Abstract
Coronavirus disease 2019 (COVID-19) is the most important global public health issue that we currently face. We aimed to explore the clinical features of patients with COVID-19 and compared them with those of hospitalized community-acquired pneumonia (CAP) patients caused by influenza virus during the same period.
From Jan 1, to Mar 4, 2020, patients with COVID-19 or CAP caused by influenza virus who were admitted to the First Affiliated Hospital of Xiamen University were consecutively screened for enrollment.
A total of 35 COVID-19 patients and 22 CAP patients caused by influenza virus were included in this study. Most of COVID-19 patients had characteristics of familial clustering (63%), however, in the other group, there was no similar finding. The percentages of patients with a high fever (the highest recorded temperature was ≥39.0°C; 11% vs 45% [COVID-19 vs CAP groups, respectively]), dyspnea (9% vs 59%), leukocytosis (3% vs 32%), elevated C-reactive protein concentrations (>10 mg/L, 48% vs 86%), elevated procalcitonin levels (>0.1 ng/ml, 15% vs 73%), PaO2/FiO2 <200 mm Hg (4% vs 22%), and infiltration on imaging (29% vs 68%) in the COVID-19 group were less than those same indices in the hospitalized CAP patients caused by influenza virus. Ground-glass opacity with reticular pattern (63%) and interlobular septal thickening (71%) in chest CT were commonly observed in the COVID-19 group.
COVID-19 and CAP caused by influenza virus appear to share some similarities in clinical manifestaions but they definitely have major distinctions. Influenza infection remains a health problem even during COVID-19 pandemic.
Keywords: community-acquired pneumonia, COVID-19, influenza, severe acute respiratory syndrome coronavirus 2
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection—which causes coronavirus disease 2019 (COVID-19)[1] —is now the most important public health issue globally. As of Sep 19, 2020, there were over 30,369,000 confirmed cases and 948,000 deaths worldwide, and the disease is found in almost all countries and regions.[1]
On Mar 11, 2020, the World Health Organization publicly characterized COVID-19 as a pandemic. In the past century, there have been 4 pandemics caused by novel influenza viruses, but this is the first pandemic known to be caused by the emergence of a new coronavirus.
Patients with COVID-19 have respiratory symptoms and occasional gastrointestinal symptoms, including fever, cough, shortness of breath, and muscle aches[2] —symptoms similar to those of influenza or pneumonia. However, at the present time, the differences between COVID-19 and community-acquired pneumonia (CAP) caused by influenza virus have rarely been reported. In the present study, we explored the clinical features of patients with COVID-19 and compared them with those of hospitalized CAP patients caused by influenza virus during the same period in Xiamen city.
2. Methods
2.1. Study design
The study was a cross-sectional retrospective study conducted at the First Affiliated Hospital of Xiamen University, which is the only designated hospital for the treatment of COVID-19 in Xiamen City, China. From Jan 1, to Mar 4, 2020, patients with COVID-19 or CAP admitted to this hospital were consecutively screened for enrollment. The study protocol was in compliance with the Declaration of Helsinki and was approved by the Ethics Committee of the First Affiliated Hospital of Xiamen University, and written informed consent was waived.
2.2. Study population
Patients were enrolled if they were
-
1.
over 14 years of age and
-
2.
diagnosed with COVID-19 or CAP caused by influenza virus.
CAP was diagnosed based upon the Infectious Diseases Society of America/American Thoracic Society consensus guidelines of 2007.[3]
Patients were excluded if they presented with a history of immunocompromised conditions—including hematologic malignancies, cancer with neutropenia (absolute neutrophil count <500/mm3), solid malignancies undergoing chemotherapy in the previous 3 months, human immunodeficiency virus infection, or solid organ or bone-marrow transplantation—or with a history of using immunosuppressive agents or systemic corticosteroids.
2.3. Pathogen detection
We obtained throat-swabs or nasopharyngeal specimen swabs from the upper respiratory tract from all the patients. SARS-CoV-2 infection was confirmed by real-time quantitative polymerase chain reaction (RT-PCR) using a previously described protocol.[2] RT-PCR detection reagents were provided by the Chinese Center for Disease Control and Prevention. Influenza infection was diagnosed by positive RT-PCR results in the central laboratory of the hospital using a Multiplex RV Detection Kit (Health Gene Technologies Co., Ltd, China).
2.4. Procedures
Epidemiologic data, demographic data, signs/symptoms, laboratory findings, initial radiographic findings, treatments, and clinical outcomes were obtained from patients electronic medical records. Clinical outcomes were followed up to Mar 4, 2020, when all patients with confirmed COVID-19 were discharged. If data were missing from the medical records, they were obtained by communicating with attending doctors directly.
The globally accepted pneumonia-severity scoring system—the pneumonia severity index (PSI)—was used to assess pneumonia severity.[4] In addition, based on the Guidelines for the Diagnosis and Treatment of Novel Coronavirus (2019-nCoV) Infection by the National Health Commission (Trial Version 5) of China,[5] we classified COVID-19 into 4 types:
-
1.
Mild,
-
2.
Moderate,
-
3.
Severe,
-
4.
Critical.
2.5. Statistical analyses
All statistical analyses were performed using SPSS 22.0 (IBM, Armonk, NY). Normally distributed continuous variables are described as means ± standard deviation (SD), and non-normally distributed continuous variables are reported as medians (interquartile range, IQR); categorical data are presented as number (percentage), unless otherwise indicated. We used a paired Student t test for comparisons between normally distributed continuous variables, a Mann–Whitney U test for comparisons between non-normally distributed continuous variables, and the χ2 or Fisher exact test for categorical data. A 2-sided P value <.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics
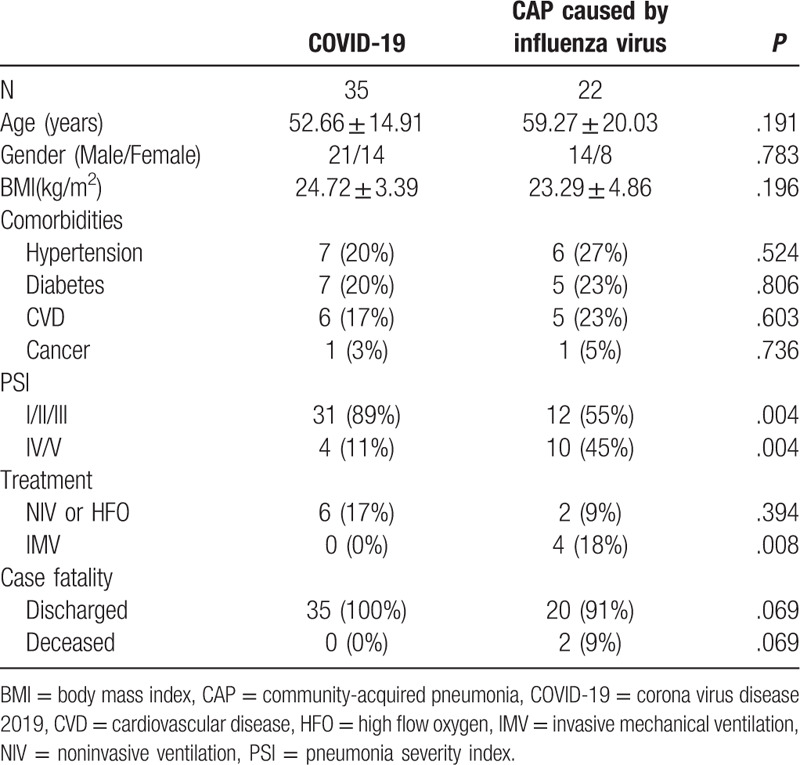
A total of 35 patients with COVID-19 and 22 CAP patients caused by influenza virus were included in our study. In the COVID-19 group, 2 patients (6%) exhibited the mild type, 26 (74%) the moderate type, 5 (14%) the severe type, and the remaining 2 patients (6%) manifested the critical type. Of the 35 patients, 22 (63%) had a history of travel or residence in Hubei Province 2 weeks before hospitalization, and we observed 8 groups of 22 familial clustering cases. In the CAP group, 16 cases (73%) were infected with H1N1 and 6 cases (27%) with H3N2, and there were no familial clustering cases. Baseline characteristics of the study population are described in Table 1. The subjects in the 2 groups were similar with regard to age, sex, body mass index, and comorbidities, with hypertension the most commonly observed comorbidity, followed by diabetes and cardiovascular disease. Concerning the PSI score, more patients in the COVID-19 group (89%) were graded as class I, II, or III, while in the CAP patients caused by influenza virus, more patients (89%) were graded as class IV/V.
Table 1.
Baseline characteristics, treatments and clinical outcomes of the study subjects.
3.2. Symptoms at admission
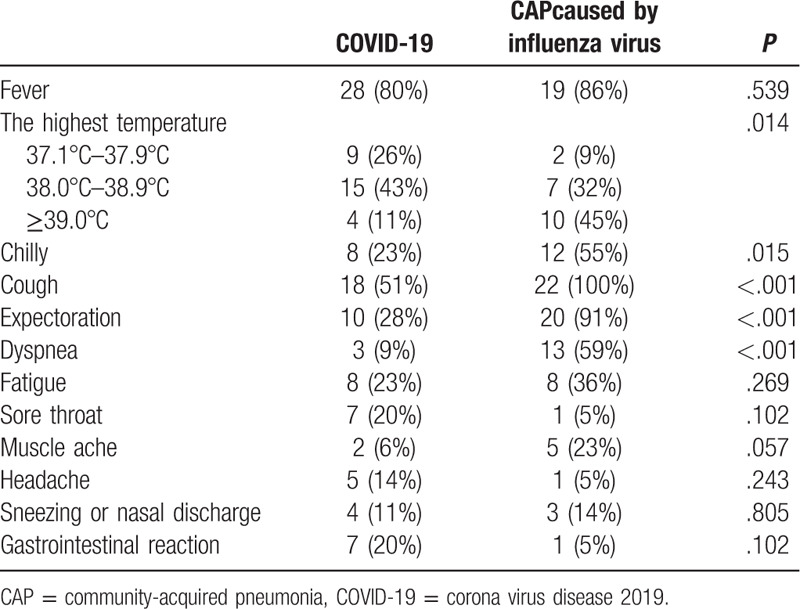
In the COVID-19 group, the median time from the first symptom to hospital admission was 2 (IQR 1–4) days. At admission, the most common signs/symptoms of patients with COVID-19 were fever (80%), cough (51%), expectoration (28%), fatigue (23%), and chills (23%). In the group of CAP patients caused by influenza virus, the most common signs/symptoms were cough (100%), expectoration (91%), fever (86%), dyspnea (59%), and chills (55%). Although fever was common to both groups, the percentage of patients with a high fever (i.e., their highest recorded temperature was ≥39.0°C) in the COVID-19 group was lower than in the group of CAP patients caused by influenza virus. Symptoms of coughing, expectoration, dyspnea, and chills were also less common in the COVID-19 group. Symptoms of the study subjects at admission are summarized in Table 2.
Table 2.
Symptoms of the study subjects at admission.
3.3. Laboratory findings at admission
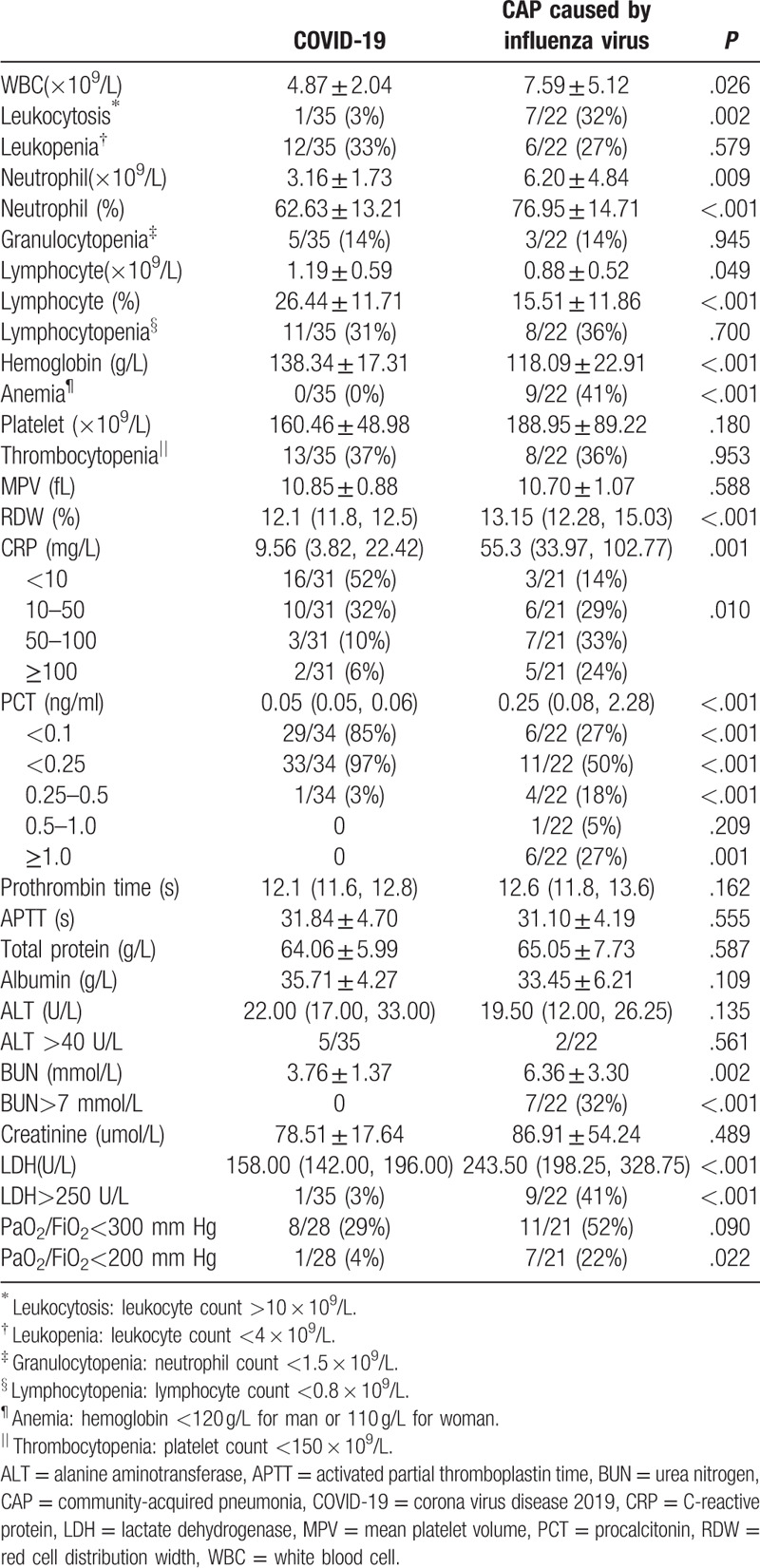
At admission, laboratory findings in the 2 groups were quite different. In terms of routine blood tests, patients with COVID-19 had lower levels of leukocytes and neutrophils—but higher levels of lymphocytes—compared to CAP patients caused by influenza virus. Leukocytosis (3% vs 32%, COVID-19 vs CAP groups, respectively) and anemia (0% vs 41%) were less commonly observed in the COVID-19 group relative to the CAP patients caused by influenza virus. We did not observe any differences in terms of leukopenia (33% vs 27%), granulocytopenia (14% vs 14%), lymphocytopenia (31% vs 36%), or thrombocytopenia (37% vs 36%) between the 2 groups.
In terms of inflammatory biomarkers, patients with COVID-19 had lower concentrations of C-reactive protein (CRP) and procalcitonin (PCT) compared to CAP patients caused by influenza virus. The percentages of patients with normal CRP (<10 mg/L) were 52% and 14%, while the percentages of patients with CRP greater than 100 mg/L were 6% and 24% for COVID-19 and CAP groups, respectively. The percentages of patients with normal PCT (<0.1 ng/ml) were 85% and 27%, while those of patients with PCT greater than 0.5 ng/ml were 0% and 32%.
Other laboratory findings were similar between the 2 groups, except that urea nitrogen >7 mmol/L, lactate dehydrogenase >250 U/L, and PaO2/FiO2 <200 mm Hg were more common in the group of CAP patients caused by influenza virus. Laboratory findings of the study subjects at admission are shown in Table 3.
Table 3.
Laboratory findings of the study subjects at admission.
3.4. Initial radiographic findings
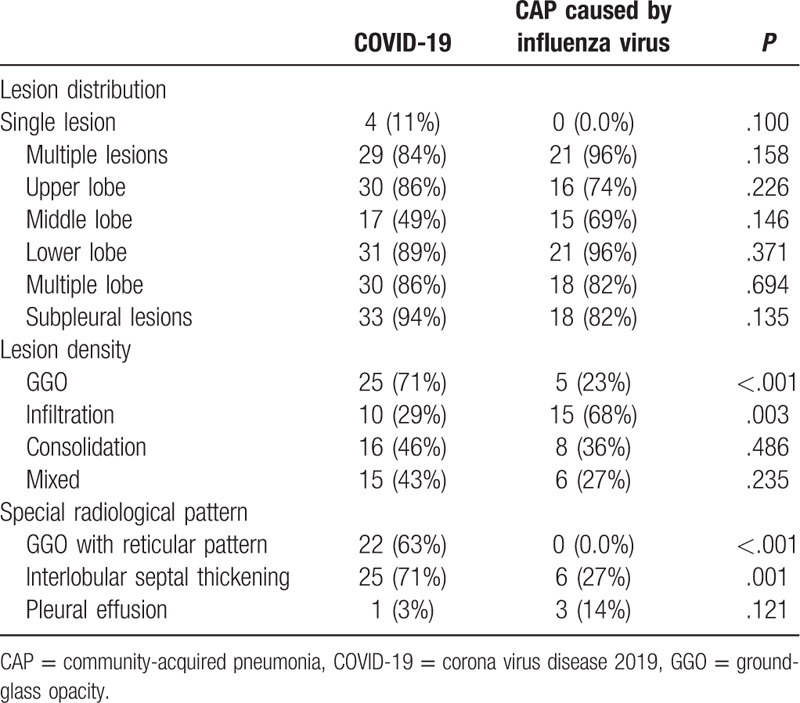
Initial radiographic findings of the study subjects are presented in Table 4. Although 2 patients (5.7%) with COVID-19 had normal initial chest CT findings upon admission, there was no difference in terms of lesion distribution between the 2 groups. Regarding lesion density, ground-glass opacity (GGO) was more common in the COVID-19 group (71%), while infiltration was more common in the group of CAP patients caused by influenza virus (68%). GGO with a reticular pattern (63%) and interlobular septal thickening (71%) were also commonly observed in the COVID-19 group.
Table 4.
Initial radiographic findings of the study subjects.
3.5. Treatments and clinical outcomes
In the COVID-19 group, 8 patients (23%) were confirmed or suspected secondary bacterial pneumonia and received antimicrobial treatment, while in the group of CAP caused by influenza virus, 21 patients (95%) received antimicrobial treatment. In the COVID-19 group, 6 patients (17%) received noninvasive ventilation (NIV) or high-flow oxygen (HFO), but none received invasive mechanical ventilation (IMV), while in the group of CAP caused by influenza virus, more patients (18%) received IMV. Additionally, no patient in either group required extracorporeal membrane oxygenation (ECMO). As of 4 Mar, all patients were discharged, except for 2 CAP patients caused by influenza virus who are deceased.
4. Discussion
COVID-19 constitutes an emerging and rapidly evolving set of circumstances, while influenza virus is the most frequently detected virus (28.4%) in non-immunocompromised, community-acquired pneumonia.[6] Meanwhile, patients with COVID-19 have symptoms similar to those of CAP caused by influenza virus. It is thus important to fully recognize and differentiate these 2 diseases in pandemic season. To the best of our knowledge, ours is the first preliminary study to describe the differences in clinical, laboratory, and radiological characteristics between COVID-19 and hospitalized CAP caused by influenza virus in the same city during the same period. It shows that COVID-19 and CAP caused by influenza share some resemblances but they definitely have major distinctions.
In this study, most of the COVID-19 patients had a characteristic of familial clustering; however, in the hospitalized CAP patients caused by influenza virus, we observed no similar finding, suggesting that COVID-19 has a higher transmissibility. Several investigators have recently estimated R0 ranges between 1.4 and 6.49, with a mean of 3.28, indicating a higher transmissibility than seasonal influenza, and even higher than severe acute respiratory syndrome.[7]
The percentages of patients with a high fever (i.e., their highest recorded temperature was ≥39.0°C; 11% vs 45% [COVID-19 vs CAP groups, respectively]), dyspnea (9% vs 59%), leukocytosis (3% vs 32%), elevated CRP concentrations (>10 mg/L, 48% vs 86%), elevated PCT (>0.1 ng/ml, 15% vs 73%), PaO2/FiO2 <200 mm Hg (4% vs 22%), and infiltration on imaging (29% vs 68%) in the COVID-19 group were less than those same indices in the hospitalized CAP patients caused by influenza virus. All of these parameters helped us to distinguish COVID-19 from CAP caused by influenza virus, in addition to molecular identification by RT-PCR.
There are several possible reasons for the differences in clinical, laboratory, and radiological characteristics between the 2 groups. First, the induced cytokine profiles and respiratory airway injury as the virus spread into the bronchial epithelium might vary with different pathogens. Second, bacterial co-infection may play a role in disease severity and mortality, which has been reported in patients with viral infections.[8] However, it was impossible to confirm all concomitant bacterial infections in our study due to limitations in traditional methods and high rates of pre-sampling antibiotic use. According to a previous study, a procalcitonin threshold of 0.1 ng/ml resulted in 80.9% (95% CI, 75.3%–85.7%) sensitivity and 51.6% (95% CI, 46.6%–56.5%) specificity for identification of any bacterial pathogen.[9] In our study, the percentages of patients with abnormal PCT (≥0.1 ng/ml) in the 2 groups were 15% and 73% for COVID and CAP groups, respectively, suggesting that different rates of bacterial co-infection may lead to different characteristics in the 2 groups. Third, all COVID-19 cases in Xiamen City were hospitalized, whereas mild-CAP patients caused by influenza virus may have been treated in the outpatient clinic—potentially leading to different disease severities in the 2 groups.
It is necessary to call attention to the fact that influenza infection remains a major health problem even during this period of high SARS-CoV-2 activity. In the group of CAP patients caused by influenza virus in the present study, the percentage of patients requiring NIV/HFO/ECMO was 27%, and the mortality rate was 9%, both of which indicated that even after active treatments, serious disease progression still occurred—consistent with previous reports.[6,10] More attention should therefore be paid to the importance of viruses as pathogens in CAP. It is also noteworthy that in areas where conventional virologic diagnostic techniques were used, the role of viruses as pneumonia pathogens has most likely been underestimated. Fortunately, the availability of molecular technologies has greatly facilitated the identification of respiratory viruses in patients with pneumonia.
Tolksdorf et al. assessed disease seriousness of COVID-19 cases in China and in German pneumonia patients between 2015 and 2019,[7] and they demonstrated a higher rate of COVID-19 patients requiring ventilation and more case fatalities among COVID-19 patients without comorbidities than in the reference cohort. Although this highlighted the importance of preparing more ventilators than usual, given the different population structures, comorbidities, onset seasons, and medical resources, these results require further verification. Therefore, additional studies with more comparable background cohorts are needed. As our study included 2 groups of patients from the same center during the same time period, we expected a reduction in the aforementioned bias.
In this study, the symptoms, laboratory findings at admission, and initial radiographic findings of COVID-19 were generally consistent with previous publications from regions outside Hubei.[11,12] Patients with COVID-19 in Xiamen had milder infections than those reported from Hubei,[2,13] as none with COVID-19 in Xiamen needed IMV or ECMO, and all recovered. In fact, the rate of IMV/ECMO use was 15.2%, and overall mortality was 4.3% in a case series of 138 hospitalized patients with COVID-19 in Hubei.[13] The fact that case series from outside Hubei showed much milder disease severities than those reported from Hubei province may be due to treating COVID-19 has become the top priority since Jan 15, 2020 in all over the China. One of the proofs was that the median time from the first symptom to hospital admission of COVID-19 patients in Xiamen was 2 days, far less than the 7 days reported for Hubei province.[13] In addition, the significant impact on the medical systems in the initially affected areas resulted in insufficient allocation of healthcare resources, possibly inhibiting adequate treatment as indicated by the strikingly high case fatality rate in Hubei.[2,13]
There were some limitations to our study. First, our data emerged from a single center during the early phase of COVID-19 pandemic with a limited number of patients and might therefore not reflect the entire spectrum of the 2 diseases. Second, we could not distinguish viruses by nasopharyngeal swabs or throat swabs as coming from lower or upper respiratory infections. Therefore, in the CAP group, only patients with radiologically confirmed pneumonia were enrolled. However, all COVID-19 phenotypes (including those with mild infections and even without pneumonia) were analyzed and compared with more severe phenotypes of influenza infection (those with pneumonia). Third, immunosuppressed patients were not included in our study, so the results cannot be generalized to that patient subset. Last but not least, the role of bacterial co-infection was not investigated in this study. All of these factors could be potential confounders that may produce significant bias.
5. Conclusions
Despite our studys retrospective nature, we described comprehensively the characteristics of COVID-19 and CAP caused by influenza virus from the same center during the identical time-period, including demographic, clinical, laboratory, and radiologic characteristics and clinical outcomes. COVID-19 and CAP caused by influenza virus appear to share some similarities in clinical manifestaions but they definitely have major distinctions. Influenza infection remains a health problem even during COVID-19 pandemic. Routine testing for newly emergent viruses may thus be warranted for patients who have been hospitalized with CAP.
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript. We thank the Science and Technology Bureau in Xiamen for its financial support.
Author contributions
Conceptualization: Yi-Hua Lin, Wen Luo, Ding-Hui Wu, Zhan-Xiang Wang, Yonghong Shi.
Data curation: Yi-Hua Lin, Wen Luo, Ding-Hui Wu.
Formal analysis: Yi-Hua Lin, Wen Luo.
Investigation: Fang Lu, Su-Xian Hu, Xiang-Yang Yao.
Methodology: Ding-Hui Wu, Fang Lu, Su-Xian Hu, Xiang-Yang Yao.
Project administration: Zhan-Xiang Wang, Yonghong Shi.
Software: Fang Lu, Su-Xian Hu.
Supervision: Xiang-Yang Yao.
Validation: Xiang-Yang Yao, Zhan-Xiang Wang, Yonghong Shi.
Writing – original draft: Yi-Hua Lin, Wen Luo, Ding-Hui Wu.
Writing – review & editing: Zhan-Xiang Wang, Yonghong Shi.
Footnotes
Abbreviations: CAP = community-acquired pneumonia, COVID-19 = coronavirus disease 2019, CRP = C-reactive protein, ECMO = extracorporeal membrane oxygenation, GGO = ground-glass opacity, HFO = high-flow oxygen, IMV = invasive mechanical ventilation, NIV = noninvasive ventilation, PCT = procalcitonin, RT-PCR = real-time quantitative polymerase chain reaction, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
How to cite this article: Lin YH, Luo W, Wu DH, Lu F, Hu SX, Yao XY, Wang ZX, Shi YH. Comparison of clinical, laboratory, and radiological characteristics between SARS-CoV-2 infection and community-acquired pneumonia caused by influenza virus: a cross-sectional retrospective study. Medicine. 2020;99:44(e23064).
YL, WL, and DW contributed equally to this work.
This study was supported by COVID-19 project (3502Z2020YJ05) of the Science and Technology Bureau in Xiamen, China.
The authors have no conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].WHO. Novel Coronavirus (COVID-19) Situation dashboard. 2020 [Available from: (2020-09-19) https://www.who.int/ [Google Scholar]
- [2].Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44: Suppl 2: S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997;336:243–50. [DOI] [PubMed] [Google Scholar]
- [5].Lin L, Li TS. Interpretation of “Guidelines for the Diagnosis and Treatment of Novel Coronavirus (2019-nCoV) Infection by the National Health Commission (Trial Version 5)”. Zhonghua Yi Xue Za Zhi 2020;100:E001. [DOI] [PubMed] [Google Scholar]
- [6].Zhou F, Wang Y, Liu Y, et al. Disease severity and clinical outcomes of community-acquired pneumonia caused by non-influenza respiratory viruses in adults: a multicentre prospective registry study from the CAP-China Network. Eur Respir J 2019;54:pii: 1802406. [DOI] [PubMed] [Google Scholar]
- [7].Tolksdorf K, Buda S, Schuler E, et al. Influenza-associated pneumonia as reference to assess seriousness of coronavirus disease (COVID-19). Euro Surveill 2020;25:2000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Voiriot G, Visseaux B, Cohen J, et al. Viral-bacterial coinfection affects the presentation and alters the prognosis of severe community-acquired pneumonia. Crit Care 2016;20:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Self WH, Balk RA, Grijalva CG, et al. Procalcitonin as a Marker of Etiology in Adults Hospitalized With Community-Acquired Pneumonia. Clin Infect Dis 2017;65:183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Al-Baadani AM, Elzein FE, Alhemyadi SA, et al. Characteristics and outcome of viral pneumonia caused by influenza and Middle East respiratory syndrome-coronavirus infections: a 4-year experience from a tertiary care center. Ann Thorac Med 2019;14:179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yang W, Cao Q, Qin L, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect 2020;80:388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tian S, Hu N, Lou J, et al. Characteristics of COVID-19 infection in Beijing. J Infect 2020;80:401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020;323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


