Abstract
Simple Summary
Patients diagnosed with endometrial cancer (EC), the most common gynaecological malignancy in women worldwide, cope with a disease associated with poor prognosis and limited treatment options after first-line therapy when it reaches an advanced or metastatic stage. Lately, small-molecule inhibitors have emerged as an alternative targeted therapy, renewing hope in the fight against this disease. The aim of this review is to shed light into the current state and future prospects of small-molecule inhibitors on EC treatment by summarizing the extensive number of clinical trials that have been performed during the last years, and to provide a comprehensive up-to-date document with the most remarkable results. Despite the great effort researchers are making to improve the molecular characterization of tumours, to unravel the underlying mechanism of EC progression, and to increase the efficacy of targeted therapy, we might say that there is still a long way to pave to efficiently treat EC patients.
Abstract
Endometrial cancer (EC) is the sixth most common cancer in women. A continued number of low-risk EC patients at diagnosis, as well as patients diagnosed with advanced-stage disease, will experience an aggressive disease. Unfortunately, those patients will present recurrence or overt dissemination. Systemic cytotoxic chemotherapy treatment on advanced, recurrent, or metastatic EC patients has shown poor results, with median survival rates of less than one year, and median progression-free survival rates of four months. Therefore, the search for innovative and alternative drugs or the development of combinatorial therapies involving new targeted drugs and standard regimens is imperative. Over the last few decades, some small-molecule inhibitors have been introduced in the clinics for cancer treatment, but only a few have been approved by the Food and Drug Administration (FDA) for EC treatment. In the present review, we present the current state and future prospects of small-molecule inhibitors on EC treatment, both alone and in combination.
Keywords: endometrial cancer, small-molecule inhibitor, PI3K/AKT/mTOR, receptor tyrosine kinase, clinical trials
1. Introduction
Endometrial cancer is the most common gynaecological malignancy and the sixth most occurring cancer in women worldwide. In terms of mortality, EC accounts for almost 90,000 deaths worldwide every year [1]. The disease is usually diagnosed in its early stages and associated with good prognosis, but approximately 20% of patients will present regionally extensive disease and 8% of them will have distant metastasis. In addition to tumour spread, measured through the International Federation of Gynaecology and Obstetrics (FIGO) staging system, other prognostic factors include histological type, histological grade, and lymphovascular space invasion (LVSI). Since 1983, EC has been classified using a dualistic classification: Type I or endometrioid subtype, which mostly includes the endometrioid histology and is associated with good prognosis; and Type II or non-endometrioid EC, which includes different minor histologies, such as serous, clear cell, mixed cell adenocarcinoma, carcinosarcomas, and other rare types, and is associated with poor prognosis [2]. In 2013, The Cancer Genome Atlas (TCGA) Research Network proposed a molecular classification that differentiates four groups of ECs: Polymerase ε (POLE) ultramutated, microsatellite stability unstable (MSI) hypermutated, copy-number low (microsatellite stable, MSS), and copy-number high (serous-like) [3]. Most of the endometrioid ECs subtypes that were classified with the dualistic classification are now classified into the POLE ultramutated, MSI hypermutated, and MSS groups, while few p53 (also known as TP53) mutated endometrioid EC and all serous EC tumours are now grouped in the serous-like group. The POLE group is characterized by having an extraordinarily good prognosis, even though it includes some high-grade tumours, and the serous-like group is the one with the worst prognosis [4].
The cornerstone treatment of EC is surgery, complemented with radiotherapy and chemotherapy upon the presence of poor prognostic factors. Unfortunately, for diagnosed women with poor prognostic factors who experience a recurrence, or develop metastatic disease, treatment options are limited. While platinum-based regimens are often administered as adjuvant therapy, this treatment has shown poor results, with median survival rates of less than one year, and median progression-free survival (PFS) rates of four months in this subgroup of patients [5]. Therefore, the search for innovative and alternative drugs or the development of combinatorial therapies involving new targeted drugs and standard regimens is imperative.
Over the last few decades, small-molecule inhibitors (SMIs) have gained acceptance as new therapeutic alternatives in cancer, since they offer a targeted approach to treatment and are expected to have fewer adverse side effects than current chemotherapy regimens. Small-molecule inhibitors are compounds of less than 500 Da in size and are often administered orally. They are developed to target any portion of a molecule, regardless of the target’s cellular location [6]. Indeed, there are SMIs that target both extracellular and intracellular proteins, such as cell surface ligand-binding receptors and anti-apoptotic proteins, among other protein types. Research on small-molecule drugs has been so far successful. Despite several drugs having been introduced in the clinic for cancer treatment, only a few have been approved for EC treatment. The present review aims to compile clinical studies that have been performed in relation to EC to evaluate the effectiveness of SMI, which targeted the phosphatidylinositol-3-kinase/Akt and the mammalian target of rapamycin (PI3K/AKT/mTOR) pathway, receptor tyrosine-kinases (RTKs), DNA damage repair (DDR) and cell cycle progression, DNA-histone modifiers, and immune checkpoints.
2. Small-Molecule Inhibitors for Endometrial Cancer Therapy
2.1. Targeting PI3K/AKT/mTOR Pathway
Increased PI3K/AKT/mTOR molecular pathway activity is seen in many different human cancers, probably because this pathway promotes cellular growth, metabolism, proliferation, survival, migration, apoptosis, and angiogenesis [7]. Among solid tumours, EC presents the highest alteration rate of the PI3K/AKT/mTOR pathway, being altered specifically in 92% of type I and 60% of type II ECs [3]. Usually, PI3K/AKT/mTOR pathway overactivation occurs as a result of overexpression of upstream tyrosine kinase growth factor receptors, phosphatase and tensin homolog (PTEN)loss-of-function (mutated in 67–84% of EC type I and 3–22% of EC type II), amplification or mutation in PI3KCA (38–55% of EC type I and 10–47% of EC type II), PIK3R1 (21–43% of EC type I and 12–17 of % of EC type II), and AKT genes (2% of EC type I) and elevated expression of mTOR (7% of EC type I) [8,9]. Overall, these data underline that the PI3K/AKT/mTOR pathway has a crucial role in EC pathogenesis, despite the slight differences in the frequency and types of alterations found in the two main histological tumour types. PTEN mutations seem to have a critical role in type I EC pathogenesis, while those found in mTOR may be primarily involved in the onset of type II EC.
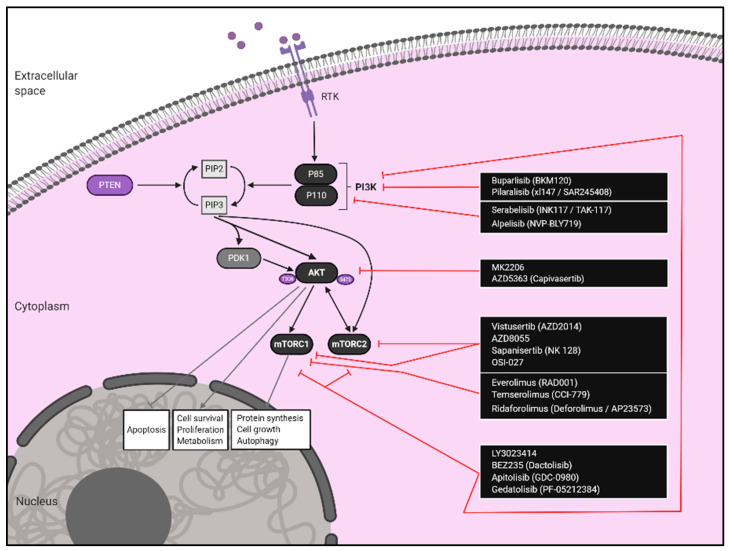
The high percentage of alterations observed in the PI3K/AKT/mTOR pathway in EC encourages the investigation towards the development of specific targeted small inhibitors acting on the three main molecular steps of this pathway (PI3K/AKT/mTOR). In particular, the inhibitors of the PI3K/AKT/mTOR pathway that will be deeply reviewed in this section fall into four main categories: mTOR inhibitors, PI3K inhibitors, AKT inhibitors, and dual mTOR/PI3K inhibitors (Figure 1).
Figure 1.
PI3K/AKT/mTOR signalling pathway SMIs for the treatment of endometrial cancer. Current SMIs under clinical investigation and their target molecules are displayed. Abbreviations: RTK, Receptor Tyrosine Kinase.
2.1.1. mTOR Inhibitors
The first studies in EC using SMI were focused on mTOR and, in particular, on Rapamycin analogues. Rapamycin and other rapalogs are classified as first-generation mTOR inhibitors, which suppress the mTORC1 but not the mTORC2 [10]. Three of these compounds—Everolimus, Temsirolimus, and Ridaforulimus—have completed phase II clinical trials tested as monotherapy in EC and are currently being evaluated in combinational trials.
Everolimus (RAD001), a specific inhibitor of mTORC1, was the first oral mTOR inhibitor studied in EC. A phase II study assessed 28 patients with persistent or recurrent type I endometrial disease. Response was determined by tumour assessments from radiological tests or physical examination using Response Evaluation Criteria in Solid Tumours (RECIST). Complete response (CR) was considered as the disappearance of all target and non-target lesions; partial response (PR) was indicated when target measurable lesions were reduced by at least 30%; progressive disease (PD) was reported when the target lesions increased 20% in size and/or new lesions appeared in the first weeks of treatment; and stable disease (SD) included any condition not meeting the above criteria. In this phase II study, no PR or CR were observed, but 43% showed SD at the first evaluation time (8 weeks) (NCT00087685) [11].
In another phase II trial testing the efficacy of Everolimus as a single agent on advanced or metastatic EC refractory to one or two previous chemotherapy regimens, the results showed that 36% of patients had a non-progressive disease in the first 3 months after treatment, 9% of patients presented PR, and 27% showed SD at 6 months. Still, no CR was observed (NCT00870337) [12]. Better results were obtained by the combination of Everolimus and the aromatase inhibitor Letrozole, which in an open phase II clinical trial obtained 9 CR and 2 PR out of 38 patients enrolled in the study (NCT01068249) [13]. A similar clinical trial, studying the combination of Everolimus, Letrozole, and Metformin, enrolling 62 patients also obtained comparable results. Data showed that patients with advanced EC present 28% PR and 22% SD. Interestingly, women presenting endometrioid histology and catenin (cadherin-associated protein), beta 1 (CTNNB1) mutations had a better response to this combinational therapy (NCT01797523) [13,14]. This could be explained by the fact that the stabilization of β-catenin, derived from mutations in CTNNB1, could result in the activation of mTORC1. Thus, Everolimus treatment would be beneficial for those patients [13].
Everolimus was evaluated with another aromatase inhibitor, Anastrozole, in a phase II trial. The combination showed promising results with a CR in 17% of EC patients. However, extensive research is imperative due to the small number of EC patients (n = 6) enrolled in this trial (NCT01197170) [15].
A phase II trial of intravenous Temsirolimus (CCI-779) in patients with recurrent and/or metastatic EC evaluated both patients who were treated with chemotherapy and patients who were not. Temsirolimus showed 14% of PR and 69% of SD among the chemotherapy-naive group. On the other hand, there were 4% of PR and 48% of SD among patients in the chemotherapy-treated group. PTEN expression, defined by immunochemistry, failed to predict response to this inhibitor (NCT00072176) [16,17]. This modest response encouraged investigators to study Temsirolimus in combination with conventional chemotherapy. A phase II study investigating this showed that Temsirolimus with Paclitaxel and Carboplatin demonstrated good tolerability, but there were no differences in terms of response or SD compared to the historical data of Paclitaxel and Carboplatin treatment (NCT00977574) [18]. In the randomized phase II GOG248 trial, the authors compared intravenous Temsirolimus regimen versus the combination of Temsirolimus plus a hormonal therapy alternating Megestrol Acetate and Tamoxifen. The combination arm was closed early because of an excess of venous thrombosis. However, the accrual of patients to single-agent Temsirolimus therapy continued. The study concluded that patients enrolled in a single Temsirolimus arm presented 28% PR. In the second stage of the study, a total of 50 patients were treated on the same arm; 22% of PR was observed and 26% presented SD (NCT00729586) [19]. Another phase II trial testing the benefit of Temsirolimus in combination with other SMIs, such as Bevacizumab, has been conducted. However, the combinational regimen of Temsirolimus plus Bevacizumab was associated with significant toxicity (NCT00723255) [20].
Results extrapolated from clinical trials on Ridaforolimus (deforolimus, AP23573) in patients with recurrent or persistent EC and disease progression after one or two lines of chemotherapy showed an 11% of PR and 18% of SD (NCT00122343) [21]. By contrast, in another phase II trial, Ridaforolimus was evaluated in 34 patients with recurrent or metastatic EC who did not receive previous systemic chemotherapy as a single agent. The results showed a modest activity of Ridaforolimus, with an observed PR of 9% and 18% SD. As also seen in clinical trials with Temsirolimus, PTEN mutational status did not correlate with PR or SD (NCT00770185) [22]. In the same line, Oza et al. reported on a randomized phase II trial of Ridaforolimus in women with advanced EC who had progressive disease following one or two lines of chemotherapy and no hormonal therapy. The trial compared Ridaforolimus treatment versus progestin or systemic chemotherapy. Ridaforolimus treatment was discontinued as a result of adverse events in 33% of patients compared to 6% in the progestin or chemotherapy group. The authors concluded that although Ridaforolimus showed encouraging activity in advanced EC, its activity was associated with significant toxicity (NCT00739830) [23].
In the last years, dual mTOR inhibitors (known also as second-generation inhibitors) have been developed. These small inhibitors can suppress both the mTORC1 and mTORC2 and their main advantage is the considerable decrease of AKT phosphorylation and better inhibition of mTORC1. Those inhibitors are developed to have an increased therapeutic effect and less resistance [9]. Preclinical studies analysing the effect of second-generation mTOR inhibitors, such as Vistusertib (AZD2014) and AZD8055, have resulted in dose-dependent growth inhibition and apoptosis in a variety of EC cell lines [24,25]. Currently, various second-generation mTOR inhibitors, such as Vistusertib (AZD2014), Sapanisertib (INK 128), and OSI-027, are in early stage clinical trials in EC (NCT02730923, NCT02725268, and NCT02208375).
2.1.2. PI3K Inhibitors
PI3K inhibitors are either pan-PI3K inhibitors (which inhibit all four classes of PI3Ks) or isoform-selective PI3K inhibitors. Buparlisib (BKM120) is an oral pure PI3K inhibitor with broad antitumor activity in preclinical studies and in a phase I clinical trial [26]. Buparlisib was tested in a phase II study including 40 patients with advanced or recurrent EC. Unfortunately, none of them presented an objective response (OR), which is a measure that compiles any response given by the patient, either complete or partial. Moreover, a huge percentage of patients (87%) experienced adverse events. Thus, the clinical trial was stopped before the end of recruitment due to toxicity (NCT01397877) [27]. Pilaralisib (XL147 or SAR245408) is an oral pan-class I PI3K inhibitor. This drug was evaluated as monotherapy in a phase II open-label study that recruited 67 patients with advanced or recurrent EC who had received one or two prior chemotherapy regimens. The results showed that Pilaralisib presents a favourable safety profile, although it has minimal antitumor activity (3% of PR and 37.3% of SD) (NCT01013324) [28]. Pilaralisib was studied in combination with a Paclitaxel and Carboplatin regimen in a phase I clinical trial. The results obtained did not appear to enhance the antitumoral efficacy of Pilaralisib in monotherapy (NCT00756847) [29].
An alternative strategy to pan-PI3K small inhibitors is to specifically target the PI3K p110 catalytic isoform, which, because of its importance and differentiated role, presents the theoretical advantage of a better safety profile. P110α selective inhibitors, such as Serabelisib (INK117 or TAK-117) and Alpelisib (NVP-BLY719), have shown preclinical antitumoral activity in EC cell lines harbouring PTEN and PI3KCA mutations [30]. The first in-human phase Ia study using Apelisib has shown a tolerable safety profile and encouraging preliminary activity in patients since among the three EC patients included in the study, one had CR and another one PR (NCT01219699) [31]. At present, a phase Ib/II study of Serabelisib in combination with Canagliflozin in patients with advanced solid tumours, including EC, is ongoing (NCT04073680).
2.1.3. Dual mTOR/PI3K Inhibitors
An important limitation to the mTOR- and PI3K-independent small inhibitors is that they are likely to present numerous signalling feedback loops. Thus, dual mTOR/PI3K inhibitors are designed to bind the ATP-binding site of both class I PI3Ks and mTORC1/2 and should lead to a more complete suppression of the PI3K/AKT/mTOR pathway. Among the most advanced dual inhibitors, LY3023414 has demonstrated a tolerable safety profile as a single agent in patients with advanced cancers including EC in a phase I clinical trial (NCT01655225) [32]. Additionally, this compound was tested in an open-label phase II study that included 28 patients with advanced EC harbouring activating mutations in the PI3K/AKT/mTOR pathway. The data obtained showed a modest activity of 14.3% of PR and 35.7% of SD (NCT02549989) [33].
BEZ235, also known as Dactolisib, is a potent, highly specific, and selective inhibitor of class I PI3K that has proven great sensitivity in preclinical studies using EC cell lines [34] and reduced tumour growth in EC xenograft models [35]. However, phase I trials administering BEZ235 as monotherapy in patients with advanced solid tumours (including EC) did not observe objective responses (NCT01343498) [36]. Finally, a clinical trial examining BEZ235 in combination with Everolimus showed limited clinical efficacy and tolerance (NCT01508104) [37].
A phase I clinical trial of Apitolisib (GDC-0980) demonstrated favourable pharmacokinetics and evidence in patients with advanced solid tumours (NCT00854152) [38]. Nonetheless, a multicentre, single-arm, and open-label phase II study of Apitolisib in patients with recurrent or persistent EC showed a relative narrow therapeutic index (5% PR) and poor tolerability, especially in diabetic patients (NCT01455493) [39].
Gedatolisib (PF-05212384) is another potent dual PI3K/mTOR inhibitor that has proved effective in two clinical trials in patients with recurrent EC following platinum-containing chemotherapy. Gedatolisib has shown manageable toxicity and was active as a single agent since out of the 38 advanced EC patients enrolled in the phase II study, 16% presented PR and 37% SD (phase I: NCT01347866 phase II: NCT01420081) [39,40]. At the moment, NCT03065062 and NCT02069158 trials have been organized for testing Gedatolisib combined with Palbociclib or systemic chemotherapy in different solid tumours, including EC, although definitive results of these studies are not yet available.
2.1.4. AKT Inhibitors
Few preliminary data are available on the clinical use of SMI targeting AKT in EC. A two-arm PI3KCA mutation stratified phase II trial on MK2206 (allosteric inhibitor of AKT) revealed a high number of toxicities and limited efficacy in recurrent EC patients, regardless of PI3KCA mutation status. Interestingly, modest clinical activity was found in serous histological patients, warranting further studies prompting a histologic-specific expansion (NCT01307631) [41].
AZD5363 (Capivasertib) is a selective inhibitor of AKT that has exhibited good results and those were strongly correlated with the presence of PIK3CA mutations in preclinical EC models [42]. A phase I trial showed that AZD5363 was well-tolerated and achieved robust AKT modulation in EC tumours (NCT01226316) [43]. Moreover, preliminary results from a phase I in recurrent EC patients that evaluated the combination of AZD5363 and Olaparib have shown 50% of PR (NCT02208375) [44]. These results encourage the undertaking of a subsequent phase II trial to evaluate the combination of Olaparib and AZD5363 in recurrent EC.
In addition, other inhibitors that indirectly modulate the PI3K/AKT/mTOR pathway, such as ABTL0812, are under investigation. In particular, ABTL0812 is being studied in a phase II clinical study on recurrent and metastatic EC patients (NCT03366480) after completing a promising phase I study (NCT02201823) that showed a highly safety profile and the second longest SD in an EC patient included in the study [45].
2.2. Targeting Receptor Tyrosine Kinases
RTKs are key actors in the normal physiology of cells, as they play critical roles by regulating a wide spectrum of processes, such as growth, motility, differentiation, and metabolism [46]. All RTKs share a similar protein structure containing an extracellular ligand-binding domain, a single transmembrane helix, and an intracellular domain with a tyrosine kinase domain (TKD) and a carboxyl-terminal tail [47]. Abnormal RTK activation in human cancers is mediated by four principal mechanisms: gain-of-function mutations, genomic amplification, chromosomal rearrangements, and/or autocrine activation [46]. Due to the RTK dysregulation being closely associated with cancer development and progression, a great effort is being made to develop novel molecules able to target RTKs. Many of these RTK inhibitors target the ATP-binding site of the intracellular TKD, impairing downstream signal transduction. In EC, RTKs are frequently mutated (Table 1) [3].
Table 1.
Mutation frequency of the most relevant RTKs in EC. Data obtained from cbioportal website (https://www.cbioportal.org) based on the following datasets: Endometrial Cancer (MSK) [48], Uterine Corpus Endometrial Carcinoma (TCGA, Firehose Legacy), Uterine Corpus Endometrial Carcinoma ([3], Uterine Corpus Endometrial Carcinoma [49].
| RTK | Mutation Frequency |
|---|---|
| FGFR2 | 12.80% |
| KIT | 7.20% |
| MET | 6.80% |
| KDR | 6.30% |
| PDGFRα | 6.00% |
| ERBB2 | 5.10% |
| EGFR | 4.90% |
| PDGFRβ | 4.70% |
| ABL1 | 3.90% |
| BRAF | 3.80% |
| BCR | 3.60% |
| FLT1 | 6.90% |
| FLT4 | 4.90% |
| SRC | 1.40% |
Here, we will analyse novel RTK inhibitors involved in EC treatment, by grouping them in different categories according to the RTKs that they target: SMIs of the epidermal growth factor receptor (EGFR), SMIs of the mitogen-activated protein kinase (MAPK) pathway, pan-TK inhibitors targeting the fibroblast growth factor receptor (FGFR), pan-TK inhibitors targeting angiogenesis (vascular endothelial growth factor receptor (VEGFR)), and pan-TK inhibitors targeting other RTKs.
2.2.1. EGFR Inhibitors
Afatinib is a potent, selective, and irreversible ErbB family blocker that covalently binds to the TKD of the receptor and blocks signalling from all ErbB family members, causing tumour growth inhibition and regression [50]. Many preclinical studies showed the efficacy of Afatinib treatment towards epidermal growth factor receptor 2 (HER2)-amplified uterine serous carcinoma (USC) models, resulting in the abrogation of cell survival, inhibition of HER2/neu autophosphorylation, and pathway blockage [48,49]. However, there are few reports showing Afatinib efficacy within EC patients. A case report showed the clinical benefit of Afatinib treatment in an endometrioid EC stage IIIC patient refractory to multiple lines of chemotherapy [51]; similarly, an ongoing phase II clinical trial (NCT02491099), which is estimated to be concluded by 2023, is also evaluating PFS on HER2-positive USC patients.
Erlotinib reversibly binds to EGFR TKD, specifically at the adenosine triphosphate (ATP)-binding site [52]. Oza et al. designed a multinomial two-stage phase II study (NCT00030485) to evaluate the activity of Erlotinib in recurrent or metastatic advanced EC women, who were chemotherapy naive and had received up to first-line hormonal therapy. Even though treatment was well tolerated, they observed a modest response rate, 12.5%, with 46.9% of the patients showing SD. Of note, from the 32 patients enrolled, only 19 were EGFR positive; thus, it was not possible to associate Erlotinib’s response to EGFR mutation or amplification [53].
Gefitinib acts similarly to Erlotinib by selectively blocking the ATP-binding site of EGFR TKD. Results from a phase II clinical trial (NCT00027690) evaluating the efficacy and safety of Gefitinib in patients with persistent/recurrent EC showed that treatment was well tolerated but lacked sufficient efficacy. From the 26 recruited patients, only 4 (15%) experienced PFS ≥ 6 months, and 1 (3.8%) had a CR [54].
Lapatinib is an HER2 and EGFR TK inhibitor, which binds to TKD, preventing receptor autophosphorylation upon ligand binding [55]. Chu et al. performed a phase I open-label study to determine the safety, optimally tolerated regimen (OTR), pharmacokinetics, and clinical activity of Lapatinib in combination with Letrozole in patients with advanced breast cancer and other solid malignancies, including two patients with EC. One of the EC patients experienced a PR after dual treatment that lasted 161 days [56]. Finally, the Gynaecologic Oncology Group performed a phase II trial (NCT00096447) to evaluate the efficacy and safety of the Lapatinib in persistent/recurrent EC and associated it to EGFR mutations. They found an insufficient overall clinical response with only 3 out of 31 patients having a PFS ≥ 6 months, 3.2% PR, 22.5% SD, and 67.7% PD. Interestingly, they were able to identify EGFR mutations, L688F, K754E, and E690K, with only E690K being associated with a Lapatinib-responder patient [57].
2.2.2. MAPK Inhibitors
Selumetinib is a non-ATP-competitive MAPK kinase 1 and 2 (MEK-1 and MEK-2) inhibitor [55,56]. A phase II single-arm open-label study (NCT01011933) demonstrated that Selumetinib was well tolerated for women with recurrent EC but lacked efficacy [58].
2.2.3. Pan-RTK Inhibitors Targeting FGFR
Dovitinib is a potent multiple-RTK inhibitor that targets the FGF, VEGF, and PDGF pathways. A multicentre, open-label, non-randomized phase II trial (NCT01379534) focused on evaluating the safety and efficacy of Dovitinib as a second-line treatment in patients with advanced and/or metastatic EC, and revealed that 32% of the FGFR2-mutated patients presented a PFS of 18 weeks and median overall survival (OS) of 20 months compared to 29% PFS and 9 months OS observed in FGFR2 non-mutated patients [59]. Another multi-targeted TK inhibitor is Brivanib, which targets VEGFR and FGFR. Brivanib was evaluated in recurrent or persistent EC and was well tolerated. However, only 18.6% of recruited patients had PR or CR in the phase II trial (NCT00888173) [60]. In this study, the authors suggested that apparently endometrioid and mixed epithelial subtypes would benefit from Brivanib therapy compared to serous histology. Lastly, Ponatinib is a novel multi-target TK inhibitor, particularly effective against T315I mutation of ABL1, but not restricted since it also inhibits VEGFR, PDGFR, FGFR, EPH receptors, and SRC kinases members, among others. This drug is currently being tested in a clinical trial (NCT01888562) focused on recurrent or persistent EC carrying FGFR mutations.
2.2.4. Pan-RTK Inhibitors Targeting Angiogenesis (VEGFR)
Cabozantinib is a non-specific TK inhibitor, acting on VEGFR-1, -2, and -3; KIT; and other kinases [61]. Cabozantinib was evaluated in a single-arm phase II clinical trial (NCT01935934) to test its efficacy and toxicity as a second-line treatment in women with advanced EC. They observed activity of Cabozantinib in both serous and endometrioid EC patients, with 6-month PFS rates of 29% to 40%, a response rate of 12% to 14%, respectively, and acceptable toxicity (17%). Nonetheless, most patients discontinued therapy due to disease progression [62]. Currently, two ongoing additional clinical trials are testing Cabozantinib in EC. The first one is a randomized phase II study (NCT03367741) in which patients with advanced, recurrent, or metastatic EC undergo Cabozantinib treatment in combination with the immunotherapeutic Nivolumab. The second is a phase Ib dose-escalation study (NCT03170960) using single or combined treatment of Cabozantinib and the immunotherapeutic Atezolizumab in patients with locally advanced or metastatic solid tumours, including EC. At present, no results have been reported yet for either of the clinical trials.
Cediranib is an orally available, highly potent, and selective VEGF signalling inhibitor of all members of the receptor [63]. A phase II study (NCT01132820) in 48 recurrent/persistent EC demonstrated that Cediranib administered as a single agent had 12.5% PR, a median PFS of 3.65 months, and median OS of 12.5 months [64]. Unfortunately, almost 30% of the patients discontinued treatment due to toxicity. Another phase II trial (NCT03660826) studied the effect of the combination of Olaparib and Cediranib in patients with recurrent, refractory, or metastatic EC. Similarly, another three-arm randomized phase II clinical trial (NCT03570437) is being performed to understand if the same combination represents a better therapeutic strategy to Paclitaxel in advanced-EC patients.
Nintedanib is another SMI, competitive, triple angiokinase inhibitor that targets multiple RTKs, including PDGFR-α and -β, FGFR1,2,3, VEGFR1,2,3, and FLT3, by binding to the ATP-binding pocket and inhibiting their activity [62,63]. A phase II clinical trial (NCT01225887) in 32 advanced, recurrent, or metastatic EC patients reported that none of them had a CR and only 3 (9.3%) patients showed PR [65]. The effectiveness of Nintedanib in combination with conventional chemotherapy is also being evaluated in a randomized phase II study (NCT02730416), which focuses on patients with primary advanced stage, or with the first relapse of EC. No data is available till now.
Sunitinib is a small-molecule multi-targeted RTK inhibitor with activity against PDGFR, VEGFR, and KIT, among others. In this regard, Castonguay et al. reported a modest activity of Sunitinib in women with recurrent EC that were recruited in a phase II study (NCT00478426) to assess the efficacy and tolerability of Sunitinib. In this study, 30% of patients showed a 6-month PFS and 21% a 12-month PFS [66]. The authors failed to identify clinico-pathological parameters linked to patients who would benefit from this therapy.
The RTK inhibitor Lenvatinib targets kinases implicated in pathogenic angiogenesis, tumour growth, and cancer progression, such as VEGFR1,2,3, PDGFR-α, FGFR1,2,3,4, KIT, and RET [67]. There are many ongoing clinical trials testing Lenvatinib in EC. Vergote et al. reported the results from a single-arm phase II study (NCT01111461) that aimed to evaluate the efficacy and safety of Lenvatinib in patients with recurrent or advanced EC after the failure of first-line platinum-based therapy. They observed that the treatment had a modest antitumor activity as second-line therapy in this cohort, with 21% of patients having an objective response, lasting 7.2–8 months, and a median PFS of 5.6 months and OS of 10.6 months [68]. On the other hand, Makker et al. designed a single-arm, phase Ib/II study (NCT02501096) to determine the maximum tolerated dose (MTD) for Lenvatinib in combination with Pembrolizumab and subsequently the safety and efficacy of the combination in solid tumours. They reported that the combinatory treatment showed antitumour activity in those patients with advanced recurrent EC, with an objective response in 35.5% (16/45) of patients with microsatellite-stable tumours [69]. As a result of these findings, they moved forward to a phase III clinical trial (NCT03517449) to evaluate Lenvatinib plus Pembrolizumab treatment in comparison to Doxorubicin or Paclitaxel in patients with advanced EC. This study is expected to conclude in 2022. In parallel, another two studies are ongoing: A randomized phase II trial (NCT03005015) that evaluates Lenvatinib compared to Doxorubicin, and NCT03884101, which compares the efficacy of Lenvatinib plus Pembrolizumab with respect to chemotherapy (Carboplatin-Paclitaxel), both in advanced and recurrent EC.
2.2.5. Pan-RTK Inhibitors of Other Targets
Dasatinib is an oral dual BCR/ABL and Src family TK inhibitor with activity against c-KIT, EPHA2, and PDGFRβ [70]. Few studies are reported on Dasatinib administration in EC. Duska et al. designed a phase 0 trial (NCT01482728) to study drug- and dose-specific changes in Src levels and activity, by analysing the tumour, normal adjacent tissue, and blood from newly diagnosed EC patients previous to surgery. They found that Dasatinib entered the solid tumour tissue and modulated Src activity. Similarly, they demonstrated that Dasatinib concentrations were higher in tumour tissue than in adjacent normal tissue, providing for the first time an insight into the biological effects and potential therapeutic efficacy of Dasatinib in EC [71]. Nowadays, there is an ongoing phase I trial (NCT01440998), which aims to evaluate Dasatinib, Paclitaxel, and Carboplatin treatment in patients with stage III-IV or recurrent EC; there is also a phase II clinical trial (NCT02059265) on patients with recurrent or persistent ovarian, fallopian tube, endometrial, and peritoneal cancers.
2.3. Targeting DNA Damage Repair and Cell Cycle Progression
Compromised DDR pathways are found in EC, resulting in uncontrolled tumour cell proliferation and accumulation of genomic alterations [72]. Moreover, around 15% of serous-like EC display homologous recombinational repair-deficient (HRR-deficient) genomic signatures [73] and loss of function of mismatch repair pathways are present in 28.6% of low-grade and 54.3% of high-grade endometrioid EC [74], suggesting that these patient populations may be good candidates for DDR small inhibitor therapies.
2.3.1. Poly ADP Ribose Polymerase (PARP) Inhibitors (PARPi)
As PARPi have emerged as a novel therapy for advanced cancers [75], several clinical trials are currently evaluating the use of Olaparib, Niraparib, or Rucaparib, the three principal PARPi, in EC patients. Among others, Olaparib was evaluated in combination with cediranib and durvalumab in a phase I study. Promising results were obtained with 44% of PR and 33% of SD (NCT02484404) [76]. Nowadays, there are several active phase I/II clinical trials evaluating the use of PARPi in combination with other therapies, such as PI3K, PD-1, and VEGF inhibitors (NCT02208375, NCT01237067, NCT03572478, and NCT03476798), and some phase II clinical trials are recruiting patients to evaluate the responses to PARPi as monotherapy or in combination with other inhibitors, such as PI3K, PD-L1, histone deacetylases (HDACs), or ATR inhibitors (NCT04171700, NCT03016338, NCT03586661, NCT03951415, NCT03924245, and NCT04065269).
2.3.2. New DDR Inhibitors (DDRi)
Recently, new DDRi are being developed against protein kinases that respond to different types of DNA damage and regulate specific cell-cycle transitions (ATM, ATR, DN-PKcs, CHK1, CHK2, and WEE1) [77]. In EC, the DNA-PK inhibitor LY3023414 was evaluated in a phase I clinical trial and demonstrated to be safe in patients with advanced cancer. A durable PR lasting 18 months was observed in a woman with EC (NCT01655225) [32]. Currently, a phase II study is ongoing to determine the effectiveness of this compound in patients with recurrent or persistent EC (NCT02549989). Another phase II study is also ongoing to test the efficacy of the ATR inhibitor AZD6738 and Olaparib in patients with EC (NCT04065269).
2.3.3. Cyclin-Dependent Kinase Inhibitors (CDKi)
Cell-cycle regulators play important roles in EC proliferation and many of these are frequently mutated, such as p53, or have altered expression patterns, such as the loss of p16 expression or cyclins A, D, and E overexpression [74]. Thus, SMIs targeting cyclin-dependent kinase (CDK) activity offer an opportunity for therapeutic intervention in these EC subgroups, since CDKi recapitulate the effects of checkpoint activation [78]. Ongoing studies are evaluating the activity of a third-generation CDKi in EC. The majority of these are active phase I or II clinical trials, which study the combination of endocrine therapies with one of the three main CDKi, Palbociclib, Abemaciclib, or Ribociclib, which inhibit CDK4/6 (NCT02657928, NCT02730429, NCT04188548, NCT03643510, NCT03675893, NCT04049227, and NCT04393285). Moreover, there are other active clinical trials in phases I and II that assess the combination of these CDKi with PI3K inhibitors (NCT03065062 and NCT03008408).
2.4. Targeting DNA and Histone Modifiers
Epigenetic regulation by covalent post-translational histone or DNA modifications, such as acetylation and methylation, represent a reversible step that regulates many biological processes by gene expression regulation [79]. Several studies have identified DNA promoter hypermethylation in several tumour suppressor genes [77,78], altered histone methyltransferase activity [80], and high levels of HDACs [81] in advanced stages of EC. Thus, histone inhibitors and DNA modifiers might be promising therapeutic agents in EC.
Among different drugs, the HDAC inhibitor (HDACi) Entinostat (MS-275) completed a phase I clinical trial that evaluated the toxicity and efficacy in patients with EC, defining an optimal dose of a schedule of every 14 days for a future phase II study (NCT00020579) [82]. Currently, several active clinical trials are evaluating the efficacy of this drug in combination with other agents in EC patients (NCT03018249 and NCT03924245). An ongoing phase I/II clinical trial is evaluating the safety and efficacy of Tinostamustine (EDO-S101) HDACi in women with EC (NCT03345485).
2.5. Targeting Immune Checkpoints
In order to escape from the immune system, EC cells can stimulate immune checkpoints by activating negative feedback mechanisms [83]. Currently, several active clinical trials are evaluating the efficacy of immune checkpoints inhibitors in EC, although none of them are completed yet. There are different active clinical trials with IDO1 inhibitors as a monotherapy (NCT03896113) or in combination with monoclonal antibody immunotherapies (NCT03277352, NCT02178722, and NCT04106414). Others phase I and II clinical trials are currently recruiting patients to evaluate the efficacy of PDL-1 inhibitors, arginase inhibitors, or agonists of adenosine receptors in combination with chemotherapy or immunotherapies (NCT04152018, NCT03314935, NCT03629756, NCT03454451, and NCT03671811); the results of these studies are eagerly awaited.
3. Conclusions
Despite the advances in the early detection and surgical management of EC patients, a considerable number of metastatic and recurrent endometrial tumours are still diagnosed. Furthermore, women with advanced disease have few treatment options and poor prognosis. The combination of platinum salts (Cisplatin and Carboplatin) is the standard regimen for treating these patients. However, platinum salt regimens have not obtained satisfactory results in recurrent or persistent EC. Therefore, the search for alternative new drugs for patients with relapsed and metastatic EC is imperative. Over the past three decades, the success of SMIs over conventional chemotherapy has been clearly proven in different types of cancer. Small-molecule inhibitors overcome the limitations of traditional cancer therapies by targeting specific molecules dysregulated in cancer cells. SMIs successfully target membrane receptors and extracellular proteins and can also translocate through plasma membranes to impair intracellular proteins’ function turning treatment more specifically than conventional chemotherapy. Even though they have wide-spectrum activity, SMIs still present certain limitations regarding toxicity. Some of the most reported toxicities include hypoglycaemia, cardiac toxicity, immunosuppression, pneumonitis, cutaneous reactions, nausea, diarrhoea, colitis, and hepatotoxic effects. However, the benefits of SMI targeted therapy widely exceeds its drawbacks (completed clinical trials summarized in Table 2).
Table 2.
Completed clinical trials of SMIs in EC.
| Drug | Target | Phase | ClinicalTrials.gov Identifier | n | Patient Inclusion | Monotherapy or Combination Therapy | Previous Chemotherapy | CR (%) | PR (%) | SD (%) | Toxicity (as % of Patients Affected by Serious Adverse Events) |
Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PI3K/AKT/mTOR SMI | ||||||||||||
| Everolimus | mTORC1 | II | NCT00087685 | 28 | Recurrent EEC | Monotherapy | Yes | 0 | 0 | 43 | 71.4 | [11] |
| II | NCT00870337 | 44 | Metastatic EC | Monotherapy | Yes | 0 | 9 | 27 | 45 | [12]] | ||
| II | NCT01068249 | 35 | Recurrent EC | Combination (Letrozole) | Yes | 26 | 6 | 9 | n/a | [13] | ||
| II | NCT01797523 | 54 | EEC | Combination (Letrozole and Metformin) | Yes | 0 | 28 | 22 | n/a | [14] | ||
| II | NCT01197170 | 50 | Advanced or metastatic EC | Combination (Anastrozole) | Yes | 4 | 6 | 24 | n/a | [15] | ||
| Temsirolimus | mTORC1 | II | NCT00072176 | 60 (33 A; 27 B) | Advanced or metastatic EC | Monotherapy | No (A)/ Yes (B) |
0 | 14 (A); 4 (B) | 69 (A); 48 (B) | 33 | [16] |
| II | NCT00977574 | 115 | Advanced or recurrent EC | Combination (Paclitaxel and Carboplatin) | No | n/a | n/a | n/a | 50.4 | [18] | ||
| II | NCT00729586 | 50 | Advanced EC | Monotherapy (A)/Combination (Megestrol Acetate and Tamoxifen) (B) | Yes (58%)/ No (42%) |
4.8 (A); 0 (B) | 14.3 (A); 20 (B) | 61.9 (A); 70 (B) | 36 (A); 61.9 (B) | [19] | ||
| II | NCT00723255 | 49 | Recurrent EC | Combination (Bevacizumab) | Yes | 2 | 22.4 | 46.9 | 63.3 | [20] | ||
| Ridaforolimus | mTORC1 | II | NCT00122343 | 45 | Recurrent EC | Monotherapy | Yes | 0 | 11 | 18 | 51 | [21] |
| II | NCT00770185 | 31 | Recurrent or metastatic EC | Monotherapy | No | 0 | 8.8 | 52.9 | n/a | [22] | ||
| II | NCT00739830 | 130 | Recurrent or metastatic EC | Monotherapy | Yes | 0 | 0 | 35 | n/a | [23] | ||
| Buparlisib | PI3K | II | NCT01397877 | 16 | Advanced or metastatic EC | Monotherapy | Yes | 0 | 0 | 25 | 75 | [27] |
| Pilaralisib | PI3K | II | NCT01013324 | 67 | EC | Monotherapy | Yes | 3 | 3 | 37.3 | n/a | [28] |
| I | NCT00756847 | 52 | Advanced EC | Combination (Paclitaxel and Carboplatin) | Yes | 0 | 13.5 | 42.3 | 48.3 | [29] | ||
| Alpelisib | PI3K | IA | NCT01219699 | 134 | Advanced EC | Monotherapy | Yes | 0.9 | 6 | 52 | n/a | [31] |
| LY3023414 | mTOR/PI3K | I | NCT01655225 | 38 | Advanced or metastatic EC | Monotherapy | Yes | 0 | 2,6 | 31.9 | n/a | [32] |
| II | NCT02549989 | 28 | Advanced EC | Monotherapy | Yes | 0 | 14.3 | 35.7 | n/a | [33] | ||
| BEZ235 | mTOR/PI3K | I | NCT01343498 | 33 | Advanced EC | Monotherapy | Yes | 0 | 0 | 45 | 39 | [36] |
| IB | NCT01508104 | 19 | Advanced or metastatic EC | Combination (Everolimus) | Yes | 0 | 0 | 9 | n/a | [37] | ||
| Apitolisib | mTOR/PI3K | I | NCT00854152 | 56 | Advanced EC | Monotherapy | Yes | 0 | 7 | 77 | n/a | [38] |
| II | NCT01455493 | 56 | Recurrent EC | Monotherapy | Yes | 3.6 | 1.8 | 49.1 | n/a | [39] | ||
| Gedatolisib | mTOR/PI3K | I | NCT01347866 | 81 | Advanced or metastatic EC | Combination (Irinotecan (A)/PD-0325901 (B)) | Yes | 0 | 4.5 (A); 11(B) | n/a | 29 | [40] |
| II | NCT01420081 | 38 | Recurrent EC | Monotherapy | Yes | 0 | 16 | 37 | 40 | [84] | ||
| MK2206 | AKT | II | NCT01307631 | 5 | High-grade EC | Monotherapy | Yes | 0 | 0 | 80 | 37.8 | [41] |
| Capivasertib | AKT | I | NCT01226316 | 90 | Advanced EC | Monotherapy | Yes | 0 | 0 | 7 | 62 | [43] |
| Receptor Tyrosine Kinase SMI | ||||||||||||
| Selumetinib | MEK1,2 | II | NCT01011933 | 54 | EC | Monotherapy | Yes | 2 | 4 | 26 | 64 | [58] |
| Erdafitinib | FGFR1,2,3,4, RET, CSF1, PDGFRα,β, KIT, VEGFR2 | I | NCT01703481 | 188 | EC | Monotherapy | Yes | --- | 11 | 16 | 64 | [85] |
| Dabrafenib | BRAF, RAF1, SIK1, NEK11, LIMK1 | I | NCT01954043 | 23 | EC | Combination (Rabeprazole and Rifampin) | Yes | n/a | n/a | n/a | n/a | * |
| Anlotinib | VEGFR2,3 | I/II | NCT02558348 | 12 | EC | Monotherapy | Yes | n/a | n/a | n/a | n/a | * |
| Brivanib Alaninate | VEGFR2, FGFR2 | II | NCT00888173 | 43 | EC | Monotherapy | Yes | 2.3 | 16.3 | n/a | 41.9 | [60] |
| Cediranib Maleate | VEGFR2 | II | NCT01132820 | 48 | EC | Monotherapy | Yes | n/a | 12.5 | n/a | 41.7 | [64] |
| Dasatinib | PDGFR, SRC, EPH, BCR, ABL | I | NCT01440998 | 18 | EC | Combination (Carboplatin and Paclitaxel) | No | n/a | n/a | n/a | n/a | * |
| I | NCT01482728 | 12 | EC | Monotherapy | No | n/a | n/a | n/a | n/a | [71] | ||
| Erlotinib hydrochloride | EGFR | II | NCT00030485 | 32 | EC | Monotherapy | Yes | 0 | 12.5 | 46.8 | 87.9 | [53] |
| Gefitinib | EGFR | II | NCT00027690 | 56 | Recurrent EC | Monotherapy | Yes | 3.8 | n/a | 26.9 | 73 | [54] |
| Lapatinib ditosylate | EGFR, ERBB2 | II | NCT00096447 | 30 | EC | Monotherapy | Yes | 0 | 3.3 | 23.3 | 33.3 | [57] |
| Lenvatinib | VEGFR1,2,3, PDGFRα, FGFR1,2,3,4, KIT, RET | II | NCT01111461 | 133 | EC | Monotherapy | Yes | 1.5 | 19.5 | 23.3 | 49 | [68] |
| Nintedanib | VEGFR1,2,3, PDGFRα,β, FGFR1,2,3, SRC | II | NCT01225887 | 32 | EC | Monotherapy | Yes | 0 | 9.4 | 21.9 | 43.7 | [65] |
| Perifosine | MAPK1, PRKCA | II | NCT00053794 | 17 | EC | Monotherapy | Yes | n/a | n/a | n/a | n/a | [86] |
| Vatalanib | VEGFR1,2,3 | I | NCT00268918 | 24 | EC | Combination (Docetaxel) | Yes | n/a | n/a | n/a | n/a | * |
| Sunitinib Malate | VEGFR1,2,3, PDGFRα,β, KIT | II | NCT00478426 | 34 | EC | Monotherapy | Yes | 0 | 18.2 | 18.2 | 90.9 | [66] |
| II | NCT00474994 | 53 | EC | Monotherapy | Yes | 0 | 2.1 | 43.7 | 17 | * | ||
| Dovitinib | FGF, VEGF, PDGF | II | NCT01379534 | 53 | Advanced EC | Monotherapy | Yes | 0 | 11.3 | 45.3 | 56.6 | [59] |
| Trametinib | MEK1,2 | I | NCT01138085 | 240 | EC | Monotherapy | Yes | n/a | n/a | n/a | n/a | * |
| DNA Damage Repair SMI | ||||||||||||
| Olaparib | PARP | I | NCT02484404 | 9 | EC | Monotherapy | Yes | n/a | 44 | 33 | n/a | [76] |
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; NM, not mentioned; n/a, not applicable; EC, endometrial cancer; EEC, endometrioid endometrial cancer; n, total amount of patients included to the clinical trial. *: Completed clinical trial with unpublished results. Data available at www.clinicaltrials.gov.
Cancer treatment with targeted therapies, such as SMIs, requires a detailed knowledge of tumour biology. Undoubtedly, in the last few years, our knowledge of EC biology has brought on the recognition of molecular pathways involved in endometrial tumorigenesis, leading to the identification of molecular targets for novel treatment strategies. In spite of it, as inferred from the studies presented and summarized above, there is no data showing irrefutable improvement of survival rates yet. The results could be explained by the fact that targeted therapies were generally administered as monotherapy, in over-treated patients, and there were few stratifications based on histology, and/or treatment-response biomarkers.
Challenges for the future are diverse and complex, and concern primarily the identification of new pathways, the early identification of resistance, and the use of SMIs with old and new chemotherapeutic and targeted agents. Combinatory treatment emerged as a feasible alternative for EC patients. The advantages of using a combinatory treatment based on SMIs and conventional chemotherapy, radiation therapy or any other agents can be promising in the management of EC patients by directly hitting altered pathways of tumour cells, potentially reducing adverse side effects caused by high or long-lasting doses of chemotherapy/radiotherapy. Further studies must be performed to exploit this strategy. However, at present, we have excellent results with targeted immunotherapy, although still only in other tumour types; therefore, it would be desirable to test these new immuno-drugs in recurrent/persistent EC, in combination with SMIs. Currently, several studies are ongoing (summarized in Table 3) and results in the next years are highly anticipated.
Table 3.
Ongoing clinical trials of SMIs in EC.
| Drug | Target | Phase | ClinicalTrials.gov Identifier | n | Patient Inclusion | Monotherapy or Combination Therapy | Expected Results |
|---|---|---|---|---|---|---|---|
| PI3K/AKT/mTOR SMI | |||||||
| Vistusertib | mTOR | I/II | NCT02730923 | 72 | Advanced EC | Combination (Anastrozole) | June 2020 |
| Ib | NCT02208375 | 159 | Recurrent EC | Combination (Olaparib) | November 2021 | ||
| Sapanisertib | mTOR | II | NCT02725268 | 241 | Advanced or recurrent EC | Combination (Paclitaxel or MLN1117) | October 2020 |
| Serabelisib | PI3K | Ib/II | NCT04073680 | 60 | Advanced EC | Combination (Canagliflozin) | December 2021 |
| Gedatolisib | mTOR/PI3K | I | NCT03065062 | 96 | Recurrent or metastatic EC | Combination (Palbociclib) | January 2023 |
| AZD5363 | AKT | Ib | NCT02208375 | 159 | Recurrent EC | Combination (Olaparib) | November 2021 |
| Receptor Tyrosine Kinase SMI | |||||||
| Afatinib, Adavosertib, Binimetinib, Capivasertib, Copanlisib, Copanlisib Hydrochloride, Crizotinib, Dabrafenib, Dasatinib, Defactinib, Erdafitinib, Ipatasertib, Larotrectinib, Nivolumab, Osimertinib, Palbociclib, Pertuzumab, GSK2636771, Sapanisertib, Sunitinib Malate, Taselisib, Trametinib, Trastuzumab, Ulixertinib and Vismodegib | EGFR | II | NCT02465060 | 6452 | Advanced or recurrent EC | Monotherapy | June 2022 |
| Anlotinib | VEGFR2-3 | I/II | NCT02584478 | 48 | Recurrent or metastatic EC | Combination (Carboplatin and Paclitaxel) | December 2020 |
| I/II | NCT04157491 | 23 | Recurrent or metastatic EC | Combination (Anlotinib) | December 2022 | ||
| BDTX-189 | EGFR, ERBB2 | I/II | NCT04209465 | 184 | Advanced EC | Monotherapy | December 2023 |
| Cabozantinib | MET, VEGFR2, RET | I/II | NCT03170960 | 1732 | Advanced or metastatic EC | Combination (Atezolizumab) | December 2021 |
| Cediranib | VEGFR2 | II | NCT03570437 | 129 | Advanced EC | Combination (Paclitaxcel and Olaparib) | September 2021 |
| CPL304110 | FGFR | I | NCT04149691 | 42 | Advanced EC | Monotherapy | July 2020 |
| Famitinib | EGFR, ERBB2, FLT1/3 | II | NCT03827837 | 265 | Advanced EC | Combination (Camrelizumab) | June 2021 |
| Pemigatinib | FGFR1,2,3,4 | I/II | NCT02393248 | 325 | Advanced EC | Combination (Gemcitabine, Cisplatin, Pembrolizumab, Docetaxel, Trastuzumab and Retifanlimab) | December 2020 |
| Rebastinib | SRC, LYN, FGR, HCK, KDR, FLT3, Tie-2, BCR, Abl1 | I/II | NCT03601897 | 201 | Advanced or metastatic EC | Combination (Paclitaxel) | November 2021 |
| Afatinib | ERBB2 | II | NCT02491099 | 50 | Recurrent EC | Monotherapy | July 2028 |
| Cabozantinib S-malate | MET, VEGFR2, RET, | II | NCT01935934 | 102 | Recurrent or metastatic EC | Monotherapy | September 2020 |
| Cediranib | VEGFR2 | I | NCT01065662 | 50 | Advanced EC | Combination (Temsirolimus) | April 2020 |
| Dasatinib | PDGFR, SRC, EPH, BCR, ABL | II | NCT02059265 | 35 | Recurrent EC | Monotherapy | November 2020 |
| Lenvatinib Mesylate | VEGFR1,2,3, PDGFRα, FGFR1,2,3,4, KIT, RET | I | NCT02788708 | 26 | Recurrent EC | Combination (Paclitaxel) | November 2020 |
| Lenvatinib | VEGFR1,2,3, PDGFRa, FGFR1,2,3,4, KIT, RET | I | NCT03006887 | 6 | EC | Combination (Pembrolizumab) | April 2020 |
| I/II | NCT02501096 | 357 | EC | Combination (Pembrolizumab) | April 2020 | ||
| III | NCT03884101 | 720 | Recurrent EC | Combination (Pembrolizumab, Paclitaxel and Carboplatin) | April 2023 | ||
| III | NCT03517449 | 780 | Advanced EC | Combination (Pembrolizumab, Paclitaxel and Doxorubicin) | January 2023 | ||
| Nintedanib | VEGFR1,2,3, PDGFRα,β, FGFR1,2,3, SRC | II | NCT02730416 | 148 | Advanced EC | Combination (Carboplatin and Paclitaxel) | July 2022 |
| Axitinib | VEGFR1,2,3 | II | NCT04197219 | 26 | Recurrent EC | Combination (Pembrolizumab) | December 2026 |
| Crizotinib | MET, ALK | II | NCT04030429 | 40 | Recurrent or metastatic EC | Monotherapy | December 2023 |
| Lapatinib | EGFR, ERBB2, TUBB3 | I | NCT01454479 | 24 | Recurrent EC | Combination (Ixempra) | April 2021 |
| DNA Damage Repair and Cell Cycle Progression SMI | |||||||
| Olaparib | PARP | I/II | NCT02208375 | 159 | Recurrent EC | Combination (Capivasertib and Vistusertib) | November 2021 |
| I | NCT01237067 | 77 | Recurrent EC | Combination (Carboplatin) | December 2020 | ||
| Rucaparib | PARP | I/II | NCT03572478 | 12 | Recurrent or metastatic EC | Combination (Nivolumab) | December 2021 |
| II | NCT03476798 | 33 | Recurrent EC | Combination (Bevacizumab) | February 2023 | ||
| II | NCT04171700 | 220 | EC | Monotherapy | May 2022 | ||
| Niraparib | PARP | II | NCT03016338 | 44 | Recurrent EC | Monotherapy and combination (TSR-042) | December 2022 |
| I | NCT03586661 | 44 | Recurrent EC | Combination (Copanlisib) | April 2022 | ||
| Olaparib | PARP | II | NCT03951415 | 55 | Advanced, recurrent or metastatic EC | Combination (Durvalumab) | July 2023 |
| I/II | NCT03924245 | 73 | High-grade EC | Combination (Entinostat) | September 2025 | ||
| II | NCT04065269 | 40 | Recurrent EC | Combination (AZD6738) | March 2023 | ||
| LY3023414 | DNA-PK | I | NCT01655225 | 156 | Advanced or metastatic EC | Monotherapy and combination (Midazolam, Fulvestrant, Pemetrexed, Cisplatin, Abemaciclib and Letrozole) | December 2020 |
| II | NCT02549989 | 31 | Recurrent EC | Monotherapy | September 2021 | ||
| AZD6738 | ATR | II | NCT04065269 | 40 | Recurrent EC | Combination (AZD6738) | March 2023 |
| Abemaciclib | CDK4/6 | I | NCT04188548 | 186 | Advanced or metastatic EC | Combination (LY3484356) | April 2023 |
| Palbociclib | CDK4/6 | II | NCT02730429 | 78 | EEC | Combination (Letrozole) | December 2022 |
| I | NCT03065062 | 96 | EC | Combination (Gedatolisib) | January 2023 | ||
| Abemaciclib | CDK4/6 | II | NCT03643510 | 25 | Recurrent EC | Combination (Fulvestrant) | August 2021 |
| II | NCT03675893 | 40 | Recurrent or metastatic EC | Combination (Letrozole) | May 2024 | ||
| I | NCT04049227 | 27 | Recurrent EC | Combination (Letrozole) | July 2023 | ||
| II | NCT04393285 | 50 | Advanced, recurrent or metastatic EC | Combination (Letrozole) | June 2023 | ||
| Ribociclib | CDK4/6 | II | NCT02657928 | 40 | Recurrent EC | Combination (Letrozole) | July 2021 |
| I | NCT03008408 | 87 | Recurrent or metastatic EC | Combination (Letrozole and Everolimus) | August 2022 | ||
| DNA and Histone Modifiers SMI | |||||||
| Entinostat | HDAC | I | NCT00020579 | 75 | Metastatic EC | Monotherapy | March 2022 |
| II | NCT03018249 | 50 | EEC | Combination (Medroxyprogesterone Acetate) | December 2020 | ||
| I/II | NCT03924245 | 73 | High-grade EC | Combination (Olaparib) | September 2025 | ||
| Tinostamustine | HDAC | I/II | NCT03345485 | 167 | Advanced or metastatic EC | Monotherapy | July 2022 |
| Celecoxib | IDO1 | II | NCT03896113 | 48 | EC | Monotherapy | June 2022 |
| Epacadostat | IDO1 | I/II | NCT03277352 | 10 | Advanced or metastatic EC | Combination (INCAGN01876 and pembrolizumab) | July 2020 |
| IDO1 | I/II | NCT02178722 | 444 | EC | Combination (MK-3475) | August 2020 | |
| BMS- 986205 | IDO1 | II | NCT04106414 | 50 | Recurrent EC | Combination (Nivolumab) | September 2021 |
| Immunocheckpoints SMI | |||||||
| PF-06801591 | PD1 | I | NCT04152018 | 104 | Advanced or metastatic EC | Combination (PF-06940434) | March 2024 |
| AB122 | PD1 | I | NCT03629756 | 44 | Advanced EC | Combination (AB298) | September 2021 |
| AB928 | Adenosin receptor | I | NCT03629756 | 44 | Advanced EC | Combination (AB298 and AB122) | September 2021 |
| INCB001158 | Arginase | I/II | NCT03314935 | 149 | Advanced or metastatic EC | Combination (Oxaliplatin, Leucovorin, 5-Fluorouracil, Gemcitabine, Cisplatin and Paclitaxel) | October 2021 |
| Ciforadenant | ADORA2A | I | NCT03454451 | 378 | Advanced EC | Combination (Pembrolizumab and CPI-006) | December 2023 |
| Pterostilbene | MUC1 | II | NCT03671811 | 36 | EC | Combination (Megestrol Acetate) | December 2020 |
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; NM, not mentioned; EC, endometrial cancer; n, total amount of patients included to the clinical trial.
In conclusion, nowadays, there are few choices of useful treatment for persistent or recurrent EC patients; however, SMIs will be central in the development of new EC treatment strategies.
Author Contributions
C.M.-L., C.P.M., C.M.-E., C.L.-G., A.G.-M., X.M.-G., E.C. and N.E. contributed equally to this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study has been funded by Ministry of Economy and Competitiveness (MINECO) through the project RTC-2017-6261-1 (co-funded by European Regional Development Fund. ERDF “A way to make Europe” and ESF “Investing in your future” by Instituto de Salud Carlos III (ISCIII) through the projects CB16/12/00328, CB16/12/00231, PI17/02071 PI17/02155 and CP19/00025; by Grups consolidats de la Generalitat de Catalunya (2017SGR1368 and 2017SGR1661); and by the Asociación Española Contra el Cáncer (AECC; Grupos Estables 2018: GCTRA1804MATI). C.M.-L and C.L.-G. hold a pre-doctoral fellowship from AGAUR- Generalitat de Catalunya. N.E. is recipient of a Miguel Servet scheme; Ministerio de Ciencia, Innovación y Universidades, Gobierno de España (ES). A PERIS grant funded E.C. (SLT002/16/00315) from Generalitat de Catalunya.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2017. CA Cancer J. Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Bokhman J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 3.Getz G., Gabriel S.B., Cibulskis K., Lander E., Sivachenko A., Sougnez C., Lawrence M., Kandoth C., Dooling D., Fulton R., et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.León-Castillo A., Gilvazquez E., Nout R., Smit V.T.H.B.M., McAlpine J.N., McConechy M., Kommoss S., Brucker S.Y., Carlson J.W., Epstein E., et al. Clinicopathological and molecular characterisation of ‘multiple-classifier’ endometrial carcinomas. J. Pathol. 2020;250:312–322. doi: 10.1002/path.5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleming G.F. Systemic chemotherapy for uterine carcinoma: Metastatic and adjuvant. J. Clin. Oncol. 2007;25:2983–2990. doi: 10.1200/JCO.2007.10.8431. [DOI] [PubMed] [Google Scholar]
- 6.Lavanya V., Adil M., Ahmed N., Rishi A.K., Jamal S. Small molecule inhibitors as emerging cancer therapeutics. Integr. Cancer Sci. Ther. 2014;1:39–46. doi: 10.15761/ICST.1000109. [DOI] [Google Scholar]
- 7.Hecht J.L., Mutter G.L. Molecular and pathologic aspects of endometrial carcinogenesis. J. Clin. Oncol. 2006;24:4783–4791. doi: 10.1200/JCO.2006.06.7173. [DOI] [PubMed] [Google Scholar]
- 8.Remmerie M., Janssens V. Targeted therapies in type II endometrial cancers: Too little, but not too late. Int. J. Mol. Sci. 2018;19:2380. doi: 10.3390/ijms19082380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barra F., Evangelisti G., Ferro Desideri L., Di Domenico S., Ferraioli D., Vellone V.G., De Cian F., Ferrero S. Investigational PI3K/AKT/mTOR inhibitors in development for endometrial cancer. Expert Opin. Investig. Drugs. 2019;28:131–142. doi: 10.1080/13543784.2018.1558202. [DOI] [PubMed] [Google Scholar]
- 10.Laplante M., Sabatini D.M. MTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slomovitz B.M., Lu K.H., Johnston T., Coleman R.L., Munsell M., Broaddus R.R., Walker C., Ramondetta L.M., Burke T.W., Gershenson D.M., et al. A phase 2 study of the oral mammalian target of rapamycin inhibitor, everolimus, in patients with recurrent endometrial carcinoma. Cancer. 2010;116:5415–5419. doi: 10.1002/cncr.25515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ray-Coquard I., Favier L., Weber B., Roemer-Becuwe C., Bougnoux P., Fabbro M., Floquet A., Joly F., Plantade A., Paraiso D., et al. Everolimus as second- or third-line treatment of advanced endometrial cancer: ENDORAD, a phase II trial of GINECO. Br. J. Cancer. 2013;108:1771–1777. doi: 10.1038/bjc.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slomovitz B.M., Jiang Y., Yates M.S., Soliman P.T., Johnston T., Nowakowski M., Levenback C., Zhang Q., Ring K., Munsell M.F., et al. Phase II study of everolimus and letrozole in patients with recurrent endometrial carcinoma. J. Clin. Oncol. 2015;33:930–936. doi: 10.1200/JCO.2014.58.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soliman P.T., Westin S.N., Iglesias D.A., Fellman B.M., Yuan Y., Zhang Q., Yates M.S., Broaddus R.R., Slomovitz B.M., Lu K.H., et al. Everolimus, letrozole, and metformin in women with advanced or recurrent endometrioid endometrial cancer: A multi-center, single arm, phase II study. Clin. Cancer Res. 2020;26:581–587. doi: 10.1158/1078-0432.CCR-19-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wheler J.J., Moulder S.L., Naing A., Janku F., Piha-Paul S.A., Falchook G.S., Zinner R., Tsimberidou A.M., Fu S., Hong D.S., et al. Anastrozole and everolimus in advanced gynecologic and breast malignancies: Activity and molecular alterations in the PI3K/AKT/mTOR pathway. Oncotarget. 2014;5:3029–3038. doi: 10.18632/oncotarget.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oza A.M., Elit L., Tsao M.S., Kamel-Reid S., Biagi J., Provencher D.M., Gotlieb W.H., Hoskins P.J., Ghatage P., Tonkin K.S., et al. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: A trial of the NCIC Clinical Trials Group. J. Clin. Oncol. 2011;29:3278–3285. doi: 10.1200/JCO.2010.34.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodwin R.A., Jamal R., Tu D., Walsh W., Dancey J., Oza A.M., Elit L., Eisenhauer E.A. Clinical and toxicity predictors of response and progression to temsirolimus in women with recurrent or metastatic endometrial cancer. Gynecol. Oncol. 2013;131:315–320. doi: 10.1016/j.ygyno.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Aghajanian C., Filiaci V., Dizon D.S., Carlson J.W., Powell M.A., Secord A.A., Tewari K.S., Bender D.P., O’Malley D.M., Stuckey A., et al. A phase II study of frontline paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/temsirolimus, or ixabepilone/carboplatin/bevacizumab in advanced/recurrent endometrial cancer. Gynecol. Oncol. 2018;150:274–281. doi: 10.1016/j.ygyno.2018.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleming G.F., Filiaci V.L., Marzullo B., Zaino R.J., Davidson S.A., Pearl M., Makker V., Burke J.J., Zweizig S.L., Van Le L., et al. Temsirolimus with or without megestrol acetate and tamoxifen for endometrial cancer: A gynecologic oncology group study. Gynecol. Oncol. 2014;132:585–592. doi: 10.1016/j.ygyno.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvarez E.A., Brady W.E., Walker J.L., Rotmensch J., Zhou X.C., Kendrick J.E., Yamada S.D., Schilder J.M., Cohn D.E., Harrison C.R., et al. Phase II trial of combination bevacizumab and temsirolimus in the treatment of recurrent or persistent endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol. Oncol. 2013;129:22–27. doi: 10.1016/j.ygyno.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 21.Colombo N., McMeekin D.S., Schwartz P.E., Sessa C., Gehrig P.A., Holloway R., Braly P., Matei D., Morosky A., Dodion P.F., et al. Ridaforolimus as a single agent in advanced endometrial cancer: Results of a single-arm, phase 2 trial. Br. J. Cancer. 2013;108:1021–1026. doi: 10.1038/bjc.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsoref D., Welch S., Lau S., Biagi J., Tonkin K., Martin L.A., Ellard S., Ghatage P., Elit L., MacKay H.J., et al. Phase II study of oral ridaforolimus in women with recurrent or metastatic endometrial cancer. Gynecol. Oncol. 2014;135:184–189. doi: 10.1016/j.ygyno.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 23.Oza A.M., Pignata S., Poveda A., McCormack M., Clamp A., Schwartz B., Cheng J., Li X., Campbell K., Dodion P., et al. Randomized phase II trial of ridaforolimus in advanced endometrial carcinoma. J. Clin. Oncol. 2015;33:3576–3582. doi: 10.1200/JCO.2014.58.8871. [DOI] [PubMed] [Google Scholar]
- 24.Shi X., Liu H., Li S., Xu H. Keratinocyte growth factor protects endometrial cells from oxygen glucose deprivation/re-oxygenation via activating Nrf2 signaling. Biochem. Biophys. Res. Commun. 2018;501:178–185. doi: 10.1016/j.bbrc.2018.04.208. [DOI] [PubMed] [Google Scholar]
- 25.English D.P., Roque D.M., Carrara L., Lopez S., Bellone S., Cocco E., Bortolomai I., Schwartz P.E., Rutherford T., Santin A.D. HER2/neu gene amplification determines the sensitivity of uterine serous carcinoma cell lines to AZD8055, a novel dual mTORC1/2 inhibitor. Gynecol. Oncol. 2013;131:753–758. doi: 10.1016/j.ygyno.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 26.Bendell J.C., Rodon J., Burris H.A., De Jonge M., Verweij J., Birle D., Demanse D., De Buck S.S., Ru Q.C., Peters M., et al. Phase I, dose-escalation study of BKM120, an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 2012;30:282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 27.Heudel P.E., Fabbro M., Roemer-Becuwe C., Kaminsky M.C., Arnaud A., Joly F., Roche-Forestier S., Meunier J., Foa C., You B., et al. Phase II study of the PI3K inhibitor BKM120 in patients with advanced or recurrent endometrial carcinoma: A stratified type I-type II study from the GINECO group. Br. J. Cancer. 2017;116:303–309. doi: 10.1038/bjc.2016.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matulonis U., Vergote I., Backes F., Martin L.P., McMeekin S., Birrer M., Campana F., Xu Y., Egile C., Ghamande S. Phase II study of the PI3K inhibitor pilaralisib (SAR245408; XL147) in patients with advanced or recurrent endometrial carcinoma. Gynecol. Oncol. 2015;136:246–253. doi: 10.1016/j.ygyno.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Wheler J., Mutch D., Lager J., Castell C., Liu L., Jiang J., Traynor A.M. Phase I Dose-Escalation Study of Pilaralisib (SAR245408, XL147) in Combination with Paclitaxel and Carboplatin in Patients with Solid Tumors. Oncologist. 2017;22:377. doi: 10.1634/theoncologist.2016-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weigelt B., Warne P.H., Lambros M.B., Reis-Filho J.S., Downward J. PI3K pathway dependencies in endometrioid endometrial cancer cell lines. Clin. Cancer Res. 2013;19:3533–3544. doi: 10.1158/1078-0432.CCR-12-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juric D., Rodon J., Tabernero J., Janku F., Burris H.A., Schellens J.H.M., Middleton M.R., Berlin J., Schuler M., Marta G.M., et al. Phosphatidylinositol 3-kinase a–selective inhibition with alpelisib (BYL719) in PIK3CA-altered solid tumors: Results from the first-in-human study. J. Clin. Oncol. 2018;36:1291–1299. doi: 10.1200/JCO.2017.72.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bendell J.C., Varghese A.M., Hyman D.M., Bauer T.M., Pant S., Callies S., Lin J., Martinez R., Wickremsinhe E., Fink A., et al. A first-in-human phase 1 study of LY3023414, an Oral PI3K/mTOR dual inhibitor, in patients with advanced cancer. Clin. Cancer Res. 2018;24:3253–3262. doi: 10.1158/1078-0432.CCR-17-3421. [DOI] [PubMed] [Google Scholar]
- 33.Rubinstein M.M., Hyman D.M., Caird I., Won H., Soldan K., Seier K., Iasonos A., Tew W.P., O’Cearbhaill R.E., Grisham R.N., et al. Phase 2 study of LY3023414 in patients with advanced endometrial cancer harboring activating mutations in the PI3K pathway. Cancer. 2020;126:1274–1282. doi: 10.1002/cncr.32677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J., Zhao K.-N., Li R., Shao R., Chen C. Activation of PI3K/Akt/mTOR Pathway and Dual Inhibitors of PI3K and mTOR in Endometrial Cancer. Curr. Med. Chem. 2014;21:3070–3080. doi: 10.2174/0929867321666140414095605. [DOI] [PubMed] [Google Scholar]
- 35.Schrauwen S., Depreeuw J., Coenegrachts L., Hermans E., Lambrechts D., Amant F. Dual blockade of PI3K/AKT/mTOR (NVP-BEZ235) and Ras/Raf/MEK (AZD6244) pathways synergistically inhibit growth of primary endometrioid endometrial carcinoma cultures, whereas NVP-BEZ235 reduces tumor growth in the corresponding xenograft models. Gynecol. Oncol. 2015;138:165–173. doi: 10.1016/j.ygyno.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 36.Bendell J.C., Kurkjian C., Infante J.R., Bauer T.M., Burris H.A., Greco F.A., Shih K.C., Thompson D.S., Lane C.M., Finney L.H., et al. A phase 1 study of the sachet formulation of the oral dual PI3K/mTOR inhibitor BEZ235 given twice daily (BID) in patients with advanced solid tumors. Investig. New Drugs. 2015;33:463–471. doi: 10.1007/s10637-015-0218-6. [DOI] [PubMed] [Google Scholar]
- 37.Wise-Draper T.M., Moorthy G., Salkeni M.A., Karim N.A., Thomas H.E., Mercer C.A., Beg M.S., O’Gara S., Olowokure O., Fathallah H., et al. A Phase Ib Study of the Dual PI3K/mTOR Inhibitor Dactolisib (BEZ235) Combined with Everolimus in Patients with Advanced Solid Malignancies. Target. Oncol. 2017;12:323–332. doi: 10.1007/s11523-017-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dolly S.O., Wagner A.J., Bendell J.C., Kindler H.L., Krug L.M., Seiwert T.Y., Zauderer M.G., Lolkema M.P., Apt D., Yeh R.F., et al. Phase I study of apitolisib (GDC-0980), dual phosphatidylinositol-3-kinase and mammalian target of rapamycin kinase inhibitor, in patients with advanced solid tumors. Clin. Cancer Res. 2016;22:2874–2884. doi: 10.1158/1078-0432.CCR-15-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Makker V., Recio F.O., Ma L., Matulonis U.A., Lauchle J.O., Parmar H., Gilbert H.N., Ware J.A., Zhu R., Lu S., et al. A multicenter, single-arm, open-label, phase 2 study of apitolisib (GDC-0980) for the treatment of recurrent or persistent endometrial carcinoma (MAGGIE study) Cancer. 2016;122:3519–3528. doi: 10.1002/cncr.30286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wainberg Z.A., Alsina M., Soares H.P., Braña I., Britten C.D., Del Conte G., Ezeh P., Houk B., Kern K.A., Leong S., et al. A Multi-Arm Phase I Study of the PI3K/mTOR Inhibitors PF-04691502 and Gedatolisib (PF-05212384) plus Irinotecan or the MEK Inhibitor PD-0325901 in Advanced Cancer. Target. Oncol. 2017;12:775–785. doi: 10.1007/s11523-017-0530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myers A.P., Konstantinopoulos P.A., Barry W.T., Luo W., Broaddus R.R., Makker V., Drapkin R., Liu J., Doyle A., Horowitz N.S., et al. Phase II, 2-stage, 2-arm, PIK3CA mutation stratified trial of MK-2206 in recurrent endometrial cancer. Int. J. Cancer. 2020;147:413–422. doi: 10.1002/ijc.32783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davies B.R., Greenwood H., Dudley P., Crafter C., Yu D.H., Zhang J., Li J., Gao B., Ji Q., Maynard J., et al. Preclinical pharmacology of AZD5363, an inhibitor of AKT: Pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic background. Mol. Cancer Ther. 2012;11:873–887. doi: 10.1158/1535-7163.MCT-11-0824-T. [DOI] [PubMed] [Google Scholar]
- 43.Banerji U., Dean E.J., Alejandro Perez-Fidalgo J., Batist G., Bedard P.L., You B., Westin S.N., Kabos P., Garrett M.D., Tall M., et al. A phase i open-label study to identify a dosing regimen of the pan-akt inhibitor azd5363 for evaluation in solid tumors and in pik3ca-mutated breast and gynecologic cancers. Clin. Cancer Res. 2018;24:2050–2059. doi: 10.1158/1078-0432.CCR-17-2260. [DOI] [PubMed] [Google Scholar]
- 44.Westin S., Litton J., Williams R., Soliman P., Frumovitz M., Schmeler K., Jazaeri A., Sood A., Lu K., Moulder S., et al. Phase I expansion of olaparib (PARP inhibitor) and AZD5363 (AKT inhibitor) in recurrent ovarian, endometrial and triple negative breast cancer. Ann. Oncol. 2017;28:v130–v131. doi: 10.1093/annonc/mdx367.025. [DOI] [Google Scholar]
- 45.Felip I., Moiola C.P., Megino-Luque C., Lopez-Gil C., Cabrera S., Solé-Sánchez S., Muñoz-Guardiola P., Megias-Roda E., Pérez-Montoyo H., Alfon J., et al. Therapeutic potential of the new TRIB3-mediated cell autophagy anticancer drug ABTL0812 in endometrial cancer. Gynecol. Oncol. 2019;153:425–435. doi: 10.1016/j.ygyno.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Du Z., Lovly C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer. 2018;17:1–13. doi: 10.1186/s12943-018-0782-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hubbard S.R. Structural analysis of receptor tyrosine kinases. Prog. Biophys. Mol. Biol. 1999;71:343–358. doi: 10.1016/S0079-6107(98)00047-9. [DOI] [PubMed] [Google Scholar]
- 48.Soumerai T.E., Donoghue M.T.A., Bandlamudi C., Srinivasan P., Chang M.T., Zamarin D., Cadoo K.A., Grisham R.N., O’Cearbhaill R.E., Tew W.P., et al. Clinical utility of prospective molecular characterization in advanced endometrial cancer. Clin. Cancer Res. 2018;24:5939–5947. doi: 10.1158/1078-0432.CCR-18-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoadley K.A., Yau C., Hinoue T., Wolf D.M., Lazar A.J., Drill E., Shen R., Taylor A.M., Cherniack A.D., Thorsson V., et al. Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell. 2018;173:291–304.e6. doi: 10.1016/j.cell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wind S., Schnell D., Ebner T., Freiwald M., Stopfer P. Clinical Pharmacokinetics and Pharmacodynamics of Afatinib. Clin. Pharmacokinet. 2017;56:235–250. doi: 10.1007/s40262-016-0440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou L., Ren Y., Wang X., Miao D., Lizaso A., Li H., Han-Zhang H., Qian J., Yang H. Efficacy of afatinib in a HER2 amplification-positive endometrioid adenocarcinoma patient—A case report. OncoTargets Ther. 2019;12:5305–5309. doi: 10.2147/OTT.S206732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raymond E., Faivre S., Armand J.P. Epidermal growth factor receptor tyrosine kinase as a target for anticancer therapy. Drugs. 2000;60:15–23. doi: 10.2165/00003495-200060001-00002. [DOI] [PubMed] [Google Scholar]
- 53.Oza A.M., Eisenhauer E.A., Elit L., Cutz J.-C., Sakurada A., Tsao M.S., Hoskins P.J., Biagi J., Ghatage P., Mazurka J., et al. Phase II study of erlotinib in recurrent or metastatic endometrial cancer: NCIC IND-148. J. Clin. Oncol. 2008;26:4319–4325. doi: 10.1200/JCO.2007.15.8808. [DOI] [PubMed] [Google Scholar]
- 54.Leslie K.K., Sill M.W., Fischer E., Darcy K.M., Mannel R.S., Tewari K.S., Hanjani P., Wilken J.A., Baron A.T., Godwin A.K., et al. A phase II evaluation of gefitinib in the treatment of persistent or recurrent endometrial cancer: A Gynecologic Oncology Group study. Gynecol. Oncol. 2013;129:486–494. doi: 10.1016/j.ygyno.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nelson M.H., Dolder C.R. Lapatinib: A novel dual tyrosine kinase inhibitor with activity in solid tumors. Ann. Pharmacother. 2006;40:261–269. doi: 10.1345/aph.1G387. [DOI] [PubMed] [Google Scholar]
- 56.Chu Q.S.C., Cianfrocca M.E., Goldstein L.J., Gale M., Murray N., Loftiss J., Arya N., Koch K.M., Pandite L., Fleming R.A., et al. A phase I and pharmacokinetic study of lapatinib in combination with letrozole in patients with advanced cancer. Clin. Cancer Res. 2008;14:4484–4490. doi: 10.1158/1078-0432.CCR-07-4417. [DOI] [PubMed] [Google Scholar]
- 57.Leslie K.K., Sill M.W., Lankes H.A., Fischer E.G., Godwin A.K., Gray H., Schilder R.J., Walker J.L., Tewari K., Hanjani P., et al. Lapatinib and potential prognostic value of EGFR mutations in a Gynecologic Oncology Group phase II trial of persistent or recurrent endometrial cancer. Gynecol. Oncol. 2012;127:345–350. doi: 10.1016/j.ygyno.2012.07.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coleman R.L., Sill M.W., Thaker P.H., Bender D.P., Street D., McGuire W.P., Johnston C.M., Rotmensch J. A phase II evaluation of selumetinib (AZD6244, ARRY-142886), a selective MEK-1/2 inhibitor in the treatment of recurrent or persistent endometrial cancer: An NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2015;138:30–35. doi: 10.1016/j.ygyno.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Konecny G.E., Finkler N., Garcia A.A., Lorusso D., Lee P.S., Rocconi R.P., Fong P.C., Squires M., Mishra K., Upalawanna A., et al. Second-line dovitinib (TKI258) in patients with FGFR2-mutated or FGFR2-non-mutated advanced or metastatic endometrial cancer: A non-randomised, open-label, two-group, two-stage, phase 2 study. Lancet Oncol. 2015;16:686–694. doi: 10.1016/S1470-2045(15)70159-2. [DOI] [PubMed] [Google Scholar]
- 60.Powell M.A., Sill M.W., Goodfellow P.J., Benbrook D.M., Lankes H.A., Leslie K.K., Jeske Y., Mannel R.S., Spillman M.A., Lee P.S., et al. A phase II trial of brivanib in recurrent or persistent endometrial cancer: An NRG Oncology/Gynecologic Oncology Group Study. Gynecol. Oncol. 2014;135:38–43. doi: 10.1016/j.ygyno.2014.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grülich C. Cabozantinib: A MET, RET, and VEGFR2 tyrosine kinase inhibitor. Recent Results Cancer Res. 2014;201:207–214. doi: 10.1007/978-3-642-54490-3_12. [DOI] [PubMed] [Google Scholar]
- 62.Dhani N.C., Hirte H.W., Wang L., Burnier J.V., Jain A., Butler M.O., Welch S., Fleming G.F., Hurteau J., Matsuo K., et al. Phase II Trial of Cabozantinib in Recurrent/Metastatic Endometrial Cancer: A Study of the Princess Margaret, Chicago, and California Consortia (NCI9322/PHL86) Clin. Cancer Res. 2020;26:2477–2486. doi: 10.1158/1078-0432.CCR-19-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wedge S.R., Kendrew J., Hennequin L.F., Valentine P.J., Barry S.T., Brave S.R., Smith N.R., James N.H., Dukes M., Curwen J.O., et al. AZD2171: A highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65:4389–4400. doi: 10.1158/0008-5472.CAN-04-4409. [DOI] [PubMed] [Google Scholar]
- 64.Bender D., Sill M.W., Lankes H.A., Reyes H.D., Darus C.J., Delmore J.E., Rotmensch J., Gray H.J., Mannel R.S., Schilder J.M., et al. A phase II evaluation of cediranib in the treatment of recurrent or persistent endometrial cancer: An NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2015;138:507–512. doi: 10.1016/j.ygyno.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dizon D.S., Sill M.W., Schilder J.M., McGonigle K.F., Rahman Z., Miller D.S., Mutch D.G., Leslie K.K. A phase II evaluation of nintedanib (BIBF-1120) in the treatment of recurrent or persistent endometrial cancer: An NRG Oncology/Gynecologic Oncology Group Study. Gynecol. Oncol. 2014;135:441–445. doi: 10.1016/j.ygyno.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Castonguay V., Lheureux S., Welch S., MacKay H.J., Hirte H., Fleming G., Morgan R., Wang L., Blattler C., Ivy P.S., et al. A phase II trial of sunitinib in women with metastatic or recurrent endometrial carcinoma: A study of the Princess Margaret, Chicago and California Consortia. Gynecol. Oncol. 2014;134:274–280. doi: 10.1016/j.ygyno.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 67.Glen H., Mason S., Patel H., Macleod K., Brunton V.G. E7080, a multi-targeted tyrosine kinase inhibitor suppresses tumor cell migration and invasion. BMC Cancer. 2011;11:309. doi: 10.1186/1471-2407-11-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vergote I., Powell M.A., Teneriello M.G., Miller D.S., Garcia A.A., Mikheeva O.N., Bidzinski M., Cebotaru C.L., Dutcus C.E., Ren M., et al. Second-line lenvatinib in patients with recurrent endometrial cancer. Gynecol. Oncol. 2020;156:575–582. doi: 10.1016/j.ygyno.2019.12.039. [DOI] [PubMed] [Google Scholar]
- 69.Makker V., Rasco D., Vogelzang N.J., Brose M.S., Cohn A.L., Mier J., Di Simone C., Hyman D.M., Stepan D.E., Dutcus C.E., et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: An interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20:711–718. doi: 10.1016/S1470-2045(19)30020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steinberg M. Dasatinib: A tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and philadelphia chromosome-positive acute lymphoblastic leukemia. Clin. Ther. 2007;29:2289–2308. doi: 10.1016/j.clinthera.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 71.Duska L.R., Petroni G.R., Lothamer H., Faust W., Beumer J.H., Christner S.M., Mills A.M., Fracasso P.M., Parsons S.J. A window-of-opportunity clinical trial of dasatinib in women with newly diagnosed endometrial cancer. Cancer Chemother. Pharmacol. 2019;83:473–482. doi: 10.1007/s00280-018-3749-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Otto T., Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer. 2017;17:93–115. doi: 10.1038/nrc.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Jonge M.M., Auguste A., Van Wijk L.M., Schouten P.C., Meijers M., Ter Haar N.T., Smit V.T.H.B.M., Nout R.A., Glaire M.A., Church D.N., et al. Frequent homologous recombination deficiency in high-grade endometrial carcinomas. Clin. Cancer Res. 2019;25:1087–1097. doi: 10.1158/1078-0432.CCR-18-1443. [DOI] [PubMed] [Google Scholar]
- 74.Giannone G., Tuninetti V., Ghisoni E., Genta S., Scotto G., Mittica G., Valabrega G. Role of cyclin-dependent kinase inhibitors in endometrial cancer. Int. J. Mol. Sci. 2019;20:2353. doi: 10.3390/ijms20092353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Minchom A., Aversa C., Lopez J. Dancing with the DNA damage response: Next-generation anti-cancer therapeutic strategies. Ther. Adv. Med. Oncol. Rev. 2018;10:1–18. doi: 10.1177/1758835918786658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zimmer A.S., Nichols E., Cimino-Mathews A., Peer C., Cao L., Lee M.-J., Kohn E.C., Annunziata C.M., Lipkowitz S., Trepel J.B., et al. A phase I study of the PD-L1 inhibitor, durvalumab, in combination with a PARP inhibitor, olaparib, and a VEGFR1-3 inhibitor, cediranib, in recurrent women’s cancers with biomarker analyses. J. Immunother. Cancer. 2019;7:197. doi: 10.1186/s40425-019-0680-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gourley C., Balmaña J., Ledermann J.A., Serra V., Dent R., Loibl S., Pujade-Lauraine E., Boulton S.J. Moving from poly (ADP-ribose) polymerase inhibition to targeting DNA repair and DNA damage response in cancer therapy. J. Clin. Oncol. 2019;37:2257–2269. doi: 10.1200/JCO.18.02050. [DOI] [PubMed] [Google Scholar]
- 78.Lin Z.P., Zhu Y.L., Ratner E.S. Targeting cyclin-dependent kinases for treatment of gynecologic cancers. Front. Oncol. 2018;8:303. doi: 10.3389/fonc.2018.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Biancotto C., Frigè G., Minucci S. Histone modification therapy of cancer. Adv. Genet. 2010;70:341–386. doi: 10.1016/B978-0-12-380866-0.60013-7. [DOI] [PubMed] [Google Scholar]
- 80.Eskander R.N. The Epigenetic Landscape in the Treatment of Gynecologic Malignancies. Am. Soc. Clin. Oncol. Educ. Book. 2018;38:480–487. doi: 10.1200/EDBK_200203. [DOI] [PubMed] [Google Scholar]
- 81.Bartosch C., Lopes J.M., Jerónimo C. Epigenetics in endometrial carcinogenesis—Part 2: Histone modifications, chromatin remodeling and noncoding RNAs. Epigenomics. 2017;9:873–892. doi: 10.2217/epi-2016-0167. [DOI] [PubMed] [Google Scholar]
- 82.Ryan Q.C., Headlee D., Acharya M., Sparreboom A., Trepel J.B., Ye J., Figg W.D., Hwang K., Chung E.J., Murgo A., et al. Phase I and pharmacokinetic study of MS-275, a histone deacetylase inhibitor, in patients with advanced and refractory solid tumors or lymphoma. J. Clin. Oncol. 2005;23:3912–3922. doi: 10.1200/JCO.2005.02.188. [DOI] [PubMed] [Google Scholar]
- 83.Di Tucci C., Capone C., Galati G., Iacobelli V., Schiavi M.C., Di Donato V., Muzii L., Panici P.B. Immunotherapy in endometrial cancer: New scenarios on the horizon. J. Gynecol. Oncol. 2019;30:1–20. doi: 10.3802/jgo.2019.30.e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Del Campo J.M., Birrer M., Davis C., Fujiwara K., Gollerkeri A., Gore M., Houk B., Lau S., Poveda A., González-Martín A., et al. A randomized phase II non-comparative study of PF-04691502 and gedatolisib (PF-05212384) in patients with recurrent endometrial cancer. Gynecol. Oncol. 2016;142:62–69. doi: 10.1016/j.ygyno.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 85.Bahleda R., Italiano A., Hierro C., Mita A., Cervantes A., Chan N., Awad M., Calvo E., Moreno V., Govindan R., et al. Multicenter phase I study of erdafitinib (JNJ-42756493), oral pan-fibroblast growth factor receptor inhibitor, in patients with advanced or refractory solid tumors. Clin. Cancer Res. 2019;25:4888–4897. doi: 10.1158/1078-0432.CCR-18-3334. [DOI] [PubMed] [Google Scholar]
- 86.Knowling M., Blackstein M., Tozer R., Bramwell V., Dancey J., Dore N., Matthews S., Eisenhauer E. A phase II study of perifosine (D-21226) in patients with previously untreated metastatic or locally advanced soft tissue sarcoma: A National Cancer Institute of Canada Clinical Trials Group trial. Investig. New Drugs. 2006;24:435–439. doi: 10.1007/s10637-006-6406-7. [DOI] [PubMed] [Google Scholar]