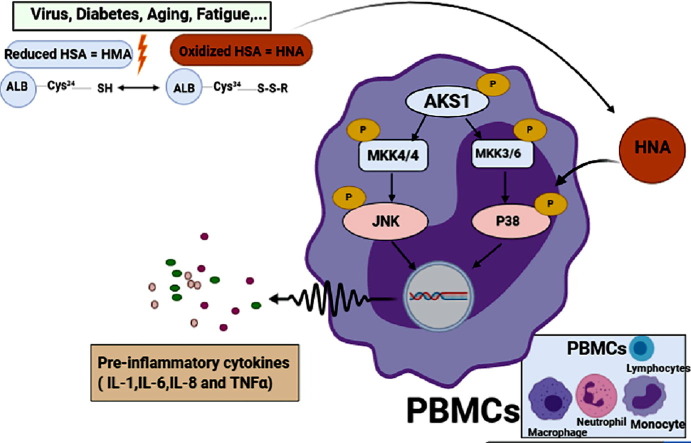
Graphical abstract
Keywords: Oxidized albumin, Diagnosis, Treatment, COVID-19
Abstract
Human serum albumin (HSA) as the most abundant protein in human blood plasma, can be a good indicator for evaluating severity of some diseases in the clinic. HSA can be find in two forms: reduced albumin (human mercaptalbumin (HMA)) and oxidized albumin (human non-mercaptalbumin (HNA)). The rate of oxidized albumin to total albumin can be enhanced in multiple diseases. Increase in HNA level have been demonstrated in liver, diabetes plus fatigue and coronary artery diseases. In liver patients, this enhancement can reach to 50–200 percent which can then lead to bacterial/viral infections and eventually death in severe conditions. Due to the induction of cytokine storm, we can say that the level of HNA in serum of coronavirus disease 2019 (COVID-19) patients may be a positive predictor of mortality, especially in patients with underlying diseases such as cardiovascular disease (CVD), diabetes, aging and other inflammatory diseases. We suggest that checking oxidized albumin in COVID-19 patients may provide new therapeutic and diagnostic opportunities to better combat COVID-19.
Human serum albumin (HSA) as the most abundant protein in human blood plasma, can be used to check the severity of some diseases in the clinic. HSA is involved in the maintaining colloidal osmotic pressure, transferring endogenous (hormones, bilirubin and fatty acids)/exogenous substances and scavenging the free radicals [1]. It can be find in two forms: reduced albumin (human mercaptalbumin (HMA)) and oxidized albumin (human non-mercaptalbumin (HNA)) [2]. In HSA, there is a free sulfhydryl group at 34 cysteine position (cys-34) which can act as an antioxidant by attacking to free radical species. In healthy people, cys-34 on 75% of HSA is in its reduced form (HMA) and in 25% of rest makes a small disulfide bond with another cysteine, homocysteine or glutathione (HNA) [3]. Conversely, the rate of oxidized albumin to the total albumin can be enhanced in multiple diseases. It has been found that HNA level increases and HMA level decreases in liver disease [4], diabetes [5] plus fatigue and coronary artery diseases [6]. In addition, the HNA level shows enhancement in some physical conditions such as aging and intense exercise [2]. In liver patients, the HNA level increases to 50–200 percent which can then lead to bacterial/viral infections and eventually death in severe conditions [7]. It has been proved that the oxidation of albumin alters its structure, function and leads to water retention and disease progression such as what in patient with viral hepatitis is found (the rate of oxidized albumin becomes significantly higher by improvement of the disease) [8]. Moreover, hypoalbuminemia and oxidation of albumin has been observed in diabetics besides systemic inflammation and oxidative stress. Therefore, It has been shown that HNA can be associated with the severity and progression of some diseases [9]. Evidence has proved that HNA triggers endothelial damage which in turn can increase the rate of cardiovascular disease and mesothelioma in elder individuals [10]. It has also been shown that the increased amount of HNA leads to an enhancement in the level of reactive oxygen species (ROS) such as H2O2, •O2 −, •OH, senescence in the cells and more importantly the risk of other diseases and infections in the elders, which can then give rise to the risk of mortality rate in these patients [11]. A study on patients with chronic obstructive pulmonary diseases has found that HSA is lower in these patients toward normal people, conversely the HNA level is higher [12]. Furthermore, in pulmonary diseases like diffuse lung disease and idiopathic pulmonary fibrosis, the amount of oxidized proteins enhances to a large extent which indicate the absence of accurate function of albumin in these patients [13].
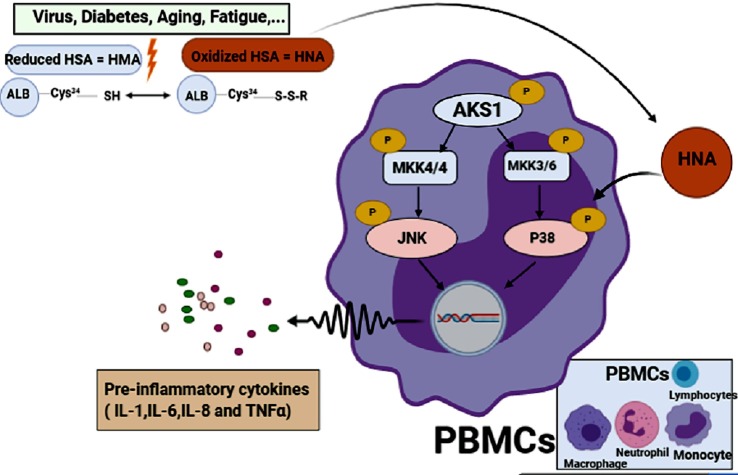
Remarkably, the high level of HNA can alter the function of immune cells in cirrhosis patients toward normal individuals. In these patients, inflammation is developed by production of inflammatory cytokines (IL-6, IL-1, TNF-α, and IL-8) and subsequent cytokine storm is triggered by activated leukocytes. Afterwards, HNA increases the inflammatory eicosanoids (PGE2, PGF2α, thromboxane B2 and leukotriene B4) as well. The effect of HNA on immune cells in this condition has been shown to be elicited by phosphorylation of p38 mitogen-activated protein kinase, followed by activation of transcription factors and ultimately increase in production/secretion of inflammatory cytokines [14]. Thus, HNA has been revealed to directly activate leukocytes and result in releasing inflammatory cytokines as are shown in Fig. 1 . It has been demonstrated that HNA in the respiratory system of patients with lung cancer activates neutrophils which in turn by accumulating ROS in these cells, plays a main role as a stimulator in enhancement neutrophils’ NETosis (neutrophil extracellular traps) function [15]. A systematic review study on laboratory publications of people with coronavirus disease 2019 (COVID-19) has recently published that 75.8% of patients had decreased amount of HSA [16]. Nevertheless, there isn’t any study which has measured the HNA in these patients yet. However, the clinical measurement can be easily done by using high-performance liquid chromatography method on whole blood samples [17] and the ratio of HNA may be a sign of systemic redox change in COVID-19 patients.
Fig. 1.
Formation of oxidized albumin and its effect on the activation of immune cells and production of inflammatory cytokines. َ Apoptosis signal-regulating kinase 1 (ASK1), mitogen activated protein kinase (MKK), c- Jun N-terminal kinase (JNK), peripheral blood monocyte cells (PBMCs).
COVID-19 is an epidemic infection disease with the responsibility of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which involved millions of people all around the world [18]. An excessive increase in ROS level due to the immune cells function (frequently macrophages and neutrophils) against virus has been identified in patients with COVID-19 [19]. On the other hand, infection with SARS-CoV-2 can lead to the damage of some cells and release of their cellular contents which in turn can initiate the epithelial cells’ toxic stress responses, accumulation of water in the lungs, uncontrolled inflammatory responses, increased ROS production and consequently generation of series problems in patients [20].
For that reasons with regard to the factors which affect the oxidized state of albumin, we can mention that in COVID-19 ill patients, albumin may further be oxidized, once as a positive feedback cycle induce the production of cytokines by activated leukocytes in the lung, increase the secretion of inflammatory cytokines and later initiate cytokine storm which can ultimately cause development of critical state or even death in these patients. These points could necessitate researchers to consider HNA level in COVID-19 patients. It has been reported that there is an association between HNA level and increase in the risk of death from cardiovascular disease (CVD), aging, diabetes, and other underlying diseases [4], [9], [10]. On the other hand, due to contribution in progression of cytokine storm, we can say that the level of HNA in serum of COVID-19 patients may be a positive predictor of mortality and intensity of infection, especially in patients with underlying diseases such as CVD, diabetes, aging and other inflammatory diseases.
It should be noted that because of reduction in HSA level of COVID-19 patients, albumin therapy may be suggested as an option by researchers. However, it is necessary to say that reports indicate that there is almost 57% heterogeneity on oxidized position of cys-34 in albumin preparation by factories [21]. This changing, significantly reduces antioxidant activity of albumin, and decreases its binding capacity to drugs [22]. On the other hand, the usage of albumin has been associated with the deposition of fatal inflammatory reactions in animal models (oxidized albumin may have been used in this study) [23]. Subsequently, COVID-19 patients may be injected with oxidized albumin, which may increase mortality rate of these patients, at least the applied drugs may not be sufficiently transferred by albumin. As a result, we suggest that checking HNA and HMA in the serum of patients may provide new diagnostic and therapeutic opportunities to better combat COVID-19. Of course, it is just a recommendation and clinical trials are needed to investigate the level of HNA in the serum of patients with COVID-19, its severity of worsening, its correlation with infection related inflammatory responses and disease progression as well as death in critically ill patients.
Funding
Given that this manuscript was a commentary, we have not received any funding from any institution.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Kragh-Hansen U. Structure and ligand binding properties of human serum albumin. Dan. Med. Bull. 1990;37(1):57–84. [PubMed] [Google Scholar]
- 2.Mera K. The structure and function of oxidized albumin in hemodialysis patients: Its role in elevated oxidative stress via neutrophil burst. Biochem. Biophys. Res. Commun. 2005;334(4):1322–1328. doi: 10.1016/j.bbrc.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 3.Era S. Age-related change in redox state of human serum albumin. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1995;1247(1):12–16. doi: 10.1016/0167-4838(94)00166-e. [DOI] [PubMed] [Google Scholar]
- 4.Kadota K. Decreased sulfhydryl groups of serum albumin in coronary artery disease. Jpn. Circ. J. 1991;55(10):937–941. doi: 10.1253/jcj.55.937. [DOI] [PubMed] [Google Scholar]
- 5.Beck J.L. Direct observation of covalent adducts with Cys34 of human serum albumin using mass spectrometry. Anal. Biochem. 2004;325(2):326–336. doi: 10.1016/j.ab.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 6.Bito R. Degradation of oxidative stress-induced denatured albumin in rat liver endothelial cells. Am. J. Physiol.-Cell Physiol. 2005;289(3):C531–C542. doi: 10.1152/ajpcell.00431.2004. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho J.R., Machado M.V. New insights about albumin and liver disease. Ann. Hepatol. 2018;17(4):547–560. doi: 10.5604/01.3001.0012.0916. [DOI] [PubMed] [Google Scholar]
- 8.Sakata M. Oxidized albumin is associated with water retention and severity of disease in patients with chronic liver diseases. e-SPEN, the Eur. e-J. Clin. Nutrition Metabolism. 2010;5(6):e247–e253. [Google Scholar]
- 9.Michelis R. Albumin oxidation leads to neutrophil activation in vitro and inaccurate measurement of serum albumin in patients with diabetic nephropathy. Free Radical Biol. Med. 2013;60:49–55. doi: 10.1016/j.freeradbiomed.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Luna C. Aging-associated oxidized albumin promotes cellular senescence and endothelial damage. Clin. Interv. Aging. 2016;11:225. doi: 10.2147/CIA.S91453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masudo R. Evaluation of human nonmercaptalbumin as a marker for oxidative stress and its association with various parameters in blood. J. Clin. Biochem. Nutrition. 2017:17–25. doi: 10.3164/jcbn.17-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hackett T.L. Oxidative modification of albumin in the parenchymal lung tissue of current smokers with chronic obstructive pulmonary disease. Respir. Res. 2010;11(1):180. doi: 10.1186/1465-9921-11-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bargagli E. Analysis of carbonylated proteins in bronchoalveolar lavage of patients with diffuse lung diseases. Lung. 2007;185(3):139–144. doi: 10.1007/s00408-007-9001-6. [DOI] [PubMed] [Google Scholar]
- 14.Alcaraz-Quiles J. Oxidized albumin triggers a cytokine storm in leukocytes through p38 Mitogen-Activated protein kinase: role in systemic inflammation in decompensated cirrhosis. Hepatology. 2018;68(5):1937–1952. doi: 10.1002/hep.30135. [DOI] [PubMed] [Google Scholar]
- 15.Inoue M. Plasma redox imbalance caused by albumin oxidation promotes lung-predominant NETosis and pulmonary cancer metastasis. Nat. Commun. 2018;9(1):1–11. doi: 10.1038/s41467-018-07550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez-Morales A.J. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2020 doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasukawa K. A simple, rapid and validated high-performance liquid chromatography method suitable for clinical measurements of human mercaptalbumin and non-mercaptalbumin. Ann. Clin. Biochem. 2018;55(1):121–127. doi: 10.1177/0004563217693257. [DOI] [PubMed] [Google Scholar]
- 18.A.C. Cismaru, et al., Game of “crowning” season 8: RAS and reproductive hormones in COVID-19–can we end this viral series? Arch. Med. Sci. 16(1). [DOI] [PMC free article] [PubMed]
- 19.Nasi A. Reactive oxygen species as an initiator of toxic innate immune responses in retort to SARS-CoV-2 in an ageing population, consider N-acetylcysteine as early therapeutic intervention. Toxicol. Rep. 2020 doi: 10.1016/j.toxrep.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schönrich G., Raftery M.J., Samstag Y. Devilishly radical NETwork in COVID-19: Oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Adv. Biol. Regulation. 2020 doi: 10.1016/j.jbior.2020.100741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bar-Or D. Heterogeneity and oxidation status of commercial human albumin preparations in clinical use. Crit. Care Med. 2005;33(7):1638–1641. doi: 10.1097/01.ccm.0000169876.14858.91. [DOI] [PubMed] [Google Scholar]
- 22.Klammt S. Albumin binding capacity (ABiC) is reduced in commercially available human serum albumin preparations with stabilizers. Zeitschrift für Gastroenterologie. 2001;39(S 2):24–27. doi: 10.1055/s-2001-919056. [DOI] [PubMed] [Google Scholar]
- 23.Humpert P.M. AGE–modified albumin containing infusion solutions boosts septicaemia and inflammation in experimental peritonitis. J. Leukoc. Biol. 2009;86(3):589–597. doi: 10.1189/jlb.1008646. [DOI] [PMC free article] [PubMed] [Google Scholar]