Abstract
There have been several reports of the incidental detection of severe acute respiratory syndrome coronavirus 2 pneumonia on positron emission tomography/computed tomography (PET/CT) studies, which represent the potential role of molecular imaging in the detection and management of coronavirus disease 2019. Here, we systematically review the value of PET/CT in this setting. We conducted a systematic search on June 23, 2020, for PET studies with findings suggestive of coronavirus disease 2019. Web of Science, PubMed, Scopus, EMBASE, and Google Scholar databases were used. Patients with at least one PET/CT imaging evaluation were included in the study. Fifty-two patients in 30 publications with a mean age of 60 ± 12.74 (age range; 27-87) were included in this study, of which 28 (53.8%) were male, and 19 (36.5%) were female. In 5 (9.7%) patients, gender was not reported. PET/CT was performed with 18F-fluorodeoxyglucose for 48 (92.3%), 18F-choline for 3 (5.8%), and 68Ga-PSMA for 1 (1.9%) patients. The mean SUV max of pulmonary lesions with 18F-fluorodeoxyglucose uptake was 4.9 ± 2.3. Moreover, 39 (75%) cases had an underlying malignancy, including 18 different type of primary cancers and 6 (11.5%) patients with metastatic disease. The most common pulmonary findings in PET/CT were bilateral hypermetabolic ground-glass opacities in 39 (75%), consolidation in 18 (34.6%), and interlobular thickening in 4 (7.6%). In addition, mediastinal 14 (27%) and hilar 10 (19.2%) lymph node involvement with increased metabolic activity was frequently identified. Early diagnosis of severe acute respiratory syndrome coronavirus 2 pneumonia is not only crucial for both appropriate patient management but also helps to ensure appropriate postexposure precautions are implemented for the department and hospital staff and those who have been in contact with the patient.
Abbreviations: COVID-19, the novel Coronavirus disease; GGOs, ground-glass opacities; PET/CT, positron emission tomography/computed tomography; 18F-FDG, 18F-fluorodeoxyglucose; 68Ga-PSMA, 68Gallium-prostate-specific membrane antigen; SUVmax, maximum standardized uptake values
Introduction
The novel Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 coronavirus), first emerged in China, and then spread to the other regions worldwide.1 Currently, almost all countries have been seriously affected by the global pandemic, with profound impacts on their healthcare and socioeconomic systems. As of July 23, 2020, a total of 15 million COVID-19 cases with around 630,000 deaths have been confirmed worldwide.2 COVID-19 typically presents with fever and lower respiratory symptoms, and the majority of infected patients display bilateral multifocal pulmonary lesions in chest imaging. No definitive therapy for the SARS-CoV-2 infection is available yet. Hence, early diagnosis, early isolation, and supportive management continue to be the crucial strategies to combat the present crisis.3
Viral RNA nucleic acid testing (RT-PCR) is currently considered the gold standard of diagnosis; however, the test suffers from insufficient sensitivity, particularly in the early time course of the disease.4 , 5 Therefore, chest imaging, specifically high-resolution CT, has been purposed as a supportive tool in the detection and management of SARS-CoV-2 pneumonia. Numerous publications have described the major imaging features of COVID-19. Multifocal ground-glass opacities (GGOs) with a peripheral distribution and later superimposition of consolidation are among the most frequently reported characteristics.6, 7, 8 However, the mentioned findings are not highly specific for COVID-19, and several other lung pathologies might also mimic similar CT manifestations.
Moreover, despite some uncertainties due to the lack of enough data, there might be a potential role for other imaging tools (eg, positron emission tomography/computed tomography or PET/CT) in the detection and management of COVID-19.9 Although 18F-fluorodeoxyglucose (18F-FDG) PET/CT is not usually employed in the diagnosis of acute pneumonia, its clinical value in the pulmonary inflammatory/infectious conditions has already been explored.10 18F-FDG-PET can assess the severity of the disease, monitor disease progression, and evaluate the post-therapy response.11 More importantly, whole-body PET/CT imaging offers a unique chance to evaluate the possible extrapulmonary manifestations of COVID-19.
Over the last few months, several case reports with incidental detection of COVID-19 on PET/CT have been published worldwide.12 , 13 However, a comprehensive study is still lacking in this regard. Hence, we conducted the present study to comprehensively review the available publications on PET/CT findings in COVID-19, which might guide us to a better understanding of the disease pathology, its long-term complications, and developing novel therapies. Indeed, the potential added value of FDG-PET/CT in depicting the underlying pathophysiological inflammatory process might offer a great chance to fight against the ongoing COVID-19 pandemic.
Materials and Methods
Search Strategy
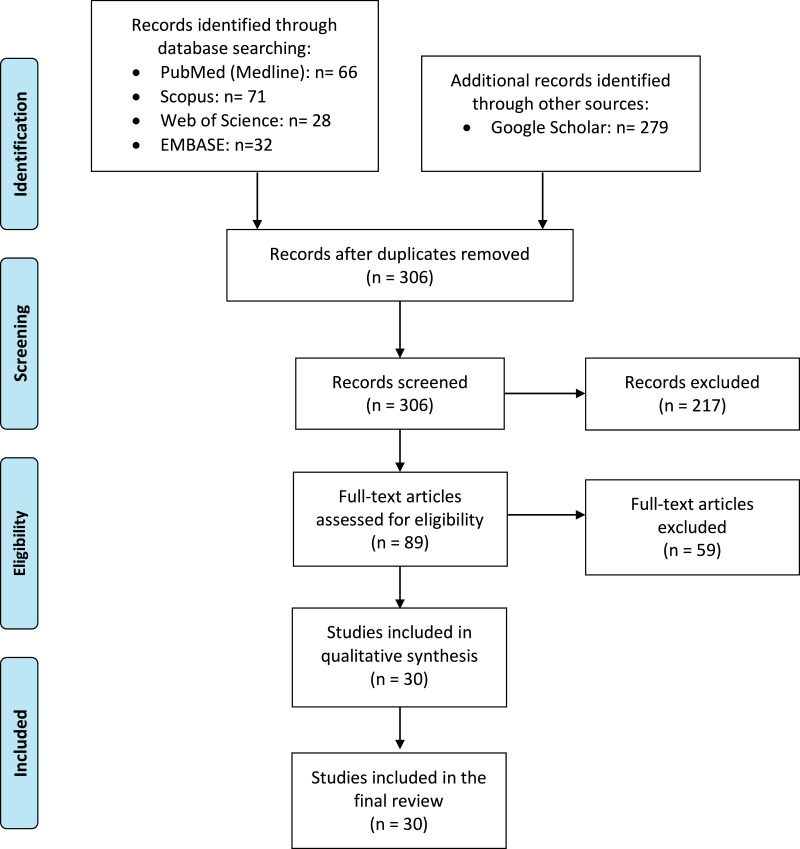
We conducted a systematic search for available published articles describing PET findings in COVID-19 patients. The search was performed on June 23, 2020, using Web of Science, PubMed, Scopus, EMBASE, and Google Scholar databases. The following search keywords were used: “COVID-19”, “coronavirus”, “coronavirus and infection”, “SARS-CoV-19”, “2019-CoV-19”, “radiolog*”, “Tomography, X-ray, Computed”, “Positron-Emission Tomography”, “PET/CT*”, and “FDG-PET”. The full list of keywords which were used in the databases is provided as Appendix 1. The study flow diagram is also presented in Figure 1 .
Figure 1.
Flow diagram of study selection process.
Eligibility Criteria
Patients with confirmed COVID-19 infection (by RT-PCR test using nasopharyngeal swabs), with at least one PET/CT imaging evaluation were included in the study. Duplicate studies, conference abstracts, irrelevant reports, and studies in any language except English were excluded.
Data Extraction and Quality Assessments
Regarding the quality assessment, two independent reviewers assessed the risk of bias of the included studies using the National Institutes of Health quality assessment tool for case series/reports,14 and cohort studies using the modified version of Newcastle-Ottawa Quality Scale15 (Supplementary Tables 1 and 2). Two blinded reviewers extracted data and then proceeded to cross-check the results. The third reviewer resolved disagreements via consensus. The following data were extracted: First author's name, study region, study type, patient's age, gender, type of cancer or reason for the exam, pulmonary PET/CT imaging findings, extrapulmonary PET/CT imaging findings, maximum standardized uptake values (SUVmax) of the target pulmonary lesions, and the radiopharmaceutical used in PET imaging. Moreover, we reported one patient in our center (Fig. 2 ) and collected a series of PET/CT images from three included studies16, 17, 18 via formal permissions obtained from their publishers (Figure 3, Figure 4, Figure 5 ).
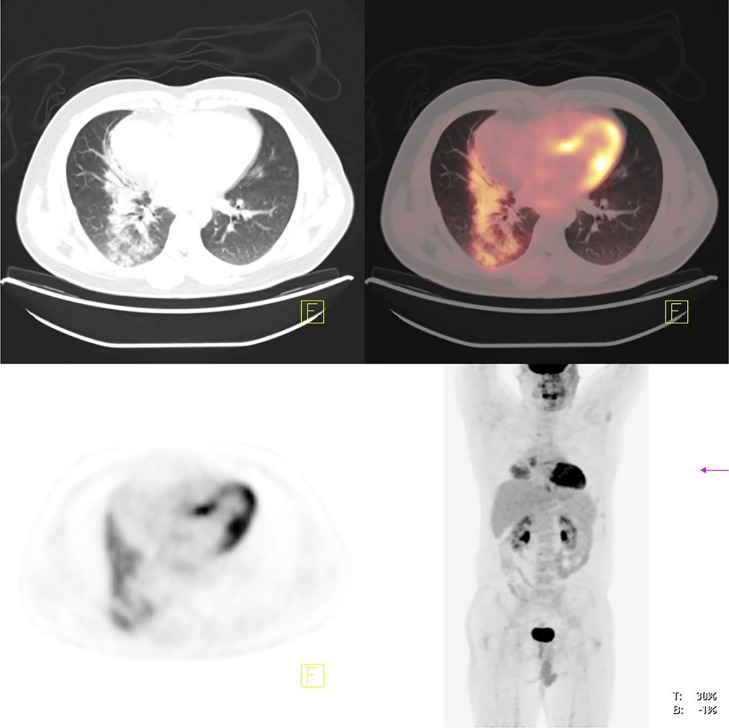
Figure 2.
A 33-year-old male with history of Hodgkin's lymphoma was referred for follow-up post-treatment imaging. 18FDG-PET/CT scan revealed mild residual metabolic activity of the mediastinal lymph nodes and focal ill defined hypermetabolic consolidation in right lung base, consistent with atypical pneumonia. The diagnosis of COVID-19 was confirmed by PCR.
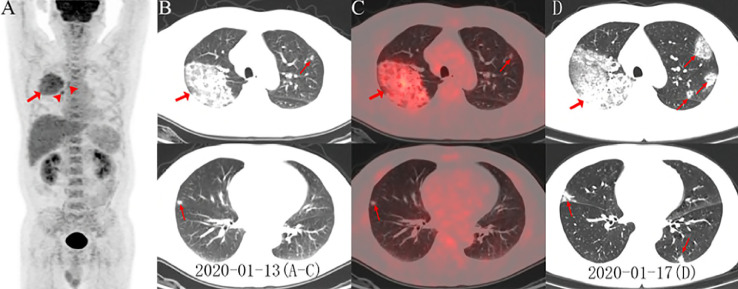
Figure 3.
(A) PET maximum intensity projection (MIP) image of patient no. 51 revealed an FDG-avid mass with a SUVmax of 4.9 in the right lung. Increased accumulation of FDG in the right paratracheal, right hilar lymph nodes (arrowheads), and bone marrow were also noted. On axial images (B, low dose CT; C, PET/CT fusion) there were ground-glass opacities (GGOs) with areas of focal consolidation primarily in the right upper lobe (arrows) and a focal opacity in the left upper and right middle lobes (arrows). Follow-up CT 4-day later demonstrated progression of lesions in bilateral upper and middle lobes with newly developed focal opacities in the left upper and lower lobes (D, arrows). Images obtained from Zou et al,16Radiology, March 6, 2020 and permission to use granted by Ashley E. Daly, Senior Manager of Radiology, Radiological Society of North America (RSNA).
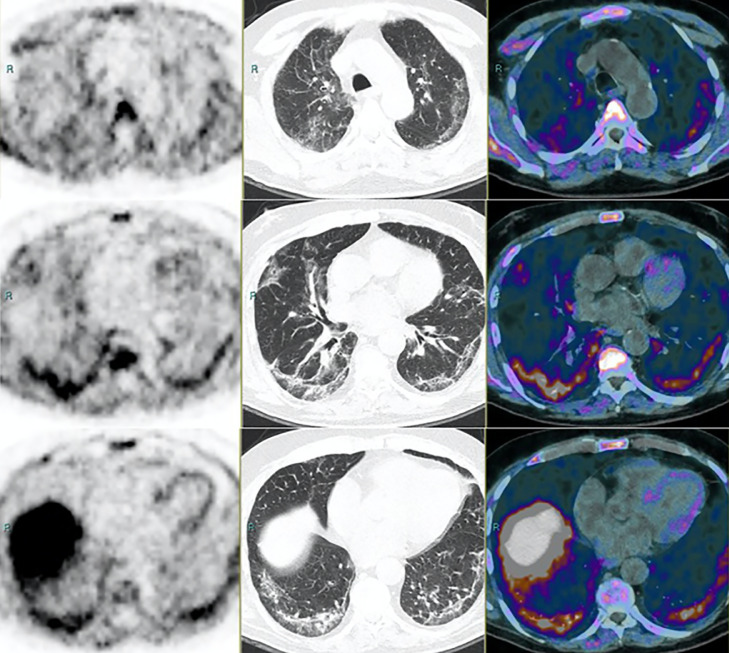
Figure 4.
PET (left panel), CT (middle), and 18-Fluorocholine PET/CT (right) of patient no. 22. First row: upper lung. Second row: middle lung. Third row: lower lung. The patient underwent examination within his routine follow-up for a prostate adenocarcinoma. PSA was 1.97 ng/mL and unchanged compared with the previous evaluation. The CT scan shows diffuse wide areas of solid-subsolid ground-glass opacities, bilateral and subpleural crazy-paving bronchovascular thickening. The patient referred with mild fever (>38°C), cough, fatigue, shortness of breath, ageusia-dysgeusia, and anosmia, all strongly suggestive symptoms for a Covid-19 infection. PET shows multiple irregular foci of mild to intense increased FDG uptake, corresponding to consolidations on CT. Images obtained from Savelli et al,17Medical Hypotheses, November 2020, Vol. 144(2020):109885, and permission to use granted by Elsevier and Copyright Clearance Center.
Figure 5.
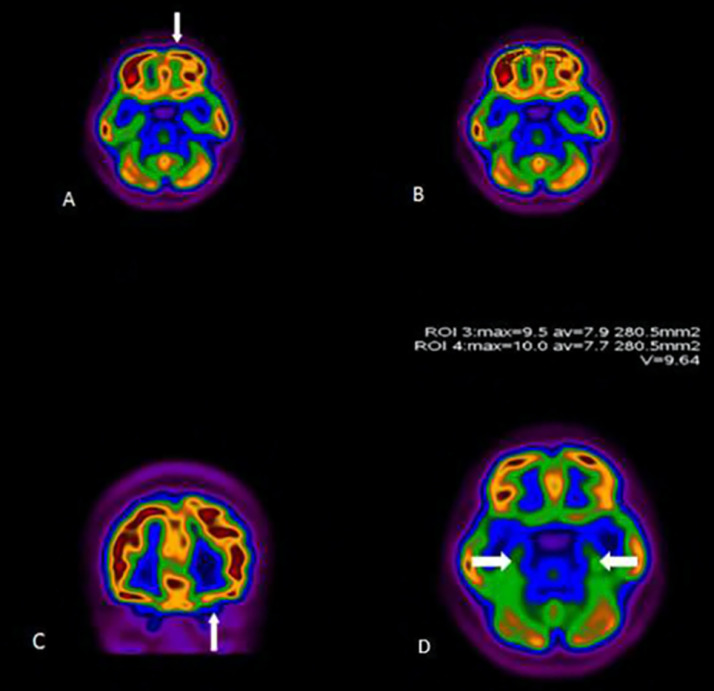
18FDG-PET/CT scan of a 27-year-old woman (patient no. 34) with persistent anosmia and positive PCR for SARS-CoV-2. Representative axial (A, B, D) and coronal (C) images are shown. There was decreased uptake in the left orbitofrontal cortex (arrows). The uptake of temporal lobes was symmetric and normal (arrows, D). Images obtained from Karimi-Galougahi et al,18Academic Radiology, July 2020, Vol. 27(7):1042-1043, and permission to use granted by Elsevier and Copyright Clearance Center.
Results
Included Studies
After excluding the duplicate search results and full-text analysis, 30 studies were included in the final review: 19 case reports, three case series, two cohort studies, and six letters to the editor and commentaries. According to the National Institutes of Health and Newcastle-Ottawa Quality Scale quality assessment tools, quality of included studies was predominantly fair (Supplementary Tables 1 and 2). Fifty-two patients with a mean age of 60 ± 12.74 (age range; 27-87, age Interquartile range; 55-67 years old) were included in this study, of which 28 (53.8%) were male, and 19 (36.5%) were female. In 5(9.6%) patients, the gender was not reported (Table 1 ). PET/CT scan was performed with 18F-FDG for 48 (92.3%), 18F-choline for 3 (5.8%), and 68Gallium-prostate-specific membrane antigen (68Ga-PSMA) for 1 (1.9%) patients. Additionally, one patient (no. 2) had both 18F-FDG-PET/CT and PET/MR. The mean SUV max of pulmonary lesions with 18F-FDG uptake was 4.9 ± 2.3, with a range of 2.2-18. Besides, mildly increased activity of pulmonary lesions was observed on 18F-choline and 68Ga-PSMA studies (SUVmax of 3.8 and 3.2, retrospectively). With regard to the patient's chronic disease, 39 (75%) cases had underlying malignancy, including 18 different types of cancer, presented in Table 2 . Five (9.6%) patients had metastases to other organs such as bone (n = 2), lung (n = 2) and liver (n = 1).
Table 1.
Characteristics of 52 Patients With COVID-19 Infection According to Pulmonary and Extrapulmonary PET/CT Imaging Findings
| Patient No./Sex/Age (y) | First Author | Country | Cancer Disease | Radiotracer Type | Extrapulmonary Findings (PET/CT Scan) | Pulmonary Findings (PET/CT Scan) |
|---|---|---|---|---|---|---|
| P1/F/65 | Castanheira19 | Portugal | Breast cancer | 18F-FDG | Ipsilateral hilar and subcarinal FDG-avid (SUVmax range: 4-5) LN | Unilateral and peripheral GGOs, Rt. Lower lobe interlobular thickening |
| P2/M/57 | Li20 | China | NR | 18F-FDG | Normal-sized LN in the mediastinum but with increased metabolic activity | Focal GGOs and bandlike opacities in both lungs without increased metabolic activity |
| P3/F/58 | Amin12 | Canada | Hodgkin's lymphoma | 18F-FDG | NR | Multifocal and bilateral peripheral GGOs, moderate18F-FDG activity (SUVmax:4.5) in Lt. lower lobe |
| P4/F/56 | Doroudinia13 | Iran | NR | 18F-FDG | NR | Bilateral and diffuse GGOs with increased metabolic activity, few bilateral hypermetabolic nodules |
| P5/M/60 | Mo21 | USA | SCC of tonsil | 18F-FDG | Foci of hilar and mediastinal FDG avidity | Irregular bilateral GGOs |
| P6/M/55 | Loforte22 | Italy | NR | 18F-FDG | NR | Multilobular and subpleural GGOs and consolidation in Rt. and Lt. inferior lobes (SUVmax:10.3-10.6) |
| P7/M/75 | Kamani23 | Switzerland | NR | 18F-FDG | Hypermetabolic lymphadenopathy in the Rt. lower paratracheal, subcarinal, and bilateral hilar stations (SUVmax:6.1) | Bilateral, hypermetabolic (SUVmax:7.6) focal GGOs with partial consolidation |
| P8/M/37 | Liu24 | China | NR | 18F-FDG | NR | Multiple bilateral 18F-FDG uptake, GGOs: at apical segment of left and right upper lobes and posterior segment of Lt. upper lobe |
| P9/M/57 | Qin25 | China | NR | 18F-FDG | NR | Peripheral GGOs with increased 18F-FDG uptake (SUVmax range:2.2-4.6) in Rt. upper lung |
| P10/M/56 | Qin25 | China | NR | 18F-FDG | Multiple FDG-avid LN in the mediastinum and the subclavian region (SUVmax range: 4.1-7.0) | Multiple FDG-positive GGOs (SUVmax range:7.9-18) in both lungs |
| P11/F/61 | Qin25 | China | NR | 18F-FDG | Multiple FDG-positive LN in the mediastinum and the Rt. subclavian region (SUVmax range: 3.4-5.4) | Multiple peripheral FDG-avid GGOs (SUVmax range: 3.7-12.2) in Rt. lung |
| P12/F/48 | Qin25 | China | NR | 18F-FDG | Multiple FDG-positive LN in the mediastinum and Rt. hilar region (SUVmax range: 3.8-5.5) | Peripheral FDG-avid GGOs (SUVmax range: 3.7-9.3) with bilateral interlobular septal thickening |
| P13/NR/53 | Czernin26 | Germany | Pancreatic cancer (NET) | 18F-FDG | NR | Hypermetabolic area in Rt. upper and lower lobe (SUVmax: 5.5) |
| P14/F/56 | Albano27 | Italy | Rectal cancer | 18F-FDG | NR | Bilateral 18F-FGD-positive GGOs and consolidation, most pronounced in the inferior lobes |
| P15/M/77 | Albano27 | Italy | Laryngeal cancer | 18F-FDG | NR | Bilateral faint 18F-FDG uptake in GGOs (not suggestive of aspiration) |
| P16/F/55 | Albano27 | Italy | Breast cancer (invasive ductal) | 18F-FDG | NR | Retrosternal 18F-FDG-avid nodal relapse, GGOs in the posterior segments of the inferior lung lobes |
| P17/F/55 | Albano27 | Italy | Hodgkin's lymphoma | 18F-FDG | Metabolically active lymphoma in the axillary LN | New interstitial opacities near the pleura of the Rt. lung |
| P18/M/65 | Albano27 | Italy | Laryngeal cancer | 18F-FDG | NR | Several GGOs with increased FDG uptake (SUVmax: 5.3) in Rt. lung |
| P19/F/65 | Albano27 | Italy | Ovarian cancer | 18F-FDG | 18F-FDG-avid mediastinal LN | Consolidative areas in both lungs with increased 18F-FDG uptake |
| P20/F/79 | Albano27 | Italy | Thyroid carcinoma (poorly differentiated) | 18F-FDG | NR | New diffuse interstitial pneumonia with peripheral GGOs |
| P21/M/75 | Sherman28 | USA | Non-Hodgkin's lymphoma | 18F-FDG | FDG-avid thoracic LN | Bilateral FDG-avid peripheral GGOs, multilobar consolidations |
| P22/NR/NR | Savelli17 | Italy | Prostate adenocarcinoma | 18F-choline | ageusia-dysgeusia and anosmia | Multiple irregular foci of increased FDG uptake |
| P23/M/73 | Polverari29 | Italy | NSCLC | 18F-FDG | Increased 18F-FDG uptake involving the Rt. lower paratracheal LN (SUVmax: 5.6) | Bilateral diffuse and intense FDG uptake (SUVmax: 5.9) in Rt. the lower and Lt. lower lobe (SUVmax: 7.9) |
| P24/M/82 | Amini30 | Iran | Colon cancer (adenocarcinoma) | 18F-FDG | Hypermetabolic mediastinal LN (SUVmax:4.5) | FDG activity in the Lt. lung (SUVmax range:1.5-8.6) and in the Rt. lung (SUV max range: 1.2-8.3) |
| P25/M/59 | Reed-Embleton31 | UK | Gastrointestinal stromal tumor | 18F-FDG | NR | FDG-avid prominently bilateral and peripheral GGOs in upper and lower lobes |
| P26/F/56 | Chuang32 | USA | SCC | 18F-FDG | NR | New bilateral multifocal hypermetabolic GGOs |
| P27/M/59 | Olivari33 | Italy | Recurrent prostate cancer | 18F-choline | NR | Bilateral subsegmental peripheral GGOs with increased choline uptake (SUV max range: 3–4) |
| P28/F/45 | Setti34 | Italy | Colon cancer | 18F-FDG | Modest mediastinal LN uptake | Consolidative opacities |
| P29/M/67 | Setti34 | Italy | Rectal cancer (adenocarcinoma) | 18F-FDG | NR | GGOs |
| P30/F/44 | Setti34 | Italy | Salivary gland carcinoma | 18F-FDG | Faint mediastinal LN uptake | Consolidative opacities and GGOs |
| P31/F/56 | Setti34 | Italy | Ovarian carcinoma (clear cell) | 18F-FDG | NR | GGOs |
| P32/M/70 | Setti34 | Italy | SCC of lung | 18F-FDG | NR | GGOs |
| P33/M/78 | Zanoni35 | Italy | Non-Hodgkin's lymphoma | 18F-FDG | Multiple FDG avid Lymphadenopathy above and below the diaphragm | Faint and diffuse uptake within a Lt. inferior lobe consolidation and a single non-FDG-avid peripheral rounded GGOs in the Rt. upper lobe |
| P34/F/27 | Karimi- Galougahi18 | Iran | NR | 18F-FDG | Lt. orbitofrontal cortex hypometabolism asymmetric confirmed to the lateral side. (SUVmax of 9.5 on the left cortex compared to 10 on the contralateral side) | NR |
| P35/M/73 | Kirienko36 | Italy | Vascular tumor of retroperitoneum | 18F-FDG | NR | Increased FDG uptake in the lungs |
| P36/F/52 | Playe37 | France | Non-Hodgkin's lymphoma (MCL) | 18F-FDG | Thoracic and subdiaphragmatic LN involvement with high FDG uptake (SUVmax:8.7) | Bilateral GGOs and curvilinear opacities, with predominance in the posterior subpleural areas associated with a moderate FDG uptake (SUVmax: 4) |
| P37/M/54 | Colandrea38 | Italy | Non-Hodgkin's lymphoma | 18F-FDG | Multiple FDG-avid LN in mediastinum and Lt. subclavian region | Intense 18F-FDG uptake on multiple bilateral subsegmental peripheral patchy GGOs with obscure boundaries and mainly subpleural distribution and areas of focal consolidation in the upper lobes |
| P38/M/61 | Colandrea38 | Italy | Lung and brain tumors (unknown origin) | 18F-FDG | Multiple FDG-positive areas in mediastinal, subcarinal and hilar LN | Intense 18F-FDG uptake on multiple peripheral GGOs in the Lt. lower lobe and areas of focal consolidation in the upper lobes |
| P39/M/48 | Colandrea38 | Italy | Lung cancer (stage IV) | 18F-FDG | Radiotracer uptake in the treated LN, due to the recent radiotherapy | Intense FDG uptake in a focal consolidation in the upper Lt. lobe, multiple peripheral GGOs with interstitial thickening and thin fibrous stripes in the Lt. lower lobe |
| P40/M/54 | Colandrea38 | Italy | Cheek melanoma | 18F-FDG | NR | Intense uptake of subpleural pseudo nodular thickenings in the Rt. lower lobe, multiple small subpleural GGOs and opacities bilaterally without18F-FDG uptake |
| P41/NR/NR | Colandrea38 | Italy | Tongue carcinoma | 18F-FDG | Focal FDG-positive area in Rt. hilar LN | Intense 18F-FDG uptake on multiple peripheral GGOs in both lower lobes and area of focal consolidation in the Rt. upper lobe |
| P42/M/80 | Scarlattei39 | Italy | Solid lung nodule | 18F-FDG | Active tracer uptake in Rt. hilar LN (SUVmax:2.5) | Increased tracer uptake (SUVmax: 2.6) corresponding to GGOs in the superior segment of the Lt. lobe with bronchovascular thickening showing mild tracer uptake (SUVmax: 2.5) |
| P43/F/57 | Scarlattei39 | Italy | Breast cancer (intraductal) | 18F-FDG | High intensity of tracer uptake in mediastinal, hilar, and carinal LN (SUVmax: 7) | Intense and diffuse uptake (SUVmax range in the Rt. Lung: 2.2-7.7; SUVmax range in the Lt. lung: 2.7-9.1) in both lungs corresponding to GGOs on CT images |
| P44/M/65 | Scarlattei39 | Italy | Prostate cancer (adenocarcinoma) | 68Ga-PSMA | NR | Mild tracer uptake (SUVmax: 3.2) in the subpleural region of both lungs, with greater extent in the Rt. lung, corresponding to CT findings of subpleural GGOs in the dependent lung |
| P45/M/70 | Scarlattei39 | Italy | Prostate cancer (adenocarcinoma) | 18F-choline | Focal tracer uptake in a hilar, carinal, and peribranchial LN (SUVmax: 3.4) | Mild focal tracer uptake (SUVmax: 3.8) in the subpleural region of both lungs and single GGOs localized in the middle lobe of the right lung |
| P46/F/57 | Scarlattei39 | Italy | Focal Splenic lesions | 18F-FDG | NR | Intense/moderate uptake (SUVmax range: 4.6-3.7) in the bilateral subpleural regions corresponding to CT findings of GGOs in the posterior segments |
| P47/NR/NR | Ajuria-Illarramendi40 | Spain | Lung cancer | 18F-FDG | NR | Heterogeneous GGOs with air bronchogram in the superior segment of the Rt. lower lobe with mild diffuse metabolic uptake (SUVmax: 3.9) |
| P48/NR/NR | Ajuria-Illarramendi40 | Spain | Lung cancer | 18F-FDG | NR | Paramedial consolidation and thickened interlobular septa in the Rt. lower lobe with high focal 18F-FDG avidity (SUVmax: 5.3) |
| P49/NR/NR | Ajuria-Illarramendi40 | Spain | Lung cancer | 18F-FDG | NR | Bibasilar opacities and fibrotic stripes with heterogeneous high metabolic activity (SUVmax:5) |
| P50/M/67 | Martineau41 | Canada | Lung cancer (adenocarcinoma) | 18F-FDG | Bilateral, hypermetabolic mediastinal lymphadenopathy, suggestive of reactive LN than metastatic disease | Relatively intense uptake (SUVs range:5.0-7.2) in the peripheral opacities |
| P51/M/55 | Zou16 | China | Suspect hilar malignancy | 18F-FDG | Increased FDG uptake of the Rt. paratracheal and Rt. hilar LN as well as bone marrow | FDG-avid mass (SUVmax:4.9) in the Rt. Lung. The GGOs with areas of focal consolidation primarily in the Rt. upper lobe and focal opacities in the Lt. upper and Rt. middle lobes |
| P52/M/87 | Krebs42 | USA | Primary salivary duct carcinoma | 18F-FDG | NR | Multiple areas of FDG-avid GGOs and patchy opacities with interlobular septal thickening in both lungs |
GGOs, ground-glass opacities; LN, lymph node; Lt., left; MCL, mantle cell lymphoma; NET, neuroendocrine tumor; NR, not reported; NSCLC, non–small-cell lung cancer; Rt., right; SCC, squamous cell carcinoma; SUV, standardized uptake values (all reported based on g/mL).
Table 2.
Types of Cancer in Study Patients
| Cancer Type (Subtypes) | Number of Patients |
|---|---|
| Breast cancer (invasive ductal, intraductal) | 3 |
| Hodgkin's lymphoma | 2 |
| Non-Hodgkin's lymphoma (mantle cell lymphoma) | 4 |
| Squamous cell carcinoma (tonsil, paratracheal lymph node) | 2 |
| Pancreas tumor (NET*) | 1 |
| Rectal cancer (adenocarcinoma of rectum, primary staging tumor) | 2 |
| Laryngeal cancer | 2 |
| Ovarian cancer (clear cells ovary carcinoma) | 2 |
| Thyroid carcinoma (poorly differentiated) | 1 |
| Prostate cancer | 4 |
| Colon adenocarcinoma | 2 |
| Gastrointestinal stromal tumor (GIST) | 1 |
| Salivary carcinoma (duct, gland carcinoma) | 2 |
| Vascular tumor of retroperitoneum | 1 |
| Lung cancer (NSCLC#, adenocarcinoma) | 8 |
| Cheek melanoma | 1 |
| Tongue carcinoma | 1 |
| Total | 39 |
NET, neuroendocrine tumor.
NSCLC, non–small-cell lung cancer.
PET/CT Findings
The most common pulmonary PET/CT findings were hypermetabolic bilateral GGOs in 39 (75%), followed by consolidation in 18 (34.6%), and interlobular thickening in 4 (7.6%). Moreover, mediastinal 14 (27%), and hilar 10 (19.2%) lymph node involvement with high metabolic activity was frequently seen (Table 3 ).
Table 3.
Types of Lymph Node Involvement in Study Patients
| Lymph Node Type | Number of Patients |
|---|---|
| Hilar | 10 |
| Subcarinal | 5 |
| Mediastinal | 14 |
| Subclavian | 3 |
| Paratracheal | 3 |
| Thoracic | 2 |
| Subdiaphragmatic | 2 |
| Total | 39 |
Four patients (no. 3, no. 8, no. 21, and no. 51) were discharged from the hospital; patient no. 3 was a known case of Hodgkin's lymphoma, with a history of diabetes and morbid obesity, who was referred for staging PET in an outpatient facility. She did not report any history of recent travel or contact with known cases of COVID-19. The PET/CT showed stage II right pelvic adenopathy with only mild 18F-FDG activity (SUVmax 2.9). Multifocal bilateral peripheral pulmonary opacities with moderate 18F-FDG activity (SUVmax 4.5) were identified in the left lower lobe. Two days later, she developed a runny nose, coughing, and fever (38°C). She was discharged uneventfully after 1 week of inpatient management, but her pelvic radiation was postponed due to the restrictions carried out during the COVID-19 pandemic.
Patient no. 8 was a Wuhan resident, who presented to the hospital with diarrhea and vertigo for 2 days. Following detection of GGOs in the upper lobe of the left lung in the chest CT scan, 18F-FDG-PET/CT was performed to differentiate between benign or malignant etiologies. The maximum intensive projection image showed 18F-FDG uptake in bilateral lungs, with the SUVmax ranging from 2.4 to 12.4 in lung GGOs. The patient received antibiotics and anti-inflammatory therapy against COVID-19. Follow-up chest CT on day nine of admission demonstrated interval progression of GGOs. Follow-up chest CT on day nineteen of admission demonstrated interval resolution of the previously identified GGOs with minimal residual consolidation. The patient was symptom-free since day 18 and was discharged from hospital on day 30.
Patient no. 51, again from Wuhan, presented with fever, fatigue and dry cough for 5 days. His previous CT suggested hilar malignancy. 18F-FDG PET/CT showed an FDG-avid mass with a SUVmax of 4.9 in the right lung and increased uptake of FDG in the right paratracheal and right hilar lymph nodes, as well as bone marrow (Fig. 3). First nasopharyngeal swab test was negative for COVID-19 infections. However, the second RT-PCR test turned positive for COVID-19 and the patient recovered and discharged from the hospital after ten days of admission.
In terms of COVID-related extrapulmonary PET/CT findings, only one patient (no. 34) with anosmia was reported. She was a 27-year-old woman with no respiratory problems, with persistent isolated anosmia for six weeks. PET/CT revealed mild asymmetric hypometabolism in the left orbitofrontal cortex (SUVmax of 9.5 on the left side, in comparison with 10 on the right side). Though, FDG uptake in the left inferior temporal cortex was normal (Fig. 5).
18F-choline PET/CT was performed for only three patients (no. 22, no. 27 and no. 45). Patient no. 27 was a 59-year-old man with a history of recurrent prostate cancer. 18F-choline PET/CT performed after radical prostatectomy for the evaluation of disease spread. This patient had no respiratory symptoms or fever. Choline PET revealed bilateral subsegmental peripheral areas of GGOs with an increased choline-PET uptake (SUV max ranging from 3 to 4). No pleural effusion or mediastinal lymph node involvement was noted. In addition, bone metastases with no pelvic nodal involvement were detected. After 36 hours of choline PET examination, the patient developed dizziness, fatigue, and respiratory failure, and thus was admitted to the hospital.
Discussion
As COVID-19 continues throughout the world, a variety of imaging findings have been reported. Chest imaging (chest X-ray and CT) has been successfully employed as a practical supplement to RT-PCR testing for COVID-19 diagnosis. Moreover, CT can be used as a valuable tool for evaluation and monitoring of lung lesions in COVID-19. Multiple bilateral GGOs with a peripheral and posterior distribution with or without superimposed consolidations are the typical CT manifestations of SARS-CoV-2 infection in the lungs.
PET imaging is commonly performed for patients with underlying inflammatory or neoplastic disease and guides the therapeutic strategies of these patients by evaluating the extent and burden of the disease and staging. Although based on the available reports, COVID-19 can be detected by PET/CT, several factors might explain why routine molecular imaging does not seem beneficial in the evaluation of COVID-19. PET is generally considered a high-cost imaging modality with prolonged acquisition time. It also has increased radiation exposure compared to chest X-ray and chest CT that are commonly employed in the diagnostic work-up of these patients. Additionally, there is a high-risk of disease transmission to the staff and other patients who have been in contact with the patient.43, 44, 45
However, in the era of COVID-19, all PET studies, no matter what the clinical indication is, should be screened by the reading nuclear radiologists for COVID-19. In fact, the interpretation of PET/CT should not be restricted to the assessment of neoplastic disease. With the growing incidence of SARS-CoV-2 pneumonia, radiologists and nuclear medicine physicians interpreting PET/CTs and PET/MRIs should be educated about the imaging features of COVID-19 on molecular imaging. COVID-19 should be considered among the differential diagnoses of suspicious imaging features, such as hypermetabolic pulmonary infiltrates and mediastinal and hilar lymphadenopathy, for which constant vigilance is crucial. More specifically, due to atypical clinical features of COVID-19 infection in immunocompromised or high-risk patients, the detection of SARS-CoV-2 pneumonia on PET imaging is of paramount clinical importance.46
Furthermore, these patients are more prone to developing complications related to SARS-CoV-2 infection, with increased rates of hospital admissions and mortality, stressing the need for early detection and management of SARS-CoV-2 pneumonia in this high-risk population. The detection of incidental COVID-19 pneumonia in FDG-PET/CT findings has been common in recent studies.16 , 17 , 23 However, the reports are so limited and sparse, and a comprehensive review of these manifestations is still lacking. Here, we systematically reviewed PET/CT findings in the available published articles describing incidental SARS-CoV-2 pneumonia features. To the best of our knowledge, this is the first systematic review in this regard. We found bilateral GGOs, consolidation, and interlobular thickening as the most common CT features in COVID-19 PET/CT imaging, consistent with previous radiologic reports.47 , 48 Furthermore, these pulmonary lesions displayed increased FDG uptake, which is not unexpected, as acute pneumonia is characterized by hypermetabolism in PET imaging. Reported SUVs have ranged from 2.2 to 18, with a mean pulmonary lesions SUVmax of 4.9. In those cases which demonstrate significantly high metabolic activity, we could suggest follow-up imaging to complete resolution of hypermetabolic space occupying pulmonary lesions to exclude underlying neoplastic lesions.
Sufficient evidence about the potential utility of FDG-PET/CT in the diagnosis and management of infectious and inflammatory diseases already exists.49 Increased glycolytic activity in the inflammatory cells enables FDG-PET to identify active inflammation sites throughout the body. Besides, the acute infectious process in COVID-19 could also be mapped via PET imaging in patients with immunocompromising underlying diseases, such as cancers. Additionally noted, even in the absence of nodal enlargement by size criteria of anatomic imaging, increased tracer uptake was frequently seen in the mediastinal and hilar lymph nodes of COVID-19 patients. This suggests that SARS-CoV-2 pneumonia causes lymphadenitis presenting with increased FDG uptake, which contradicts previous radiologic studies about rare nodal involvement in COVID-19 pneumonia.50 , 51
Moreover, the extrapulmonary findings of COVID-19 have been reported recently in literature, causing complications in the brain, gastrointestinal tract, heart and kidneys.52 , 53 In this era, a unique advantage of whole-body PET/CT over competing imaging modalities is the potential for detection of additional incidental metabolically active lesions throughout the rest of the body in a single acquisition. Karimi et al18 (no. 34) have reported reduced activity in the orbitofrontal cortex in COVID-19-associated anosmia, which might indicate an impaired neural function as the underlying pathology of anosmia, due to SARS-CoV-2 neurotropism. Also, Zou et al16 (no. 51) reported bone marrow uptakes in COVID-19, similar to the previous reports of Middle East respiratory syndrome in nonhuman models.54 This suggests that FDG-PET might be able to assess the end-organ damages of COVID-19. Increased uptake of COVID-19 pulmonary lesions is not limited to FDG-PET studies. Other radiopharmaceuticals have also shown mild increased activity in the pulmonary lesions and lymphadenopathy of COVID-19. Scarlattei et al39 reported cases (no. 44 and no. 45) of mildly increased activity of pulmonary consolidation in 68Ga-PSMA (SUVmax of 3.2). They also reported mildly increased uptake of pulmonary consolidation (SUVmax of 3.8) and reactive mediastinal lymphadenopathy (SUVmax of 3.4) in 18F- choline study.
As mentioned above, FDG-PET/CT holds an important role in monitoring the disease activity, and response evaluation of lung inflammatory pathologies. Deng et al55 suggested that higher FDG uptake in pulmonary lesions is positively correlated with both longer healing time and the erythrocyte sedimentation rate value. They reported a patient with a pulmonary lesions SUVmax of 4.6 recovered faster than a patient with a SUVmax of 12.2 (17 days compared to 26 days after the symptom onset). However, these case findings should be confirmed in larger studies before drawing a definite conclusion. Due to some disadvantages of PET imaging as mentioned above, no serial PET imaging is also present to date. However, it is believed that molecular imaging with PET is potentially able to quantify the lung inflammation during the course treatment of lung inflammatory diseases.
Our study has some limitations. First, since per the international guidelines, the elective nuclear medicine operations are not usually performed in patients with confirmed COVID-19, and most of the current reports on the detection of COVID-19 in molecular imaging studies have been obtained in asymptomatic cases. Hence, the imaging manifestations of patients with known infection and/or severe complications have not been studied yet. Second, the limited number of reports in this era might limit the interpretation, and thus further studies are warranted.
Overall, the potential value of PET imaging to better understand and characterize the disease might help us improve our coping strategies during this ongoing pandemic. However, although contested by many the fact that PET can be a noninvasive beneficial imaging tool in the early detection and monitoring COVID-19 pneumonia in high-risk patients, we believe that PET should not be recommended in the diagnostic work-up of COVID-19 and other infectious types of pneumonia, as poses the risk of the disease spreading in nuclear medicine units and other imaging modalities are available that can provide sufficient clinical and diagnostic information.56 Thus, more investigations with modified protocols are needed in future, to fully explore the possible clinical indications of molecular imaging for characterizing the disease severity and its prognosis.57 Incidental detection of different abnormalities in nuclear medicine studies has been widely discussed in the literature. Accordingly, we strongly recommend the evaluation of PET images for previously unsuspected pathologies in daily practice, as this may eventually lead to uncovering of crucial pathologies that significantly change the patient management and prognosis. This is particularly important with the evolving diseases of the 21st century.45 , 46 , 58
Conclusion
This systematic review gains a deep insight over the diagnostic value of detecting COVID-19 on PET imaging performed for other clinical indications. As patients undergoing PET imaging usually suffer from immunocompromising underlying diseases, such as cancers, early detection of potentially life-threatening and devastating pneumonia in these patients is of paramount importance to minimize the disease complications and limit the progression. This not only helps to uncover clinically unsuspected COVID-19 in this sensitive population, but also helps ensure appropriate postexposure precautions are implemented for the department and hospital staff and those who have been in contact with the patient.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was not required because it was a literature study.
Ethical Approval
Institutional Review Board approval was not required because it was a literature study.
Authors’ Contributions
All authors designed the study and wrote the manuscript. FR, PK, SFN, FE performed literature research. FR, PK, AGH conducted manuscript editing. FR, PK, and AGH significantly supported conception and design of the study.
Acknowledgments
The authors would like to thank Dr S. Zou, Dr G. Savelli, and Dr M. Karimi-Galougahi for providing the images and figures for this study and also, thank to publisher holder of Radiology (RSNA), Medical Hypotheses, and Academic Radiology Journal for granting permission to use the images.
Footnotes
Funding information: The authors state that this work has not received any funding.
Supplementary material associated with this article can be found, in the online version, at doi:10.1053/j.semnuclmed.2020.10.002.
Appendix. Supplementary materials
References
- 1.Yang X, Yu Y, Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Resp Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Organization WH . 2020. Coronavirus Disease (COVID-2019) Situation Reports.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ Available at: Accessed July 23, 2020. [Google Scholar]
- 3.Salehi S, Abedi A, Balakrishnan S. Coronavirus disease 2019 (COVID-19): A systematic review of imaging findings in 919 patients. AJR Am J of Roentgenol. 2020;250:87–93. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 4.Ai T, Yang Z, Hou H. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology. 2020;293:E2–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashraf MA, Keshavarz P, Hosseinpour P. Coronavirus disease 2019 (COVID-19): a systematic review of pregnancy and the possibility of vertical transmission. J Reprod Infertil. 2020;21:157–168. [PMC free article] [PubMed] [Google Scholar]
- 6.Katal S, Aghaghazvini L, Gholamrezanezhad A. Chest-CT findings of COVID-19 in patients with pre-existing malignancies; a pictorial review. Clin Imaging. 2020;67:121–129. doi: 10.1016/j.clinimag.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye Z, Zhang Y, Wang Y. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): A pictorial review. Eur Radiol. 2020;30:4081–4089. doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salehi S, Reddy S, Gholamrezanezhad A. Long-term pulmonary consequences of coronavirus disease 2019 (COVID-19): What we know and what to expect. J Thorac Imaging. 2020;35:W87–W89. doi: 10.1097/RTI.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 9.Manna S, Wruble J, Maron SZ. COVID-19: A multimodality review of radiologic techniques, clinical utility, and imaging features. Radiol: Cardiothorac Imaging. 2020;2 doi: 10.1148/ryct.2020200210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haroon A, Zumla A, Bomanji J. Role of fluorine 18 fluorodeoxyglucose positron emission tomography–computed tomography in focal and generalized infectious and inflammatory disorders. Clin Infect Dis. 2020;54:1333–1341. doi: 10.1093/cid/cis193. [DOI] [PubMed] [Google Scholar]
- 11.Giraudo C, Evangelista L, Fraia AS. Molecular imaging of pulmonary inflammation and infection. Int J Mol Sci. 2020;21:894–915. doi: 10.3390/ijms21030894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amin R, Grinblat L, Husain M. Incidental COVID-19 on PET/CT imaging. CMAJ. 2020;192 doi: 10.1503/cmaj.200831. E631-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doroudinia A, Tavakoli M. A case of coronavirus infection incidentally found on FDG PET/CT scan. Clin Nucl Med. 2020;45:e303–e304. doi: 10.1097/RLU.0000000000003084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.aBIw NHL. Study Quality Assessment Tools. Available at: http://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed 6 April 2020.
- 15.Margulis AV, Pladevall M, Riera-Guardia N. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle–Ottawa scale and the RTI item bank. Clin Epidemiol. 2014;6:359–365. doi: 10.2147/CLEP.S66677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou S, Zhu X. FDG PET/CT of COVID-19. Radiology. 2020;296:E118. doi: 10.1148/radiol.2020200770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savelli G, Bonacina M, Rizzo A. Activated macrophages are the main inflammatory cell in COVID-19 interstitial pneumonia infiltrates. Is it possible to show their metabolic activity and thus the grade of inflammatory burden with 18F-Fluorocholine PET/CT. Med Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.109885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karimi-Galougahi M, Yousefi-Koma A, Bakhshayeshkaram M. 18FDG PET/CT scan reveals hypoactive orbitofrontal cortex in anosmia of COVID-19. Acad Radiol. 2020;27:1042–1043. doi: 10.1016/j.acra.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castanheira J, Gaivão AM, Teixeira SM. Asymptomatic COVID-19 positive patient suspected on FDG-PET/CT. Nucl Med Commun. 2020;41:598–599. doi: 10.1097/MNM.0000000000001221. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Wang Y, Bai Y. PET/MR and PET/CT in a severe COVID-19 patient. Eur J Nucl Med Mol Imaging. 2020;47:2078–2079. doi: 10.1007/s00259-020-04887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mo A, Brodin NP, Tomé WA. COVID-19 incidentally detected on PET/CT during work-up for locally advanced head and neck cancer. In Vivo. 2020;34(3 suppl):1681–1684. doi: 10.21873/invivo.11961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loforte A, Gliozzi G, Suarez SM. Positron emission tomography for detecting COVID-19 in asymptomatic left ventricular assist device recipients. Asaio J. 2020;66:599–602. doi: 10.1097/MAT.0000000000001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamani CH, Jreige M, Pappon M. Added value of 18F-FDG PET/CT in a SARS-CoV-2-infected complex case with persistent fever. Eur J Nucl Med Mol Imaging. 2020;47:2036-37:2036–2037. doi: 10.1007/s00259-020-04860-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu C, Zhou J, Xia L. 18F-FDG PET/CT and serial chest CT findings in a COVID-19 patient with dynamic clinical characteristics in different period. Clin Nucl Med. 2020;45:495–496. doi: 10.1097/RLU.0000000000003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin C, Liu F, Yen T-C. 18 F-FDG PET/CT findings of COVID-19: A series of four highly suspected cases. Eur J Nucl Med Mol Imaging. 2020;47:1281–1286. doi: 10.1007/s00259-020-04734-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Czernin J, Fanti S, Meyer PT. Nuclear medicine operations in the times of COVID-19: Strategies, precautions, and experiences. J Nucl Med. 2020;61:626–629. doi: 10.2967/jnumed.120.245738. [DOI] [PubMed] [Google Scholar]
- 27.Albano D, Bertagna F, Bertoli M. Incidental findings suggestive of COVID-19 in asymptomatic patients undergoing nuclear medicine procedures in a high-prevalence region. J Nucl Med. 2020;61:632–636. doi: 10.2967/jnumed.120.246256. [DOI] [PubMed] [Google Scholar]
- 28.Sherman FT, Lechich AJ. Nursing home physician with normal chest X-ray and probable COVID-19 based on 18F-FDG PET/CT imaging. J Am Geriatr Soc. 2020;68:1918–1920. doi: 10.1111/jgs.16680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polverari G, Arena V, Ceci F. 18F-fluorodeoxyglucose uptake in patient with asymptomatic severe acute respiratory syndrome coronavirus 2 (coronavirus disease 2019) referred to positron emission tomography/computed tomography for NSCLC restaging. J Thorac Oncol. 2020;15:1078–1080. doi: 10.1016/j.jtho.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amini H, Divband G, Montahaei Z. A case of COVID-19 lung infection first detected by [18F] FDG PET-CT. Eur J Nucl Med Mol Imaging. 2020;47:1771–1772. doi: 10.1007/s00259-020-04821-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reed-Embleton H, Khan KS, Mathias N. Case report: Incidental findings of COVID-19 infection on positron emission tomography/computed tomography for staging of a giant gastric gastrointestinal stromal tumour. Pan Afr Med J. 2020;35:28. doi: 10.11604/pamj.supp.2020.35.2.23167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chuang HH, Emery DJ, Campbell RM. FDG PET/CT in diagnosing COVID-19 infection in a cancer patient with exposure history but minimal symptoms. Clin Nucl Med. 2020;45:656–658. doi: 10.1097/RLU.0000000000003137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olivari L, Riccardi N, Rodari P. COVID-19 pneumonia: Increased choline uptake with 18F-choline PET/CT. Eur J Nucl Med Mol Imaging. 2020;47:2476–2477. doi: 10.1007/s00259-020-04870-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Setti L, Kirienko M, Dalto SC. FDG-PET/CT findings highly suspicious for COVID-19 in an Italian case series of asymptomatic patients. Eur J Nucl Med Mol Imaging. 2020;47:1649–1656. doi: 10.1007/s00259-020-04819-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zanoni L, Mosconi C, Cervati V. [18F]-FDG PET/CT for suspected lymphoma relapse in a patient with concomitant pneumococcal pneumonia during COVID-19 outbreak: Unexpected SARS-Cov-2 co-infection despite double RT-PCR negativity. Eur J Nucl Med Mol Imaging. 2020:2038–2039. doi: 10.1007/s00259-020-04838-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirienko M, Padovano B, Serafini G. CT,[18F] FDG-PET/CT and clinical findings before and during early Covid-19 onset in a patient affected by vascular tumour. Eur J Nucl Med Mol Imaging. 2020;47:1769–1770. doi: 10.1007/s00259-020-04822-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Playe M, Siavellis J, Braun T. FDG PET/CT in a patient with mantle cell lymphoma and COVID-19: Typical findings. Clin Nucl Med. 2020;45:e305–e306. doi: 10.1097/RLU.0000000000003113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colandrea M, Gilardi L, Travaini LL. 18F-FDG PET/CT in asymptomatic patients with COVID-19: The submerged iceberg surfaces. Researchsquare. 2020;38:1007–1011. doi: 10.1007/s11604-020-01006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scarlattei M, Baldari G, Silva M. Unknown SARS-CoV-2 pneumonia detected by PET/CT in patients with cancer. Tumori. 2020;106:325–332. doi: 10.1177/0300891620935983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ajuria-Illarramendi O, Martinez Lorca A, Orduña-Diez M. [18F]FDG-PET/CT in different COVID-19 phases. IDCases. 2020;21:e00869. doi: 10.1016/j.idcr.2020.e00869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martineau P, Kidane B. FDG PET/CT findings in an asymptomatic case of confirmed COVID-19. Clin Nucl Med. 2020;45:647–648. doi: 10.1097/RLU.0000000000003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krebs S, Petkovska I, Ho AL. Laboratory-proven asymptomatic SARS-CoV-2 (COVID-19) infection on 18F-FDG PET/CT. Clin Nucl Med. 2020;45:654–655. doi: 10.1097/RLU.0000000000003141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Uden D, Prins M, Siesling S. [18F] FDG PET/CT in the staging of inflammatory breast cancer: A systematic review. Crit Rev Oncol Hematol. 2020;15 doi: 10.1016/j.critrevonc.2020.102943. [DOI] [PubMed] [Google Scholar]
- 44.Alavi A, Hess S, Werner TJ. An update on the unparalleled impact of FDG-PET imaging on the day-to-day practice of medicine with emphasis on management of infectious/inflammatory disorders. Eur J Nucl Med Mol Imaging. 2020;47:18–27. doi: 10.1007/s00259-019-04490-6. [DOI] [PubMed] [Google Scholar]
- 45.Gholamrezanezhad A, Guermazi A, Salavati A. PET-computed tomography and PET-MR imaging and their applications in the twenty-first century. PET Clin. 2019;14 doi: 10.1016/j.cpet.2018.09.001. xv-xvii. [DOI] [PubMed] [Google Scholar]
- 46.Behzad S, Velez E, Najafi MH. Coronavirus disease 2019 (COVID-19) pneumonia incidentally detected on coronary CT angiogram: a do-not-miss diagnosis. Emerg Radiol. 2020:4. doi: 10.1007/s10140-020-01802-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hosseiny M, Kooraki S, Gholamrezanezhad A. Radiology perspective of coronavirus disease 2019 (COVID-19): Lessons from severe acute respiratory syndrome and Middle East respiratory syndrome. AJR Am J Roentgenol. 2020;214:1078–1082. doi: 10.2214/AJR.20.22969. [DOI] [PubMed] [Google Scholar]
- 48.Hu Q, Guan H, Sun Z. Early CT features and temporal lung changes in COVID-19 pneumonia in Wuhan, China. Eur J Radiol. 2020;128 doi: 10.1016/j.ejrad.2020.109017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Treglia G. Diagnostic performance of 18F-FDG PET/CT in infectious and inflammatory diseases according to published meta-analyses. Contrast Media Mol Imaging. 2019;2019:1–12. doi: 10.1155/2019/3018349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han R, Huang L, Jiang H. Early clinical and CT manifestations of coronavirus disease 2019 (COVID-19) pneumonia. AJR Am J Roentgenol. 2020;215:338–343. doi: 10.2214/AJR.20.22961. [DOI] [PubMed] [Google Scholar]
- 51.Wu J, Wu X, Zeng W. Chest CT findings in patients with coronavirus disease 2019 and its relationship with clinical features. Invest Radiol. 2020;55:257–261. doi: 10.1097/RLI.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katal S, Balakrishnan S, Gholamrezanezhad A. Neuroimaging findings in COVID-19 and other coronavirus infections: A systematic review in 116 patients. Neuroradiology. 2020;922:1–8. doi: 10.1016/j.neurad.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Behzad S, Aghaghazvini L, Radmard AR. Extrapulmonary manifestations of COVID-19: Radiologic and clinical overview. Clin Imaging. 2020;66:35–41. doi: 10.1016/j.clinimag.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chefer S, Thomasson D, Seidel J. Modeling [18 F]-FDG lymphoid tissue kinetics to characterize nonhuman primate immune response to Middle East respiratory syndrome-coronavirus aerosol challenge. Eur J Nucl Med Mol Imaging. 2015;5:1–11. doi: 10.1186/s13550-015-0143-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deng Y, Lei L, Chen Y. The potential added value of FDG PET/CT for COVID-19 pneumonia. Eur J Nucl Med Mol Imaging. 2020;47:1634–1635. doi: 10.1007/s00259-020-04767-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joob B, Wiwanitkit V. 18F-FDG PET/CT and COVID-19. Eur J Nucl Med Mol Imaging. 2020;47:1348. doi: 10.1007/s00259-020-04762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guedj E, Verger A, Cammilleri S. PET imaging of COVID-19: The target and the number. Eur J Nucl Med Mol Imaging. 2020;47:1636–1637. doi: 10.1007/s00259-020-04820-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gholamrezanezhad A, Mirpour S. An important but easily forgettable review: Extracardiac activity in myocardial perfusion scans. Int Cardiovasc Imaging. 2007;23:207–208. doi: 10.1007/s10554-006-9137-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.