Abstract
Background
Patients with pre-existing heart failure (HF) are likely at higher risk for adverse outcomes in coronavirus disease-2019 (COVID-19), but data on this population are sparse.
Objectives
This study described the clinical profile and associated outcomes among patients with HF hospitalized with COVID-19.
Methods
This study conducted a retrospective analysis of 6,439 patients admitted for COVID-19 at 1 of 5 Mount Sinai Health System hospitals in New York City between February 27 and June 26, 2020. Clinical characteristics and outcomes (length of stay, need for intensive care unit, mechanical ventilation, and in-hospital mortality) were captured from electronic health records. For patients identified as having a history of HF by International Classification of Diseases-9th and/or 10th Revisions codes, manual chart abstraction informed etiology, functional class, and left ventricular ejection fraction (LVEF).
Results
Mean age was 63.5 years, and 45% were women. Compared with patients without HF, those with previous HF experienced longer length of stay (8 days vs. 6 days; p < 0.001), increased risk of mechanical ventilation (22.8% vs. 11.9%; adjusted odds ratio: 3.64; 95% confidence interval: 2.56 to 5.16; p < 0.001), and mortality (40.0% vs. 24.9%; adjusted odds ratio: 1.88; 95% confidence interval: 1.27 to 2.78; p = 0.002). Outcomes among patients with HF were similar, regardless of LVEF or renin-angiotensin-aldosterone inhibitor use.
Conclusions
History of HF was associated with higher risk of mechanical ventilation and mortality among patients hospitalized for COVID-19, regardless of LVEF.
Key Words: coronavirus, COVID-19, heart failure, left ventricular ejection fraction, outcome, renin-angiotensin-aldosterone system inhibitor
Abbreviations and Acronyms: AdjOR, adjusted odds ratio; CI, confidence interval; COVID-19, coronavirus disease-2019; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFmrEF, heart failure with mid-range ejection fraction; HFrEF, heart failure with reduced ejection fraction; ICD, International Classification of Disease; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; LVEF, left ventricular ejection fraction; RAASi, renin-angiotensin-aldosterone inhibitor; SARS-CoV-2, severe acute respiratory syndrome- coronavirus-2
Central Illustration
Coronavirus disease-2019 (COVID-19), caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), is a rapidly expanding pandemic associated with overwhelming morbidity and mortality across the globe (1). History of cardiovascular disease has repeatedly been associated with worse prognosis (2,3), whereas de novo cardiovascular involvement in its various forms, from myocardial injury to myocarditis and shock, has also been amply described (4, 5, 6, 7). Among patients hospitalized with COVID-19, patients with heart failure (HF) represent a population at the highest potential risk for complications due to a high prevalence of underlying frailty or renal dysfunction among other comorbidities (8). Yet data as to the clinical course and outcomes of COVID-19 among patients with a history of HF are scarce (9, 10, 11, 12). Furthermore, it is unknown as to whether the clinical course of COVID-19 differs according to left ventricular ejection fraction (LVEF) or background medications, including renin-angiotensin-aldosterone system inhibitors (RAASi) (13).
The Mount Sinai Healthcare System is a large academic health care institution that serves a racially and ethnically diverse patient population in New York City, once the global epicenter of the disease. Here, we present the clinical characteristics, hospital course, and outcomes of the largest cohort to date of patients with a history of HF hospitalized with laboratory-confirmed COVID-19.
Methods
Study population and design
We conducted a retrospective cohort study of consecutive patients at least 18 years or older hospitalized with confirmed COVID-19 infection by positive reverse transcription polymerase chain reaction at 1 of 5 Mount Sinai Healthcare System hospitals (Mount Sinai Hospital, Mount Sinai Morningside, and Mount Sinai West located in Manhattan; Mount Sinai Brooklyn located in Brooklyn; and Mount Sinai Queens located in Queens). Patients were admitted from February 27, 2020 to June 26, 2020, and they were followed-up until July 18, 2020. The Mount Sinai Institutional Review Board approved this research under a broad regulatory protocol that allowed for analysis of limited patient-level data.
Data collection and outcomes
Demographics, laboratory measurements, disease diagnoses, comorbidities, procedures, and outcomes (death, need for intensive care unit [ICU], intubation and mechanical ventilation, length of stay [LOS], and hospital discharge) were collected from electronic health records. Patients were considered right-censored if they were discharged from the hospital alive or remained admitted at the time of data freeze (July 18th). Comorbidities were extracted using the International Classification of Disease-9th and/or 10th (ICD-9/10) Revision codes for atrial fibrillation, asthma, obesity, coronary artery disease, cancer, chronic kidney disease, chronic obstructive pulmonary disease, diabetes, HF, and hypertension (Supplemental Appendix).
Manual chart review was performed for patients identified as having a history of HF by ICD-9/10 codes, to collect historic variables of interest, including etiology of HF, date of HF diagnosis, baseline New York Heart Association functional class, and LVEF before index COVID-19 admission. Laboratory values and cardiovascular procedures performed during admission, as well as specific outcomes (need for vasopressors or vasodilators, acute kidney injury, shock, thromboembolic events, arrhythmias, causes of death, and 30-day readmission rate) were also abstracted. Patients with a history of HF were classified into 3 groups according to LVEF category: HF with reduced EF (HFrEF) (≤40%); HF with mid-range EF (HFmrEF) (41% to 49%); and HF with preserved EF (HFpEF) (≥50%) (14).
Statistical analysis
Continuous variables are presented as mean ± SD or median (interquartile range [IQR]) when they did not show a normal distribution. Categorical variables are expressed as absolute number of patients (percentage). Variables were compared between patients with and without a history of HF as well as between LVEF categories and survivors and nonsurvivors using the Fisher exact test or chi-square test for categorical variables, and the Student’s t-test, analysis of variance, Wilcoxon, or Kruskal-Wallis, as appropriate, for continuous variables. Multiple imputation by chained equation (m = 20) was applied whenever necessary, and variables with >20% of missing data were not included in the models (Supplemental Appendix) (15).
To determine the impact of HF history on outcomes, a multivariable logistic regression analysis was performed, adjusted by age, sex, race, obesity, hypertension, diabetes, coronary artery disease, atrial fibrillation, chronic kidney disease, chronic obstructive pulmonary disease, previous treatment with RAASi, systolic blood pressure, heart rate, oxygen saturation, white blood count, lymphocytes, creatinine, and albumin. In addition, we calculated the adjusted odds ratio (adjOR) in the subgroup of patients with available values of D-dimer and troponin (n = 1,777).
To evaluate the impact of LVEF category and previous treatment with RAASi on in-hospital mortality, a multivariable Cox regression analysis was performed, adjusted by age, sex, race, body mass index, hypertension, diabetes, atrial fibrillation, chronic kidney disease, chronic obstructive pulmonary disease, baseline New York Heart Association functional class, previous mitral regurgitation, systolic blood pressure, heart rate, oxygen saturation, lymphocytes, creatinine, brain natriuretic peptide, and troponin.
All statistical tests were 2-tailed, and statistical significance was defined as a p value <0.05. Analyses were performed using Stata version 14 (StataCorp, College Station, Texas).
Results
Clinical characteristics
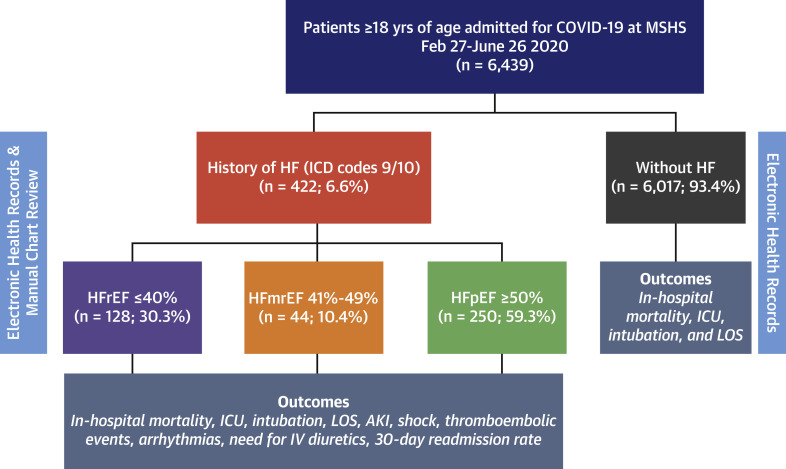
A total of 6,439 patients were admitted for COVID-19 during the study period, and 422 (6.6%) had a history of HF (Figure 1 ). Overall, the mean age was 63.5 ± 18 years, 45% were women, and the mean body mass index was 29.0 ± 7.5 kg/m2. Hypertension (34.5%), obesity (27.9%), and diabetes mellitus (22.8%) were the most frequent comorbidities, and one-third of patients were treated with RAASi before COVID-19 admission. Table 1 summarizes the clinical characteristics of the study population stratified by history of HF. Compared with patients without HF, those with a history of HF were older, had a higher prevalence of comorbidities, and were receiving a greater number of medications for cardiovascular disease. Patients with a history of HF presented with higher systolic blood pressure (126 mm Hg vs. 119 mm Hg; p < 0.001) and lower oxygen saturation (91% vs. 94%; p < 0.001); however, respiratory rate and temperature were similar to those without HF. Patients with a history of HF had lower lymphocyte count, hemoglobin, platelet count, sodium, and alanine aminotransferase, but had higher median values of creatinine, total bilirubin, lactate, D-dimer, troponin, natriuretic peptides, and inflammatory markers (e.g., C-reactive protein or interleukin-6). In terms of in-hospital management, patients with HF received supplemental oxygen by nasal cannula (72.0% vs. 51.8%; p < 0.001) and anticoagulation (82.2% vs. 55.0%; p < 0.001) more frequently compared with patients without a history of HF, with no major differences in the administration of antiviral or steroid therapy.
Figure 1.
Consort Diagram of the Study Population
A total of 6,439 patients were admitted for coronavirus disease-2019 (COVID-19) during the study period and 422 (6.6%) patients had a history of heart failure (HF). AKI = acute kidney injury; HFmrEF = heart failure with mid-range ejection fraction; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; ICD = International Classification of Diseases; ICU = intensive care unit; IV = intravenous; LOS = length of stay; MSHS = Mount Sinai Health System.
Table 1.
Clinical Characteristics, Management, and Outcomes of the Study Population According to HF History
| Total (N = 6,439) | HF (n = 422; 6.6%) | Non-HF (n = 6,017; 93.4%) | p Value | |
|---|---|---|---|---|
| Age, yrs | 63.5 ± 17.6 | 72.5 ± 13.3 | 62.9 ± 17.7 | <0.001 |
| Female | 2,892 (44.9) | 186 (44.1) | 2,706 (45.0) | 0.720 |
| BMI, kg/m2 | 29.0 ± 7.5 | 29.5 ± 8.4 | 28.9 ± 7.3 | 0.207 |
| Race | <0.001 | |||
| Black | 1,614 (25.1) | 134 (31.8) | 1,480 (24.6) | |
| Hispanic/Latino | 1,738 (27.0) | 120 (28.4) | 1,618 (26.9) | |
| White | 1,481 (23.0) | 105 (24.9) | 1,376 (22.9) | |
| Asian | 321 (5.0) | 21 (5.0) | 300 (5.0) | |
| Other | 963 (15.0) | 34 (8.1) | 929 (15.4) | |
| Unknown | 322 (5.0) | 8 (1.9) | 314 (5.2) | |
| Comorbidities | ||||
| Obesity | 1,796 (27.9) | 169 (40.0) | 1,627 (27.0) | <0.001 |
| Hypertension | 2,222 (34.5) | 382 (90.5) | 1,840 (30.6) | <0.001 |
| Diabetes mellitus | 1,470 (22.8) | 269 (63.7) | 1,201 (20.0) | <0.001 |
| Dyslipidemia | 1,139 (17.7) | 228 (54.0) | 911 (15.1) | <0.001 |
| CAD | 901 (14.0) | 235 (55.7) | 666 (11.1) | <0.001 |
| Stroke | 379 (5.9) | 114 (27.0) | 265 (4.4) | <0.001 |
| Atrial fibrillation | 464 (7.2) | 160 (37.9) | 304 (5.1) | <0.001 |
| CKD | 436 (6.8) | 177 (41.9) | 259 (4.3) | <0.001 |
| COPD | 292 (4.5) | 94 (22.3) | 198 (3.3) | <0.001 |
| Asthma | 378 (5.9) | 58 (13.7) | 320 (5.3) | <0.001 |
| OSA | 193 (3.0) | 57 (13.5) | 136 (2.3) | <0.001 |
| Background treatment | ||||
| RAAS inhibitors | 1,927 (29.9) | 260 (61.6) | 1,667 (27.7) | <0.001 |
| Beta-blockers | 1,781 (27.7) | 354 (83.9) | 1,427 (23.7) | <0.001 |
| MRA | 175 (2.7) | 60 (14.2) | 115 (1.9) | <0.001 |
| Loop diuretics | 993 (15.4) | 318 (75.4) | 675 (11.2) | <0.001 |
| Thiazides | 635 (9.9) | 64 (15.2) | 571 (9.5) | <0.001 |
| Antiplatelet | 1,793 (27.9) | 327 (77.5) | 1,466 (24.5) | <0.001 |
| Anticoagulant | 613 (9.5) | 175 (41.5) | 438 (7.3) | <0.001 |
| Statins | 1,848 (28.7) | 351 (83.2) | 1,497 (24.9) | <0.001 |
| Clinical presentation | ||||
| Systolic BP, mm Hg | 120 ± 25 | 126 ± 30 | 119 ± 24 | <0.001 |
| Diastolic BP, mm Hg | 69 ± 15 | 68 ± 17 | 69 ± 15 | 0.408 |
| Heart rate, beats/min | 86 ± 18 | 87 ± 20 | 86 ± 18 | 0.181 |
| Respiratory rate, rpm | 20 ± 5 | 21 ± 5 | 20 ± 5 | <0.001 |
| Saturation O2, % | 94 ± 10 | 91 ± 9 | 94 ± 10 | <0.001 |
| Temperature, ºF | 98.2 ± 1.5 | 98.5 ± 1.7 | 98.2 ± 1.5 | <0.001 |
| Laboratory data | ||||
| WBC, k/μl | 7.9 (5.8−11.5) | 7.0 (5.2−10.3) | 8.0 (5.8−11.6) | <0.001 |
| Neutrophils, % | 72 (61−83) | 76 (66−84) | 72 (61−83) | <0.001 |
| Lymphocytes, % | 16 (9−25) | 14 (8−20) | 17 (9−25) | <0.001 |
| Hemoglobin, g/dl | 11.6 (9.7−13.2) | 10.9 (9.3−13.0) | 11.7 (9.7−13.2) | <0.001 |
| Platelets, k/μl | 254 (183−359) | 199 (144−281) | 260 (187−364) | <0.001 |
| INR | 1.2 (1.1−1.4) | 1.2 (1.1−1.5) | 1.2 (1.1−1.4) | <0.001 |
| Fibrinogen, mg/dl | 581 (450−718) | 524 (429−645) | 589 (454−725) | <0.001 |
| D-dimer, Ug/ml | 1.70 (0.83−3.44) | 1.97 (0.97−3.42) | 1.68 (0.82−3.44) | 0.049 |
| Glucose, mg/dl | 106 (88−154) | 118 (90−185) | 106 (88−151) | <0.001 |
| Sodium, mmol/l | 140 (137−142) | 139 (135−141) | 140 (137−142) | <0.001 |
| Potassium, mmol/l | 4.4 (4.0−4.8) | 4.5 (4.1−5.0) | 4.4 (4.0−4.8) | 0.004 |
| Creatinine, mg/dl | 0.9 (0.7−1.8) | 2.1 (1.2−4.9) | 0.9 (0.7−1.6) | <0.001 |
| BUN, mg/dl | 19 (12−42) | 36 (20−60) | 18 (12−38) | <0.001 |
| ALT, U/l | 34 (20−66) | 23 (14−41) | 36 (20−68) | <0.001 |
| Bilirubin, mg/dl | 0.6 (0.4−0.8) | 0.6 (0.4−0.9) | 0.5 (0.4−0.8) | <0.001 |
| Albumin, g/dl | 2.7 (2.3−3.2) | 2.9 (2.5−3.3) | 2.7 (2.3−3.2) | <0.001 |
| Troponin I∗, ng/ml | 0.06 (0.02−0.19) | 0.07 (0.03−0.19) | 0.05 (0.02−0.18) | 0.022 |
| BNP, pg/ml | 123 (42−456) | 514 (154−1383) | 86 (32−262) | <0.001 |
| Lactate, mmol/l | 1.5 (1.1−2.2) | 1.6 (1.1−2.4) | 1.5 (1.1−2.2) | 0.373 |
| CRP, mg/l | 58.9 (19.1−137.6) | 75.2 (32.2−148.5) | 57.8 (18.4−136.9) | <0.001 |
| Ferritin, ng/ml | 746 (348−1593) | 759 (330−2107) | 745 (350−1570) | 0.535 |
| Procalcitonin, ng/ml | 0.17 (0.06−0.79) | 0.38 (0.10−1.44) | 0.16 (0.06−0.72) | <0.001 |
| Interleukin-6, pg/ml | 54.4 (22.0−126.0) | 66.1 (30.3−131.0) | 53.7 (21.8−125.0) | 0.051 |
| ECG at admission | ||||
| QT interval | 379 (53) | 401 (60) | 377 (53) | <0.001 |
| QT corrected interval | 453 (43) | 474 (46) | 452 (42) | <0.001 |
| Treatment | ||||
| Hydroxychloroquine | 3,758 (58.4) | 249 (59.0) | 3,509 (58.3) | 0.782 |
| Azithromycin | 3,305 (51.3) | 227 (53.8) | 3,078 (51.2) | 0.295 |
| Hydroxy+azithrom | 2,850 (44.3) | 182 (43.1) | 2,668 (44.3) | 0.628 |
| Remdesivir | 166 (2.6) | 6 (1.4) | 160 (2.7) | 0.121 |
| Tocilizumab | 291 (4.5) | 13 (3.1) | 278 (4.6) | 0.141 |
| Steroids | 1,869 (29.0) | 140 (33.2) | 1,729 (28.7) | 0.052 |
| Anticoagulant† | 3,655 (56.8) | 347 (82.2) | 3,308 (55.0) | <0.001 |
| Nasal cannula | 2,755 (53.5) | 304 (72.0) | 2451 (51.8) | <0.001 |
| Outcomes | ||||
| ICU | 1,098 (17.1) | 98 (23.2) | 1,000 (16.6) | <0.001 |
| LOS ICU, days | 7 (3−15) | 5 (2−11) | 7 (3−15) | 0.057 |
| ICU mortality | 636 (57.9) | 72 (73.5) | 564 (56.4) | 0.001 |
| LOS, days | 6 (3−12) | 8 (4−13) | 6 (3−12) | <0.001 |
| Intubation | 813 (12.6) | 96 (22.8) | 717 (11.9) | <0.001 |
| Still admitted | 228 (3.5) | 0 (0.0) | 228 (3.8) | <0.001 |
| In-hospital mortality | 1,664 (25.8) | 169 (40.0) | 1,495 (24.9) | <0.001 |
Values are mean ± SD, n (%), or median (interquartile range).
ALT = alanine transaminase; BMI = body mass index; BNP = brain natriuretic peptide; BP = blood pressure; BUN = blood urea nitrogen; CAD = coronary artery disease; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; CRP = C-reactive protein; ECG = electrocardiogram; HF = heart failure; ICU = intensive care unit; INR = international normalized ratio; LOS = length of stay; MRA = mineraloid receptor antagonist; OSA = obstructive sleep apnea; RAAS = renin-angiotensin-aldosterone system; rpm = respirations per minute; WBC = white blood cells.
N = 2,264.
In those patients without previous anticoagulation.
Outcomes in patients with HF compared with patients without HF
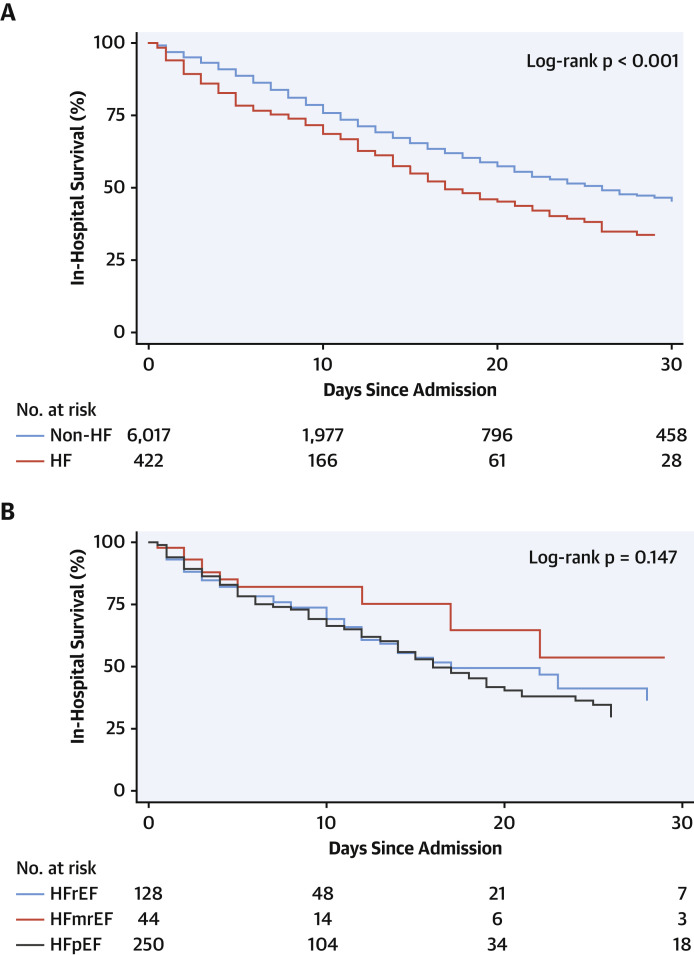
Median LOS for the overall cohort was 6 days (IQR: 3 to 12 days), whereas median LOS among patients with a history of HF was longer (8 days; IQR: 4 to 13 days). A requirement for ICU care was observed in nearly one-fifth (17.1%) of patients, whereas intubation with mechanical ventilation was observed in 12.6% in the study population. Both outcomes were more likely among patients with a history of HF compared with those without HF (odds ratio [OR]: 1.52; 95% confidence interval [CI]: 1.20 to 1.92; p = 0.001, and OR: 2.18; 95% CI: 1.71 to 2.77; p < 0.001; respectively). Overall mortality was 25.8%; however, the risk of mortality among patients with HF was twice that of patients without HF (40.0% vs. 24.9%; OR: 2.02; 95% CI: 1.65 to 2.48; p < 0.001) (Figure 2A ).
Figure 2.
Kaplan-Meier Survival Curves
(A) Kaplan-Meier survival curves in patients hospitalized with COVID-19 according to HF history. (B) Kaplan-Meier survival curves in patients with HF hospitalized with COVID-19 according to left ventricular ejection fraction (LVEF) category. Abbreviations as in Figure 1.
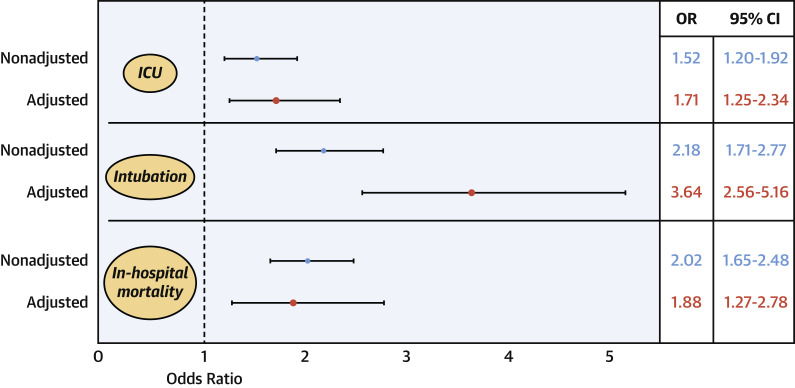
After a multivariable logistic regression that adjusted for relevant demographic variables, comorbidities, previous treatment with RAASi, and markers of clinical severity on admission, history of HF persisted as an independent risk factor for the need for ICU care (adjOR: 1.71; 95% CI: 1.25 to 2.34; p = 0.001), intubation and mechanical ventilation (adjOR: 3.64; 95% CI: 2.56 to 5.16; p < 0.001), and in-hospital mortality (adjOR: 1.88; 95% CI: 1.27 to 2.78; p = 0.002) (Figure 3 ). In the subgroup of patients who had both D-dimer and troponin assessed on admission (n = 1,777), the increased risk was sustained despite adjustment for these markers (Supplemental Figure 1).
Figure 3.
Forest Plot of the Effect of a History of HF on Outcomes in Patients Admitted for COVID-19
After a multivariable logistic regression adjusting for age, sex, race, obesity, hypertension, diabetes, coronary artery disease, atrial fibrillation, chronic kidney disease, chronic obstructive pulmonary disease, previous treatment with renin-angiotensin-aldosterone inhibitors, systolic blood pressure, heart rate, oxygen saturation, white blood count, lymphocytes, creatinine, and albumin on admission, history of HF persisted as an independent risk factor for the need for intensive care unit (ICU) care, intubation and mechanical ventilation, and in-hospital mortality. CI = confidence interval; OR = odds ratio; other abbreviations as in Figure 1.
Clinical profile, management, and echocardiography in patients with HF stratified by LVEF
Of 422 patients with a history of HF, 250 (59.3%), 128 (30.3%), and 44 (10.4%) had HFpEF, HFrEF, and HFmrEF, respectively. Table 2 summarizes the clinical characteristics and outcomes of the study population according to the LVEF. Overall, patients with HFpEF were older, more frequently women, with a higher body mass index and prevalence of previous lung disease than patients with HFrEF, whereas those with HFmrEF fell in between (Supplemental Figure 2). Patients with HFpEF had less frequent ischemic heart disease, smaller left ventricular diameters, less mitral regurgitation, lower previous 1-year HF admission rate, less frequent left bundle branch block, or presence of defibrillators and cardiac resynchronization devices. Expectedly, neurohormonal therapy was also less frequently prescribed in patients with HFpEF compared with those with HFrEF or HFmrEF. On hospital presentation, there were no significant differences in symptoms among groups. Patients with HFpEF presented with lower oxygen saturation and lower median values of hemoglobin, D-dimer, alanine aminotransferase, bilirubin, and natriuretic peptides compared with those with HFrEF. They were also treated with hydroxychloroquine or macrolides and noninvasive ventilation more frequently than the other 2 groups, whereas antiplatelet and neurohormonal therapies were more common among patients with HFrEF.
Table 2.
Clinical Characteristics of the Patients With HF Admitted for COVID-19 According to the LVEF Category
| HFrEF (n = 128; 30.3%) | HFmrEF (n = 44; 10.4%) | HFpEF (n = 250; 59.3%) | p Value | |
|---|---|---|---|---|
| Age, yrs | 69.9 ± 13.7 | 71.2 ± 15.3 | 74.1 ± 12.5 | 0.013 |
| Female | 37 (28.9) | 18 (40.9) | 131 (52.4) | <0.001 |
| BMI, kg/m2 | 27.4 ± 6.7 | 31.3 ± 12.0 | 30.2 ± 8.2 | 0.002 |
| Race | 0.207 | |||
| Black | 46 (35.9) | 12 (27.3) | 76 (30.4) | |
| Hispanic/Latino | 41 (32.0) | 16 (36.4) | 63 (25.2) | |
| White | 28 (21.9) | 12 (27.3) | 65 (26.0) | |
| Asian | 2 (1.6) | 1 (2.3) | 18 (7.2) | |
| Other | 10 (7.8) | 3 (6.8) | 21 (8.4) | |
| Unknown | 1 (0.8) | 0 (0.0) | 7 (2.8) | |
| Comorbidities | ||||
| Obesity | 41 (32.0) | 23 (52.3) | 105 (42.0) | 0.038 |
| Hypertension | 114 (89.1) | 39 (88.6) | 229 (91.6) | 0.657 |
| Diabetes mellitus | 74 (57.8) | 28 (63.4) | 167 (66.8) | 0.228 |
| Dyslipidemia | 73 (57.0) | 25 (56.8) | 130 (52.0) | 0.601 |
| CAD | 86 (67.2) | 26 (59.1) | 123 (49.2) | 0.003 |
| Stroke | 35 (27.3) | 10 (22.7) | 69 (27.6) | 0.794 |
| AF/flutter | 48 (37.5) | 23 (52.3) | 89 (35.6) | 0.109 |
| CKD | 49 (38.3) | 18 (40.9) | 110 (44.0) | 0.560 |
| COPD | 19 (14.8) | 10 (22.7) | 65 (26.0) | 0.048 |
| Asthma | 12 (9.4) | 8 (18.2) | 38 (15.2) | 0.198 |
| OSA | 8 (6.3) | 7 (15.9) | 42 (16.8) | 0.016 |
| HF history | ||||
| Ischemic HF | 70 (54.7) | 21 (47.7) | 67 (26.8) | <0.001 |
| HF duration, yrs | 3.9 ± 3.9 | 4.5 ± 2.7 | 4.2 ± 3.4 | 0.036 |
| LVEF, % | 30 ± 9 | 45 ± 2 | 61 ± 6 | <0.001 |
| LVEDD, mm | 55 ± 9 | 50 ± 7 | 46 ± 8 | <0.001 |
| Septum, mm | 11 (3) | 12 (3) | 12 (3) | 0.014 |
| Mod/severe MR | 37 (32.5) | 10 (23.8) | 21 (9.0) | <0.001 |
| Baseline NYHA functional class | 0.942 | |||
| I | 9 (7.2) | 3 (7.1) | 21 (8.7) | |
| II | 65 (52.0) | 26 (61.9) | 128 (53.1) | |
| III | 46 (36.8) | 12 (28.6) | 83 (34.4) | |
| IV | 5 (4.0) | 1 (2.4) | 9 (3.7) | |
| Past 1-yr HF admission | 58 (45.3) | 18 (40.9) | 90 (36.1) | 0.221 |
| No. of 1-yr HF admissions | 1.2 (2.7) | 0.6 (0.8) | 0.7 (1.5) | 0.025 |
| LBBB | 22 (17.2) | 3 (6.8) | 9 (3.6) | <0.001 |
| ICD | 44 (34.4) | 3 (6.8) | 6 (82.4) | <0.001 |
| CRT | 15 (11.7) | 1 (2.3) | 1 (0.4) | <0.001 |
| Background treatment | ||||
| RAAS inhibitors | 96 (75.0) | 32 (72.7) | 132 (52.8) | <0.001 |
| Beta-blockers | 116 (90.6) | 38 (86.4) | 200 (80.0) | 0.026 |
| MRA | 26 (20.3) | 8 (18.2) | 26 (10.4) | 0.024 |
| SGLT2i | 5 (3.9) | 1 (2.3) | 6 (2.4) | 0.819 |
| Loop diuretics | 96 (75.0) | 33 (75.0) | 189 (75.5) | 0.990 |
| Thiazides | 13 (10.2) | 6 (13.6) | 45 (18.0) | 0.126 |
| Antiplatelet | 104 (81.3) | 36 (81.8) | 187 (74.8) | 0.280 |
| Anticoagulant | 55 (43.0) | 19 (43.2) | 101 (40.4) | 0.865 |
| Statins | 115 (89.8) | 37 (84.1) | 199 (79.6) | 0.041 |
| Clinical presentation | ||||
| Fever | 41 (32.0) | 21 (47.7) | 100 (40.0) | 0.130 |
| Cough | 50 (39.1) | 25 (56.8) | 108 (43.2) | 0.122 |
| Shortness of breath | 76 (59.4) | 27 (61.4) | 151 (60.4) | 0.968 |
| Weakness/fatigue | 38 (29.7) | 15 (34.1) | 61 (24.4) | 0.294 |
| Systolic BP, mm Hg | 122 ± 27 | 128 ± 27 | 127 ± 32 | 0.313 |
| Diastolic BP, mm Hg | 70 ± 15 | 71 ± 17 | 67 ± 17 | 0.140 |
| Heart rate, beats/min | 86 ± 20 | 87 ± 23 | 88 ± 20 | 0.657 |
| Respiratory rate, rpm | 20 ± 5 | 21 ± 5 | 21 ± 5 | 0.818 |
| Saturation O2, % | 92 ± 9 | 94 ± 6 | 91 ± 10 | 0.045 |
| Temperature, ºF | 98.5 ± 1.8 | 98.2 ± 1.2 | 98.6 ± 1.8 | 0.403 |
| Any sign of congestion | 61 (47.7) | 16 (36.4) | 101 (40.4) | 0.285 |
| Laboratory data | ||||
| WBC, k/μl | 6.7 (4.6−9.8) | 6.4 (4.8−11.6) | 7.3 (5.3−10.6) | 0.164 |
| Neutrophils, % | 77 (65−85) | 70 (62−84) | 76 (68−84) | 0.379 |
| Lymphocytes, % | 13 (8−20) | 16 (9−24) | 13 (8−20) | 0.232 |
| Hemoglobin, g/dl | 11.6 (9.9−13.6) | 10.5 (9.4−13.3) | 10.7 (8.9−12.7) | 0.005 |
| Platelets, k/μl | 192 (137−258) | 213 (142−318) | 203 (145−284) | 0.450 |
| INR | 1.2 (1.1−1.6) | 1.3 (1.1−1.4) | 1.2 (1.1−1.5) | 0.377 |
| Fibrinogen, mg/dl | 520 (410−633) | 565 (457−651) | 519 (432−650) | 0.578 |
| D-dimer, UG/ml | 2.15 (1.22−3.59) | 1.14 (0.77−2.18) | 1.97 (1.01−3.67) | 0.014 |
| Glucose, mg/dl | 120 (93−189) | 109 (87−170) | 119 (90−186) | 0.572 |
| Sodium, mmol/l | 139 (136−142) | 139 (136−141) | 138 (135−141) | 0.864 |
| Potassium, mmol/l | 4.5 (4.1−5.1) | 4.5 (4.2−4.8) | 4.5 (4.0−4.9) | 0.508 |
| Creatinine, mg/dl | 1.7 (1.2−3.4) | 1.8 (1.1−3.3) | 2.2 (1.2−5.5) | 0.162 |
| BUN, mg/dl | 38 (21−59) | 29 (16−49) | 37 (21−64) | 0.131 |
| ALT, U/l | 28 (18−52) | 18 (12−28) | 22 (14−34) | 0.001 |
| Bilirubin, mg/dl | 0.7 (0.5−1.1) | 0.6 (0.4−0.8) | 0.6 (0.4−0.8) | 0.045 |
| Albumin, g/dl | 2.9 (2.4−3.3) | 3.2 (2.5−3.5) | 2.9 (2.5−3.3) | 0.361 |
| Troponin I, ng/ml | 0.07 (0.03−0.22) | 0.07 (0.02−0.16) | 0.08 (0.03−0.19) | 0.627 |
| Peak troponin, ng/ml | 0.10 (0.03−0.25) | 0.09 (0.03−0.42) | 0.13 (0.04−0.39) | 0.183 |
| BNP, pg/ml | 678 (235−1862) | 585 (177−1121) | 378 (125−1271) | 0.018 |
| Lactate, mmol/l | 1.6 (1.1−2.7) | 1.6 (1.1−2.2) | 1.6 (1.1−2.3) | 0.590 |
| CRP, mg/l | 93.4 (41.0−160.7) | 67.6 (27.3−131.7) | 73.7 (32.2−131.7) | 0.363 |
| Ferritin, ng/ml | 960 (319−2811) | 508 (183−861) | 760 (348−2017) | 0.126 |
| Procalcitonin, ng/ml | 0.33 (0.08−1.23) | 0.19 (0.11−0.56) | 0.46 (0.10−1.77) | 0.109 |
| Interleukin-6, pg/ml | 71.4 (36.6−144.2) | 66.8 (31.3−126.3) | 60.4 (26.2−124.0) | 0.943 |
| CV tests during admission | ||||
| ECG | 126 (98.4) | 43 (97.7) | 235 (94.0) | 0.102 |
| Sinusal | 83 (65.9) | 25 (58.1) | 174 (74.0) | 0.005 |
| AF/flutter | 20 (15.9) | 13 (30.2) | 45 (19.2) | |
| Other | 23 (18.3) | 5 (11.6) | 16 (6.8) | |
| LBBB | 15 (12.5) | 3 (7.3) | 10 (4.5) | 0.020 |
| QT interval | 412 (62) | 398 (55) | 395 (59) | 0.030 |
| QTc interval | 487 (45) | 475 (53) | 466 (43) | <0.001 |
| Echocardiography | 30 (23.4) | 9 (20.5) | 41 (16.5) | 0.254 |
| LVEF, % | 34 ± 14 | 41 ± 18 | 58 ± 11 | <0.001 |
| Mod/severe MR | 10 (33.3) | 1 (11.1) | 10 (25.6) | 0.481 |
| Mod/severe TR | 10 (33.3) | 2 (22.2) | 8 (20.5) | 0.464 |
| Cardiac CT | 6 (4.7) | 0 (0.0) | 2 (0.8) | 0.031 |
| RHC | 3 (2.3) | 0 (0.0) | 0 (0.0) | 0.057 |
| LHC | 3 (2.3) | 0 (0.0) | 1 (0.4) | 0.141 |
| Treatment | ||||
| Hydroxychloroquine | 65 (50.8) | 21 (47.7) | 163 (65.2) | 0.007 |
| Azithromycin | 59 (46.1) | 20 (45.5) | 148 (59.2) | 0.027 |
| Hydroxy+azithrom | 46 (35.9) | 15 (34.1) | 121 (48.4) | 0.030 |
| Remdesivir | 1 (0.8) | 1 (2.3) | 4 (1.6) | 0.576 |
| Tocilizumab | 4 (3.1) | 2 (4.6) | 7 (2.8) | 0.763 |
| Steroids | 37 (28.9) | 13 (29.6) | 90 (36.0) | 0.331 |
| Anticoagulant∗ | 59 (80.8) | 20 (80.0) | 126 (84.6) | 0.718 |
| Antiplatelet | 72 (56.3) | 19 (43.2) | 105 (42.0) | 0.028 |
| RAAS inhibitor (only if present at baseline) | ||||
| Continued | 25 (26.0) | 11 (34.4) | 20 (15.4) | 0.028 |
| Stopped | 71 (74.0) | 21 (65.6) | 110 (84.6) | |
| Beta-blockers | 74 (57.8) | 23 (52.3) | 105 (42.0) | 0.012 |
| MRA | 10 (7.8) | 2 (4.6) | 6 (2.4) | 0.044 |
| IV diuretics | 50 (39.1) | 14 (31.8) | 92 (36.8) | 0.689 |
| Statins | 67 (52.3) | 25 (56.8) | 120 (48.0) | 0.475 |
| Nasal cannula | 93 (72.7) | 30 (68.2) | 181 (72.4) | 0.833 |
| CPAP/BIPAP | 34 (26.6) | 10 (22.7) | 93 (37.2) | 0.039 |
| Inotropes | 10 (7.9) | 1 (2.3) | 7 (2.8) | 0.078 |
| Vasopressors | 25 (19.5) | 6 (13.6) | 41 (16.4) | 0.608 |
| MCS | 2 (1.6) | 0 (0.0) | 0 (0.0) | 0.166 |
| RRT (excluding pts with long-term dialysis) | 5 (3.9) | 1 (2.3) | 16 (6.4) | 0.382 |
| Outcomes | ||||
| ICU | 27 (21.1) | 11 (25.0) | 60 (24.0) | 0.783 |
| LOS ICU, days | 7 (3−13) | 3 (1−5) | 5 (2−13) | 0.117 |
| LOS, days | 8 (3−14) | 7 (3−12) | 8 (4−13) | 0.682 |
| Intubation | 28 (21.9) | 8 (18.2) | 60 (24.0) | 0.670 |
| AKI | 57 (44.5) | 15 (34.1) | 102 (40.8) | 0.468 |
| Shock | 34 (26.6) | 5 (11.4) | 52 (20.8) | 0.096 |
| Cardiogenic | 10 (7.8) | 1 (2.3) | 5 (2.0) | 0.019 |
| Septic | 24 (18.8) | 3 (6.8) | 47 (18.8) | 0.134 |
| Hypovolemic | 5 (3.9) | 1 (2.3) | 6 (2.4) | 0.819 |
| Thromboembolic events | 8 (6.3) | 1 (2.3) | 10 (4.0) | 0.207 |
| ACS | 5 (3.9) | 0 (0.0) | 5 (2.0) | 0.383 |
| Stroke | 1 (0.8) | 0 (0.0) | 1 (0.4) | 1.000 |
| PE | 0 (0.0) | 0 (0.0) | 3 (1.2) | 0.680 |
| Others | 2 (1.6) | 1 (2.3) | 1 (0.4) | 0.210 |
| Arrhythmias | 23 (18.0) | 9 (20.5) | 32 (12.8) | 0.243 |
| AF/SVT | 17 (13.3) | 9 (20.5) | 31 (12.4) | 0.352 |
| NSVT | 2 (1.6) | 1 (2.3) | 0 (0.0) | 0.086 |
| VT | 3 (2.3) | 0 (0.0) | 0 (0.0) | 0.057 |
| VF | 2 (1.6) | 0 (0.0) | 0 (0.4) | 0.473 |
| 30-day readmission rate | 17 (17.7) | 3 (8.3) | 35 (18.6) | 0.347 |
| Non-CV | 6 (35.3) | 2 (66.7) | 23 (65.7) | 0.019 |
| CV non-HF | 3 (17.7) | 1 (33.3) | 9 (25.7) | |
| CV HF related | 8 (47.1) | 0 (0.0) | 3 (8.6) | |
| Death | 49 (38.3) | 10 (22.7) | 110 (44.0) | 0.026 |
| Non-CV | 40 (81.6) | 9 (90.0) | 102 (92.7) | 0.157 |
| CV non-HF | 5 (10.2) | 0 (0.0) | 5 (4.6) | |
| CV HF related | 4 (8.2) | 1 (10.0) | 3 (2.7) | |
Values are mean ± SD, n (%), or median (interquartile range).
ACS = acute coronary syndrome; AF = atrial fibrillation; AKI = acute kidney injury; BiPAP = bilevel positive airway pressure; CPAP = continuous positive airway pressure; CRP = C-reactive protein; CRT = cardiac resynchronization therapy; CT = computed tomography; CV = cardiovascular; ICD = implantable cardioverter defibrillator; LBBB = left bundle branch block; LHC = left heart catheterization; LVEDD = left ventricular end-diastolic diameter; MCS = mechanical circulatory support; MR = mitral regurgitation; NSVT = non-supraventricular tachycardia; NYHA = New York Heart Association; PE = pulmonary embolism; RHC = right heart catheterization; RRT = renal replacement therapy; SGLT2i = sodium-glucose co-transporter-2 inhibitors; SVT = supraventricular tachycardia; TR = tricuspid regurgitation; VF = ventricular fibrillation; VT = ventricular tachycardia; other abbreviations as Table 1.
In those patients without previous anticoagulation.
Echocardiography was performed in 80 of 422 (19.0%) patients with history of HF during the COVID-19 hospitalization (Supplemental Table 1). Interestingly, 14 (17.5%) presented with worsening LVEF of ≥10 points. De novo severe tricuspid and mitral regurgitation was encountered in 9 (11.3%), and 6 (7.5%) patients, respectively, in comparison with the study before admission. Other cardiovascular tests such as cardiac computed tomography and left or right heart catheterization were performed rarely on a case-by-case basis during the COVID-19 hospitalization (Table 2).
Outcomes among patients with HF stratified by LVEF
Among the 422 patients with a history of HF hospitalized for COVID-19, there were no significant differences in LOS, need for ICU care, intubation and mechanical ventilation, acute kidney injury, shock, thromboembolic events, arrhythmias, or 30-day readmission rates across LVEF strata. However, cardiogenic shock (7.8% vs. 2.3% vs. 2%; p = 0.019) and HF-related causes for 30-day readmission (47.1% vs. 0% vs. 8.6%) were significantly higher in patients with HFrEF than in those with HFmrEF or HFpEF. Finally, although this was a smaller group of patients, mortality was observed to be lower among patients with HFmrEF (22.7%) compared with the 2 other HF categories (38.3% in HFrEF and 44% in HFpEF). Figure 2B shows the Kaplan-Meier survival curves of the HF population according to LVEF category.
Risk factors for in-hospital mortality among patients with HF by multivariable Cox regression included older age, more severe HF (baseline New York Heart Association functional classes III and IV), previous mitral regurgitation, lower systolic blood pressure, lower oxygen saturation, lower lymphocyte count, and increased troponin concentrations. Again, neither LVEF category nor previous treatment with RAASi were independently associated with worse prognosis (Table 3 ). Remarkably, race was not associated with worse outcomes.
Table 3.
Risk Factors for In-Hospital Mortality in Patients With HF Admitted for COVID After Cox Proportional Hazards Regression Analysis
| HR | 95% CI | p Value | aHR | 95% CI | p Value | |
|---|---|---|---|---|---|---|
| Age (for each increase of 5 yrs) | 1.18 | 1.10−1.26 | <0.001 | 1.15 | 1.05−1.25 | 0.002 |
| Female | 1.08 | 0.79−1.47 | 0.642 | 1.13 | 0.77−1.64 | 0.538 |
| Race | ||||||
| White (ref) | — | — | — | — | — | — |
| Black | 0.62 | 0.41−0.94 | 0.025 | 0.84 | 0.51−1.36 | 0.467 |
| Hispanic/Latino | 0.76 | 0.50−1.14 | 0.179 | 1.10 | 0.69−1.75 | 0.679 |
| Asian | 0.87 | 0.43−1.77 | 0.698 | 1.25 | 0.58−2.71 | 0.575 |
| Other | 0.87 | 0.47−1.60 | 0.649 | 1.15 | 0.59−2.23 | 0.684 |
| Unknown | 2.18 | 0.78−6.07 | 0.137 | 1.67 | 0.55−5.04 | 0.365 |
| BMI (for each increase of 1 kg/m2) | 1.00 | 0.98−1.02 | 0.822 | 1.01 | 0.99−1.03 | 0.274 |
| Hypertension | 0.82 | 0.50−1.34 | 0.432 | 1.05 | 0.61−1.82 | 0.860 |
| Diabetes mellitus | 0.75 | 0.55−1.03 | 0.071 | 1.02 | 0.70−1.50 | 0.915 |
| AF/flutter | 1.28 | 0.94−1.75 | 0.113 | 0.91 | 0.63−1.31 | 0.597 |
| Chronic kidney disease | 0.70 | 0.51−0.97 | 0.032 | 0.75 | 049−1.14 | 0.175 |
| COPD | 1.28 | 0.90−1.81 | 0.164 | 1.09 | 0.74−1.60 | 0.676 |
| LVEF category | ||||||
| HFmrEF (ref) | — | — | — | — | — | — |
| HFrEF | 1.68 | 0.82−3.43 | 0.157 | 1.44 | 0.67−3.11 | 0.347 |
| HFpEF | 1.98 | 1.00−3.92 | 0.049 | 1.54 | 0.74−3.22 | 0.250 |
| NYHA functional class III/IV | 1.53 | 1.11−2.11 | 0.009 | 1.61 | 1.13−2.30 | 0.009 |
| Past moderate/severe MR | 1.65 | 1.13−2.40 | 0.009 | 1.62 | 1.04−2.51 | 0.033 |
| Previous RAAS inhibitors | 0.80 | 0.59−1.09 | 0.152 | 0.84 | 0.59−1.19 | 0.319 |
| Systolic BP (for each increase of 10 mm Hg) | 0.90 | 0.85−0.95 | <0.001 | 0.93 | 0.88−0.99 | 0.015 |
| Heart rate, beats/min (for each increase of 1 beats/min) | 1.01 | 1.00−1.01 | 0.070 | 1.01 | 0.99−1.01 | 0.114 |
| Saturation O2. % (for each increase of 1%) | 0.96 | 0.95−0.97 | <0.001 | 0.97 | 0.96−0.99 | 0.001 |
| Lymphocytes, % (for each increase of 1%) | 0.95 | 0.93−0.97 | <0.001 | 0.97 | 0.95−0.99 | 0.005 |
| Creatinine, mg/dl (for each increase of 1 mg/dl) | 1.00 | 0.95−1.04 | 0.946 | 1.04 | 0.98−1.11 | 0.191 |
| BNP (for each increase of 100 pg/ml) | 1.00 | 0.99−1.01 | 0.427 | 1.00 | 0.99−1.01 | 0.356 |
| Troponin, ng/ml (for each increase of 1 ng/ml) | 1.07 | 1.01−1.13 | <0.001 | 1.08 | 1.01−1.16 | 0.017 |
Discussion
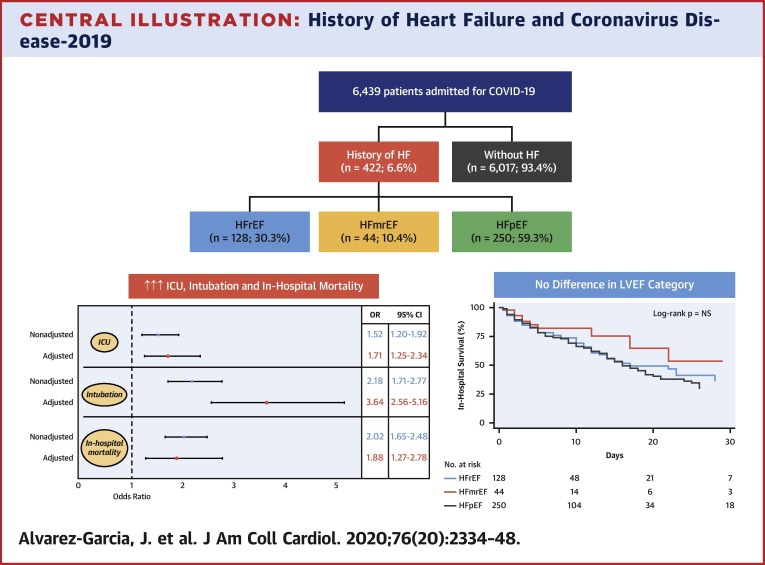
Patients with HF represent a population at particularly high risk for worse outcomes with COVID-19. In this multihospital retrospective cohort study from New York City, which was once the global epicenter of COVID-19, we showed that approximately 7% of patients had a history of HF. Compared with patients without HF, history of HF was associated with a nearly 2-fold higher risk of death, >3 times higher risk of mechanical ventilation, and longer LOS despite adjustment for relevant clinical factors. Interestingly, no major differences were noted in the clinical course and outcomes among patients with HFpEF, HFmrEF, or HFrEF (Central Illustration ). Finally, previous RAASi use was not associated with a worse prognosis among patients with a history of HF. These simple yet powerful findings revealed the substantially increased risk patients with HF face once hospitalized with COVID-19, regardless of EF, and also pointed to the importance of maintaining RAASi in patients in whom these medications are strongly indicated.
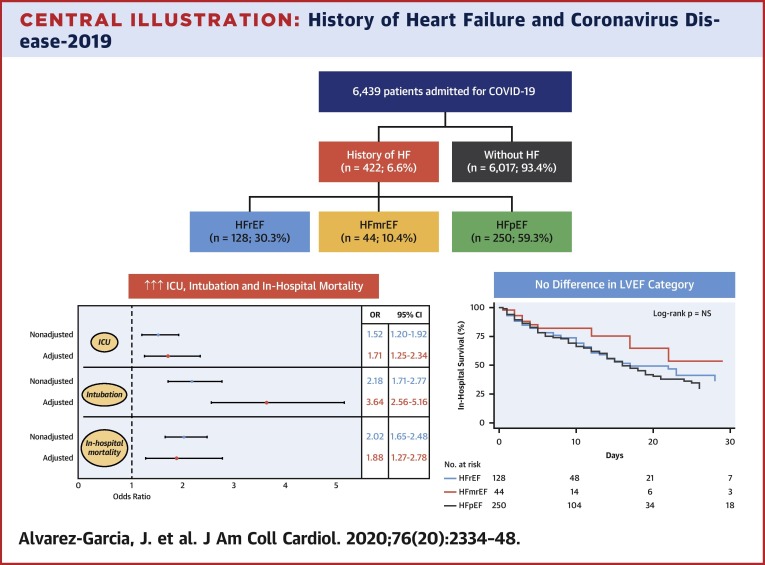
Central Illustration.
History of Heart Failure and Coronavirus Disease-2019
Patients with pre-existing heart failure (HF) are at nearly twice the risk of mortality and 3 times the risk of mechanical ventilation compared with patients without HF when hospitalized for coronavirus disease-2019 (COVID-19), yet outcomes among patients with HF were similar regardless of left ventricular ejection fraction (LVEF). (Top panel) Consort diagram of the study population. (Bottom right panel) Kaplan-Meier survival curves in patients hospitalized with COVID-19 according to LVEF category. (Bottom left panel) Forest plot of the effect of history of HF on outcomes in patients admitted for COVID-19. CI = confidence interval; HFmrEF = heart failure with mid-range ejection fraction; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; HR = hazard ratio; ICU = intensive care unit.
Prognostic impact of history of HF
Although cardiovascular disease, including HF, has been identified as a risk factor for worse outcomes in COVID-19 (16, 17, 18, 19), specific data on the clinical profile, hospital course, and prognosis of patients with a history of HF, particularly in the United States, have been limited (10,11). Specifically, 2 smaller studies (<100 patients each) from Italy and Denmark showed mortality rates of 36% to 37% among patients with cardiovascular disease (wherein HF was well represented) compared with 26% in the overall cohorts. The present analysis included a diverse cohort of >400 patients with HF and included detailed information on comorbid conditions, severity of HF, medications, LVEFs, and specific outcomes.
Patients with HF frequently have a high number of comorbid conditions that contribute to the increased risk of adverse outcomes encountered in the face of acute illness. However, our results revealed that a history of HF itself was associated with a near doubling risk of mortality despite adjustment for comorbid conditions. The systemic effects of COVID-19, particularly on the cardiovascular system, have been increasingly recognized (20). In particular, SARS-CoV-2 has been found within macrophages, endothelial cells, and pericytes (21,22), with a recent study demonstrating evidence of active viral replication in the myocardium on autopsy (23). Widespread inflammation, as well as increased micro- and macrovascular thrombosis, may underlie the cardiac manifestations of arrhythmias, myocarditis, and de novo LV dysfunction that have been reported (20,22). Our group previously showed that the degree of myocardial injury, reflected by increased troponin concentrations, correlated with increasing risk of mortality in the setting of COVID-19 (4). In the present analysis, we saw higher mean troponin concentrations among patients with HF compared with those without HF. Specific mechanisms by which patients with pre-existing HF are more susceptible to deleterious cardiac manifestations and subsequent increased mortality related to infection with SARS-CoV-2 remains to be further elucidated.
Impact of LVEF and RAASi among patients with HF hospitalized with COVID-19
It was particularly interesting to note the lack of difference in LOS, ICU requirement, intubation and mechanical ventilation, acute renal failure, intravenous diuretic requirement, and mortality among patients with HF based on LVEF. Despite substantial evidence pointing to equivalent outcomes in other settings, patients with HFpEF are often considered at lower risk for mortality compared with their HFrEF counterparts. The present analysis added to this mounting body of literature (24,25), which demonstrated similar outcomes among patients with HFpEF and HFrEF, even in the setting of acute COVID-19. In contrast, our results suggested that patients with HFmrEF could have a better prognosis, because they can represent a distinct and more favorable HF phenotype (26,27).
Similarly, in the early stages of the pandemic, RAASi were thought to confer increased risk due to increased angiotensin-converting enzyme 2 expression, hence facilitating increased viral entry into host cells (13,21,28). Among patients with HF, particularly those with reduced EFs, RAASi form the essential cornerstone of management, and as such, discontinuation of these medications could lead to deleterious effects in the long term. In accordance with subsequent papers that disproved the postulated adverse effects of angiotensin-converting enzyme/angiotensin receptor blockers in the setting of COVID-19 (29,30), our analysis also showed no association between RAASi and adverse events but specifically in the patient population who benefitted from them the most. As such, we offer additional support for continuation of these life-saving medications in patients with HF amidst the COVID-19 pandemic.
Clinical implications
The present analysis of patients with HF with COVID-19 can entail several clinical implications. First, the strong association with increased risk of mechanical ventilation and mortality may help triage patients upon presentation to the hospital. Furthermore, because of this increased risk, the utmost caution must also be taken to prevent exposure for patients with HF. Several centers have reported a reduction of HF hospitalization during the pandemic (31, 32, 33, 34), and as such, the reliance on telemonitoring and telemedicine may increase for patients where COVID-19 is rampant (35, 36, 37, 38). Future studies are needed to understand the impact of telemonitoring on long-term care and outcomes for this population. Among patients with severe HF, weighing the risk of exposure to COVID-19 against the benefit of life-saving therapies, such as mechanical circulatory support and heart transplantation, is particularly relevant and must be carefully considered on a case-by-case basis (39). Finally, understanding the mechanisms that underlie the high risk of complications and mortality among patients with HF begs the question of whether specific therapies to combat acute infection in COVID-19 should be used based on the history of HF. Recent studies have pointed to the potential benefits of corticosteroids and anticoagulation, as well as antiviral therapies in the treatment of more severe COVID-19 cases (40, 41, 42). Because inflammation underlies both chronic HF (43) and acute COVID-19, it may be that anti-inflammatory drugs are particularly effective in mitigating adverse events in this population. This hypothesis and others will warrant further longitudinal follow-up studies.
Study limitations
First, the use of electronic health records for patient-level data in such a large sample size was subject to error. Because history of HF was identified using ICD-9/10 codes, it was possible that some patients with history of HF were not appropriately classified. However, for those patients identified as having a history of HF, we manually verified history, clinical data, and outcomes to ensure accuracy. Second, it was not possible to ascertain causes of death nor 30-day readmission rate in the overall cohort. In addition, we did not capture readmissions to other hospitals; however, the Mount Sinai Health system is large and far-reaching within New York City, and as such, it was more likely that most rehospitalizations were reflected. Finally, because of the small number of patients with echocardiographic studies performed during the hospitalization for COVID-19, related imaging findings should be interpreted with caution.
Conclusions
History of HF is associated with an almost 2-fold increased risk of death among patients hospitalized with COVID-19, despite adjustment for other prognostic and clinically relevant factors. Importantly, neither LVEF category nor previous treatment with RAASi were associated with worse prognosis among patients with HF and COVID-19. If these findings are confirmed in other populations, history of HF may help guide triage upon hospital presentation and potentially dictate aggressive therapies in the treatment of COVID-19.
Perspectives.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: Patients with a history of HF hospitalized for COVID-19 face nearly 3 times the risk of mechanical ventilation and twice the risk of mortality compared with patients without HF. Outcomes of patients with HF are independent of LVEF or use of RAASi medications.
TRANSLATIONAL OUTLOOK: Prospective studies are warranted to elucidate the mechanisms responsible for the association of HF and adverse outcomes in patients with COVID-19 and to identify management strategies that improve survival.
Author Relationship With Industry
Dr. Alvarez-Garcia received a mobility grant from Private Foundation Daniel Bravo Andreu (Spain). Dr. Rivas-Lasarte received a “Magda Heras” mobility grant from Spanish Society of Cardiology (Spain). Dr. Mitter has received personal fees from Abbott Laboratories, Cowen & Co., and the Heart Failure Society of America. Dr. Nadkarni has received grants, personal fees, and nonfinancial support from Renalytix AI; has received nonfinancial support from Pensieve Health; and has received personal fees from AstraZeneca, Variant Bio, BioVie, and GLG Consulting, outside the submitted work. Dr. Fayad has received grants from Daiichi-Sankyo, Amgen, Bristol Myers Squibb, and Siemens Healthineers; has received personal fees from Alexion, GlaxoSmithKline, and Trained Therapeutix Discovery, outside the submitted work; and holds patents licensed to Trained Therapeutix Discovery. Dr. Lala has received personal fees from Zoll, outside the submitted work. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Lisa A. Mendes, MD, served as Guest Associate Editor for this paper. Athena Poppas, MD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACCauthor instructions page.
Appendix
For an expanded Methods section as well as supplemental figures and a table, please see the online version of this paper.
Appendix
References
- 1.John Hopkins University COVID-19 Map. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html Available at:
- 2.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lala A., Johnson K.W., Januzzi J.L. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giustino G., Croft L.B., Oates C.P. Takotsubo cardiomyopathy in COVID-19. J Am Coll Cardiol. 2020;76:628–629. doi: 10.1016/j.jacc.2020.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatla A., Mayer M.M., Adusumalli S. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17:1439–1444. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried J.A., Ramasubbu K., Bhatt R. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141:1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeFilippis E.M., Reza N., Donald E., Givertz M.M., Lindenfeld J.A., Jessup M. Considerations for heart failure care during the COVID-19 pandemic. J Am Coll Cardiol HF. 2020;8:681–691. doi: 10.1016/j.jchf.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inciardi R.M., Adamo M., Lupi L. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur. Heart J. 2020;41:1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersson C., Andersson C., Gerds T. Incidence of new-onset and worsening heart failure before and after the COVID-19 epidemic lockdown in Denmark: a nationwide cohort study. Circ Hear Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007274. [DOI] [PubMed] [Google Scholar]
- 12.Jiménez-Blanco Bravo M., Cordero Pereda D., Sánchez Vega D. Heart failure in the time of COVID-19. Cardiology. 2020;145:481–484. doi: 10.1159/000509181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 15.Little R.J.A., Rubin D.B. 3rd Edition. Wiley; New York: 1991. Statistical Analysis with Missing Data. [Google Scholar]
- 16.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi S., Qin M., Cai Y. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41:2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrilli C.M., Jones S.A., Yang J. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atri D., Siddiqi H.K., Lang J.P., Nauffal V., Morrow D.A., Bohula E.A. COVID-19 for the cardiologist: basic virology, epidemiology, cardiac manifestations, and potential therapeutic strategies. J Am Coll Cardiol Basic Trans Science. 2020;5:518–536. doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116:1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindner D., Fitzek A., Bräuninger H. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020 July 27 doi: 10.1001/jamacardio.2020.3551. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lam C.S.P., Gamble G.D., Ling L.H. Mortality associated with heart failure with preserved vs. reduced ejection fraction in a prospective international multi-ethnic cohort study. Eur Heart J. 2018;39:1770–1780. doi: 10.1093/eurheartj/ehy005. [DOI] [PubMed] [Google Scholar]
- 25.Shiga T., Suzuki A., Haruta S. Clinical characteristics of hospitalized heart failure patients with preserved, mid-range, and reduced ejection fractions in Japan. ESC Heart Fail. 2019;6:475–486. doi: 10.1002/ehf2.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalogeropoulos A.P., Fonarow G.C., Georgiopoulou V. Characteristics and outcomes of adult outpatients with heart failure and improved or recovered ejection fraction. JAMA Cardiol. 2016;1:510–518. doi: 10.1001/jamacardio.2016.1325. [DOI] [PubMed] [Google Scholar]
- 27.Nadruz W., West E., Santos M. Heart failure and midrange ejection fraction: implications of recovered ejection fraction for exercise tolerance and outcomes. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bozkurt B., Kovacs R., Harrington B. Joint HFSA/ACC/AHA Statement Addresses Concerns Re: Using RAAS Antagonists in COVID-19. J Card Fail. 2020;26:370. doi: 10.1016/j.cardfail.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y., Coats A.J.S., Zheng Z. Management of heart failure patients with COVID-19: a joint position paper of the Chinese Heart Failure Association & National Heart Failure Committee and the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020;22:941–956. doi: 10.1002/ejhf.1915. [DOI] [PubMed] [Google Scholar]
- 31.Cox Z.L., Lai P., Lindenfeld J.A. Decreases in acute heart failure hospitalizations during COVID-19. Eur J Heart Fail. 2020;22:1045–1046. doi: 10.1002/ejhf.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall M.E., Vaduganathan M., Khan M.S. Reductions in heart failure hospitalizations during the COVID-19 pandemic. J Card Fail. 2020;26:462–463. doi: 10.1016/j.cardfail.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McIlvennan C.K., Allen L.A., DeVore A.D., Granger C.B., Kaltenbach L.A., Granger B.B. Changes in care delivery for patients with heart failure during the COVID-19 pandemic: results of a multicenter survey. J Card Fail. 2020;26:635–636. doi: 10.1016/j.cardfail.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bromage D.I., Cannatà A., Rind I.A. The impact of COVID-19 on heart failure hospitalization and management: report from a heart failure unit in London during the peak of the pandemic. Eur J Heart Fail. 2020;22:978–984. doi: 10.1002/ejhf.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorodeski E.Z., Goyal P., Cox Z.L. Virtual visits for care of patients with heart failure in the era of COVID-19: a statement from the Heart Failure Society of America. J Card Fail. 2020;26:448–456. doi: 10.1016/j.cardfail.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abraham W.T., Fiuzat M., Psotka M.A., O’Connor C.M. Heart failure collaboratory statement on heart failure remote monitoring in the landscape of COVID-19 and social distancing. J Am Coll Cardiol HF. 2020;8:692–694. doi: 10.1016/j.jchf.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Food and Drug Administration Enforcement policy for non-invasive remote monitoring devices used to support patient monitoring during the coronavirus disease 2019 (COVID-19) public health emergency (Revised) https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-policy-non-invasive-remote-monitoring-devices-used-support-patient-monitoring-during Available at: Accessed October 20, 2020.
- 38.Virani S.A., Clarke B., Ducharme A. Optimizing access to heart failure care in Canada during the COVID-19 pandemic. Can J Cardiol. 2020;36:1148–1151. doi: 10.1016/j.cjca.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singhvi A., Barghash M., Lala A. Challenges in heart transplantation during COVID-19: a single center experience. J Heart Lung Transplant. 2020;39:894–903. doi: 10.1016/j.healun.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.RECOVERY Collaborative Group. Horby P., Lim W.S. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020 July 17 [E-pub ahead of print] [Google Scholar]
- 41.Paranjpe I., Fuster V., Lala A. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76:122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beigel J.H., Tomashek K.M., Dodd L.E. Remdesivir for the treatment of Covid-19 — preliminary report. N Engl J Med. 2020 May 20 doi: 10.1056/NEJMc2022236. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43.Murphy S.P., Kakkar R., McCarthy C.P., Januzzi J.L. Inflammation in heart failure: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:1324–1340. doi: 10.1016/j.jacc.2020.01.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.