Abstract
Remdesivir has seen extensive use during the coronavirus disease-2019 pandemic given its clinically proven efficacy against severe acute respiratory syndrome coronavirus type 2. There has been little cited regarding adverse effects. Here we present the case of a patient with marked sinus bradycardia that began acutely on initiation of remdesivir and resolved almost immediately on cessation of the drug. (Level of Difficulty: Beginner.)
Key Words: bradycardia, cancer, cardiovascular disease, chest pain, electrocardiogram, electrophysiology, lethargy, physical examination, shortness of breath
Abbreviations and Acronyms: AV, atrioventricular; COVID-19, coronavirus disease-2019; ECG, electrocardiogram; h-mtRNAP, human mitochondrial RNA polymerase; LBBB, left bundle branch block
Graphical abstract
History of Presentation
A 54-year-old woman with a medical history notable for left bundle branch block (LBBB) of unknown origin, hypertension, and B-cell lymphoma being treated with lenalidomide and rituximab presented with a 3-week duration of worsening shortness of breath, fevers, and chills. On presentation she was febrile to 102.7°F, with other vital signs remaining within normal limits and oxygen saturations maintained at 95% on room air. Physical examination demonstrated bilateral diffuse expiratory wheezing.
Learning Objectives
-
•
To be aware of a possible rare adverse effect of remdesivir.
-
•
To understand the importance of assessing for ECG changes in patients with COVID-19.
-
•
To be vigilant for cardiac complications of COVID-19 (and its therapy) in patients with underlying cardiovascular disease.
Past Medical History
Her past medical history included LBBB, hypertension, and B-cell lymphoma.
Investigations
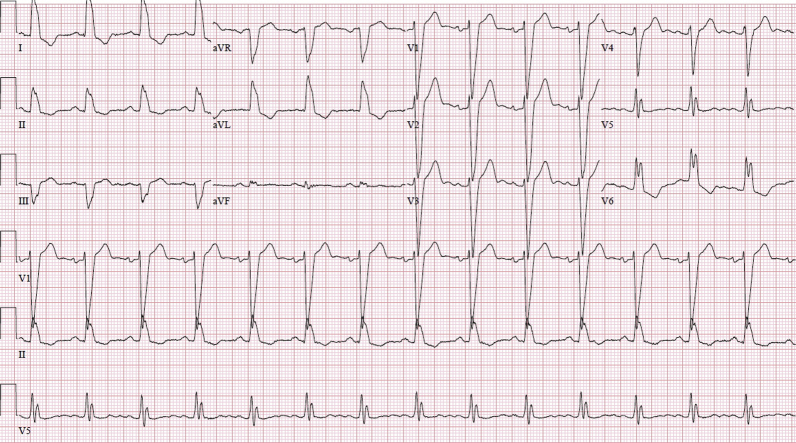
Laboratory test results were significant for the following: lymphopenia; ferritin, 1,125 ng/ml; lactate dehydrogenase, 324 U/l; aspartate transaminase, 58 U/l; and alanine transaminase, 58 U/l. The chest radiograph demonstrated new bilateral pulmonary infiltrates superimposed on pre-existing pulmonary nodules from her B-cell lymphoma. Computed tomography of the chest with contrast enhancement was performed to rule out pulmonary embolism. The scan demonstrated new ground-glass opacities present in addition to advanced lung infiltration from B-cell lymphoma. The electrocardiogram (ECG) demonstrated mild QRS complex widening from baseline (168 ms from 150 ms) (Figure 1). She was subsequently found to be positive for coronavirus disease-2019 (COVID-19) by real-time polymerase chain reaction. The patient’s last cycle of rituximab was several weeks before admission, and lenalidomide was held on the day of her admission.
Figure 1.
Admission Electrocardiogram Before, Remdesivir Administration, Demonstrates Normal Sinus Rhythm and Chronic Left Bundle Branch Block
Management
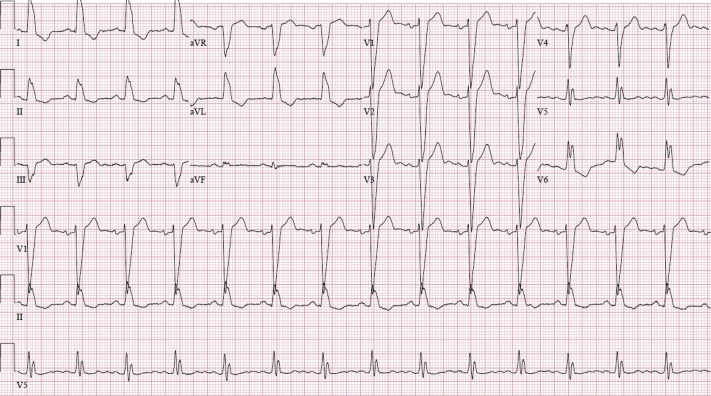
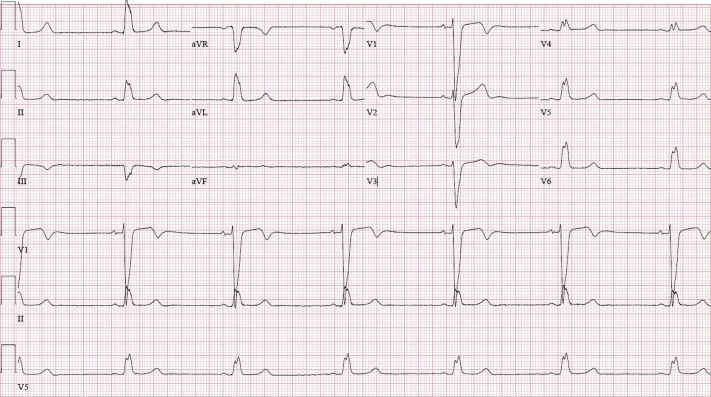
Oxygen delivered through a nasal cannula at 2 l/min resolved the patient’s sensation of dyspnea. Remdesivir was started on day 2 of her hospitalization, starting with a loading dose of 200 mg and proceeding with 100 mg daily for 5 additional days. Within 24 h, the patient was noted to be in sinus bradycardia to 38 beats/min on telemetry monitoring (baseline heart rate 60 to 70 beats/min). Other vital signs remained normal. Cardiac enzyme test results were negative. Her medications were monitored, and no known pro-bradycardic, arrhythmogenic, or cardiotoxic agents were identified. Her bradycardia was attributed to COVID-19 at the time. The patient also exhibited chronotropic responsiveness, and the decision was made to monitor the patient cautiously. In the following 2 days, her sinus bradycardia persisted, with her heart rate decreasing to 34 beats/min. She began to complain of dizziness and progressive, persistent crushing chest pressure accompanied by associated shortness of breath. Blood pressure had decreased, and she was now hypotensive. Cardiac enzyme test results remained negative. Subsequent ECG demonstrated sinus bradycardia and QRS complex widening to 170 ms (Figure 2). The decision was made to discontinue remdesivir and administer atropine at the bedside. Over the following days, the patient’s heart rate returned to her baseline of 60 to 70 beats/min, and her QRS complex decreased to 168 ms (Figure 3). Chest pressure and dizziness rapidly resolved as well.
Figure 2.
Electrocardiogram During Remdesivir Treatment Demonstrates Sinus Bradycardia, Chronic Left Bundle Branch Block, Worsening QRS Complex Widening, and QTc Interval Prolongation
Figure 3.
Electrocardiogram After Withdrawal of Remdesivir Demonstrates Normal Sinus Rhythm and Chronic Left Bundle Branch Block, With Resolution of QRS Complex Widening and QTc Interval Prolongation
Discussion
Remdesivir is a nucleoside analogue prodrug. Its triphosphorylated form resembles adenosine triphosphate, and it is used as a substrate of several viral RNA-dependent RNA polymerases, which it then subsequently inhibits. It demonstrates in vivo and in vitro activity against nonsegmented negative-sense RNA viruses, including the Filoviridae, Paramyxoviridae, Pneumoviridae, and Coronaviridae (Middle East respiratory syndrome coronavirus, severe acute respiratory syndrome coronavirus, severe acute respiratory syndrome coronavirus type 2) families (1). In vitro, remdesivir is a substrate of cytochrome P 450 (CYP) 2C8, CYP2D6, and CYP3A4, as well as organic anion transporting polypeptide 1B1 (OATP1B1) and P-glycoprotein (P-gp) transporters. It inhibits CYP3A4, OATP1B1, OATP1B3, bile salt export pump (BSEP), multidrug resistance protein 4 (MRP4), and sodium and taurocholate cotransporting polypeptide (NTCP). Inactive ingredients within the remdesivir injection include sulfobutylether-β-cyclodextrin sodium salt (SBECD) and water and may include hydrochloric acid and/or sodium hydroxide for pH adjustment (2).
Despite the emergent and widespread use of remdesivir during the COVID-19 pandemic, little information exists regarding the drug’s cardiac side effects. During the Ebola pandemic of 2016, a randomized controlled trial of various antiviral agents including remdesivir was performed on 673 subjects. One incident of hypotension and cardiac arrest that occurred during transfusion of the loading dose of remdesivir was recorded (3). In the initial compassionate use trial of remdesivir in COVID-19 (n = 53), hypertension developed in 8 patients, and atrial fibrillation developed in 6 patients. This study was limited in that it did not have a control group (4). A subsequent randomized controlled trial of 233 patients cited 1 patient with cardiac arrest during remdesivir therapy and none noted in the placebo group (5). The ACTT-1 (Adaptive COVID-19 Treatment Trial) demonstrated a 1.1% incidence of cardiac arrest in the experimental group compared with 1.0% in the control group, as well as a 4% incidence of serious atrial fibrillation (2% control) (6).
Despite the rare incidence of cardiac side effects of remdesivir, a possible mechanism exists on review of existing literature. The inherent safety and efficacy of remdesivir, like that many other antiviral nucleotide analogues, partly depend on its affinity for viral RNA polymerase compared with human mitochondrial RNA polymerase (h-mtRNAP). In studies performed during the Ebola pandemic, remdesivir was found to have an affinity for viral polymerases >500 times that of h-mtRNAP (7). Although this affinity for viral RNA polymerase over h-mtRNAP is significant, the possibility of h-mtRNAP involvement and subsequent mitochondrial dysfunction is still plausible, with drug-induced mitochondrial dysfunction from other agents being a well-known cause of observed cardiotoxicity (8).
It is known that the endogenous nucleoside adenosine has intrinsic electrophysiological properties, given its use as an antiarrhythmic drug. Specifically, adenosine is most commonly used in atrioventricular (AV) nodal inhibition in the setting of supraventricular tachycardias and works by binding to the A1 receptor on cardiac cells, thereby causing a cascade of changes that blocks AV nodal conduction (9). Given remdesivir’s status as a nucleoside analogue that resembles adenosine triphosphate, investigation into expression of similar effects on the AV node may be warranted. A theoretical partial affinity for the A1 receptor may explain the transient QRS complex prolongation observed in our patient. The other components of the remdesivir injection (SBECD, water, and small amounts of hydrochloric acid or sodium hydroxide) are not known to cause clinically evident cardiac dysfunction.
During her hospitalization, our patient was not given any other medications known to cause cardiac dysfunction. She did have some underlying form of cardiovascular disease in the form of a chronic LBBB of unknown origin, however. An echocardiogram performed 2 years earlier demonstrated an ejection fraction of 64% with no other significant abnormality. A nuclear stress test the following year demonstrated a fixed defect in the anteroseptal segments that was thought to be the result of overlying breast tissue, otherwise without abnormality.
Follow-Up
The patient had an unremarkable hospital course after discontinuation of the remdesivir and was discharged home with outpatient follow up. In clinic a month after discharge, it was confirmed that her bradycardia, chest pain, and dizziness had not returned.
Conclusions
Although the data from remdesivir’s initial use indicate a clear clinical benefit for most patients, little information exists on the drug’s potential side effects, specifically those effects on the cardiovascular system. The presence of bradycardia in our patient that began briskly with the administration of remdesivir and subsided immediately after its discontinuation raises significant suspicion that remdesivir was the causative factor. Further surveillance and research are needed to assess for possible mechanisms of cardiotoxicity in the general population, as well as in patients with underlying cardiovascular disease. It is our hope that these data will increase the overall safety and efficacy of remdesivir, to the global benefit of all who receive it.
Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.Gordon C.J., Tchesnokov E.P., Woolner E. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J Biol Chem. 2020;295:6785–6797. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilead Sciences . U.S. Food and Drug Administration; 2020. Remdesivir [package insert] [Google Scholar]
- 3.Mulangu S., Dodd L.E., Davey R.T. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381:2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grein J., Ohmagari N., Shin D. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y., Zhang D., Du G. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beigel J.H., Tomashek K.M., Dodd L.E. Remdesivir for the treatment of Covid-19 — preliminary report. N Engl J Med. 2020;383:994. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 7.Tchesnokov E.P., Feng J.Y., Porter D.P., Götte M. Mechanism of inhibition of Ebola virus RNA-dependent RNA polymerase by remdesivir. Viruses. 2019;11:326. doi: 10.3390/v11040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varga Z.V., Ferdinandy P., Liaudet L., Pacher P. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am J Physiol Heart Circ Physiol. 2015;309:H1453–H1467. doi: 10.1152/ajpheart.00554.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zipes D.P., Libby P., Bonow R.O., Mann D.L., Tomaselli G.F., Braunwald E., editors. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 11th edition. Elsevier/Saunders; Philadelphia, PA: 2018. [Google Scholar]