Abstract
Background
Hydroxychloroquine (HCQ) poisoning is a life-threatening but treatable toxic ingestion. The scale of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) and the controversial suggestion that HCQ is a treatment option have led to a significant increase in HCQ use. HCQ poisoning should be at the top-of-mind for emergency providers in cases of toxic ingestion. Treatment for HCQ poisoning includes sodium bicarbonate, epinephrine, and aggressive electrolyte repletion. We highlight the use of hypertonic saline and diazepam.
Case Report
We describe the case of a 37-year-old man who presented to the emergency department after the ingestion of approximately 16 g of HCQ tablets (initial serum concentration 4270 ng/mL). He was treated with an epinephrine infusion, hypertonic sodium chloride, high-dose diazepam, sodium bicarbonate, and aggressive potassium repletion. Persistent altered mental status necessitated intubation, and he was managed in the medical intensive care unit until his QRS widening and QTc prolongation resolved. After his mental status improved and it was confirmed that his ingestion was not with the intent to self-harm, he was discharged home with outpatient follow-up.
Why Should an Emergency Physician Be Aware of This?
For patients presenting with HCQ overdose and an unknown initial serum potassium level, high-dose diazepam and hypertonic sodium chloride should be started immediately for the patient with widened QRS. The choice of hypertonic sodium chloride instead of sodium bicarbonate is to avoid exacerbating underlying hypokalemia which may in turn potentiate unstable dysrhythmia. In addition, early intubation should be a priority in vomiting patients because both HCQ toxicity and high-dose diazepam cause profound sedation.
Keywords: arrhythmia, chloroquine, COVID-19, diazepam, dysrhythmia, ECG, epinephrine, hydroxychloroquine, hypertonic saline, hypertonic sodium chloride, overdose, plaquenil, SARS-CoV-2, sodium bicarbonate, toxicology
Introduction
Hydroxychloroquine (HCQ) poisoning is a life-threatening but treatable toxic ingestion. The scale of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) and the controversial suggestion that HCQ is a treatment option have led to a significant increase in HCQ use (1). HCQ poisoning should therefore be at the top-of-mind for emergency providers in cases of toxic ingestion. Treatment for HCQ poisoning includes sodium bicarbonate, epinephrine, and aggressive electrolyte repletion (2, 3, 4, 5). In this case report we highlight the use of hypertonic saline and diazepam.
As of early August 2020, the SARS-CoV-2 or COVID-19 pandemic had led to >715,000 deaths and 19.1 million confirmed cases (6). Early in the pandemic, there was widespread interest in the potential therapeutic role of HCQ (7,8). HCQ is a 4-aminoquinoline derivative approved as treatment for malaria and systemic lupus erythematosus (9). HCQ has been investigated as a potential treatment for COVID-19 with no strong data to suggest a therapeutic benefit despite being mentioned frequently in the media (10, 11, 12, 13). As a result, HCQ poisoning, which can cause life-threatening illness, may become more common as the number of prescriptions has risen substantially (1,14). We describe the case of a previously healthy man who presented to the emergency department (ED) after ingesting approximately 16 g of HCQ.
Case Report
A 37-year-old man was brought into the ED by emergency medical services with acute-onset chills, lightheadedness, and malaise. The patient reported taking “a handful” of HCQ tablets, which his wife quantified as up to eighty 200-mg tablets based on the number of missing tablets from a recently filled prescription written for the patient's mother. Upon learning of his ingestion, his wife encouraged him to vomit without success. He developed altered mental status approximately 90 min after ingestion, at which point his wife called 911. En route, emergency medical services obtained a 12-lead electrocardiogram (ECG) significant for complete heart block and prolonged corrected QT interval (QTc) of 611 ms (goal ≤450 ms).
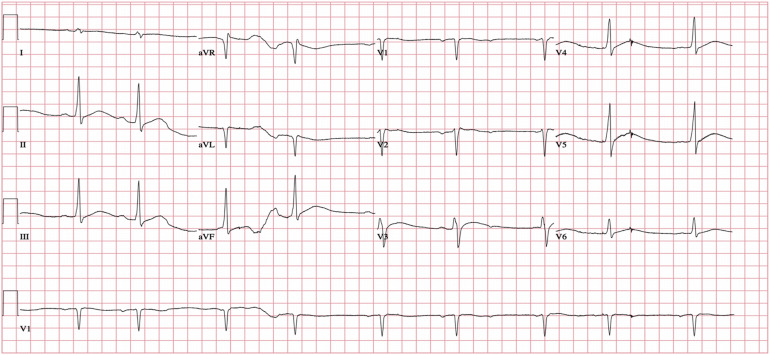
The patient arrived to the ED approximately 2 h postingestion and was immediately roomed and seen by providers within 3 min. The patient was drowsy but oriented to person, place, date, and situation. Before obtaining vital signs, he had several episodes of nonbloody, nonbilious emesis without tablets or fragments. His temporal temperature was 30°C (86°F), he was bradycardic to 56 beats/min, and his initial blood pressure (BP) could not be obtained with an automatic BP cuff; manual BP measurement was deferred to facilitate the placement of large bore intravenous (IV) access as the patient maintained his mental status and had palpable femoral pulses without distal cyanosis or duskiness of the extremities. The remainder of his physical examination was remarkable for shivering, cool and clammy skin, and irregular bradycardia. Initial ECG in the ED revealed complete heart block, QRS interval of 124 ms, and QTc of 548 ms (Figure 1 ). Fifteen minutes after arrival, an epinephrine infusion was initiated at 10 μg/min with improvement in his heart rate to 72 beats/min and BP to 90/48 mm Hg. Central IV access was obtained and the patient was placed on an involuntary psychiatric hold out of concern for possible self-harm.
Figure 1.
Initial electrocardiogram in the emergency department showing bradycardia with complete AV block, wide QRS of 124 ms, and prolonged QTc of 548 ms.
HCQ poisoning causes significant hypokalemia (15,16). While awaiting the results of initial laboratory work, we administered 100 mL IV 3% sodium chloride 30 min after arrival and 50 mg IV diazepam (approximately 1 mg/kg) approximately 1 h after arrival to address the widened QRS (goal ≤100 ms) and cardiac ion channel dysfunction, respectively (17). His laboratory values were remarkable for serum potassium of 2.9 mEq/L (reference range 3.5–5.0 mEq/L) and lactate 10.2 mEq/L (reference range <2.0 mEq/L). We administered 4 g IV magnesium sulfate and started the patient on aggressive IV potassium repletion at 20 mEq/h to achieve a potassium level of ≥4.0 mEq/L; he received a total of 130 mEq IV potassium chloride in the ED. Following potassium repletion, 100 mEq IV 8.4% sodium bicarbonate was administered and he was started on an isotonic sodium bicarbonate infusion at 15 mEq/h. Serial ECGs were obtained every 15 to 30 min and his QRS and QTc narrowed to 118 ms and 383 ms, respectively. His epinephrine infusion was increased to a rate of 15 μg/min for intermittent hypotension with mean arterial pressure of 55 mm Hg with subsequent improvement to 67 mm Hg before transfer to the medical intensive care unit (MICU) 5 h after arrival to the ED. At that time his heart rate was noted to be 107 beats/min.
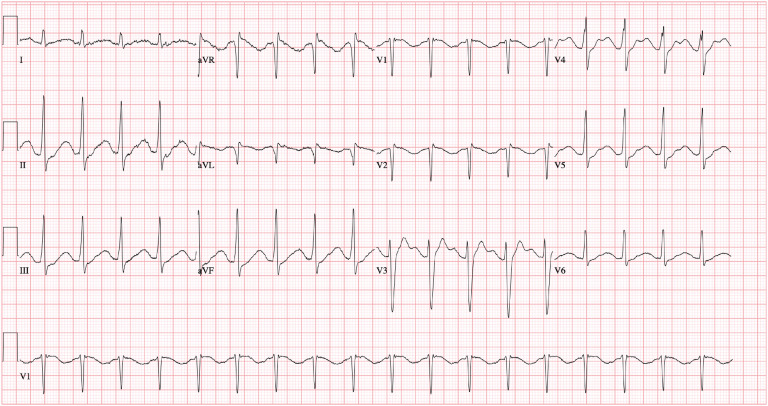
He was admitted to the MICU where he was intubated for airway protection in the setting of altered mental status. Epinephrine infusion was titrated to maintain a mean arterial pressure >65 mm Hg and a heart rate >100 beats/min, the latter to reduce the risk of torsades de pointes. ECG shortly after arrival to the MICU showed a QRS of 114 ms, TU-fusion waves, and QTc of 666 ms (Figure 2 ). In the setting of a widened QRS and prolonged QTc, he was given an additional bolus of 100 mL IV 3% sodium chloride; he also received 2 g IV magnesium sulfate and 2 g IV calcium gluconate to promote cardiac myocyte stability. ECGs were obtained every 2 h and we planned to give additional boluses of 100 mL IV 3% sodium chloride as needed for widened QRS or prolonged QTc. The patient's hypokalemia persisted while in the MICU and an additional 80 mEq IV potassium chloride was administered for an initial serum potassium of 2.3 mEq/L. Repeat laboratory values were drawn every 2 h and IV potassium chloride was given as needed to achieve normokalemia. Diazepam 6 mg IV was administered every 2 h for 24 h after the initial 50-mg IV bolus to antagonize cardiac myocyte toxicity and for seizure prophylaxis. We chose intermittent diazepam dosing over an infusion based on clinical pharmacist and poison control recommendations.
Figure 2.
Electrocardiogram obtained early in the intensive care unit course. The patient had recurrence of QTc prolongation of 666 ms with TU-fusion waves.
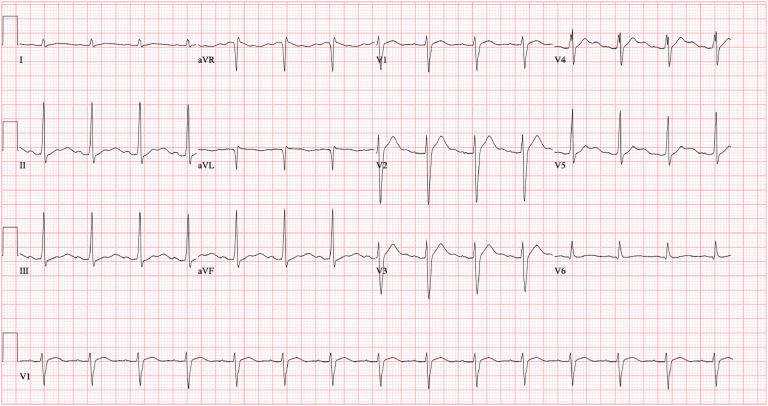
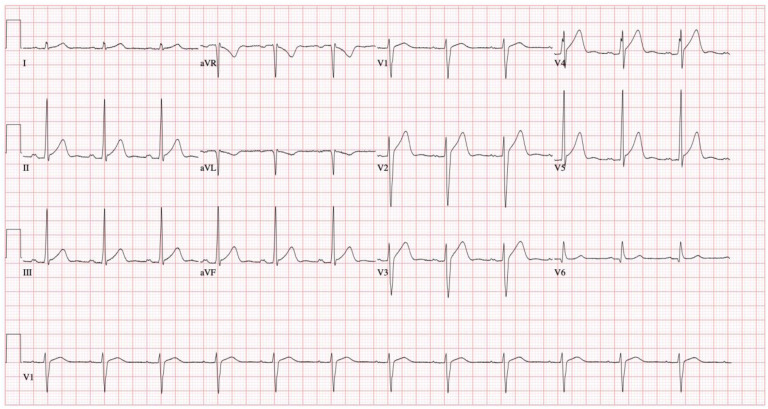
Over the next 24 h, the patient received 1 additional bolus of 100 mL IV 3% sodium chloride for a prolonged QTc of 534 ms. Serial ECGs showed narrowing of the QRS and QTc to 100 ms and 440 ms, respectively, with persistent U waves (Figure 3 ). Once the QRS and QTc remained within normal limits without additional 3% sodium chloride boluses at approximately the 24-h mark, epinephrine was weaned off over 12 h while monitoring closely for rebound hyperkalemia (none observed). We discontinued diazepam after 24 h both because this was the expected window of toxicity and to avoid propylene glycol toxicity from prolonged diazepam administration (18,19). An ECG 37 h after presentation showed resolution of U waves, a normal QTc (434 ms), and diffuse J point elevations (Figure 4 ) with high-sensitivity troponin-T levels within normal limits. He received an empiric course of ceftriaxone and doxycycline for possible aspiration pneumonia based on bilateral infiltrates present on his admission chest radiograph and 2 nasopharyngeal swabs sent for COVID-19 reverse transcription polymerase chain reaction testing were negative. He was extubated on hospital day 3 and transferred to the medical floor on hospital day 4.
Figure 3.
Electrocardiogram obtained later in the intensive care unit course. The patient's QTc interval narrowed to 440 ms with prominent U waves.
Figure 4.
Electrocardiogram obtained before discharge. The patient had a narrow QRS of 92 ms and a normal QTc of 424 ms.
On the medical floor, he remained hemodynamically stable without return of electrolyte derangements or ECG changes. Once his mental status was back to baseline, he described taking HCQ to protect himself and his family, including a newborn, from contracting COVID-19. He acknowledged that he did not know the proper HCQ dosage and took it as if it was a supplement. He expressed no intention for self-harm, and his wife corroborated this narrative. He was discharged home on hospital day 5 with planned outpatient psychiatry follow-up.
After discharge, his serum HCQ level from admission returned at 4270 ng/mL (typical therapeutic levels: 10 ng/mL for malarial prophylaxis; ≤1000 ng/mL for systemic lupus erythematosus) (20).
Discussion
The diagnosis of HCQ overdose relies on history, physical examination, laboratory abnormalities, and ECG findings. The main clinical effects of HCQ toxicity are cardiac and neurologic. HCQ blocks sodium channels and potassium human-ether-a-go-go-related gene (hERG) channels, slowing cardiac intraventricular conduction and lowering the seizure threshold (16). Additional potassium efflux pump blockade leads to hypokalemia by inhibiting potassium egress from the intracellular space, which can be exacerbated by vomiting, bicarbonate-containing fluids, and epinephrine (15,21). The delayed cardiac myocyte repolarization and resulting prolonged refractory period prolongs the QRS and the QTc intervals, which may increase the risk of fatal dysrhythmias including torsades de pointes. These mechanisms culminate in hypotension secondary to bradycardia, negative inotropy, and dysrhythmias (22, 23, 24).
HCQ toxicity management depends upon the interval between the ingestion and presentation to medical care. Activated charcoal can be administered to prevent gastric absorption if the patient presents early after the ingestion (25). Similar to the management of tricyclic antidepressant overdose, sodium-containing fluids should be started immediately in patients with a widened QRS to overcome sodium channel blockade by increasing serum sodium concentrations (26, 27, 28). However, sodium bicarbonate infusion can decrease serum potassium level through intracellular shift, which may worsen QTc prolongation and precipitate malignant dysrhythmias. Based on data showing efficacy in tricyclic antidepressant overdose with wide QRS, hypertonic sodium chloride can be used as an alternative if the patient's serum potassium level is unknown, as was done in this case before the first potassium concentration resulted (28,29).
At normal serum potassium concentrations, the sodium-containing fluid of choice for HCQ toxicity is sodium bicarbonate, administered in boluses of 50 to 150 mEq followed by an isotonic sodium bicarbonate infusion at 10 to 20 mEq/h. The infusion is continued until the QRS has corrected to ≤100 ms and QTc has corrected to ≤450 ms, and then weaned off slowly. Once the above treatments have been initiated, clinicians should monitor QRS and QTc intervals with frequent ECGs and follow serum potassium levels every 1 to 2 h, targeting a value of ≥4.0 mEq/L. We recommend close ECG and serum potassium monitoring for 36 h to include the expected window of HCQ toxicity (24 h) and assess for rebound hyperkalemia after toxicity resolves (19,25). If serum potassium concentrations begin to decrease, consider decreasing the sodium bicarbonate infusion rate or changing to hypertonic sodium chloride boluses. Potentially fatal dysrhythmias that may result from HCQ toxicity itself or potassium shifts resulting in hypo- or hyperkalemia include torsades de pointes, ventricular tachycardia, and ventricular fibrillation (2,24,30). If hypotensive, epinephrine is recommended preferentially for HCQ toxicity for its inotropic effects secondary to beta agonism; norepinephrine is a second choice agent (31,32). Experts generally recommend titrating the beta-agonist to a target heart rate of ≥90 beats/min while the QTc is prolonged to avoid R-on-T phenomenon from an ectopic beat on a preceding T wave, which may precipitate malignant dysrhythmias (31).
Diazepam administration is the mainstay of seizure prophylaxis in HCQ overdose, and was associated with reduced mortality when used in combination with epinephrine in a retrospective analysis, potentially related to effects on cardiac ion channels (4,33). Generally, a 1 to 2 mg/kg bolus of IV diazepam is administered followed by either a continuous infusion or frequent boluses. Diazepam should be continued for 24 h, as this is the typical window of toxicity based on the rapid absorption and large volume of distribution of HCQ (19). Diazepam contains a significant amount of propylene glycol; it is relatively insoluble in water and not compatible for long periods in other solutions (18). Many hospitals do not use continuous infusions for these reasons, in which case scheduled boluses are a reasonable alternative (5). In addition to its role in preventing seizures, diazepam also directly stabilizes cardiac myocytes (17). Administering diazepam may exacerbate the sedating effects of HCQ toxicity and necessitate intubation for airway protection.
Why Should an Emergency Physician Be Aware of This?
HCQ toxicity poses several distinct challenges in management. HCQ toxicity causes widened QRS, prolonged QTc, and altered mental status which when left untreated may progress to malignant dysrhythmia, seizures, and death. In addition to early initiation of an epinephrine infusion and high-dose (1–2 mg/kg) diazepam, we advocate for the use of 3% sodium chloride to overcome sodium channel blockade and avoid exacerbating hypokalemia. When hypokalemia has been addressed, sodium bicarbonate should be administered to address cardiac myocyte channel toxicity. We also encourage early intubation to address the sedating effects of HCQ and high-dose diazepam.
Footnotes
Reprints are not available from the authors.
References
- 1.Shehab N., Lovegrove M., Budnitz D.S. US hydroxychloroquine, chloroquine, and azithromycin outpatient prescription trends, October 2019 through March 2020. JAMA Intern Med. 2020;180:1384–1386. doi: 10.1001/jamainternmed.2020.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gunja N., Roberts D., McCoubrie D. Survival after massive hydroxychloroquine overdose. Anaesth Intensive Care. 2009;37:130–133. doi: 10.1177/0310057X0903700112. [DOI] [PubMed] [Google Scholar]
- 3.Demaziere J., Saissy J.M., Vitris M. Effects of diazepam on mortality from acute choloroquine poisoning [in French] Ann Fr Anesth Reanim. 1992;11:164–167. doi: 10.1016/s0750-7658(05)80009-7. [DOI] [PubMed] [Google Scholar]
- 4.Riou B., Barriot P., Rimailho A., Baud F.J. Treatment of severe chloroquine poisoning. N Engl J Med. 1988;318:1–6. doi: 10.1056/NEJM198801073180101. [DOI] [PubMed] [Google Scholar]
- 5.Chary M.A., Barbuto A.F., Izadmehr S., Hayes B.D., Burns M.M. COVID-19: therapeutics and their toxicities. J Med Toxicol. 2020;16:284–294. doi: 10.1007/s13181-020-00777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johns Hopkins Coronavirus Resource Center website COVID-19 dashboard. https://coronavirus.jhu.edu/map.html Available at:
- 7.Baker P., Rogers K., Enrich D., Haberman M. Trump’s aggressive advocacy of malaria drug for treating coronavirus divides medical community. The New York Times. https://www.nytimes.com/2020/04/06/us/politics/coronavirus-trump-malaria-drug.html Available at:
- 8.Centers for Disease Control and Prevention website Therapeutic options for COVID-19 patients. https://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html Available at:
- 9.UpToDate Hydroxychloroquine. https://www.uptodate.com/contents/hydroxychloroquine-drug-information?search=hydroxychloroquine&source=panel_search_result&selectedTitle=1∼148&usage_type=panel&kp_tab=drug_general&display_rank=1#references Available at:
- 10.Voss A. Statement on IJAA paper. https://www.isac.world/news-and-publications/official-isac-statement Available at:
- 11.Gautret P., Lagier J.-C., Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Colson P., Rolain J.M., Lagier J.C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020;55:105932. doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincent M.J., Bergeron E., Benjannet S. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shepherd K. Fish tank cleaner chloroquine phosphate killed a man who ingested it as coronavirus cure. The Washington Post. https://www.washingtonpost.com/nation/2020/03/24/coronavirus-chloroquine-poisoning-death/ Available at:
- 15.Clemessy J.L., Borron S.W., Baud F.J., Favier C., Hantson P.E., Vicaut E. Hypokalaemia related to acute chloroquine ingestion. Lancet. 1995;346:877–880. doi: 10.1016/s0140-6736(95)92711-5. [DOI] [PubMed] [Google Scholar]
- 16.de Olano J., Howland M.A., Su M.K., Hoffman R.S., Biary R. Toxicokinetics of hydroxychloroquine following a massive overdose. Am J Emerg Med. 2019;37:2264.e5–2264.e8. doi: 10.1016/j.ajem.2019.158387. [DOI] [PubMed] [Google Scholar]
- 17.Koudogbo B., Asseko M.C., Nguemby Mbina C., Laguerret-Atadou V. Mode of antidotal action of diazepam in the treatment of chloroquine poisoning [in French] J Toxicol Clin Exp. 1986;6:307–312. [PubMed] [Google Scholar]
- 18.Morris M.E. Compatibility and stability of diazepam injection following dilution with intravenous fluids. Am J Hosp Pharm. 1978;35:669–672. [PubMed] [Google Scholar]
- 19.IBM Micromedex. IBM Corporation; Greenwood Village, CO: 2020. Hydroxychloroquine. [Google Scholar]
- 20.Mayo Clinic Laboratories website Test ID: HCQ. Hydroxychloroquine, serum. https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/64947 Available at:
- 21.Isbister G.K., Dawson A., Whyte I.M. Hydroxychloroquine overdose: a prospective case series. Am J Emerg Med. 2002;20:377–378. doi: 10.1053/ajem.2002.33775. [DOI] [PubMed] [Google Scholar]
- 22.Michael T.A.D., Aiwazzadeh S. The effects of acute chloroquine poisoning with special reference to the heart. Am Heart J. 1970;79:831–842. doi: 10.1016/0002-8703(70)90371-6. [DOI] [PubMed] [Google Scholar]
- 23.Clemessy J.L., Lapostolle F., Borron S.W., Baud F.J. Acute chloroquine poisoning. Presse Med. 1996;25:1435–1439. [in French] [PubMed] [Google Scholar]
- 24.Messant I., Jérémie N., Lenfant F., Freysz M. Massive chloroquine intoxication: Importance of early treatment and pre-hospital treatment. Resuscitation. 2004;60:343–346. doi: 10.1016/j.resuscitation.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Marquardt K., Albertson T.E. Treatment of hydroxychloroquine overdose. Am J Emerg Med. 2001;19:420–424. doi: 10.1053/ajem.2001.25774. [DOI] [PubMed] [Google Scholar]
- 26.Bruccoleri R.E., Burns M.M. A literature review of the use of sodium bicarbonate for the treatment of QRS widening. J Med Toxicol. 2016;12:121–129. doi: 10.1007/s13181-015-0483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pentel P., Benowitz N. Efficacy and mechanism of action of sodium bicarbonate in the treatment of desipramine toxicity in rats. J Pharmacol Exp Ther. 1984;230:12–19. [PubMed] [Google Scholar]
- 28.McKinney P.E., Rasmussen R. Reversal of severe tricyclic antidepressant-induced cardiotoxicity with intravenous hypertonic saline solution. Ann Emerg Med. 2003;42(1):20–24. doi: 10.1067/mem.2003.233. [DOI] [PubMed] [Google Scholar]
- 29.McCabe J.L., Cobaugh D.J., Menegazzi J.J., Fata J. Experimental tricyclic antidepressant toxicity: a randomized, controlled comparison of hypertonic saline solution, sodium bicarbonate, and hyperventilation. Ann Emerg Med. 1998;32:329–333. doi: 10.1016/s0196-0644(98)70009-5. [DOI] [PubMed] [Google Scholar]
- 30.Mégarbane B., Résière D., Sonneville R., Guerrier G., Deye N., Baud F. Acute hydroxychloroquine poisoning. The danger of rapid or excessive correction of initial hypokalemia. Presse Med. 2005;34:933–934. doi: 10.1016/s0755-4982(05)84083-7. [in French] [DOI] [PubMed] [Google Scholar]
- 31.Engel T.R., Meister S.G., Frankl W.S. The “R-on-T” phenomenon: an update and critical review. Ann Intern Med. 1978;88:221–225. doi: 10.7326/0003-4819-88-2-221. [DOI] [PubMed] [Google Scholar]
- 32.Riou B., Rimailho A., Galliot M., Bourdon R., Huet Y. Protective cardiovascular effects of diazepam in experimental acute chloroquine poisoning. Intensive Care Med. 1988;14:610–616. doi: 10.1007/BF00256764. [DOI] [PubMed] [Google Scholar]
- 33.Crouzette J., Vicaut E., Palombo S., Girre C., Fournier P.E. Experimental assessment of the protective activity of diazepam on the acute toxicity of chloroquine. Clin Toxicol. 1983;20:271–279. doi: 10.3109/15563658308990070. [DOI] [PubMed] [Google Scholar]