Abstract
Efforts to control and eliminate human schistosomiasis have accelerated over the past decade. In a number of endemic countries and settings, interruption of schistosome transmission has been achieved. In others, Schistosoma infections continue to challenge program managers at different levels, from the complexity of the transmission cycle, over limited treatment options and lack of field-friendly accurate diagnostics, to controversy around adequate intervention strategies. We conducted a landscape analysis on parasitic and vector-borne disease elimination approaches with the aim to identify evidence-based strategies, core components and key concepts for achieving and sustaining schistosomiasis control and for progressing elimination efforts towards interruption of transmission in sub-Saharan Africa. A total of 118 relevant publications were identified from Web of Science, Pubmed and the grey literature and reviewed for their content. In addition, we conducted in-depth interviews with 23 epidemiologists, program managers, policymakers, donors and field researchers. Available evidence emphasizes the need for comprehensive, multipronged and long-term strategies consisting of multiple complementary interventions that must be sustained over time by political commitment and adequate funding in order to reach interruption of transmission. Based on the findings of this landscape analysis, we propose a comprehensive set of intervention strategies for schistosomiasis control and elimination. Before deployment, the proposed interventions will require review, evaluation and validation in the frame of an expert consultation as a step towards adaptation to specific contexts, conditions and settings. Field testing to ensure local relevance and effectiveness is paramount given the diversity of socio-ecological and epidemiological contexts.
Author summary
This landscape analysis explored successful concepts, approaches and interventions of past and ongoing parasitic and vector-borne disease elimination efforts and programs with regard to relevance for progress in the elimination of human schistosome infections. Schistosomiasis is a disabling, water borne parasitic disease of public health concern with an estimated 250 million people infected worldwide. The long-term morbidity of this neglected tropical disease significantly impacts growth, cognition and socioeconomic development at all ages. Despite increased global efforts to control morbidity and advance elimination, challenges in view of the complex life cycle which involves freshwater sources, intermediate snail hosts and humans, remain. This calls for targeted interventions and concerted programs. According to the evidence from the literature and as proposed by a wide range of key informants, comprehensive, multipronged and long-term strategies supported by strong political commitment and adequate funding are required in order to achieve and sustain the set goals. Based on the findings, we propose here a comprehensive set of intervention strategies for schistosomiasis control and elimination for review and evaluation to inform implementation research needs and elimination program design.
Introduction
Schistosomiasis is a neglected tropical disease (NTD) with considerable impact on global health [1]. Five different Schistosoma (blood fluke) species have been described to affect humans, all of which depend on either aquatic or amphibious snails as intermediate hosts. Transmission to the human host occurs during exposure to water bodies infested with cercariae (schistosome larvae). Infection can result in acute and chronic disease manifestations in all age groups [2, 3].
Historically, endemic countries mainly relied on snail control and environmental modification in their efforts to control schistosome transmission, since treatment options were limited due to concerns about the safety of then available drugs [4]. Since the mid-1980s, praziquantel allows safe treatment [5]. As a consequence of the introduction of praziquantel, efforts in endemic countries were refocused on controlling the morbidity due to schistosomiasis, mainly through periodic mass drug administration (MDA) of praziquantel to the at-risk population without prior diagnosis, a concept also known as “preventive chemotherapy”[6]. A paradigm shift occurred in 2012 when the declared goal of the World Health Organization (WHO) was revised from morbidity control by 2020 to eliminating schistosomiasis as a public health problem, and to interrupt transmission in selected areas by 2025 [7]. Control of morbidity was suggested to be achieved through large-scale administration of praziquantel to populations at risk using defined thresholds of prevalence as criteria for selecting the appropriate interval of re-treatment. It was also emphasized that for elimination as a public health problem and interruption of transmission, preventive chemotherapy intervals needed to be adjusted and intensified, and complementary public health interventions were strongly recommended if not deemed essential [7]. In 2020, the WHO Roadmap for NTD control 2021–2030 was published. It measures progress in schistosomiasis control in terms of number of countries where elimination as a public health problem (<1% heavy intensity infections) has been validated. It also states that continued actions are required to maintain these achievements and to further advance elimination through interruption of transmission in defined geographical areas [8].
In several countries, most notably Japan, the goal of breaking schistosome transmission has long been achieved [4, 9]. Nationwide efforts in P.R. China and several other countries including in the Caribbean, have succeeded in eliminating schistosomiasis as a public health problem through integrated intervention efforts [9–11]. Multipronged strategies tailored to the local microepidemiology are needed to interrupt transmission. This was again highlighted by recent studies from the Zanzibar islands of the United Republic of Tanzania where ten rounds of biannual MDA implemented over 5 years were not sufficient to interrupt Schistosoma haematobium transmission [12, 13]. This is in contrast to estimates from mathematical models suggesting MDA alone could result in transmission interruption if maintained over sufficient time at moderate to high coverage of school-aged children and other key community groups [14, 15].
Despite considerable progress and scaling-up of interventions over the past decades, a high burden of disease due to schistosomiasis persists in sub-Saharan Africa. Sustained transmission has been attributed to factors related to socio-economic development, weak health systems, a lack of country ownership and behavioral aspects in regards to dependency on open water sources and livelihoods [16–18]. In addition to these endogenous factors, limitations in field-applicable and affordable, highly sensitive and specific diagnostic tools, scalable biological vector control methods and a lack of novel drugs present ongoing bottlenecks and threaten global elimination efforts [11].
To improve the effectiveness of interventions that can contribute to realizing the goals set by WHO, it is necessary to review current intervention strategies, tools and guidance for treatment and control, and to identify innovations to overcome past and ongoing program failures, compliance and acceptance issues [19]. Consequently, we conducted a landscape analysis on interventions and strategies for parasitic and vector-borne disease elimination including a literature review and key informant interviews. The objective was to identify evidence-based components, concepts and intervention strategies that can help to advance schistosomiasis control towards elimination as a public health problem and interruption of Schistosoma spp. transmission across a variety of settings, with a focus on sub-Saharan Africa.
Methods
Literature review
The scientific literature databases PubMed and Web of Science were explored using the string search ((elimination OR eradication) AND vector-borne) OR ((elimination OR eradication) AND parasit*). No restrictions were applied with regard to study location, date of publication, article type, or language. Hits were imported into the online software “Covidence” for title and abstract screening. Two independent reviewers then stratified potentially relevant references into high- and low priority based on the title. Important criteria for the classification as high-priority articles were i) being a review or opinion piece on innovative intervention strategies; or ii) the description of comprehensive control or elimination programs. Overviews over research needs and program updates were regarded as lower priority articles. High-priority references underwent a detailed full text screening by two independent reviewers using a data extraction matrix in Microsoft Excel to capture relevant information for further assessment and final inclusion. Eligibility disagreements were resolved by discussion and reference to a third reviewer. Final inclusion of an article was based on the description of interventions and concepts relevant for vector-borne disease elimination. Findings were summarized in an excel table (see S1 Table) and stratified into 6 thematic groupings, namely i) treatment, ii) vector control, iii) water, sanitation and hygiene (WASH), iv) information, education and communication (IEC), v) surveillance and information systems, and vi) conceptual design of program implementation (see S2 Table). Complementary evidence was obtained from the grey literature underlining the findings from the peer-reviewed literature.
Key informant interviews (KII)
In-depth interviews were conducted with key informants with first-hand knowledge on parasitic and vector-borne diseases, disease elimination and public health programs, field researchers, program managers, policymakers and donors. The interviews were conducted in person, via skype or by telephone. Sampling was purposive and informants were first identified through personal and professional networks. In addition, a snowball sampling technique was used during interviews for experts to nominate additional relevant contacts for recruitment. Information on the background and life cycle of schistosomiasis was provided before the interview and in addition, a semi-structured questionnaire guided by a specific scenario (schistosomiasis elimination efforts in Zanzibar: a setting with a long history of control and elimination activities, with a now very low prevalence and predominantly light-intensity infections) was available upon request. A semi-structured interview guide was developed by the authors and sent to the key informants (see S1 Appendix). After verbal consent of the interviewees had been obtained, interviews were audio-recorded and transcribed for further analysis by two researchers. The interview transcripts were analyzed by summarizing them in a data extraction matrix modelled on the example used for the literature review (i.e., stratified into 6 thematic groupings, namely i) treatment, ii) vector control, iii) water, sanitation and hygiene (WASH), iv) information, education and communication (IEC), v) surveillance and information systems, and vi) conceptual design of program implementation, with a view to identify key topics and components (see S3 Table).
Results
Literature review and key informant interviews
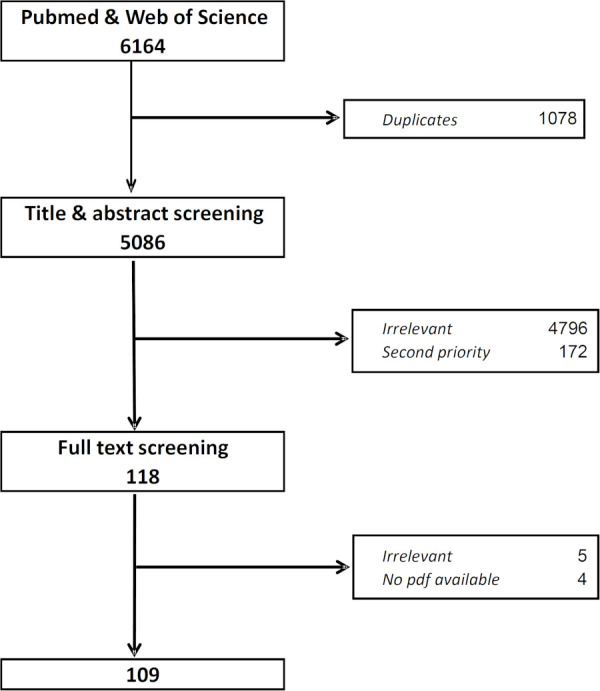
The final literature database search was conducted on 12 March 2019. As shown in Fig 1, applying the search algorithm in the two databases yielded a total of 5’086 hits. After exclusion of duplicates and irrelevant articles, 290 titles were retained and subsequently stratified into high- and low-priority references based on the above-mentioned criteria. A total of 118 articles were classified as high-priority and selected for full-text screening. Among them, four full texts were not obtainable from online archives and through the scientific library services. Among the remaining 114 articles, five were excluded because upon review of the full text it appeared they did not describe interventions of potential relevance for parasitic and vector-borne disease elimination. The final list included 109 articles that underwent content analysis.
Fig 1. Flow-chart of article selection for inclusion in the literature review on vector-borne disease elimination strategies.
Key informant interviews were conducted with 23 participants with a background in parasitic and vector-borne diseases and elimination. Included were representatives of international organizations, academic and implementation research experts, program managers, policymakers and donors from 6 continents including endemic regions in Africa, Asia, and the Americas, between 18.03.2019 and 20.06.2019.
The combined findings from the literature review and key informant interviews are presented below, stratified by the 6 thematic groupings (i.e., i) treatment, ii) vector control, iii) water, sanitation and hygiene (WASH), iv) information, education and communication (IEC), v) surveillance and information systems, and vi) conceptual design of program implementation).
Treatment
Periodic praziquantel-based MDA to school-aged children is the mainstay of most current schistosomiasis control programs worldwide. The key informants highlighted a series of strategies, which in their opinion could improve MDA treatment coverage and compliance with a view to cover all at-risk populations. Strategies ranged from large-scale risk assessments over focused mapping of risk groups to inform targeted and selective treatment efforts, to tailored and adaptive strategies which address residual high transmission foci amid generally low prevalence populations. Tailoring interventions and targeted treatment strategies entails a need for clear guidance on which populations and individuals at risk to screen, and when to implement focal or large-scale mass drug administration, or, potentially, test-and-treat approaches.
Targeted treatment approaches were suggested to avoid unnecessary treatment of healthy people (overtreatment) and to enhance acceptance, compliance and coverage in the population. In consideration of a potential risk of drug resistance and to improve clearance of parasites, it was referred to studies indicating that varying the frequency of treatment and a combination of praziquantel with artemether might offer alternatives to the current standard of periodic treatment with praziquantel for schistosomiasis. Artemether, a derivative of artemisinin and key component of artemisinin-based combination therapy (ACT) against malaria, has been shown to kill immature schistosome stages that are not susceptible to praziquantel. It was suggested to (re)-considered using artemether, but only in settings where malaria is not endemic to avoid the promotion of resistance development to antimalarials.
To avoid the formation of persisting “reservoirs” of infected individuals maintaining transmission, it was highlighted that treatment efforts should target the entire at-risk population at high coverage. To this end, key informants emphasized that availability and access to treatment beyond school-based programs need to be strengthened. Thus, for targeting schistosomiasis, praziquantel must be made available through routine health services and drug distribution be integrated into existing platforms such as child health days.
The views of key informants were confirmed by the findings from the literature review, where countries including Oman, Venezuela, Lao PDR, Cambodia and P.R. China reported the successful combination of targeted schistosomiasis screening and selective/targeted treatment with the aim to avoid the treatment of non-infected individuals and to increase compliance for treatment against Schistosoma infections in endemic areas [20–29]. The value for decision-making of tailored interventions to target asymptomatic carriers contributing to transmission through community-wide mass drug treatment and active case finding, in addition to mapping spatial and temporal transmission dynamics, has been established for malaria [30–34].
In terms of praziquantel alternatives, limited experience with combining artemether and praziquantel for targeting both the juvenile schistosomula and adult parasites has been published [35, 36].
Vector control
Many successful efforts to eliminate schistosomiasis have included snail control and environmental modification as key interventions [4]. Both the interviewed experts and the literature emphasized the importance of vector control when considering disease elimination targets. Vector control has been identified as a key factor for the successful elimination of schistosomiasis in Japan, and national programs in P.R. China and Puerto Rico relied in an important way on snail control supported by WASH and health education [29, 37–42]. Similarly, vector control has been instrumental for the control and elimination of other parasitic and vector-borne diseases such as malaria, dengue, onchocerciasis and Guinea worm [43–57].
In summary, as expressed by key informants and described in the literature, snail control is a valuable component of schistosomiasis control efforts, and mapping infested water sources to inform small-scale and focal mollusciciding (by hand or through GPS-guided drones) is considered essential where biological methods are not available [4, 11, 58]. Better training and community engagement were recommended by the key informants to reduce environmental and monetary costs and to increase sustainability and acceptance of vector control activities in the face of toxic molluscicides. The guide on field use of molluscicides for schistosomiasis control issued by WHO serves as an operational manual for programme managers on snail control and informs decision-making [59]. Engaging and training communities in vector control activities to foster ownership and sustainability have proven feasible in community-based integrated vector control programs for dengue, malaria and lymphatic filariasis (LF) [43, 46, 60–63]. Reported mainly in the Chinese literature, large-scale interventions such as changes in land use and farming practices, civil engineering and water resource development projects highlight the need for engagement with multiple sectors for investments, coordination and risk assessment to ensure sustainability and impact. Most recent published experience in the domains of environmental, agricultural and ecological modifications is concentrated in P.R. China and relates to S. japonicum in humans, animals and snails [38–42]. Mollusciciding has not been widely implemented in sub-Saharan Africa, and biological control approaches and trials are limited to specific settings. However, gene-drive technologies, cost-effectiveness analyses, modelling and innovations to assess snail abundance for targeted snail population management offer promising future pathways [4, 64, 65].
Water, sanitation and hygiene (WASH)
WASH infrastructure and behavior are key for the control of water-borne and water-related diseases including NTDs, and are critical for schistosomiasis control and elimination efforts [66]. The WHO, in 2019, published a “how-to”-guide that offers practical advice for NTD programs interested in establishing ties with the WASH sector [67].
As stressed by several interviewed key informants and documented in the literature, safe water and improved sanitation installations must be locally and culturally appropriate and tailored to environmental conditions and the socio-economic context [68]. Key informants emphasized that community involvement in the planning and implementation of interventions is crucial to ensure uptake, acceptance and sustainability of WASH interventions. Successful examples of comprehensive community-led MDA combined with community-led WASH (CL-WASH) interventions for schistosomiasis control in Cambodia and Lao PDR have been published [24, 38].
Information, education and communication (IEC)
Effective and well-executed IEC and behavior change (BC) interventions are imperative for disease control and elimination in terms of acceptance by the target population and sustainability of interventions, but have long been neglected due to difficult standardization and the scarcity of proven models [69–72]. Interviewed key informants highlighted that a focus should be placed on raising awareness for the disease and interventions in the target population and among relevant stakeholders to facilitate diagnosis, treatment and surveillance activities. Several key informants pointed out that early childhood education has proven effective in adapting and improving WASH behavior and is applied for trachoma and soil-transmitted helminth (STH) control and prevention through habit formation. Community participation was identified by key informants and in the literature as crucial to maximize acceptance, gain a sense of ownership and empowerment and achieve sustainability of interventions and activities including vector control, WASH, surveillance, treatment, health education and behavior change communication in programmes targeting malaria, dengue, schistosomiasis, Guinea worm and other parasitic and vector-borne diseases [11, 24, 43, 46, 53, 60–62, 72–76]. Caregivers, social groups, religious or community leaders, teachers and health staff should be engaged in control activities, intervention planning and design of tools [77].
Health education and health promotion have been documented to play an integral role in national schistosomiasis control program efforts in P.R. China [69, 71] and Brazil [72]. Comprehensive and well-executed activities around disease awareness, knowledge and risk mitigation improved intervention acceptance and compliance rates, and reduced water exposure for parasitic and vector-borne diseases [70, 71, 75, 78]. Toolkits, as developed during the Zanzibar Elimination of Schistosomiasis Transmission (ZEST) program and adapted to local context offer guidance and educational material for engaging with pupils and communities in activities related to prevention, control and treatment (https://www.eliminateschisto.org/resources/teacher-toolkits-for-schistosomiasis). Community participation in sensitization and awareness raising also increased acceptance of preventative measures (vector control) for malaria control in Rwanda [53].
Surveillance and information systems
The essential basis for planning interventions for parasitic and vector-borne disease elimination is accurate knowledge on the spatial distribution of infected individuals and transmission sites to identify implementation gaps and inform interventions needs. Interviewed key informants stressed that mapping (including spatio-temporal, precision, fine-scale and micro-mapping) of human and animal sources of infection with pathogenic organisms and associated factors including vectors, transmission sites and relevant human behavior are key for planning targeted interventions. They also indicated that Geographic information system (GIS), remote sensing (RS) and other GeoHealth technologies are well developed for schistosomiasis control and that network modeling and tracking human mobility can inform interventions on a larger scale and across regions.
High-quality data and rigorous surveillance response systems are critical for an effective control and elimination program as was mentioned with particular reference to Guinea worm eradication efforts. It was pointed out that this requires early and sustained investments in a comprehensive surveillance system including routine (periodic, integrated into the primary health system) and active surveillance (i.e. active case detection, tracing and investigations). Key informants stressed that surveillance activities should be performed at all levels (community, district, national, regional) and are an essential component in the “endgame” for monitoring residual human infections as well as for detecting emerging zoonotic reservoirs. Surveillance must be maintained throughout the different stages of elimination programs, as presented in the Transmission Assessment Survey (TAS) framework for LF [79, 80]. As recommended by key informants and in the literature, surveillance should involve community members and primary health care systems [48, 52]. Surveillance strategies call for integration across several diseases to maximize cost-effectiveness [81].
Concepts for schistosomiasis surveillance systems designed for elimination programs, including early warning systems [25, 58, 82–85], mapping of hot spots and modelling for targeted approaches [58, 83, 86] are covered in several reviewed articles. Challenges due to the lack of sensitive diagnostic tools for monitoring and validating stages of elimination in regards to low-prevalence settings and low intensity infections were discussed in articles pertaining to schistosomiasis control programs in Morocco [87], Venezuela [21, 23], Oman [20] and P.R. China [28]. High-quality surveillance and mapping requires sensitive tools for assessing the epidemiology and detecting re-emergence in humans, animals and vectors. Relevant diagnostic tools for schistosomiasis surveillance include molecular diagnostic such as quantitative PCR (qPCR) and loop-mediated isothermal amplification techniques (LAMP) and intermediate host surveillance methods or the detection of environmental DNA, some of which are currently not widely available [88–90].
Several identified articles discussed strategies for surveillance and response systems to achieve elimination of malaria. For example, P.R. China’s comprehensive 1-3-7-strategy for malaria elimination [48, 50] monitors and guides elimination activities including case reporting (e.g. cell-phone based alert system) within 1 day through the disease surveillance information report management system. As outlined in China’s national malaria elimination strategy, case investigation and laboratory confirmation occur ideally within 3 days while response actions including preventive chemotherapy to individuals or populations at risk, community health education, and vector control in at-risk houses are implemented within 7 days. Nearing elimination in many settings, LF control is also facing increased surveillance needs. In response, specific protocols have been developed that offer stepwise guidance on targeted responses for different stages of elimination and highlight the importance of adequate post-intervention surveillance tools to detect re-emergence of infections [79, 80].
Conceptual design of program implementation
The interviewed key informants stressed that interruption of schistosome transmission is only feasible with an integrated, multipronged, cross-sectoral and locally adapted strategy. The basis for such comprehensive interventions is collaboration including joint planning, central coordination of different sectors and effective community engagement. Regular progress reviews and strategy updates are essential. Barriers around the establishment of inter-sectoral collaborations are manifold despite common goals, and long-term political commitment to schistosomiasis elimination is paramount to ensure focus and adequate funding [91]. Key informants emphasized that the lack of technical and management capacity at national and local level must be addressed through capacity building and health systems strengthening through training of program managers, health and laboratory staff and community members.
Most of the identified articles that focus on strategies for implementation of interventions refer to the Chinese schistosomiasis control programme. They describe a combination of tools and comprehensive strategies [11, 20, 21, 23, 36, 90, 92–94] implemented through collaborations between multiple sectors but under central coordination [26, 29, 39, 95, 96]. This approach has been identified as important to reduce schistosomiasis japonica levels across P.R. China [22, 26, 27, 29, 39, 42, 95–97]. The value of integrated One Health approaches has been described in the context of Schistosoma mekongi elimination efforts in Lao PDR and Cambodia [24]. Other Asian countries and Puerto Rico reported similar experiences [37, 38, 90].
The need for multipronged elimination strategies has also been highlighted for other parasitic and vector-borne diseases including malaria [32, 44, 49, 98–100], dengue [43, 46, 56, 60], onchocerciasis [57] and Guinea worm [45, 52, 54]. Examples of integrated, inter-programmatic and cross-sectoral initiatives have been outlined in detail for prevention, control and elimination of NTDs in the Americas [63]. Community-based integrated strategies were also used for polio and measles elimination [101]. Integration across MDA programs and diseases in regards to concurrent administration of multiple drugs exist for schistosomiasis, soil-transmitted helminthiasis and LF [63, 102]. Beyond exploiting synergies from distributing drug combinations, malaria and LF control programs have paired MDA with vector control activities to ensure sustainability of transmission suppression [47].
Challenges, gaps and needs for achieving interruption of schistosome transmission
The following challenges, gaps and needs were identified from the literature or highlighted by the interviewed key informants.
Treatment
There is little evidence in the peer-reviewed literature on schistosomiasis treatment strategies targeting populations beyond school-aged children, despite recommendations by WHO to offer regular treatment to all at-risk populations. However, epidemiological considerations and modelling results suggest that a focus on MDA for schoolchildren risks ignoring a potentially substantial reservoir (and morbidity burden) [14, 36, 75, 92, 93, 103, 104]. Risk factors related to occupation, behavior, and proximity to, as well as dependency on, open water sources have long been identified as challenges to elimination efforts, further underscoring the need to treat also other at-risk populations [105, 106] at high coverage [92, 93, 103].
Mass drug administration may also lead to treatment fatigue, non-compliance and therefore low coverage [36, 107, 108]. Investment in research and development for new anti-schistosomal drugs is needed in view of concerns related to emerging drug resistance and availability of a pediatric formulation of praziquantel suitable for administration to infants [109, 110]. The factors above as well as the opinions of key informants, call for mapping of populations or individuals at risk to facilitate a targeted approach, following clear and comprehensive treatment guidelines, and supported by robust monitoring and evaluation (M&E) adapted to stages in elimination. Guidance on the design of effective test and treat strategies is currently lacking as are affordable, field-ready point-of-care tests, especially for S. haematobium, that are needed to facilitate test-and-treat options.
Vector control
Since the discovery of praziquantel in the 1980s snail control has been increasingly neglected as a key component for many schistosomiasis control and elimination strategies. The toxicity, costs and operational issues associated with chemical mollusciciding also present an ongoing challenge in the face of elusive alternatives. The main issues related to snail control include the contamination of water and food sources resulting in niclosamide being banned in certain locations such as in rice fields in the Philippines. The inaccessibility of certain snail breeding sites, e.g. in the Mekong river, also presents challenges [38]. In March 2020, the WHO has urged member states to include snail control as an integral and crucial component for reaching elimination of schistosomiasis [111]. Chemical mollusciciding, unlike former Dichlorodiphenyldichloroethane (DDT) spraying or long-lasting insecticidal bed nets and indoor residual spraying (LLIN/IRS) for malaria control, is neither sustainable and cost-effective in the long-term, nor feasible for application at large scale [29].
Several articles identified a need for innovative biological snail control and suggested microbial pathogens for the control of B. glabrata [112] and snail “diagnostics” surveillance for risk mapping with LAMP [88].
Information, education and communication (IEC)
Many countries’ efforts and success in eliminating schistosomiasis include strategies relying, among else, on comprehensive health education and communication activities. Well executed, locally appropriate and culturally sensitive IEC can improve community acceptance and participation in all dimensions of a control or elimination program considerably, and facilitates behavioral change in the population. Many current health educational interventions are ineffective and do not lead to behavior change due to a lack of local relevance, effective models, implementation guidance and skilled personnel [113]. IEC and behavior change activities require a time consuming process, and limited interest among control programs and donors so far resulted in little investment in innovative IEC and BC interventions [77].
Water, sanitation and hygiene (WASH)
WASH interventions essentially aim at preventing contamination of fresh water bodies with urine and stool while reducing human exposure to unsafe water sources. Successful WASH programs include sectors such as engineering, water authorities, non-governmental organizations (NGOs) and environmental health agencies for the installation of sanitary and hygiene infrastructure, making safe water accessible, and providing health education. Hardware-based solutions have shown limited impact on the local epidemiology of schistosomiasis unless they are accompanied by sustained changes in water contact behaviors [12, 68]. Monitoring of integrated WASH/NTD implementation and generating evidence on the impact of strategies need to be enforced in view of long term investment and sustainability [66].
Surveillance and information systems
The paramount importance of understanding local disease and transmission patterns, context and structures using a systems epidemiology approach is described by Krauth et al. [114]. Mapping and surveillance are essential activities for planning and managing schistosomiasis at different stages of elimination efforts [76]. Interviewed key informants highlighted that ecological and human surveillance systems needed to be established to detect transmission-related factors (e.g. zoonotic hybrid schistosomes, animal and human reservoirs, transmission foci) and inform responses. A lack of point-of-care, low-cost and sensitive diagnostic tools contributes to the challenge of defining and understanding the epidemiology of low-intensity and asymptomatic infections and the relevance of animal reservoirs. In addition, cases with low- or asymptomatic clinical presentation challenge traditional case detection approaches and suggest a need for better guidance including test-and-treat algorithms. Last, adequate resources and capacity at local and national levels of endemic countries are required to appropriately interpret data and design timely response activities.
Conceptual design of program implementation
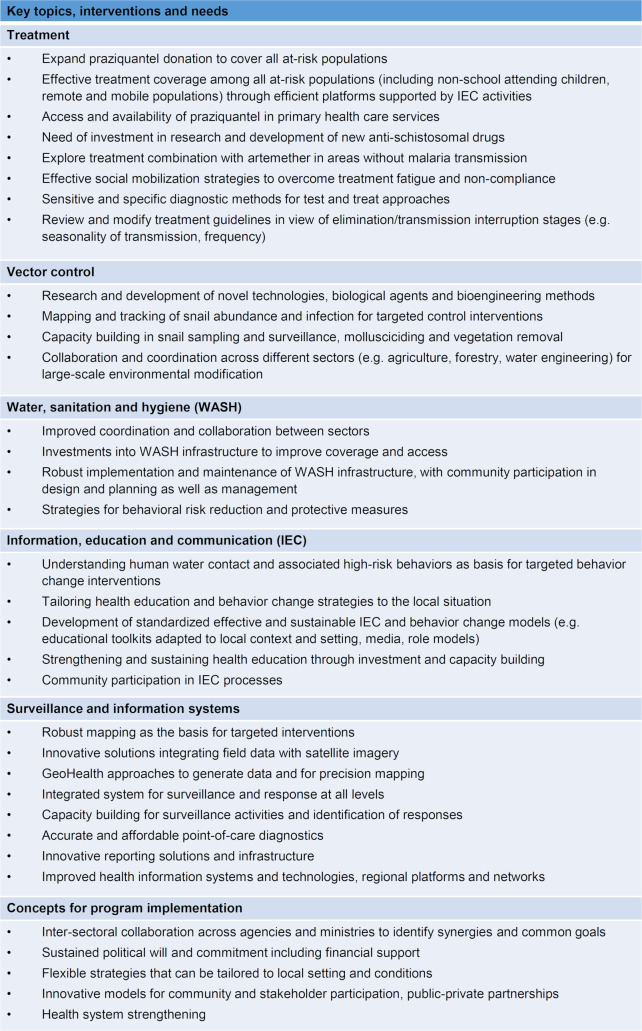
The rationale for integrated schistosomiasis control and elimination efforts to advance towards interruption of transmission as opposed to the current approach, mainly relying on MDA for elimination of schistosomiasis as a public health problem, has been discussed repeatedly for non-African settings [11, 21, 24, 36, 38, 55, 63, 90, 115, 116], but also with reference to Nigeria [75], Zanzibar [117] and Egypt [105]. Low acceptance of MDA, vector control or WASH interventions [103, 116] as well as insufficient resources, weak health systems and lack of coordination are a reality in many programs [11, 21, 38, 55, 63, 66, 95, 97, 115, 116]. Impact modeling studies have shown that strategies combining different interventions are more likely to result in successful disease control and elimination [64, 65]. More generally, a human-centered design approach is key for acceptance of interventions, community ownership and successful and sustainable implementation of programs [77]. Fig 2 presents priorities, needs and considerations in addition to identified key topics and interventions from key informants and the reviewed literature.
Fig 2. Key topics, interventions and needs for advancing towards breaking schistosomiasis transmission.
Discussion
Solid evidence demonstrates that sustainable control and particularly interruption of transmission and elimination of human schistosomiasis is only possible through multipronged long-term strategies that are adapted to local conditions, sustained by political commitment and funded adequately [22, 27, 63, 74, 92]. Intervention strategies need to be based on (predictive) risk mapping and combine different interventions that are tailored to the local socio-economic and epidemiological context through close consultation with the local population. Costs and other economic factors associated with implementation of interventions represent a critical aspect needed for intervention prioritization and policy change. Targeted cost-effectiveness analyses are valuable tools to guide country programmes and inform strategies and intervention design [118–120]. Mapping must go beyond epidemiological parameters such as disease prevalence and infection intensity in humans. Instead, it must also cover abundance of snails and snail infection rates, transmission sites, access to safe water sources, and human behaviors to guide interventions, as proposed in a systems modelling approach to evaluate and predict effectiveness of interventions [114]. The current focus on school-aged children of the treatment programs is of concern in regards to reaching the set elimination targets. This calls for in-depth analysis on implementation gaps, barriers and needs to establish or improve access to treatment for all at-risk populations. Intervention packages need to cover treatment of infected individuals, snail control in infested water bodies, WASH and human behavior as well as strengthening relevant knowledge, and are ideally implemented by communities and the local health system under the strong leadership of an elimination program also coordinating activities of other sectors. As emphasized by many interviewed key informants and reflected in the literature, understanding perspectives and involvement of the affected communities [53, 77, 101, 121] in the planning of targeted interventions through a bottom-up approach is critical for acceptance and ownership and thus sustainability of most interventions. Engaging communities in vector control activities, treatment campaigns (e.g. through community-directed treatment strategies), behavior change activities, and WASH (such as CL-WASH) has been shown to be feasible, (cost)-effective [121] and sustainable when prioritizing locally appropriate interventions [53, 61, 77, 101, 122–125]. A strong surveillance and response framework ensures quality control and appropriate adaptation of the interventions to changing epidemiological and risk profiles.
Biological, epidemiological, public health and health system considerations suggest a need for a pluralistic governance framework for comprehensive programs targeting human schistosomiasis elimination. Strong global and national partnerships are essential in advocacy for sustained financial and political commitment. This entails the transition from donor-driven programmes to country ownership for sustainability [126]. Based on the successful implementation of such programs in P.R. China and Japan, the following basic tenets have been formulated [74]. Their relevance in terms of success and sustainability has been confirmed by the findings of our literature review and key informant interviews:
Local policy development, strong political commitment
Use of multiple interventions in integrated fashion through cross-sectoral action
Adaptation of interventions for specific eco-epidemiological settings and over time
Active participation of the community and other stakeholders (both private and public) underpinned by country ownership
Strong linkages to research and learning activities using surveillance and monitoring data
The aim of the landscape analysis was to identify components, concepts and intervention strategies used in past and ongoing parasitic and vector-borne parasitic disease elimination programs in diverse settings that might be applied for advancing interruption of schistosome transmission. Our study did not result in an exhaustive list of potential innovative tools and stand-alone intervention approaches but rather identified crucial components and concepts of strategies to drive progress towards breaking schistosome transmission. The opinions of key informants and evidence from the peer-reviewed literature presented here are largely reflected in the final draft of the new WHO roadmap for NTD control 2021–2030 [8]. All sources call for adequate efforts to address critical gaps and concerted action across sectors to maximize synergies for effective implementation and sustained impact. The proposed strategies and recommendations should be assessed in regards to country perspectives and experiences in the frame of a consultation to inform future implementation research needs and elimination program design. Recognizing the heterogeneity of schistosome transmission settings in terms of ecological and social context, health infrastructure, human capacity and material as well as financial resources, a focused literature review, qualitative research for exploring community perspectives and detailed evaluation of each individual key intervention approach are required. Such intervention-focused reviews can help to identify the range of available options, tools and intervention modalities and their individual strengths and weaknesses before selecting a particular combination of approaches to be included into a strategy for any given setting. Crucially, and as emphasized in many key informant interviews conducted in the frame of this landscape analysis: consistent and high-quality implementation of available tools and interventions, regularly reviewed and pragmatically adapted, is critical to achieve a sustained impact, and can potentially succeed in interruption of schistosome transmission [127].
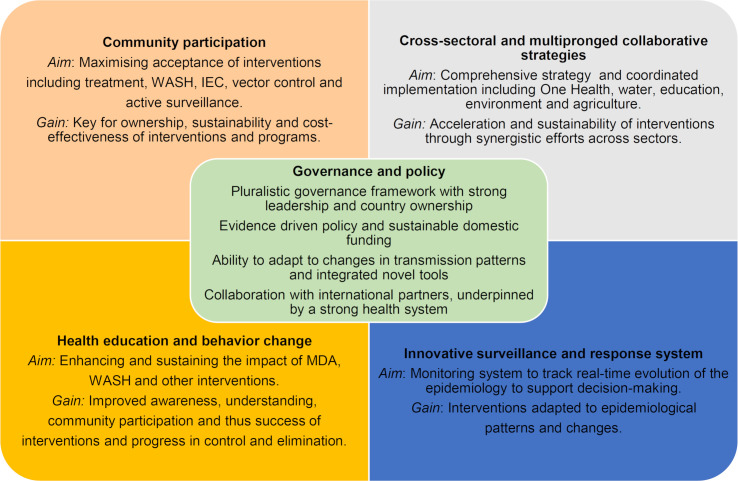
To summarize key messages from our landscape review, five key components, complementing treatment and underpinning integrated comprehensive interventions for parasitic disease elimination, highlight the core dimensions for planning a comprehensive intervention and for defining the concrete approaches that make up an elimination strategy (Fig 3).
Fig 3. Key components of parasitic disease elimination strategies.
Recommendations and conclusions
Our landscape analysis reviewed concepts and intervention strategies of past and current parasitic and vector-borne disease elimination programs and identified five key components needed for achieving and sustaining schistosomiasis control and for progressing elimination efforts towards interruption of transmission in sub-Saharan Africa. Based on the evidence from a literature review and information obtained through a series of key informant interviews, we propose three strategies (Fig 4) of escalating complexity and ambition towards interruption of schistosome transmission and elimination, namely i) “Improved treatment strategy for morbidity control”, ii) “Advanced intervention strategy for nearing elimination” and iii) “Ultimate intervention strategy for gaining and sustaining elimination”. For each of the three strategies, we define the aim and list a set of tools and core interventions to address and accommodate the complexity of endemic settings and schistosome transmission dynamics that must be overcome in order to break transmission and advance towards true elimination. Importantly, the decision on the strategy to pursue and the final selection of tools and interventions must be based on a thorough assessment of the local context and take into account resources and timelines.
Fig 4. Strategies for strengthening efforts of escalating complexity and ambition towards interruption of schistosome transmission and elimination.
The “Improved treatment strategy for morbidity control” focuses on MDA and IEC. Through locally appropriate IEC, awareness, knowledge and acceptance is increased in the target population, which translates into high MDA coverage and compliance. The strategy aims for a rapid reduction of infection intensity and thus morbidity due to schistosomiasis in the at-risk population. It also contributes to the reduction of schistosome transmission. Relatively low costs are incurred and implementation is within the capacity of well-established schistosomiasis control programs. This strategy is in line with the WHO recommended strategy for morbidity control [7].
The “Advanced intervention strategy for nearing elimination” includes a comprehensive multi-sectoral approach, where MDA and IEC including behavior change activities are combined with WASH and vector control activities at large-scale and applied with high intensity. The strategy aims to achieve low morbidity and to advance the impact of programs towards elimination of schistosomiasis as a public health problem. Costs are considerably higher than in the first scenario but might be shared by a wider range of sectors and stakeholders. Relying on this strategy, an optimal medium-term cost-benefit ratio might be achieved in all but the most complicated endemic areas. This strategy is in line with the WHO strategy for elimination and WHA resolution 65.21 [7, 128].
The “Ultimate intervention strategy for gaining and sustaining elimination” builds on the set of interventions highlighted under scenario 2. However, MDA, IEC and behavior change, WASH, and snail control are applied focally to areas where the prevalence is above a to-be defined threshold that sustains local transmission. The focal application is guided by the results of extensive precision mapping efforts. In areas where infection levels are below the threshold, extensive surveillance and response efforts will help to avoid the re-introduction and resurgence of transmission and morbidity. Sensitive and specific point-of-care diagnostic tools will be essential for informing interventions and monitoring impact. The aim of the strategy is to gain and sustain interruption of schistosome transmission in all foci and populations, including persistent hotspots and migrant populations. While post-elimination surveillance is suggested by WHO, specific guidance and thresholds on when and where to adapt intervention strategies in near-to-elimination settings is yet to be developed.
Supporting information
(XLSX)
(DOCX)
Summary of key topics from key informant interviews.
(DOCX)
(DOCX)
Data Availability
In order to protect participant confidentiality the interview transcripts are not provided, but the thematic analysis is available as supporting information. All other relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The study was funded by the Bill & Melinda Gates Foundation (Investment ID: OPP1198086; https://www.gatesfoundation.org/). SK was additionally supported by a PRIMA grant from the Swiss National Science Foundation (PR00P3_179753; www.snf.ch/en/Pages/default.aspx). The funders played no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hotez PJ, Alvarado M, Basanez MG, Bolliger I, Bourne R, Boussinesq M, et al. The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl Trop Dis. 2014;8(7):e2865 10.1371/journal.pntd.0002865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383(9936):2253–64. 10.1016/S0140-6736(13)61949-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6(7):411–25. 10.1016/S1473-3099(06)70521-7 [DOI] [PubMed] [Google Scholar]
- 4.Sokolow SH, Wood CL, Jones IJ, Lafferty KD, Kuris AM, Hsieh MH, et al. To reduce the global burden of human schistosomiasis, use 'old fashioned' snail control. Trends Parasitol. 2018;34(1):23–40. 10.1016/j.pt.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doenhoff MJ, Cioli D, Utzinger J. Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr Opin Infect Dis. 2008;21(6):659–67. 10.1097/QCO.0b013e328318978f [DOI] [PubMed] [Google Scholar]
- 6.WHO. Preventive Chemotherapy in Human Helminthiasis. Coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. Geneva, Switzerland; 2006.
- 7.WHO. Schistosomiasis: progress report 2001–2011, strategic plan 2012–2020. Geneva: World Health Organization; 2013. [Google Scholar]
- 8.WHO. Ending the neglect to attain the sustainable development goals: A road map for neglected tropical diseases 2021–2030 2020 February 2020.
- 9.Tanaka H, Tsuji M. From discovery to eradication of schistosomiasis in Japan: 1847–1996. Int J Parasitol. 1997;27(12):1465–80. 10.1016/s0020-7519(97)00183-5 [DOI] [PubMed] [Google Scholar]
- 10.Song LG, Wu XY, Sacko M, Wu ZD. History of schistosomiasis epidemiology, current status, and challenges in China: on the road to schistosomiasis elimination. Parasitol Res. 2016;115(11):4071–81. 10.1007/s00436-016-5253-5 [DOI] [PubMed] [Google Scholar]
- 11.Rollinson D, Knopp S, Levitz S, Stothard JR, Tchuem Tchuente LA, Garba A, et al. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2013;128(2):423–40. 10.1016/j.actatropica.2012.04.013 [DOI] [PubMed] [Google Scholar]
- 12.Knopp S, Ame SM, Person B, Hattendorf J, Rabone M, Juma S, et al. A 5-Year intervention study on elimination of urogenital schistosomiasis in Zanzibar: Parasitological results of annual cross-sectional surveys. PLoS Negl Trop Dis. 2019;13(5):e0007268 10.1371/journal.pntd.0007268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knopp S, Person B, Ame SM, Ali SM, Hattendorf J, Juma S, et al. Evaluation of integrated interventions layered on mass drug administration for urogenital schistosomiasis elimination: a cluster-randomised trial. Lancet Glob Health. 2019;7(8):e1118–e29. 10.1016/S2214-109X(19)30189-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toor J, Alsallaq R, Truscott JE, Turner HC, Werkman M, Gurarie D, et al. Are we on our way to achieving the 2020 goals for schistosomiasis morbidity control using current World Health Organization guidelines? Clin Infect Dis. 2018;66(suppl_4):S245–S52. 10.1093/cid/ciy001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner HC, Truscott JE, Bettis AA, Farrell SH, Deol AK, Whitton JM, et al. Evaluating the variation in the projected benefit of community-wide mass treatment for schistosomiasis: Implications for future economic evaluations. Parasit Vectors. 2017;10(1):213 10.1186/s13071-017-2141-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray DJ, McManus DP, Li Y, Williams GM, Bergquist R, Ross AG. Schistosomiasis elimination: lessons from the past guide the future. Lancet Infect Dis. 2010;10(10):733–6. 10.1016/S1473-3099(10)70099-2 [DOI] [PubMed] [Google Scholar]
- 17.Adenowo AF, Oyinloye BE, Ogunyinka BI, Kappo AP. Impact of human schistosomiasis in sub-Saharan Africa. Braz J Infect Dis. 2015;19(2):196–205. 10.1016/j.bjid.2014.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotez PJ, Kamath A. Neglected tropical diseases in sub-saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis. 2009;3(8):e412 10.1371/journal.pntd.0000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weerakoon KG, Gobert GN, Cai P, McManus DP. Advances in the diagnosis of human schistosomiasis. Clin Microbiol Rev. 2015;28(4):939–67. 10.1128/CMR.00137-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al Abaidani I, Al-Abri S, Shaban M, Ghugey SL, Al Kathery S, Al-Mashikhi K, et al. Decline in transmission of schistosomiasis mansoni in Oman. Infect Dis Poverty. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alarcon de Noya B, Balzan C, Arteaga C, Cesari I, Noya O. The last fifteen years of schistosomiasis in Venezuela: features and evolution. Mem Inst Oswaldo Cruz. 1999;94(2):139–46. 10.1590/s0074-02761999000200002 [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Xu J, Bergquist R, Li SZ, Zhou XN. "Farewell to the God of Plague": The importance of political commitment towards the elimination of schistosomiasis. Trop Med Infect Dis. 2018;3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Noya BA, Noya O, Balzan C, Cesari IM. New approaches for the control and eradication of schistosomiasis in Venezuela. Mem Inst Oswaldo Cruz. 1992;87 Suppl 4:227–31. [DOI] [PubMed] [Google Scholar]
- 24.Khieu V, Sayasone S, Muth S, Kirinoki M, Laymanivong S, Ohmae H, et al. Elimination of schistosomiasis mekongi from endemic areas in Cambodia and the Lao People's Democratic Republic: Current status and plans. Trop Med Infect Dis. 2019;4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang YS, Huang YX, Hong QB, Yang K, Sun LP, Dai JR, et al. [Novel strategies and technologies to achieve the transmission control of schistosomiasis in Jiangsu Province]. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2012;24(2):119–22. [PubMed] [Google Scholar]
- 26.Sun LP, Wang W, Zuo YP, Hong QB, Du GL, Ma YC, et al. A multidisciplinary, integrated approach for the elimination of schistosomiasis: a longitudinal study in a historically hyper-endemic region in the lower reaches of the Yangtze River, China from 2005 to 2014. Infect Dis Poverty. 2017;6(1):56 10.1186/s40249-017-0270-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian C, Zhang Y, Zhang X, Yuan C, Gao Z, Yuan H, et al. Effectiveness of the new integrated strategy to control the transmission of Schistosoma japonicum in China: a systematic review and meta-analysis. Parasite. 2018;25:54 10.1051/parasite/2018058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spear RC, Seto EY, Carlton EJ, Liang S, Remais JV, Zhong B, et al. The challenge of effective surveillance in moving from low transmission to elimination of schistosomiasis in China. Int J Parasitol. 2011;41(12):1243–7. 10.1016/j.ijpara.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Wang W, Wang P. Long-term effectiveness of the integrated schistosomiasis control strategy with emphasis on infectious source control in China: a 10-year evaluation from 2005 to 2014. Parasitol Res. 2017;116(2):521–8. 10.1007/s00436-016-5315-8 [DOI] [PubMed] [Google Scholar]
- 30.Maude RJ, Socheat D, Nguon C, Saroth P, Dara P, Li G, et al. Optimising strategies for Plasmodium falciparum malaria elimination in Cambodia: primaquine, mass drug administration and artemisinin resistance. PLoS One. 2012;7(5):e37166 10.1371/journal.pone.0037166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosha JF, Sturrock HJW, Greenhouse B, Greenwood B, Sutherland CJ, Gadalla N, et al. Epidemiology of subpatent Plasmodium falciparum infection: implications for detection of hotspots with imperfect diagnostics. Malar J. 2013;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.malERA: An updated research agenda for characterising the reservoir and measuring transmission in malaria elimination and eradication. PLoS Med. 2017;14(11):e1002452 10.1371/journal.pmed.1002452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sturrock HJW, Hsiang MS, Cohen JM, Smith DL, Greenhouse B, Bousema T, et al. Targeting asymptomatic malaria infections: Active surveillance in control and elimination. PLoS Med. 2013;10(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang D, Chaki P, Mlacha Y, Gavana T, Michael MG, Khatibu R, et al. Application of community-based and integrated strategy to reduce malaria disease burden in southern Tanzania: the study protocol of China-UK-Tanzania pilot project on malaria control. Infect Dis Poverty. 2019;8(1):4 10.1186/s40249-018-0507-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergquist R, Elmorshedy H. Artemether and praziquantel: Origin, mode of action, impact, and suggested application for effective control of human schistosomiasis. Trop Med Infect Dis. 2018;3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ross AG, Chau TN, Inobaya MT, Olveda RM, Li Y, Harn DA. A new global strategy for the elimination of schistosomiasis. Int J Infect Dis. 2017;54:130–7. 10.1016/j.ijid.2016.09.023 [DOI] [PubMed] [Google Scholar]
- 37.Negron-Aponte H, Jobin WR. Schistosomiasis control in Puerto Rico: twenty-five years of operational experience. Am J Trop Med Hyg. 1979;28(3):515–25. 10.4269/ajtmh.1979.28.515 [DOI] [PubMed] [Google Scholar]
- 38.Gordon CA, Kurscheid J, Williams GM, Clements ACA, Li Y, Zhou XN, et al. Asian schistosomiasis: Current status and prospects for control leading to elimination. Trop Med Infect Dis. 2019;4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun LP, Wang W, Zuo YP, Zhang ZQ, Hong QB, Yang GJ, et al. An integrated environmental improvement of marshlands: impact on control and elimination of schistosomiasis in marshland regions along the Yangtze River, China. Infect Dis Poverty. 2017;6(1):72 10.1186/s40249-017-0287-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang X, Zhang Y, Sun QX, Zhou JX, Zhou XN. SWOT analysis on snail control measures applied in the national schistosomiasis control programme in the People's Republic of China. Infect Dis Poverty. 2019;8(1):13 10.1186/s40249-019-0521-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Y, Zhou YB, Song XX, Li SZ, Zhong B, Wang TP, et al. Integrated control strategy of schistosomiasis in the People's Republic of China: Projects involving agriculture, water conservancy, forestry, sanitation and environmental modification. Adv Parasitol. 2016;92:237–68. 10.1016/bs.apar.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 42.Spear R, Zhong B, Liang S. Low transmission to elimination: Rural development as a key determinant of the end-game dynamics of Schistosoma japonicum in China. Trop Med Infect Dis. 2017;2(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abbas A, Abbas RZ, Khan JA, Iqbal Z, Bhatti MMH, Sindhu ZUD, et al. Integrated strategies for the control and prevention of Dengue vectors with particular reference to Aedes aegypti. Pakistan Veterinary Journal. 2014;34(1):1–10. [Google Scholar]
- 44.Agrawal VK. Plasmodium falciparum containment strategy. Med J Armed Forces India. 2008;64(1):57–60. 10.1016/S0377-1237(08)80150-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beyene HB, Bekele A, Shifara A, Ebstie YA, Desalegn Z, Kebede Z, et al. Elimination of Guinea worm disease in Ethiopia; Current status of the disease's eradication strategies and challenges to the end game. Ethiop Med J. 2017;55(Suppl 1):15–31. [PMC free article] [PubMed] [Google Scholar]
- 46.Bos R, Fevrier M, Knudsen AB. Saint Lucia revisited. Parasitol Today. 1988;4(10):295–8. 10.1016/0169-4758(88)90029-4 [DOI] [PubMed] [Google Scholar]
- 47.Burkot TR, Durrheim DN, Melrose WD, Speare R, Ichimori K. The argument for integrating vector control with multiple drug administration campaigns to ensure elimination of lymphatic filariasis. Filaria J. 2006;5:10 10.1186/1475-2883-5-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao J, Sturrock HJ, Cotter C, Zhou S, Zhou H, Liu Y, et al. Communicating and monitoring surveillance and response activities for malaria elimination: China's "1-3-7" strategy. PLoS Med. 2014;11(5):e1001642 10.1371/journal.pmed.1001642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Enayati A, Hemingway J. Malaria management: past, present, and future. Annu Rev Entomol. 2010;55:569–91. 10.1146/annurev-ento-112408-085423 [DOI] [PubMed] [Google Scholar]
- 50.Feng XY, Xia ZG, Vong S, Yang WZ, Zhou SS. Surveillance and response to drive the national malaria elimination program. Adv Parasitol. 2014;86:81–108. 10.1016/B978-0-12-800869-0.00004-4 [DOI] [PubMed] [Google Scholar]
- 51.Hopkins DR. Disease eradication. N Engl J Med. 2013;368(1):54–63. 10.1056/NEJMra1200391 [DOI] [PubMed] [Google Scholar]
- 52.Hopkins DR, Azam M, Ruiz-Tiben E, Kappus KD. Eradication of dracunculiasis from Pakistan. Lancet. 1995;346(8975):621–4. 10.1016/s0140-6736(95)91442-0 [DOI] [PubMed] [Google Scholar]
- 53.Ingabire CM, Hakizimana E, Kateera F, Rulisa A, Van Den Borne B, Nieuwold I, et al. Using an intervention mapping approach for planning, implementing and assessing a community-led project towards malaria elimination in the Eastern Province of Rwanda. Malar J. 2016;15(1):594 10.1186/s12936-016-1645-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Molyneux DH. Vector-borne parasitic diseases—an overview of recent changes. Int J Parasitol. 1998;28(6):927–34. 10.1016/s0020-7519(98)00067-8 [DOI] [PubMed] [Google Scholar]
- 55.Molyneux DH. Control of human parasitic diseases: Context and overview. Adv Parasitol. 2006;61:1–45. 10.1016/S0065-308X(05)61001-9 [DOI] [PubMed] [Google Scholar]
- 56.Naranjo DP, Qualls WA, Jurado H, Perez JC, Xue RD, Gomez E, et al. Vector control programs in Saint Johns County, Florida and Guayas, Ecuador: successes and barriers to integrated vector management. BMC Public Health. 2014;14 10.1186/1471-2458-14-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodriguez-Perez MA, Lara-Ramirez EE, Real-Najarro O, Unnasch TR, FrancoParedes C, SantosPreciado JI. A roadmap followed: The path towards the elimination of onchocerciasis in Latin America. 2015:155–73. [Google Scholar]
- 58.Malone J, Bergquist R, Rinaldi L, Zhou XN, Halounova L, Safar V, et al. Schistosomiasis: Geospatial surveillance and response systems in Southeast Asia. 2016;41(B8):1409–11. [Google Scholar]
- 59.WHO. Field use of molluscicides in schistosomiasis control programmes: an operational manual for programme managers. Geneva, Switzerland: World Health Organization, diseases Docont; 2017. [Google Scholar]
- 60.Barbazan P, Tuntaprasart W, Souris M, Demoraes F, Nitatpattana N, Boonyuan W, et al. Assessment of a new strategy, based on Aedes aegypti (L.) pupal productivity, for the surveillance and control of dengue transmission in Thailand. Ann Trop Med Parasitol. 2008;102(2):161–71. 10.1179/136485908X252296 [DOI] [PubMed] [Google Scholar]
- 61.Das PK. Community participation in vector borne disease control: facts and fancies. Ann Soc Belg Med Trop. 1991;71 Suppl 1:233–42. [PubMed] [Google Scholar]
- 62.Pi-Bansa S, Osei JHN, Joannides J, Woode ME, Agyemang D, Elhassan E, et al. Implementing a community vector collection strategy using xenomonitoring for the endgame of lymphatic filariasis elimination. Parasit Vectors. 2018;11(1):672 10.1186/s13071-018-3260-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holveck JC, Ehrenberg JP, Ault SK, Rojas R, Vasquez J, Cerqueira MT, et al. Prevention, control, and elimination of neglected diseases in the Americas: Pathways to integrated, inter-programmatic, inter-sectoral action for health and development. BMC Public Health. 2007;7 10.1186/1471-2458-7-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lo NC, Gurarie D, Yoon N, Coulibaly JT, Bendavid E, Andrews JR, et al. Impact and cost-effectiveness of snail control to achieve disease control targets for schistosomiasis. Proc Natl Acad Sci U S A. 2018;115(4):E584–e91. 10.1073/pnas.1708729114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang H, Harvim P, Georgescu P. Preventing the spread of schistosomiasis in Ghana: Possible outcomes of integrated optimal control strategies. J Bio Sys. 2017;25(4):625–55. [Google Scholar]
- 66.Campbell SJ, Biritwum NK, Woods G, Velleman Y, Fleming F, Stothard JR. Tailoring water, sanitation, and hygiene (WASH) targets for soil-transmitted helminthiasis and schistosomiasis control. Trends Parasitol. 2018;34(1):53–63. 10.1016/j.pt.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 67.WASH and Health working together: a ‘how-to’ guide for neglected tropical disease programmes. Geneva: World Health Organization; 2019. [Google Scholar]
- 68.Grimes JE, Croll D, Harrison WE, Utzinger J, Freeman MC, Templeton MR. The roles of water, sanitation and hygiene in reducing schistosomiasis: a review. Parasit Vectors. 2015;8:156 10.1186/s13071-015-0766-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen L, Zhong B, Xu J, Li RZ, Cao CL. Health education as an important component in the National Schistosomiasis Control Programme in the People's Republic of China. Adv Parasitol. 2016;92:307–39. 10.1016/bs.apar.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 70.Gillette HP. Health education of the public: its role in the eradication of vector-borne disease. Bull World Health Organ. 1963;29 Suppl:183–7. [PMC free article] [PubMed] [Google Scholar]
- 71.Guang-Han H, Jing X, Chun-Li C, Jia-Ning J, Shan L, Shi-Zhu L, et al. Challenges and strategies of health education and health promotion in stage of schistosomiasis elimination. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2018;30(2):117–20. 10.16250/j.32.1374.2018075 [DOI] [PubMed] [Google Scholar]
- 72.Schall VT. Health education, public information, and communication in schistosomiasis control in Brazil: a brief retrospective and perspectives. Mem Inst Oswaldo Cruz. 1995;90(2):229–34. 10.1590/s0074-02761995000200018 [DOI] [PubMed] [Google Scholar]
- 73.Adhikari B, Phommasone K, Pongvongsa T, Soundala X, Koummarasy P, Henriques G, et al. Perceptions of asymptomatic malaria infection and their implications for malaria control and elimination in Laos. PLoS One. 2018;13(12):e0208912 10.1371/journal.pone.0208912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Michael E, Madon S. Socio-ecological dynamics and challenges to the governance of neglected tropical disease control. Infect Dis Poverty. 2017;6(1):35 10.1186/s40249-016-0235-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Noriode RM, Idowu ET, Otubanjo OA, Mafe MA. Urinary schistosomiasis in school aged children of two rural endemic communities in Edo State, Nigeria. J Infect Public Health. 2018;11(3):384–8. 10.1016/j.jiph.2017.09.012 [DOI] [PubMed] [Google Scholar]
- 76.Tambo E, Ai L, Zhou X, Chen JH, Hu W, Bergquist R, et al. Surveillance-response systems: the key to elimination of tropical diseases. Infect Dis Poverty. 2014;3:17 10.1186/2049-9957-3-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Person B, Knopp S, Ali SM, A'Kadir F M, Khamis AN, Ali JN, et al. Community co-designed schistosomiasis control interventions for school-aged children in Zanzibar. J Biosoc Sci. 2016;48 Suppl 1:S56–73. [DOI] [PubMed] [Google Scholar]
- 78.Msyamboza K, Ngwira B, Banda R, Mkwanda S, Brabin B. Sentinel surveillance of lymphatic filariasis, schistosomiasis soil transmitted helminths and malaria in rural southern Malawi. Malawi Med J. 2010;22(1):12–4. 10.4314/mmj.v22i1.55901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Budge PJ, Dorkenoo AM, Sodahlon YK, Fasuyi OB, Mathieu E. Ongoing surveillance for lymphatic filariasis in Togo: assessment of alternatives and nationwide reassessment of transmission status. Am J Trop Med Hyg. 2014;90(1):89–95. 10.4269/ajtmh.13-0407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kelly-Hope LA, Stanton MC, Zoure HGM, Kinvi BE, Mikhailov A, Tekle A, et al. A practical approach for scaling up the alternative strategy for the elimination of lymphatic filariasis in Loa loa endemic countries—developing an action plan. Glob Health Res Policy. 2017;2:12 10.1186/s41256-017-0032-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lammie PJ, Moss DM, Brook Goodhew E, Hamlin K, Krolewiecki A, West SK, et al. Development of a new platform for neglected tropical disease surveillance. Int J Parasitol. 2012;42(9):797–800. 10.1016/j.ijpara.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 82.Liang S, Yang C, Zhong B, Guo J, Li H, Carlton EJ, et al. Surveillance systems for neglected tropical diseases: global lessons from China's evolving schistosomiasis reporting systems, 1949–2014. Emerg Themes Epidemiol. 2014;11:19 10.1186/1742-7622-11-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Malone JB, Bergquist R, Martins M, Luvall JC. Use of geospatial surveillance and response systems for vector-borne diseases in the elimination phase. Trop Med Infect Dis. 2019;4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang LJ, Li SZ, Wen LY, Lin DD, Abe EM, Zhu R, et al. The establishment and function of schistosomiasis surveillance system towards elimination in the People's Republic of China. Adv Parasitol. 2016;92:117–41. 10.1016/bs.apar.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 85.Yang K, Xu JF, Zhang JF, Li W, He J, Liang S, et al. Establishing and applying a schistosomiasis early warning index (SEWI) in the lower Yangtze River Region of Jiangsu Province, China. PLoS One. 2014;9(4):e94012 10.1371/journal.pone.0094012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Magalhaes RJS, Salamat MS, Leonardo L, Gray DJ, Carabin H, Halton K, et al. Geographical distribution of human Schistosoma japonicum infection in The Philippines: tools to support disease control and further elimination. Int J Parasitol. 2014;44(13):977–84. 10.1016/j.ijpara.2014.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Balahbib A, Amarir F, Corstjens PL, de Dood CJ, van Dam GJ, Hajli A, et al. Selecting accurate post-elimination monitoring tools to prevent reemergence of urogenital schistosomiasis in Morocco: a pilot study. Infect Dis Poverty. 2017;6(1):75 10.1186/s40249-017-0289-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tong QB, Chen R, Zhang Y, Yang GJ, Kumagai T, Furushima-Shimogawara R, et al. A new surveillance and response tool: risk map of infected Oncomelania hupensis detected by Loop-mediated isothermal amplification (LAMP) from pooled samples. Acta Trop. 2015;141(Pt B):170–7. 10.1016/j.actatropica.2014.01.006 [DOI] [PubMed] [Google Scholar]
- 89.Carlton EJ, Bates MN, Zhong B, Seto EYW, Spear RC. Evaluation of mammalian and intermediate host surveillance methods for detecting schistosomiasis reemergence in Southwest China. PLoS Negl Trop Dis. 2011;5(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sato MO, Adsakwattana P, Fontanilla IKC, Kobayashi J, Sato M, Pongvongsa T, et al. Odds, challenges and new approaches in the control of helminthiasis, an Asian study. Parasite Epidemiol Control. 2019;4:e00083 10.1016/j.parepi.2018.e00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nakagawa J, Ehrenberg JP, Nealon J, Fürst T, Aratchige P, Gonzales G, et al. Towards effective prevention and control of helminth neglected tropical diseases in the Western Pacific Region through multi-disease and multi-sectoral interventions. Acta Trop. 2015;141(Pt B):407–18. 10.1016/j.actatropica.2013.05.010 [DOI] [PubMed] [Google Scholar]
- 92.Bergquist R, Zhou XN, Rollinson D, Reinhard-Rupp J, Klohe K. Elimination of schistosomiasis: the tools required. Infect Dis Poverty. 2017;6(1):158 10.1186/s40249-017-0370-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tchuem Tchuenté LA, Rollinson D, Stothard JR, Molyneux D. Moving from control to elimination of schistosomiasis in sub-Saharan Africa: time to change and adapt strategies. Infect Dis Poverty. 2017;6(1):42 10.1186/s40249-017-0256-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu J, Xu JF, Li SZ, Zhang LJ, Wang Q, Zhu HH, et al. Integrated control programmes for schistosomiasis and other helminth infections in P.R. China. Acta Trop. 2015;141(Pt B):332–41. 10.1016/j.actatropica.2013.11.028 [DOI] [PubMed] [Google Scholar]
- 95.Seto EY, Remais JV, Carlton EJ, Wang S, Liang S, Brindley PJ, et al. Toward sustainable and comprehensive control of schistosomiasis in China: lessons from Sichuan. PLoS Negl Trop Dis. 2011;5(10):e1372 10.1371/journal.pntd.0001372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang SQ, Sun CS, Wang M, Lin DD, Zhou XN, Wang TP. Epidemiological features and effectiveness of schistosomiasis control programme in lake and marshland region in The People's Republic of China. Adv Parasitol. 2016;92:39–71. 10.1016/bs.apar.2016.02.018 [DOI] [PubMed] [Google Scholar]
- 97.Zhu H, Yap P, Utzinger J, Jia TW, Li SZ, Huang XB, et al. Policy support and resources mobilization for the National Schistosomiasis Control Programme in the People's Republic of China. Adv Parasitol. 2016;92:341–83. 10.1016/bs.apar.2016.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Karunaweera ND, Galappaththy GN, Wirth DF. On the road to eliminate malaria in Sri Lanka: lessons from history, challenges, gaps in knowledge and research needs. Malar J. 2014;13:59 10.1186/1475-2875-13-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bruce-Chwatt LJ. Malaria and its control: present situation and future prospects. Annu Rev Public Health. 1987;8:75–110. 10.1146/annurev.pu.08.050187.000451 [DOI] [PubMed] [Google Scholar]
- 100.A research agenda for malaria eradication: monitoring, evaluation, and surveillance. PLoS Med. 2011;8(1):e1000400 10.1371/journal.pmed.1000400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tambo E, Ngogang JY, Ning X, Xiao-Nong Z. Strengthening community support, resilience programmes and interventions in infectious diseases of poverty. East Mediterr Health J. 2018;24(6):598–603. 10.26719/2018.24.6.598 [DOI] [PubMed] [Google Scholar]
- 102.Fenwick A. Host-parasite relations and implications for control. Adv Parasitol. 2009;68:247–61. 10.1016/S0065-308X(08)00610-6 [DOI] [PubMed] [Google Scholar]
- 103.Ndao B, Senghor CS, Sy I, Diedhiou K, Talla I, Barbier D, et al. [Can we overcome schistosomiasis? A Senegalese example]. Bull Soc Pathol Exot. 2015;108(1):17–20. 10.1007/s13149-014-0370-9 [DOI] [PubMed] [Google Scholar]
- 104.Korir HK, Riner DK, Kavere E, Omondi A, Landry J, Kittur N, et al. Young adults in endemic areas: An untreated group in need of school-based preventive chemotherapy for schistosomiasis control and elimination. Trop Med Infect Dis. 2018;3(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Elmorshedy H, Bergquist R, El-Ela NE, Eassa SM, Elsakka EE, Barakat R. Can human schistosomiasis mansoni control be sustained in high-risk transmission foci in Egypt? Parasit Vectors. 2015;8:372 10.1186/s13071-015-0983-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gazzinelli A, Oliveira-Prado R, Matoso LF, Veloso BM, Andrade G, Kloos H, et al. Schistosoma mansoni reinfection: Analysis of risk factors by classification and regression tree (CART) modeling. PLoS One. 2017;12(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Knopp S, Person B, Ame SM, Ali SM, Muhsin J, Juma S, et al. Praziquantel coverage in schools and communities targeted for the elimination of urogenital schistosomiasis in Zanzibar: a cross-sectional survey. Parasit Vectors. 2016;9:5 10.1186/s13071-015-1244-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seto EY, Wong BK, Lu D, Zhong B. Human schistosomiasis resistance to praziquantel in China: should we be worried? Am J Trop Med Hyg. 2011;85(1):74–82. 10.4269/ajtmh.2011.10-0542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ismail M, Botros S, Metwally A, William S, Farghally A, Tao LF, et al. Resistance to praziquantel: direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am J Trop Med Hyg. 1999;60(6):932–5. 10.4269/ajtmh.1999.60.932 [DOI] [PubMed] [Google Scholar]
- 110.Wang W, Wang L, Liang YS. Susceptibility or resistance of praziquantel in human schistosomiasis: a review. Parasitol Res. 2012;111(5):1871–7. 10.1007/s00436-012-3151-z [DOI] [PubMed] [Google Scholar]
- 111.WHO. Schistosomiasis elimination: refocusing on snail control to sustain progress: World Health Organization; 2020. [ [Google Scholar]
- 112.Duval D, Galinier R, Mouahid G, Toulza E, Allienne JF, Portela J, et al. A novel bacterial pathogen of Biomphalaria glabrata: a potential weapon for schistosomiasis control? PLoS Negl Trop Dis. 2015;9(2):e0003489 10.1371/journal.pntd.0003489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kloos H. Human behavior, health education and schistosomiasis control: a review. Soc Sci Med. 1995;40(11):1497–511. 10.1016/0277-9536(94)00310-p [DOI] [PubMed] [Google Scholar]
- 114.Krauth SJ, Balen J, Gobert GN, Lamberton PHL. A call for systems epidemiology to tackle the complexity of schistosomiasis, its control, and its elimination. Trop Med Infect Dis. 2019;4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lustigman S, Prichard RK, Gazzinelli A, Grant WN, Boatin BA, McCarthy JS, et al. A research agenda for helminth diseases of humans: The problem of helminthiases. PLoS Negl Trop Dis. 2012;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhou XN, Bergquist R, Leonardo L, Yang GJ, Yang K, Sudomo M, et al. Schistosomiasis japonica control and research needs. Adv Parasitol. 2010;72:145–78. 10.1016/S0065-308X(10)72006-6 [DOI] [PubMed] [Google Scholar]
- 117.Knopp S, Stothard JR, Rollinson D, Mohammed KA, Khamis IS, Marti H, et al. From morbidity control to transmission control: time to change tactics against helminths on Unguja Island, Zanzibar. Acta Trop. 2013;128(2):412–22. 10.1016/j.actatropica.2011.04.010 [DOI] [PubMed] [Google Scholar]
- 118.Turner HC, French MD, Montresor A, King CH, Rollinson D, Toor J. Economic evaluations of human schistosomiasis interventions: a systematic review and identification of associated research needs. Wellcome Open Res. 2020;5:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Salari P, Fürst T, Knopp S, Utzinger J, Tediosi F. Cost of interventions to control schistosomiasis: A systematic review of the literature. PLoS Negl Trop Dis. 2020;14(3):e0008098 10.1371/journal.pntd.0008098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Salari P, Fürst T, Knopp S, Rollinson D, Kabole F, Khamis MI, et al. Financial costs of the Zanzibar elimination and schistosomiasis transmission project. Am J Trop Med Hyg. 2020. (in press) 10.4269/ajtmh.20-0252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ndyomugyenyi R, Kabatereine N. Integrated community-directed treatment for the control of onchocerciasis, schistosomiasis and intestinal helminths infections in Uganda: advantages and disadvantages. Trop Med Int Health. 2003;8(11):997–1004. 10.1046/j.1360-2276.2003.01124.x [DOI] [PubMed] [Google Scholar]
- 122.Boelee E, Laamrani H. Environmental control of schistosomiasis through community participation in a Moroccan oasis. Trop Med Int Health. 2004;9(9):997–1004. 10.1111/j.1365-3156.2004.01301.x [DOI] [PubMed] [Google Scholar]
- 123.Katabarwa MN, Habomugisha P, Richards FO Jr., Hopkins D. Community-directed interventions strategy enhances efficient and effective integration of health care delivery and development activities in rural disadvantaged communities of Uganda. Trop Med Int Health. 2005;10(4):312–21. 10.1111/j.1365-3156.2005.01396.x [DOI] [PubMed] [Google Scholar]
- 124.Parker M, Allen T, Hastings J. Resisting control of neglected tropical diseases: dilemmas in the mass treatment of schistosomiasis and soil-transmitted helminths in north-west Uganda. J Biosoc Sci. 2008;40(2):161–81. 10.1017/S0021932007002301 [DOI] [PubMed] [Google Scholar]
- 125.Aagaard-Hansen J, Mwanga JR, Bruun B. Social science perspectives on schistosomiasis control in Africa: past trends and future directions. Parasitology. 2009;136(13):1747–58. 10.1017/S0031182009006404 [DOI] [PubMed] [Google Scholar]
- 126.Whittaker MA, Dean AJ, Chancellor A. Advocating for malaria elimination—learning from the successes of other infectious disease elimination programmes. Malar J. 2014;13:221 10.1186/1475-2875-13-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Utzinger J, Raso G, Brooker S, De Savigny D, Tanner M, Ornbjerg N, et al. Schistosomiasis and neglected tropical diseases: towards integrated and sustainable control and a word of caution. Parasitology. 2009;136(13):1859–74. 10.1017/S0031182009991600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.WHO. Resolution on schistosomiasis WHA65.21. Geneva, Switzerland: World Health Organization; 2012. 28 May 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(DOCX)
Summary of key topics from key informant interviews.
(DOCX)
(DOCX)
Data Availability Statement
In order to protect participant confidentiality the interview transcripts are not provided, but the thematic analysis is available as supporting information. All other relevant data are within the manuscript and its Supporting Information files.