Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Gastrointestinal symptoms in COVID-19 are being reported increasingly.1 Data on the involvement of the pancreas in COVID-19 have been emerging, and multiple case reports of SARS-CoV-2–induced acute pancreatitis have been published in the literature.2 Angiotensin-converting enzyme-2 is the target receptor of SARS-CoV-2 and is expressed abundantly by both the exocrine and endocrine pancreatic tissues.3 The presence of angiotensin-converting enzyme-2 in the pancreas could make it susceptible to SARS-CoV-2, resulting in interstitial leakage of pancreatic lipase, adipose tissue lipolysis, and potentially toxic fatty acid–induced damage. These changes can at least contribute to cytokine storm, multiorgan dysfunction, and COVID-19 morbidity.4 Due to its nonspecificity, lipase could be elevated in a myriad of conditions, such as infections, renal dysfunction, medication-related, gastrointestinal, and hepatobiliary disease.5 Given this, it is critical to evaluate the prevalence of hyperlipasemia in COVID-19 and predict clinical outcomes.
Methods
We conducted a systematic search using PubMed, Embase, Ovid, and Google Scholar databases from December 1, 2019 to October 9, 2020, to evaluate hyperlipasemia in patients with COVID-19. The following search terms were used: COVID-19, SARS-CoV-2, lipase, pancreatic injury, and pancreas. The articles with relevant data on the prevalence of hyperlipasemia and its effect on COVID-19 severity were examined. All adult patients with nasopharyngeal reverse transcription polymerase chain reaction positive for SARS-CoV-2 were included in the analysis. Severe COVID-19 was defined as clinical deterioration resulting in adverse clinical outcomes, such as admission to the intensive care unit (ICU), need for mechanical ventilation, or death. Hyperlipasemia was defined as any elevation in lipase levels above the upper limit of the normal reference level. The definition of hyperlipasemia varied among the studies because of differences in the range of lipase levels, as did the definition of severe COVID-19.
OpenMeta[Analyst] software was used to estimate the pooled prevalence of lipase elevations among patients with COVID-19 and the pooled odds ratio for severe COVID-19 among this subset of patients. Results were reported with a 95% confidence interval, and a P value of <.05 was considered statistically significant. Heterogeneity was assessed using the I 2 test, and I 2 >50% was taken as a measure of moderate inter-study variation.
Results
The initial search yielded 52 articles. After excluding duplicates and review articles, 7 studies3 , 6, 7, 8, 9, 10 (6 retrospective observational studies and 1 prospective observational study11) were included in the pooled analysis. A flow chart depicting the study screening and selection process is presented in Supplementary Figure 1.
Supplementary Figure 1.
Study flow chart.
Data on the point prevalence of hyperlipasemia were available in all 7 studies, whereas only 4 reported clinical outcomes, ICU admission status, and need for mechanical ventilation or death. The normal range of serum lipase levels differed among the studies. Although 4 studies had an average upper limit of 50–60 U/L, 2 had a higher cutoff (>300 U/L) for lipase levels. Data for 756 patients with COVID-19 were reported in these studies, of which 92 had hyperlipasemia. All of the studies were single-center experiences except two. Two studies defined severe COVID-19 as patients needing ICU admission, 1 study as a need for mechanical ventilation, and only 1 study used a combination of all of the above three to define severe COVID-19. Supplementary Table 1 outlines the baseline characteristics of the included studies.
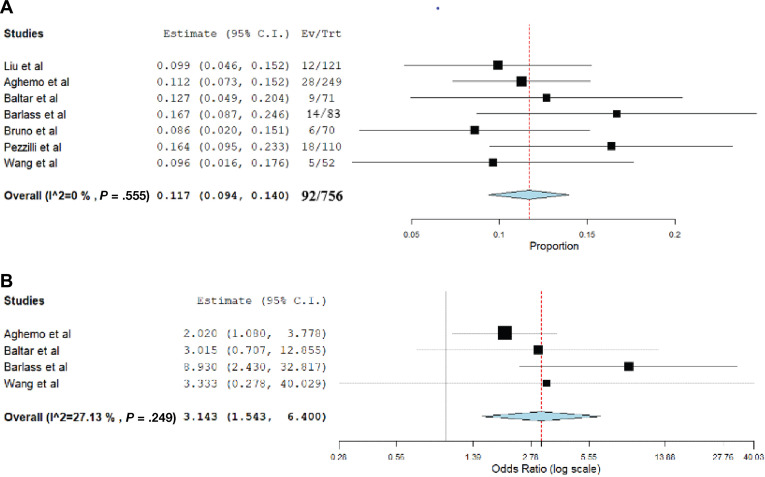
The results of this pooled analysis are shown in Figure 1 A and B. Among 756 patients with COVID-19 with available lipase levels, the pooled prevalence of hyperlipasemia was 11.7% (95% confidence interval, 0.094–0.140; P = .001; I 2 = 0%). The pooled odds ratio for severe COVID-19 in these patients was 3.143 (95% confidence interval, 1.543–6.400; P = .003); mild inter-study heterogeneity was observed (I 2 = 27%).
Figure 1.
(A) Pooled incidence rate of hyperlipasemia in patients with COVID-19. (B) Pooled odds ratio of severe COVID-19 in patients with hyperlipasemia.
Discussion
Based on the results of our pooled analysis, hyperlipasemia was found in 11.7% of patients affected by SARS-CoV-2. Patients with COVID-19 with hyperlipasemia are at an approximately 3-fold higher risk of poor clinical outcomes, including need for ICU admission, mechanical ventilation, or death.
Although multiple mechanisms have been proposed for pancreatic injury in COVID-19, the exact etiology remains unclear. Some of the mechanisms include direct pancreatic tissue damage by SARS-CoV-2 and intense inflammatory response (interleukin 1β, interleukin 6, and tumor necrosis factors) with cytokine storm–mediated tissue injury. Furthermore, studies found that patients with COVID-19 with pancreatic injury had a higher prevalence of severe illness on admission; lower levels of CD3+ and CD4+ T cells; and higher levels of aspartate aminotransferase, γ-glutamyl transferase, creatinine, lactate dehydrogenase, and erythrocyte sedimentation rate.6
Limitations of this study include a modest sample size of 756 patients with COVID-19, of which 92 patients had elevated serum lipase levels. The degree of hyperlipasemia was not uniform across all studies. Potential confounders, such as age, comorbidities, and medication use, could alter the results of this study. Furthermore, a lack of high-quality randomized controlled trials with adjustment for potential confounders is a notable limitation. Given the inclusion of observational studies, selection bias, information bias, and confounding bias are possible. The prevalence of hyperlipasemia could be underestimated due to a lack of testing and nonreporting of the data for many patients.
Severe pancreatic injury resulting in acute pancreatitis might not be a common event in COVID-19. As evidenced by lipase elevation, mild to moderate pancreatic injury is a clinically significant finding in these patients. Future prospective studies are warranted to ascertain the exact impact of lipase elevation in COVID-19 and guide management strategies for these patients.
Acknowledgments
CRediT Authorship Contributions
Hemant Goyal, MBBS, MD, FACP (Methodology: Lead; Supervision: Lead; Writing – review & editing: Equal).
Sonali Sachdeva, MBBS (Data curation: Equal; Formal analysis: Equal; Writing – original draft: Lead).
Abhilash Perisetti, MD, FACP (Supervision: Supporting; Writing – review & editing: Equal).
Rupinder Mann, MD (Writing – original draft: Supporting; Writing – review & editing: Supporting).
Sumant Inamdar, MD, MPH (Supervision: Equal; Writing – review & editing: Supporting).
Benjamin Tharian, MD, MRCP, FACP, FRACP (Supervision: Equal; Writing – review & editing: Lead).
Footnotes
Conflicts of interest The authors disclose no conflicts.
Author names in bold designate shared co-first authorship.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://doi.org/10.1053/j.gastro.2020.10.037.
Supplementary Material
Supplementary Table 1.
Baseline Characteristics of Included Studies With Hyperlipasemia in Coronavirus Disease 2019
| First author | Type of study | Region | Sample size | Male patients, n | Definition of hyperlipasemia (serum lipase levels, U/L) |
|---|---|---|---|---|---|
| McNabb-Baltar7 | Retrospective multicenter cohort study | United States | 71 patients with COVID-19 | 32 | >60 |
| Barlass8 | Retrospective cohort single-center study | United States | 1003 patients with COVID-19; 83 with available lipase levels | 11 | >3 × ULN; >156 |
| Liu3 | Retrospective observational multicenter study | China | 121 patients with COVID-19 | 75 | >78 |
| Aghemo9 | Retrospective observational single-center study | Italy | 292 patients with COVID-19; lipase levels available in 249 patients | 199 | >68 |
| Wang6 | Retrospective observational single-center study | China | 52 patients with COVID-19 | 24 | >70 |
| Bruno10 | Retrospective observational single-center study | Italy | 70 patients with COVID-19 | Not reported | >393 |
| Pezzilli11 | Prospective observational single-center study | Italy | 110 patients with COVID-19 | 69 | >300 |
ULN, upper limit of normal.
References
- 1.Aziz M. Gastroenterology. 2020;159:1132–1133. doi: 10.1053/j.gastro.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aloysius M.M. Pancreatology. 2020;20:1026–1027. doi: 10.1016/j.pan.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu F., Long X., Zhang B., Zhang W., Chen X., Zhang Z. Clin Gastroenterol Hepatol. 2020;18:2128–2130.e2. doi: 10.1016/j.cgh.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hegyi P., Szakács Z., Sahin-Tóth M. Gastroenterology. 2020;159:824–827. doi: 10.1053/j.gastro.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hameed A.M. HPB (Oxford) 2015;17:99–112. doi: 10.1111/hpb.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang F., Wang H., Fan J., Zhang Y. Gastroenterology. 2020;159:367–370. doi: 10.1053/j.gastro.2020.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNabb-Baltar J., Jin D.X., Grover A.S. Lipase Elevation in Patients With COVID-19. Official journal of the American College of Gastroenterology. ACG. 2020:115. doi: 10.14309/ajg.0000000000000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barlass U., Wiliams B., Dhana K. Marked Elevation of Lipase in COVID-19 Disease: A Cohort Study. Clin Transl Gastroenterol. 2020;11 doi: 10.14309/ctg.0000000000000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aghemo A., Piovani D., Parigi T.L. Clin Gastroenterol Hepatol. 2020;18:2366–2368.e3. doi: 10.1016/j.cgh.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruno G., Fabrizio C., Santoro C.R., Buccoliero G.B. J Med Virol. 2020 Jun 4 doi: 10.1002/jmv.26134. 10.1002/jmv.26134. doi: 10.1002/jmv.26134. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pezzilli R., Centanni S., Mondoni M. Patients with COVID-19 Interstitial Pneumonia Exhibit Pancreatic Hyperenzymemia and Not Acute Pancreatitis. 2020 doi: 10.21203/rs.3.rs-50275/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]