Abstract
Background
Animal models and few clinical reports suggest the involvement of the complement system in the onset of severe manifestations of coronavirus disease-2019 (COVID-19). However, complement contribution to endotheliopathy and hypercoagulability has not been elucidated yet.
Objective
To evaluate the association among complement activation, endothelial damage and disease severity or activity in COVID-19 patients.
Methods
In this single-centre cohort study, 148 patients with COVID-19 of different severity were evaluated upon hospital admission and 30 days later. Markers of complement activation (SC5b-9 and C5a) and endothelial perturbation (von Willebrand factor [vWF], tissue-type plasminogen activator [t-PA], plasminogen activator inhibitor-1 [PAI-1], soluble thrombomodulin [sTM], and soluble endothelial selectin [sE-selectin]) were measured in plasma.
Results
The patients had high plasma levels of SC5b-9 and C5a (p = 0.0001 for both) and vWF, t-PA and PAI-1 (p = 0.0001 for all). Their SC5b-9 levels correlated with those of vWF (r = 0.517, p = 0.0001) and paralleled disease severity (severe vs mild p = 0.0001, severe vs moderate p = 0.026 and moderate vs mild p = 0.001). The levels of sE-selectin were significantly increased only in the patients with severe disease. After 30 days, plasma SC5b-9, C5a and vWF levels had significantly decreased (p = 0.0001 for all), and 43% of the evaluated patients had normal levels.
Conclusions
Complement activation is boosted during the progression of COVID-19 and dampened during remission, thus indicating its role in the pathophysiology of the disease. The association between complement activation and the biomarkers of endothelial damage suggests that complement may contribute to tissue injury and could be the target of specific therapy.
Keywords: Complement, von Willebrand factor, SC5b-9, C5a, COVID-19, Endothelium
Highlights
-
•
Levels of complement activation products increase with COVID19 severity and activity.
-
•
Complement activation is correlated with endothelium damage in COVID19.
-
•
Complement mediates the response to SARSCoV2 and gives a rationale for target therapy.
1. Introduction
Coronavirus disease 2019 (COVID-19) is due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and characterised by clinical symptoms ranging from minor upper airway manifestations to severe and life-threatening acute respiratory distress syndrome (ARDS), which is observed in approximately 15% of patients [[1], [2], [3]]. Increased levels of pro-inflammatory cytokines suggest that a sustained inflammatory response to the virus plays a role in the clinical manifestations of the disease [4], and these are associated with increased levels of markers of coagulation activation that justify the use of anticoagulant therapy [[4], [5], [6]]. This thrombophilic state has been linked to endothelial damage that is mediated by the cytokine storm and SARS-CoV-2 endothelial cell tropism via surface angiotensin-converting enzyme 2 (ACE2). Preliminary evidence of an association between increased circulating levels of biomarkers of endothelial damage and clinical outcomes suggests that an endotheliopathy may be critical for COVID-19 associated coagulopathy [[7], [8], [9]].
The pro-inflammatory and pro-thrombotic state associated with COVID-19 resembles that observed in patients with various autoimmune/inflammatory disorders in which the complement system is involved [10,11], and suggests that it is worth looking for signs of complement activation in COVID-19 patients [[12], [13], [14], [15]]. The complement system is a key mediator of innate immune responses but, in addition to protecting against infectious agents [16,17], it also contributes to the hyperinflammatory response in concert with coagulation [11,17]. The activation peptide of complement component 5 (C5a) and the membrane attack complex (MAC/C5b-9) drive neutrophil activation and the inflammation that eventually leads to endothelial activation/damage without necessarily mediating cell lysis [11,18,19].
However, although the role of complement in the acute respiratory distress syndrome (ARDS) caused by previous SARS coronaviruses has been established by experimental studies [20,21], its contribution to COVID-19 has been less widely investigated. Magro et al. found the tissue deposition of complement activation products (C5b-9, C4d, and MASP2 [mannose-binding protein-associated serine protease 2]) in five COVID-19 patients [12], and we reported for the first time significantly increased plasma levels of C5a and C5b-9 in a series of 31 patients with moderate or severe COVID-19 [13], a finding that has recently been confirmed by Carvelli et al. in 67 patients [14] and by Peffault de Latour et al. in 95 patients [22]. Carvelli et al. also suggested that the C5a-C5aR1 axis may play a role in the inflammatory mechanisms underlying the development of ARDS [14]. Finally, the findings of a quite recent multimodal analytical study further support the view that complement and coagulation hyperactivation predisposes COVID-19 patients to adverse outcomes [15]. However, there is still no published information concerning the relationship between complement activation and endothelial damage in COVID-19 patients. With this as background, we evaluated markers of complement activation and of endothelial damage in a cohort of 148 patients with COVID-19 of different severity during the acute phase of the disease at admission and during remission after 30 days of follow-up.
2. Materials and methods
2.1. Patients
We studied 148 patients (87 males and 61 females; median age 63 years, range 26–92) who were admitted to our hospital between March 1 and April 15, 2020. The inclusion criteria were an age of >18 years and a diagnosis of COVID-19 confirmed by means of a positive RT-PCR for SARS-CoV-2 in at least one biological sample; the exclusion criteria were an infectious disease other than SARS-CoV2, previous or current autoimmune diseases, and pregnancy.
On the basis of the clinical, radiological and laboratory data, the severity of COVID-19 was considered to be mild when the patients were kept under observation with low intensity care, moderate when they required intermediate care with non-invasive ventilation, or severe when they were admitted to an intensive care unit for mechanical ventilation. The disease was mild in 58 patients, moderate in 44, and severe in 46. The controls were 27 healthy subjects: (19 men and eight women with a median age of 55 years, range 34–78).
On the day of admission or the following day, antecubital vein blood samples were collected into EDTA tubes for the measurement of soluble C5b-9 (SC5b-9) and C5a, and sodium citrate tubes for the measurement of endothelial markers. The samples were processed within 2 h by means of centrifugation at 2000×g for 15 min at room temperature, and the plasma aliquots were immediately frozen and stored at −80C° until testing.
In addition to the plasma levels of SC5b-9 and C5a as markers of complement activation, we measured the following markers of endothelial damage: von Willebrand factor (vWF), tissue-type plasminogen activator (t-PA), plasminogen activator inhibitor-1 (PAI-1), soluble thrombomodulin (sTM), and soluble E-selectin (sE-selectin). The laboratory parameters collected from the patients’ clinical records were fibrin fragment D-dimer, C-reactive protein (CRP), interleukin-6 (IL-6), ferritin, white blood cells, neutrophils, lymphocytes, platelets, prothrombin time (PT), activated partial thromboplastin time (aPTT), and fibrinogen.
The study was approved by the Ethics Committee of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico in Milan (No. 360_2020) and was carried out in conformity with the 2013 revision of the Declaration of Helsinki and the code of Good Clinical Practice.
2.2. SARS-CoV-2 detection in nasopharingeal swabs
Nasopharingeal swabs were tested for the presence of SARS-CoV-2 using a One-Step Reverse Transcription Real-Time PCR kit (GeneFinder COVID-19 Plus RealAmp kit. ELITe, platform InGenius (ELITech, Torino, Italy) according with manufacturer instructions. The assay detects SARS-CoV-2 nucleocapsid (N) proteins, RNA Polymerase (RdRp) gene fragments, and envelope (E) gene fragment. The InGenius platform displays a sensibility of 1000 cp/ml and a specificity of 100%. A gene was considered detected when the cycle threshold (Ct) value resulted ≤ 45 while the assay was considered negative for a Ct value > 45. Ct values lower than the Ct median of our patients (i.e. < 28.4) were considered highly positive indicating a higher viral load. Values higher than the Ct median were considered moderate or weak positive indicating a lower viral load.
2.3. Complement activation products
The plasma levels of soluble C5b-9 (SC5b-9) were measured using a solid-phase assay (MicroVue Complement SC5b-9 Plus EIA kit, Quidel Corporation, San Diego, CA, USA) whose intra- and inter-assay coefficients of variation (CVs) were respectively 6.8% and 13.1%; the lower detection limit was 3.7 ng/mL.
Plasma C5a levels were measured using an immunoenzymatic method (MicroVue Complement C5a EIA, Quidel Corporation) with intra- and inter-assay CVs of <12%; the lower detection limit was 0.01 ng/mL.
2.4. Detection of endothelial biomarkers
Plasma levels of von Willebrand factor (vWF) antigen were measured using a commercial method (HemosIL Von Willebrand Factor Antigen, Instrumentation Laboratory, Bedford, MA, USA) with intra- and inter-assay CVs of <2.3%; the lower detection limit was 2.2%.
Tissue-type plasminogen activator (t-PA) antigen was measured in plasma using a commercially available ELISA (Zymutest t-PA antigen, Hyphen BioMed, Neuville sur Oise, France) in accordance with the manufacturer's instructions. The intra- and inter-assay CVs were <10%, and the lower detection limit was 0.5 ng/mL.
Plasminogen activator inhibitor-1 (PAI-1) antigen was measured in plasma using a commercially available ELISA (Zymutest PAI-1 antigen, Hyphen BioMed) whose intra- and inter-assay CVs were 8% and 13%, respectively; the lower detection limit was 0.5 ng/mL.
Soluble plasma thrombomodulin (sTM) plasma levels were measured using a commercial sandwich ELISA (Human Thrombomodulin/BDCA-3 Quantikine ELISA Kit, R&D Systems, Minneapolis, MN, USA) whose intra- and inter-assay CVs were both <10%; the lower detection limit was 7.82 pg/mL.
Soluble endothelial selectin (sE-selectin) was measured in plasma using a sandwich ELISA (Human sE-Selectin/CD62E Quantikine ELISA Kit, R&D Systems Inc.) whose intra- and inter-assay CVs were respectively 5% and 8.8%; the lower detection limit was 0.009 ng/mL.
2.5. Statistical analysis
On the basis of the findings of our previous study [13], the sample size gave a statistical power of >80%, with an alpha error of <5%. The Kolmogorov-Smirnov test was used to assess normal distribution. The data are given as median values and ranges (min–max). The between-group differences were analysed using the Mann-Whitney test for independent samples; the within-group differences were analysed using Wilcoxon's test for paired data. The correlations between the parameters were evaluated using Spearman's test. A P value of <0.05 was considered significant. The data were analysed using the SPSS PC statistical package v.25.0 (IBM, Armonk, NY, USA).
3. Results
3.1. Complement activation
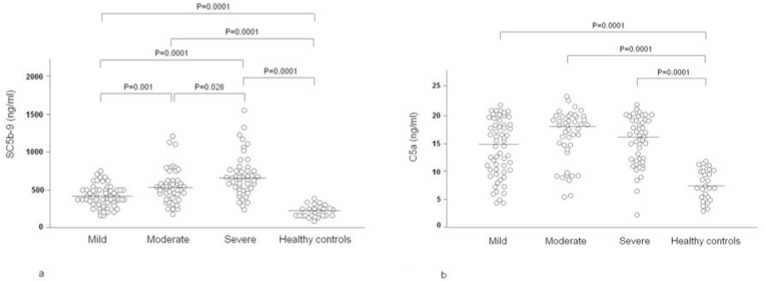
Fig. 1 shows the plasma levels of SC5b-9 and C5a in 148 COVID-19 patients grouped on the basis of disease severity. In comparison with the normal controls (median 217 ng/mL, range 106–499), the median levels of SC5b-9 were higher in all three patient groups: 434 ng/mL, range 30–755 ng/mL in those with mild disease (p = 0.0001), 545 ng/mL, range 31–1223 ng/mL, in those with moderate disease (p = 0.0001) and 635 ng/mL, range 182–1555 ng/mL, in those with severe disease (p = 0.0001). There were statistically significant differences in SC5b-9 levels between the patients with mild and moderate disease (p = 0.001), moderate and severe disease (p = 0.026), and between mild and severe (p = 0.0001). The median levels of C5a were also higher in the three patient groups (mild disease: 15.4 ng/mL, range 4.2–21.8 ng/mL (p = 0.0001); moderate disease: 18.2 ng/mL, range 5.5–23.0 ng/mL (p = 0.0001); severe disease: 16.0 ng/mL, range 2.6–21.9 ng/mL (p = 0.0001) than in the controls: 7.4 ng/mL, range 3.1–11.8 ng/mL, without any significant differences between the patient groups.
Fig. 1.
Plasma levels of soluble complement terminal complex (SC5b-9) (1a) and activated complement component 5 (C5a) (1b) in 58 COVID-19 patients kept under observation and receiving low-intensity care (mild), 44 patients requiring intermediate care with non-invasive ventilation (moderate), 46 patients admitted to an intensive care unit for mechanical ventilation (severe), and 27 healthy controls.
The data distribution does not change when the patients were subdivided via sex, age or BMI. Complement activation products did not display any correlation with age or BMI.
Patients with high viral load at nasopharingeal swabs as detected by low RT-PCR Ct values displayed statistically significant higher levels of SC5b-9 (median 560, range 255–1555 ng/mL) than patients with low viral load (median 465, range 30–781 ng/mL; p < 0.005). The levels of C5a were also higher in patients with high viral load (17.6 [5.8–23] vs 16.5 [5.5–21.5], but the difference was not statistically significant.
3.2. Plasma von Willebrand factor levels and their correlations with complement activation products
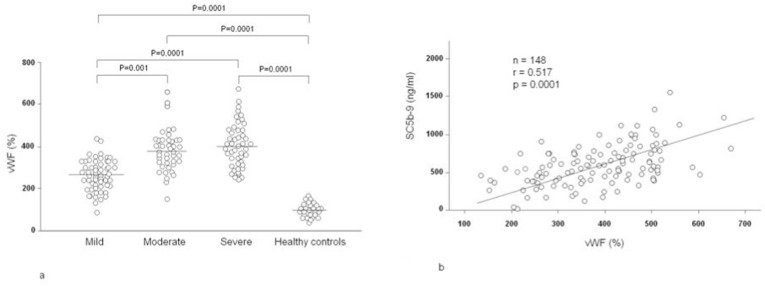
Fig. 2 shows the plasma levels of vWF in 148 patients with COVID-19 grouped on the basis of disease severity, and their correlations with SC5b-9. In comparison with the controls (97%, range 67–166%), the levels were significantly higher in the patients with mild disease: (263%, range 90–435%; p = 0.0001, moderate disease (374%, range 153–652%; p = 0.0001), or severe disease (395%, range 251–667%; p = 0.0001). There were statistically significant differences in vWF levels between the patients with mild and moderate disease (p = 0.001), and between mild and severe patients (p = 0.0001). The highest levels were found in moderate or severe disease, thus mirroring the association between disease severity and the complement activation products. However, it is worth noting that vWF levels significantly correlated with SC5b-9 levels (r = 0.517, p = 0.0001), but not with C5a levels (r = 0.132).
Fig. 2.
a) Plasma levels of von Willebrand factor (vWF) in 58 COVID-19 patients kept under observation and receiving low-intensity care (mild), 44 patients requiring intermediate care with non-invasive ventilation (moderate), 46 patients admitted to an intensive care unit for mechanical ventilation (severe), and 27 healthy controls; b) Correlation between the plasma levels of vWF and soluble complement terminal complex (SC5b-9) in 148 COVID-19 patients.
The data distribution does not change when the patients were subdivided via sex, age or BMI. Plasma vWF levels did not display any correlation with age or BMI.
The levels of vWF were also higher in patients with high viral load than with low viral load (369%, range 219–667% vs 341%, range 90–601%), but the difference was not statistically significant.
3.3. Other biomarkers of endothelial activation/damage
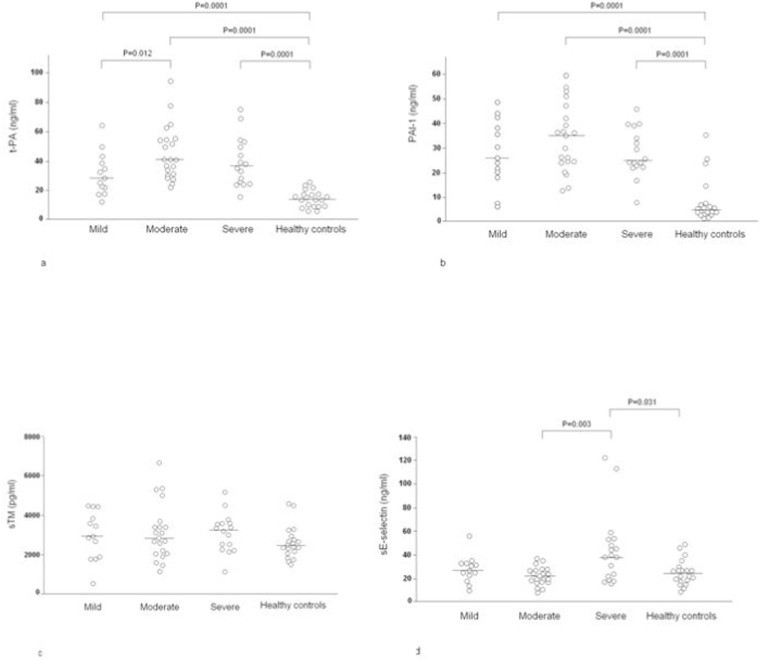
Given the limited nature of the sampling, t-PA antigen, PAI-1 antigen, sTM and sE-selectin could only be measured in a subgroup of 50 patients (13 with mild, 21 with moderate, and 16 with severe disease) (Fig. 3 a-d). In comparison with the controls (13.4 ng/mL, range 6.1–26.0 ng/mL), plasma levels of t-PA antigen were higher in the patients with mild (28.5 ng/mL, range 12.6–63.9 ng/mL; p = 0.0001), moderate (41.0 ng/mL, range 22.2–94.2 ng/mL; p = 0.0001) and severe disease (35.7 ng/mL, range 16.0–75.2 ng/mL; p = 0.0001), with a statistically significant difference in levels between the patients with mild and moderate disease (p = 0.012) (Fig. 3a).
Fig. 3.
Plasma levels of tissue-type plasminogen activator (t-PA) (3a), plasminogen activator inhibitor-1 (PAI-1) (3b), soluble thrombomodulin (sTM) (3c), and soluble E-selectin (sE-selectin) (3d) in 13 COVID-19 patients kept under observation and receiving low-intensity care (mild), 21 patients requiring intermediate care with non-invasive ventilation (moderate), 16 patients admitted to an intensive care unit for mechanical ventilation (severe), and 20 healthy controls.
The levels of PAI-1 antigen were also higher in the three patient groups: mild disease (25.4 ng/mL, range 6.2–48.7 ng/mL [p = 0.0001]), moderate disease (34.6 ng/mL, range 12.9–59.5 ng/mL [p = 0.0001]) and severe disease (25.1 ng/mL, range 8.1–46.0 ng/mL [p = 0.0001]) than in the controls (4.2 ng/mL, range 1.5–35.3 ng/mL) without any significant difference between the patient groups (Fig. 3b).
There was no significant difference in the plasma levels of sTM between the patients with mild disease (2947 pg/mL, range 553–4499 pg/mL), moderate disease (2827 pg/mL, range 1170–6662 pg/mL) or severe disease (3224 pg/mL, range 1154–5188 pg/mL) and the controls (2426 pg/mL, range 1520–4599 pg/mL) (Fig. 3c). Finally, sE-selectin levels were significantly higher in the patients with severe disease (37.5 ng/mL, range 16.0–122.0 ng/mL) than in the controls (p = 0.031) and patients with moderate disease (p = 0.003), but there was no significant difference between the patients with mild (28.0 ng/mL, range 10.0–56.0 ng/mL) or moderate disease (20.5 ng/mL, range 8.0–37.0 ng/mL) and the normal controls (22.0 ng/mL, range 9.0–49.0 ng/mL) (Fig. 3d).
3.4. Hematological and inflammatory parameters and their correlations with complement activation and endothelial activation/damage
Table 1 shows that our cohort of COVID-19 patients had increased levels of acute phase proteins, and hematological and coagulation system abnormalities. The plasma levels of fibrin fragment D-dimer increased with disease severity, and correlated with plasma vWF (r = 0.358, p = 0.001), CRP (r = 0.315, p = 0.0001) and IL-6 levels (r = 0.307, p = 0.008), as well as with neutrophil counts (r = 0.466, p = 0.0001). CRP levels increased with disease severity, and correlated positively with SC5b-9 (r = 0.288, p = 0.0001), C5a (r = 0,259, p = 0.002, vWF (0.382, p = 0.0001), ferritin (r = 0.447, p = 0.0001) and IL-6 levels (r = 0.454, p = 0.0001), and inversely with lymphocyte counts (r = −0.428, p = 0.0001). Ferritin levels increased with disease severity and correlated positively with SC5b-9 (r = 0.318, p = 0.0001) and vWF levels (r = 0.450, p = 0.0001), and inversely with lymphocyte counts (r = −0.325, p = 0.0001). IL-6 levels were high in all three groups and highest in the patients with severe disease but did not correlate with plasma SC5b-9 (r = 0.133) or C5a levels (r = 0.181). Lymphocyte counts decreased with the increase of disease severity, and inversely correlated with plasma vWF levels (r = −0.376, p = 0.0001).
Table 1.
Coagulation and inflammation parameters expressed as median values and ranges (min-max) in 58 patients with mild COVID-19 (requiring only observation and low-intensity care), 44 with moderately severe COVID-19 (requiring intermediate care with non-invasive ventilation), and 46 with severe COVID-19 (requiring intensive care with mechanical ventilation).
| D-dimer μg/L | CRP mg/dL | Ferritin μg/L | IL-6 ng/L | White cells n/μL | Neutrophils n/μL | Lymphocytes n/μL | Platelets n x 103/μL | PT ratio | aPTT ratio |
Fibrinogen mg/dL | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mild | 810 | 5.35 | 483 | 41.0 | 6080 | 3740 | 1170 | 250 | 1.12 | 0.96 | 500 |
| (203–12638) | (0.20–26.99) | (40–6384) | (2.1–176.0) | (2320–21290) | (300–17800) | (300–4550) | (79–1699) | (0.94–1.58) | (0.69–1.37) | (192–982) | |
| Moderate | 1038 | 7.40 | 1284 | 38.6 | 7045 | 5415 | 915 | 235 | 1.13 | 0.98 | 562 |
| (290–21639) | (0.55–26.37) | (69–8633) | (1.5–268.0) | (2180–18900) | (1560–17380) | (130–3330) | (70–799) | (0.96–5.43) | (0.71–1.25) | (229–961) | |
| Severe | 1667 | 10.95 | 1301 | 61.2 | 7470 | 5900 | 660 | 249 | 1.12 | 0.95 | 517 |
| (229–199872) | (1.61–34.15) | (206–11366) | (3.8–5805) | (1650–51410) | (1280–49680) | (180–2140) | (5–608) | (1.02–1.54) | (0.70–2.17) | (110–1035) | |
| Normal ranges | <500 | 0.00–0.05 | 30–400 | <10.0 | 4800–10800 | 1500–6500 | 1200–3400 | 130–430 | 0.84–1.20 | 0.86–1.20 | 165–350 |
3.5. Follow-up
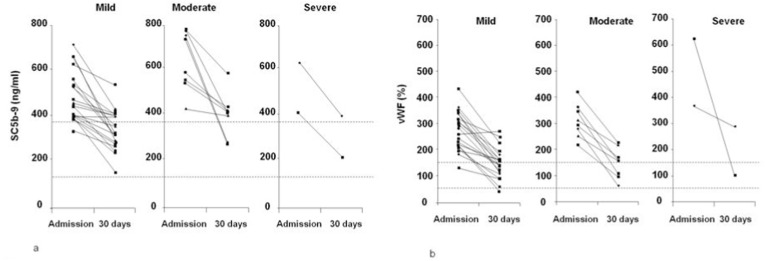
Thirty days after admission, plasma samples were obtained from 30 patients in remission after experiencing different degrees of disease severity (20 with mild, eight with moderate, and two with severe disease) and were used to measure SC5b-9, C5a and vWF levels, which had respectively decreased from 511 ng/mL (range 256–783 ng/mL) to 373 ng/mL (range 151–640 ng/mL) (p = 0.0001), from 16.4 ng/mL (range 6.1–20.9 ng/mL) to 11.7 ng/mL (range 2.5–18.8 ng/mL) (p = 0.001), and from 310% (range 133–623%) to 145% (range 46–286%) (p = 0.0001). Fig. 4 shows the reduction in plasma SC5b-9 and vWF levels by disease severity, and it is interesting to note that both normalised in 43% of all cases and 50% of the cases of mild disease.
Fig. 4.
Plasma levels of soluble complement terminal complex (SC5b-9) (4a) and von Willebrand factor (vWF) (4b) upon admission and 30 days later in 30 patients who developed COVID-19 of different severity: 20 patients who had been kept under observation and received low-intensity care (mild); eight patients who had required intermediate care with non-invasive ventilation (moderate); and two patients who had been admitted to an intensive care unit for mechanical ventilation (severe). The dotted lines represent the lower and upper limits of normal.
A treatment with subcutaneous low molecular weight heparin at a daily dose of 100–200 IU/Kg and oral hydroxychloroquine at a daily dose 400 mg was carried out in all the patients enrolled in the study. Additional drugs were added depending on the clinical course of the patients and according to the physicians’ opinion. SC5b-9, C5a and vWF levels at 30 days displayed a statistically significant reduction in all the patients in remission. Treatment with i.v. methylprednisolone in 12 patients at daily dose of 80 mg did not show a significant difference in the levels of these biomarkers in comparison with the 18 COVID-19 patients that did not receive methylprednisolone. The addition of s.c. Anakinra in 6 patients on methylprednisolone at a daily dose of 300–600 mg did not affect C5b-9, C5a and vWF values at the end of the follow up.
4. Discussion
The complement system is emerging as a major player in the pathophysiology of COVID-19: it has been recognized that it played an important role in the development of respiratory dysfunction in mouse models of infections due to previous coronaviruses (SARS-CoV1 [20] and MERS-CoV [23]), and there have been recent reports of complement activation [13,14,22] and tissue deposition in the lung and skin of a few COVID-19 patients [12].
Our findings show that C5a and SC5b-9 levels were increased in 148 patients with mild, moderate or severe disease, thus extending our previous observations [13] and revealing the concomitant activation of endothelial cells. The increase was proportional to the severity of the disease, and the levels decreased after clinical improvement. These findings support the view that SC5b-9 plasma levels may be considered sensitive indicators of COVID-19 severity and activity. Furthermore, their detection in COVID-19 patients suggests that the pathogenic role of complement is not restricted to the action of C5a but also involves SC5b-9. This has important therapeutic implications insofar as it implies that the aim of treating such patients with complement inhibitors should not be limited to controlling the C5a-C5aR1 axis [14], but should also be extended to blocking the assembly of the terminal complex.
It is difficult to identify the factors responsible for in vivo complement activation in COVID-19 patients, but the observations that the spike protein activates the alternative pathway [24] make it likely that SARS-CoV-2 is the initial trigger. Our data showing the association of higher viral load in the nasopharyngeal tissues with higher SC5b-9 levels are in line with the recent report that viral load predicts mortality [25] and further supports a direct role of the virus in complement activation. Reduced interferon activity due to impaired function of critical genes or production of interferon blocking autoantibodies may contribute to a defective response to SARS-CoV-2 [26,27] and eventually to higher viral load in turn associated with disease severity [25] and complement activation. However, the activation of the classical pathway by antibodies against the different viral epitopes cannot be excluded. Moreover, interferon may regulate the synthesis of complement components by various cell types [28,29], and the larger availability of complement components may fuel the increase in the levels of activation products. Interferon may activate complement in a rare vasculopathy, Degos Disease [30] but to date there is no evidence for such mechanisms in COVID-19.
COVID-19 has been primarily conceptualised as an acute respiratory viral disease, but it is now accepted that it is a systemic disease in which a hyper-inflammatory response to the virus and generalised coagulopathy are thought to mediate damage to a number of tissues and organs. Together with pro-inflammatory cytokines, complement activation contributes to the induction and amplification of the inflammatory process as a result of the ability of C5a and SC5b-9 to recruit phagocytic cells in the lung and other infected organs, activate endothelial cells, and stimulate vascular permeability [19,31].
There is growing evidence that a common pathogenic denominator is endotheliopathy, as supported by the recent finding of increased plasma biomarkers of vascular injury [7,32]. In line with this, we found a significant increase in the plasma levels of vWF in all three of our patient groups, with the highest values being observed in those with moderate and severe disease. In order to support the presence of endotheliopathy further, we also measured a number of biomarkers of endothelial activation/damage in a subgroup of 50 patients. Tissue-type plasminogen activator and PAI-1 levels were increased in all of the patients regardless of disease severity, and both are widely accepted markers of endothelial damage in cardiovascular disorders and other vasculopathies [33]. It can be speculated that the increase in t-PA levels may also be related to activation of the fibrinolytic system, as shown by the high levels of the fibrin degradation product D-dimer [5]. Furthermore, increased secretion of PAI-1 by the activated endothelium may contribute to thrombophilia [34], but the techniques we used to detect t-PA and PAI-1 does not measure the functional activity of either, and so we cannot say whether their activity is enhanced. The soluble isoform of E-selectin, an endothelium-specific adhesion molecule released by activated or damaged cells, was also increased in the patients with severe COVID-19. Taken together, the presence of all these biomarkers speaks in favor of endotheliopathy in our patients, in line with the findings of a previous study [7] which, like ours, did not reveal any increase in plasma sTM. The soluble variant of TM is the result of proteolytic cleavage of the molecule from the endothelial cell surface after vascular injury, and plasma sTM has been detected in heterogeneous forms of endotheliopathy such as during the course of sepsis, TNF-induced endothelial activation and ARDS. However, normal or reduced levels of sTM have been found in patients with other vasculopathies, which raises doubts as to whether it is a sensitive enough marker of endothelial damage [35].
The correlation between circulating SC5b-9 levels and vWF during the course of active disease and their similar decline during remission suggest that complement activation plays an active role in the pathophysiology of COVID-19. It has been reported that the terminal complement complex may induce a pro-thrombotic and pro-inflammatory endothelium phenotype and mediate vWF release not necessarily as a result of cell death [36]. Although these studies used in vitro models, it is possible to speculate that similar mechanisms take place in vivo and, if so, this would explain the correlation between complement activation and circulating vWF levels. Our data strongly support the hypothesis that, in addition to the effect of pro-inflammatory cytokines and direct SARSCoV-2 endothelial tropism via ACE2 receptors [8], activation of the complement cascade plays a key role in mediating COVID-19 endotheliopathy.
It is worth noting that, as serum IL-6 levels did not have any relationship with those of C5a, SC5b-9 and vWF, complement activation may play an independent role in mediating the release of vWF from damaged endothelial cells [18,36]. Consequently, measuring complement activation products (particularly SC5b-9) may provide a sensitive and useful biomarker of COVID-19 severity and activity. Interestingly, both complement activation products and vWF levels tend to decrease or normalize during remission. Ad hoc clinical trials should be planned to validate the same biomarkers for evaluating the effect of the therapy.
5. Conclusions
In conclusion, complement should be accepted as another key pathogenic player in COVID-19 endotheliopathy and coagulation dysfunction because it mediates both vascular injury and coagulopathy as a result of its close crosstalk with the coagulation cascade. Drugs blocking complement activation are therefore potential means of controlling the severe manifestations of COVID-19, alone or in combination with other anti-inflammatory and anti-viral drugs [22,37].
Author contributions
Massimo Cugno: Conceptualization, Investigation, Data curation, Writing - Original Draf. Pier Luigi Meroni: Conceptualization, Investigation, Data curation, Writing - Original Draf. Roberta Gualtierotti: Investigation, Writing - review & editing. Samantha Griffini: Investigation, Writing - review & editing. Elena Grovetti: Investigation, Writing - review & editing. Adriana Torri: Investigation, Writing - review & editing. Paola Lonati: Investigation, Writing - review & editing. Claudia Grossi: Investigation, Writing - review & editing. Maria Orietta Borghi: Investigation, Writing - review & editing. Cristina Novembrino: Investigation, Writing - review & editing. Massimo Boscolo: Investigation, Writing - review & editing. Sara Colonia Uceda Renteria: Investigation, Writing - review & editing. Luca Valenti: Investigation, Writing - review & editing; Giuseppe Lamorte: Investigation, Writing - review & editing. Maria Manunta: Investigation, Writing - review & editing. Daniele Prati: Investigation, Writing - review & editing. Antonio Pesenti: Investigation, Writing - review & editing. Francesco Blasi: Investigation, Writing - review & editing. Giorgio Costantino: Investigation, Writing - review & editing. Andrea Gori: Investigation, Writing - review & editing; Alessandra Bandera: Investigation, Writing - review & editing. Francesco Tedesco: Conceptualization, Writing - review & editing. Flora Peyvandi: Investigation, Writing - review & editing, Supervision.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no relevant conflicts of interest.
References
- 1.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H. Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemostasis. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemostasis. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goshua G., Pine A.B., Meizlish M.L., Chang C.H., Zhang H., Bahel P. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/s0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henderson L.A., Canna S.W., Schulert G.S., Volpi S., Lee P.Y., Kernan K.F. On the alert for cytokine storm: immunopathology in COVID-19. Arthritis Rheum. 2020;72:1059–1063. doi: 10.1002/art.41285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riedl M., Fakhouri F., Le Quintrec M., Noone D.G., Jungraithmayr T.C., Fremeaux-Bacchi V. Spectrum of complement-mediated thrombotic microangiopathies: pathogenetic insights identifying novel treatment approaches. Semin. Thromb. Hemost. 2014;40:444–464. doi: 10.1055/s-0034-1376153. [DOI] [PubMed] [Google Scholar]
- 12.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cugno M., Meroni P.L., Gualtierotti R., Griffini S., Grovetti E., Torri A. Complement activation in patients with COVID-19: a novel therapeutic target. J. Allergy Clin. Immunol. 2020;146:215–217. doi: 10.1016/j.jaci.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carvelli J., Demaria O., Vély F., Batista L., Benmansour N.C., Fares J. Association of COVID-19 inflammation with activation of the C5a–C5aR1 axis. Nature. 2020 doi: 10.1038/s41586-020-2600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramlall V., Thangaraj P.M., Meydan C., Foox J., Butler D., Kim J. Immune complement and coagulation dysfunction in adverse outcomes of SARS-CoV-2 infection. Nat. Med. 2020 doi: 10.1038/s41591-020-1021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reis E.S., Mastellos D.C., Hajishengallis G., Lambris J.D. New insights into the immune functions of complement. Nat. Rev. Immunol. 2019;19:503–516. doi: 10.1038/s41577-019-0168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holers V.M. Complement and its receptors: new insights into human disease. Annu. Rev. Immunol. 2014;32:433–459. doi: 10.1146/annurev-immunol-032713-120154. [DOI] [PubMed] [Google Scholar]
- 18.Hattori R., Hamilton K.K., McEver R.P., Sims P.J. Complement proteins C5b-9 induce secretion of high molecular weight multimers of endothelial von Willebrand factor and translocation of granule membrane protein GMP-140 to the cell surface. J. Biol. Chem. 1989;264:9053–9060. [PubMed] [Google Scholar]
- 19.Tedesco F., Pausa M., Nardon E., Introna M., Mantovani A., Dobrina A. The cytolytically inactive terminal complement complex activates endothelial cells to express adhesion molecules and tissue factor procoagulant activity. J. Exp. Med. 1997;185:1619–1627. doi: 10.1084/jem.185.9.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gralinski L.E., Sheahan T.P., Morrison T.E., Menachery V.D., Jensen K., Leist S.R. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio. 2018;9 doi: 10.1128/mBio.01753-18. e01753-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang Y., Zhao G., Song N., Li P., Chen Y., Guo Y. Blockade of the C5a-C5aR axis alleviates lung damage in hDPP4-transgenic mice infected with MERS-CoV, Emerg. Microb. Infect. 2018;7:77. doi: 10.1038/s41426-018-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peffault de Latour R., Bergeron A., Lengline E., Dupont T., Marchal A., Galicier L. Complement C5 inhibition in patients with COVID-19 - a promising target? Haematologica. 2020 doi: 10.3324/haematol.2020.260117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang Y., Li J., Teng Y., Sun H., Tian G., He L. Complement receptor C5aR1 inhibition reduces pyroptosis in hDPP4-transgenic mice infected with MERS-CoV. Viruses. 2019;11:39. doi: 10.3390/v11010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu J., Yuan X., Chen H., Chaturvedi S., Braunstein E.M., Brodsky R.A. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition, Blood. 2020. [DOI] [PMC free article] [PubMed]
- 25.Pujadas E., Chaudhry F., McBride R., Richter F., Zhao S., Wajnberg A. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet Respir Med. 2020;8:e70. doi: 10.1016/S2213-2600(20)30354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Q., Bastard P., Liu Z., Le Pen J., Moncada-Velez M., Chen J. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.H., Zhang Y. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lubbers R., van Essen M.F., van Kooten C., Trouw L.A. Production of complement components by cells of the immune system. Clin. Exp. Immunol. 2017;188:183–194. doi: 10.1111/cei.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jodele S., Medvedovic M., Luebbering N., Chen J., Dandoy C.E., Laskin B.L. Interferon-complement loop in transplant-associated thrombotic microangiopathy. Blood Adv. 2020;4:1166–1177. doi: 10.1182/bloodadvances.2020001515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magro C.M., Poe J.C., Kim C., Shapiro L., Nuovo G., Crow M.K. Degos disease: a C5b-9/interferon-α-mediated endotheliopathy syndrome. Am. J. Clin. Pathol. 2011;135:599–610. doi: 10.1309/AJCP66QIMFARLZKI. [DOI] [PubMed] [Google Scholar]
- 31.Bossi F., Fischetti F., Pellis V., Bulla R., Ferrero E., Mollnes T.E. Platelet-activating factor and kinin-dependent vascular leakage as a novel functional activity of the soluble terminal complement complex. J. Immunol. 2004;173:6921–6927. doi: 10.4049/jimmunol.173.11.6921. [DOI] [PubMed] [Google Scholar]
- 32.Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V. Hypercoagulability of COVID-19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemostasis. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cugno M., Borghi M.O., Lonati L.M., Ghiadoni L., Gerosa M., Grossi C. Patients with antiphospholipid syndrome display endothelial perturbation. J. Autoimmun. 2010;34:105–110. doi: 10.1016/j.jaut.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Whyte C.S., Morrow G.B., Mitchell J.L., Chowdary P., Mutch N.J. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. J. Thromb. Haemostasis. 2020;18:1548–1555. doi: 10.1111/jth.14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin F.A., Murphy R.P., Cummins P.M. Thrombomodulin and the vascular endothelium: insights into functional, regulatory, and therapeutic aspects. Am. J. Physiol. Heart Circ. Physiol. 2013;304:H1585–H1597. doi: 10.1152/ajpheart.00096.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riedl Khursigara M., Schlam D., Noone D.G., Bruno V., Ortiz-Sandoval C.G., Pluthero F.G. Vascular endothelial cells evade complement-mediated membrane injury via Weibel-Palade body mobilization. J. Thromb. Haemostasis. 2020;18:1484–1494. doi: 10.1111/jth.14767. [DOI] [PubMed] [Google Scholar]
- 37.Jodele S., Köhl J. Tackling COVID-19 infection through complement-targeted immunotherapy. Br. J. Pharmacol. 2020 doi: 10.1111/bph.15187. [DOI] [PMC free article] [PubMed] [Google Scholar]