Abstract
According to the latest reports, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which caused coronavirus disease 2019 (COVID-19), was successfully isolated from the excreta (stool and urine) of COVID-19 patients, suggesting SARS-CoV-2 could be transmitted through excreta contaminated water. As pit latrines and the use of untreated excreta as fertilizer were common in rural China, we surveyed 27 villages of Jiangxi and Hubei provinces and found that pit latrines could be a potential source of SARS-CoV-2 water pollution. Recently, bats have been widely recognized as the source of SARS-CoV-2. There were many possible intermediate hosts of SARS-CoV-2, including pangolin, snake, bird and fish, but which one was still not clear exactly. Here, we proposed a hypothesis to illustrate the mechanism that SARS-CoV-2 might spread from the excreta of infected humans in pit latrines to potential animal hosts, thus becoming a sustainable source of infection in rural China. Therefore, we believe that abolishing pit latrines and banning the use of untreated excreta as fertilizer can improve the local living environment and effectively prevent COVID-19 and other potential waterborne diseases that could emanate from the excreta of infected persons. Although this study focused on rural areas in China, the results could also be applied to low-income countries, especially in Africa.
Keywords: COVID-19, SARS-CoV-2, Excreta, Pit latrines, Rural China, Low-income countries
Graphical abstract
1. Introduction
The ongoing pandemic of coronavirus disease 2019 (COVID-19) is an emerging respiratory infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which was named 2019-nCoV previously (Zhu et al., 2020), posing an unprecedented challenge to global public health. The most common symptoms of COVID-19 patients include fever (80%–99%, 90% on average), cough (59%–82%, 69% on average), fatigue (70%), sore throat (5%–40%, 21% on average), shortness of breath (31%–55%,39% on average), rhinorrhea (4%–20%, 12% on average) and muscle ache (11%–35%, 23% on average) (Cai et al., 2020; Chen et al., 2020a; Huang et al., 2020; Wang et al., 2020a), while diarrhea (2%–10%, 5% on average), nausea (10%), vomiting (4%) and abdominal pain (2%) were also reported (Huang et al., 2020, Chen et al., 2020a, Wang et al., 2020a). Significantly, SARS-CoV-2 ribonucleic acid (RNA) was detected in the stool of 53%–83% (64% on average) COVID-19 patients (including patients with severe symptoms, asymptomatic status, and treated patients with no further sign of the symptoms), and the duration of positive stool ranged from one day to more than a month (Cai et al., 2020; Chen et al., 2020b; Holshue et al., 2020; Lescure et al., 2020; Pan et al., 2020; Tang et al., 2020; Wang et al., 2020b; Wu et al., 2020c; Xiao et al., 2020a; Xu et al., 2020; Zheng et al., 2020). Among all patients, 23%–43% (33% on average) of all age groups and 80% of children were still positive in stool even after the respiratory tract samples were negative (Chen et al., 2020b; Wu et al., 2020c; Xiao et al., 2020a; Xu et al., 2020), suggesting that SARS-CoV-2 might be excreted from gastrointestinal tract and the fecal-oral transmission was possible (Cai et al., 2020; Gao et al., 2020; Xiao et al., 2020a). To investigate the possibility of enteric infection by SARS-CoV-2 as reported previously in severe acute respiratory syndrome coronavirus (SARS-CoV) and middle east respiratory syndrome coronavirus (MERS-CoV) infections (Leung et al., 2003; Zhou et al., 2017), Zhou et al. (2020a) isolated SARS-CoV-2 from differentiated bat small intestinal organoids and human intestinal organoids, an in vitro model of the human intestinal epithelium. They found that both bat and human intestinal organoids developed progressive cytopathic changes after SARS-CoV-2 inoculation and accompanied by a substantially increased viral load in the culture media (Zhou et al., 2020a), suggesting that active replication of SARS-CoV-2 in human intestinal organoids. Notably, infectious SARS-CoV-2 was successfully isolated from the stool specimen of a patient with diarrheal COVID-19 (Zhou et al., 2020a) and 2 patients without diarrhea (Wang et al., 2020d). Another study showed that infectious SARS-CoV-2 virus were successfully isolated from 2 of 3 patients with viral RNA–positive, indicating that infectious virus in feces was a common manifestation of COVID-19 and confirmed the potential of fecal–oral or fecal–respiratory transmission (Xiao et al., 2020b). In addition, recent studies showed that human angiotensin-converting enzyme II (ACE2), which had been proved to be a cell receptor for SARS-CoV-2 (Lu et al., 2020; Zhou et al., 2020b), was highly expressed in glandular cells of human gastric, duodenal, and rectal epithelia, supporting the entry of SARS-CoV-2 into host cells (Xiao et al., 2020a). Moreover, viral nucleocapsid protein (NP)-positive cells were not only observed in the cytoplasm of gastric, duodenal, and rectum glandular epithelial cells from the biopsy specimens of COVID-19 patients (Xiao et al., 2020a), but also in human intestinal organoids (Zhou et al., 2020a). Collectively, the available evidence demonstrated that the occurrence of SARS-CoV-2 infection in human intestinal organoids might recapitulate enteric infection of COVID-19 patients, and the human intestinal tract might represent an additional route of SARS-CoV-2 transmission (Zhou et al., 2020a). In the meantime, SARS-CoV-2 RNA was also detected in the urine sample (Sun et al., 2020), sink, and toilet (Ong et al., 2020) of COVID-19 patients, and the positive urine duration was more than one month (Sun et al., 2020). Notably, Sun et al. (2020) successfully isolated infectious SARS-CoV-2 from the urine of a COVID-19 patient, suggesting that SARS-CoV-2 might be secreted through the human urinary system.
Considering that infectious SARS-CoV-2 virus was found in urine and stool samples from COVID-19 patients on days 11–15 of the clinical process (Jeong et al., 2020), and the duration of SARS-CoV-2 RNA positive in stool and urine would last more than one month (Cai et al., 2020; Chen et al., 2020b; Holshue et al., 2020; Lescure et al., 2020; Pan et al., 2020; Tang et al., 2020; Wang et al., 2020b; Wu et al., 2020c; Xiao et al., 2020a; Xu et al., 2020; Zheng et al., 2020; Sun et al., 2020), we believed that SARS-CoV-2 might exist in the stool and urine of patients with COVID-19 for a long time. According to the available literature, SARS-CoV-2 RNA was detected in wastewater samples from the Netherlands (Lodder and de Roda Husman, 2020), Australia (Ahmed et al., 2020), France (Wurtzer et al., 2020), USA (Wu et al., 2020a), Italy (La Rosa et al., 2020b), and Spain (Randazzo et al., 2020), we inferred that SARS-CoV-2 contaminated water might be a potential sustainable source of infection, thus threatening the local individuals' health. Previous studies have shown that water contaminated by bacteria, viruses, and chemicals was closely related to the use of pit latrines and septic tanks (Gerba, 1999; Graham and Polizzotto, 2013; Hammoud et al., 2018; Ngasala et al., 2019). Herein, this study aims to comprehensively evaluate the potential risks of COVID-19 in rural China through the investigation of rural geographical environment, the use of pit latrines, and the villagers' daily life, then try to find out the solutions. Although this study focused on rural areas in China, the results could also be applied to other low-income countries, especially in Africa.
2. Materials and methods
2.1. Field survey
In order to prevent the spread of COVID-19, several versions of the prevention guidelines were issued in China, suggesting that people with a history of living or traveling in epidemic areas should carry out self-health monitoring twice a day for 14 days from the time of leaving the epidemic area, and try to live alone or in a single room with good ventilation to minimize close contact with their families (National Health Commission of the People's Republic of China, 2020). Zhang et al. (2020a) proposed a home confinement guideline for rural areas, emphasizing that people from the epidemic areas of COVID-19 should use tableware and sanitary items alone to avoid or control the suspicious transmission of SARS-CoV-2. However, the living conditions of some rural residents were difficult to meet the criteria for home confinement during the outbreak response. For example, some villagers who did not have flush toilets at home must leave their rooms and use the nearby shared pit latrines for defecation, which might increase the close contact between people and also increase the transmission of SARS-CoV-2. In addition, SARS-CoV-2 could be transmitted through the human excreta as mentioned above.
A field investigation was carried out in 3 villages of Jiangxi province, China, which contained returnees from Wuhan. The survey included the sources of domestic water, the structure, distribution and use of pit latrines among villages, and the excreta management pattern in rural China, to find out whether there was a systematic disinfection process and analyze the possibility of fecal contamination of drinking water. In addition, we also conducted a detailed survey on the distribution of family houses and daily life routes of villagers with a history of being in epidemic areas in Wuhan and other cities of Hubei province to identify the impact of pit latrines on the risks of COVID-19 transmission.
2.2. Source of data
Village committees are grass-roots mass organizations of self-governance elected by villagers of the administrative village under the jurisdiction of a township in mainland China, a village committee shall be composed of 3–7 members, including the chairman, vice-chairmen and members (Chinese Government, 2010). Their functions mainly include self-management, self-education, self-service by villagers, and carry out democratic elections, democratic decision-making, democratic management and democratic supervision. The village committees handle the village's public affairs and public welfare undertakings, mediate civil disputes, assist in maintaining public order, and report to the people's government the opinions, requirements and suggestions of the villagers (Chinese Government, 2010). Thus, the village committees know the basic situation of the village very well.
To understand the prevalence of pit latrines in rural China, we contacted to the village committees from 27 villages in Jiangxi and Hubei provinces by phone, email and WeChat to survey the number of households, the total population, the average household size, whether there are flush toilets at family, whether villagers use pit latrines, and whether villagers use untreated excreta as fertilizer among the rural households. Here, we define flush toilets as flushing human excreta (urine and feces) into the septic-tanks through drainpipes, and then the excreta in septic-tanks can be made harmless by bacterial fermentation or centralized disinfection when necessary (for example: to kill potential SARS-CoV-2). This definition is consistent with the Joint Monitoring Programme (JMP) classification of improved sanitation facilities that flush or pour flush toilets connecting to piped sewer systems (WHO and UNICEF, 2017).
3. Results and discussions
3.1. Pit latrines and excreta management in rural China
Chinese villages have long been known for their small bridges and flowing water, which means that Chinese villages are usually built near streams, where great rivers originated. During the field investigation of 3 villages in Jiangxi province, China, we found that they all located upstream of the river, and many pit latrines are scattered around the rural houses throughout the villages. As shown in Fig. 1 , a typical pit latrine in rural China consists of 3 main parts: a shelter for creating a private space, a slab or floor with a small hole for villagers to defecate, and a cesspool for storing feces and urine. Besides, there was usually a small window on the wall of cesspool for farmers to take the excreta as a natural fertilizer. To make it easier for farmers to get the excreta, some cesspools were built on the open next to the shelter. As described in available researches (Heinonen-Tanski and van Wijk-Sijbesma, 2005; Lam et al., 2020a; Mamera et al., 2020), human urine and feces as fertilizer could meet the needs of plants for potassium and phosphorus, and improve soil structure, and using human excreta as fertilizer is free, which leads to the villagers like to use excreta as fertilizer. Through surveying 27 villages in Jiangxi and Hubei provinces by contacting the village committees through phone, email and WeChat, it was found that about 0%–60% (31% on average) of rural households used the excreta directly from the pit latrines as crop fertilizer in recent 3 years. In addition, according to the villagers, we learned that the excreta in the open cesspools may be washed to everywhere by rainwater, or be carried to everywhere by animal like dogs, cats, or field mouse, eventually polluting the local water. Fortunately, the villagers' domestic water is all diverted from the mountain stream by water pipes rather than groundwater. Although the water from mountain stream was not treated before using, it was not polluted by excreta as the source of water was far away from the pit latrines.
Fig. 1.
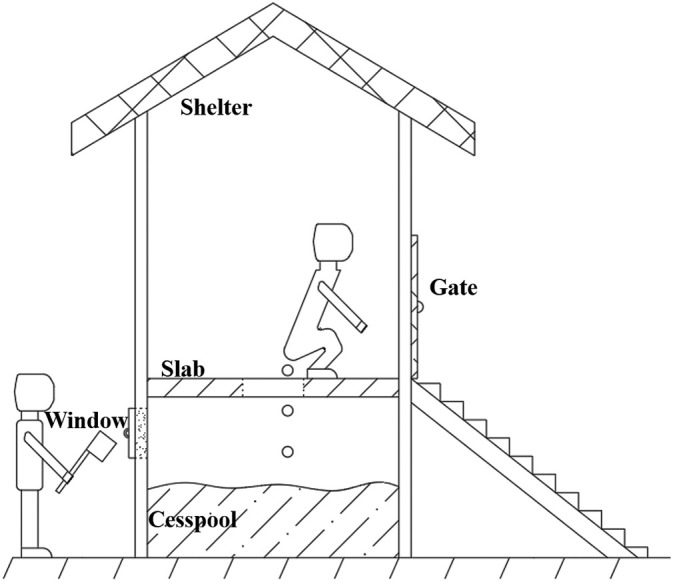
Schematic diagram of a typical pit latrine in rural China.
It was well documented that the bacteria, viruses, and chemicals within drinking water sources or agricultural soil posed a great threat to human health (Gerba, 1999; Gerba and Bitton, 1984; Graham and Polizzotto, 2013; Hammoud et al., 2018; Jamieson et al., 2002; Leung et al., 2020; Mamera and Van Tol, 2018; Mamera et al., 2020; Nganje et al., 2020; Tallon et al., 2005). For example, wells in nearby septic tanks and pit latrines were found to be significantly contaminated in Dar es Salaam, Tanzania, leading to more than 80% of wells contained with Escherichia coli and 58% of wells with nitrate levels higher than WHO guidelines (Ngasala et al., 2019). It has been recognized that the consequent movement of pathogens along with subsurface drainage systems to surface water systems was the main route of pathogen transport (Jamieson et al., 2002; Prüss-Ustün et al., 2019), and it was possible that slimy bacteria form a thin coat over the pipelines to aid in the spread of SARS-CoV-2 (Naddeo and Liu, 2020). Thus, SARS-CoV-2, as a virus, may also be transmitted through water.
A study from Australia (Ahmed et al., 2020) showed that the number of infected individuals in the catchment areas could be reasonably estimated by detecting the copy numbers of SARS-CoV-2 RNA in the wastewater, which verified that early detection of coronavirus in wastewater might be a viable surveillance strategy for COVID-19 infections (Daughton, 2020; Orive et al., 2020; Wu et al., 2020b) as previously demonstrated for hepatitis A virus, norovirus (Hellmér et al., 2014) and poliovirus (Asghar et al., 2014; Lodder et al., 2012). Although there was no sufficient evidence that fecal-oral transmission of COVID-19 was viable, while there was evidence showed that SARS-CoV-2 could be easily and sustainably transmitted in the community in Shenzhen, China, because the proportion of COVID-19 patients without definite exposure from January 25 through February 5 (11%) was much higher than that before January 24 (6%) (Liu et al., 2020). This suggests that there may be other potential routes of transmission, such as exposure to SARS-CoV-2 that survive in community environment. Given that the infectious SARS-CoV-2 was found to be secreted through the human urinary system (Sun et al., 2020) and intestinal tract (Wang et al., 2020d; Xiao et al., 2020b; Zhou et al., 2020a), and SARS-CoV-2 RNA was detected in wastewater worldwide (Randazzo et al., 2020), many studies have suggested the possibility of wastewater transmission of COVID-19 disease (Adelodun et al., 2020; Arslan et al., 2020; Foladori et al., 2020). However, the direct use of untreated excreta as a fertilizer and the flushing of excreta from open cesspools into the waters on rainy days might lead to serious water pollution, including the SARS-CoV-2 within human excreta. Thus, the possibility of SARS-CoV-2 transmission through water contaminated with human excreta cannot be ignored.
3.2. SARS-CoV-2 survives in nature could be a potentially sustainable source of infection
Coronaviruses are present in a variety of animals and can cause respiratory, enteric, hepatic, and nervous system diseases of varying severity (Lau et al., 2005). The members of the coronavirus family, SARS-CoV (Drosten et al., 2003), MERS-CoV (Chan et al., 2015) and the current SARS-CoV-2 (Zhu et al., 2020) have caused severe respiratory illness and high mortality to humans since 2002. Studies have shown that both SARS-CoV and MERS-CoV were likely originated from bats and then crossed species barriers to infect humans through an amplification mammalian host, the Paguma larvata for SARS-CoV and the Camelus dromedarius for MERS-CoV (Chan et al., 2015; Cheng et al., 2007; Lau et al., 2005). Similarly, recent studies have shown that SARS-CoV-2 might originated from bats with the genome of SARS-CoV-2 has 88%–96% (91% on average) nucleotide identity with several bat coronaviruses and 79%–82% (80% on average) with human SARS-CoV, but might with more proximal origins from a potential intermediate animal host (Chan et al., 2020; Ji et al., 2020; Lu et al., 2020; Zhou et al., 2020b). It has been reported that pangolins (Lam et al., 2020b; Lopes et al., 2020; Wahba et al., 2020; Xiao et al., 2020c; Zhang et al., 2020b) and snakes (Ji et al., 2020) might be the intermediate hosts of SARS-CoV-2. However, the possibility of snakes as intermediate hosts of SARS-CoV-2 was questioned by the scientific community (Zhang et al., 2020b), and the existing evidence was not sufficient to either confirm or rule out the role of pangolins as an intermediate host (Tiwari et al., 2020; Wahba et al., 2020). In other words, it is still not clear which animal is the intermediate hosts that brings SARS-CoV-2 to human hosts.
Bats and birds are natural reservoirs for providing coronavirus and influenza virus genes during the evolution of new virus species and viruses for interspecies transmission (Chan et al., 2013). As described in the latest review (Tiwari et al., 2020), many coronaviruses have bats, birds, or pigs as the primary host, in addition to infecting animals such as civets, pangolins, and camels, coronaviruses also could be harbored by a range of animal species, such as fish, snake, cattle, horse, dog, cat, rabbit, rodent, ferret, minks, frog, marmot, and hedgehog. Moreover, Damas et al. (2020) had greatly expanded the potential number of intermediate hosts that might be infected by SARS-CoV-2 through ACE2 receptors by protein structural analysis, which means that plenty of wild animals might be novel SARS-CoV-2 hosts. The results highlight the importance of wildlife and biosecurity in farms and wet markets, which may serve as the potential source and amplification centers for emerging infections (Cheng et al., 2007; Tiwari et al., 2020).
Previous studies have elucidated that SARS-CoV could survive for 4 days in diarrheal stool samples with an alkaline pH (Lai et al., 2005), 14 days in sewage at 4 degrees Celsius and 2 days at 20 degrees Celsius (Wang et al., 2005), and retained its viability for over 5 days at temperatures of 22 °C -25 °C and relative humidity of 40%–50% (Chan et al., 2011). Kampf et al. (2020) analyzed 22 studies and found that human coronaviruses such as SARS-CoV, MERS-CoV or endemic human coronaviruses could persist on inanimate surfaces like metal, glass or plastic for up to 9 days. Another study (Casanova et al., 2009) found that two surrogate coronaviruses, transmissible gastroenteritis (TGEV) and mouse hepatitis (MHV) remained infectious in water and sewage for days to weeks at 25 degrees Celsius, the time for 99% reduction was 9 days for TGEV and 7 days for MHV even in the pasteurized settled sewage, suggesting that contaminated water might a potential vehicle for human exposure if aerosol was generated. Notably, recent studies demonstrated that SARS-CoV-2 was highly stable at 4 °C with only around a 0.7 log-unit reduction of infectious titre on day 14, but was sensitive to heat as the time for virus inactivation was reduced to 5 min (Chin et al., 2020). Besides, no infectious virus was detected from treated smooth surfaces on day 4 (glass and banknote) or day 7 (stainless steel and plastic), but a detectable level of infectious virus could still be present on the outer layer of a surgical mask on day 7 (Chin et al., 2020). SARS-CoV-2 could be highly stable in a favorable environment (van Doremalen et al., 2020), for example, SARS-CoV-2 is extremely stable in a wide range of pH values at room temperature (pH 3–10) (Chin et al., 2020).
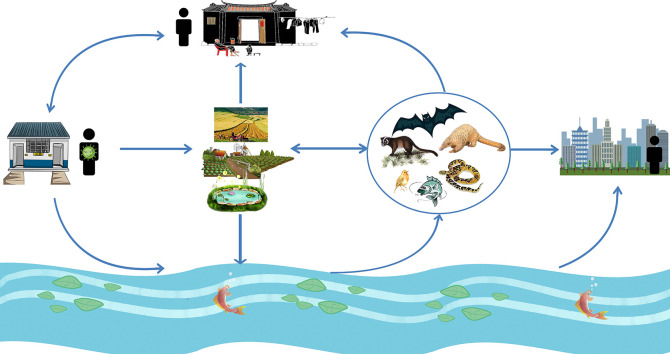
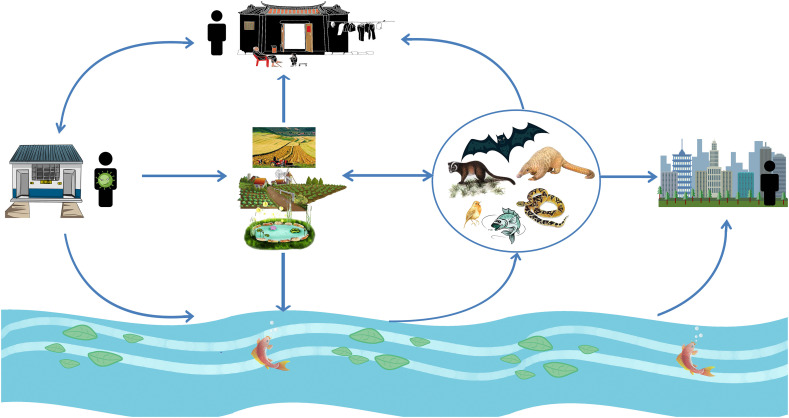
Infectious SARS-CoV-2 has been found in human excreta samples of COVID-19 patient in many previous studies (Sun et al., 2020; Wang et al., 2020d; Xiao et al., 2020b; Zhou et al., 2020a), and the SARS-CoV-2 embedded in stool particles in septic tanks could escape from disinfection and slowly release into aqueous phase, behaving as a secondary source of SARS-CoV-2 and potentially contributing to its spread through drainage pipelines (Zhang et al., 2020c). Former study has found that particles (kaolin clay, humic acid powder, and activated sludge) <2 μm in diameter were large enough to protect viruses from 254-nm ultraviolet (UV) light (Templeton et al., 2005), suggesting that the protection of fecal particles might make the survival of SARS-CoV-2 more easier. The SARS-CoV-2 within the excreta of COVID-19 patients can be released into the water (natural environment) (Zhang et al., 2020c) when farmers use untreated excreta as fertilizer in agricultural fields, and the excreta been washed into water by rainwater or be carried to water by animal. Then the SARS-CoV-2 survived in the natural environment would be available absorbed by wild animals (such as bats, birds, fishes, and snakes) by drinking or contacting contaminated water, and become a secondary source of human SARS-CoV-2 infection. This hypothesis was consistent with another study that SARS-CoV-2 spilled into novel wild hosts in North America, as demonstrated by Franklin and Bevins (2020) through a conceptual model for the perpetuation of the pathogen. Owing to the typically high mutation rates of RNA viruses, coronaviruses (including SARS-CoV-2) can rapidly increase their virulence and adapt to new hosts (Duffy, 2018; Elena and Sanjuán, 2005). Besides, the potential aquatic animals that may be infected with SARS-CoV-2 in the river may reach cities downstream of the river, then may be caught and eaten by city dwellers, further expanding the range of transmission. As excreta was directly used as fertilizer, fruits and vegetables grown in rural areas might be contaminated by SARS-CoV-2 and then eaten by wild animals, or purchased and eaten by rural and urban residents through wet markets. Long-term exposure to contaminated environmental sources, such as the air pollutants, extended exposure to aerosols produced by contaminated water, and the inadequate cleaning processes of food and the surfaces of some materials, may resulted in an increased risk of SARS-CoV-2 transmission (Adelodun et al., 2020; Carraturo et al., 2020). Thus, it was seemly reasonable that the SARS-CoV-2 survives in nature could be a potential sustainable source of infection (Fig. 2 ), particularly for those people in rural areas who relied on untreated drinking groundwater sources (Hammoud et al., 2018; Mamera and Van Tol, 2018; Rosa and Clasen, 2010) and who practiced open defecation (Adzawla et al., 2020), the increased SARS-CoV-2 load in the natural environment might increase the possibility of human infection.
Fig. 2.
SARS-CoV-2 may spread from the excreta of infected humans in pit latrines to potential animal hosts and then become a sustainable source of infection. Detailed description in the text.
3.3. The prevalence of pit latrines in rural China and other low-income countries
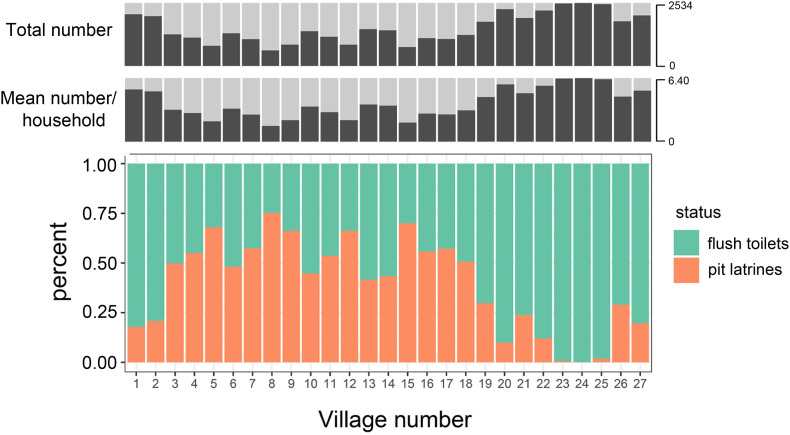
Through contacting the village committees by phone, email and WeChat, a total of 9606 households were investigated in 27 villages of Jiangxi and Hubei provinces, China, involving 34,101 persons with an average household size of 3.6 persons (1–12 persons per household) and an average number of 355.8 ± 126.4 households per village. The average proportion of households using pit latrines was 40% (0%–75%) (Fig. 3 , Table 1 ).
Fig. 3.
The population situation and the prevalence of pit latrines in rural China. The top row was the total population of each village, the middle row was the average household size of each village, the bottom row was the proportion of households using flush toilets or using pit latrines.
Table 1.
The population situation in rural China and the proportion of households with or without flush toilets.
| Villages in rural China | Num of households | Total population | Average household size | Num of the households using flush toilets | Percent of the households using flush toilets | Num of the households using pit latrines | Percent of the households using pit latrines |
|---|---|---|---|---|---|---|---|
| 1 | 235 | 1146 | 4.876595745 | 193 | 0.821276596 | 42 | 0.178723404 |
| 2 | 225 | 795 | 3.533333333 | 178 | 0.791111111 | 47 | 0.208888889 |
| 3 | 237 | 481 | 2.029535865 | 119 | 0.502109705 | 118 | 0.497890295 |
| 4 | 422 | 1201 | 2.845971564 | 190 | 0.450236967 | 232 | 0.549763033 |
| 5 | 412 | 1213 | 2.944174757 | 132 | 0.32038835 | 280 | 0.67961165 |
| 6 | 185 | 595 | 3.216216216 | 96 | 0.518918919 | 89 | 0.481081081 |
| 7 | 507 | 1295 | 2.554240631 | 216 | 0.426035503 | 291 | 0.573964497 |
| 8 | 323 | 1164 | 3.60371517 | 80 | 0.247678019 | 243 | 0.752321981 |
| 9 | 562 | 2054 | 3.65480427 | 190 | 0.338078292 | 372 | 0.661921708 |
| 10 | 154 | 986 | 6.402597403 | 85 | 0.551948052 | 69 | 0.448051948 |
| 11 | 332 | 1289 | 3.88253012 | 154 | 0.463855422 | 178 | 0.536144578 |
| 12 | 290 | 957 | 3.3 | 98 | 0.337931034 | 192 | 0.662068966 |
| 13 | 265 | 705 | 2.660377358 | 155 | 0.58490566 | 110 | 0.41509434 |
| 14 | 374 | 1330 | 3.556149733 | 212 | 0.56684492 | 162 | 0.43315508 |
| 15 | 503 | 1281 | 2.546719682 | 151 | 0.300198807 | 352 | 0.699801193 |
| 16 | 181 | 482 | 2.662983425 | 80 | 0.44198895 | 101 | 0.55801105 |
| 17 | 308 | 829 | 2.691558442 | 132 | 0.428571429 | 176 | 0.571428571 |
| 18 | 286 | 1040 | 3.636363636 | 141 | 0.493006993 | 145 | 0.506993007 |
| 19 | 547 | 2035 | 3.720292505 | 384 | 0.702010969 | 163 | 0.297989031 |
| 20 | 425 | 1410 | 3.317647059 | 383 | 0.901176471 | 42 | 0.098823529 |
| 21 | 374 | 1539 | 4.114973262 | 285 | 0.762032086 | 89 | 0.237967914 |
| 22 | 574 | 2534 | 4.414634146 | 505 | 0.879790941 | 69 | 0.120209059 |
| 23 | 433 | 1722 | 3.976905312 | 430 | 0.993071594 | 3 | 0.006928406 |
| 24 | 539 | 2192 | 4.066790353 | 539 | 1 | 0 | 0 |
| 25 | 385 | 1654 | 4.296103896 | 378 | 0.981818182 | 7 | 0.018181818 |
| 26 | 320 | 1296 | 4.05 | 227 | 0.709375 | 93 | 0.290625 |
| 27 | 208 | 876 | 4.211538462 | 167 | 0.802884615 | 41 | 0.197115385 |
| Total/average | 9606 | 34,101 | 3.583953791 | 5900 | 0.604342392 | 3706 | 0.395657608 |
Existing reports indicated that pit latrines were one of the most common human excreta disposal systems in low-income countries (Heinonen-Tanski and van Wijk-Sijbesma, 2005; Mamera and Van Tol, 2018; Mamera et al., 2020) with approximately 1.77 billion of the world's population (Graham and Polizzotto, 2013). According to a recent survey (Mutyambizi et al., 2020), pit latrines was the most common toilet facilities in informal settlements in South Africa (53%), followed by flush toilets (24%), with approximately 6% reported not having access to any toilet facilities. Similarly, the most common type of toilet facilities was shared pit latrine with a slab (67%) with no hand-washing facilities in Kampala, Uganda (Ssemugabo et al., 2020). In Ghana, 40% of commonly used toilet facilities were dry toilets (pit latrines), and between 2 and 21 households, or 4 and 84 people shared one facility, (Antwi-Agyei et al., 2020), and more than 25% of households engaged in open defecation (Adzawla et al., 2020). In short, toilet facilities in low-income countries are critical and need to be improved.
In contrast to pit latrines, flush toilets have septic tanks that collect excreta through water-closed drainpipes, allowing for microbial degradation and disinfectant treatment of excreta when necessary (for example: to kill potential SARS-CoV-2). As generally considered that coronaviruses were very sensitive to oxidants, such as chlorine, ozonation, and UV irradiation (La Rosa et al., 2020a; Quevedo et al., 2020; Wang et al., 2020c; Zhang et al., 2020c), the SARS-CoV-2 released from human excreta during flush toilets-drainpipes-septic tanks could be inactivated by conventional disinfection. However, pit latrines with an open cesspool are not easy to be disinfected, because the excreta may be washed to everywhere by rainwater or be carried to everywhere by dogs or field mouse. Coupled with the fact that villagers usually use untreated excreta as agricultural fertilizer, we believe that the use of pit latrines in rural China and other low-income countries increases the possibility of SARS-CoV-2 contaminating the surrounding natural environment and ultimately harms human health.
3.4. The use of pit latrines increases the potential risk of transmission of COVID-19 by increasing the contact between human
Human to human contact is the most important factor to increase the transmission rate of COVID-19. According to recent reports, the transmission routes of SARS-CoV-2 mainly include the respiratory tract by droplets or respiratory secretions, and contact with infected persons or contaminated surface with poor hygiene practice (Arslan et al., 2020; Saeed et al., 2020). In order to prevent the spread of COVID-19 between people, several measures were widely accepted by many countries, such as social distancing, handwashing, mask-wearing, isolation, quarantine, and community containment (Ataguba and Ataguba, 2020; Mahase, 2020; Teslya et al., 2020; Wilder-Smith and Freedman, 2020), and these measures were effective and have been confirmed by multiple reports (Mwalili et al., 2020; Ngonghala et al., 2020; Teslya et al., 2020; Tian et al., 2020).
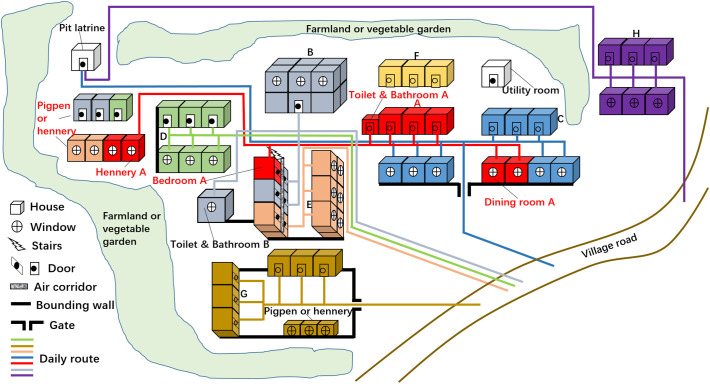
During the field investigation of 3 villages in Jiangxi province, China, we found that it was common for villagers to live with scattered houses and share pit latrines, with 1 to 5 households (an average of 2.2) sharing one pit latrines. As shown in Fig. 4 , of the 8 households in A-H, only household A included a member with a history of being in epidemic areas (Wuhan), family members of both C and H households all defecated in pit latrines, other households' members defecated in flush toilets at their home and occasionally used the pit latrines. The houses of A, B, and C households were scattered, naturally, the daily life routes of family members were significantly larger than those of other families. Notably, the use of pit latrines expanded the daily life routes of C and H households' members and increased the possibility of close contact and exposure to SARS-CoV-2 between individuals. Ultimately, it might increase the risk of COVID-19 transmission. Given that plentiful households shared toilet facilities (most of them were pit latrines) with poor ancillary facilities (such as hand-washing facilities) were common in low-income countries (Antwi-Agyei et al., 2020; Ssemugabo et al., 2020), we believed that the use of pit latrines in rural China and low-income countries would increase the contact between human, thus increasing the risk of COVID-19 transmission to a certain extent.
Fig. 4.
The household houses distribution of some households in rural China and the daily life routes of family members. Of the 8 households in A–H, houses marked with the same color were owned by the same household, for example, all the houses marked with red were owned by household A. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Conclusion
We proposed this hypothesis to illustrate the mechanism that SARS-COV-2 might spread from the excreta of infected humans in pit latrines to potential animal hosts and then become a sustainable source of infection in rural China and other low-income countries. The widely use of pit latrines and open cesspools coupled with agricultural fields application of untreated excreta could act as a potential route for the spread of COVID-19 disease and other possible waterborne diseases that could emanate from the excreta of infected persons. We suggest for further implementation of the toilets revolution in rural China and also low-income countries in the world, completely replace pit latrines by flush toilets. Next, according to the division of the residential area, using drainage systems to collect human excreta centrally and then used as agricultural fertilizer after unified disinfection treatment. Through these preventive measures, the local living environment will be significantly improved and SARS-CoV-2 and other potential waterborne diseases will be effectively prevented.
CRediT authorship contribution statement
Lilong Liu, Junyi Hu, and Yaxin Hou contributed equally to this work. Lilong Liu: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Roles/Writing - original draft. Junyi Hu: Data curation, Formal analysis, Investigation, Methodology, Roles/Writing - original draft. Yaxin Hou: Investigation, Methodology. Zhen Tao: Conceptualization, Investigation, Methodology, Writing - review & editing. Zhaohui Chen: Conceptualization, Formal analysis, Methodology, Writing - review & editing. Ke Chen: Conceptualization, Formal analysis, Investigation, Methodology, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Graduates' Innovation Fund of Huazhong University of Science and Technology (2019ygscxcy069).
Editor: Jay Gan
References
- Adelodun B., Ajibade F.O., Ibrahim R.G., Bakare H.O., Choi K.S. Snowballing transmission of COVID-19 (SARS-CoV-2) through wastewater: any sustainable preventive measures to curtail the scourge in low-income countries? Sci. Total Environ. 2020;742 doi: 10.1016/j.scitotenv.2020.140680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adzawla W., Alhassan H., Jongare A.I. Explaining the effects of socioeconomic and housing characteristics on the choice of toilet facilities among Ghanaian households. J. Environ. Public Health. 2020;2020:4036045. doi: 10.1155/2020/4036045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., et al. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antwi-Agyei P., Dwumfour-Asare B., Adjei K.A., Kweyu R., Simiyu S. Understanding the barriers and opportunities for effective management of shared sanitation in low-income settlements-the case of Kumasi, Ghana. Int. J. Environ. Res. Public Health. 2020;17 doi: 10.3390/ijerph17124528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan M., Xu B., Gamal El-Din M. Transmission of SARS-CoV-2 via fecal-oral and aerosols-borne routes: environmental dynamics and implications for wastewater management in underprivileged societies. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar H., Diop O.M., Weldegebriel G., Malik F., Shetty S., El Bassioni L., et al. Environmental surveillance for polioviruses in the global polio eradication initiative. J. Infect. Dis. 2014;210(Suppl. 1):S294–S303. doi: 10.1093/infdis/jiu384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataguba O.A., Ataguba J.E. Social determinants of health: the role of effective communication in the COVID-19 pandemic in developing countries. Glob. Health Action. 2020;13:1788263. doi: 10.1080/16549716.2020.1788263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J., Xu J., Lin D., Yang Z., Xu L., Qu Z., et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin. Infect. Dis. 2020;71:1547–1551. doi: 10.1093/cid/ciaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraturo F., Del Giudice C., Morelli M., Cerullo V., Libralato G., Galdiero E., et al. Persistence of SARS-CoV-2 in the environment and COVID-19 transmission risk from environmental matrices and surfaces. Environ. Pollut. 2020;265 doi: 10.1016/j.envpol.2020.115010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova L., Rutala W.A., Weber D.J., Sobsey M.D. Survival of surrogate coronaviruses in water. Water Res. 2009;43:1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.H., Peiris J.S., Lam S.Y., Poon L.L., Yuen K.Y., Seto W.H. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv Virol. 2011;2011:734690. doi: 10.1155/2011/734690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., To K.K., Tse H., Jin D.Y., Yuen K.Y. Interspecies transmission and emergence of novel viruses: lessons from bats and birds. Trends Microbiol. 2013;21:544–555. doi: 10.1016/j.tim.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., Lau S.K., To K.K., Cheng V.C., Woo P.C., Yuen K.Y. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin. Microbiol. Rev. 2015;28:465–522. doi: 10.1128/CMR.00102-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., Kok K.H., Zhu Z., Chu H., To K.K., Yuan S., et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Chen L., Deng Q., Zhang G., Wu K., Ni L., et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J. Med. Virol. 2020;92:833–840. doi: 10.1002/jmv.25825. [DOI] [PubMed] [Google Scholar]
- Cheng V.C., Lau S.K., Woo P.C., Yuen K.Y. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin. Microbiol. Rev. 2007;20:660–694. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.L., Chan M.C.W., et al. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1:e10. doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese Government Organic Law of the Villagers' Committees of the People's Republic of China. Govnet of China, October 28. 2010. http://www.gov.cn/flfg/2010-10/28/content_1732986.htm Available at.
- Damas J., Hughes G.M., Keough K.C., Painter C.A., Persky N.S., Corbo M., et al. 2020. Broad Host Range of SARS-CoV-2 Predicted by Comparative and Structural Analysis of ACE2 in Vertebrates. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C.G. Wastewater surveillance for population-wide Covid-19: the present and future. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Günther S., Preiser W., van der Werf S., Brodt H.R., Becker S., et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Duffy S. Why are RNA virus mutation rates so damn high? PLoS Biol. 2018;16 doi: 10.1371/journal.pbio.3000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elena S.F., Sanjuán R. Adaptive value of high mutation rates of RNA viruses: separating causes from consequences. J. Virol. 2005;79:11555–11558. doi: 10.1128/JVI.79.18.11555-11558.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foladori P., Cutrupi F., Segata N., Manara S., Pinto F., Malpei F., et al. SARS-CoV-2 from faeces to wastewater treatment: what do we know? A review. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin A.B., Bevins S.N. Spillover of SARS-CoV-2 into novel wild hosts in North America: a conceptual model for perpetuation of the pathogen. Sci. Total Environ. 2020;733 doi: 10.1016/j.scitotenv.2020.139358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q.Y., Chen Y.X., Fang J.Y. 2019 novel coronavirus infection and gastrointestinal tract. J. Dig. Dis. 2020;21:125–126. doi: 10.1111/1751-2980.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C. Virus survival and transport in groundwater. J. Ind. Microbiol. Biotechnol. 1999;22:535–539. [Google Scholar]
- Gerba C., Bitton G. Microbial pollutants: their survival and transport pattern to groundwater. Groundwater pollution microbiology. 1984:39–54. [Google Scholar]
- Graham J.P., Polizzotto M.L. Pit latrines and their impacts on groundwater quality: a systematic review. Environ. Health Perspect. 2013;121:521–530. doi: 10.1289/ehp.1206028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammoud A., Leung J., Tripathi S., Butler A., Sule M., Templeton M. The impact of latrine contents and emptying practices on nitrogen contamination of well water in Kathmandu Valley, Nepal. AIMS Environmental Science. 2018;5:143–153. [Google Scholar]
- Heinonen-Tanski H., van Wijk-Sijbesma C. Human excreta for plant production. Bioresour. Technol. 2005;96:403–411. doi: 10.1016/j.biortech.2003.10.036. [DOI] [PubMed] [Google Scholar]
- Hellmér M., Paxéus N., Magnius L., Enache L., Arnholm B., Johansson A., et al. Detection of pathogenic viruses in sewage provided early warnings of hepatitis a virus and norovirus outbreaks. Appl. Environ. Microbiol. 2014;80:6771–6781. doi: 10.1128/AEM.01981-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., et al. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson R., Gordon R., Sharples K.E., Stratton G., Madani A. Movement and persistence of fecal bacteria in agricultural soils and subsurface drainage water: a review. Canadian Biosystems Engineering / Le Genie des biosystems au Canada. 2002;44:1.1–1.9. [Google Scholar]
- Jeong H.W., Kim S.M., Kim H.S., Kim Y.I., Kim J.H., Cho J.Y., et al. Viable SARS-CoV-2 in various specimens from COVID-19 patients. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.07.020. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W., Wang W., Zhao X., Zai J., Li X. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J. Med. Virol. 2020;92:433–440. doi: 10.1002/jmv.25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods - a scoping review. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., et al. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.Y., Cheng P.K., Lim W.W. Survival of severe acute respiratory syndrome coronavirus. Clin. Infect. Dis. 2005;41:e67–e71. doi: 10.1086/433186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam K.L., Zlatanović L., van der Hoek J.P. Life cycle assessment of nutrient recycling from wastewater: a critical review. Water Res. 2020;173 doi: 10.1016/j.watres.2020.115519. [DOI] [PubMed] [Google Scholar]
- Lam T.T., Jia N., Zhang Y.W., Shum M.H., Jiang J.F., Zhu H.C., et al. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature. 2020;583:282–285. doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- Lau S.K., Woo P.C., Li K.S., Huang Y., Tsoi H.W., Wong B.H., et al. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc. Natl. Acad. Sci. U. S. A. 2005;102:14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescure F.X., Bouadma L., Nguyen D., Parisey M., Wicky P.H., Behillil S., et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect. Dis. 2020;20:697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung W.K., To K.F., Chan P.K., Chan H.L., Wu A.K., Lee N., et al. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011–1017. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A.K.C., Leung A.A.M., Wong A.H.C., Hon K.L. Human ascariasis: an updated review. Recent Patents Inflamm. Allergy Drug Discov. 2020 doi: 10.2174/1872213X14666200705235757. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Liu J., Liao X., Qian S., Yuan J., Wang F., Liu Y., et al. Community transmission of severe acute respiratory syndrome coronavirus 2, Shenzhen, China, 2020. Emerg. Infect. Dis. 2020;26:1320–1323. doi: 10.3201/eid2606.200239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder W., de Roda Husman A.M. SARS-CoV-2 in wastewater: potential health risk, but also data source. Lancet Gastroenterol Hepatol. 2020;5:533–534. doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder W.J., Buisman A.M., Rutjes S.A., Heijne J.C., Teunis P.F., de Roda Husman A.M. Feasibility of quantitative environmental surveillance in poliovirus eradication strategies. Appl. Environ. Microbiol. 2012;78:3800–3805. doi: 10.1128/AEM.07972-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes L.R., de Mattos Cardillo G., Paiva P.B. Molecular evolution and phylogenetic analysis of SARS-CoV-2 and hosts ACE2 protein suggest Malayan pangolin as intermediary host. Braz. J. Microbiol. 2020:1–7. doi: 10.1007/s42770-020-00321-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahase E. Covid-19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction. Bmj. 2020;368:m1036. doi: 10.1136/bmj.m1036. [DOI] [PubMed] [Google Scholar]
- Mamera M., Van Tol J.J. Application of hydropedological information to conceptualize pollution migration from dry sanitation systems in the Ntabelanga Catchment Area, South Africa. Air, Soil and Water Research. 2018;11 [Google Scholar]
- Mamera M., van Tol J.J., Aghoghovwia M.P., Mapetere G.T. Community faecal management strategies and perceptions on sludge use in agriculture. Int. J. Environ. Res. Public Health. 2020;17 doi: 10.3390/ijerph17114128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutyambizi C., Mokhele T., Ndinda C., Hongoro C. Access to and satisfaction with basic services in informal settlements: results from a baseline assessment survey. Int. J. Environ. Res. Public Health. 2020;17 doi: 10.3390/ijerph17124400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwalili S., Kimathi M., Ojiambo V., Gathungu D., Mbogo R. SEIR model for COVID-19 dynamics incorporating the environment and social distancing. BMC Res Notes. 2020;13:352. doi: 10.1186/s13104-020-05192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naddeo V., Liu H. Editorial perspectives: 2019 novel coronavirus (SARS-CoV-2): what is its fate in urban water cycle and how can the water research community respond? Environ. Sci. Water Res. Technol. 2020;6:1213–1216. doi: 10.1039/D0EW90015J. [DOI] [Google Scholar]
- National Health Commission of the People'’s Republic of China Guidelines for the prevention of novel coronavirus-infected pneumonia, January 29. 2020. http://www.chinanews.com/gn/2020/01-29/9072587.shtml Available at.
- Nganje T.N., Agbor E.E., Adamu C.I., Ukpong A.J., Katte B.F., Edet A.E., et al. Public health challenges as a result of contaminated water sources in Kumba, Cameroon. Environ. Geochem. Health. 2020;42:1167–1195. doi: 10.1007/s10653-019-00375-7. [DOI] [PubMed] [Google Scholar]
- Ngasala T.M., Masten S.J., Phanikumar M.S. Impact of domestic wells and hydrogeologic setting on water quality in peri-urban Dar es Salaam, Tanzania. Sci. Total Environ. 2019;686:1238–1250. doi: 10.1016/j.scitotenv.2019.05.202. [DOI] [PubMed] [Google Scholar]
- Ngonghala C.N., Iboi E., Eikenberry S., Scotch M., MacIntyre C.R., Bonds M.H., et al. Mathematical assessment of the impact of non-pharmaceutical interventions on curtailing the 2019 novel coronavirus. Math. Biosci. 2020;108364:325. doi: 10.1016/j.mbs.2020.108364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. Jama. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orive G., Lertxundi U., Barcelo D. Early SARS-CoV-2 outbreak detection by sewage-based epidemiology. Sci. Total Environ. 2020;732 doi: 10.1016/j.scitotenv.2020.139298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prüss-Ustün A., Wolf J., Bartram J., Clasen T., Cumming O., Freeman M.C., et al. Burden of disease from inadequate water, sanitation and hygiene for selected adverse health outcomes: an updated analysis with a focus on low- and middle-income countries. Int. J. Hyg. Environ. Health. 2019;222:765–777. doi: 10.1016/j.ijheh.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo R., Bastías-Montes J., Espinoza-Tellez T., Ronceros B., Balic I., Muñoz O. Inactivation of coronaviruses in food industry: the use of inorganic and organic disinfectants, ozone, and UV radiation. Scientia Agropecuaria. 2020;11:257–266. [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa G., Clasen T. Estimating the scope of household water treatment in low- and medium-income countries. Am J Trop Med Hyg. 2010;82:289–300. doi: 10.4269/ajtmh.2010.09-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed H., Shahi J., Khadka S., Bajgain Y., Gupta R., Yadav T.P. vol. 2. 2020. Different Modes of Transmission and Containment Strategies for COVID-19. [Google Scholar]
- Ssemugabo C., Wafula S.T., Ndejjo R., Osuret J., Musoke D., Halage A.A. Characteristics of sanitation and hygiene facilities in a slum community in Kampala, Uganda. Int. Health. 2020 doi: 10.1093/inthealth/ihaa011. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Zhu A., Li H., Zheng K., Zhuang Z., Chen Z., et al. Isolation of infectious SARS-CoV-2 from urine of a COVID-19 patient. Emerg Microbes Infect. 2020;9:991–993. doi: 10.1080/22221751.2020.1760144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon P., Magajna B., Lofranco C., Leung K.T. Microbial indicators of faecal contamination in water: a current perspective. Water Air Soil Pollut. 2005;166:139–166. [Google Scholar]
- Tang A., Tong Z.D., Wang H.L., Dai Y.X., Li K.F., Liu J.N., et al. Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerg. Infect. Dis. 2020;26:1337–1339. doi: 10.3201/eid2606.200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton M.R., Andrews R.C., Hofmann R. Inactivation of particle-associated viral surrogates by ultraviolet light. Water Res. 2005;39:3487–3500. doi: 10.1016/j.watres.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Teslya A., Pham T.M., Godijk N.G., Kretzschmar M.E., Bootsma M.C.J., Rozhnova G. Impact of self-imposed prevention measures and short-term government-imposed social distancing on mitigating and delaying a COVID-19 epidemic: a modelling study. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H., Liu Y., Li Y., Wu C.-H., Chen B., Kraemer M.U.G., et al. 2020. The Impact of Transmission Control Measures During the First 50 Days of the COVID-19 Epidemic in China. medRxiv. (2020.01.30.20019844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari R., Dhama K., Sharun K., Iqbal Yatoo M., Malik Y.S., Singh R., et al. COVID-19: animals, veterinary and zoonotic links. Vet Q. 2020;40:169–182. doi: 10.1080/01652176.2020.1766725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahba L., Jain N., Fire A.Z., Shoura M.J., Artiles K.L., McCoy M.J., et al. An extensive meta-metagenomic search identifies SARS-CoV-2-homologous sequences in pangolin lung Viromes. mSphere. 2020;5 doi: 10.1128/mSphere.00160-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Li J., Guo T., Zhen B., Kong Q., Yi B., et al. Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan Hospital and the 309th Hospital of the Chinese People’s Liberation Army. Water Sci. Technol. 2005;52:213–221. [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Jama. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Feng H., Zhang S., Ni Z., Ni L., Chen Y., et al. SARS-CoV-2 RNA detection of hospital isolation wards hygiene monitoring during the coronavirus disease 2019 outbreak in a Chinese hospital. Int. J. Infect. Dis. 2020;94:103–106. doi: 10.1016/j.ijid.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Shen J., Ye D., Yan X., Zhang Y., Yang W., et al. Disinfection technology of hospital wastes and wastewater: suggestions for disinfection strategy during coronavirus disease 2019 (COVID-19) pandemic in China. Environ. Pollut. 2020;262 doi: 10.1016/j.envpol.2020.114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., et al. Detection of SARS-CoV-2 in different types of clinical specimens. Jama. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, UNICEF . World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF); Geneva: 2017. Progress on Drinking Water, Sanitation and Hygiene: 2017 Update and SDG Baselines. [Google Scholar]
- Wilder-Smith A., Freedman D.O. Isolation, quarantine, social distancing and community containment: pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak. J Travel Med. 2020;27 doi: 10.1093/jtm/taaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Xiao A., Zhang J., Gu X., Lee W.L., Kauffman K., et al. 2020. SARS-CoV-2 Titers in Wastewater Are Higher Than Expected From Clinically Confirmed Cases. medRxiv. (2020.04.05.20051540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Xiao A., Zhang J., Moniz K., Endo N., Armas F., et al. 2020. SARS-CoV-2 Titers in Wastewater Foreshadow Dynamics and Clinical Presentation of New COVID-19 Cases. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Marechal V., Mouchel J.-M., Maday Y., Teyssou R., Richard E., et al. 2020. Evaluation of Lockdown Impact on SARS-CoV-2 Dynamics Through Viral Genome Quantification in Paris Wastewaters. medRxiv. (2020.04.12.20062679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Sun J., Xu Y., Li F., Huang X., Li H., et al. Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerg. Infect. Dis. 2020;26:1920–1922. doi: 10.3201/eid2608.200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K., Zhai J., Feng Y., Zhou N., Zhang X., Zou J.J., et al. Isolation of SARS-CoV-2-related coronavirus from Malayan pangolins. Nature. 2020;583:286–289. doi: 10.1038/s41586-020-2313-x. [DOI] [PubMed] [Google Scholar]
- Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y., et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Zheng W., Huang X., Bell E.W., Zhou X., Zhang Y. Protein structure and sequence reanalysis of 2019-nCoV genome refutes snakes as its intermediate host and the unique similarity between its spike protein insertions and HIV-1. J. Proteome Res. 2020;19:1351–1360. doi: 10.1021/acs.jproteome.0c00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Ling H., Huang X., Li J., Li W., Yi C., et al. Potential spreading risks and disinfection challenges of medical wastewater by the presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral RNA in septic tanks of Fangcang Hospital. Sci. Total Environ. 2020;741 doi: 10.1016/j.scitotenv.2020.140445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Yao M., Wang J., Ye D., Chen Q., Guo F., et al. Guidance on the control and prevention of SARS-CoV-2 infection in primary healthcare institutions in rural China(first edition) Chinese General Practice. 2020;23:763–769. [Google Scholar]
- Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q., et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January–March 2020: retrospective cohort study. Bmj. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Li C., Zhao G., Chu H., Wang D., Yan H.H., et al. Human intestinal tract serves as an alternative infection route for Middle East respiratory syndrome coronavirus. Sci. Adv. 2017;3:eaao4966. doi: 10.1126/sciadv.aao4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Li C., Liu X., Chiu M.C., Zhao X., Wang D., et al. Infection of bat and human intestinal organoids by SARS-CoV-2. Nat. Med. 2020;26:1077–1083. doi: 10.1038/s41591-020-0912-6. [DOI] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]