B.W., Lilyblade A.L. The visual pigments of birds: I. The turkey. Vis. Res. 1964;4:275–280. doi: 10.1016/0042-6989(64)90018-5. [DOI] [PubMed] [Google Scholar]
- Elkomy H.E., Taha A.E., Basha H.A., Abo-Samaha M.I., Sharaf M.M. Growth and reproduction performance of Japanese quails (Coturnix coturnix japonica) under various environments of light colors. Slov. Vet. Res. 2019;56:119–127. [Google Scholar]
- Er D., Wang Z., Cao J., Chen Y. Effect of monochromatic light on the egg quality of laying hens. J. Appl. Poult. Res. 2007;16:605–612. [Google Scholar]
- Gongruttananun N. Influence of red light on reproductive performance, eggshell ultrastructure, and eye morphology in Thai-native hens. Poult. Sci. 2011;90:2855–2863. doi: 10.3382/ps.2011-01652. [DOI] [PubMed] [Google Scholar]
- Hart N. Variations in cone photoreceptor abundance and the visual ecology of birds. J. Comp. Physiol. A. 2001;187:685–697. doi: 10.1007/s00359-001-0240-3. [DOI] [PubMed] [Google Scholar]
- Hartwig H.G., van Veen T. Spectral characteristics of visible radiation penetrating into the brain and stimulating extraretinal photoreceptors. J. Comp. Physiol. 1979;130:277–282. [Google Scholar]
- Hassan M.R., Sultana S., Choe H.S., Ryu K.S. Effect of monochromatic and combined light colour on performance, blood parameters, ovarian morphology and reproductive hormones in laying hens. Ital. J. Anim. Sci. 2013;12:359–364. [Google Scholar]
- Hassan M.R., Sultana S., Choe H.S.S., Ryu K.S. Effect of combinations of monochromatic LED light color on the performance and behavior of laying hens. J. Poult. Sci. 2014;51:321–326. [Google Scholar]
- Hester P.Y., Wilson D.A., Settar P., Arango J.A., O’Sullivan N.P. Effect of lighting programs during the pullet phase on skeletal integrity of egg-laying strains of chickens. Poult. Sci. 2011;90:1645–1651. doi: 10.3382/ps.2011-01411. [DOI] [PubMed] [Google Scholar]
- Huber-Eicher B., Suter A., Spring-Stähli P. Effects of colored light-emitting diode illumination on behavior and performance of laying hens. Poult. Sci. 2013;92:869–873. doi: 10.3382/ps.2012-02679. [DOI] [PubMed] [Google Scholar]
- Jácome I.M.T.D., Rossi L.A., Borille R. Influence of artificial lighting on the performance and egg quality of commercial layers: a review. Braz. J. Poult. Sci. 2014;16:337–344. [Google Scholar]
- Lewis P.D., Morris T.R. Responses of domestic poultry to various light sources. Worlds Poult. Sci. J. 1998;54:7–25. [Google Scholar]
- Lewis P.D., Morris T.R. Light intensity and performance of domestic pullets. Worlds Poult. Sci. J. 1999;55:241–250. [Google Scholar]
- Lewis P.D., Morris T.R. Poultry and coloured light. World. Poult. Sci. J. 2000;56:189–207. [Google Scholar]
- Li D., Zhang L., Yang M., Yin H., Xu H., Trask J.S., Zhu Q. The effect of monochromatic light-emitting diode light on reproductive traits of laying hens. J. Appl. Poult. Res. 2014;23:367–375. [Google Scholar]
- Li M., Gao Y., Lan G., Gu Z.B. Effects of ultraviolet-B radiation on immunity and carcass characteristics in quail. J. Appl. Poult. Res. 2014;23:429–436. [Google Scholar]
- Li G., Li B., Zhao Y., Shi Z., Liu Y., Zheng W. Layer pullet preferences for light colors of light-emitting diodes. Animal. 2018;13:1245–1251. doi: 10.1017/S1751731118002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Xin H., Sekhon J., Wang T. Effect of fluorescent vs. poultry-specific light-emitting diode lights on production performance and egg quality of W-36 laying hens. Poult. Sci. 2017;97:834–844. doi: 10.3382/ps/pex371. [DOI] [PubMed] [Google Scholar]
- Manser C.E. Effects of lighting on the welfare of domestic poultry: a review. Anim. Welf. 1996;5:341–360. [Google Scholar]
- Mccoy M.A., Reilly G.A.C., Kilpatrick D.J. Density and breaking strength of bones of mortalities among caged layers. Res. Vet. Sci. 1996;60:185–186. doi: 10.1016/s0034-5288(96)90017-x. [DOI] [PubMed] [Google Scholar]
- Min J.K., Hossan M.S., Nazma A., Jae C.N., Han T.B., Hwan K.K., Ok S.S. Effect of monochromatic light on sexual maturity, production performance and egg quality of laying hens. Avian Biol. Res. 2012;5:69–74. [Google Scholar]
- Mobarkey N., Avital N., Heiblum R., Rozenboim I. The role of retinal and extra-retinal photostimulation in reproductive activity in broiler breeder hens. Domest. Anim. Endocrinol. 2010;38:235–243. doi: 10.1016/j.domaniend.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Mobarkey N., Avital N., Heiblum R., Rozenboim I. The effect of parachlorophenylalanine and active immunization against vasoactive intestinal peptide on reproductive activities of broiler breeder hens photostimulated with green light. Biol. Reprod. 2013;88:83. doi: 10.1095/biolreprod.112.103697. [DOI] [PubMed] [Google Scholar]
- Morris T.R. The Effects of ahemeral light and dark cycles on egg production in the fowl. Poult. Sci. 1973;52:423–445. doi: 10.3382/ps.0520423. [DOI] [PubMed] [Google Scholar]
- Narat M. Production of antibodies in chickens. Food Technol. Biotechnol. 2003;41:259–267. [Google Scholar]
- Newberry R.C., Hunt J.R., Gardiner E.E. Influence of light intensity on behavior and performance of broiler chickens. Poult. Sci. 1988;67:1020–1025. doi: 10.3382/ps.0671020. [DOI] [PubMed] [Google Scholar]
- Olanrewaju H.A., Wongpichet S., Thaxton J.P., Dozier W.A., Branton S.L. Stress and acid-base balance in chickens. Poult. Sci. 2006;85:1266–1274. doi: 10.1093/ps/85.7.1266. [DOI] [PubMed] [Google Scholar]
- Osorio D., Vorobyev M. A review of the evolution of animal colour vision and visual communication signals. Vis. Res. 2008;48:2042–2051. doi: 10.1016/j.visres.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Pang S.F., Ralph C.L., Reilly D.P. Melatonin in the chicken brain: its origin, diurnal variation, and regional distribution. Gen. Comp. Endocrinol. 1974;22:499–506. doi: 10.1016/0016-6480(74)90026-4. [DOI] [PubMed] [Google Scholar]
- Park S., Birkhold S., Kubena L., Nisbet D., Ricke S. Effect of storage condition on bone breaking strength and bone ash in laying hens at different stages in production cycles. Poult. Sci. 2003;82:1688–1691. doi: 10.1093/ps/82.11.1688. [DOI] [PubMed] [Google Scholar]
- Parvin R., Mushtaq M.M.H., Kim M.J., Choi H.C. Light emitting diode (LED) as a source of monochromatic light: a novel lighting approach for behaviour, physiology and welfare of poultry. World Poult. Sci. J. 2014;70:543–556. [Google Scholar]
- Prayitno D., Phillips C., Stokes D. The effects of color and intensity of light on behavior and leg disorders in broiler chickens. Poult. Sci. 1997;76:1674–1681. doi: 10.1093/ps/76.12.1674. [DOI] [PubMed] [Google Scholar]
- Pyrzak R., Siopes T.D. The effect of light color on egg quality of turkey hens in cages. Poult. Sci. 1986;65:1262–1267. [Google Scholar]
- Pyrzak R., Snapir N., Goodman G., Perek M. The effect of light wavelength on the production and quality of eggs of the domestic hen. Theriogenology. 1987;28:947–960. [Google Scholar]
- Reddy I.J., David C.G., Selvaraju S., Mondal S., Ravi Kiran G. GnRH-1 mRNA, LH surges, steroid hormones, egg production, and intersequence pause days alter in birds exposed to longer wavelength of light in the later stages of production in Gallus gallus domesticus. Trop. Anim. Health Prod. 2012;44:1311–1317. doi: 10.1007/s11250-012-0073-9. [DOI] [PubMed] [Google Scholar]
- Renema R.A., Robinson F.E. Effects of light intensity from photostimulation in four strains of commercial egg layers: 1. ovarian morphology and carcass parameters. Poult. Sci. 2001;80:1112–1120. doi: 10.1093/ps/80.8.1112. [DOI] [PubMed] [Google Scholar]
- Renema R.A., Robinson F.E., Oosterhoff H.H., Feddes J.J.R., Wilson J.L. Effects of photostimulatory light intensity on ovarian morphology and carcass traits at sexual maturity in modern and antique egg-type pullets. Poult. Sci. 2001;80:47–56. doi: 10.1093/ps/80.1.47. [DOI] [PubMed] [Google Scholar]
- Rennie J.S., Fleming R.H., McCormack H.A., McCorquodale C.C., Whitehead C.C. Studies on effects of nutritional factors on bone structure and osteoporosis in laying hens. Br. Poult. Sci. 1997;38:417–424. doi: 10.1080/00071669708418012. [DOI] [PubMed] [Google Scholar]
- Riczu C.M., Saunders-Blades J.L., Yngvesson A.K., Robinson F.E., Korver D.R. End-of-cycle bone quality in white- and brown-egg laying hens. Poult. Sci. 2004;83:375–383. doi: 10.1093/ps/83.3.375. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Biran I., Chaiseha Y., Yahav S., Rosenstrauch A., Sklan D., Halevy O. The effect of a green and blue monochromatic light combination on broiler growth and development. Poult. Sci. 2004;83:842–845. doi: 10.1093/ps/83.5.842. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Biran I., Uni Z., Robinzon B., Halevy O. The effect of monochromatic light on broiler growth and development. Poult. Sci. 1999;78:135–138. doi: 10.1093/ps/78.1.135. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Zilberman E., Gvaryahu G. New monochromatic light source for laying hens. Poult. Sci. 1998;77:1695–1698. doi: 10.1093/ps/77.11.1695. [DOI] [PubMed] [Google Scholar]
- Scott R., Siopes T. Light color: effect on blood cells, immune function and stress status in turkey hens. Comp. Biochem. Physiol. A Physiol. 1994;108:161–168. doi: 10.1016/0300-9629(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Shi H.P., Li B.M., Tong Q., Zheng W.C., Zeng D., Feng G.B. Effects of LED light color and intensity on feather pecking and fear responses of layer breeders in natural mating colony cages. Animals. 2019;9:814–828. doi: 10.3390/ani9100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana S., Hassan M.R., Choe H.S., Kang M.I., Ryu K.S. Effect of various LED light color on the behavior and stress response of laying hens. Indian J. Anim. Sci. 2013;83:829–833. [Google Scholar]
- Sultana S., Hassan M.R., Choe H.S., Ryu K.S. The effect of monochromatic and mixed LED light colour on the behaviour and fear responses of broiler chicken. Avian Biol. Res. 2013;6:207–214. [Google Scholar]
- Whitehead C.C., Fleming R.H. Osteoporosis in cage layers. Poult. Sci. 2000;79:1033–1041. doi: 10.1093/ps/79.7.1033. [DOI] [PubMed] [Google Scholar]
- Xie D., Wang Z.X., Dong Y.L., Cao J., Wang J.F., Chen J.L., Chen Y.X. Effects of monochromatic light on immune response of broilers. Poult. Sci. 2008;87:1535–1539. doi: 10.3382/ps.2007-00317. [DOI] [PubMed] [Google Scholar]
- Xie D., Wang Z., Cao J., Dong Y., Chen Y. Effects of monochromatic light on proliferation response of splencyte in broilers. Anat. Histol. Embryol. 2008;37:332–337. doi: 10.1111/j.1439-0264.2008.00849.x. [DOI] [PubMed] [Google Scholar]
- Yang Y.F., Jiang J.S., Pan J.M., Ying Y.B., Wang X.S., Zhang M.L., Chen X.H. The relationship of spectral sensitivity with growth and reproductive response in avian breeders (Gallus gallus) Sci. Rep. 2016;6 doi: 10.1038/srep19291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz Dikmen B., İpek A., Şahan Ü., Petek M., Sözcü A. Egg production and welfare of laying hens kept in different housing systems (conventional, enriched cage, and free range) Poult. Sci. 2016;95:1564–1572. doi: 10.3382/ps/pew082. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Cao J., Wang Z., Dong Y., Chen Y. Effect of a combination of green and blue monochromatic light on broiler immune response. J. Photochem. Photobiol. B Biol. 2014;138:118–123. doi: 10.1016/j.jphotobiol.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Zhang L., Shi Z., Wang X., Geng A., Li B. Effects of ultraviolet radiation on skeleton development of broiler chickens. Agr. Sci. China. 2006;5:313–317. [Google Scholar]