The beta coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infects lung alveolar type II epithelial calls by attaching to angiotensin-converting enzyme 2 (ACE-2) expressed at the cells’ surface via its viral spike protein.1 A transmembrane serum protease (TMPRSS2) activates the viral spike protein and enables cell entry. The more severe manifestations of the resultant inflammatory response include dry cough, dyspnea, tachypnea, a feeling of drowning, pulmonary edema, unilateral or bilateral pneumonia, mottling and ground-glass opacifies on computed tomography scan, and progression to the acute respiratory distress syndrome requiring ventilatory support.2 Hypoxemia is particularly prominent throughout, and a hyaline membrane of dead cells can be observed at autopsy. Once infection takes hold, a cascade of inflammatory events is initiated including the release of cytokines such as IL-1, IL-6, IP-10, MCP-1, TNF-α,3 and many more, which has been referred to as a “cytokine storm.” In addition, the prominent edema seen throughout the lung and the association of ACE inhibition with severe angioedema has focused attention on another innate inflammatory cascade, namely, the overproduction of bradykinin,3 which is the focus of this editorial.
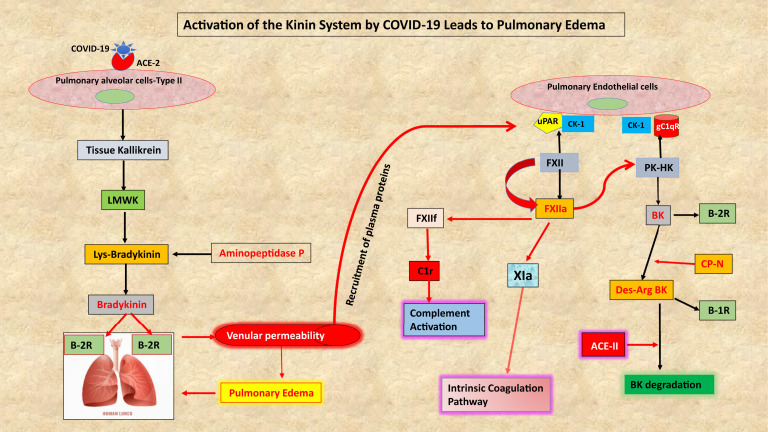
There are 2 general pathways for the production of bradykinin, the first being the release of cellular tissue kallikrein, which cleaves low-molecular weight kininogen (LK or LMWK) to release lys-bradykinin (Fig 1 ). Tissue kallikrein is secreted as an active enzyme (ie, processed intracellularly) and is a particularly prominent product of the lung, pancreas, kidney, salivary glands, and the prostate. There are 15 homologous gene products, 3 of which can produce bradykinin (KLK 1, 2, and 12), with KLK1 being the most prominent.
Fig 1.
A diagram of important interrelationships involving the bradykinin-forming pathways in patients with COVID-19. Pulmonary epithelial cells release tissue kallikrein which cleaves low-molecular weight kininogen to release lys-bradykinin that is rapidly converted to bradykinin. Both are ligands of the B-2 receptor. The plasma cascade is recruited into the surrounding lung tissue, and all the requisite proteins for bradykinin formation exist bound to endothelial cells where activation can proceed along the surface as well as in the fluid phase. In addition, activation of endothelial cells by IL-1 or TNF-α can release HSP-90 and prolylcarboxypeptidase. Both convert PK to plasma kallikrein if PK is bound to HK. Formation of bradykinin and its des-arg9 degradation products stimulate B2 and B1 receptors, respectively, leading to lung edema. PK, Plasma prekallikrein.
The second pathway is present in plasma and consists of factor XII, plasma prekallikrein, and high-molecular weight kininogen (HK).4 Prekallikrein circulates primarily as a bimolecular complex with HK (about 75%-80% bound) as does coagulation factor XI (95% is bound). They compete for a single overlapping binding site, but there is sufficient HK present to bind both. Both factor XII and prekallikrein possess minute levels of proteolytic activity relative to their respective active enzymes, which may be the initial spark needed for activation to proceed. All 3 proteins are also bound to bimolecular sites on the surface of endothelial cells (Fig 1). Factor XII binds primarily to urokinase plasminogen activator receptor-cytokeratin 1, whereas HK binds to the receptor for the globular heads of C1q (gC1qR)-cytokeratin 1 with plasma prekallikrein attached to the HK (Fig 1). Once activation proceeds, factor XII is converted to 2 forms of the activated enzyme, factor XIIa (80 Kd) and factor XIIf (28.5-30 Kd; β FXIIa). Both can convert prekallikrein to kallikrein, and kallikrein digests HK to release bradykinin (Arg-pro-pro-gly-phe-ser-pro-phe-arg). Factor XII activation proceeds by a relatively slow autoactivation process to produce a small amount of factor XIIa and a very rapid positive feedback in which the initial kallikrein formed activates all remaining factor XII in seconds to yield factor XIIa and then factor XIIf. Tissue kallikrein does not activate factor XII. The larger 80-Kd factor XIIa (also known as alpha F X11a) is the clotting factor that converts factor XI to factor XIa to continue the intrinsic coagulation pathway (Fig 1). Factor XIIf lacks a surface binding site, loses 96% to 98% of the clotting activity, but gains a new function, that is, activation of C1r to initiate the classical complement cascade (Fig 1). This is not surprising because cross-activation by enzymes of the complement system and the coagulation pathway proteins is known to occur when either pathway is activated. For example, plasmin, factor Xa, and factor XIa are able to cleave C3 and C5 to generate the potent chemoattractants C3a and C5a, which in turn are able to recruit and activate leukocytes to produce proinflammatory cytokines. This contributes to the cytokine storm that is the hallmark of many inflammatory processes including those induced by SARS-CoV-2.
Bradykinin causes vasodilation and increases vascular permeability by interacting with constitutively expressed B-2 receptors (B-2R) on small venules. The same is true of lys-bradykinin produced by tissue kallikrein (Fig 1) although the lys is rapidly removed by aminopeptidase P. Bradykinin is degraded primarily by ACE, a dipeptidase that removes the C-terminal phe-arg, which inactivates it, followed by removal of ser-pro. An alternative process requires carboxypeptidase activity (carboxypeptidase N in plasma and carboxypeptidase M on pulmonary vascular endothelial cells) to first remove the C-terminal arg from either bradykinin (plasma cascade) or lys-bradykinin (tissue kallikrein product) (Fig 1). This leaves des-arg9 bradykinin (Arg-pro-pro-gly-phe-ser-pro-phe), which is minimally reactive with B-2. However, this peptide binds to the B-1 receptor (B-1R), which also mediates vasodilation and vascular permeability. The B-1 receptor is not normally present but is induced by IL-1 or TNF-α (produced by febrile viral illnesses such as coronavirus disease 2019 [COVID-19]) as well as gC1qR. Its ligands are des-arg9 bradykinin3 as well as des-arg9 lys-bradykinin (Fig 1).
There are many observations and theories regarding a prominent role for bradykinin and perhaps des-arg9 bradykinin in the pathogenesis of the pulmonary dysfunction of COVID-19, which is linked in part to changes in the renin-angiotensin system. Studies of gene expression in bronchoalveolar lavage specimens of patients with COVID-19,5 when compared with normal control specimens, reveal upregulation of multiple components that lead to bradykinin production and downregulation of factors that control the process. All “kallikreins and kininogens” are upregulated, the B-2 receptor was increased 207-fold and the B-1 receptor, 2945-fold. The gene expression for C1 Inhibitor (C1-INH) was decreased 33-fold, which would render the plasma bradykinin cascade labile and overreactive as we see in C1-INH deficiency (types I and II HAE) in which enzymes not adequately inhibited by C1-INH include both forms of activated factor XII, plasma kallikrein, and C1r. In contrast, gene expression for ACE was decreased 8-fold so that bradykinin would not be inactivated normally. Although viral binding to ACE-2 limits its enzymatic activity3 so that des-arg9 bradykinin is not degraded (ACE-2 removes C-terminal phe) and lowered ACE levels also limit des-arg9 bradykinin degradation (it removes C-terminal ser-pro-phe acting then as a tripeptidase rather than a dipeptidase). With the markedly augmented bradykinin receptor production, a “bradykinin storm” can result.
Our own preliminary observations (unpublished, 2020) reveal upregulation and secretion of gC1qR by infected cells, which creates the cell surface platform for activation of the bradykinin cascade, and the secreted gC1qR also upregulates the B-1 receptor.6 The renin-angiotensin system7 can also be contributory in that decreased ACE limits formation of the vasoconstrictor angiotensin II from angiotensin I. As angiotensin I accumulates, ACE-2 removes C-terminal phe to produce angiotensin 1-9. This moiety stimulates angiotensin-2 receptors to cause vasodilation and can do so synergistically with bradykinin.5 If significant amounts of angiotensin II were produced, ACE-2 can then convert it to another vasodilator, angiotensin 1-7 active through the MAS receptor.7 Here, the balance of decreased ACE-2 via viral binding and internalization3 and increased ACE-2, as seen when COVID-2 bronchoalveolar lavage fluids are examined,5 needs to be quantified at the protein level (cell surface and interstitial fluid) rather than at the DNA level to determine the net enzymatic effect.
There are numerous reports of a possible therapeutic role for antagonists of cytokines such as IL-1 (anakinra) or IL-6 (tocilizumab) to treat COVID-19.2 , 7 , 8 We suggest use of lanadelumab to block plasma kallikrein9 and icatibant to inhibit B-2 receptors as possible therapy for the severe pulmonary manifestations of COVID-19. Preliminary observations using icatibant (uncontrolled) indicate improved oxygenation. Antagonists of tissue kallikrein and the B-1 receptors have been used for research purposes but are not approved for clinical use, but there is a need for such agents. Simultaneous inhibition of B-1 and B-2 receptors is also possible. Finally, an mAb to gC1qR to disrupt bradykinin formation along endothelial cell surfaces would be a novel, additional approach.10
Footnotes
Disclosure of potential conflict of Interest: A. P. Kaplan has no ties to pharmaceutical companies who have products related to this subject. B. Ghebrehiwet receives royalties from the sale of detection kit for gC1qR and mAbs 60.11 and 74.5.2.
References
- 1.Zhou P., Yang K., Wang X., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.St John A., Rathore A. Early insights into immune responses during COVID-19. J Immunol. 2020;705:555–564. doi: 10.4049/jimmunol.2000526. [DOI] [PubMed] [Google Scholar]
- 3.van de Veerdonk F., Netea M., van Deuren M., van der Meer J., de Mast Q., Brüggemann R., et al. Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. eLife. 2020;9 doi: 10.7554/eLife.57555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan A., Ghebrihiwet B. The plasma bradykinin-forming pathways and its interrelationships with complement. Mol Immunol. 2010;47:2161–2169. doi: 10.1016/j.molimm.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Garvin M., Alvarez C., Miller J., Prates E., Walker A., Amos B., et al. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. ELife. 2020;9 doi: 10.7554/eLife.59177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghebrehiwet B., Ji Y., Valentino A., Pednekar L., Ramadass M., Habiel D., et al. Soluble gC1qR is an autocrine signal that induces B1R expression on endothelial cells. J Immunol. 2014;192:377–384. doi: 10.4049/jimmunol.1302031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franco R., Rivas-Santisteban R., Serrano-Marín J., Rodríguez-Pérez A., Labandeira-García J., Navarro G. SARS-CoV-2 as a factor to disbalance the renin–angiotensin system: a suspect in the case of exacerbated IL-6 production. J Immunol. 2020;205:1198–1206. doi: 10.4049/jimmunol.2000642. [DOI] [PubMed] [Google Scholar]
- 8.Manjili R., Zarei M., Habibi M., Manjili M. COVID-19 as an acute inflammatory disease. J Immunol. 2020;205:12–19. doi: 10.4049/jimmunol.2000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busse P., Christiansen S. Hereditary angioedema. N Engl J Med. 2020;382:1136–1148. doi: 10.1056/NEJMra1808012. [DOI] [PubMed] [Google Scholar]
- 10.Ghebrehiwet B., Jesty J., Xu S., Vinayagasundaram R., Vinayagasundaram U., Ji Y., et al. Structure–function studies using deletion mutants identify domains of gC1qR/p33 as potential therapeutic targets for vascular permeability and inflammation. Front Immunol. 2011;2:1–9. doi: 10.3389/fimmu.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]