Abstract
Background
A seroprevalence study can estimate the percentage of people with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies in the general population; however, most existing reports have used a convenience sample, which may bias their estimates.
Methods
We sought a representative sample of Connecticut residents, ages ≥18 years and residing in noncongregate settings, who completed a survey between June 4 and June 23, 2020, and underwent serology testing for SARS-CoV-2-specific immunoglobulin G (IgG) antibodies between June 10 and July 29, 2020. We also oversampled non-Hispanic black and Hispanic subpopulations. We estimated the seroprevalence of SARS-CoV-2-specific IgG antibodies and the prevalence of symptomatic illness and self-reported adherence to risk-mitigation behaviors among this population.
Results
Of the 567 respondents (mean age 50 [± 17] years; 53% women; 75% non-Hispanic white individuals) included at the state level, 23 respondents tested positive for SARS-CoV-2-specific antibodies, resulting in weighted seroprevalence of 4.0 (90% confidence interval [CI] 2.0-6.0). The weighted seroprevalence for the oversampled non-Hispanic black and Hispanic populations was 6.4% (90% CI 0.9-11.9) and 19.9% (90% CI 13.2-26.6), respectively. The majority of respondents at the state level reported following risk-mitigation behaviors: 73% avoided public places, 75% avoided gatherings of families or friends, and 97% wore a facemask, at least part of the time.
Conclusions
These estimates indicate that the vast majority of people in Connecticut lack antibodies against SARS-CoV-2, and there is variation by race and ethnicity. There is a need for continued adherence to risk-mitigation behaviors among Connecticut residents to prevent resurgence of COVID-19 in this region.
Keywords: Antibodies, Connecticut, COVID-19, SARS-CoV-2, Seroprevalence
Clinical Significance.
-
•
Our results show that despite Connecticut having an early outbreak of coronavirus disease 2019 (COVID-19), a majority of people in Connecticut lack detectable antibodies to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and, as such, remain vulnerable to infection.
-
•
There is continued need for strong public health efforts encouraging Connecticut residents to continue adherence to risk-mitigation behaviors so as to prevent resurgence of the virus in the region.
Alt-text: Unlabelled box
Introduction
Connecticut was one of the first states in the United States to be severely affected by coronavirus disease 2019 (COVID-19), with its first confirmed case of COVID-19 in early March. While almost 43,000 cases and 4000 deaths were reported by June,1 a seroprevalence study, which estimates the percentage of people with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies, may provide a more accurate estimate of the percentage of Connecticut population with evidence of a prior infection from COVID-19.
Prior seroprevalence studies have estimated the spread of COVID-19 in the United States.2, 3, 4, 5, 6, 7, 8 However, the majority have taken advantage of blood samples collected for other reasons or used a convenience sample, which limits their representativeness. The Centers for Disease Control and Prevention (CDC) conducted a seroprevalence survey in Connecticut using blood specimens collected at commercial laboratories.8 However, these specimens were produced as part of routine or sick visits, representing a biased sample. Moreover, this effort did not provide the reason for the blood collection or information about recent symptomatic illness, underlying conditions, or relevant risk-mitigation behaviors, which may help predict detection of antibodies against SARS-CoV-2.
Accordingly, with support from the Connecticut Department of Public Health (DPH) and the CDC, we conducted the Post-Infection Prevalence (PIP) Study, a public health surveillance project to determine the seroprevalence of SARS-CoV-2 among adults residing in community noncongregate settings in Connecticut before June. Specifically, we sought to understand prior spread at the state level; collect information about symptomatic illness, risk factors for virus infection, and self-reported adherence to risk-mitigation behaviors; compare our seroprevalence estimates to available Connecticut estimates; and provide targeted estimates for the non-Hispanic black and Hispanic populations.
Methods
Study Cohort
For the state-level seroprevalence estimate, from June 4 to June 23, 2020, we enrolled 735 adults residing in noncongregate settings (ie, excluding individuals living in long-term care facilities, assisted living facilities, nursing homes, and prisons or jails) in Connecticut, ages ≥18 years, using a dual-frame Random Digit Dial (RDD) methodology.9 Additionally, from June 23 to July 22, 2020, we oversampled non-Hispanic black (n = 269) and Hispanic (n = 341) individuals to provide more accurate estimates for these subpopulations. Details of the sample size calculation and RDD methodology are described in eMethods 1, available online. Details of participant recruitment are described in eMethods 2, available online. We contacted a total of 7305 respondents at the state level and successfully completed 735 interviews. We contacted a total of 12,508 respondents for the oversampled subpopulations, of whom 457 completed interviews.
The study was deemed not to be research by the institutional review board at Yale University because of the public health surveillance activity exclusion and was approved by the institutional review board at Gallup.
Survey Components
Individuals selected were provided study details, and informed consent was obtained from all participants by trained interviewers. Participants were interviewed using a questionnaire that collected information on demographics, social determinants of health, history of influenza-like-illness, symptoms experienced, and other COVID-19-related topics. The average survey time was 15 minutes.
Specimen Collection and Serology Testing
Within 24-48 hours of completing the interview, respondents were contacted to schedule their blood draw appointment at their nearest Quest Diagnostics Patient Service Center (PSC). Up to 5 attempts were made to each household where the participant agreed to be tested. On confirmation that the participant had completed the test, an incentive payment of $50 was sent as a gift card via email or mail. Beginning July 17, 2020, we offered participants an additional $50 (for a total compensation of $100) to incentivize completion of the serology test.
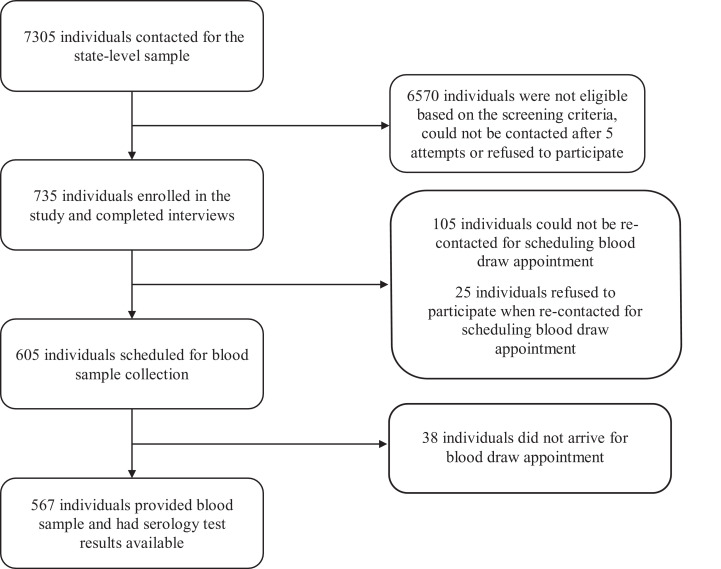
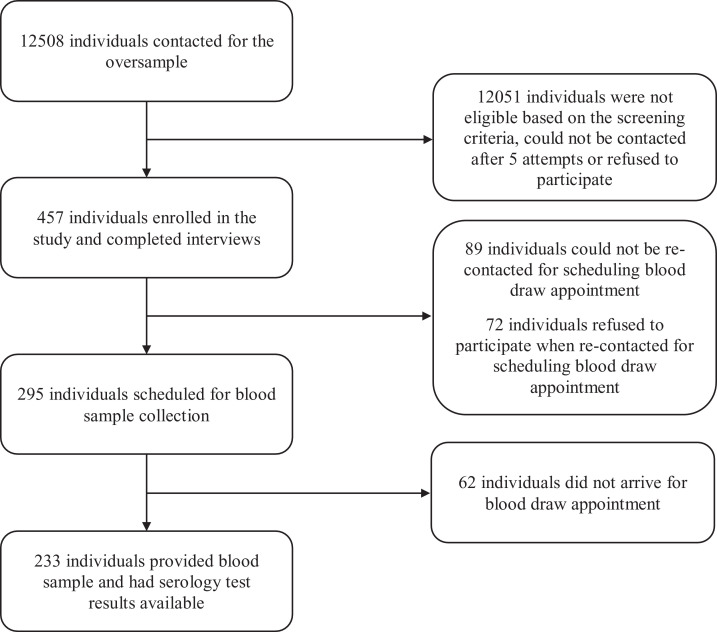
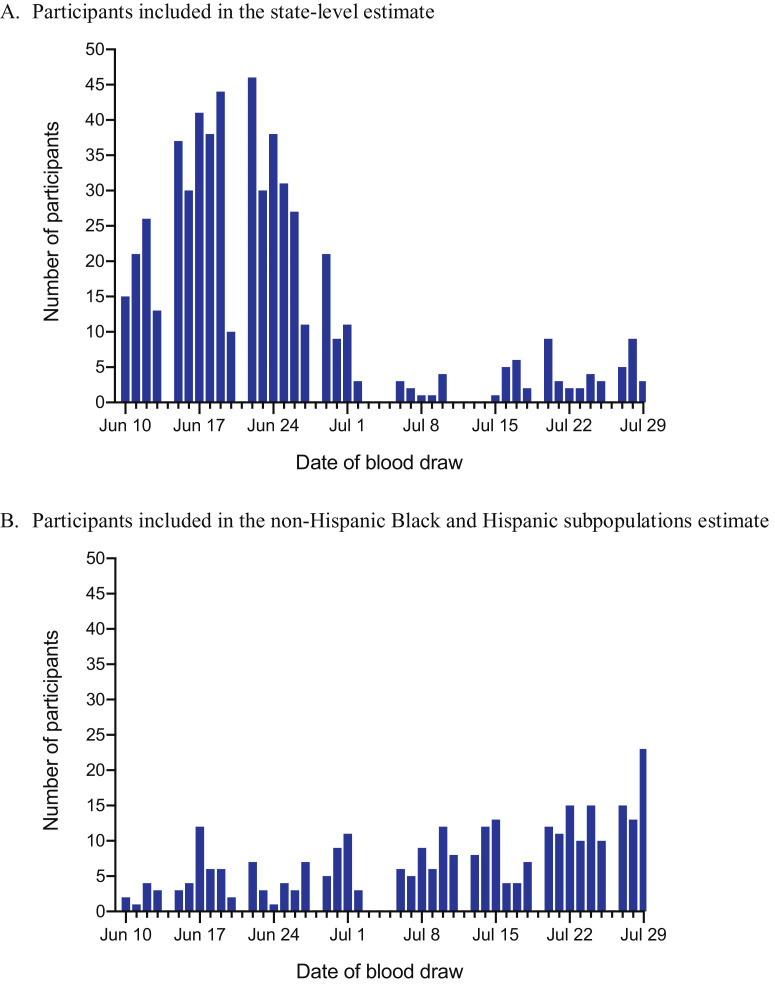
Of the 735 participants enrolled in the state-level estimate, 25 participants refused to participate when recontacted for scheduling and 567 participants completed serology testing at 93 Quest Diagnostics PSCs throughout Connecticut between June 10 and July 29, 2020 (eFigure 1 , available online). Of the total 341 Hispanic and 269 non-Hispanic black participants enrolled for the oversample estimate, 171 and 148 participants, respectively, completed serology testing (eFigure 2 , available online). The distribution of the timing of the blood draws is shown in eFigure 3 , available online.
eFigure 1.
Flowchart showing sample selection for the state-level estimate.
eFigure 2.
Flowchart showing sample selection for the oversample estimate.
eFigure 3.
Distribution of the timing of the blood draws between June 10 and July 29, 2020, for the state-level estimate (A) and the subpopulation estimate (B).
Sera were obtained from samples collected in BD Hemogard serum separator tubes. All samples were processed at the Quest Diagnostics Marlborough Laboratory. Samples were run at room temperature using the primary collection tube. We measured immunoglobulin G (IgG) SARS-CoV-2 antibodies using the Ortho-Clinical Diagnostics Vitros anti-SARS-CoV-2 IgG test, which detects antibodies against the spike glycoprotein of the virus.10 Antibody levels were expressed as the ratio of the chemiluminescence signal over the cutoff value, with a value ≥1.00 reported as positive.11 The Ortho Vitros IgG test had a reported sensitivity and specificity of 90% and 100%, respectively.10 We validated the sensitivity of this test in a small subset of patients who were positive for SARS-CoV-2 (n = 36) with variable disease severity, using reverse transcription polymerase chain reaction testing as the gold standard.12
Additionally, given the concern about the accuracy of serology tests,13 we retested the negative samples from 5 high-risk cities of Connecticut (ie, Bridgeport, Hartford, New Haven, Stamford, and Waterbury) with the Abbott Architect SARS-CoV-2 IgG test that detects antibodies aimed at a different SARS-CoV-2 antigen (nucleocapsid protein).14
Finally, Quest Diagnostics provided results for all SARS-CoV-2 serology tests conducted throughout Connecticut in the same time period (ie, June 10 and July 29, 2020) for comparison.
Statistical Analysis
The sample data were weighted to approximate the Connecticut population (details described in eMethods 3, available online). Briefly, the base weight assigned to each completed survey was derived as the product of the inverse of the probability of selection and nonresponse adjustment. Next, poststratification weighting adjustments were made to account for residual nonresponse and to match the weighted sample estimates to known population characteristics for Connecticut. Poststratification weighting for the state-level sample was carried out using ranking (or Iterative Proportional Fitting) procedures to adjust for age, gender, race or ethnicity, and education. The categories chosen for weighting the oversample subpopulations were different from what was used for the state-level adjustments due to lower available sample sizes. To reduce the effect of extreme weights on sampling variance, final weights were trimmed. The margin of error (MOE) for this study was calculated at the 90% confidence level (CI) taking into consideration the design effect introduced by variability of weights on each survey estimate. Overall study design effect as estimated by the Kish approximation equals 1.83; however, it varies by each survey estimate.
Next, the unweighted seroprevalence was calculated for both the overall state-level sample and the oversampled non-Hispanic black and Hispanic subgroups. Finally, we estimated the weighted state-level seroprevalence and the MOE of these estimates, both overall and for subgroups with sufficient sample size. Subgroups with sample sizes < 30 were too small to calculate accurate estimates and were thus not reported. We also estimated the MOE at 95% CI for the state-level estimates as a secondary outcome. We reported the weighted seroprevalence for non-Hispanic black and Hispanic subgroups separately.
All statistical analyses were performed using SPSS 24.0 (SPSS, Inc. Chicago, Ill.) and R version 4.0.2. We considered 2-sided P values < 0.05 as statistically significant.
Results
Population Characteristics for the State-Level Sample
The final state-level sample included 567 respondents who completed both the survey and the serology test. The mean age of the weighted sample was 50.1 (± 17.2) years, 53% were women, and the majority (75%) were non-Hispanic white individuals. Other weighted and unweighted characteristics of the study sample are reported in Table 1 .
Table 1.
Sociodemographic and Clinical Characteristics of Adults Included in the Study for the State-Level Estimate
| Characteristics | Unweighted N | Unweighted Proportion, % | Weighted Proportion, % | Target Percentage,* % |
|---|---|---|---|---|
| Overall | 567 | — | 567 | — |
| Age group, years | ||||
| 18-29 | 41 | 7.2 | 13.1 | 19.9 |
| 30-44 | 90 | 15.9 | 26.7 | 22.9 |
| 45-54 | 113 | 19.9 | 18.6 | 17.5 |
| 55-64 | 134 | 23.6 | 18.6 | 18.1 |
| ≥65 | 187 | 33.0 | 23.0 | 21.6 |
| Sex | ||||
| Men | 244 | 43.0 | 46.6 | 48.1 |
| Women | 323 | 57.0 | 53.4 | 51.9 |
| Race/ethnicity | ||||
| Hispanic | 49 | 8.6 | 13.0 | 14.4 |
| Non-Hispanic White | 470 | 82.9 | 74.9 | 69.4 |
| Non-Hispanic Black | 37 | 6.5 | 9.6 | 9.8 |
| Non-Hispanic Asian | 9 | 1.6 | 1.2 | 4.7 |
| Non-Hispanic Other | 5 | 0.9 | 1.7 | 1.7 |
| Education level | ||||
| Less than high school | 7 | 1.2 | 3.7 | 9.3 |
| High school or GED | 79 | 13.9 | 33.2 | 27.4 |
| Some college | 131 | 23.1 | 23.9 | 26.5 |
| Bachelor's degree or more | 350 | 61.7 | 39.2 | 36.8 |
| Income level | ||||
| <$24,000 | 40 | 7.1 | 11.3 | — |
| $24,000-$59,999 | 104 | 18.3 | 25.0 | — |
| $60,000-$119,999 | 178 | 31.4 | 30.1 | — |
| $120,000+ | 195 | 34.4 | 26.8 | — |
| Don't know/Refused | 50 | 8.8 | 6.8 | — |
| Health insurance | ||||
| Yes | 554 | 97.7 | 95.3 | 94.0 |
| No | 13 | 2.3 | 4.7 | 6.0 |
| Employment status | ||||
| Employed full-time | 263 | 46.4 | 45.2 | 63.8 |
| Employed part-time | 56 | 9.9 | 10.0 | |
| Unemployed | 43 | 7.6 | 10.6 | 3.5 |
| Retired/Student/Homemaker | 175 | 30.9 | 25.5 | — |
| Disabled | 0 | 0.0 | 0.0 | — |
| Unknown | 30 | 5.3 | 8.6 | — |
| Essential job (exempt from stay-at-home orders) | ||||
| Yes | 140 | 24.7 | 27.5 | — |
| No | 169 | 29.8 | 24.8 | — |
| Don't know/Refused/Not employed | 258 | 45.5 | 47.7 | — |
| Region/county | ||||
| Fairfield | 126 | 22.2 | 25.2 | 25.8 |
| Hartford | 157 | 27.7 | 24.1 | 24.9 |
| Litchfield | 42 | 7.4 | 5.5 | 5.2 |
| Middlesex | 34 | 6.0 | 5.0 | 4.7 |
| New Haven | 131 | 23.1 | 24.3 | 24.1 |
| New London | 41 | 7.2 | 7.8 | 7.6 |
| Tolland | 20 | 3.5 | 4.5 | 4.4 |
| Windham | 16 | 2.8 | 3.6 | 3.3 |
| Type of home | ||||
| Mobile home | 2 | 0.4 | 1.0% | — |
| Single family house or townhouse | 447 | 78.8 | 69.7% | — |
| Apartment or condo | 112 | 19.8 | 28.1 | — |
| Group facility | 2 | 0.4 | 0.3 | — |
| Don't know/Refused | 4 | 0.7 | 0.9 | — |
| Self-reported health status | — | |||
| Excellent | 177 | 31.2 | 29.7 | — |
| Very good | 223 | 39.3 | 32.0 | — |
| Good | 128 | 22.6 | 26.9 | — |
| Fair | 33 | 5.8 | 9.2 | — |
| Poor | 6 | 1.1 | 2.3 | — |
| Chronic conditions | — | |||
| Diabetes | 64 | 11.3 | 12.2 | — |
| Asthma, COPD, or another lung disease | 63 | 11.1 | 16.7 | — |
| Heart disease | 37 | 6.5 | 6.9 | — |
| Cancer | 72 | 12.7 | 10.7 | — |
| High blood pressure | 171 | 30.2 | 30.5 | — |
| Immune compromised | 46 | 8.1 | 8.5 | — |
| Lived in Connecticut in past 12 weeks | ||||
| <6 weeks | 9 | 1.6 | 1.0 | — |
| 6-10 weeks | 13 | 2.3 | 1.9 | — |
| 11-12 weeks | 543 | 95.8 | 96.5 | — |
| Don't know/Refused | 2 | 0.4 | 0.6 | — |
Source for age, sex, race, ethnicity, education, employment, county targets: American Community Survey 2018. Source for health insurance: the Current Population Survey estimates, 2018. Target percentage is based on expected proportions for a perfectly random sample, based on credible external sources.
COPD = chronic obstructive pulmonary disease; GED = general educational development test.
Comparison of the unweighted demographic distribution of individuals who completed only the survey with those who completed both the survey and the antibody test has been provided in eTable 1, available online. Although the 2 groups were not significantly different in regional representation, a significantly higher number of younger, Hispanic and non-Hispanic black individuals did not complete blood testing. However, our weighted study sample was closer to the target sample in the distribution of subgroups by age, sex, race and ethnicity, education level, and health insurance (Table 1, available online).
eTable 1.
Comparison of Demographics (Age-Group, Race/Ethnicity, and Geographic Region) of Those Who Completed Only the Survey with Those Who Completed the Survey and the Serology Test for the State-Level Population
| Completed survey but not blood test (N = 168) |
Completed survey and blood test (N = 567) |
||||
|---|---|---|---|---|---|
| N | Unweighted % | N | Unweighted % | P Value† | |
| Region | |||||
| Total | 168 | — | 567 | — | — |
| Fairfield | 38 | 22.6 | 126 | 22.2 | 0.91 |
| Hartford | 41 | 24.4 | 157 | 27.7 | 0.40 |
| Litchfield | 10 | 6.0 | 42 | 7.4 | 0.52 |
| Middlesex | 12 | 7.1 | 34 | 6.0 | 0.59 |
| New Haven | 41 | 24.4 | 131 | 23.1 | 0.73 |
| New London | 16 | 9.5 | 41 | 7.2 | 0.33 |
| Tolland | 9 | 5.4 | 20 | 3.5 | 0.28 |
| Windham | 1 | 0.6 | 16 | 2.8 | 0.09 |
| Race/Ethnicity | |||||
| Total* | 176 | — | 570 | — | |
| Hispanic | 36 | 21.4 | 49 | 8.6 | <0.001 |
| Non-Hispanic White | 94 | 56.0 | 470 | 82.9 | <0.001 |
| Non-Hispanic Black | 32 | 19.0 | 37 | 6.5 | <0.001 |
| Non-Hispanic Asian | 10 | 6.0 | 9 | 1.6 | 0.002 |
| Non-Hispanic Other | 4 | 2.4 | 5 | 0.9 | 0.12 |
| Age | |||||
| Total | 167 | — | 565 | — | |
| 18-29 years | 34 | 20.2 | 41 | 7.2 | <0.001 |
| 30-44 years | 48 | 28.6 | 90 | 15.9 | <0.001 |
| 45-54 years | 23 | 13.7 | 113 | 19.9 | 0.07 |
| 55-64 years | 26 | 15.5 | 134 | 23.6 | 0.02 |
| ≥ 65 years | 36 | 21.4 | 187 | 33.0 | 0.004 |
Can have multiresponse for race/ethnicity so this sum may be higher than the total N.
P value at 95% confidence level.
Symptoms and Risk-Mitigation Behaviors at the State Level
As shown in Table 2 , fever, cough, sore throat, diarrhea, and new loss of taste or smell was reported by 9%, 18%, 10%, 16%, and 5% respondents, respectively, at some point between March and June. About 16% of individuals reported being tested for coronavirus previously, and of these, 12% reported testing positive.
Table 2.
Prevalence of Symptomatic Illness, Risk Factors for Possible Exposure, and Adherence to Social-Distancing Behaviors Since March 1, 2020, Among the State-Level Population
| Characteristics | Unweighted N | Unweighted Proportion, % | Weighted Proportion, % (MOE) |
|---|---|---|---|
| Symptoms | |||
| Fever | 43 | 7.6 | 8.7 (±2.7) |
| Cough | 84 | 14.8 | 18.3 (±3.5) |
| Sore throat | 54 | 9.5 | 10.1 (±2.7) |
| New loss of taste or smell | 25 | 4.4 | 4.8 (±2.0) |
| Diarrhea | 70 | 12.3 | 15.9 (±3.2) |
| Risk Factors/Behaviors | |||
| Received coronavirus test | 90 | 15.9 | 15.7 (±3.5) |
| Tested positive for coronavirus | 11 | 1.9 | 1.9 (±1.4) |
| Anyone in household (other than respondent) had symptoms of coronavirus | 54 | 9.5 | 10.2 (±2.7) |
| Anyone in household (other than respondent) tested positive for coronavirus | 16 | 2.8 | 3.7 (±1.8) |
| Avoided going to public places, such as stores or restaurants | 422 | 74.4 | 72.8 (±4.1) |
| Avoided small gatherings of people, with family or friends | 426 | 75.1 | 75.3 (±4.0) |
| Worked from home (among all respondents, regardless of employment status) | 223 | 39.3 | 31.4 (±4.1) |
| Worn a mask on your face when outside your home | 557 | 98.2 | 96.9 (±1.6) |
| Traveled by airplane | 39 | 6.9 | 6.5 (±2.6) |
| Traveled using public transportation, such as bus or train | 19 | 3.4 | 5.2 (±2.0) |
MOE = margin of error at the 90% confidence level.
The majority of respondents reported following risk-mitigation practices, at least some of the time, since March, with 73% reporting having avoided public places and 75% reporting having avoided gatherings of family and friends. Notably, 97% of respondents reported wearing a mask outside their home at least part of the time. About 31% of all respondents reported having worked from home at least part of the time, representing 57% of working respondents. We compared the prevalence of symptomatic illness and risk-mitigation behaviors among individuals who completed only the survey with those who completed the survey and the antibody test in eTable 2.
eTable 2.
Comparison of Prevalence of Symptomatic Illness, Risk Factors for Possible Exposure, and Adherence to Social-Distancing Behaviors Since March 1, 2020, Among Those Who Completed Only the Survey with Those Who Completed the Survey and the Serology Test for the State-Level Population
| Completed survey but not blood draw, N = 168 |
Completed survey and blood draw, N = 567 |
||||
|---|---|---|---|---|---|
| Characteristics | Unweighted N* | Unweighted % | Unweighted N* | Unweighted % | P Value† |
| Symptoms | |||||
| Fever | 16 | 9.5 | 43 | 7.6 | 0.42 |
| Cough | 20 | 11.9 | 84 | 14.8 | 0.34 |
| Sore throat | 18 | 10.7 | 54 | 9.5 | 0.65 |
| New loss of taste or smell | 3 | 1.8 | 25 | 4.4 | 0.12 |
| Diarrhea | 19 | 11.3 | 70 | 12.3 | 0.72 |
| Risk Factors/Behaviors | |||||
| Received coronavirus test | 32 | 19.0 | 90 | 15.9 | 0.33 |
| Tested positive for coronavirus | 7 | 4.2 | 11 | 1.9 | 0.10 |
| Anyone in household (other than respondent) had symptoms of coronavirus | 17 | 10.1 | 54 | 9.5 | 0.82 |
| Avoided going to public places, such as stores or restaurants | 129 | 76.8 | 422 | 74.4 | 0.54 |
| Avoided small gatherings of people, with family or friends | 129 | 76.8 | 426 | 75.1 | 0.66 |
| Worked from home (among all respondents, regardless of employment status) | 35 | 20.8 | 223 | 39.3 | <0.001 |
| Worn a mask on your face when outside your home | 159 | 94.6 | 557 | 98.2 | 0.01 |
| Traveled by airplane | 15 | 8.9 | 39 | 6.9 | 0.37 |
| Traveled using public transportation, such as bus or train | 15 | 8.9 | 19 | 3.4 | <0.001 |
N for “yes” response.
P value at 95% confidence level.
Seroprevalence of SARS-CoV-2 Antibodies at the State Level
Seroprevalence estimates are shown in Table 3 . Overall, 23 respondents tested positive for SARS-CoV-2 antibodies, yielding a weighted seroprevalence of 4.0% (90% CI 2.0-6.0). Among individuals who reported having symptomatic illness, those with fever, cough, sore throat, and diarrhea had a weighted seroprevalence of 32.4% (90% CI 15.1-49.7), 11.4% (90% CI 2.8-20.0), 10.3% (90% CI 0.0-21.0), and 6.9% (90% CI 0.0-14.4), respectively. Among the 25 individuals who reported loss of taste or smell, 14 tested positive for SARS-CoV-2-specific antibodies.
Table 3.
Unweighted and Weighted State-Level Seroprevalence of SARS-CoV-2-Specific IgG Antibodies Among Adults in Connecticut, Overall and by Symptoms and Risk Factors and Behaviors
| Characteristics | Sample Size, N | Unweighted Seroprevalence, N (%) | Weighted Seroprevalence, % (MOE) |
|---|---|---|---|
| Overall | 567 | 23 (4.1) | 4.0 (±2.0) |
| Race/Ethnicity | |||
| Hispanic | 49 | 3 (6.1) | 12.8 (±8.0) |
| Non-Hispanic White | 470 | 16 (3.4) | 2.7 (±1.7) |
| Non-Hispanic Black | 37 | 3 (8.1) | 2.6 (±4.7) |
| Non-Hispanic Asian | 9 | * | * |
| Non-Hispanic Other | 5 | * | * |
| Symptoms | |||
| Fever | 43 | 14 (32.6) | 32.4 (±17.3) |
| Cough | 84 | 11 (13.1) | 11.4 (±8.6) |
| Sore throat | 54 | 5 (9.3) | 10.3 (±10.7) |
| New loss of taste or smell† | 25 | * | * |
| Diarrhea | 70 | 5 (7.1) | 6.9 (±7.5) |
| Symptoms Aggregate | |||
| Asymptomatic | 410 | 5 (1.2) | 0.6 (±0.7) |
| 1 or more symptoms | 157 | 18 (11.5) | 11.3 (±5.9) |
| 2 or more symptoms | 67 | 13 (19.4) | 16.1 (±11.2) |
| Risk Factors/Behaviors | |||
| Received coronavirus test | 90 | 13 (14.4) | 19.5 (±9.5) |
| Tested positive for coronavirus† | 11 | * | * |
| Anyone in household (other than respondent) had symptoms of coronavirus | 54 | 12 (22.2) | 19.8 (±11.8) |
| Anyone in household (other than respondent) tested positive for coronavirus | 16 | * | * |
| Avoided going to public places, such as stores or restaurants | 422 | 17 (4.0) | 4.8 (±2.4) |
| Avoided small gatherings of people, with family or friends | 426 | 17 (4.0) | 4.6 (±2.4) |
| Worked from home (among all respondents, regardless of employment status) | 223 | 14 (6.3) | 4.2 (±2.3) |
| Worn a mask on your face when outside your home | 557 | 23 (4.1) | 4.1 (±2.0) |
| Traveled by airplane | 39 | 0 (0.0) | 0.0 |
| Traveled using public transportation, such as bus or train | 19 | * | * |
Sample size is < 30 and too small to report.
Though the sample size was too small to report seroprevalence estimates, all 11 of these individuals tested positive for SARS-CoV-2-specific IgG antibodies. Among the 25 individuals who reported loss of taste or smell, 14 tested positive for SARS-CoV-2-specific IgG antibodies.
IgG = immunoglobulin G; MOE = margin of error at the 90% confidence level.
Asymptomatic individuals had significantly lower weighted seroprevalence 0.6% (90% CI 0.0-1.3) compared with the overall state estimate, whereas those with ≥ 1 and ≥ 2 symptoms had a seroprevalence of 11.3% (90% CI 5.4-17.2) and 16.1% (90% CI 4.9-27.3), respectively (Table 3). The comparisons between other subgroups and the state estimates are presented in eTable 3, available online. Additionally, seroprevalence estimates at 95% MOE have also been shown in eTable 3, available online.
eTable 3.
Unweighted and Weighted State-Level Seroprevalence of SARS-Cov-2-Specific IgG Antibodies Among Adults in Connecticut, by Sociodemographic and Clinical Subgroups
| Characteristics | Sample Size, N | Unweighted Seroprevalence, N (%) | Weighted Seroprevalence %, (MOE at 90% CI) | Weighted Seroprevalence %, (MOE at 95% CI) |
|---|---|---|---|---|
| Overall | 567 | 23 (4.1) | 4.0 (±2.0) | 4.0 (±2.3) |
| Age group, years | ||||
| 18-29 | 41 | 2 (4.9) | 6.4 (±7.7) | 6.4 (±9.2) |
| 30-44 | 90 | 4 (4.4) | 4.9 (±4.6) | 4.9 (±5.5) |
| 45-54 | 113 | 9 (8.0) | 6.6 (±4.7) | 5.5 (±5.6) |
| 55-64 | 134 | 6 (4.5) | 2.6 (±2.4) | 2.6 (±2.9) |
| ≥65 | 187 | 2 (1.1) | 0.8 (±1.2) | 0.8 (±1.4) |
| Sex | ||||
| Men | 244 | 8 (3.3) | 2.5 (±2.4) | 2.5 (±2.9) |
| Women | 323 | 15 (4.6) | 5.3 (±2.9) | 5.3 (±3.5) |
| Race/ethnicity | ||||
| Hispanic | 49 | 3 (6.1) | 12.8 (±8.0) | 12.8 (±9.6) |
| Non-Hispanic White | 470 | 16 (3.4) | 2.7 (±1.7) | 2.7 (±2.0) |
| Non-Hispanic Black | 37 | 3 (8.1) | 2.6 (±4.7) | 2.6 (±5.6) |
| Non-Hispanic Asian | 9 | * | * | * |
| Education level | ||||
| Less than high school | 7 | * | * | * |
| High school or GED | 79 | 3 (3.8) | 5.0 (±4.1) | 5.0 (±4.9) |
| Some college | 131 | 4 (3.1) | 4.0 (±3.6) | 4.0 (±4.3) |
| Bachelor's degree or more | 350 | 16 (4.6) | 3.6 (±1.7) | 3.6 (±2.1) |
| Income level | ||||
| <$24,000 | 40 | 2 (5.0) | 8.5 (±7.4) | 8.5 (±8.8) |
| $24,000 to $59,999 | 104 | 2 (1.9) | 3.0 (±3.7) | 3.0 (±4.4) |
| $60,000 to $119,999 | 178 | 9 (5.1) | 4.7 (±3.5) | 4.7 (±4.2) |
| $120,000+ | 195 | 10 (5.1) | 3.3 (±2.2) | 3.3 (±2.6) |
| Don't know/Refused | 50 | 0 (0.0) | 0.0 | 0.0 |
| Health insurance | ||||
| Yes | 554 | 20 (3.6) | 3.1 (±1.8) | 3.1 (±2.2) |
| No | 13 | * | * | * |
| Employment status | ||||
| Employed full-time | 263 | 13 (4.9) | 2.6 (±1.7) | 2.6 (±2.0) |
| Employed part-time | 56 | 7 (12.5) | 15.1 (±10.1) | 15.1 (±12.0) |
| Unemployed | 43 | 1 (2.3) | 5.4 (±5.7) | 5.4 (±6.8) |
| Retired | 152 | 1 (0.7) | 0.2 (±0.6) | 0.2 (±0.7) |
| Homemaker | 15 | * | * | * |
| Student | 8 | * | * | * |
| Disabled | 0 | * | * | * |
| Unknown | 30 | * | * | * |
| Essential job (exempt from stay-at-home orders) | ||||
| Yes | 140 | 8 (5.7) | 5.3 (±4.3) | 5.3 (±5.2) |
| No | 169 | 12 (7.1) | 5.0 (±2.8) | 5.0 (±3.4) |
| Don't know/refused | 10 | * | * | * |
| Not employed | 248 | 3 (1.2) | 3.0 (±2.1) | 3.0 (±2.5) |
| Region/County | ||||
| Fairfield | 126 | 9 (7.1) | 5.7 (±5.4) | 5.7 (±6.4) |
| Hartford | 157 | 5 (3.2) | 4.0 (±3.6) | 4.0 (±4.3) |
| Litchfield | 42 | 2 (4.8) | 1.6 (±3.2) | 1.6 (±3.8) |
| Middlesex | 34 | * | * | * |
| New Haven | 131 | 5 (3.8) | 3.4 (±3.2) | 3.4 (±3.8) |
| New London | 41 | 1 (2.4) | 1.7 (±3.3) | 1.7 (±4.0) |
| Tolland | 20 | * | * | * |
| Windham | 16 | * | * | * |
| Type of home | ||||
| Mobile home | 2 | * | * | * |
| Single family house/townhouse | 447 | 20 (4.5) | 4.1 (±2.2) | 4.1 (±2.6) |
| Apartment or condo | 112 | 2 (1.8) | 3.9 (±3.1) | 3.9 (±3.7) |
| Group facility | 2 | * | * | * |
| Unknown | 4 | * | * | * |
| Self-reported health status | ||||
| Excellent | 177 | 5 (2.8) | 1.3 (±1.5) | 1.3 (±1.7) |
| Very good | 223 | 11 (4.9) | 3.3 (±2.1) | 3.3 (±2.5) |
| Good | 128 | 4 (3.1) | 3.4 (±3.5) | 3.4 (±4.1) |
| Fair | 33 | 1 (3.0) | 4.3 (±5.8) | 4.3 (±6.9) |
| Poor | 6 | * | * | * |
| Chronic conditions | ||||
| Diabetes | 64 | 2 (3.1) | 4.3 (±4.7) | 4.3 (±5.6) |
| Asthma, COPD, or another lung disease | 63 | 2 (3.2) | 4.2 (±4.9) | 4.2 (±5.9) |
| Heart disease | 37 | 0 (0.0) | 0.0 | 0.0 |
| Cancer | 72 | 3 (4.2) | 7.5 (±7.9) | 7.5 (±9.4) |
| High blood pressure | 171 | 4 (2.3) | 2.9 (±2.8) | 2.9 (±3.4) |
| Immune compromised | 46 | 1 (2.2) | 8.4 (±6.7) | 8.4 (±8.0) |
| Individual symptoms | ||||
| Fever | 43 | 14 (32.6) | 32.4 (±17.3) | 32.4 (±20.6) |
| Cough | 84 | 11 (13.1) | 11.4 (±8.6) | 11.4 (±10.3) |
| Sore throat | 54 | 5 (9.3) | 10.3 (±10.7) | 10.3 (±12.7) |
| New loss of taste or smell | 25 | * | * | * |
| Diarrhea | 70 | 5 (7.1) | 6.9 (±7.5) | 6.9 (±9.0) |
| Symptoms aggregate | ||||
| Asymptomatic | 410 | 5 (1.2) | 0.6 (±0.7) | 0.6 (±0.8) |
| 1 or more symptoms | 157 | 18 (11.5) | 11.3 (±5.9) | 11.3 (±7.0) |
| 2 or more symptoms | 67 | 13 (19.4) | 16.1 (±11.2) | 16.1 (±13.4) |
| Risk factors/behaviors | ||||
| Received coronavirus test | 90 | 13 (14.4) | 19.5 (±9.5) | 19.5 (±11.3) |
| Tested positive for coronavirus† | 11 | * | * | * |
| Anyone in household (other than respondent) had symptoms | 54 | 12 (22.2) | 19.8 (±11.8) | 19.8 (±14.0) |
| Anyone in household (other than respondent) tested positive for coronavirus | 16 | * | * | * |
| Avoided going to public places, such as stores or restaurants | 422 | 17 (4.0) | 4.8 (±2.4) | 4.8 (±2.8) |
| Avoided small gatherings of people, with family or friends | 426 | 17 (4.0) | 4.6. (±2.4) | 4.6 (±2.8) |
| Worked from home (among all respondents, regardless of employment status) | 223 | 14 (6.3) | 4.2 (±2.3) | 4.2 (±2.8) |
| Worn a mask on your face when outside your home | 557 | 23 (4.1) | 4.1 (±2.0) | 4.1 (±2.4) |
| Traveled by airplane | 39 | 0 (0.0) | 0.0 | 0.0 |
| Traveled using public transportation, such as bus or train | 19 | * | * | * |
Sample size is < 30 and too small to report
Though the sample size was too small to report seroprevalence estimates, all 9 of these individuals tested positive for SARS-Cov-2-specific IgG antibodies.
Note 1: The rows highlighted in grey indicate estimates where we are confident at the 90% level that there is not a null result (estimate is not equal to 0). Though the results for the other rows are presented, the sample size is inadequate to be able to detect any significance.
CI = confidence interval; COPD = chronic obstructive pulmonary disease; GED = general educational development test; IgG = immunoglobulin G; MOE = margin of error.
Among the 143 negative samples from 5 high-risk cities in Connecticut that were retested with Abbott Architect serology assay, 142 (99.3%) samples tested negative. Additionally, of the total 25,274 antibody tests conducted by Quest Diagnostics in Connecticut during this time period, 2072 (8.4%) samples tested positive. Of the 11 respondents who reported testing positive for coronavirus, all tested positive for antibodies.
Characteristics and Seroprevalence Estimates Among Non-Hispanic Black and Hispanic Subpopulations
For the subpopulation estimate, the final sample included 171 Hispanic (39.9 [± 15.5] years and 51% women) and 148 non-Hispanic Black (46.4 [± 13.0] years and 56% women) adults (eTable 4, available online). Fever, cough, sore throat, diarrhea, and new loss of taste or smell was reported by 11%, 17%, 15% 10%, and 8%, respectively, of Hispanic participants and 4%, 10%, 5%, 4%, and 6%, respectively, of black participants (Table 4 ). About 37% of Hispanic and 31% of non-Hispanic black individuals reported receiving a coronavirus test previously and nearly 6% of Hispanic and 4% non-Hispanic black individuals reported testing positive for coronavirus. The prevalence of symptomatic illness and risk-mitigation behaviors among individuals who completed only the survey has been compared with those who completed both the survey and the antibody test in eTable 5 , available online.
eTable 4.
Sociodemographic and Clinical Characteristics of the Non-Hispanic Black and Hispanic Subpopulations Included in the Study
| Hispanic Subpopulation |
Non-Hispanic Black Subpopulation |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Unweighted N | Unweighted Proportion, % | Weighted Proportion, % | Target Percentage,* % | Unweighted N | Unweighted Proportion, % | Weighted Proportion, % | Target Percentage,* % |
| N | 171 | 148 | ||||||
| Age group, years | ||||||||
| 18-29 | 35 | 20.5 | 28.6 | 29.5 | 4 | 2.7 | 9.1 | 26.9 |
| 30-44 | 54 | 31.6 | 29.0 | 33.5 | 28 | 18.9 | 37.7 | 26.6 |
| 45-54 | 28 | 16.4 | 18.2 | 17.5 | 41 | 27.7 | 20.9 | 18.3 |
| 55-64 | 35 | 20.5 | 17.8 | 10.9 | 56 | 37.8 | 28.2 | 14.5 |
| ≥65 | 18 | 10.5 | 4.9 | 8.7 | 19 | 12.8 | 4.1 | 13.7 |
| Sex | ||||||||
| Men | 68 | 39.8 | 48.6 | 49.4 | 53 | 35.8 | 44.4 | 46.5 |
| Women | 103 | 60.2 | 51.4 | 50.6 | 95 | 64.2 | 55.6 | 53.5 |
| Don't know/Refused | 0 | 0.0 | 0.0 | — | 0 | 0.0 | 0.0 | — |
| Education level | ||||||||
| Less than high school | 20 | 11.7 | 19.0 | 26.5 | 2 | 1.4 | 1.0 | 12.4 |
| High school or GED | 45 | 26.3 | 44.4 | 33.1 | 31 | 20.9 | 30.6 | 35.0 |
| Some College | 40 | 23.4 | 21.1 | 25.6 | 50 | 33.8 | 47.1 | 33.2 |
| Bachelor's degree or more | 65 | 38.0 | 15.1 | 14.9 | 63 | 42.6 | 20.2 | 19.4 |
| Don't know/Refused | 1 | 0.6 | 0.4 | — | 2 | 1.4 | 1.0 | — |
| Income level | ||||||||
| <$24,000 | 37 | 21.6 | 30.6 | — | 15 | 10.1 | 13.9 | — |
| $24,000 to $59,999 | 59 | 34.5 | 37.9 | — | 50 | 33.8 | 41.5 | — |
| $60,000 to $119,999 | 33 | 19.3 | 12.1 | — | 45 | 30.4 | 22.7 | — |
| $120,000+ | 28 | 16.4 | 9.7 | — | 29 | 19.6 | 12.5 | — |
| Don't know/Refused | 14 | 8.2 | 9.7 | — | 9 | 6.1 | 9.3 | — |
| Health insurance | ||||||||
| Yes | 155 | 90.6 | 80.5 | 81.4 | 141 | 95.3 | 91.8 | 91.6 |
| No | 16 | 9.4 | 19.5 | 18.6 | 7 | 4.7 | 8.2 | 8.4 |
| Unknown | 0 | 0.0 | 0.0 | — | 0 | 0.0 | 0.0 | — |
| Employment status | ||||||||
| Employed full-time | 80 | 46.8 | 40.1 | 64.7 | 72 | 48.6 | 39.3 | 62.1 |
| Employed part-time | 21 | 12.3 | 17.3 | 19 | 12.8 | 13.2 | ||
| Unemployed | 24 | 14.0 | 18.1 | 6.6 | 18 | 12.2 | 25.2 | 7.4 |
| Retired/Student/Homemaker | 33 | 19.3 | 16.7 | — | 21 | 14.2 | 8.7 | — |
| Disabled | 0 | 0.0 | 0.0 | — | 0 | 0.0 | 0.0 | — |
| Unknown | 13 | 7.6 | 7.8 | — | 18 | 12.2 | 13.7 | — |
| Essential job (exempt from stay-at-home orders) | ||||||||
| Yes | 65 | 38.0 | 37.9 | — | 43 | 29.1 | 27.6 | — |
| No | 33 | 19.3 | 17.9 | — | 47 | 31.8 | 24.4 | — |
| Don't know/Refused/Not Employed | 73 | 42.7 | 44.2 | — | 58 | 39.2 | 48.1 | — |
| Region/county | ||||||||
| Fairfield | 40 | 23.4 | 29.8 | 33.2 | 25 | 16.9 | 22.1 | 27.7 |
| Hartford | 63 | 36.8 | 28.4 | 27.9 | 64 | 43.2 | 34.2 | 32.2 |
| Litchfield | 3 | 1.8 | 4.2 | 1.9 | 1 | 0.7 | 1.8 | 0.9 |
| Middlesex | 2 | 1.2 | 0.3 | 1.7 | 0 | 0.0 | 0.0 | 2.3 |
| New Haven | 49 | 28.7 | 26.9 | 26.5 | 55 | 37.2 | 41.3 | 29.7 |
| New London | 5 | 2.9 | 0.8 | 4.9 | 1 | 0.7 | 0.4 | 4.9 |
| Tolland | 4 | 2.3 | 1.2 | 1.6 | 2 | 1.4 | 0.1 | 1.5 |
| Windham | 5 | 2.9 | 8.3 | 2.3 | 0 | 0.0 | 0.0 | 0.8 |
| Unknown | 0 | 0.0 | 0.0 | — | 0 | 0.0 | 0.0 | — |
| Type of home | ||||||||
| Mobile home | 0 | 0.0 | 0.0 | 0.1 | 1 | 0.7 | 0.4 | 0.1 |
| Single family house or townhouse | 89 | 52.0 | 42.7 | 41.7 | 95 | 64.2 | 49.3 | 44.8 |
| Apartment or condo | 78 | 45.6 | 55.9 | 54.8 | 46 | 31.1 | 43.7 | 48.3 |
| Group facility | 1 | 0.6 | 0.2 | 3.3 | 1 | 0.7 | 0.4 | 6.8 |
| Don't know/Refused | 3 | 1.8 | 1.2 | — | 5 | 3.4 | 6.2 | — |
| Self-reported health status | ||||||||
| Excellent | 41 | 24.0 | 26.7 | — | 25 | 16.9 | 25.2 | — |
| Very good | 46 | 26.9 | 20.8 | — | 50 | 33.8 | 28.5 | — |
| Good | 64 | 37.4 | 35.3 | — | 60 | 40.5 | 37.2 | — |
| Fair | 17 | 9.9 | 15.5 | — | 12 | 8.1 | 7.1 | — |
| Poor | 3 | 1.8 | 1.8 | — | 1 | 0.7 | 2.0 | — |
| Unknown | 0 | 0.0 | 0.0 | — | 0 | 0.0 | 0.0 | — |
| Chronic conditions | ||||||||
| Diabetes | 34 | 19.9 | 19.3 | — | 40 | 27.0 | 26.6 | — |
| Asthma, COPD, or another lung disease | 28 | 16.4 | 15.6 | — | 29 | 19.6 | 21.8 | — |
| Heart disease | 10 | 5.8 | 4.1 | — | 8 | 5.4 | 3.0 | — |
| Cancer | 8 | 4.7 | 5.6 | — | 7 | 4.7 | 4.1 | — |
| High blood pressure | 42 | 24.6 | 25.4 | — | 69 | 46.6 | 44.7 | — |
| Immune compromised | 12 | 7.0 | 6.9 | — | 7 | 4.7 | 6.1 | — |
| Lived in Connecticut in past 12 weeks | ||||||||
| <6 weeks | 1 | 0.6 | 0.2 | — | 1 | 0.7 | 0.5 | — |
| 6-10 weeks | 6 | 3.5 | 1.8 | — | 0 | 0.0 | 0.0 | — |
| 11-12 weeks | 162 | 94.7 | 97.3 | — | 145 | 98.0 | 98.5 | — |
| Don't know/Refused | 2 | 1.2 | 0.7 | — | 2 | 1.4 | 1.0 | — |
Source for age, sex, race, ethnicity, education, employment, county targets: American Community Survey 2018. Source for health insurance: Reference information for health insurance coverage is obtained from the Current Population Survey estimates, 2018. Target percentage is based on expected proportions for a perfectly random sample, based on credible external sources.
COPD = chronic obstructive pulmonary disorder; GED = general educational development test.
Table 4.
Prevalence of Symptomatic Illness, Risk Factors for Possible Exposure, and Adherence to Social-Distancing Behaviors Since March 1, 2020, Among Non-Hispanic Black and Hispanic Subpopulation
| Hispanic Subpopulation |
Non-Hispanic Black Subpopulation |
|||||
|---|---|---|---|---|---|---|
| Characteristics | Unweighted N | Unweighted Proportion, % | Weighted Proportion, % (MOE) | Unweighted N | Unweighted Proportion, % | Weighted Proportion, % (MOE) |
| Overall | 171 | — | — | 148 | — | — |
| Symptoms | ||||||
| Fever | 16 | 9.4 | 10.8 (±5.6) | 7 | 4.7 | 3.9 (±3.6) |
| Cough | 31 | 18.1 | 17.4 (±6.4) | 18 | 12.2 | 10.1 (±6.4) |
| Sore throat | 30 | 17.5 | 15.0 (±6.1) | 8 | 5.4 | 4.7 (±4.6) |
| New loss of taste or smell | 15 | 8.8 | 7.8 (±4.3) | 8 | 5.4 | 4.2 (±3.8) |
| Diarrhea | 25 | 14.6 | 10.2 (±5.5) | 11 | 7.4 | 5.7 (±4.7) |
| Risk Factors/Behaviors | ||||||
| Received coronavirus test | 64 | 37.4 | 36.9 (±9.6) | 54 | 36.5 | 31.0 (±10.5) |
| Tested positive for coronavirus (out of all participants, regardless of prior testing) | 10 | 5.8 | 6.2 (±3.8) | 9 | 6.1 | 3.9 (±4.7) |
| Anyone in household (other than respondent) had symptoms of coronavirus | 28 | 16.4 | 20.6 (±6.8) | 6 | 4.1 | 2.1 (±2.1) |
| Anyone in household (other than respondent) tested positive for coronavirus | 15 | 8.8 | 9.3 (±4.6) | 3 | 2.0 | 2.0 (±2.7) |
| Avoided going to public places, such as stores or restaurants | 139 | 81.3 | 79.2 (±7.6) | 96 | 64.9 | 63.8 (±10.5) |
| Avoided small gatherings of people, with family or friends | 140 | 81.9 | 81.6 (±6.9) | 108 | 73.0 | 75.4 (±9.2) |
| Worked from home (among all respondents, regardless of employment status) | 42 | 24.6 | 11.8 (±5.7) | 48 | 32.4 | 17.8 (±9.0) |
| Worn a mask on your face when outside your home | 168 | 98.2 | 97.7 (±2.8) | 145 | 98.0 | 96.5 (±4.0) |
| Traveled by airplane | 11 | 6.4 | 4.8 (±3.3) | 6 | 4.1 | 4.0 (±4.8) |
| Traveled using public transportation, such as bus or train | 11 | 6.4 | 13.1 (±5.5) | 16 | 10.8 | 23.7 (±7.5) |
MOE = margin of error at the 90% confidence level.
eTable 5.
Comparison of Prevalence of Symptomatic Illness, Risk Factors for Possible Exposure, and Adherence to Social-Distancing Behaviors Since March 1, 2020, Among Those Who Completed Only the Survey with Those Who Completed the Survey and the Serology Test for the Hispanic and Non-Hispanic Black Subpopulations
| Hispanic Subpopulation |
Non-Hispanic Black Subpopulation |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Completed survey but not blood draw, N = 170 |
Completed survey and blood draw, N = 171 |
Completed survey but not blood draw, N = 121 |
Completed survey and blood draw, N = 148 |
|||||||
| Characteristics | Unweighted N* | Unweighted % | Unweighted N* | Unweighted % | P value† | Unweighted N* | Unweighted % | Unweighted N* | Unweighted % | P value† |
| Symptoms | ||||||||||
| Fever | 14 | 8.2 | 16 | 9.4 | 0.71 | 8 | 6.6 | 7 | 4.7 | 0.50 |
| Cough | 19 | 11.2 | 31 | 18.1 | 0.07 | 15 | 12.4 | 18 | 12.2 | 0.95 |
| Sore throat | 19 | 11.2 | 30 | 17.5 | 0.09 | 10 | 8.3 | 8 | 5.4 | 0.35 |
| New loss of taste or smell | 11 | 6.5 | 15 | 8.8 | 0.42 | 7 | 5.8 | 8 | 5.4 | 0.89 |
| Diarrhea | 13 | 7.6 | 25 | 14.6 | 0.04 | 17 | 14.0 | 11 | 7.4 | 0.08 |
| Risk Factors/Behaviors | ||||||||||
| Received coronavirus test | 40 | 23.5 | 64 | 37.4 | 0.01 | 32 | 26.4 | 54 | 36.5 | 0.08 |
| Tested positive for coronavirus | 6 | 3.5 | 10 | 5.8 | 0.31 | 4 | 3.3 | 9 | 6.1 | 0.29 |
| Anyone in household (other than respondent) had symptoms of coronavirus | 14 | 8.2 | 28 | 16.4 | 0.02 | 7 | 5.8 | 6 | 4.1 | 0.51 |
| Avoided going to public places, such as stores or restaurants | 134 | 78.8 | 139 | 81.3 | 0.57 | 79 | 65.3 | 96 | 64.9 | 0.94 |
| Avoided small gatherings of people, with family or friends | 139 | 81.8 | 140 | 81.9 | 0.98 | 88 | 72.7 | 108 | 73.0 | 0.96 |
| Worked from home (among all respondents, regardless of employment status) | 31 | 18.2 | 42 | 24.6 | 0.15 | 22 | 18.2 | 48 | 32.4 | 0.01 |
| Worn a mask on your face when outside your home | 165 | 97.1 | 168 | 98.2 | 0.47 | 117 | 96.7 | 145 | 98.0 | 0.51 |
| Traveled by airplane | 8 | 4.7 | 11 | 6.4 | 0.49 | 8 | 6.6 | 6 | 4.1 | 0.35 |
| Traveled using public transportation, such as bus or train | 19 | 11.2 | 11 | 6.4 | 0.12 | 17 | 4.0 | 16 | 10.8 | 0.42 |
N for “yes” response.
P value at 95% confidence level.
The weighted seroprevalence among the Hispanic and non-Hispanic black subpopulation, derived from both the random state sample and the oversample, was 19.9% (90% CI 13.2-26.6) and 6.4% (90% CI 0.9-11.9), respectively. The seroprevalence estimate for the Hispanic group was significantly higher than the overall state-level estimate.
Discussion
Our study primarily shows that despite Connecticut being an early COVID-19 hotspot, the vast majority of people in Connecticut lack detectable antibodies to SARS-CoV-2. In addition, individuals who reported having symptomatic illness between March and June of 2020 had higher seroprevalence rates, but more than 90% of these individuals did not have SARS-CoV-2-specific IgG antibodies. Also, a high percentage of people interviewed reported following risk-mitigation strategies, which may be partly responsible for the reduction in the number of new COVID-19 cases being reported in Connecticut. Finally, the Hispanic subpopulation had a higher prevalence of SARS-CoV-2-specific antibodies as compared with the overall state-level estimate, suggesting that the burden of disease was higher in this subgroup.
Our findings are consistent with other reports of more selected Connecticut populations. The CDC conducted a seroprevalence study using commercial laboratory data and reported a seroprevalence of 4.9% (95% CI 3.6-6.5) between April 26 and May 3 and 5.2% (95% CI 3.8-6.6) between May 21 and May 26 in Connecticut.2 , 8 However, these estimates were from people who had blood specimens tested for reasons unrelated to COVID-19, such as for a routine or sick visit, and as such would be expected to be biased higher than estimates for the general population. Similarly, data for all antibody tests conducted by Quest Diagnostics in Connecticut between June 10 and July 29 showed a seropositivity rate of 8.4%. Because these estimates were also among people who had a serology test done at a commercial laboratory, it is likely that these specimens were drawn from individuals who were more likely to suspect prior disease exposure than the general population.
Overall, our findings are consistent with other reports of population-level seroprevalence of SARS-CoV-2 in Europe and the United States, although the burden of disease in these regions may have varied. Recent data report from Spain15 indicated a seroprevalence of 4.6% (95% CI 4.3-5.0), and a population-based study from Switzerland16 reported SARS-CoV-2 antibodies in <10% of the population. Reports from regions within the United States have also shown similar numbers. A recent report from Indiana5 found a seropositivity rate of 1.0% (95% CI, 0.8-1.5), and a community seroprevalence survey from Atlanta4 estimated seroprevalence of 2.5% (95% CI 1.4-4.5). Our findings of a higher burden of SARS-CoV-2 antibodies among Hispanic subgroups is also consistent with reports demonstrating that minority populations have been disproportionately affected by COVID-19.5 , 17
There are several explanations for why our state-level estimates are lower than what one might expect given that Connecticut had nearly 43,000 positive cases and 4000 COVID-19 deaths by June 1, 2020. First, the majority of those deaths were among residents of congregate facilities. Second, the response and serology testing rates may have influenced the result. Only 7% of those contacted by phone completed the survey and blood test, and the recruited population differed from the targets. However, this is a standard response rate in studies seeking representative populations and was considered in weighting the data. It is also possible that those who were more likely to have a positive test failed to complete the blood draw in higher proportions. However, this nonresponse was taken into account while weighting the sample. Third, there is some evidence suggesting a short-lived antibody response, especially among individuals with mild or asymptomatic illness,18 , 19 and it is possible that more people were infected who lost antibodies over time. However, recent studies suggest that the decline in this timeframe is small and antibody levels can remain stable for up to 120 days,20 , 21 and all 11 people who reported receiving a positive coronavirus test previously, tested positive for antibodies in our study. Fourth, the accuracy of the serology tests has been a concern.13 However, 99% of the negative serology samples from the highest-risk regions of Connecticut that we retested with Abbott Architect serology assay tested negative a second time.
Nevertheless, our findings are concordant with other studies in indicating that the vast majority of the population in Connecticut does not have detectable levels of antibodies against SARS-CoV-2. At present, we do not know whether anti-SARS-CoV-2 antibodies confer immunity. If such antibodies, as detected by enzyme-linked immunosorbent assay (ELISA), are a marker of immunity, then more than 95% of the people in Connecticut would be susceptible to the virus. Given low infection rates over the summer, these general estimates are still reasonable. As such, there is continued need for strong public health efforts encouraging Connecticut residents to adhere to risk-mitigation behaviors so as to prevent a second wave of spread in the region.
Conclusion
Our findings indicate that even in one of the early hotspots of the SARS-CoV-2 outbreak in the United States, most of the population does not have detectable antibodies against SARS-CoV-2 and, as such, remains vulnerable to infection. Also, there is notable variation by race and ethnicity. People likely need to continue to be vigilant about practices that can slow the spread to prevent resurgence of the virus in these regions.
Acknowledgments
We are thankful to Matt Cartter, Josh Geballe, and Deidre Gifford from the Connecticut Department of Public Health for their assistance with the funding. We are also thankful to Michael F. Murray, Saad B. Omer, Alan Gerber, Adam Wisnewski, Richard Torres, Nathan Grubaugh, Wade Schulz, Tesheia Johnson, Cesar Caraballo-Cordovez, Yuan Lu, Erica S. Spatz, and Karthik Murugiah from Yale University for their support. Finally, we are in debt to those who participated in the surveys and completed the serology test and the many people at our organizations who spent countless hours ensuring the success of the project and contributing to the public's health.
Footnotes
Funding: This project was supported by the Centers for Disease Control and Prevention through the CARES Act and the Beatrice Kleinberg Neuwirth Fund.
Conflicts of Interest: CL is an Adjunct Professor at The First Affiliated Hospital of Xi'an Jiaotong University. AIK reports grants from Bristol Myers Squibb Foundation, Regeneron, and Serimmune, outside the submitted work. In the past three years, HMK received expenses and/or personal fees from UnitedHealth, IBM Watson Health, Element Science, Aetna, Facebook, the Siegfried and Jensen Law Firm, Arnold and Porter Law Firm, Martin/Baughman Law Firm, F-Prime, and the National Center for Cardiovascular Diseases in Beijing. He is an owner of Refactor Health and HugoHealth, and had grants and/or contracts from the Centers for Medicare & Medicaid Services, Medtronic, the U.S. Food and Drug Administration, Johnson & Johnson, and the Shenzhen Center for Health Information. SM, RS, CAR, SKH, KMA, LC, DSM, AD, DW, JM, S-XL, ZL, DH, MC, MDA, LVR, CS, and KK report none.
Authorship: All authors had access to the data and a role in writing this manuscript.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amjmed.2020.09.024.
Supplemental Appendix
eMethods 1. Calculation of sample size.
In designing the study, sample size calculation has an important role to detect an effect and to achieve the desired precision in estimates of the prevalence of (SARS-CoV-2). Early research has shown that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is more prevalent in minority groups and in older populations, which needs to be accounted for while estimating sample size requirements. For the purposes of baselining, we estimated having to recruit a total of 1460 respondents to go through testing. The assumptions underlying the state-level estimate is:
-
•
For state-level estimates: required sample size = 609 assuming state-level prevalence of 10%, precision requirement of 2%, and a confidence level of 90%.
-
•
For estimates among non-Hispanic black and Hispanic subpopulations: combined required sample size of 960, including those completed as part of the state sample, assuming a subpopulation prevalence of 20%, precision requirement of 3%; and confidence level of 90%.
eMethods 2. Details of data source and participant enrollment.
Data source: To achieve the required sample size for generating state-level estimates of seroprevalence, Gallup used a dual-frame Random Digit Dial (RDD)9 methodology to select a random sample of adults residing in noncongregate settings (ie, excluding individuals living in long-term care facilities, assisted living facilities, nursing homes, and prisons or jails), ages 18 years and older, living in Connecticut. Briefly, this involves drawing a random sample of landline and cell phone numbers from among all potential landline and cell phone numbers with valid area codes assigned to Connecticut. The source of the telephone numbers was the North American Numbering Plan that specifies which area codes and exchanges are assigned to Connecticut. A simple random sample of telephone numbers was drawn from the landline and cellphone frame with a goal to complete roughly 30% of the sample from the landline frame and the remaining 70% from the cellphone frame. The proportion in which landline and cell phone interviews were completed was designed to maximize coverage and representation of those with access to landline telephone only, cell phone only, and those having access to both landline and cell phones. Interviews were available to be conducted in either English or Spanish depending on the preference of the respondent.
To minimize the undercoverage resulting from residents having cell phone numbers that are not assigned to Connecticut, Gallup supplemented the RDD sample with a random sample of listed cellular numbers of residents with an address in Connecticut but a cell phone number with area code that is not assigned to Connecticut. This was done to ensure that residents of Connecticut who moved from another area in the country and ported their cellphone number were still eligible to participate in this survey. The combination of the different sample types was designed to ensure high coverage and representativeness of the state population and subgroups in an efficient manner.
To ensure adequate sample sizes for subpopulations of interest (black and Hispanic subpopulations), additional RDD samples were drawn from the cellphone frame of geographic areas with relatively higher expected incidence of these racial/ethnic groups. The incidence of these groups in the statewide RDD samples was not high enough to produce the corresponding targeted number of interviews, and hence, it was necessary to carry out targeted oversampling of these ethnic groups. Respondents recruited through these oversamples were screened to confirm that they actually belonged to these targeted groups. Although the plan was to complete 430 surveys for the black subpopulation and 320 surveys for the Hispanic subpopulation, the study was closed once 269 non-Hispanic black interviews and 341 Hispanic interviews were completed by cellphone using the oversamples.
Participant enrollment: Within households reached by landline, a random adult 18 or older from among all eligible adults in that household was selected using the “next birthday” method. For those reached via cell phone, we confirmed that we are speaking with an adult 18 years or older, living in Connecticut, and for the oversample that they meet the race and ethnicity requirements. No respondent selection was done in the case of cell phone respondents, where 1 user per number was assumed.
To maximize the opportunity for the inclusion of harder to reach individuals, a multicall design was implemented whereby up to 5 attempts were made to each randomly selected telephone number, spread over different days of the week, including the weekend and different times of day to ensure we achieved a representative sample of adults. Scheduled “call-backs” to complete an interview with an eligible respondent at a later time or date agreed on between the interviewer and respondent were also executed.
If after 5 attempts, we were unable to make a human contact or encountered a refusal to participate, the number was retired. New sample replicates were released as numbers were retired and the selection of the respondent was started afresh with the new number.
For the state-level sample, out of the 7305 participants contacted, 1875 were via landline and 5430 via cell phone. Of the 735 participants who completed the survey, 224 were contacted via landline and 511 via cell phone. Out of 567 participants who completed the survey and the blood draw, 186 were contacted via landline and 381 via cell phone.
For the non-Hispanic black and Hispanic subpopulations, out of the 12,508 participants contacted, all 12,508 were over cell phone.
State-level response rate: We contacted a total of 7305 respondents at the state-level between June 4 and June 23 of 2020, and successfully completed 735 interviews resulting in a combined dual-frame Response Rate of 7.3%. Response rate calculated was RR3 as specified by the American Association of Public Opinion Research standard definition (p. 61): https://www.aapor.org/AAPOR_Main/media/publications/Standard-Definitions20169theditionfinal.pdf.
Response rate formula was defined as “completes” divided by “eligible” plus “presumed eligible” participants, and as such, the denominator contained more individuals than those actually contacted. Among those whom we could not contact, we estimate a certain proportion to be eligible based on what we observed among those we contacted. So, the actual denominator is more than 7305. We estimate, had we been able to continue calling indefinitely, we would have found a total of 9959 individuals eligible to participate in the study, given the number of phone numbers we were dialing.
eMethods 3. Details of weighting of study sample.
The sample data were weighted to minimize bias and thereby ensure that the results were representative of the target population of all adults in Connecticut. The base weight (or selection probability weight) assigned to each completed survey was derived as the inverse of the probability of selection of that respondent in the sample.
As described in the section on Sampling Methodology, a dual-frame (landline and cell phone) sample design was employed for this study. In addition, Gallup supplemented the Random Digit Dial (RDD) sample with a random sample of listed cellular numbers (hereafter referred to as the listed sample) of residents with an address in Connecticut but a cell phone number with area code that is not assigned to Connecticut.
The calculation of selection probabilities for each respondent was done taking into consideration his or her access to landline or cell phones. If someone had access to landline only, then selection probability was based on the landline frame count and the sample size drawn from that frame. If someone had access to cell phone only, similar calculations based on cell frame count and sample size were used. For dual users (with access to both landline and cell phones), their chances of selection from both frames were taken into consideration. If a respondent from the listed cell sample had access to landline phones, the selection probability calculation for that respondent included his or her chances of selection from the landline frame also. In other words, the selection probability was dependent on all possible ways that person could be selected in the sample and not based on how that respondent was actually selected in this sample.
The oversamples were weighted to minimize bias in the survey-based estimates. As described, specific geographic areas with relatively higher incidence of these groups were targeted for sampling, and hence, the selection probabilities were unequal. For every respondent, the selection probability was calculated taking into consideration the various ways that a respondent could be selected in the oversample. For any specific area, the total number of cell phone numbers in the sampling frame (frame count) and the corresponding sample size actually selected was known, and hence, it was possible to calculate the selection probability as the ratio of the sample size and the frame count. The base weight assigned to each respondent was the inverse of the selection probability.
Because not every respondent who completed the survey ended up going through the test, a separate nonresponse weighting step was first carried out (after computation of base weights) before using the poststratification process. For all respondents who completed the survey, a response propensity model was used to estimate the probability of them completing the test using a logistic regression model. The dependent variable in the logistic regression set up was the probability of completing the test (P) whereas the independent (or explanatory) variables included demographic and other survey variables such as symptoms, risk factors and behaviors, self-reported results of polymerase chain reaction (PCR) test, etc. For the state sample consisting of 735 respondents, 567 took the test and hence the information on whether they took the test or not was available for each respondent. Once the model was fit (using the stepwise regression method with inclusion criteria of 0.10 P value and stay criteria of 0.15 P value), the inverse of the estimated probability (P) was used as the nonresponse adjustment weighting factor. For each respondent who took the test, the weight up to this point (at the end of nonresponse adjustment process) was the product of base weight and the nonresponse adjustment factor and that was used as the input weight for the final poststratification weighting step.
For the Hispanic subpopulation consisting of 341 respondents, 171 took the test, and for the non-Hispanic black subpopulation consisting of 269 respondents, 148 took the test, and hence, information on whether they took the test or not was available for each respondent. Separate response propensity models were fit for the Hispanic and non-Hispanic black subpopulations and following the previously given description, the nonresponse adjustment weighting factor was estimated for each subpopulation.
The next step involved poststratification weighting adjustments to account for any residual nonresponse and to match the weighted sample estimates to known population characteristics for the state of Connecticut. For the state-level sample, poststratification weighting was carried out using raking (or iterative proportional fitting) procedures to adjust for demographic variables such as age, gender, race and ethnicity, and education. The different categories for these variables that were used for poststratification were as follows: age group (18-39, 40-49, 50-59, 60-69, and 70+), gender (male, female) race/ethnicity, (Hispanic, non-Hispanic white/Other, non-Hispanic black), education (high school or less, some college/no 4-year college degree, college graduate, postgraduate degree), and county of residence.
The distribution of the final weights (obtained after the poststratification weighting) were examined and some trimming of weights (7th percentile at the bottom and 97th percentile at the top) was carried out to avoid extreme weights and thereby reduce the effect of such weights on sampling variance.
For the oversamples, poststratification weighting adjustments were carried out to account for any residual nonresponse and to match the weighted sample estimates to known population characteristics for those subpopulations in the state of Connecticut. The categories chosen are different from what was used for the state-level adjustments due to lower available sample sizes.
For the Hispanic oversample, poststratification weighting was carried out using ranking as described previously. The categories that were used for poststratification were: age group (18-29, 30-39, 40-49, 50-59, 60+), gender (male, female), race (white Hispanic, other), education (high school graduate or less, some college/no 4-year college degree, college graduate and higher) and county of residence. The distribution of the final weights (obtained after the poststratification weighting) was examined and some trimming of weights (3rd percentile at the bottom and 98th percentile at the top) was carried out to avoid extreme weights and thereby reduce the effect of such weights on sampling variance.
For the non-Hispanic black oversample, poststratification weighting was carried out using ranking as described previously. The categories that were used for poststratification were: age group (18-39, 40-49, 50-59, 60+), gender (male, female), education (college degree or higher, rest), and county of residence. The distribution of the final weights (obtained after the poststratification weighting) was examined and some trimming of weights (3rd percentile at the bottom and 98th percentile at the top) was carried out to avoid extreme weights and thereby reduce the effect of such weights on sampling variance.
The margin of error (MOE) for a simple random sample, also known as precision for estimating the unknown population proportion, P, at the 90% confidence level can be derived based on the following formula:
where n is the sample size (ie, the number of individuals who completed the survey and the blood test).
The MOE at the 95% confidence level can be derived based on the following formula:
where n is the sample size (ie, the number of individuals who completed the survey and the blood test).
The weighting process resulted in unequal weights to correct for household selection with unequal probability of selection and nonresponse adjustments through poststratification weighting. This introduces a design effect that needs to be taken into account while computing precision of estimates. The design effect is defined as the ratio of the design-based sample variance to the sample variance obtained from a simple random sample of the same size. Overall study design effect as estimated by the Kish approximation equals 1.83. In addition, the design effect for every estimate is calculated and used in the construction of 90% confidence intervals using the below formula.
The design effect for every estimate was calculated and used in the construction of 95% confidence intervals using the below formula:
The design effect was defined formally by Kish as “the ratio of the actual variance of a sample to the variance of a simple random sample of the same number of elements.” Based on Kish's approximate formula:
References
- 1.Connecticute Department of Public Health. COVID-19 data resources. Available at: https://data.ct.gov/stories/s/COVID-19-data/wa3g-tfvc/. Accessed September 22, 2020.
- 2.Havers FP, Reed C, Lim T, et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-May 12, 2020 [e-pub ahead of print]. JAMA Intern Med. Accessed September 22, 2020. [DOI] [PMC free article] [PubMed]
- 3.Sood N, Simon P, Ebner P, et al. Seroprevalence of SARS-CoV-2-specific antibodies among adults in Los Angeles County, California, on April 10-11, 2020. JAMA. 2020;323:2425–2427. doi: 10.1001/jama.2020.8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biggs HM, Harris JB, Breakwell L, et al. Estimated community seroprevalence of SARS-CoV-2 antibodies — two Georgia counties, April 28-May 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:965–970. doi: 10.15585/mmwr.mm6929e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menachemi N, Yiannoutsos CT, Dixon BE, et al. Population point prevalence of SARS-CoV-2 infection based on a statewide random sample — Indiana, April 25-29, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:960–964. doi: 10.15585/mmwr.mm6929e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg ES, Tesoriero JM, Rosenthal EM, et al. Cumulative incidence and diagnosis of SARS-CoV-2 infection in New York. Ann Epidemiol. 2020;48:23–29.e4. doi: 10.1016/j.annepidem.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryan A, Pepper G, Wener MH, et al. Performance characteristics of the Abbott Architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.00941-20. e00941-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Commercial laboratory seroprevalence survey data. Coronavirus Disease 2019 (COVID-19): serology surveillance. Available at:https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/commercial-lab-surveys.html. Accessed September 22, 2020.
- 9.Cummings KM. Random digit dialing: a sampling technique for telephone surveys. Public Opinion Quarterly. 1979;43(2):233–244. [Google Scholar]
- 10.US Food & Drug Administration. EUA authorized serology test performance. Available at: https://www.fda.gov/medical-devices/emergency-situations-medical-devices/eua-authorized-serology-test-performance. Accessed September 22, 2020.
- 11.US Food and Drug Administration. Instructions to use. Ortho-Clinical Diagnostics VITROS Anti-SARS-CoV-2 IgG test. EUA authorized serology test performance. Available at: https://www.fda.gov/media/136967/download. Accessed September 22, 2020.
- 12.Mahajan S, Redlich CA, Wisnewski AV, et al. Performance of Abbott Architect, Ortho Vitros, and Euroimmun assays in detecting prior SARS-CoV-2 infection [e-pub ahead of print]. medRxiv. Accessed September 22, 2020.
- 13.Deeks JJ, Dinnes J, Takwoingi Y, et al. Antibody tests for identification of current and past infection with SARS‐CoV‐2. Cochrane Database of Systematic Reviews. 2020;6 doi: 10.1002/14651858.CD013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration. Instructions to use. Abbott Architect SARS-CoV-2 IgG. EUA authorized aerology test performance. Available at: https://www.fda.gov/media/137383/download. Accessed September 22, 2020.
- 15.Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396:535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stringhini S, Wisniak A, Piumatti G, et al. Seroprevalence of Anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396:313–319. doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with COVID-19. N Engl J Med. 2020;382(26):2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long Q-X, Tang X-J, Shi Q-L, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 19.Ibarrondo FJ, Fulcher JA, Goodman-Meza D, et al. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild COVID-19. N Engl J Med. 2020;383:1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wajnberg A, Amanat F, Firpo A, et al. SARS-CoV-2 infection induces robust, neutralizing antibody responses that are stable for at least three months [e-pub ahead of print]. medRxiv. Accessed September 22, 2020
- 21.Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immuneresponse to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383:1724–1734. doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]