Abstract
Angiotensin-converting enzyme 2 (ACE 2) is a membrane-bound enzyme that cleaves angiotensin II (Ang II) into angiotensin (1–7). It also serves as an important binding site for SARS-CoV-2, thereby, facilitating viral entry into target host cells. ACE 2 is abundantly present in the intestine, kidney, heart, lungs, and fetal tissues. Fetal ACE 2 is involved in myocardium growth, lungs and brain development. ACE 2 is highly expressed in pregnant women to compensate preeclampsia by modulating angiotensin (1–7) which binds to the Mas receptor, having vasodilator action and maintain fluid homeostasis. There are reports available on Zika, H1N1 and SARS-CoV where these viruses have shown to produce fetal defects but very little is known about SARS-CoV-2 involvement in pregnancy, but it might have the potential to interact with fetal ACE 2 and enhance COVID-19 transmission to the fetus, leading to fetal morbidity and mortality. This review sheds light on a path of SARS-CoV-2 transmission risk in pregnancy and its possible link with fetal ACE 2.
Keywords: SARS-CoV-2, ACE 2, Pregnancy and fetal transmission
Graphical abstract

1. Introduction
Angiotensin-converting enzyme (ACE) is an ectoenzyme with a molecular weight of 195 kDa, which plays a crucial role in the renin-angiotensin system (RAS) pathway. The ACE enzyme converts angiotensin I (Ang I), a decapeptide to angiotensin II (Ang II), an octapeptide that binds to the AT1 receptor to induce vasoconstrictor response, which is a well-known target for the treatment of cardiovascular complications [1,2]. ACE 2 is a type I transmembrane metallocarboxypeptidase, composed of a single HEXXH zinc-binding domain and is a homologue of ACE with 40% similarity. ACE 2 is able to hydrolyze angiotensin I (Ang I) to produce angiotensin (1–9) and inactivates potent vasoconstrictor Ang II to produce angiotensin-(1–7) (Ang I-7). The enzyme is able to cleave several peptides from other systems such as the kinin metabolites, neurotensin 1–13, apelin 13, dynorphin 1–13 [3]. The enzyme has vasodilator property as an endogenous ligand for the G protein-coupled receptor Mas, stimulates prostaglandin synthesis and inhibits proliferation of vascular smooth muscles [[4], [5], [6]]. ACE 2 is a membrane-bound enzyme and is most abundantly present in the intestine, kidney and heart compared to other organs such as lungs and arteries [7,8]. Several reports from the literature, have indicated that ACE 2 is highly expressed in the reproductive organs, placenta, uterus and, maternal-fetal interface during pregnancy; which is important for normal fetal growth and also for regulation of the Ang II level. Besides, renal ACE 2 is also upregulated in pregnant women, further in comparison; the placenta shows the highest expression of renal ACE 2 mRNA, followed by kidney and, then the uterus; whereas, the ACE 2 activity is higher in kidney in comparison to placenta and uterus (Table 1 ) [[11], [12], [13], [14]].
Table 1.
Distribution of ACE 2 in different tissues and SARS-COV-2 has great potential to interact with ACE 2 where it presents in abundant form.
| Expression | Tissue | Reference |
|---|---|---|
| mRNA expression of ACE 2 in tissue | Small intestine>Colon>Duodenum>Kidney>Testes>Gall bladder> Heart muscle>Thyroid gland>Adipose tissue>Epididymis (Consensus data set) | ([9], [10]) |
| Protein expression of ACE 2 in tissue | Duodenum>Gall bladder>Kidney>Small intestine>Testes>Adrenal gland>Colon>Rectum>Seminal vesicle | [10] |
| ACE 2 mRNA in the pregnancy | placenta > kidneys > or = uterus | [11] |
| ACE 2 activity in the pregnancy | kidney > placenta > uterus | [11] |
| ACE 2 expression in fetal tissues | Heart, liver and lung, but not in fetal kidney | [12] |
| High expression of ACE 2 | At maternal-fetal interface cells, stromal cells, perivascular cells of decidua, cytotrophoblast and syncytiotrophoblast in placenta | [12] |
Recently, it is documented that transmembrane ACE 2 also serves as an entry point into cells for human pathogenic human coronavirus NL63 (HCoV-NL63), severe acute respiratory syndrome-related coronavirus (SARS-CoV), and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [[15], [16], [17], [18]]. The RAAS inhibitors in the pathogenesis of Covid-19 are overly complex and controversial. Recently, Saavedra et al. reported that treatment with AT1 receptor blocker protects the lungs from the viral infection including coronavirus through the modulation of inflammation and ACE 2 upregulation. Hence, the AT1 receptor blocker can be used in cormobid conditions including diabetes, hypertension and renal diseases among COVID-19 patients. Contrary, the expression of ACE 2 is upregulated in patients with heart failure, arterial hypertension and diabetes mellitus when treating with ACE inhibitors & AT1R blockers and thereby, increased chances of COVID-19 disease [19,20]. In addition, higher expression of ACE 2 in fetal tissues such as heart, liver and lung but not in the kidney, may also increase the risk to neonates in pregnant women, infected with COVID-19, could facilitate the disease transmission to developing fetus and may affect different organ systems which have high expression of ACE 2 [12,21,22].
Preeclampsia is a pregnancy complication characterized by high blood pressure and proteinuria and usually begins after 20 weeks of gestation. It is one of the foremost reasons for maternal and fetal morbidity and mortality [23]. Reports suggest that the levels of angiotensinogen, Ang II, and mineralocorticoids are increased in pregnancy and lead to preeclampsia [11,24]. Further, to regulate this increased blood pressure, a compensatory increase in ACE 2 activity leads to the production of Ang (1–7) which causes vasodilation, reduces the production of aldosterone by acting on adrenal glomerulosa cells [23,[25], [26], [27], [28]]. ACE 2 has an antihypertrophic activity that plays a pivotal role in cardiac tissue during the gestational period and may modulate myocardial tissue growth [29,30]. Additionally, a report shows that ACE 2 is also involved in fetal brain and lung development in the gestation period [29]. Experimental evidence shows that Murine coronavirus infects placenta and uterus in pregnancy/challenge of susceptible BALB/cByJ mice. In addition, a porcine Betaarterivirus suid 1 infection induces fetal death in pigs at early stage of pregnancy [31]. Moreover, MERS CoV and SARS-CoV infection during pregnancy is also linked with maternal illness, abortion, maternal deaths and preterm birth but little is known about SARS-CoV-2 transmission in pregnancy and its impact on the fetus [[31], [32], [33]]. The lacuna in the availability of reports in literature regarding the vertical transmission of SARS-CoV-2 infection from mother to fetus has raised the concern for the potential interaction of ACE 2 with SARS-CoV-2 virus. In this review, we focus on the importance of the ACE 2 receptor in SARS-CoV-2 transmission from pregnant women to fetuses and neonates.
2. SARS-CoV-2 and ACE 2
SARS-CoV-2 is a positive-sense single-stranded RNA virus and causes respiratory illness. SARS-CoV-2 genome is identical to SARS-CoV (80%) and BatCoV RaTG13 (96%) [18]. There are four major proteins in the structure of SARS-CoV; namely N protein (nucleocapsid), M protein (membrane), E protein (envelope), and S protein (spikes that lead to virus entry) [[34], [35], [36], [37], [38]]. N protein functions largely to bind to the CoV RNA genome and the nucleocapsid formation. In endoplasmic reticulum (ER)-Golgi, it helps in assembling and budding of SARS-CoV [39]. M Protein is an ample structural protein that maintains the shape of the viral envelope [40,41]. E protein is the smallest of all the proteins available and is abundantly produced during viral infection and then incorporated in the viral envelope to mediate membrane fusion [[42], [43], [44]]. Cleavage of S protein (Trimer) leads to the formation of S1 and S2 subunits. S1 subunit is primarily composed of the receptor-binding domain and is liberated during the phase of post-transfusion conformation [[44], [45], [46], [47]]. It has a tendency to bind directly to the angiotensin-converting enzyme 2 (ACE 2) at its peptidase domain (PD) site [48,49]. Whereas the S2 subunit facilitates membrane fusion which is a paramount step for viral infection. S2 comprises a cleavage site for host proteases [45,50,51]. The ectodomain of the SARS-CoV-2 S protein binds to the peptidase domain (PD) of ACE 2 [52,53].
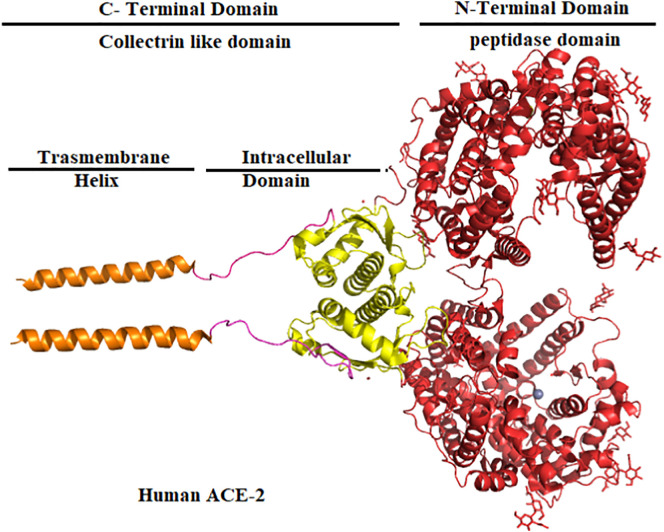
ACE 2 consists of the N-terminal peptidase domain (PD) and a C-terminal collectrin-like domain (CLD) which is comprised of 40 intracellular residue segments; including chain and the single transmembrane helix at the end of C-Terminal [54,55]. PD of ACE 2 cleaves Ang I, resulting in the formation of Ang (1–9), which are then further converted to Ang (1–7). However, ACE 2 performs the direct cleavage of Ang II to produce Ang (1–7) [54,56,57]. The dimer structure of the ACE 2 domain structure is shown in Fig. 1 [58].
Fig. 1.
Structure of the ACE 2 N-Terminal which contains peptidase domain (red) and C-Terminal domain contains collectrin like a domain that includes intracellular domain (yellow) with chain (pink) and transmembrane helix (orange). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
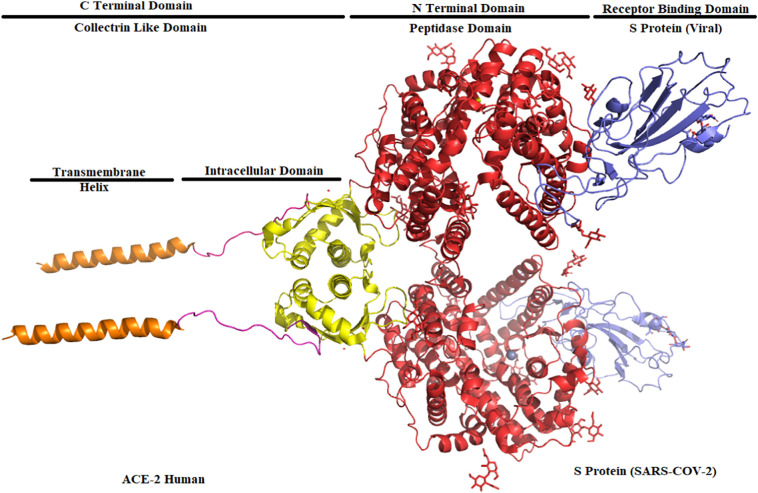
ACE 2 seats the receptor-binding domain (S Protein of SARS-CoV-2) in its peptidase domain (Fig. 2 ). The presence of polar interactions between the ACE 2 and SARS-CoV-2 enabled the binding efficacy [47,49,[59], [60], [61]]. An arch-shaped helix of the peptidase domain of ACE 2 interacts with the loop region of the Receptor Binding Domain of the S protein. The other helix and loops connect the antiparallel strands and co-ordinate the peptidase domain to the receptor-binding domain.
Fig. 2.
Structure of the peptidase domain (red) (ACE 2) binding to the receptor-binding domain (blue) (SARS-CoV-2. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Based on data and analysis published by Statistical Research Department for Italy, there can be inferences that men may be more at risk than women (53.1% vs. 46.9% among total COVID-19 cases) [62]. Further, growing evidence shows that there is an increased mortality rate in male COVID-19 patients as compared to females underlying with chronic illness such as hypertension, which is the foremost reason for the comorbidity and mortality followed by diabetes mellitus, renal disorder, chronic obstructive pulmonary disease and cancer [[63], [64], [65]]. The infection and fatality rate is less in females, possibly due to strong immune response; less susceptibility to viral infections; high level of the protective hormone estrogen, progesterone and, presence of ACE 2 which is X-linked [[66], [67], [68], [69]].
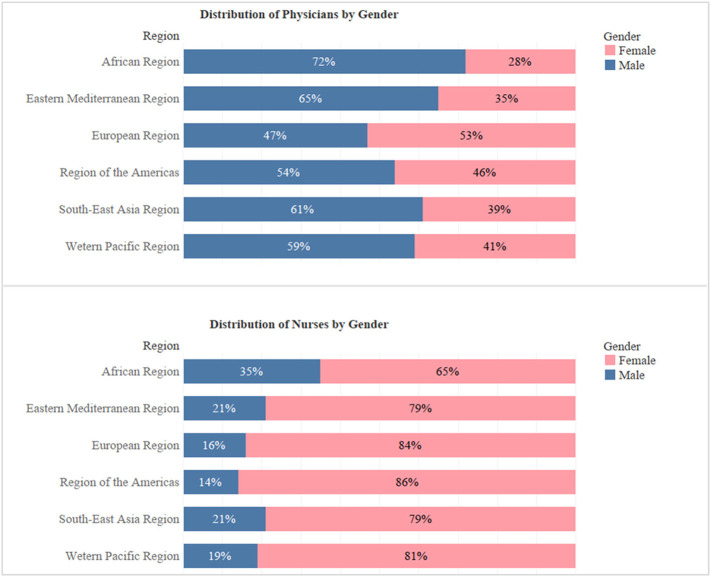
However, in an article by World Economic Forum, it is suggested that COVID-19 fallout may be worse in women compared to men since i) women are on the front lines of the fight against SARS-CoV-2 infection as they form the majority of health and social care workers (Fig. 3 ) [70] ii) women are specifically affected by mass school closures as they still bear most of the responsibility for childcare iii) Women already do three-times as much unpaid care work than men [71].
Fig. 3.
Distribution of physicians and nurses by gender.
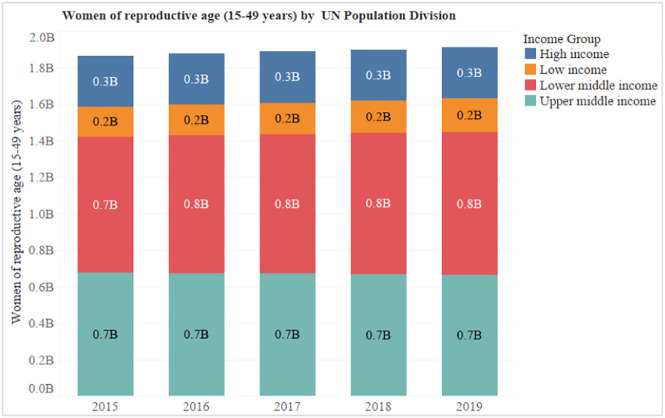
Globally, we have analyzed the last five years data of Women of reproductive age (15–49 years) population made available by UN Population Division and found that each year there are around 1.9 billion women of childbearing age (Fig. 4 ) [72].
Fig. 4.
Women of reproductive age (15–49 years) by UN population division.
Furthermore, these numbers were segregated based on Income groups (High, Low, Low middle and Upper middle). According to the article published by the Center on Society and Health, income is a leading force behind the striking health disparities and low-income adults are approximately five folds more likely to report being in poor health [73]. Therefore, especially women belonging to low and lower-middle-income groups (approximately 1.1 billion; Fig. 4) are much more susceptible to these COVID-19 infections.
3. Current cases of COVID-19 in pregnancy and fetal risk
Due to the presence of limited data, COVID-19 transmission in pregnancy and its effect on the fetus is still not so clear. ACE2 is extensively expressed in human placenta and chiefly in the syncytiotrophoblast, cytotrophoblast, endothelium and vascular smooth muscle of primary and secondary villi. In the maternal stroma, ACE2 is expressed in the invading and intravascular trophoblast and decidual cells. ACE2 is also found in arterial and venous endothelium and smooth muscle of the umbilical cord [74,75]. The placenta and decidua are the main maternal-fetal interface during pregnancy, and virus receptors expression in placenta and decidual cells may play an important role in promoting the transmission of SARS-CoV-2.
At the gestation week of (6–14) ACE 2 gene is expressed in stromal cells, perivascular cells in decidua, and villous cytotrophoblast and syncytiotrophoblast in placenta however the extravillous trophoblast did not express ACE2 at this time. TMPRSS2 was expressed in villous cytotrophoblast and epithelial glandular cells and also had low expression in syncytiotrophoblast. Extravillous trophoblast cells had extremely low level of ACE2 at early placenta (8 week) while the ACE2 expression was significantly increased in Extravillous trophoblast cells at later stage of pregnancy (24 week) [12]. Further, the high levels of Ang II, ACE2 and Ang-(1–7) expression may be involved in hypertension of pregnancy, preeclampsia and eclampsia. In addition preeclamptic women presented plasma Ang-(1–7) suppressed levels when compared with normal pregnancy subjects [76].
Recently, Chen et al. reported that nine women in the third trimester of pregnancy are infected with SARS-CoV-2 and all had pneumonia. Women underwent cesarean delivery and all infants are born with good health, having Apgar scores “between” 8–10 [77]. In addition, a report also shows that a 30-week pregnant woman infected with SARS-CoV-2 gave birth to a baby without any symptoms of COVID-19 and after testing the neonate swab, the results were negative [78]. A study also reports eleven pregnant with the disease gave birth without neonatal respiratory illness, abortion and deaths [79]. Schwartz et al. analyzed the thirty-eight pregnant women infected with SARS-CoV-2 in China and reported that there is no evidence of intrauterine transmission of the virus, no maternal deaths and all neonates found negative for COVID-19 test [80]. In support of these reports, Fan. C et al. show that newborns are safe and there are no abnormalities observed in babies of SARS-CoV-2 infected mothers [81].
Contrary to these reports, Wang. S et al. reveal that pregnant women infected with SARS-CoV-2 delivered a COVID-19 positive baby in China. This was confirmed in the neonatal pharyngeal swabs which were tested positive with SARS-CoV-2 after 36 h; this shows the still unexplored vertical transmission of the virus from mother to fetus [82]. Zhu H et al. reported that prenatal SARS-CoV-2 exposure might have adverse effects on neonates such as fetal distress, premature labor, respiratory distress, thrombocytopenia associated with abnormal liver function, and death. However, swab testing reports of infants were negative, thus challenging the intrauterine COVID-19 transmission to the fetus. Further, one infant born preterm died due to multi-organ failure, refractory shock and disseminated intravascular coagulation [31,83]. Lam CM et al. reported that COVID-19 infection along with pregnancy might result in intrauterine growth restriction, preterm birth, intrauterine death, and neonatal death [84]. Zeng et al. reported outcomes of six pregnant women with COVID-19 admitted to Zhongnan Hospital of Wuhan University and all mothers underwent cesarean deliveries in their third trimester in negative pressure isolation rooms with all infection control measures. After delivery, all six infants were immediately isolated from the mother and show negative swab testing reports. However, out of six, two infants showed a high level of IgM antibodies with SARS-CoV-2 in the serum samples with no symptoms, later all infants were tested for viral RNA and showed negative reports [85].
Nan Yu and colleagues in Wuhan, China reported the assessment of obstetric and neonatal outcomes of pregnancy with COVID-19 pneumonia in seven pregnant women. All patients were kept in isolation and on Antiviral treatment and oxygen therapy. The onset symptoms were identical to the non-pregnant individuals. All patients went under the cesarean section and three neonates were positive for SARS-CoV-2 [86]. A report from Wuhan hospital mothers with COVID-19 gave birth to 33 neonates, out of whom, 3 neonates were infected with the SARS-CoV-2 and showed symptoms of pneumonia; from all 3 neonates, nasopharyngeal and anal swabs were taken and confirmed with COVID-19 on day 2 and 4, after birth. However, later one infant was tested negative for the disease on day 7, whose mother underwent cesarean delivery at 31 weeks of the gestational period [87]. In addition, the mother underwent a Cesarean section with all infection control measures, in a negative pressure isolation room. She gave birth to neonate presenting positive serum IgM, high level of cytokine level after 2 h of birth, indicating the possibility of vertical transmission of the virus from mother to fetus [31,88]. Zambrano et al. reported that 31 weeks pregnant woman suffering from gestational hypertension and hypothyroidism with symptoms of fever, dry cough, headache and myalgias admitted to the hospital. An obstetric ultrasounds report shows dysplastic and multicystic right kidney in fetuses. However, these defects are not confirmed due to COVID-19 [33].
Here, we hypothesized that high expression of ACE 2 might be responsible for the fetal defects, as there are chances of binding of SARS-CoV-2 with fetal ACE 2. However, further research is required to claim any information. The report shows that pregnancy leads to an immunosuppressive state and may increase susceptibility towards respiratory pathogens [33,89,90]. Na Li, MD et al. compare the effect of SARS-CoV-2 infection in maternal and neonatal outcomes in pregnant women with and without COVID-19 pneumonia. Nine pregnant women infected with SARS-CoV-2 went under the cesarean section and two pregnant women had a normal delivery with a higher risk of premature delivery (33.3%) but none was due to severe maternal respiratory failure. [91]. A mother infected with SARS-CoV-2 during the third trimester of pregnancy experienced decreased fetal movement, anemia, dyspnea, and newborns infected with SARS-CoV-2 [92]. In total, SARS-CoV-2, induced fetal abnormalities reported are miscarriage (2%), intrauterine growth restriction (10%) and pre-term birth (39%). However, there is no evidence of vertical transmission of SARS-CoV-2 from mother to fetus and as well under cesarean section and vaginal delivery [77,91,93].
Recent report shows that both S-protein and N-protein of SARS-CoV-2 presented positive to immunostains in the cytoplasm of the syncytiotrophoblast and the presence of N protein in rare intervillous macrophages and Hofbauer cells however, SARS-CoV-2 proteins were not detected in villous capillaries. In addition, in situ hybridization technique showed intense signal positivity for SARS-CoV-2 in syncytiotrophoblast lining with a distribution similar to that detected for the S-protein immunohistochemistry. Further, nucleic acid analysis, ultrastructural examination studies revealed the presence of coronavirus-like particles within the cytoplasm of syncytiotrophoblast and within chorionic villous fibroblasts and fetal capillary endothelial cells. Therefore, this could be the possible mechanism of SARS-CoV-2 may enter the placenta and passed to the fetus prior to delivery [[94], [95], [96]]. However, presently the data suggest that there is little evidence of vertical transmission to the newborn; hence; more studies will require to prove vertical transmission from the pregnant woman to the fetus.
4. Conclusion
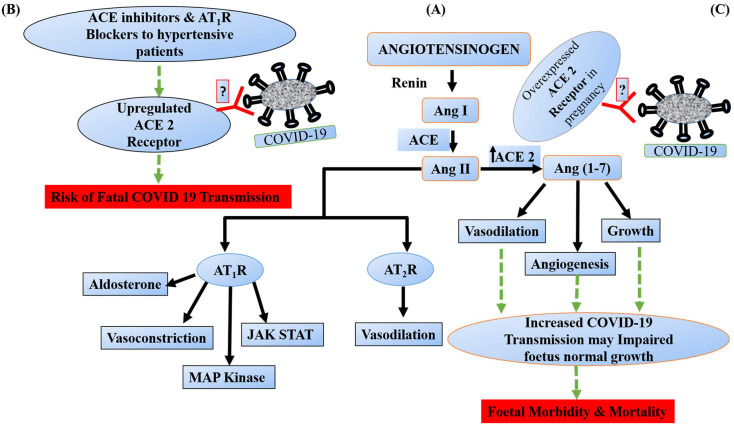
The literature studies and data support that there is no clear information regarding the infection and its intrauterine transmission of COVID-19 to the fetus. However, a wide range of trials needs to be conducted to warrant fetuses' safety from COVID-19 disease in pregnancy. There is no clear evidence, it might be due to the small population size of pregnant women infected with the disease and thus, further prospective studies need to be carried out worldwide. However, we hypothesize that fetal ACE 2 may interact with SARS-CoV-2 and increase the potential for fetal morbidity and mortality. Further, there is a lack of evidence about whether SARS-CoV-2 can cross the placenta and causes intrauterine infection through vertical transmission. If it crosses the placenta, it might interact with the fetal ACE 2, which is abundantly present in fetal tissues such as lungs, heart, liver, brain and may induce fetal death and abortion (Fig. 5 ). The incidence of SARS-CoV-2 infection to the fetus is really debated therefore careful monitoring of pregnant women is warranted to prevent neonatal infection.
Fig. 5.
Renin-Angiotensin pathway & possible mechanism by which SARS-CoV-2 binds to fetal ACE 2 and might induce Fetal Morbidity and Mortality. (A) Briefly, renin is an enzyme that act on angiotensinogen to produce decapeptide angiotensin I (Ang I) which is further cleaved by angiotensin converting enzyme (ACE) to catalyze the formation of octapeptide angiotensin II (Ang II). Ang II binds to angiotensin 1 receptor (AT1R) to produce vasoconstriction, activates mitogen activate protein kinase (MAP) kinase, JAK-STAT pathway and induces aldosterone production on the other side AT2R has vasodilator property. (B) Further, ACE inhibitors and AT1R blockers upregulates the expression of ACE 2 to produce Ang (1–7). This upregulated ACE 2 act as a binding site for SARS-CoV-2 and may facilitate the COVID 19 infection. (C) During pregnancy condition, fetal ACE 2 is highly expressed and so we are hypothesized that if SARS-CoV-2 crosses placenta similar to SARS-CoV then it would interact with fetal ACE 2 and may induce fetal deaths and abortions. (Note: Green dotted arrow indicates proposed/hypothetical pathway). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Acknowledgments
Acknowledgements
Dr. Sourav Kalra is thankful to ICMR, Delhi (Ref. no. 3/2/3/12/2019/NCDIII), for the ICMR- Research Associate Fellowship. We are thankful to Business Intelligence software tableau® from Sales force for extending the free student license for creating all the data charts in this article. The authors would like to thank Puja Kumari, Snehal Sainath Jawalekar and Dr. Gaurav Chauhan for their scientific inputs and manuscript proofreading.
Lastly, we would like to pay our tribute to all frontline medical professionals who are working tirelessly to bring the COVID-19 pandemic under control.
Declaration of competing interest
All authors declare that they have no conflicts of interest.
References
- 1.Turner A.J., Tipnis S.R., Guy J.L., Rice G., Hooper N.M. ACEH/ACE2 is a novel mammalian metallocarboxypeptidase and a homologue of angiotensin-converting enzyme insensitive to ACE inhibitors. Can. J. Physiol. Pharmacol. 2002;80:346–353. doi: 10.1139/y02-021. [DOI] [PubMed] [Google Scholar]
- 2.Warner F.J., Smith A.I., Hooper N.M., Turner A.J. Angiotensin-converting enzyme-2: a molecular and cellular perspective. Cell. Mol. Life Sci. 2004;61:2704–2713. doi: 10.1007/s00018-004-4240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vickers C., Hales P., Kaushik V., Dick L., Gavin J., Tang J. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 4.Chappell M.C. Of diabetic mice and ACE2: a new biomarker of renal disease? Am J Physiol Renal Physiol. 2013;305:F970–F972. doi: 10.1152/ajprenal.00403.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santos R.A., e Silva A.C.S., Maric C., Silva D.M., Machado R.P., de Buhr I. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor mas. Proc. Natl. Acad. Sci. U. S. A. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tikoo K., Patel G., Kumar S., Karpe P.A., Sanghavi M., Malek V. Tissue specific up regulation of ACE2 in rabbit model of atherosclerosis by atorvastatin: role of epigenetic histone modifications. Biochem. Pharmacol. 2015;93:343–351. doi: 10.1016/j.bcp.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Crackower M.A., Sarao R., Oudit G.Y., Yagil C., Kozieradzki I., Scanga S.E. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 8.Riviere G., Michaud A., Breton C., VanCamp G., Laborie C., Enache M. Angiotensin-converting enzyme 2 (ACE2) and ACE activities display tissue-specific sensitivity to undernutrition-programmed hypertension in the adult rat. Hypertension. 2005;46:1169–1174. doi: 10.1161/01.HYP.0000185148.27901.fe. [DOI] [PubMed] [Google Scholar]
- 9.Dai Y.-J., Hu F., Li H., Huang H.-Y., Wang D.-W., Liang Y. A profiling analysis on the receptor ACE2 expression reveals the potential risk of different type of cancers vulnerable to SARS-CoV-2 infection. Ann Transl Med. 2020;8:481. doi: 10.21037/atm.2020.03.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020:1–8. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy A., Yagil Y., Bursztyn M., Barkalifa R., Scharf S., Yagil C. ACE2 expression and activity are enhanced during pregnancy. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1953–R1961. doi: 10.1152/ajpregu.90592.2008. [DOI] [PubMed] [Google Scholar]
- 12.Li M., Chen L., Zhang J., Xiong C., Li X. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS One. 2020;15:e0230295. doi: 10.1371/journal.pone.0230295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bharadwaj M.S., Strawn W.B., Groban L., Yamaleyeva L.M., Chappell M.C., Horta C. Angiotensin-converting enzyme 2 deficiency is associated with impaired gestational weight gain and fetal growth restriction. Hypertension. 2011;58:852–858. doi: 10.1161/HYPERTENSIONAHA.111.179358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He J., Lu Y.P., Li J., Li T.Y., Chen X., Liang X.J. Fetal but not maternal angiotensin converting enzyme (ACE)-2 gene Rs2074192 polymorphism is associated with increased risk of being a small for gestational age (SGA) newborn. Kidney Blood Press Res. 2018;43:1596–1606. doi: 10.1159/000494449. [DOI] [PubMed] [Google Scholar]
- 15.Allawadhi P., Khurana A., Allwadhi S., Navik U.S., Joshi K., Banothu A.K. Potential of electric stimulation for the management of COVID-19. Med. Hypotheses. 2020;144:110259. doi: 10.1016/j.mehy.2020.110259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol. Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mourad J.J., Levy B.I. Interaction between RAAS inhibitors and ACE2 in the context of COVID-19. Nat. Rev. Cardiol. 2020;17:313. doi: 10.1038/s41569-020-0368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navik U., Bhatti J., Sheth V., Jawalekar S., Bhatti G., Kalra S. Multi-organ failure in COVID-19 patients: a possible mechanistic approach. Authorea Preprints. 2020 doi: 10.22541/au.159110399.94076751. [DOI] [Google Scholar]
- 22.Taylor D.M., Aronow B.J., Tan K., Bernt K., Salomonis N., Greene C.S. The pediatric cell atlas: defining the growth phase of human development at single-cell resolution. Dev. Cell. 2019;49:10–29. doi: 10.1016/j.devcel.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merrill D.C., Karoly M., Chen K., Ferrario C.M., Brosnihan K.B. Angiotensin-(1-7) in normal and preeclamptic pregnancy. Endocrine. 2002;18:239–245. doi: 10.1385/ENDO:18:3:239. [DOI] [PubMed] [Google Scholar]
- 24.Shah D.M. Role of the renin-angiotensin system in the pathogenesis of preeclampsia. Am J Physiol Renal Physiol. 2005;288:F614–F625. doi: 10.1152/ajprenal.00410.2003. [DOI] [PubMed] [Google Scholar]
- 25.Kalra S., Kumar A., Gupta M.K. Modeling of antitubercular activity of biphenyl analogs of 2-nitroimidazo [2, 1-b][1,3] oxazine to rationalize their activity profile. Med. Chem. Res. 2013;22:3444–3451. [Google Scholar]
- 26.Marcus Y., Shefer G., Sasson K., Kohen F., Limor R., Pappo O. Angiotensin 1-7 as means to prevent the metabolic syndrome: lessons from the fructose-fed rat model. Diabetes. 2013;62:1121–1130. doi: 10.2337/db12-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neves L.A., Stovall K., Joyner J., Valdes G., Gallagher P.E., Ferrario C.M. ACE2 and ANG-(1-7) in the rat uterus during early and late gestation. Am J Physiol Regul Integr Comp Physiol. 2008;294:R151–R161. doi: 10.1152/ajpregu.00514.2007. [DOI] [PubMed] [Google Scholar]
- 28.Shefer G., Marcus Y., Knoll E., Dolkart O., Foichtwanger S., Nevo N. Angiotensin 1-7 is a negative modulator of aldosterone secretion in vitro and in vivo. Hypertension. 2016;68:378–384. doi: 10.1161/HYPERTENSIONAHA.116.07088. [DOI] [PubMed] [Google Scholar]
- 29.Song R., Preston G., Yosypiv I.V. Ontogeny of angiotensin-converting enzyme 2. Pediatr. Res. 2012;71:13–19. doi: 10.1038/pr.2011.7. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto K., Ohishi M., Katsuya T., Ito N., Ikushima M., Kaibe M. Deletion of angiotensin-converting enzyme 2 accelerates pressure overload-induced cardiac dysfunction by increasing local angiotensin II. Hypertension. 2006;47:718–726. doi: 10.1161/01.HYP.0000205833.89478.5b. [DOI] [PubMed] [Google Scholar]
- 31.Muldoon K.M., Fowler K.B., Pesch M.H., Schleiss M.R. SARS-CoV-2: is it the newest spark in the TORCH? J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong S.F., Chow K.M., de Swiet M. Severe acute respiratory syndrome and pregnancy. BJOG. 2003;110:641–642. doi: 10.1046/j.1471-0528.2003.03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zambrano LI, Fuentes-Barahona IC, Bejarano-Torres DA, Bustillo C, Gonzales G, Vallecillo-Chinchilla G, et al. A pregnant woman with COVID-19 in Central America. Travel Med. Infect. Dis.. 2020;101639:101639. [DOI] [PMC free article] [PubMed]
- 34.Chokkar N., Kalra S., Chauhan M., Kumar R. A review on quinoline derived scaffolds as anti-hiv agents. Mini Reviews in Medicinal Chemistry. 2019;19:510–526. doi: 10.2174/1389557518666181018163448. [DOI] [PubMed] [Google Scholar]
- 35.Liu D.X., Fung T.S., Chong K.K., Shukla A., Hilgenfeld R. Accessory proteins of SARS-CoV and other coronaviruses. Antivir. Res. 2014;109:97–109. doi: 10.1016/j.antiviral.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masters P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mortola E., Roy P. Efficient assembly and release of SARS coronavirus-like particles by a heterologous expression system. FEBS Lett. 2004;576:174–178. doi: 10.1016/j.febslet.2004.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang C., Zheng X., Gai W., Zhao Y., Wang H., Wang H. MERS-CoV virus-like particles produced in insect cells induce specific humoural and cellular imminity in rhesus macaques. Oncotarget. 2017;8:12686. doi: 10.18632/oncotarget.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Haan C.A., Rottier P.J. Molecular interactions in the assembly of coronaviruses. Adv. Virus Res. 2005;64:165–230. doi: 10.1016/S0065-3527(05)64006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neuman B.W., Kiss G., Kunding A.H., Bhella D., Baksh M.F., Connelly S. A structural analysis of M protein in coronavirus assembly and morphology. J. Struct. Biol. 2011;174:11–22. doi: 10.1016/j.jsb.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tooze J., Tooze S., Warren G. Replication of coronavirus MHV-A59 in sac-cells: determination of the first site of budding of progeny virions. Eur. J. Cell Biol. 1984;33:281–293. [PubMed] [Google Scholar]
- 42.Gallagher T.M., Buchmeier M.J. Coronavirus spike proteins in viral entry and pathogenesis. Virology. 2001;279:371–374. doi: 10.1006/viro.2000.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalra S., Joshi G., Munshi A., Kumar R. Structural insights of cyclin dependent kinases: implications in design of selective inhibitors. Eur. J. Med. Chem. 2017;142:424–458. doi: 10.1016/j.ejmech.2017.08.071. [DOI] [PubMed] [Google Scholar]
- 44.Simmons G., Zmora P., Gierer S., Heurich A., Pöhlmann S. Proteolytic activation of the SARS-coronavirus spike protein: cutting enzymes at the cutting edge of antiviral research. Antivir. Res. 2013;100:605–614. doi: 10.1016/j.antiviral.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belouzard S., Chu V.C., Whittaker G.R. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc. Natl. Acad. Sci. U. S. A. 2009;106:5871–5876. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simmons G., Reeves J.D., Rennekamp A.J., Amberg S.M., Piefer A.J., Bates P. Characterization of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) spike glycoprotein-mediated viral entry. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4240–4245. doi: 10.1073/pnas.0306446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song W., Gui M., Wang X., Xiang Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Path. 2018;14 doi: 10.1371/journal.ppat.1007236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar N., Mishra S.S., Sharma C.S., Singh H.P., Kalra S. In silico binding mechanism prediction of benzimidazole based corticotropin releasing factor-1 receptor antagonists by quantitative structure activity relationship, molecular docking and pharmacokinetic parameters calculation. J. Biomol. Struct. Dyn. 2018;36:1691–1712. doi: 10.1080/07391102.2017.1332688. [DOI] [PubMed] [Google Scholar]
- 49.Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309:1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- 50.Millet J.K., Whittaker G.R. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015;202:120–134. doi: 10.1016/j.virusres.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simmons G., Gosalia D.N., Rennekamp A.J., Reeves J.D., Diamond S.L., Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. U. S. A. 2005;102:11876–11881. doi: 10.1073/pnas.0505577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chhokar N., Kalra S., Chauhan M., Munshi A., Kumar R. Quinoline-based protein–protein interaction inhibitors of LEDGF/p75 and HIV Integrase: an in Silico study. Curr. Top. Med. Chem. 2018;18:2800–2815. doi: 10.2174/1568026619666190208164801. [DOI] [PubMed] [Google Scholar]
- 53.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N. A novel angiotensin-converting enzyme–related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000;87:e1–e9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 55.Zhang H., Wada J., Hida K., Tsuchiyama Y., Hiragushi K., Shikata K. Collectrin, a collecting duct-specific transmembrane glycoprotein, is a novel homolog of ACE2 and is developmentally regulated in embryonic kidneys. J. Biol. Chem. 2001;276:17132–17139. doi: 10.1074/jbc.M006723200. [DOI] [PubMed] [Google Scholar]
- 56.Chappell M.C. Biochemical evaluation of the renin-angiotensin system: the good, bad, and absolute? Am. J. Physiol. Heart Circ. Physiol. 2016;310:H137–H152. doi: 10.1152/ajpheart.00618.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamming I., Cooper M.E., Haagmans B.L., Hooper N.M., Korstanje R., Osterhaus A.D. The emerging role of ACE2 in physiology and disease. J. Pathol. 2007;212:1–11. doi: 10.1002/path.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barnes B.B., Steindorf K., Hein R., Flesch-Janys D., Chang-Claude J. Population attributable risk of invasive postmenopausal breast cancer and breast cancer subtypes for modifiable and non-modifiable risk factors. Cancer Epidemol. 2011;35:345–352. doi: 10.1016/j.canep.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 59.Allawadhi P., Khurana A., Allwadhi S., Joshi K., Packirisamy G., Bharani K.K. Nanoceria as a possible agent for the management of COVID-19. Nano Today. 2020;35:100982. doi: 10.1016/j.nantod.2020.100982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chauhan G., Madou M.J., Kalra S., Chopra V., Ghosh D., Martinez-Chapa S.O. Nanotechnology for COVID-19: therapeutics and vaccine research. ACS Nano. 2020;14:7760–7782. doi: 10.1021/acsnano.0c04006. [DOI] [PubMed] [Google Scholar]
- 61.Mishra S.S., Ranjan S., Sharma C.S., Singh H.P., Kalra S., Kumar N. Computational investigation of potential inhibitors of novel coronavirus 2019 through structure-based virtual screening, molecular dynamics and density functional theory studies. J. Biomol. Struct. Dyn. 2020:1–13. doi: 10.1080/07391102.2020.1791957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Floyd C.N., Ferro A. Mechanisms of aspirin resistance. Pharmacol. Ther. 2014;141:69–78. doi: 10.1016/j.pharmthera.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 63.Hussain A., Bhowmik B., do Vale Moreira N.C. COVID-19 and diabetes: knowledge in progress. Diabetes Res. Clin. Pract. 2020;162:108142. doi: 10.1016/j.diabres.2020.108142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun P., Lu X., Xu C., Sun W., Pan B. Understanding of COVID-19 based on current evidence. J. Med. Virol. 2020;92:548–551. doi: 10.1002/jmv.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valencia D.N. Brief review on COVID-19: the 2020 pandemic caused by SARS-CoV-2. Cureus. 2020;12:e7386. doi: 10.7759/cureus.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England) 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Conti P., Younes A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J. Biol. Regul. Homeost. Agents. 2020:34. doi: 10.23812/Editorial-Conti-3. [DOI] [PubMed] [Google Scholar]
- 68.Hanff T.C., Harhay M.O., Brown T.S., Cohen J.B., Mohareb A.M. Is there an association between COVID-19 mortality and the renin-angiotensin system-a call for epidemiologic investigations. Clin. Infect. Dis. 2020;71:870–874. doi: 10.1093/cid/ciaa329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kumar D., Chauhan G., Kalra S., Kumar B., Gill M.S. A perspective on potential target proteins of COVID-19: comparison with SARS-CoV for designing new small molecules. Bioorg. Chem. 2020:104326. doi: 10.1016/j.bioorg.2020.104326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoelder S., Clarke P.A., Workman P. Discovery of small molecule cancer drugs: successes, challenges and opportunities. Mol. Oncol. 2012;6:155–176. doi: 10.1016/j.molonc.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Curt G.A. Cancer drug development: new targets for Cancer treatment. Oncologist. 1996;1:II–III. [PubMed] [Google Scholar]
- 72.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2015. CA Cancer J. Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 73.Woolf S.H. 2015. How are Income and Wealth Linked to Health and Longevity? [Google Scholar]
- 74.Pringle K.G., Tadros M.A., Callister R.J., Lumbers E.R. The expression and localization of the human placental prorenin/renin-angiotensin system throughout pregnancy: roles in trophoblast invasion and angiogenesis? Placenta. 2011;32:956–962. doi: 10.1016/j.placenta.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 75.Valdés G., Neves L.A., Anton L., Corthorn J., Chacón C., Germain A.M. Distribution of angiotensin-(1-7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta. 2006;27:200–207. doi: 10.1016/j.placenta.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 76.Brosnihan K.B., Neves L.A., Anton L., Joyner J., Valdes G., Merrill D.C. Enhanced expression of Ang-(1-7) during pregnancy. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas. 2004;37:1255–1262. doi: 10.1590/s0100-879x2004000800017. [DOI] [PubMed] [Google Scholar]
- 77.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet (London, England) 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang X., Zhou Z., Zhang J., Zhu F., Tang Y., Shen X. A case of 2019 novel coronavirus in a pregnant woman with preterm delivery. Clin. Infect. Dis. 2020;71:844–846. doi: 10.1093/cid/ciaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu D., Li L., Wu X., Zheng D., Wang J., Yang L. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. Am. J. Roentgenol. 2020:1–6. doi: 10.2214/AJR.20.23072. [DOI] [PubMed] [Google Scholar]
- 80.Schwartz D.A. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med. 2020;144:799–805. doi: 10.5858/arpa.2020-0901-SA. [DOI] [PubMed] [Google Scholar]
- 81.Fan C., Lei D., Fang C., Wang M., Liu Y. Perinatal transmission of COVID-19 associated SARS-CoV-2: should we worry? Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa226. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang S., Guo L., Chen L., Liu W., Cao Y., Zhang J. A case report of neonatal COVID-19 infection in China. Clin. Infect. Dis. 2020;71:853–857. doi: 10.1093/cid/ciaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu H., Wang L., Fang C., Peng S., Zhang L., Chang G. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9:51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lam C.M., Wong S.F., Leung T.N., Chow K.M., Yu W.C., Wong T.Y. A case-controlled study comparing clinical course and outcomes of pregnant and non-pregnant women with severe acute respiratory syndrome. BJOG. 2004;111:771–774. doi: 10.1111/j.1471-0528.2004.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zeng H., Xu C., Fan J., Tang Y., Deng Q., Zhang W. Antibodies in infants born to mothers with COVID-19 pneumonia. J. Am. Med. Assoc. 2020;323:1848–1849. doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu N., Li W., Kang Q., Xiong Z., Wang S., Lin X. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-Centre, descriptive study. Lancet Infect. Dis. 2020;20:559–564. doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zeng L., Xia S., Yuan W., Yan K., Xiao F., Shao J. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020;174:722–725. doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dong L., Tian J., He S., Zhu C., Wang J., Liu C. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. J. Am. Med. Assoc. 2020;323:1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kourtis A.P., Read J.S., Jamieson DjJNEJoM. Pregnancy and infection. N. Engl. J. Med. 2014;370:2211–2218. doi: 10.1056/NEJMra1213566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Warning J.C., McCracken S.A., Morris J.M. A balancing act: mechanisms by which the fetus avoids rejection by the maternal immune system. Reproduction (Cambridge, England) 2011;141:715–724. doi: 10.1530/REP-10-0360. [DOI] [PubMed] [Google Scholar]
- 91.Li N., Han L., Peng M., Lv Y., Ouyang Y., Liu K., Yue L., Li. Q., Sun G., Chen L., Yang L. Maternal and neonatal outcomes of pregnant women with coronavirus disease 2019 (COVID-19) pneumonia: a case-control study. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen Y., Peng H., Wang L., Zhao Y., Zeng L., Gao H. Infants born to mothers with a new coronavirus (COVID-19) Front. Pediatr. 2020;8:104. doi: 10.3389/fped.2020.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dashraath P., Jing Lin Jeslyn W., Mei Xian Karen L., Li Min L., Sarah L., Biswas A. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am. J. Obstet. Gynecol. 2020;222:521–531. doi: 10.1016/j.ajog.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Facchetti F., Bugatti M., Drera E., Tripodo C., Sartori E., Cancila V. SARS-CoV2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of placenta. EBioMedicine. 2020;59 doi: 10.1016/j.ebiom.2020.102951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schwartz D.A., Morotti D., Beigi B., Moshfegh F., Zafaranloo N., Patanè L. Confirming vertical fetal infection with COVID-19: neonatal and pathology criteria for early onset and transplacental transmission of SARS-CoV-2 from infected pregnant mothers. Archives of pathology & laboratory medicine. 2020 doi: 10.5858/arpa.2020-0442-SA. Epub ahead of print. PMID: 32886737. [DOI] [PubMed] [Google Scholar]
- 96.Schwartz D.A., Thomas K.M. Characterizing COVID-19 maternal-fetal transmission and placental infection using comprehensive molecular pathology. EBioMedicine. 2020;60 doi: 10.1016/j.ebiom.2020.102983. [DOI] [PMC free article] [PubMed] [Google Scholar]